Advanced Nanomedicine Delivery Systems for Cardiovascular Diseases: Viral and Non-Viral Strategies in Targeted Therapy
Abstract
1. Introduction
2. Conventional Delivery Strategies Targeting the Cardiovascular System
2.1. Passive Targeted Delivery
2.2. Active Targeted Delivery
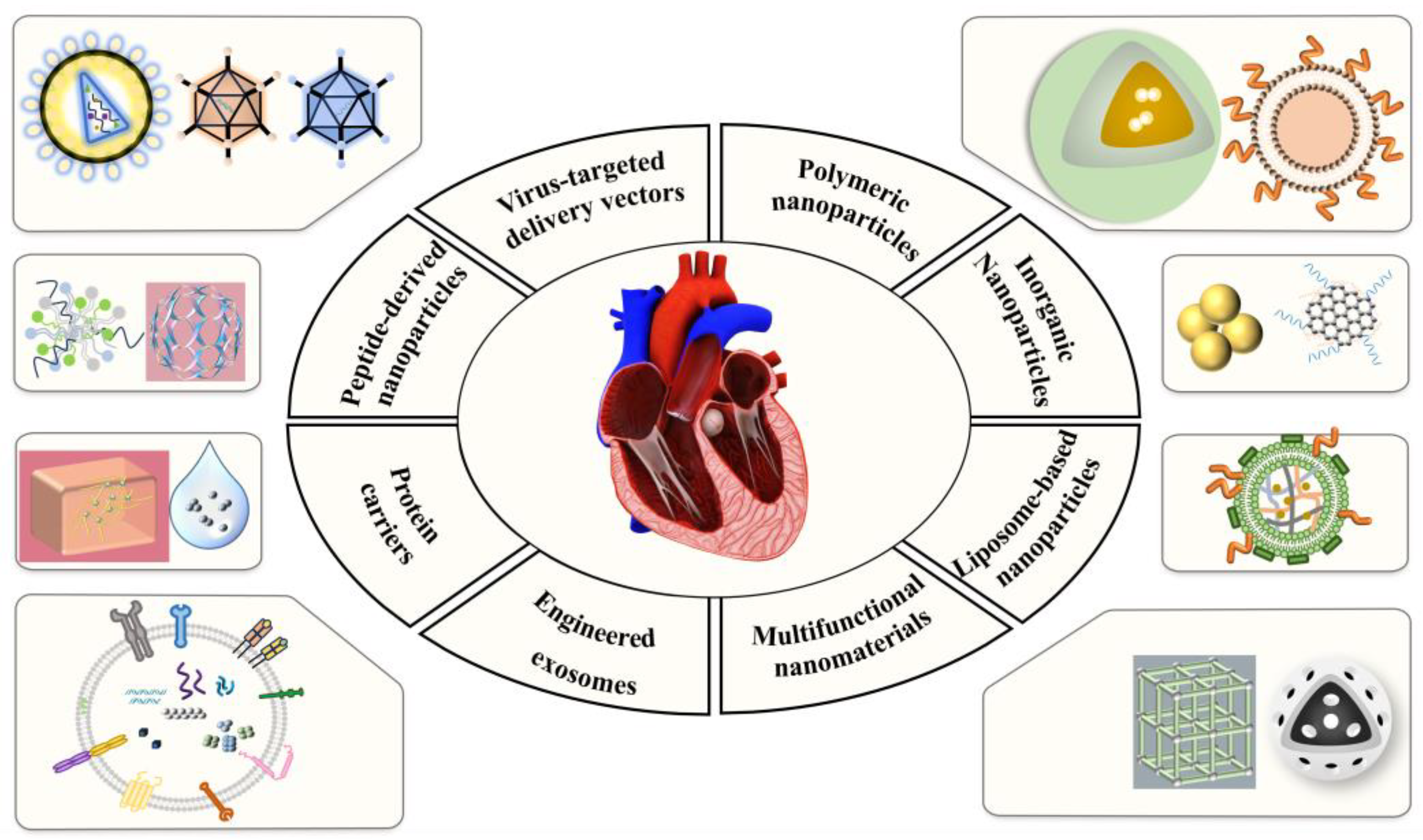
3. Delivery Vehicles Targeting Cardiovascular Disease
3.1. Virus-Targeted Delivery Vectors
3.1.1. Lentiviral Vectors
3.1.2. Adenoviral Vectors
3.1.3. Adeno-Associated Viral Vectors
3.2. Non-Viral Targeted Delivery Vectors
3.2.1. Liposome-Based Nanoparticles
3.2.2. Polymer Nanoparticles
3.2.3. Inorganic Nanoparticles
3.3. Engineered Exosomes
| Category | Types | Advantages | Model | Applications | Contents | Biological Functions | Refs. |
|---|---|---|---|---|---|---|---|
| Liposome | BB-lip | Passive | In vitro and in vivo (LAD) | MI | Berberine | Improved ejection fraction and reduced adverse remodeling | [70] |
| FA-liposomes | Passive | In vitro and in vivo (Apoe−/− mice) | Atherosclerosis (AS) | Fluoroketone Acetonide (FA) | Anti-inflammatory, promotes cholesterol efflux | [71] | |
| cT-21-LIPs | Active targeting of cTnT | In vitro and in vivo (LAD) | Acute myocardial infarction (AMI) | miR-21 | Reduces apoptosis and infarct size | [72] | |
| Fe@PLP-TR-A | Active targeting of thrombin peptides | In vitro and in vivo (LAD for 30 min) | MI/RI | ANGPTL4 and Fe3O4 | ROS scavenging effect and protection of endothelial cells from apoptosis | [73] | |
| LNPs | LNPs | Passive | In vitro and in vivo (LAD for 60 min) | MI/RI | modRNA | Reprogramming to reduce fibrosis and promote cardiac repair | [74] |
| CD5/LNP-FAPCAR | Active targeting of the T-cell surface protein CD5 | In vitro and in vivo (Angiotensin II and Phenylephrine for a week) | Myocardial fibrosis | mRNA | Reduces vascular gap and attenuates fibrosis | [78] | |
| ECM-NPs | Active (In situ injection) | In vitro and in vivo (LAD) | MI | Colchicine | Improved cardiac function and reduced fibrosis | [75] | |
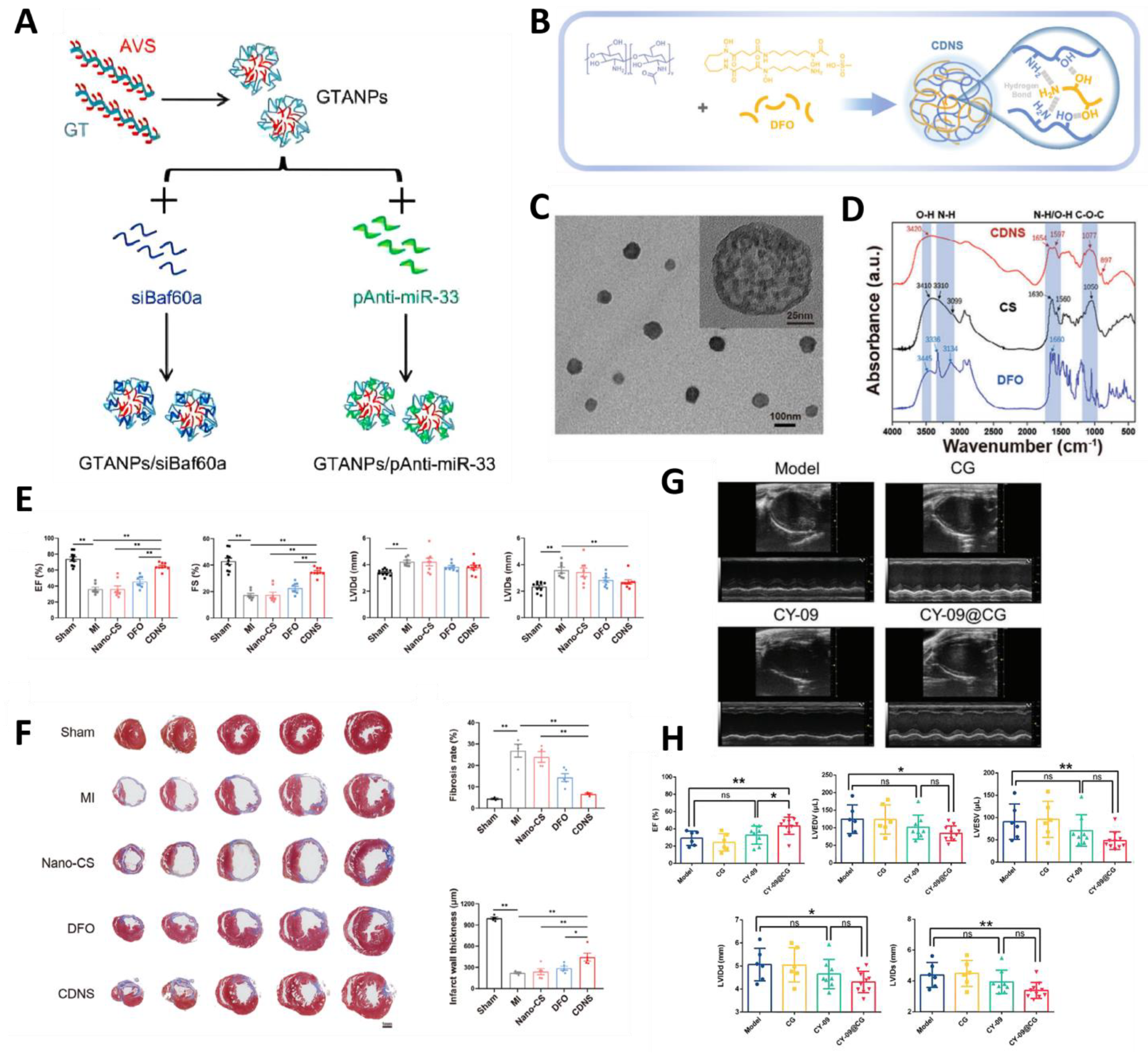
| Natural Polymer NPs | GTANPs | Active targeting, galactose modification | In vitro and in vivo (ApoE KO mice) | AS | Atorvastatin (AVS) and siBaf60a and pAnti-miR-33 | Reduces plasma cholesterol and inhibits plaque formation | [87] |
| CDNS | Active targeting, chelates iron ions | In vitro and in vivo (LAD) | MI | Desferrioxamine | Reduced oxidative stress and promoted angiogenesis. | [88] | |
| CY-09@CG | Active targeting of Dectin-1 | In vitro and in vivo (LAD for 30 min) | MI/RI | CY-09 | Suppressing Inflammation | [89] | |
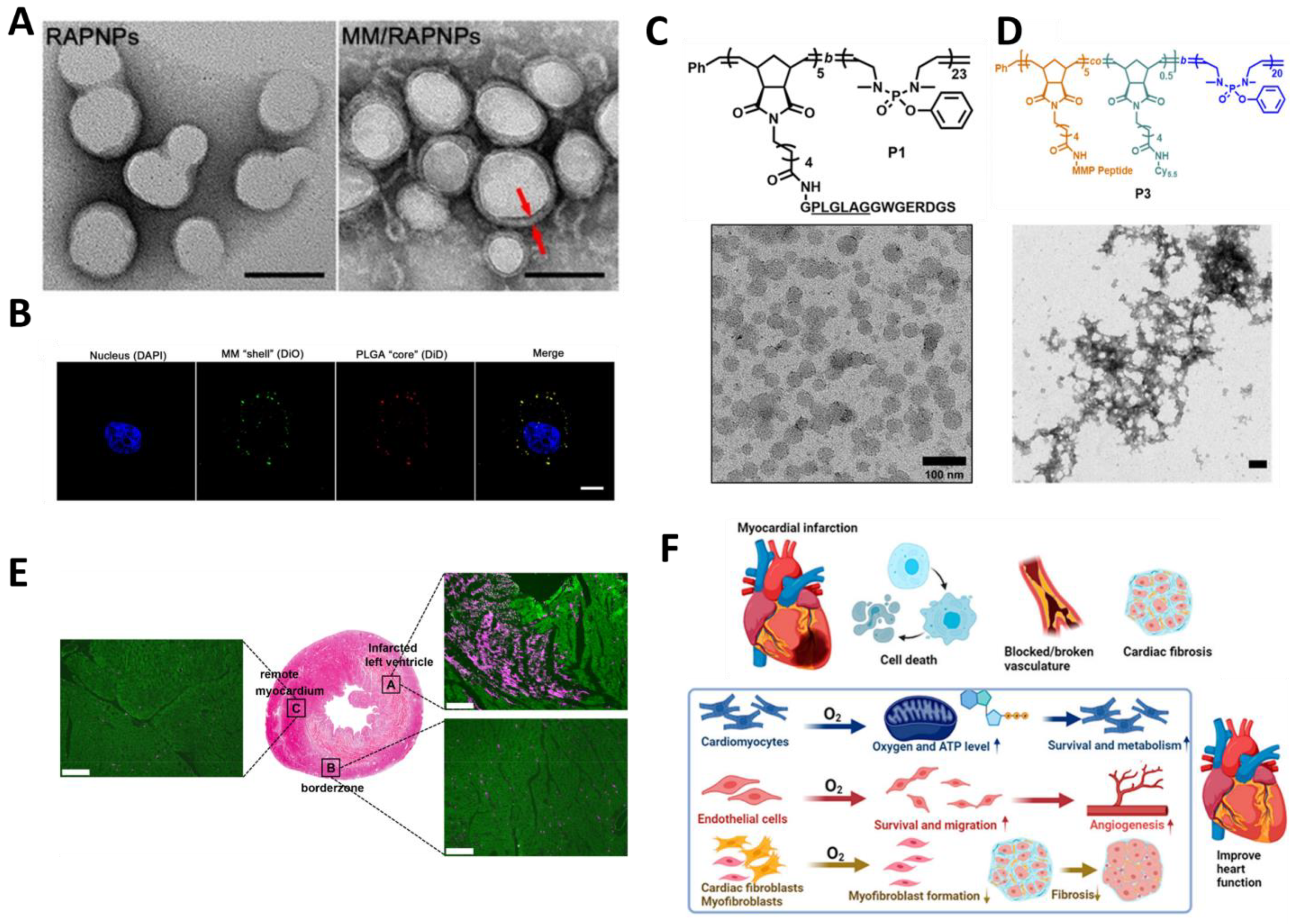
| Synthetic polymer NPs | MM/RAPNPs | Active (Macrophage membrane modification) | In vitro and in vivo (Apoe −/− mice) | AS | Rapamycin | Inhibition of macrophage and smooth muscle cell proliferation and reduction in plaque growth | [91] |
| MePTDO | Responding to matrix metalloproteinases | In vitro and in vivo (LAD) | MI | Phosphate NPs | Improve inflammation | [26] | |
| PCNP/O2 | Active (Platelet membrane modification) | In vitro and in vivo (LAD) | AMI | Oxygen-releasing NPs | Promotes angiogenesis and inhibits fibrosis | [92] | |
| Inorganic NPs | PEG-AuNPs | Active targeting of β-adrenergic receptors | In vivo (Isoprotereno-induced myocardial hypertrophy) | Myocardial hypertrophy | / | Reduces cardiac hypertrophy and inflammation | [95] |
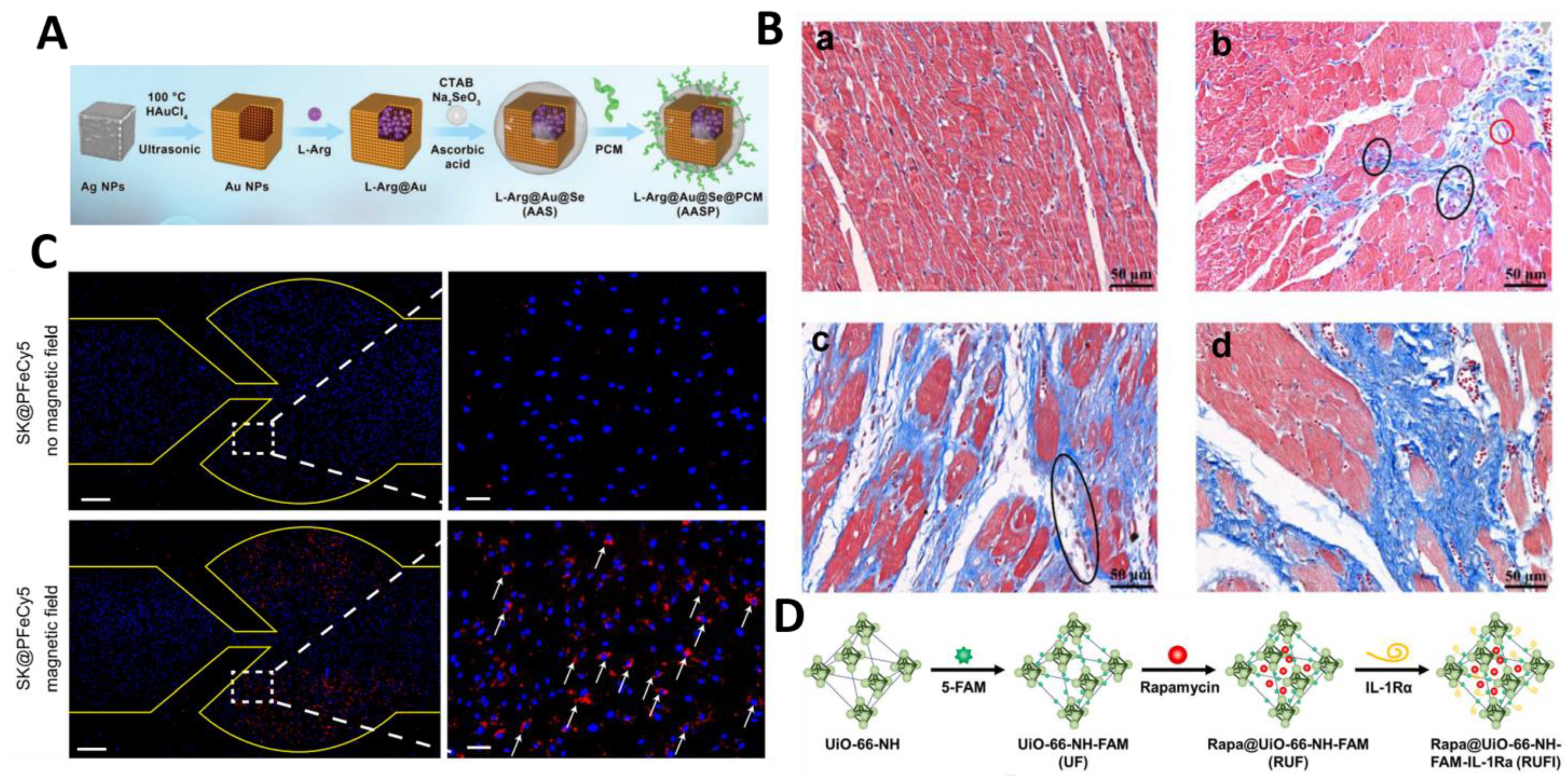
| AASP | Active (Cardiomyocyte-targeted peptide modification) | In vitro and in vivo (LAD for 30 min) | MI/RI | L-Arginine | Maintains mitochondrial function and inhibits fibrosis | [96] | |
| Inorganic NPs | AuNPs-zwit-glucose | GLUT-1 transporter protein | In vitro and in vivo (Isoprotereno-induced MI) | MI | / | / | [97] |
| SR@PFeXCT | Active targeting of protease-activated receptor 2 | In vitro and in vivo (①Ldlr −/− mice; ②Wire injury) | Calcific Aortic Valve Disease | XCT790 | Inhibits osteogenic differentiation of VICs and inhibits calcium deposition | [98] | |
