The Effect of Farming Systems and Cultivars on the Qualitative and Quantitative Composition of Bioactive Compounds in Winter Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Results and Discussion
2.1. Results of the Organic Farming System
2.1.1. Biometric Analyses, Grain Yield, Evaluation of Plant Infestation Using Pathogen and Fusarium spp. Occurrence
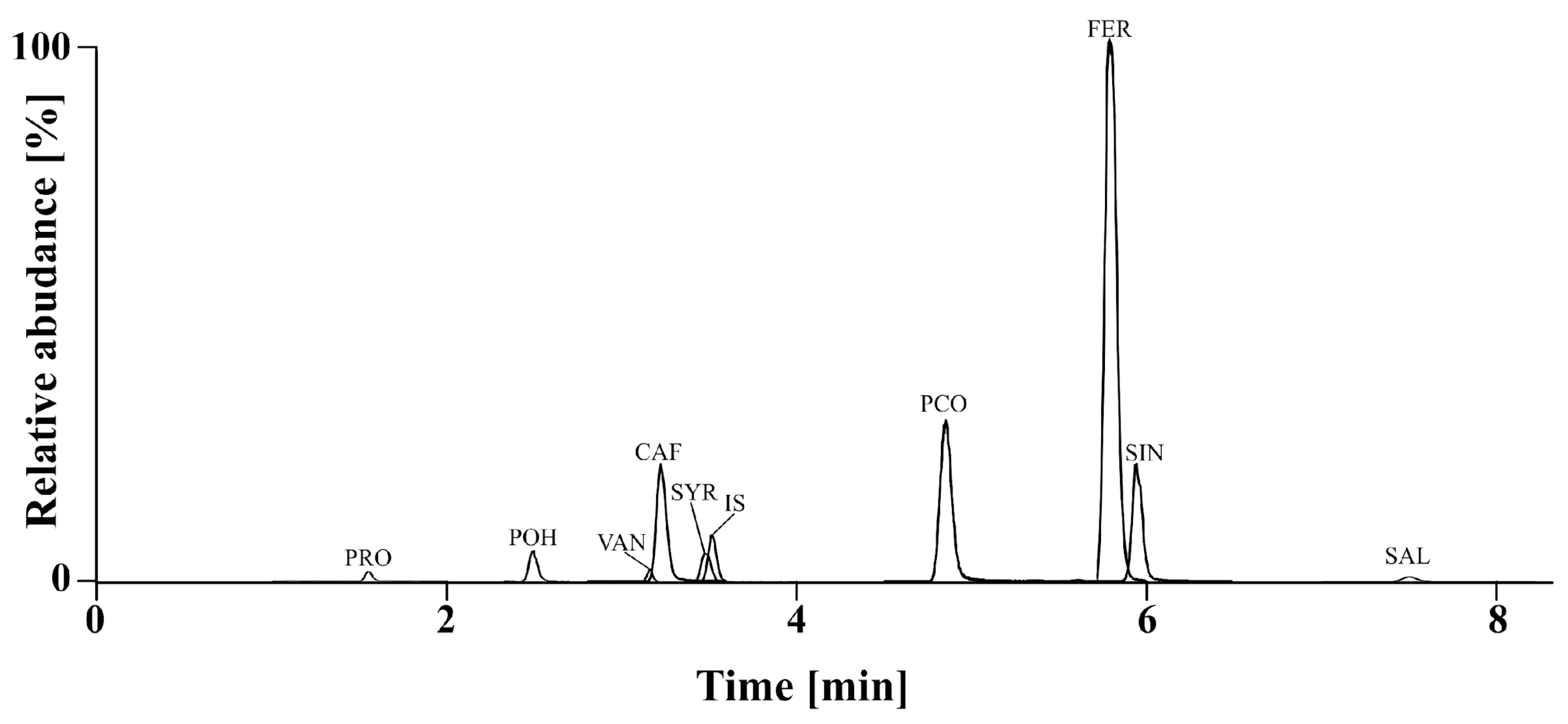
2.1.2. Chemical Analysis of Phenolic Acids (PAs)
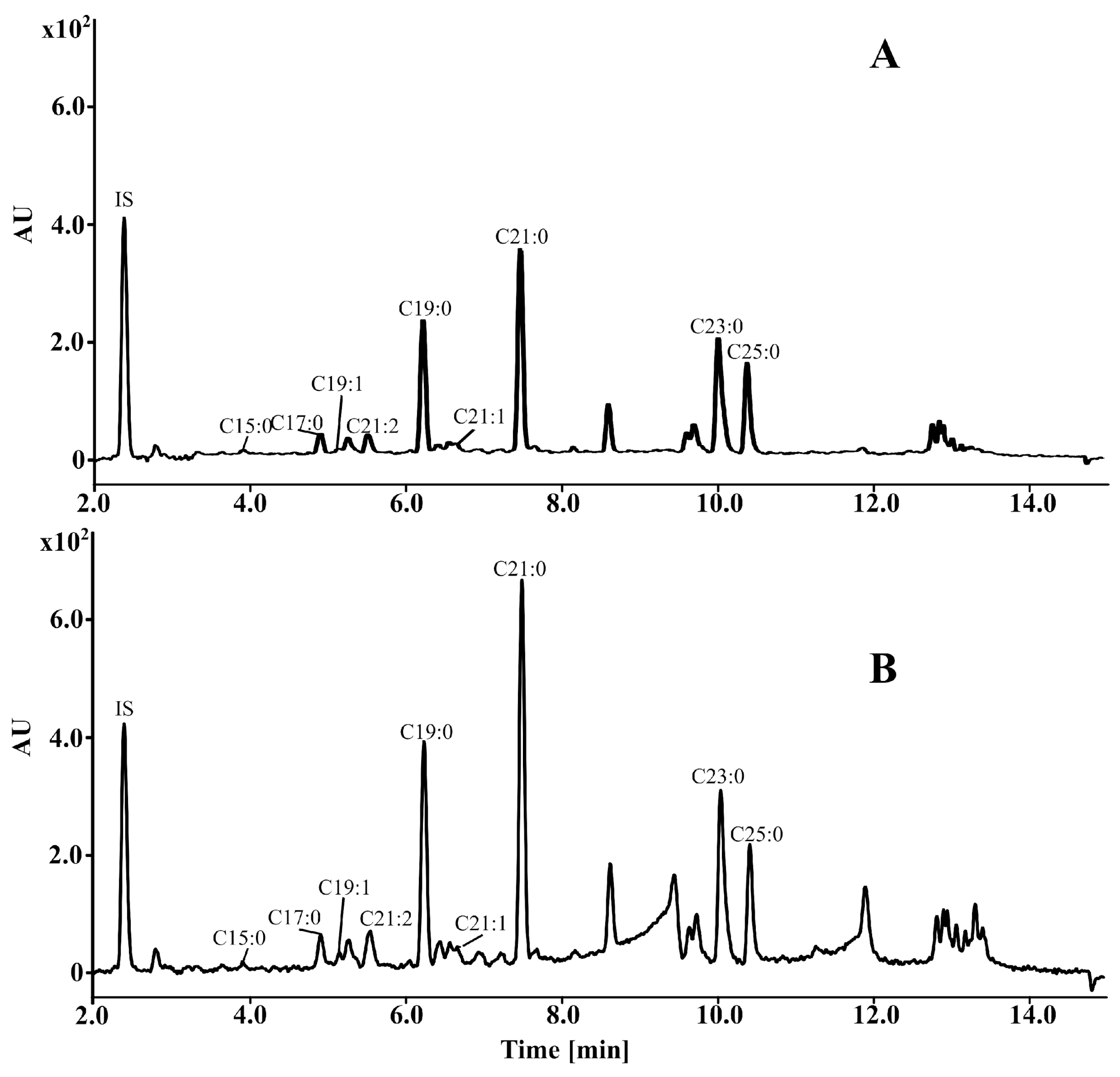
2.1.3. Identification, Quantification, and Biological Activity of Alkylresorcinols (ARs, Resorcinolic Lipids)
2.2. Comparison of Four Different Farming Systems
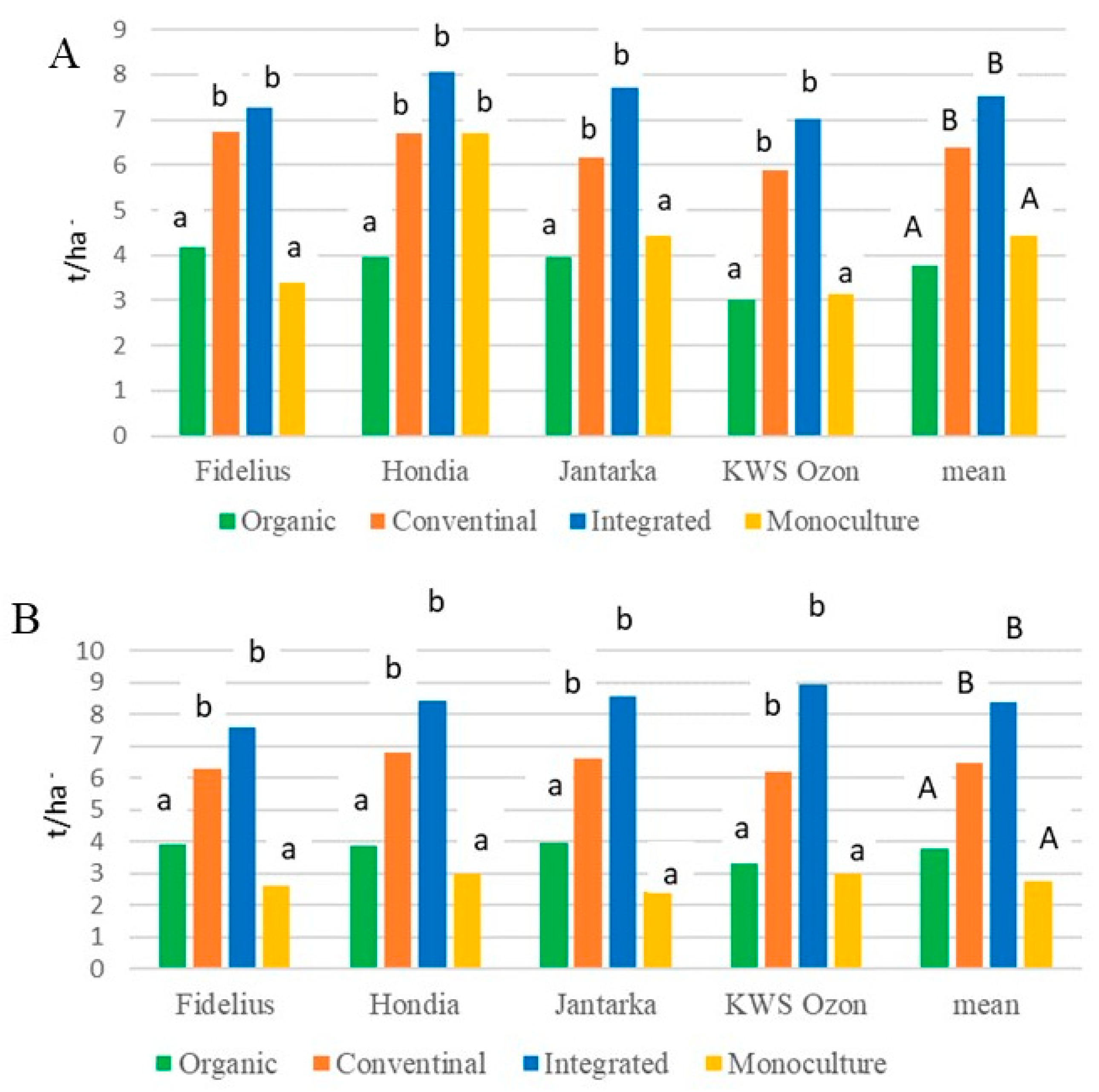
2.2.1. Grain Yield, Ear Density, Thousand-Grain Mass, and Colonization of Grain by Fungi
2.2.2. Qualitative and Quantitative Analyses and Antioxidant Activity of PA Extracts
2.2.3. Characterization, Quantification, and Antiradical Activity of AR Extracts
3. Materials and Methods
3.1. Plant Material
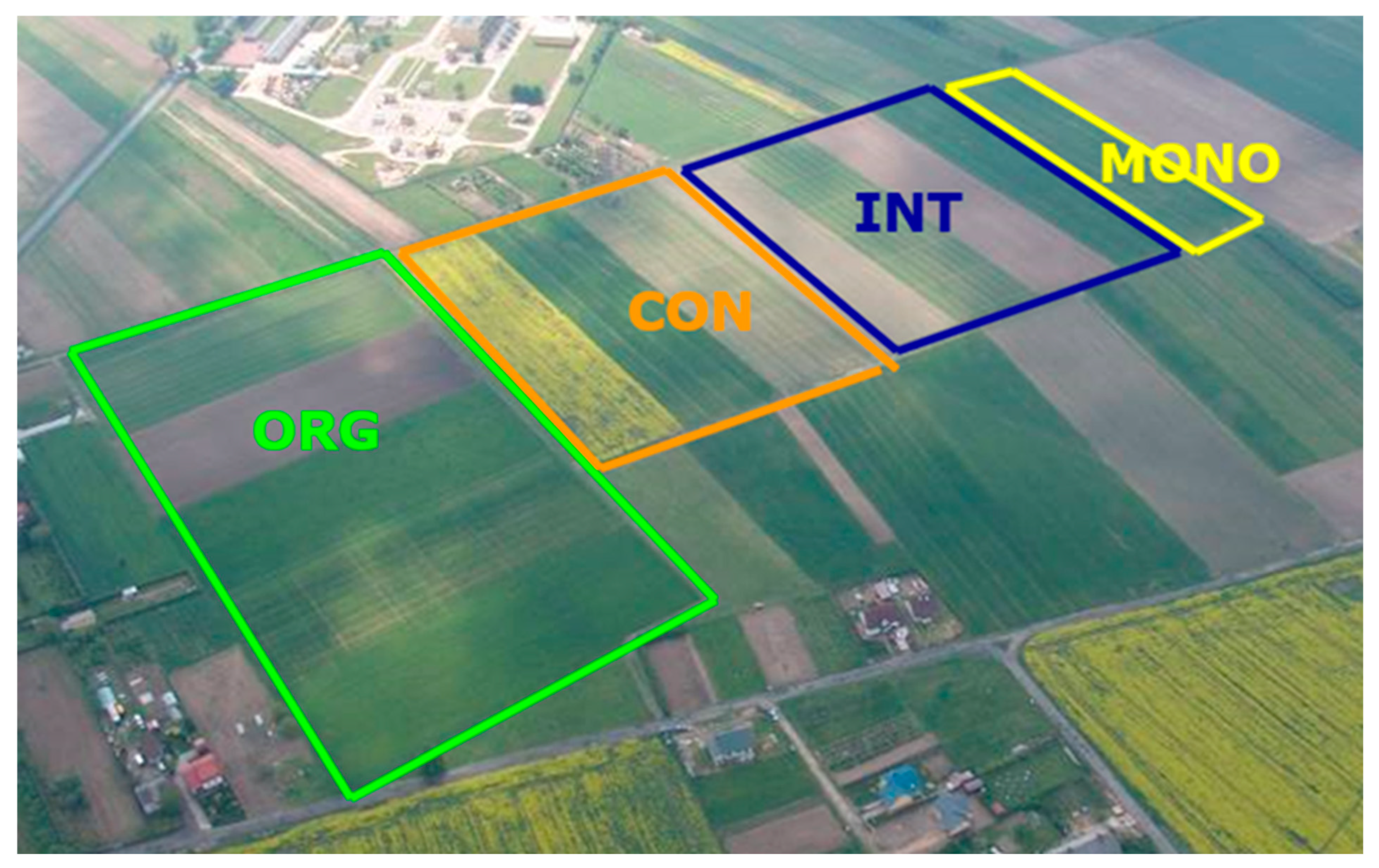
3.2. Characteristics of the Experimental Plots Under Different Farming Systems
3.3. Meteorological Conditions
3.4. Biometric Analyses, Grain Yield, and Assessment of Plant Infestation by Pathogens
3.5. Assessment of Fusarium spp. Occurrence
3.6. Reagents
3.7. Phenolic Acid Analysis
3.7.1. Extraction of PAs from Winter Wheat Cultivars
3.7.2. UPLC Conditions for Quantitative Analysis of PAs
3.7.3. Quantification of Individual PAs
3.7.4. Antiradical Activity of PA Extracts
3.8. Alkylresorcinol Analysis
3.8.1. Extraction of ARs from Winter Wheat
3.8.2. UPLC Conditions for Quantitative Analysis
3.8.3. Quantification of Individual ARs
3.8.4. Antioxidant Activity of ARs Extracts
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Buczek, J.; Jańczak-Pieniążek, M.; Harasim, E.; Kwiatkowski, C.A.; Kapusta, I. Effect of cropping systems and environment on phenolic acid profiles and yielding of hybrid winter wheat genotypes. Agriculture 2023, 13, 834. [Google Scholar] [CrossRef]
- Górska-Warsewicz, H.; Rejman, K.; Ganczewski, G.; Kwiatkowski, B. Economic importance of nutritional and healthy cereals and/or cereal products. In Developing Sustainable and Health Promoting Cereals and Pseudocereals; Academic Press: Cambridge, MA, USA, 2023; pp. 433–450. [Google Scholar]
- Czaban, J.; Sułek, A.; Pecio, Ł.; Żuchowski, J.; Podolska, G. Effect of genotype and crop management systems on phenolic acid content in winter wheat grain. J. Food Agric. Environ. 2013, 11, 1201–1206. [Google Scholar]
- Mason, H.E.; Spaner, D. Competitive ability of wheat in conventional and organic management systems: A review of the literature. Can. J. Plant Sci. 2006, 86, 333–343. [Google Scholar] [CrossRef]
- Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Gródek-Szostak, Z.; Kapusta-Duch, J.; Kuboń, M.; Komorowska, M.; Karcz, J. Impact of integrated and conventional plant production on selected soil parameters in carrot production. Sustainability 2019, 11, 5612. [Google Scholar] [CrossRef]
- Korbas, M.; Horoszkiewicz-Janka, J.; Mrówczyński, M.; Roślin, I.O. (Eds.) Metodyka Integrowanej Ochrony Pszenicy Ozimej i Jarej Dla Doradców: Opracowanie Zbiorowe; OIR-PIB: Poznań, Poland, 2017. [Google Scholar]
