Co-Improvement in Electrocatalytic Hydrogen Evolution Performance of MoS2 by Ni Doping and Graphene Oxide Compounding
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Preparation Temperature on Structure and HER Properties of Ni-MoS2
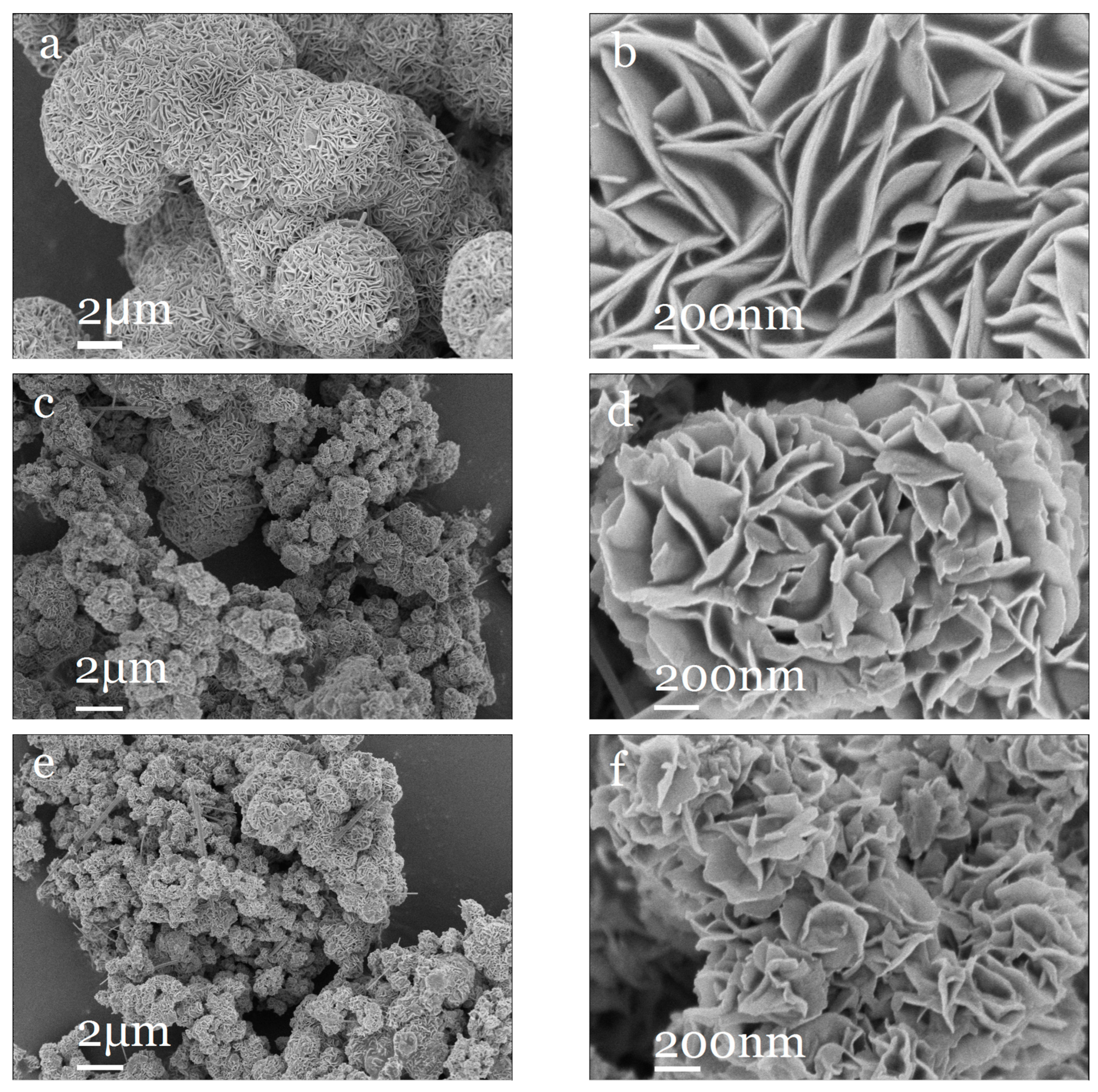
2.1.1. Material Structure and Morphology
2.1.2. Electrocatalytic Hydrogen Evolution Performance
2.2. Effect of Ni Doping Amount on Structure and HER Properties of Ni-MoS2
2.2.1. Material Structure and Morphology
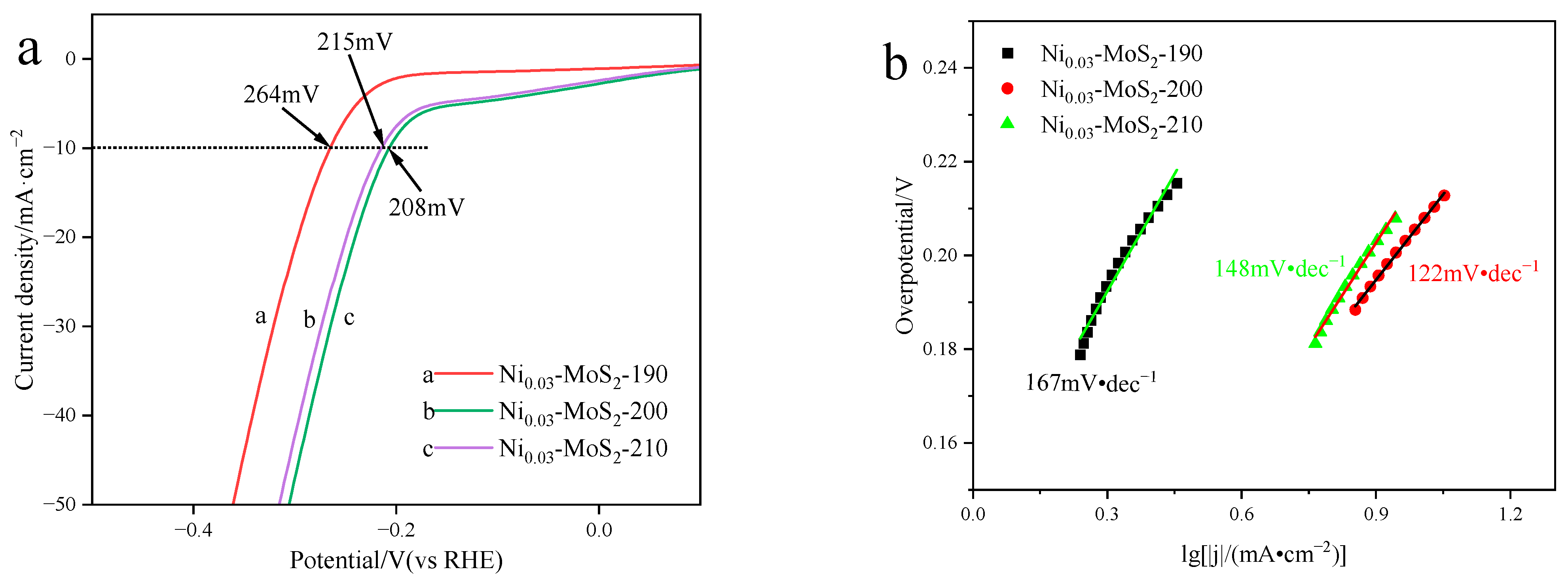
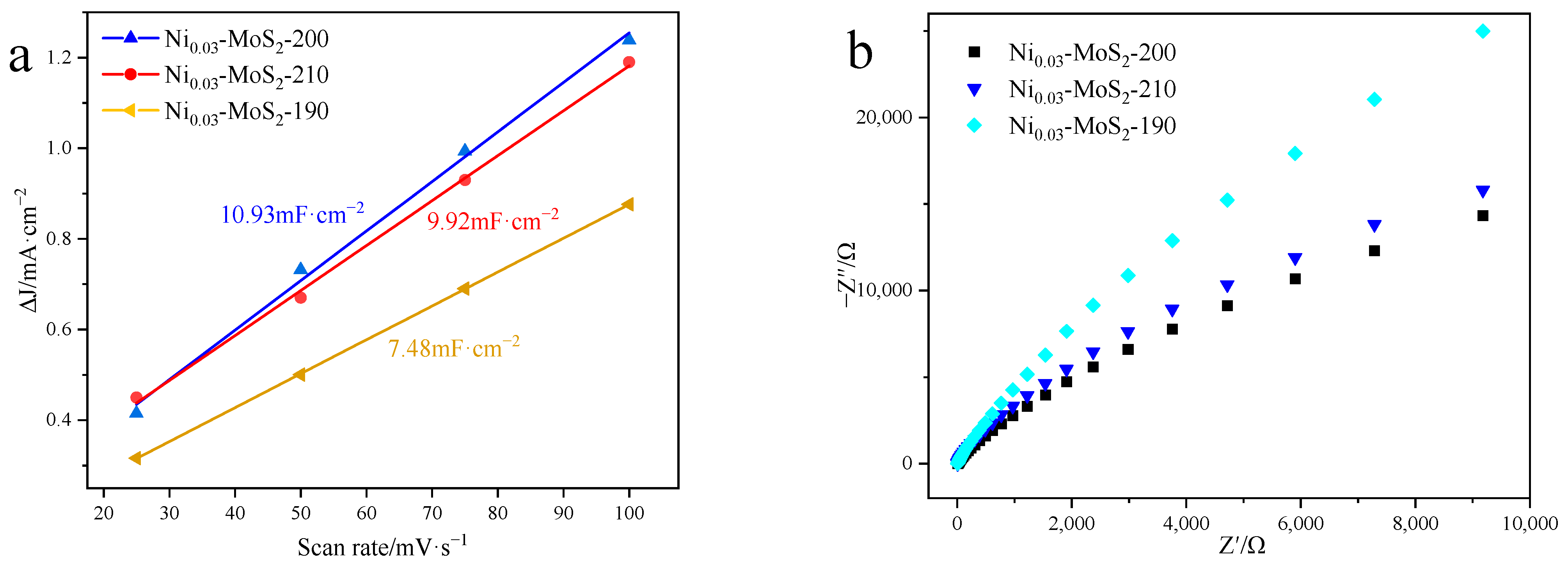
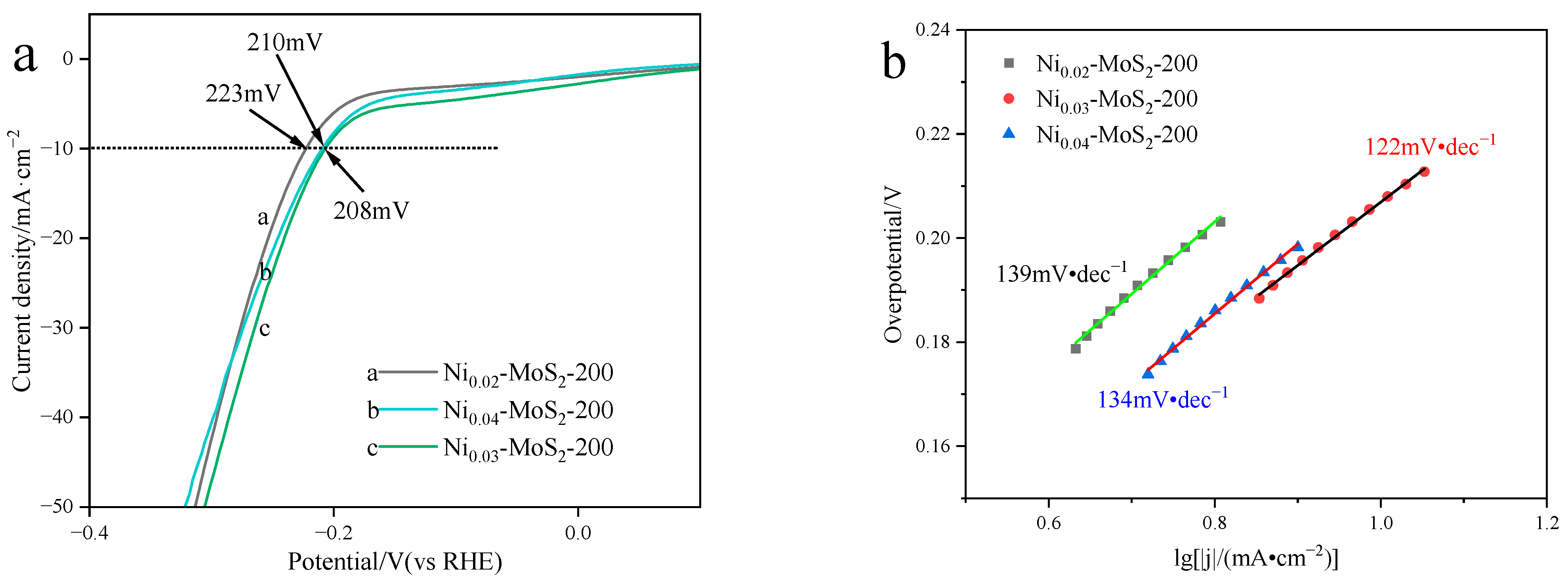