| Rapa@UiO-66-NH-FAM-IL-1Ra (RUFI) | Active targeting Interleukin-1 receptor | In vitro and in vivo (Apoe −/− mice and carotid artery ligation or carotid collar placement) | AS | Rapamycin and IL-1Ra | Immunomodulation, Cellular Targeting | [99] | |
| MSN-NGR1-CD11b | Active targeting of macrophage surface antibodies | In vitro and in vivo (LAD) | MI | Notoginsenoside R1 (NGR1) | Promotes angiogenesis and regulates macrophage phenotype | [100] | |
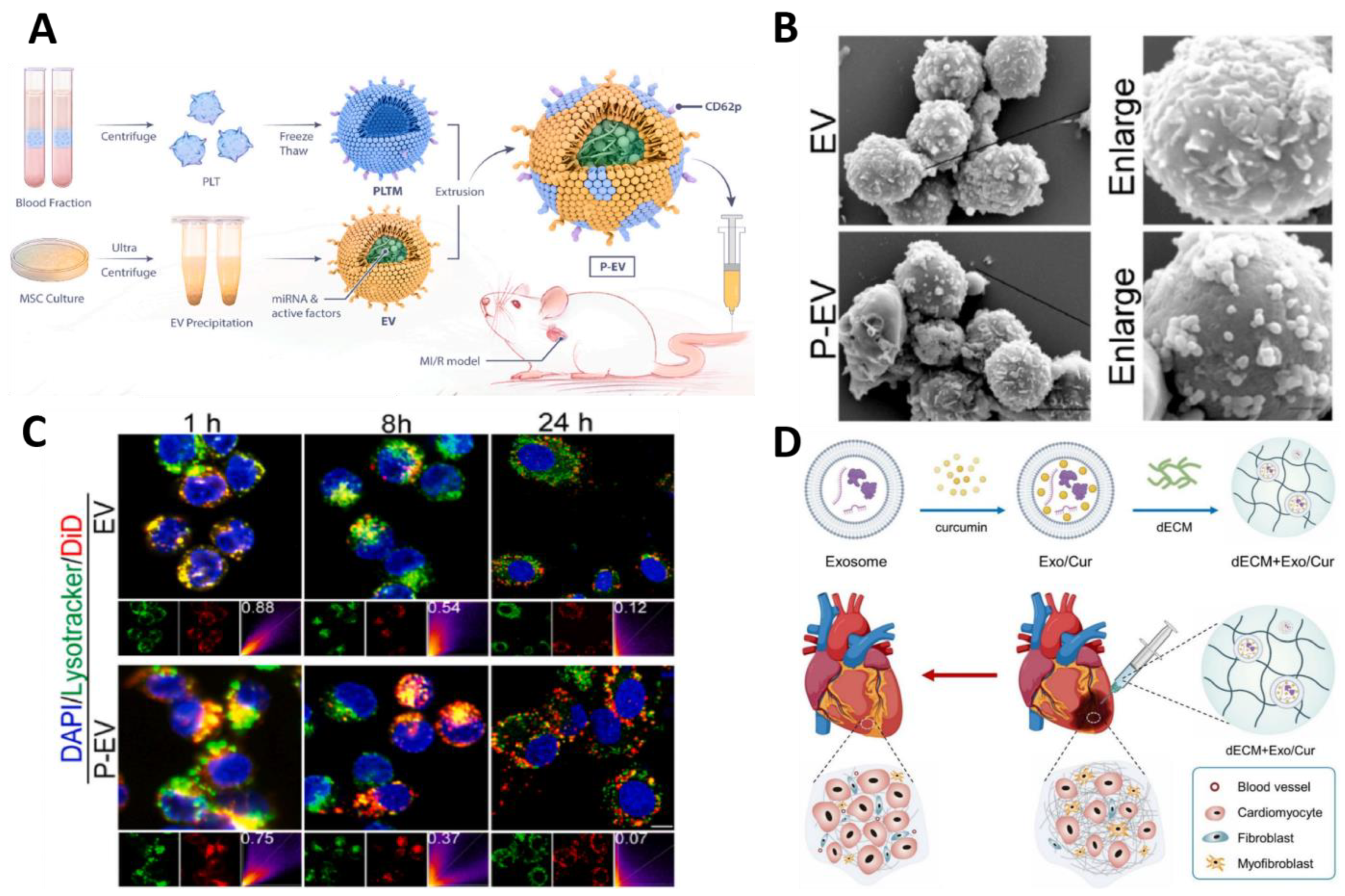
| Engineered exosomes | P-EVs | Active targeting of monocytes | In vitro and in vivo (LAD for 45 min) | MI/RI | miRNA | Regulation of macrophage phenotype, immunomodulation | [104] |
| dECM + Exo/Cur | Passive | In vitro and in vivo (LAD) | MI | curcumin | Reduces collagen deposition, fibrosis, and infarct size | [105] |
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L. The Global Burden of Disease Study at 30 Years. Nat. Med. 2022, 28, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of Cardiovascular Disease in China: Current Features and Implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [PubMed]
- Gao, C.; He, X.; Ouyang, F.; Zhang, Z.; Shen, G.; Wu, M.; Yang, P.; Ma, L.; Yang, F.; Ji, Z.; et al. Drug-Coated Balloon Angioplasty with Rescue Stenting versus Intended Stenting for the Treatment of Patients with de Novo Coronary Artery Lesions (REC-CAGEFREE I): An Open-Label, Randomised, Non-Inferiority Trial. Lancet 2024, 404, 1040–1050. [Google Scholar] [CrossRef]
- Feldmann, A.; Nitschke, Y.; Linß, F.; Mulac, D.; Stücker, S.; Bertrand, J.; Buers, I.; Langer, K.; Rutsch, F. Improved Reversion of Calcifications in Porcine Aortic Heart Valves Using Elastin-Targeted Nanoparticles. Int. J. Mol. Sci. 2023, 24, 16471. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Cheng, C.; Chen, W.; Lin, R.; Wang, Y.; Cui, W.; Meng, J.; Du, J.; Wang, Y. Platelet and Erythrocyte Membranes Coassembled Biomimetic Nanoparticles for Heart Failure Treatment. ACS Nano 2024, 18, 26614–26630. [Google Scholar] [CrossRef]
- Ji, X.; Meng, Y.; Wang, Q.; Tong, T.; Liu, Z.; Lin, J.; Li, B.; Wei, Y.; You, X.; Lei, Y.; et al. Cysteine-Based Redox-Responsive Nanoparticles for Fibroblast-Targeted Drug Delivery in the Treatment of Myocardial Infarction. ACS Nano 2023, 17, 5421–5434. [Google Scholar] [CrossRef]
- Gelosa, P.; Castiglioni, L.; Camera, M.; Sironi, L. Drug Repurposing in Cardiovascular Diseases: Opportunity or Hopeless Dream? Biochem. Pharmacol. 2020, 177, 113894. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Tzolos, E.; Fletcher, A.J.; Nash, J.; Meah, M.N.; Cadet, S.; Adamson, P.D.; Grodecki, K.; Joshi, N.; Williams, M.C.; et al. Bypass Grafting and Native Coronary Artery Disease Activity. JACC Cardiovasc. Imaging 2022, 15, 875–887. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, T.; Xu, M.; Wang, S.; Wu, A.; Zhang, M.; Zhou, Y.L.; Shi, J. Platelet Membrane-Camouflaged Nanoparticles Carry microRNA Inhibitor against Myocardial Ischaemia–Reperfusion Injury. J. Nanobiotechnol. 2022, 20, 434. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The Evolution of Commercial Drug Delivery Technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xue, J.; Wang, G.; Diao, Q. Nanoparticle-Based Drug Delivery Systems for the Treatment of Cardiovascular Diseases. Front. Pharmacol. 2022, 13, 999404. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Advanced Drug Delivery Micro- and Nanosystems for Cardiovascular Diseases. Molecules 2022, 27, 5843. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Marei, I.; Crovella, S.; Abou-Saleh, H. Recent Developments in Nanomaterials-Based Drug Delivery and Upgrading Treatment of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1404. [Google Scholar] [CrossRef]
- Meng, X.; Yao, J.; Gu, J. Advanced Bioanalytical Techniques for Pharmacokinetic Studies of Nanocarrier Drug Delivery Systems. J. Pharm. Anal. 2025, 15, 101070. [Google Scholar] [CrossRef]