- Haliniarz, M.; Nowak, A.; Woźniak, A.; Sekutowski, T.R.; Kwiatkowski, C.A. Production and Economic Effects of Environmentally Friendly Spring Wheat Production Technology. Pol. J. Environ. Stud. 2018, 27, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Soane, B.D.; Ball, B.C.; Arvidsson, J.; Basch, G.; Moreno, F.; Roger-Estrade, J. No-till in northern, western and south-western Europe: A review of problems and opportunities for crop production and the environment. Soil Tillage Res. 2012, 118, 66–87. [Google Scholar] [CrossRef]
- Sieling, K.; Stahl, C.; Winkelmann, C.; Christen, O. Growth and yield of winter wheat in the first 3 years of a monoculture under varying N fertilization in NW Germany. Eur. J. Agron. 2005, 22, 71–84. [Google Scholar] [CrossRef]
- Kowalska, I.; Pawelec, S.; Pecio, Ł.; Feledyn-Szewczyk, B. The Effects of a Cultivar and Production System on the Qualitative and Quantitative Composition of Bioactive Compounds in Spring Wheat (Triticum sp.). Molecules 2024, 29, 4106. [Google Scholar] [CrossRef] [PubMed]
- Durham, T.C.; Mizik, T. Comparative economics of conventional, organic, and alternative agricultural production systems. Economies 2021, 9, 64. [Google Scholar] [CrossRef]
- Augšpole, I.; Liniņa, A.; Cinkmanis, I.; Vucāne, S. Phenolic Compounds in Organic and Conventional Winter Wheat (L.) Wholemeal. In Proceedings of the Latvian Academy of Sciences. Sect. B Nat. Ex. Appl. Sci. 2021, 75, 444–448. [Google Scholar]
- Jaśkiewicz, B.; Szczepanek, M. Crop management and variety have influence on alkylresolcinol content in triticale grain. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2016, 66, 570–574. [Google Scholar] [CrossRef]
- Kowalska, I.; Pecio, L.; Ciesla, L.; Oleszek, W.; Stochmal, A. Isolation, chemical characterization, and free radical scavenging activity of phenolics from Triticum aestivum L. aerial parts. J. Agric. Food Chem. 2014, 62, 11200–11208. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Vieites-Álvarez, Y.; Otero, P.; Prieto, M.A.; Simal-Gandara, J.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Weed pressure determines the chemical profile of wheat (Triticum aestivum L.) and its allelochemicals potential. Pest Manag. Sci. 2022, 78, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.K.; Hanafi, R.S.; Choucry, M.A.; Handoussa, H. Structural, functional, nutritional composition and analytical profiling of Triticum aestivum L. Appl. Biol. Chem. 2023, 66, 48. [Google Scholar] [CrossRef]
- Stracke, B.A.; Eitel, J.; Watzl, B.; Mäder, P.; Rüfer, C.E. Influence of the production method on phytochemical concentrations in whole wheat (Triticum aestivum L.): A comparative study. J. Agric. Food Chem. 2009, 57, 10116–10121. [Google Scholar] [CrossRef] [PubMed]
- Mwendwa, J.M.; Weston, P.A.; Weidenhamer, J.D.; Fomsgaard, I.S.; Wu, H.; Gurusinghe, S.; Weston, L.A. Metabolic profiling of benzoxazinoids in the roots and rhizosphere of commercial winter wheat genotypes. Plant Soil. 2021, 466, 467–489. [Google Scholar] [CrossRef]
- Żuchowski, J.; Jończyk, K.; Pecio, Ł.; Oleszek, W. Phenolic acid concentrations in organically and conventionally cultivated spring and winter wheat. J. Sci. Food Agric. 2011, 91, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acids in wheat varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Nawracała, J.; Tomkowiak, A.; Perkowski, J. Phenolic acid content in wheat grain (Triticum spp.) of different genotypes. Rev. Fac. Cienc. Agrar. UNCuyo 2016, 48, 1–7. [Google Scholar]