2.2.2. Electrocatalytic Hydrogen Evolution Performance
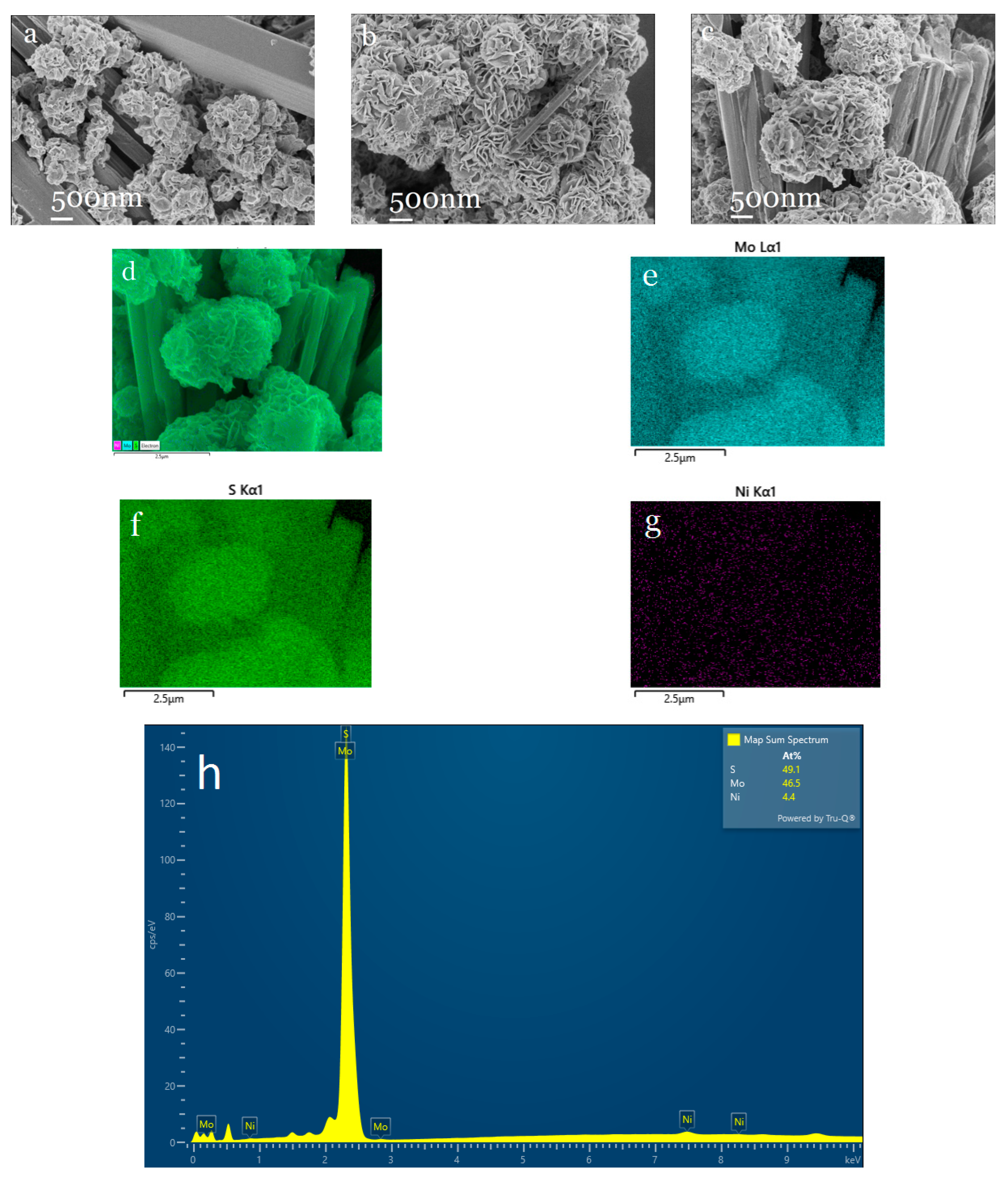
2.3. Characterization of Ni0.03-MoS2-200/GO and Study of HER Performance
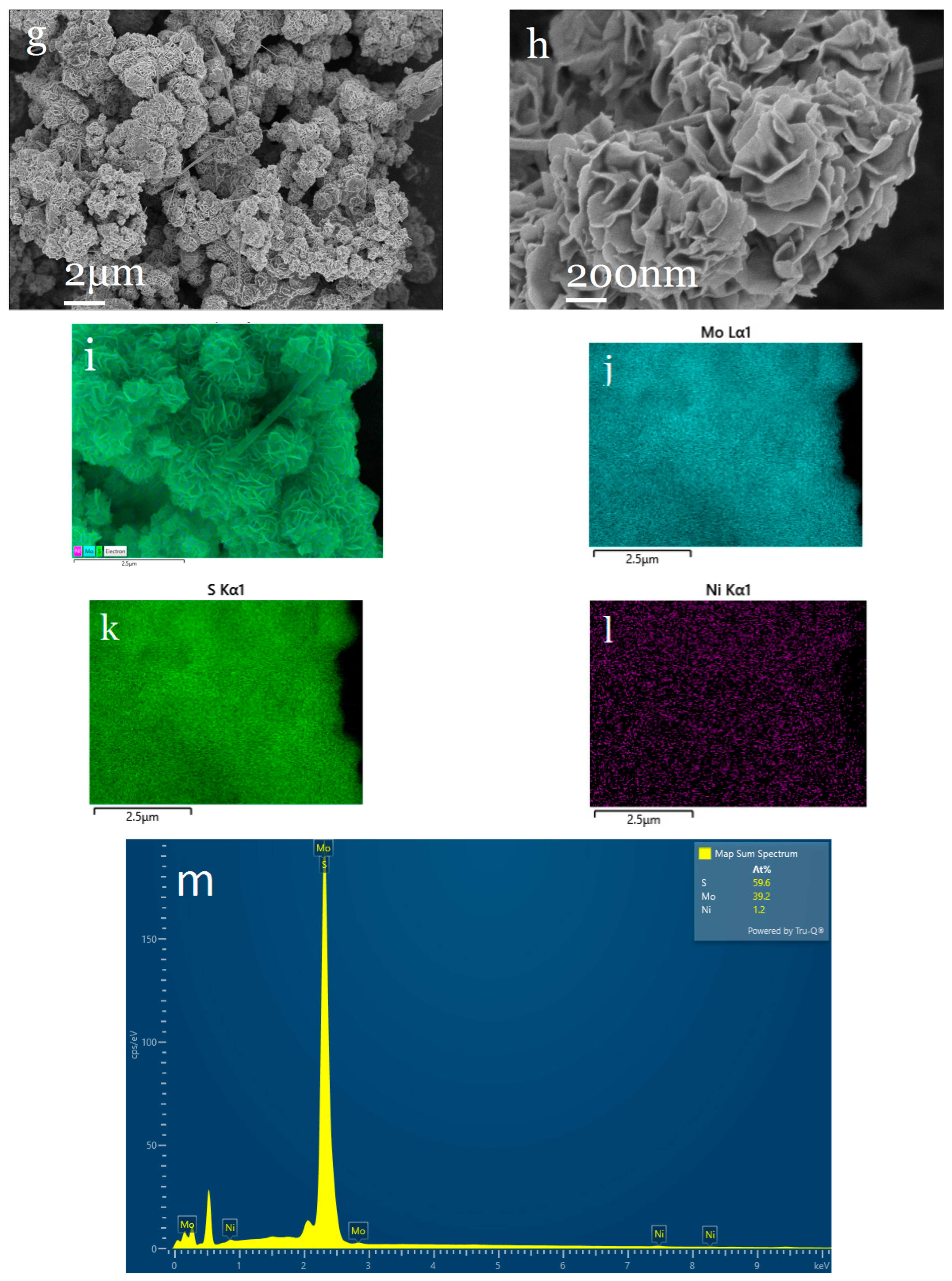
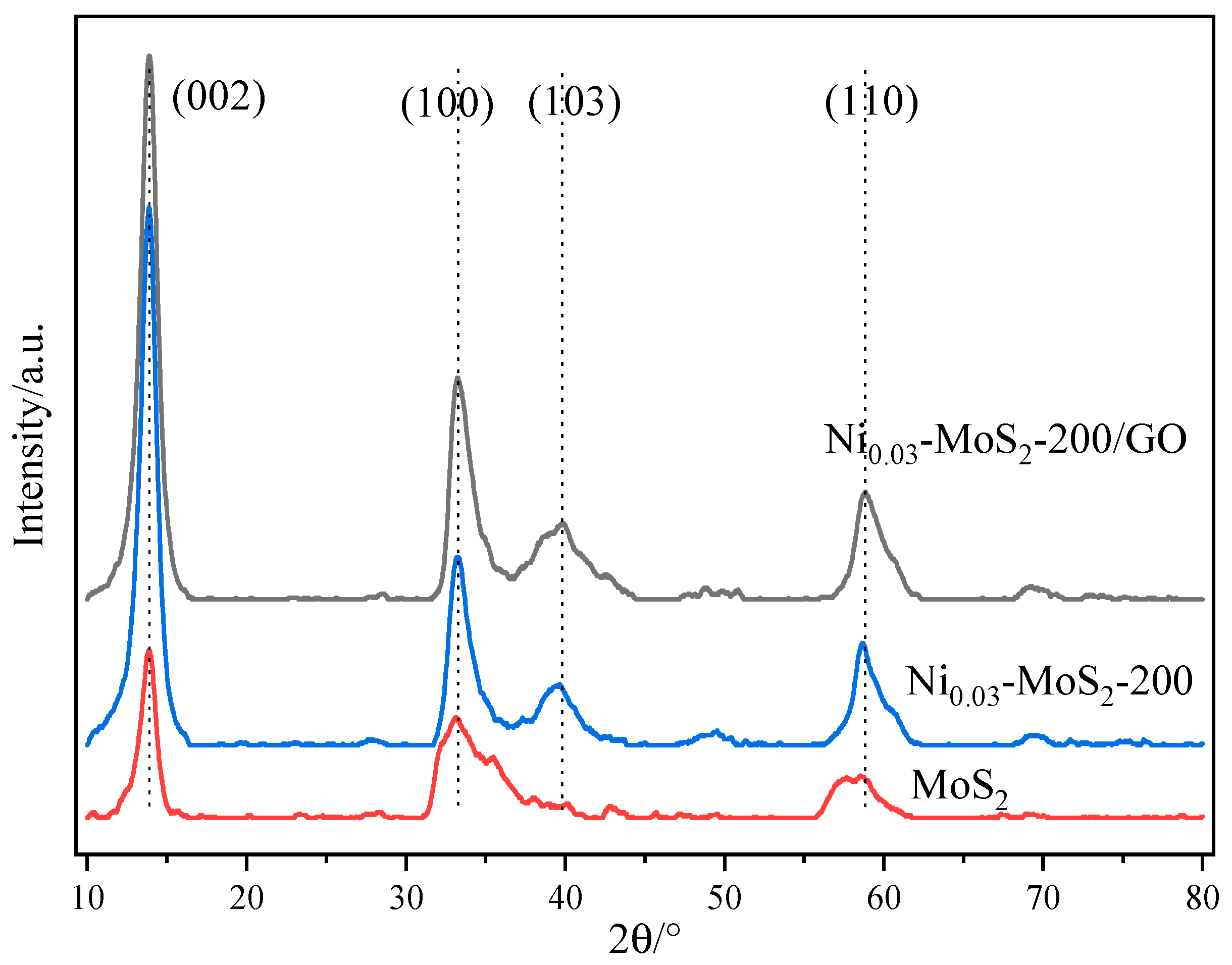
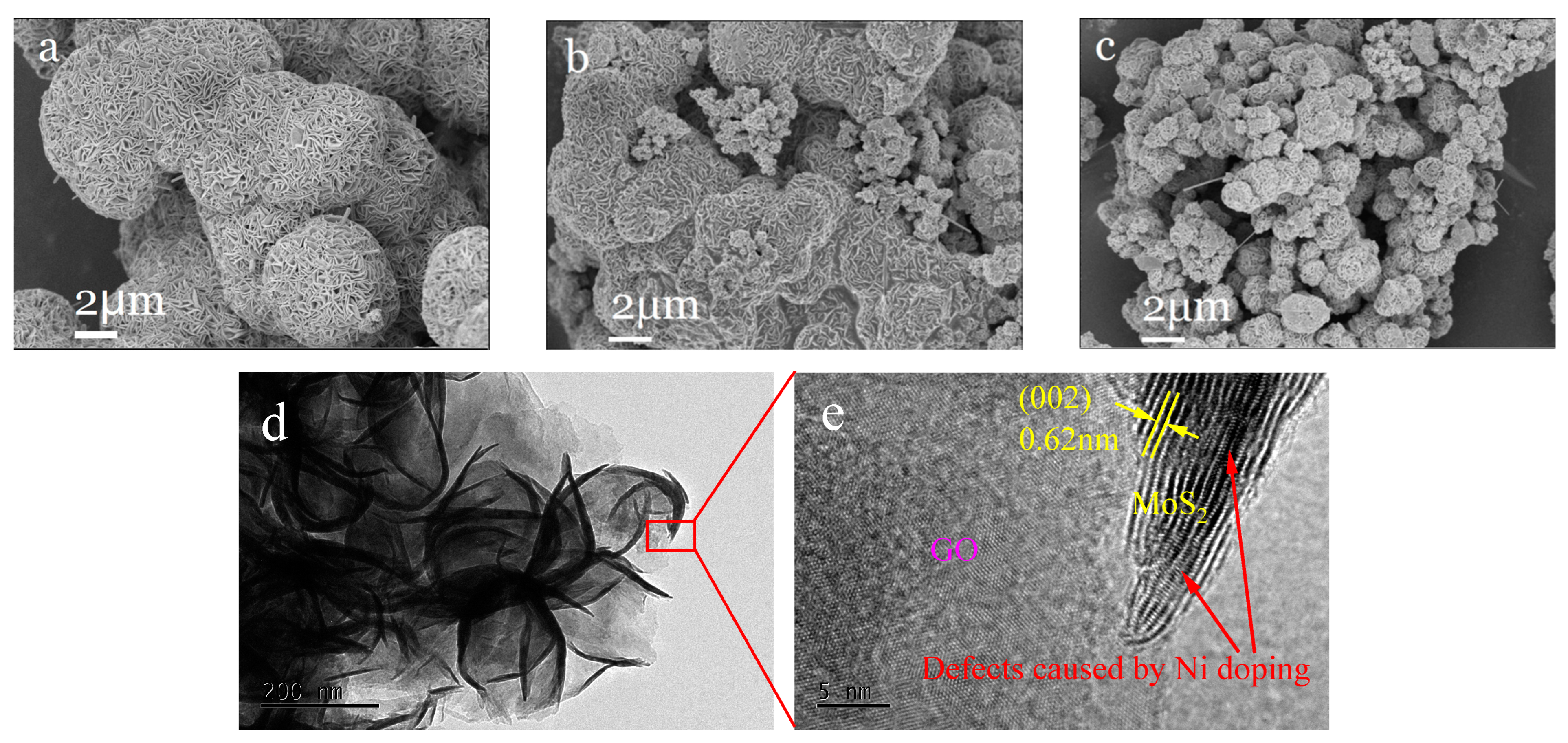
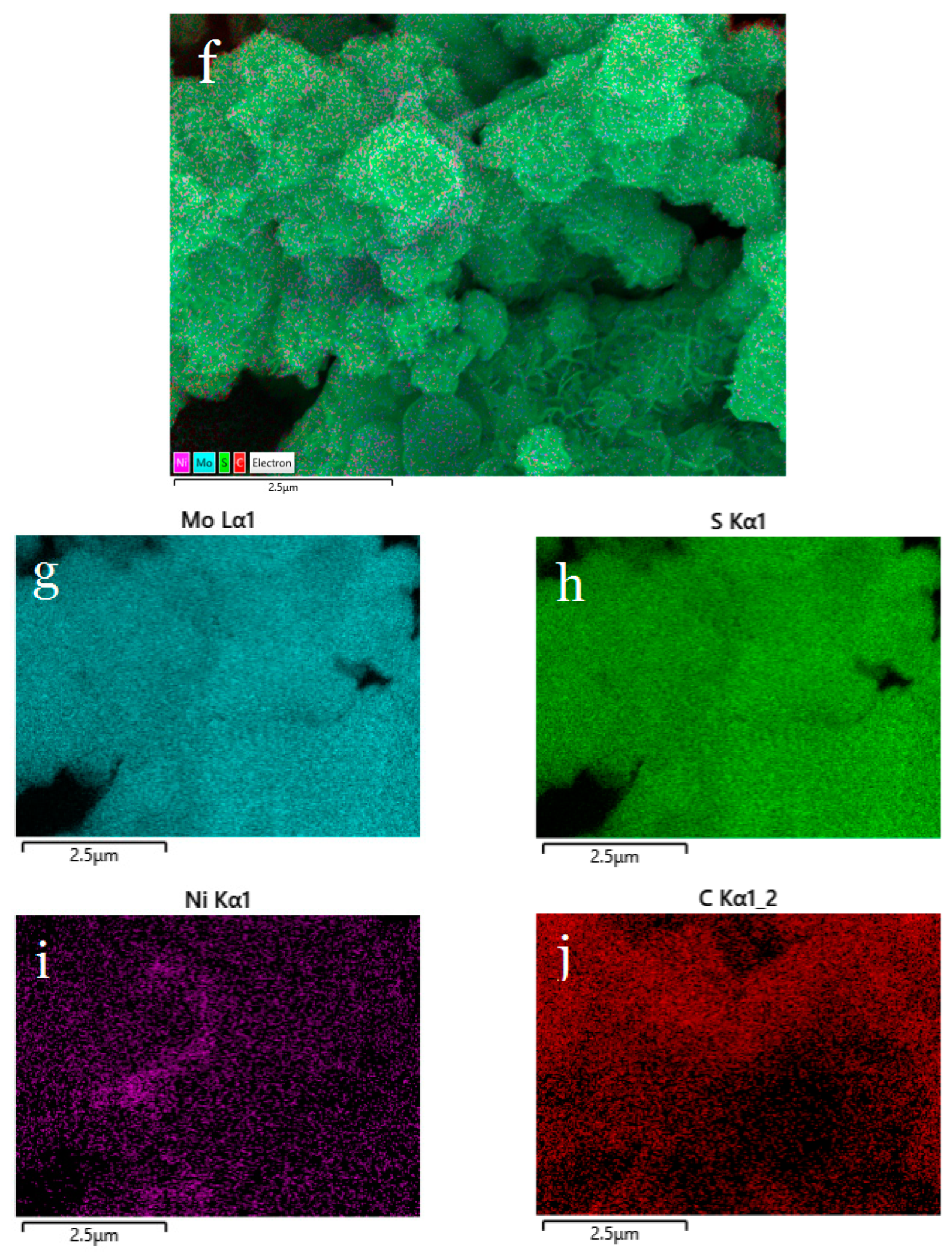
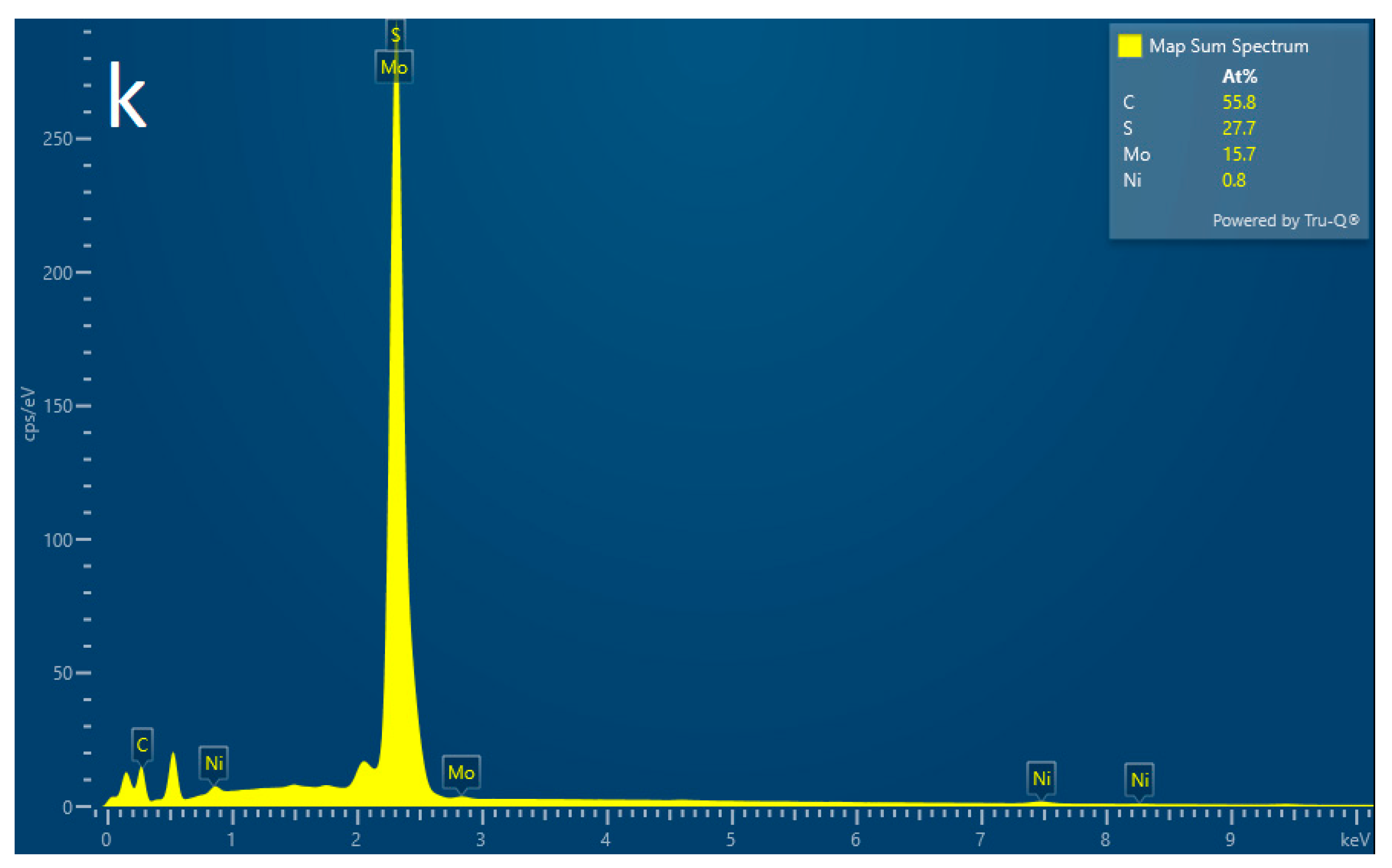
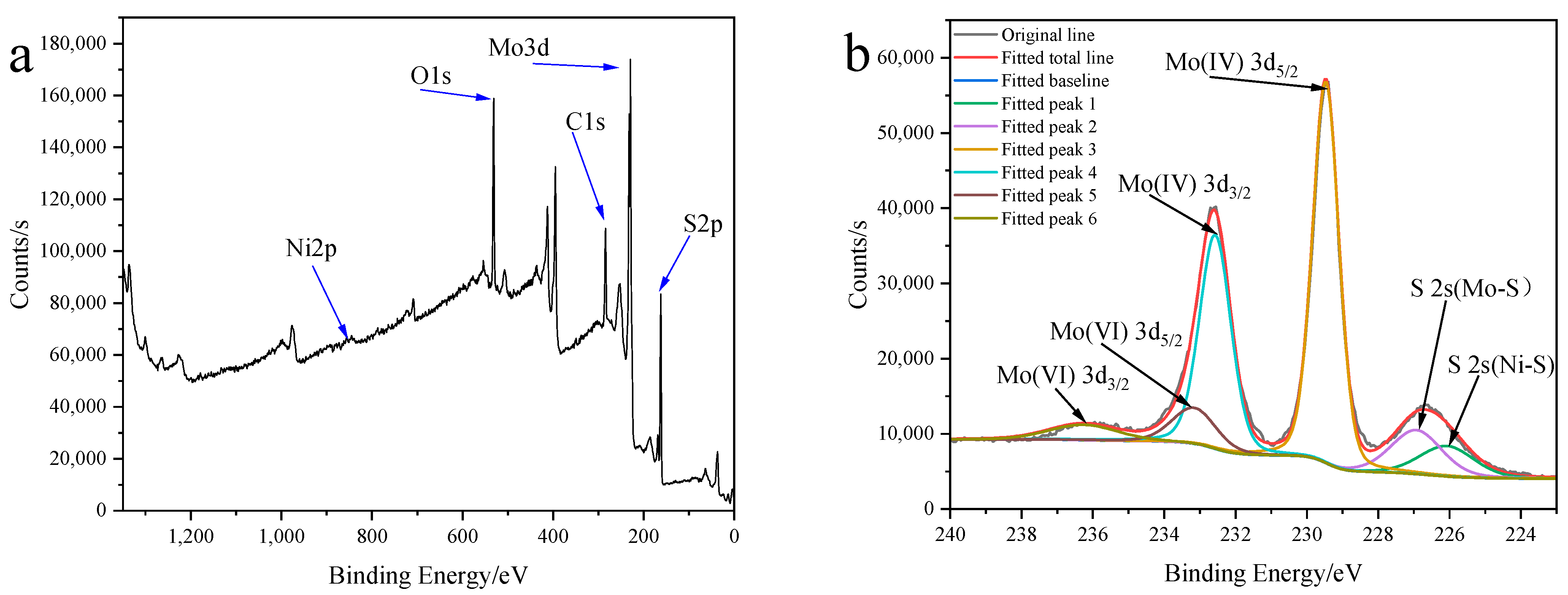
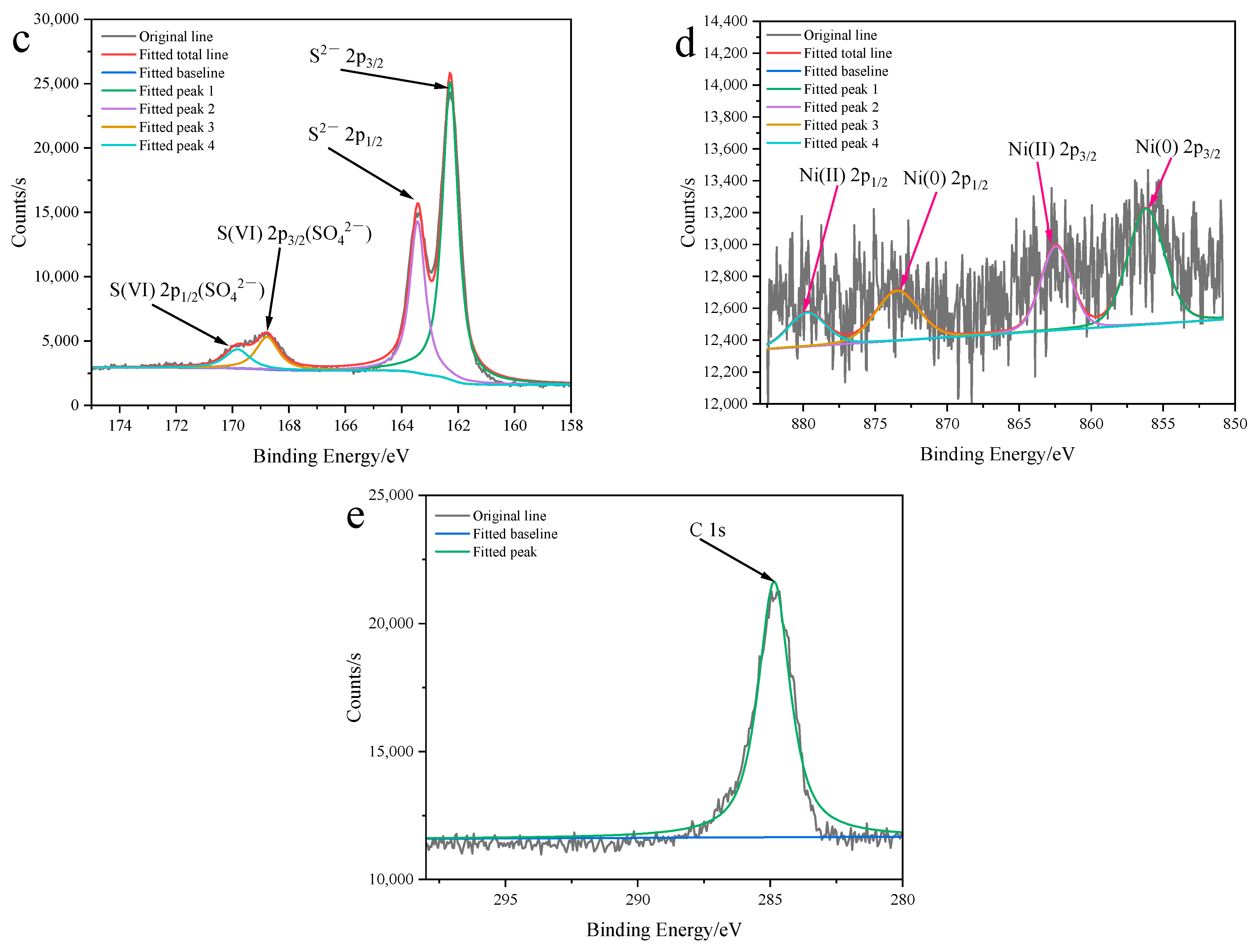
2.3.1. Characterization of Structure and Morphology of Ni0.03-MoS2-200/GO
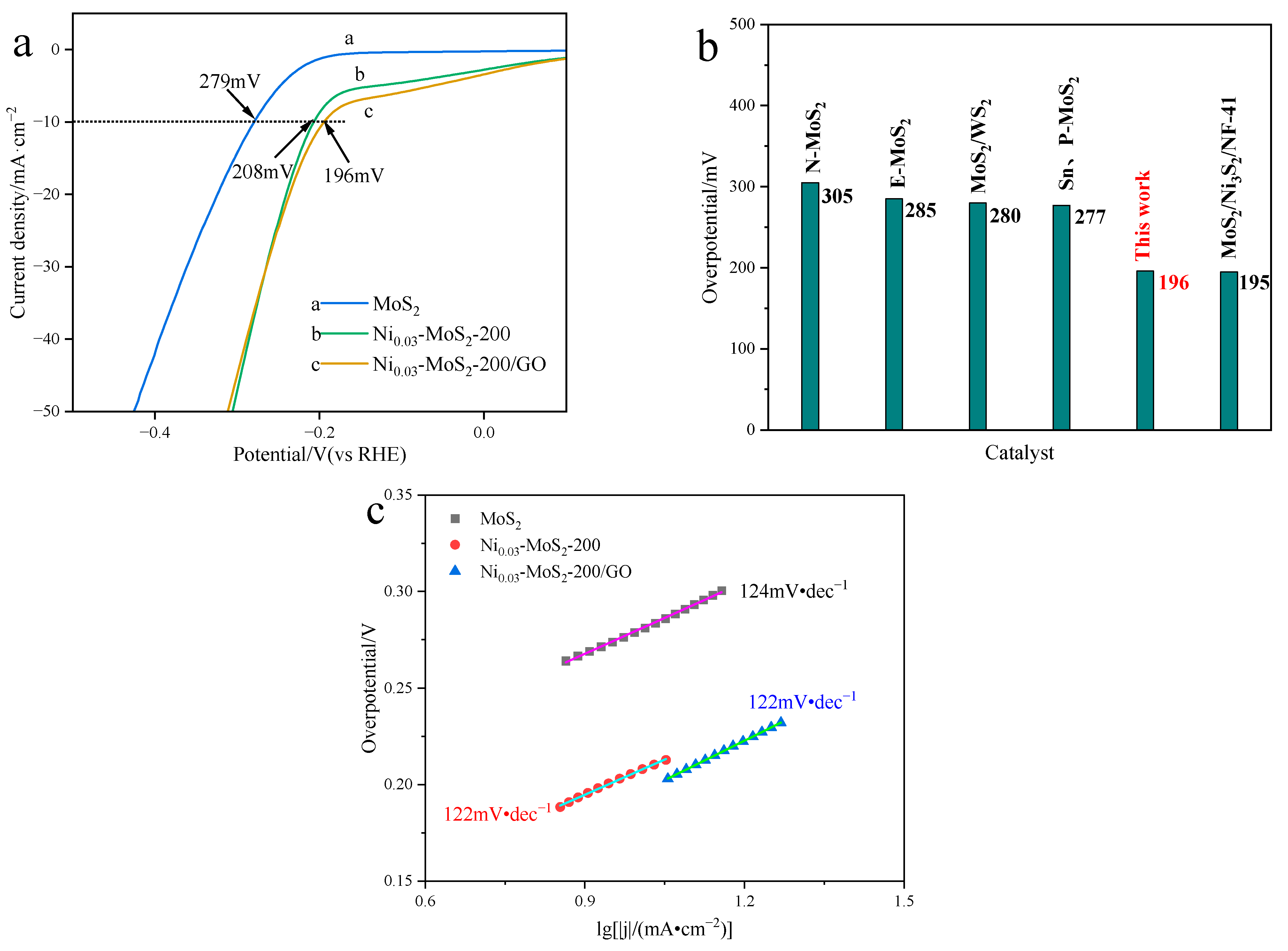
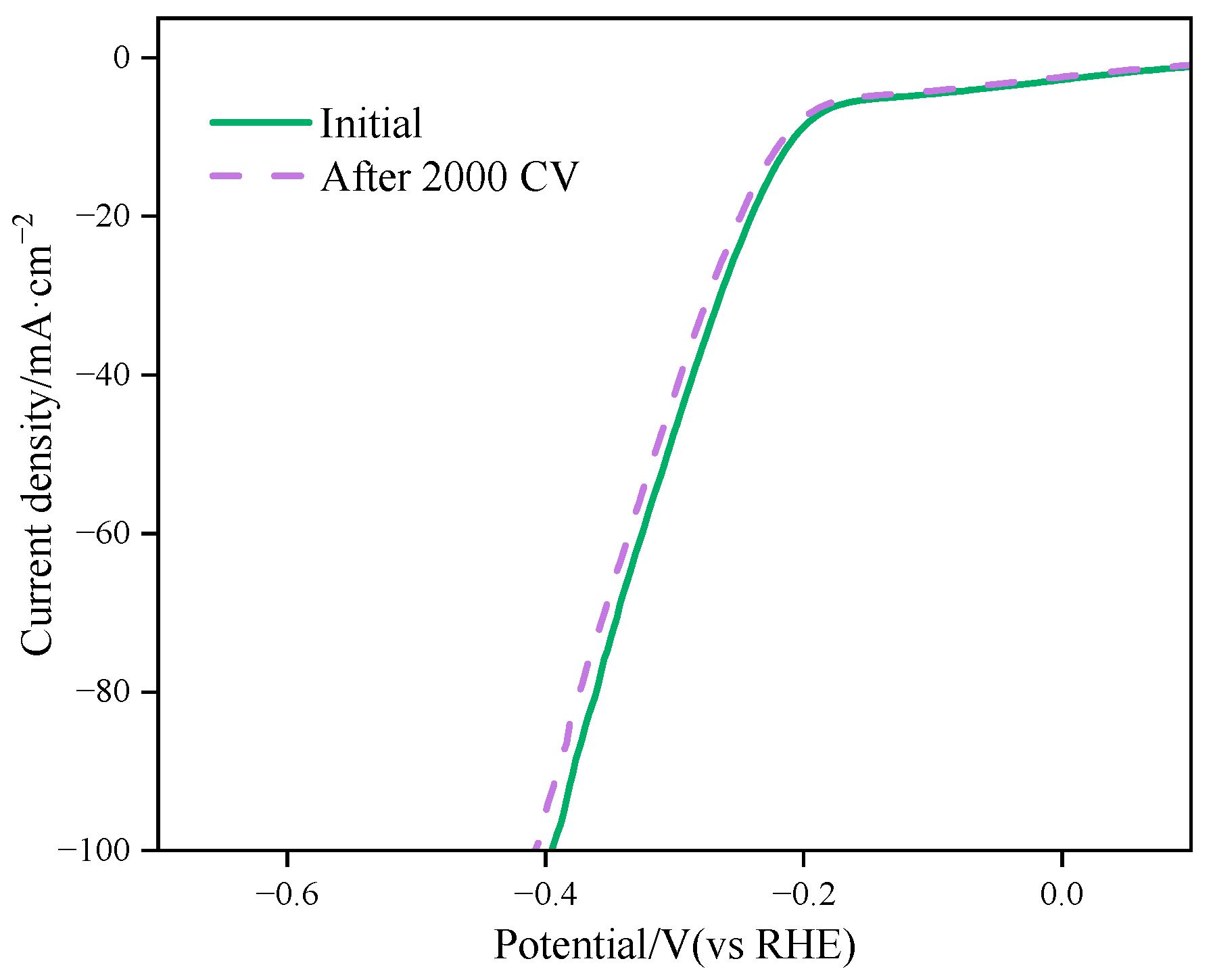