- Gupta, P.; Garcia, E.; Sarkar, A.; Kapoor, S.; Rafiq, K.; Chand, H.S.; Jayant, R.D. Nanoparticle Based Treatment for Cardiovascular Diseases. Cardiovasc. Hematol. Disord. Drug Targets 2019, 19, 33–44. [Google Scholar] [CrossRef]
- Li, J.; Lu, K.; Sun, S.; Peng, J.; Zhao, L. Long-Circulating Nanoparticles as Passive Targeting Nanocarriers for the Treatment of Thrombosis. Nanoscale 2024, 16, 6132–6141. [Google Scholar] [CrossRef]
- Chen, Z.; Kankala, R.K.; Long, L.; Xie, S.; Chen, A.; Zou, L. Current Understanding of Passive and Active Targeting Nanomedicines to Enhance Tumor Accumulation. Coord. Chem. Rev. 2023, 481, 215051. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, J.; Chong, S.Y.; Ting, H.J.; Tang, X.; Yang, L.; Zhang, S.; Qi, X.; Pei, P.; Yi, Z.; et al. Dual-Function Nanoscale Coordination Polymer Nanoparticles for Targeted Diagnosis and Therapeutic Delivery in Atherosclerosis. Small 2024, 20, 2401659. [Google Scholar] [CrossRef]
- Skourtis, D.; Stavroulaki, D.; Athanasiou, V.; Fragouli, P.G.; Iatrou, H. Nanostructured Polymeric, Liposomal and Other Materials to Control the Drug Delivery for Cardiovascular Diseases. Pharmaceutics 2020, 12, 1160. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Siegwart, D.J. Passive, Active and Endogenous Organ-Targeted Lipid and Polymer Nanoparticles for Delivery of Genetic Drugs. Nat. Rev. Mater. 2023, 8, 282–300. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the Dynamics of the EPR Effect and Strategies to Improve the Therapeutic Effects of Nanomedicines by Using EPR Effect Enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Zhang, Z.; Li, H.; Yang, Y.-G.; Zhang, Y.; Sun, T. Nanocarriers for Targeted Drug Delivery in the Vascular System: Focus on Endothelium. J. Nanobiotechnol. 2024, 22, 620. [Google Scholar] [CrossRef] [PubMed]
- England, C.G.; Im, H.-J.; Feng, L.; Chen, F.; Graves, S.A.; Hernandez, R.; Orbay, H.; Xu, C.; Cho, S.Y.; Nickles, R.J.; et al. Re-Assessing the Enhanced Permeability and Retention Effect in Peripheral Arterial Disease Using Radiolabeled Long Circulating Nanoparticles. Biomaterials 2016, 100, 101–109. [Google Scholar] [CrossRef]
- Glassman, P.M.; Myerson, J.W.; Ferguson, L.T.; Kiseleva, R.Y.; Shuvaev, V.V.; Brenner, J.S.; Muzykantov, V.R. Targeting Drug Delivery in the Vascular System: Focus on Endothelium. Adv. Drug Deliv. Rev. 2020, 157, 96–117. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, C.; Zhu, S.; Wu, J.; Sun, C.; Huang, S.; Li, G.; Yang, W.; Zhang, T.; Ma, X.-L.; et al. One Endotheli-um-Targeted Combined Nucleic Acid Delivery System for Myocardial Infarction Therapy. ACS Nano 2024, 18, 8107–8124. [Google Scholar] [CrossRef]
- Chin, D.D.; Poon, C.; Wang, J.; Joo, J.; Ong, V.; Jiang, Z.; Cheng, K.; Plotkin, A.; Magee, G.A.; Chung, E.J. miR-145 Mi-celles Mitigate Atherosclerosis by Modulating Vascular Smooth Muscle Cell Phenotype. Biomaterials 2021, 273, 120810. [Google Scholar] [CrossRef]
- Mao, J.; Wu, C.; Zheng, L.; Li, Y.; Yang, R.; Yuan, P.; Jiang, J.; Li, C.; Zhou, X. Advances in Stimulus-Responsive Na-nomedicine for Treatment and Diagnosis of Atherosclerosis. Colloids Surf. B Biointerfaces 2025, 245, 114298. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Shen, M.; Hao, Y.; Wu, X.; Yao, Y.; Li, Y.; Yang, Q. Hyaluronic Acid Targeted and pH-Responsive Multifunctional Nanoparticles for Chemo-Photothermal Synergistic Therapy of Atherosclerosis. J. Mater. Chem. B 2022, 10, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sullivan, H.L.; Carrow, K.; Mesfin, J.M.; Korpanty, J.; Worthington, K.; Luo, C.; Christman, K.L.; Gianneschi, N.C. Inflammation-Responsive Micellar Nanoparticles from Degradable Polyphosphoramidates for Targeted Delivery to Myocardial Infarction. J. Am. Chem. Soc. 2023, 145, 11185–11194. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Ni, Y.; Yu, H.; Yin, H.; Yang, F.; Li, C.; Sun, D.; Pei, T.; Ma, J.; Deng, L.; et al. Inflammatory Endothelium-Targeted and Cathepsin Responsive Nanoparticles Are Effective against Atherosclerosis. Theranostics 2022, 12, 4200–4220. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Chen, L.; Li, F.; Cao, Y.; Li, D.; Xiong, Q.; Ling, Z. Biomimetic Nanoparticles Loaded Lutein Functionalized by Macrophage Membrane for Targeted Amelioration Pressure Overload-Induced Cardiac Fibrosis. Biomed. Pharmacother. 2023, 167, 115579. [Google Scholar] [CrossRef]