- Moore, J.; Hao, Z.; Zhou, K.; Luther, M.; Costa, J.; Yu, L. Carotenoid, tocopherol, phenolic acid and antioxidant properties of Maryland-grown soft wheat. J. Agric. Food Chem. 2005, 53, 6649–6657. [Google Scholar] [CrossRef]
- Tian, W.; Wilson, T.L.; Chen, G.; Guttieri, M.J.; Nelson, N.O.; Fritz, A.; Smith, G.; Li, Y. Effects of environment, nitrogen, and sulfur on total phenolic content and phenolic acid composition of winter wheat grain. Cereal Chem. 2021, 98, 903–911. [Google Scholar] [CrossRef]
- Gasztonyi, M.N.; Farkas, R.T.; Berki, M.; Petróczi, I.M.; Daood, H.G. Content of phenols in wheat as affected by varietal and agricultural factors. J. Food Comp. Anal. 2011, 24, 785–789. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Li, Y.; Wang, C.; Xie, Y.; Guo, T. Effect of nitrogen fertilisation and irrigation on phenolic content, phenolic acid composition, and antioxidant activity of winter wheat grain. J. Sci. Food Agric. 2015, 95, 1039–1046. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.S. Antioxidant and antiproliferative activities in immature and mature wheat kernels. Food Chem. 2016, 196, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, B.; Turksoy, S.; Özkaya, H.; Baumgartner, B.; Özkeser, İ.; Köksel, H. Changes in the functional constituents and phytic acid contents of firiks produced from wheats at different maturation stages. Food Chem. 2018, 246, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Barros Santos, M.C.; Ribeiro da Silva Lima, L.; Ramos Nascimento, F.; Pimenta do Nascimento, T.; Cameron, L.C.; Simões Larraz Ferreira, M. Metabolomic approach for characterization of phenolic compounds in different wheat genotypes during grain development. Food Res. Int. 2019, 124, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, I.; Mołdoch, J.; Pawelec, S.; Podolska, G.; von Cossel, M.; Derycke, V.; Haesaert, G.; Lana, M.A.; da Silva Lopes, M.; Riche, A.B.; et al. Environmental and cultivar variability in composition, content and biological activity of phenolic acids and alkylresorcinols of winter wheat grains from a multi-site field trial across Europe. J. Cereal Sci. 2022, 107, 103527. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Stumpf, B.; Yan, F.; Honermeier, B. Influence of nitrogen fertilization on yield and phenolic compounds in wheat grains (Triticumm aestivum L. ssp. aestivum). J. Plant Nutr. Soil Sci. 2018, 182, 111–118. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Vanara, F.; Bhandari, D.; Bruni, R.; Spengler, B.; Blandino, M.; Righetti, L. 5-n-Alkylresorcinol Profiles in Different Cultivars of Einkorn, Emmer, Spelt, Common Wheat, and Tritordeum. J. Agric. Food Chem. 2021, 69, 14092–14102. [Google Scholar] [CrossRef]
- Skrajda-Brdak, M.; Konopka, I.; Tańska, M.; Sulewska, H. Phenolic nutrient composition and grain morphology of winter spelt wheat (Triticum aestivum ssp. spelta) cultivated in Poland. Qual. Assur. Saf. Crops Foods 2018, 10, 285–295. [Google Scholar] [CrossRef]
- L-Baeckstrom, G.; Hanell, U.; Svensson, G. Baking quality of winter wheat grown in different cultivating systems, 1992–2001: A holistic approach. J. Sustain. Agric. 2004, 24, 53–79. [Google Scholar] [CrossRef]