2.3.2. Performance Test of Electrocatalytic Hydrogen Evolution
3. Experimental Section
3.1. Reagents
3.2. Catalyst Synthesis
3.2.1. Preparation of MoS2 and Ni-MoS2
3.2.2. Preparation of GO
3.2.3. Preparation of Composite Material Ni0.03-MoS2-200/GO
3.3. Catalyst Characterization
3.4. Electrocatalytic Hydrogen Evolution Test of Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wickramaratne, K.M.K.; Ramezanipour, F. Electrocatalytic properties of quasi-2D oxides LaSrMn0.5M0.5O4 (M = Co, Ni, Cu, and Zn) for hydrogen and oxygen evolution reactions. Molecules 2024, 29, 3107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Tian, S.Q.; Liu, X.N.; An, X.G.; Zhang, J.X.; Xu, L.H.; Yao, W.T.; Kong, Q.Q. Morphology-controlled synthesis of V1.11S2 for electrocatalytic hydrogen evolution reaction in acid media. Molecules 2022, 27, 8019. [Google Scholar] [CrossRef]
- Meng, C.; Chen, X.D.; Gao, Y.F.; Zhao, Q.Q.; Kong, D.; Lin, M.C.; Chen, X.M.; Li, Y.X.; Zhou, Y. Recent modification strategies of MoS2 for enhanced electrocatalytic hydrogen evolution. Molecules 2020, 25, 1136. [Google Scholar] [CrossRef] [PubMed]
- Abdelghafar, F.; Xu, X.M.; Jiang, S.P.; Shao, Z.P. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep.-Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Pehlivan, L.B.; Atak, G.; Niklasson, G.A. Electrochromic solar water splitting using a cathodic WO3 electrocatalyst. Nano Energy 2021, 81, 105620. [Google Scholar] [CrossRef]
- Jiang, X.L.; Jang, H.; Liu, S.G. Heterostructure of Ru2P/WO3/NPC synergistically promotes H2O dissociation for improved hydrogen evolution. Angew. Chem. Int. Edit. 2021, 60, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczuk, M.; Poks, P. The HER performance of colloidal Pt nanoparticles incorporated in polyaniline. Electrochim. Acta 2000, 45, 4171–4177. [Google Scholar] [CrossRef]
- Liu, X.T.; Wang, C.W. In situ wet etching of MoS2@dWO3 heterostructure as ultra-stable highly active electrocatalyst for hydrogen evolution reaction. Catalysts 2020, 10, 977. [Google Scholar] [CrossRef]
- Xu, X.M.; Pan, Y.L.; Zhong, Y.J.; Ge, L.; Jiang, S.P.; Shao, Z.P. From scheelite BaMoO4 to perovskite BaMoO3: Enhanced electrocatalysis toward the hydrogen evolution in alkaline media. Compos. Part B-Eng. 2020, 198, 108214. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, K.; Jiang, B. Emerging two dimensional nanomaterials for electrochemical hydrogen evolution. J. Mater. Chem. A 2017, 5, 187–208. [Google Scholar] [CrossRef]