- Jiang, F.; Wu, G.; Yang, H.; Zhang, Y.; Shen, X.; Tao, L. Diethylaminoethyl-Dextran and Monocyte Cell Membrane Coated 1,8-Cineole Delivery System for Intracellular Delivery and Synergistic Treatment of Atherosclerosis. Int. J. Biol. Macromol. 2023, 253, 127365. [Google Scholar] [CrossRef]
- Rezaie, J.; Nejati, V.; Mahmoodi, M.; Ahmadi, M. Mesenchymal Stem Cells Derived Extracellular Vesicles: A Promising Nanomedicine for Drug Delivery System. Biochem. Pharmacol. 2022, 203, 115167. [Google Scholar] [CrossRef]
- Li, T.; Feng, J.; Ding, W.; Zhang, Z. Targeted Treatment of Myocardial Infarction by Macrophage Membrane Coated with Resveratrol Nanoparticles. ACS Omega 2024, 9, 47145–47155. [Google Scholar] [CrossRef]
- Russo, V.; Young, S.; Hamilton, A.; Amsden, B.G.; Flynn, L.E. Mesenchymal Stem Cell Delivery Strategies to Promote Cardiac Regeneration Following Ischemic Injury. Biomaterials 2014, 35, 3956–3974. [Google Scholar] [CrossRef]
- Greco, S.J.; Rameshwar, P. Mesenchymal Stem Cells in Drug/Gene Delivery: Implications for Cell Therapy. Ther. Deliv. 2012, 3, 997–1004. [Google Scholar] [CrossRef]
- Lin, L.; Su, K.; Cheng, Q.; Liu, S. Targeting Materials and Strategies for RNA Delivery. Theranostics 2023, 13, 4667–4693. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of Engineered Nanoparticles for Drug Delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Říhová, B. Immunocompatibility and Biocompatibility of Cell Delivery Systems. Adv. Drug Deliv. Rev. 2000, 42, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, T. A Brief History of Gene Therapy. Nat Genet. 1992, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Li, X.; Le, Y.; Zhang, Z.; Nian, X.; Liu, B.; Yang, X. Viral Vector-Based Gene Therapy. Int. J. Mol. Sci. 2023, 24, 7736. [Google Scholar] [CrossRef]
- Lamsfus-Calle, A.; Daniel-Moreno, A.; Ureña-Bailén, G.; Raju, J.; Antony, J.S.; Handgretinger, R.; Mezger, M. Hematopoietic Stem Cell Gene Therapy: The Optimal Use of Lentivirus and Gene Editing Approaches. Blood Rev. 2020, 40, 100641. [Google Scholar] [CrossRef]
- Jiang, M.; Feng, J.; Fu, R.; Pan, Y.; Liu, X.; Dai, J.; Jiang, C.; Hao, Y.; Ren, M. Transfection of STAT3 Overexpression Plasmid Mediated through Recombinant Lentivirus Promotes Differentiation of Bone Marrow Mesenchymal Stem Cells into Neural Cells in Fetal Rats with Spina Bifida Aperta. Aging 2021, 13, 21778–21790. [Google Scholar] [CrossRef]
- Di Pasquale, E.; Latronico, M.V.; Jotti, G.S.; Condorelli, G. Lentiviral Vectors and Cardiovascular Diseases: A Genetic Tool for Manipulating Cardiomyocyte Differentiation and Function. Gene Ther. 2012, 19, 642–648. [Google Scholar] [CrossRef]
- Zhao, J.; Pettigrew, G.J.; Bolton, E.M.; Murfitt, C.R.; Carmichael, A.; Bradley, J.A.; Lever, A.M.L. Lentivirus-Mediated Gene Transfer of Viral Interleukin-10 Delays but Does Not Prevent Cardiac Allograft Rejection. Gene Ther. 2005, 12, 1509–1516. [Google Scholar] [CrossRef]
- Merentie, M.; Lottonen-Raikaslehto, L.; Parviainen, V.; Huusko, J.; Pikkarainen, S.; Mendel, M.; Laham-Karam, N.; Kärjä, V.; Rissanen, R.; Hedman, M.; et al. Efficacy and Safety of Myocardial Gene Transfer of Adenovirus, Adeno-Associated Virus and Lentivirus Vectors in the Mouse Heart. Gene Ther. 2016, 23, 296–305. [Google Scholar] [CrossRef]
- Berkner, K.L. Development of Adenovirus Vectors for the Expression of Heterologous Genes. Biotechniques 1988, 6, 616–629. [Google Scholar] [PubMed]