- Żuchowski, J.; Kapusta, I.; Szajwaj, B.; Jończyk, K.; Oleszek, W. Phenolic acid content of organic and conventionally grown winter wheat. Cereal Res. Commun. 2009, 37, 189–197. [Google Scholar] [CrossRef]
- Hildermann, I.; Thommen, A.; Dubois, D.; Boller, T.; Wiemken, A.; Mader, P. Yield and baking quality of winter wheat cultivars in different farming systems of the DOK long-term trial. J. Sci. Food Agric. 2009, 89, 2477–2491. [Google Scholar] [CrossRef]
- Mazzoncini, M.; Antichi, D.; Silvestri, N.; Ciantelli, G.; Sgherri, C. Organically vs conventionally grown winter wheat: Effects on grain yield, technological quality, and on phenolic composition and antioxidant properties of bran and refined flour. Food Chem. 2015, 175, 445–451. [Google Scholar] [CrossRef]
- Zrcková, M.; Capouchová, I.; Eliášová, M.; Paznocht, L.; Pazderu, K.; Dvorák, P.; Konvalina, P.; Orsák, M.; Šterba, Z. The effect of genotype, weather conditions and cropping system on antioxidant activity and content of selected antioxidant compounds in wheat with coloured grain. Plant Soil Environ. 2018, 64, 530–538. [Google Scholar] [CrossRef]
- Moitzi, G.; Neugschwandtner, R.W.; Kaul, H.-P.; Wagentristl, H. Efficiency of mineral nitrogen fertilization in winter wheat under Pannonian climate conditions. Agriculture 2020, 10, 541. [Google Scholar] [CrossRef]
- Kosik, T.; Lacko-Bartošová, M.; Kobida, L. Free phenol content and antioxidant activity of winter wheat in sustainable farming systems. J. Microbiol. Biotech. Food Sci. 2014, 3, 247–249. [Google Scholar]
- Ma, D.; Wang, C.; Feng, J.; Xu, B. Wheat grain phenolics: A review on composition, bioactivity, and influencing factors. J. Sci. Food Agric. 2021, 101, 6167–6185. [Google Scholar] [CrossRef]
- Rembiałkowska, E. Quality of plant products from organic agriculture. J. Sci. Food Agric. 2007, 87, 2757–2762. [Google Scholar] [CrossRef]
- Kowalska, I.; Jędrejek, D. Benzoxazinoid and alkylresorcinol content, and their antioxidant potential, in a grain of spring and winter wheat cultivated under different production systems. J. Cereal Sci. 2020, 95, 103063. [Google Scholar] [CrossRef]
- Kwaśna, H.; Chełkowski, J.; Zajkowski, P. Fungi, XII; Polish Academy of Sciences: Warsaw, Poland; Cracow, Poland, 1991; p. 136. [Google Scholar]
- Hill, T.; Lewicki, P. Statistics—Methods and Applications: A Comprehensive Reference for Science, Industry, and Data Mining, 1st ed.; StatSoft Inc.: Tulsa, OK, USA, 2006; p. 832. [Google Scholar]
| Phenolic Acid | Year | Cultivar | |||||
| 2017 | 2018 | Fidelius | Hondia | Jantarka | KWS Ozon | Arktis | |
| Protocatechuic acid | 5.89 ±0.14 a | 5.19 ±0.11 b | 5.90 ±0.25 b | 4.71 ±0.13 de | 4.92 ±0.22 de | 5.32 ±0.20 b–d | 5.57 ±0.16 bc |
| p-OH-Benzoic acid | 3.17 ±0.30 b | 3.35 ±0.24 a | 2.03 ±0.11 g | 2.15 ±0.09 fg | 2.11 ±0.10 fg | 2.73 ±0.09 ef | 2.20 ±0.12 e–g |
| Vanillic acid | 26.02 ±1.18 a | 24.37 ± 0.78 b | 24.13 ±0.68 c–e | 21.74 ±0.50 ef | 20.44 ±0.52 f | 24.73 ±0.71 cd | 23.09 ±0.65 c–e |
| Caffeic acid | 38.88 ±1.80 a | 33.98 ±0.89 b | 31.16 ±0.80 de | 26.83 ±0.6 e | 37.72 ±0.58 a–d | 44.97 ±8.46 a | 40.70 ±1.43 a–c |
| Syringic acid | 14.54 ±0.74 b | 14.98 ±0.67 a | 16.02 ±0.32 bc | 15.91 ±0.35 bc | 11.40 ±0.05 hi | 13.99 ±0.27 de | 11.13 ±0.22 i |
| p-Coumaric acid | 65.87a ±13.05 a | 57.04b ±7.89 b | 47.56 ±4.43 b | 34.28 a ±2.22 b | 37.51 ±2.38 b | 50.33 ±4.39 b | 36.07 ±1.24 b |
| Ferulic acid | 829.01 ±13.19 a | 826.28 ±14.78 a | 712.77 ±23.40 e | 757.30 ±17.41 de | 824.73 ±16.69 b–d | 851.01 ±23.60 a–c | 795.34 ±9.46 c–e |
| Sinapic acid | 44.61 ±1.32 b | 45.90 ±1.68 a | 38.08 ±0.84 fg | 39.50 ±0.80 ef | 57.40 ±1.14 b | 46.79 ±2.00 cd | 41.89 ±0.66 d–f |
| Salicylic acid | 1.63 ±0.02 b | 1.68 ±0.02 a | 1.54 ±0.01 e | 1.53 ±0.02 e | 1.55 ±0.02 e | 1.66 ±0.04 cd | 1.62 ±0.05 d |
| Total | 1029.63 ±24.49 a | 1012.78 ±19.40 a | 879.19 ±24.31 f | 903.96 ±20.54 ef | 997.78 ±20.34 c–e | 1041.53 ±25.13 b–d | 957.62 ±11.03 d–f |
| Antiradical acitvity | 0.220 ±0.005 a | 0.217 ±0.004 a | 0.188 ±0.005 f | 0.193 ±0.004 e | 0.213 ±0.004 c–e | 0.223 ±0.005 bc | 0.205 ±0.002 de |
| Phenolic Acid | Cultivar | ||||||
| Belissa | Estivus | Linus | Markiza | Ostka Strzelecka | Pokusa | Rokosz | |
| Protocatechuic acid | 6.77 ±0.13 a | 5.53 ±0.28 bc | 5.15 ±0.03 c–e | 5.53 ±0.14 bc | 5.68 ±0.28 bc | 4.64 ±0.11 e | 6.74 ±0.45 a |
| p-OH-Benzoic acid | 5.36 ±0.18 b | 2.78 ±0.23 e | 3.74 ±0.28 d | 4.56 ±0.19 c | 2.39 ±0.14 e–g | 2.08 ±0.15 g | 7.02 ±0.33 a |
| Vanillic acid | 23.91 ±0.36 c–e | 22.75 ±0.39 c–e | 25.00 ±1.31 c | 27.84 ±0.46 b | 24.01 ±0.63 c–e | 22.05 ±0.50 d–f | 42.68 ±2.58 a |
| Caffeic acid | 34.91 ±1.15 b–e | 36.23 ±1.71 a–e | 40.87 ±2.16 a–c | 33.10 ±0.97 c–e | 37.56 ±2.43 a–d | 29.27 ±0.74 de | 43.85 ±4.67 ab |
| Syringic acid | 12.74 ±0.87 f–g | 13.26 ±0.35 ef | 14.93 ±0.42 cd | 26.73 ±0.59 a | 12.12 ±0.22 g–i | 11.76 ±0.31 g–i | 17.15 ±0.87 b |
| p-Coumaric acid | 41.18 ±3.97 b | 37.56 ±2.90 b | 48.51 ±0.67 b | 41.63 ±3.04 b | 49.43 ±3.46 b | 48.48 ±1.76 b | 264.94 ±25.88 a |
| Ferulic acid | 830.61 ±14.81 b–d | 858.14 ±16.75 a–c | 929.64 ±26.73 a | 905.40 ±26.51 ab | 872.42 ±23.89 a–c | 710.39 ±20.68 e | 883.97 ±24.24 a–c |
| Sinapic acid | 44.68 ±1.63 c–e | 48.59 ±0.77 c | 65.47 ±3.07 a | 43.14 ±1.96 d–f | 39.50 ±1.20 ef | 43.90 ±1.01 c–e | 34.09 ±0.70 g |
| Salicylic acid | 1.64 ±0.04 cd | 1.63 ±0.03 d | 1.75 ±0.01 b | 1.68 ±0.03 cd | 1.70 ±0.03 bc | 1.67 ±0.04 cd | 1.92 ±0.03 a |
| Total | 1001.91 ±19.14 c–e | 1026.50 ±19.14 b–d | 1135.07 ±31.96 b | 1089.60 ±29.92 bc | 1044.82 ±30.03 b–d | 874.24 ±23.25 f | 1302.35 ±57.02 a |
| Antiradical acitvity | 0.214 ±0.004 cd | 0.220 ±0.004 bc | 0.243 ±0.007 b | 0.233 ±0.006 b | 0.223 ±0.006 b–d | 0.187 ±0.005 f | 0.279 ±0.012 a |
| Alkyl- Resorcinol | Year | Cultivar | |||||
| 2017 | 2018 | Fidelius | Hondia | Jantarka | KWS Ozon | Arktis | |
| C15:0 | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ |
| C17:0 | 28.31 ± 1.14 a | 28.12 ±1.02 a | 36.32 ±3.45 ab | 28.51 ±1.21 a–d | 19.35 ±0.62 e | 37.20 ±1.61 a | 22.38 ±1.65 de |
| C19:1 | LOQ | 22.56 ±2.41 a | 11.11 ±5.04 ef | LOQ | 12.93 ±5.84 d–f | LOQ | 10.85 ±5.04 f |
| C21:2 | 42.57 ±1.63 a | 37.54 ±1.26 b | 36.12 ±4.03 b–d | 41.16 ±3.60 a–d | 46.00 ±1.51 ab | 46.25 ±2.47 ab | 22.50 ±1.59 e |
| C19:0 | 221.48 ±7.33 a | 224.60 ±6.54 a | 289.68 ±11.94 a | 228.83 ±6.80 cd | 161.26 ±3.16 g | 259.34 ±7.77 b | 201.43 ±2.40 ef |
| C21:1 | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ |
| C21:0 | 405.83 ±9.20 b | 421.29 ±8.75 a | 507.50 ±21.51 a | 452.97 ±12.47 b | 385.18 ±8.10 d–f | 448.84 ±14.38 b | 421.19 ±1.79 b–d |
| C23:0 | 87.13 ±2.37 b | 96.96 ±2.46 a | 122.73 ±8.04 a | 89.82 ±7.75 b | 88.86 ±2.52 b | 95.79 ±2.79 b | 95.02 ±0.92 b |
| C25:0 | 27.01 ±2.52 b | 35.45 ±2.15 a | 40.66 ±4.54 ab | 32.97 ±3.28 ab | 30.64 ±2.63 bc | 32.88 ±1.14 b | 37.07 ±6.07 ab |
| Total | 812.32 ±18.43 b | 866.51 ±17.90 a | 1053.11 ±46.89 a | 874.24 ±24.09 b–d | 744.22 ±15.29 fg | 920.31 ±29.12 bc | 810.45 ±6.61 d–g |
| Antiradical acitvity | 0.286 ±0.006 b | 0.305 ±0.006 a | 0.371 ±0.017 a | 0.308 ±0.008 bc | 0.262 ±0.005 f | 0.324 ±0.010 bc | 0.286 ±0.002 fg |
| Alkyl- Resorcinol | Cultivar | ||||||
| Belissa | Estivus | Linus | Markiza | Ostka Strzelecka | Pokusa | Rokosz | |
| C15:0 | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ |
| C17:0 | 32.92 ± 2.01 a–c | 25.85 ±0.72 c–e | 30.41 ±3.05 a–d | 24.89 ±0.55 c–e | 25.98 ±0.98 c–e | 28.28 ±0.47 b–d | 26.48 ±1.21 c–e |
| C19:1 | 18.76 ± 8.39 ab | 13.90 ± 6.24 c–f | 17.33 ±7.79 a–c | LOQ | 14.98 ±6.80 b–e | 20.31 ±9.10 a | 15.22 ±6.86 b–d |
| C21:2 | 35.03 ±1.72 cd | 46.72 ±0.98 a | 33.13 ±3.52 d | 48.94 ±2.16 a | 41.64 ±1.60 a–d | 44.62 ±2.11 a–c | 38.52 ±1.48 a–d |
| C19:0 | 269.11 ±8.05 b | 196.93 ±5.66 ef | 249.02 ±13.33 bc | 185.79 ±2.48 f | 212.16 ±7.02 de | 201.80 ±3.86 ef | 212.08 ±8.26 de |
| C21:1 | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ |
| C21:0 | 459.76 ±8.92 b | 434.72 ±14.72 bc | 378.23 ±8.18 ef | 349.76 ±5.57 f | 372.68 ±10.42 ef | 352.91 ±8.43 f | 398.99 ±16.04 c–e |
| C23:0 | 95.03 ±5.75 b | 94.55 ±3.78 b | 88.31 ±7.72 b | 83.60 ±2.30 b | 87.18 ±3.07 b | 77.26 ±2.54 b | 86.36 ±2.87 b |
| C25:0 | 46.91 ±3.36 a | 29.00 ±2.64 bc | 31.32 ±3.11 b | 37.34 ±2.22 ab | 39.17 ±2.21 ab | 16.77 ±7.50 c | LOQ |
| Total | 957.51 ±16.46 b | 841.66 ±32.84 c–e | 827.75 ±17.92 c–f | 730.32 ±11.03 f | 793.81 ±29.05 d–g | 741.96 ±28.99 fg | 777.64 ±34.01 e–g |
| Antiradical acitvity | 0.337 ±0.006 b | 0.297 ±0.012 cd | 0.292 ±0.006 ef | 0.257 ±0.004 f | 0.280 ±0.010 ef | 0.261 ±0.010 g | 0.274 ±0.012 e–g |
| Cultivar | Farming System | Average for a Cultivar | |||
|---|---|---|---|---|---|
| ORG | INT | CON | MONO | ||
| 2017 | |||||
| Fidelius | 5.0 b 1 B | 17.0 b A | 5.7 b B | 5.0 a B | 8.2 c |
| Hondia | 8.3 ab B | 23.1 ab A | 3.5 b C | 8.5 a B | 10.9 ab |
| Jantarka | 6.2 ab C | 24.0 a A | 13.6 a B | 8.5 a C | 13.1 a |
| KWS Ozon | 9.8 a A | 10.7 c A | 11.5 a A | 8.2 a A | 10.1 bc |
| Mean | 7.3 B 2 | 18.7 A | 8.6 B | 7.6 B | 10.5 |
| 2018 | |||||
| Fidelius | 18.5 b B | 36.0 a A | 9.5 b C | 14.0 b BC | 19.5 c |
| Hondia | 24.0 b A | 18.5 c A | 5.0 b C | 12.5 b B | 15.0 d |
| Jantarka | 46.5 a A | 28.0 b C | 27.0 a C | 36.0 a B | 34.4 a |
| KWS Ozon | 43.5 a A | 33.0 ab B | 29.5 a B | 11.0 b C | 29.3 b |
| Mean | 33.1 A | 28.9 B | 17.8 C | 18.4 C | 24.5 |
| Phenolic Acid | Source of Variability | V (%) # | ||||||
|---|---|---|---|---|---|---|---|---|
| Year (Y) | Cultivar (C) | System (S) | Y × C | Y × S | C × S | Y × C × S | ||
| Protocatechuic acid | *** | *** | *** | ns | *** | *** | ns | 5.95 |
| p-OH-Benzoic acid | ns | *** | *** | ns | *** | *** | ns | 8.21 |
| Vanillic acid | *** | *** | *** | ns | ** | *** | ns | 4.29 |