- Salauddin, M.; Saha, S.; Hossain, M.G.; Okuda, K.; Shimada, M. Clinical Application of Adenovirus (AdV): A Comprehensive Review. Viruses 2024, 16, 1094. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Henry, T.D.; Traverse, J.H.; Latter, D.A.; Mokadam, N.A.; Answini, G.A.; Williams, A.R.; Sun, B.C.; Burke, C.R.; Bakaeen, F.G.; et al. Angiogenic Gene Therapy for Refractory Angina: Results of the EXACT Phase 2 Trial. Circ. Cardiovasc. Interv. 2024, 17, e014054. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.-M.R.; Xu, Y.; Yang, Z.; Acton, S.T.; French, B.A. Robust Cardiomyocyte-Specific Gene Expression Following Systemic Injection of AAV: In Vivo Gene Delivery Follows a Poisson Distribution. Gene Ther. 2011, 18, 43–52. [Google Scholar] [CrossRef]
- Piras, B.A.; Tian, Y.; Xu, Y.; Thomas, N.A.; O’Connor, D.M.; French, B.A. Systemic Injection of AAV9 Carrying a Periostin Promoter Targets Gene Expression to a Myofibroblast-like Lineage in Mouse Hearts after Reperfused Myocardial Infarction. Gene Ther. 2016, 23, 469–478. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Liu, L.; Wang, Y.; Zheng, J.; Li, L.; Li, S.; Zhang, H.; Ni, J.; Ma, C.; et al. SUMO1 Regulates Post-Infarct Cardiac Repair Based on Cellular Heterogeneity. J. Pharm. Anal. 2023, 13, 170–186. [Google Scholar] [CrossRef]
- Liu, M.; Peng, T.; Hu, L.; Wang, M.; Guo, D.; Qi, B.; Ren, G.; Wang, D.; Li, Y.; Song, L.; et al. N-Glycosylation-Mediated CD147 Accumulation Induces Cardiac Fibrosis in the Diabetic Heart through ALK5 Activation. Int. J. Biol. Sci. 2023, 19, 137–155. [Google Scholar] [CrossRef]
- Knezevic, T.; Myers, V.D.; Su, F.; Wang, J.; Song, J.; Zhang, X.-Q.; Gao, E.; Gao, G.; Madesh, M.; Gupta, M.K.; et al. Adeno-Associated Virus Serotype 9–Driven Expression of BAG3 Improves Left Ventricular Function in Murine Hearts with Left Ventricular Dysfunction Secondary to a Myocardial Infarction. JACC Basic Transl. Sci. 2016, 1, 647–656. [Google Scholar] [CrossRef]
- Yan, H.; Wang, H.; Zhu, X.; Huang, J.; Li, Y.; Zhou, K.; Hua, Y.; Yan, F.; Wang, D.-Z.; Luo, Y. Adeno-Associated Virus-Mediated Delivery of Anti-miR-199a Tough Decoys Attenuates Cardiac Hypertrophy by Targeting PGC-1alpha. Mol. Ther. Nucleic Acids 2021, 23, 406–417. [Google Scholar] [CrossRef]
- Jarrett, K.E.; Lee, C.; De Giorgi, M.; Hurley, A.; Gillard, B.K.; Doerfler, A.M.; Li, A.; Pownall, H.J.; Bao, G.; Lagor, W.R. Somatic Editing of Ldlr with Adeno-Associated Viral-CRISPR Is an Efficient Tool for Atherosclerosis Research. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1997–2006. [Google Scholar] [CrossRef]
- Kim, M.-A.; Ryu, N.; Kim, H.-M.; Kim, Y.-R.; Lee, B.; Kwon, T.-J.; Bok, J.; Kim, U.-K. Targeted Gene Delivery into the Mammalian Inner Ear Using Synthetic Serotypes of Adeno-Associated Virus Vectors. Mol. Ther. Methods Clin. Dev. 2019, 13, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, X.-Y.; Lu, A.; Wang, X.-Y.; Jiang, L.-X.; Wang, J.-C. Non-Viral Vectors for RNA Delivery. J. Control. Release 2022, 342, 241–279. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Aftab, S.; Nisar, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for Targeted Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 62, 102426. [Google Scholar] [CrossRef]
- Thapa Magar, K.; Boafo, G.F.; Li, X.; Chen, Z.; He, W. Liposome-Based Delivery of Biological Drugs. Chin. Chem. Lett. 2022, 33, 587–596. [Google Scholar] [CrossRef]
- Khan, M.S.; Baskoy, S.A.; Yang, C.; Hong, J.; Chae, J.; Ha, H.; Lee, S.; Tanaka, M.; Choi, Y.; Choi, J. Lipid-Based Colloidal Nanoparticles for Applications in Targeted Vaccine Delivery. Nanoscale Adv. 2023, 5, 1853–1869. [Google Scholar] [CrossRef]
- Peng, T.; Xu, W.; Li, Q.; Ding, Y.; Huang, Y. Pharmaceutical Liposomal Delivery—Specific Considerations of Innovation and Challenges. Biomater. Sci. 2023, 11, 62–75. [Google Scholar] [CrossRef]
- Allen, T.M.; Chonn, A. Large Unilamellar Liposomes with Low Uptake into the Reticuloendothelial System. FEBS Lett. 1987, 223, 42–46. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Romberg, B.; Hennink, W.E.; Storm, G. Sheddable Coatings for Long-Circulating Nanoparticles. Pharm. Res. 2008, 25, 55–71. [Google Scholar] [CrossRef]
- Allijn, I.E.; Czarny, B.M.S.; Wang, X.; Chong, S.Y.; Weiler, M.; Da Silva, A.E.; Metselaar, J.M.; Lam, C.S.P.; Pastorin, G.; De Kleijn, D.P.V.; et al. Liposome Encapsulated Berberine Treatment Attenuates Cardiac Dysfunction after Myocardial Infarction. J. Control. Release 2017, 247, 127–133. [Google Scholar] [CrossRef]
- Darwitan, A.; Wong, Y.S.; Nguyen, L.T.H.; Czarny, B.; Vincent, A.; Nedumaran, A.M.; Tan, Y.F.; Muktabar, A.; Tang, J.K.; Ng, K.W.; et al. Liposomal Nanotherapy for Treatment of Atherosclerosis. Adv. Healthc. Mater. 2020, 9, 2000465. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, X.; Liu, X.; Cui, X.; Lian, M.; Zhao, M.; Peng, H.; Han, X. Targeted miR-21 Loaded Liposomes for Acute Myocardial Infarction. J. Mater. Chem. B 2020, 8, 10384–10391. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Song, Y.; Wang, Q.; Chen, J.; Li, Q.; Tan, H.; Yakufu, W.; Zhang, N.; Li, S.; Zhang, J.; et al. Precisely Co-Delivery of Protein and ROS Scavenger with Platesomes for Enhanced Endothelial Barrier Preservation against Myocardial Ischemia Reperfusion Injury. Chem. Eng. J. 2022, 446, 136960. [Google Scholar] [CrossRef]
- Evers, M.J.W.; Du, W.; Yang, Q.; Kooijmans, S.A.A.; Vink, A.; Van Steenbergen, M.; Vader, P.; De Jager, S.C.A.; Fuchs, S.A.; Mastrobattista, E.; et al. Delivery of Modified mRNA to Damaged Myocardium by Systemic Administration of Lipid Nanoparticles. J. Control. Release 2022, 343, 207–216. [Google Scholar] [CrossRef]
- Wang, X.; Shi, H.; Huang, S.; Zhang, Y.; He, X.; Long, Q.; Qian, B.; Zhong, Y.; Qi, Z.; Zhao, Q.; et al. Localized Delivery of Anti-Inflammatory Agents Using Extracellular Matrix-Nanostructured Lipid Carriers Hydrogel Promotes Cardiac Repair Post-Myocardial Infarction. Biomaterials 2023, 302, 122364. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Wang, Y.; Si, X.; Feng, Y.; Feng, D.; Xu, X.; Zhang, Y. Ionizable Lipids with Triazole Moiety from Click Reaction for LNP-Based mRNA Delivery. Molecules 2023, 28, 4046. [Google Scholar] [CrossRef]
- Rurik, J.G.; Tombácz, I.; Yadegari, A.; Méndez Fernández, P.O.; Shewale, S.V.; Li, L.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; et al. CAR T Cells Produced in Vivo to Treat Cardiac Injury. Science 2022, 375, 91–96. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Leong, J.; Teo, J.Y.; Aakalu, V.K.; Yang, Y.Y.; Kong, H. Engineering Polymersomes for Diagnostics and Therapy. Adv Healthc. Mater. 2018, 7, 1701276. [Google Scholar] [CrossRef]
- Lefley, J.; Waldron, C.; Becer, C.R. Macromolecular Design and Preparation of Polymersomes. Polym. Chem. 2020, 11, 7124–7136. [Google Scholar] [CrossRef]
- Matoori, S.; Leroux, J.-C. Twenty-Five Years of Polymersomes: Lost in Translation? Mater. Horiz. 2020, 7, 1297–1309. [Google Scholar] [CrossRef]
- Matalqah, S.M.; Aiedeh, K.; Mhaidat, N.M.; Alzoubi, K.H.; Bustanji, Y.; Hamad, I. Chitosan Nanoparticles as a Novel Drug Delivery System: A Review Article. Curr. Drug Targets 2020, 21, 1613–1624. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a Potential Natural Compound to Manage Plant Diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef]
- Cohen, E.; Poverenov, E. Hydrophilic Chitosan Derivatives: Synthesis and Applications. Chem. Eur. J. 2022, 28, e202202156. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, L.; Zhao, M.; Kong, F.; Lu, X.; Tang, C.; Yin, C. Dual Targeted Delivery of Statins and Nucleic Acids by Chitosan-Based Nanoparticles for Enhanced Antiatherosclerotic Efficacy. Biomaterials 2022, 280, 121324. [Google Scholar] [CrossRef]