| Caffeic acid | ns | *** | *** | *** | *** | *** | ns | 7.74 |
| Syringic acid | *** | *** | *** | ** | *** | *** | ns | 5.05 |
| p-Coumaric acid | *** | *** | *** | *** | *** | *** | ns | 7.44 |
| Ferulic acid | ns | *** | *** | ns | ns | *** | ns | 5.85 |
| Sinapic acid | ns | *** | *** | ** | ** | *** | ns | 5.67 |
| Salicylic acid | *** | *** | ** | ** | *** | *** | ns | 1.64 |
| Total | ns | *** | *** | ns | ns | *** | ns | 5.78 |
| Antiradical acitvity | ns | *** | *** | ns | ns | *** | ns | 5.93 |
| Phenolic Acid | Year | Cultivar | System | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | Fidelius | Hondia | Jantarka | KWS Ozon | ORG | CON | INT | MONO | |
| Protocatechuic acid | 4.49 ±0.11 a | 3.91 ±0.10 b | 4.64 ±0.17 a | 4.13 ±0.16 b | 3.99 ±0.14 b | 4.03 ±0.18 b | 5.21 ±0.13 a | 3.75 ±0.09 c | 3.98 ±0.11 b | 3.87 ±0.08 b |
| p-OH-Benzoic acid | 1.88 ±0.06 a | 1.92 ±0.08 a | 1.58 ±0.11 c | 1.99 ±0.06 b | 1.71 ±0.09 c | 2.33 ±0.07 a | 2.25 ±0.07 a | 1.59 ±0.10 c | 1.73 ±0.11 c | 2.05 ±0.07 b |
| Vanillic acid | 22.16 ±0.34 a | 21.17 ±0.31 b | 23.82 ±0.33 a | 22.05 ±0.26 c | 19.19 ±0.26 b | 21.60 ±0.45 c | 22.76 ±0.46 a | 21.32 ±0.39 bc | 20.85 ±0.40 c | 21.73 ±0.55 b |
| Caffeic acid | 24.25 ±1.70 a | 22.76 ±0.85 a | 21.71 ±1.23 b | 20.92 ±0.83 b | 26.36 ±1.43 a | 25.03 ±3.11 a | 35.17 ±2.45 a | 19.66 ±0.40 b | 19.73 ±0.59 b | 19.46 ±0.62 b |
| Syringic acid | 15.34 ±0.34 a | 14.77 ±0.39 b | 16.74 ±0.22 b | 17.55 ±0.29 a | 11.71 ±0.21 d | 14.22 ±0.17 c | 14.33 ±0.41 b | 15.61 ±0.56 a | 15.03 ±0.55 a | 15.26 ±0.55 a |
| p-Coumaric acid | 35.83 ±1.48 a | 26.22 ±1.75 b | 27.26 ±2.76 b | 34.28 ±2.41 a | 30.86 ±1.98 ab | 31.69 ±2.65 a | 42.42 ±2.15 a | 26.28 ±1.94 b | 26.12 ±1.73 b | 29.27 ±2.51 b |
| Ferulic acid | 726.23 ±10.14 a | 722.98 ±10.43 a | 671.22 ±11.46 b | 739.00 ±12.30 a | 728.97 ±13.70 a | 759.23 ±14.46 a | 786.45 ±14.87 a | 704.61 ±13.44 b | 713.30 ±14.33 b | 694.06 ±5.36 b |
| Sinapic acid | 43.61 ±1.07 a | 44.26 ±1.13 a | 34.95 ±0.56 d | 42.00 ±0.78 c | 52.76 ±1.01 a | 46.02 ±0.89 b | 45.44 ±1.70 a | 42.90 ±1.52 b | 46.22 ±1.74 a | 41.17 ±1.01 b |
| Salicylic acid | 1.53 ±0.01 b | 1.64 ±0.01 a | 1.56 ±0.02 c | 1.60 ±0.02 b | 1.57 ±0.02 c | 1.62 ±0.02 a | 1.57 ±0.02 b | 1.60 ±0.02 a | 1.58 ±0.02 ab | 1.59 ±0.02 a |
| Total | 875.33 ±12.74 a | 859.63 ±12.76 a | 803.49 ±14.67 b | 883.52 ±13.84 a | 877.13 ±17.40 a | 905.79 ±19.45 a | 955.62 ±17.43 a | 837.31 ±15.62 b | 848.54 ±17.25 b | 828.46 ±5.87 b |
| Antiradical acitvity | 0.187 ±0.003 a | 0.184 ±0.003 a | 0.172 ±0.003 b | 0.189 ±0.003 a | 0.188 ±0.004 a | 0.194 ±0.004 a | 0.204 ±0.004 a | 0.179 ±0.003 b | 0.182 ±0.004 b | 0.177 ±0.001 b |
| Alkyl- Resorcinol | Year | Cultivar | System | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | Fidelius | Hondia | Jantarka | KWS Ozon | ORG | CON | INT | MONO | |
| C15:0 | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ |
| C17:0 | 27.77 ±1.18 a | 27.68 ±0.81 a | 32.17 ±1.15 a | 27.46 ±0.75 b | 20.32 ±1.03 c | 30.96 ±1.30 a | 30.34 ±1.77 a | 27.11 ±1.42 b | 25.99 ±1.12 b | 27.47 ±1.21 ab |
| C19:1 | LOQ | 26.15 ± 1.70 a | 15.99 ±3.50 a | 13.21 ±3.61 b | 13.17 ±2.82 b | 9.93 ±2.74 c | 6.01 ± 2.20 b | 15.60 ±3.44 a | 14.60 ± 3.16 a | 16.10 ± 3.46 a |
| C21:2 | 42.24 ±1.20 a | 36.65 ±1.15 b | 34.48 ±1.26 b | 39.81 ±1.49 a | 41.89 ±1.90 a | 41.60 ±1.92 a | 42.38 ±1.67 a | 38.87 ±2.08 ab | 36.83 ±1.83 b | 39.71 ±1.21 ab |
| C19:0 | 212.85 ±6.83 a | 218.47 ±5.24 a | 250.67 ±7.55 a | 213.77 ±4.03 c | 168.82 ±6.72 d | 229.37 ±5.40 b | 237.03 ±11.13 a | 209.46 ±7.46 b | 204.52 ±6.95 b | 211.63 ±7.01 b |
| C21:1 | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ | LOQ |
| C21:0 | 375.19 ±9.16 b | 421.16 ±6.70 a | 413.08 ±14.87 a | 392.49 ±10.34 b | 370.38 ±12.39 c | 416.76 ±8.79 a | 448.62 ±11.40 a | 385.76 ±11.11 b | 373.37 ±10.29 b | 384.94 ±10.37 b |
| C23:0 | 79.27 ±2.18 b | 95.44 ±1.96 a | 94.20 ±4.35 a | 82.08 ±2.93 c | 83.74 ±3.07 bc | 89.40 ±2.35 ab | 99.30 ±3.98 a | 85.76 ±2.69 b | 81.17 ±2.70 b | 83.19 ±2.72 b |
| C25:0 | 24.48 ±0.90 b | 34.16 ±1.21 a | 34.74 ±2.10 a | 28.98 ±1.55 b | 25.52 ±1.38 c | 28.05 ±1.65 bc | 34.29 ±1.67 a | 27.21 ±1.23 b | 24.11 ±1.23 b | 31.68 ±2.23 a |
| Total | 761.81 ±18.75 b | 859.71 ±13.43 a | 875.34 ±27.66 a | 797.81 ±18.47 b | 723.83 ±25.10 c | 846.07 ±16.83 a | 897.97 ±27.20 a | 789.78 ±22.40 b | 760.59 ±19.35 b | 794.71 ±22.47 b |
| Antiradical acitvity | 0.266 ±0.007 b | 0.300 ±0.005 a | 0.306 ±0.010 a | 0.279 ±0.006 b | 0.253 ±0.009 c | 0.295 ±0.006 a | 0.314 ±0.009 a | 0.276 ±0.008 b | 0.266 ±0.007 b | 0.278 ±0.008 b |
| Alkyl- Resorcinol | Source of Variability | V (%) # | ||||||
|---|---|---|---|---|---|---|---|---|
| Year (Y) | Cultivar (C) | System (S) | Y × C | Y × S | C × S | Y × C × S | ||
| C15:0 | ns | ns | ns | ns | ns | ns | ns | 0.00 |
| C17:0 | ns | *** | ** | *** | ns | ns | * | 9.36 |
| C19:1 | *** | *** | *** | *** | *** | *** | *** | 7.22 |
| C21:2 | *** | *** | ** | * | ** | *** | *** | 8.36 |
| C19:0 | ns | *** | *** | *** | ns | *** | *** | 8.07 |
| C21:1 | ns | ns | ns | ns | ns | ns | ns | 0.00 |
| C21:0 | *** | *** | *** | ** | *** | *** | *** | 6.75 |
| C23:0 | *** | *** | *** | ns | ns | *** | ** | 10.30 |
| C25:0 | *** | *** | *** | ns | *** | ns | ns | 8.38 |
| Total | *** | *** | *** | *** | ** | *** | *** | 7.06 |
| Antiradical acitvity | *** | *** | *** | *** | ** | *** | *** | 5.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, I.; Soluch, A.; Mołdoch, J.; Jończyk, K. The Effect of Farming Systems and Cultivars on the Qualitative and Quantitative Composition of Bioactive Compounds in Winter Wheat (Triticum aestivum L.). Molecules 2025, 30, 902. https://doi.org/10.3390/molecules30040902
Kowalska I, Soluch A, Mołdoch J, Jończyk K. The Effect of Farming Systems and Cultivars on the Qualitative and Quantitative Composition of Bioactive Compounds in Winter Wheat (Triticum aestivum L.). Molecules. 2025; 30(4):902. https://doi.org/10.3390/molecules30040902
Chicago/Turabian StyleKowalska, Iwona, Agata Soluch, Jarosław Mołdoch, and Krzysztof Jończyk. 2025. "The Effect of Farming Systems and Cultivars on the Qualitative and Quantitative Composition of Bioactive Compounds in Winter Wheat (Triticum aestivum L.)" Molecules 30, no. 4: 902. https://doi.org/10.3390/molecules30040902
APA StyleKowalska, I., Soluch, A., Mołdoch, J., & Jończyk, K. (2025). The Effect of Farming Systems and Cultivars on the Qualitative and Quantitative Composition of Bioactive Compounds in Winter Wheat (Triticum aestivum L.). Molecules, 30(4), 902. https://doi.org/10.3390/molecules30040902