- Lv, Q.; Lin, J.; Huang, H.; Ma, B.; Li, W.; Chen, J.; Wang, M.; Wang, X.; Fu, G.; Xiao, Y. Nanosponge for Iron Chelation and Efflux: A Ferroptosis-Inhibiting Approach for Myocardial Infarction Therapy. Adv. Sci. 2024, 11, 2305895. [Google Scholar] [CrossRef]
- Liu, Z.; Lian, W.; Long, Q.; Cheng, R.; Torrieri, G.; Zhang, B.; Koivuniemi, A.; Mahmoudzadeh, M.; Bunker, A.; Gao, H.; et al. Promoting Cardiac Repair through Simple Engineering of Nanoparticles with Exclusive Targeting Capability toward Myocardial Reperfusion Injury by Thermal Resistant Microfluidic Platform. Adv. Funct. Mater. 2022, 32, 2204666. [Google Scholar] [CrossRef]
- Sofini, S.P.S.; Balasubramanian, D.; Girigoswami, A.; Girigoswami, K. Biomedical Applications of Natural and Synthetic Polymer Based Nanocomposites. J. Biomater. Sci. Polym. Ed. 2024, 35, 269–294. [Google Scholar]
- Wang, Y.; Zhang, K.; Li, T.; Maruf, A.; Qin, X.; Luo, L.; Zhong, Y.; Qiu, J.; McGinty, S.; Pontrelli, G.; et al. Macrophage Membrane Functionalized Biomimetic Nanoparticles for Targeted Anti-Atherosclerosis Applications. Theranostics 2021, 11, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Niu, H.; Wen, J.; Dang, Y.; Zayed, M.; Guan, J. Rescuing Cardiac Cells and Improving Cardiac Function by Targeted Delivery of Oxygen-Releasing Nanoparticles after or Even before Acute Myocardial Infarction. ACS Nano 2022, 16, 19551–19566. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, G.; Joy, A.; Megha, M.; Kolanthai, E.; Senthilkumar, M. Exploration of Inorganic Nanoparticles for Revolutionary Drug Delivery Applications: A Critical Review. Discov. Nano 2023, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Scafa Udriște, A.; Burdușel, A.; Niculescu, A.-G.; Rădulescu, M.; Grumezescu, A. Metal-Based Nanoparticles for Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 1001. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhu, B.; Tian, A.; Li, Z. PEG-Coated Gold Nanoparticles Attenuate β;-Adrenergic Receptor-Mediated Cardiac Hypertrophy. Int. J. Nanomed. 2017, 12, 4709–4719. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, N.; Hou, Y.; Li, Y.; Yin, C.; Yang, E.; Cao, H.; Hu, G.; Xue, J.; Yang, J.; et al. L-Arginine-Loaded Gold Nanocages Ameliorate Myocardial Ischemia/Reperfusion Injury by Promoting Nitric Oxide Production and Maintaining Mitochondrial Function. Adv. Sci. 2023, 10, 2302123. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, T.; Lio, C.; Yu, X.; Chen, X.; Liu, L.; Wu, Y.; Huang, H.; Qing, L.; Luo, P. Surface Hydrolysis-Designed AuNPs-Zwitterionic-Glucose as a Novel Tool for Targeting Macrophage Visualization and Delivery into Infarcted Hearts. J. Control. Release 2023, 356, 678–690. [Google Scholar] [CrossRef]
- Chen, J.; Ren, T.; Xie, L.; Hu, H.; Li, X.; Maitusong, M.; Zhou, X.; Hu, W.; Xu, D.; Qian, Y.; et al. Enhancing Aortic Valve Drug Delivery with PAR2-Targeting Magnetic Nano-Cargoes for Calcification Alleviation. Nat. Commun. 2024, 15, 557. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Z.; Huang, S.; Ye, K.; Jiang, Y.; Liu, J.; Liu, J.; Lu, X.; Li, B. A Metal-Organic Framework-Based Immunomodulatory Nanoplatform for Anti-Atherosclerosis Treatment. J. Control. Release 2023, 354, 615–625. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; Xu, Y.; Mou, F.; Shan, X.; Wang, Q.; Liu, B.; Ning, K.; Liu, J.; Wang, Y.; et al. Notoginsenoside R1-Loaded Mesoporous Silica Nanoparticles Targeting the Site of Injury through Inflammatory Cells Improves Heart Repair after Myocardial Infarction. Redox Biol. 2022, 54, 102384. [Google Scholar] [CrossRef]
- Barjesteh, T.; Mansur, S.; Bao, Y. Inorganic Nanoparticle-Loaded Exosomes for Biomedical Applications. Molecules 2021, 26, 1135. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal Stromal/Stem Cell (MSC)-Derived Exosomes in Clinical Trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Ma, Z.-J.; Yang, J.-J.; Lu, Y.-B.; Liu, Z.-Y.; Wang, X.-X. Mesenchymal Stem Cell-Derived Exosomes: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. World J. Stem Cells 2020, 12, 814–840. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Z.; Wang, Q.; Gao, J.; Chen, J.; Tan, H.; Li, S.; Wang, Z.; Weng, X.; Yang, H.; et al. Targeted Immunomodulation Therapy for Cardiac Repair by Platelet Membrane Engineering Extracellular Vesicles via Hitching Peripheral Monocytes. Biomaterials 2022, 284, 121529. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Liu, C.; Li, J.; Lu, K.; Yu, Q.; Zhang, Y.; Shen, Z. Injectable Decellularized Extracellular Matrix Hydrogel Loaded with Exosomes Encapsulating Curcumin for Prevention of Cardiac Fibrosis after Myocardial Infarction. J. Mater. Sci. Technol. 2023, 167, 50–58. [Google Scholar] [CrossRef]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Bhardwaj, H.; Jangde, R.K. Current Updated Review on Preparation of Polymeric Nanoparticles for Drug Delivery and Biomedical Applications. Nanotechnology 2023, 2, 100013. [Google Scholar] [CrossRef]
- Komsthöft, T.; Bovone, G.; Bernhard, S.; Tibbitt, M.W. Polymer Functionalization of Inorganic Nanoparticles for Biomedical Applications. Curr. Opin. Chem. Eng. 2022, 37, 100849. [Google Scholar] [CrossRef]
- Guo, D.; Xu, Y.; Ding, J.; Dong, J.; Jia, N.; Li, Y.; Zhang, M. Roles and Clinical Applications of Exosomes in Cardiovascular Disease. BioMed Res. Int. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Joyce, P.; Allen, C.J.; Alonso, M.J.; Ashford, M.; Bradbury, M.S.; Germain, M.; Kavallaris, M.; Langer, R.; Lammers, T.; Peracchia, M.T.; et al. A Translational Framework to DELIVER Nanomedicines to the Clinic. Nat. Nanotechnol. 2024, 19, 1597–1611. [Google Scholar] [CrossRef]
- Shan, X.; Gong, X.; Li, J.; Wen, J.; Li, Y.; Zhang, Z. Current Approaches of Nanomedicines in the Market and Various Stage of Clinical Translation. Acta Pharm. Sin. B 2022, 12, 3028–3048. [Google Scholar] [CrossRef] [PubMed]
- Matkar, P.N.; Leong-Poi, H.; Singh, K.K. Cardiac Gene Therapy: Are We There Yet? Gene Ther. 2016, 23, 635–648. [Google Scholar] [CrossRef] [PubMed]

| Characterization | LV | AdV | AAV |
|---|---|---|---|
| Genome Type | RNA | dsDNA | ssDNA |
| Host Genome Integration | Integration | Non-integration | Non-integration |
| Duration of Expression | Long | Short or medium | Medium or long |
| Immunogenicity | Low to medium | Medium to high | Low |
| Gene Carrying Capacity | Larger (up to 9 kb) | Larger (up to 7.5–8 kb) | Smaller (5 kb) |
| Dominance | Long-term stable expression | High-efficiency transfection; broad tissue tropism; vaccine development | Low immunogenicity; High safety; cardiac affinity |
| Challenge | Mutations | Immune response; transient gene expression | Limited carrying capacity; pre-existing immunity; high cost |
| Applications | Cardiac transplantation | Myocardial perfusion; angina symptoms | Myocardial infarction, Myocardial hypertrophy and atherosclerosis |
| References | [49] | [53] | [55,56,57,58,59,60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Yu, T.; Gong, J.; Shan, H. Advanced Nanomedicine Delivery Systems for Cardiovascular Diseases: Viral and Non-Viral Strategies in Targeted Therapy. Molecules 2025, 30, 962. https://doi.org/10.3390/molecules30040962
Chen Q, Yu T, Gong J, Shan H. Advanced Nanomedicine Delivery Systems for Cardiovascular Diseases: Viral and Non-Viral Strategies in Targeted Therapy. Molecules. 2025; 30(4):962. https://doi.org/10.3390/molecules30040962
Chicago/Turabian StyleChen, Qian, Tong Yu, Jingyi Gong, and Hongli Shan. 2025. "Advanced Nanomedicine Delivery Systems for Cardiovascular Diseases: Viral and Non-Viral Strategies in Targeted Therapy" Molecules 30, no. 4: 962. https://doi.org/10.3390/molecules30040962
APA StyleChen, Q., Yu, T., Gong, J., & Shan, H. (2025). Advanced Nanomedicine Delivery Systems for Cardiovascular Diseases: Viral and Non-Viral Strategies in Targeted Therapy. Molecules, 30(4), 962. https://doi.org/10.3390/molecules30040962