- Gupta, U.; Rao, C. Hydrogen generation by water splitting using MoS2 and other transition metal dichalcogenides. Nano Energy 2017, 41, 49–65. [Google Scholar] [CrossRef]
- Xu, X.M.; Zhong, Y.J.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z.P. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Wang, X.; Yao, X.L.; Hou, J.F. Non-isothermal crystallization of aqueous graphene oxide suspensions. J. Zhejiang Univ. (Eng. Sci.) 2014, 48, 1273–1277. [Google Scholar]
- Li, Y.G.; Wang, H.L.; Xie, L.M. MoS2 nanoparticles grown on graphene: An advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Miao, P.; Zhang, Y. Significantly increased raman enhancement on MoX2 (X = S, Se) monolayers upon phase transition. Adv. Funct. Mater. 2017, 27, 16066–16094. [Google Scholar] [CrossRef]
- Tang, L.H.; Wang, Y.; Li, Y.M. Preparation, structure, and electrochemical properties of reduced graphene sheet films. Adv. Funct. Mater. 2009, 19, 2782–2789. [Google Scholar] [CrossRef]
- Lin, W.J.; Liao, C.S.; Jhang, J.H. Graphene modified basal and edge plane pyrolytic graphite electrodes for electrocatalytic oxidation of hydrogen peroxide and β-nicotinamide adenine dinucleotide. Electrochem. Commun. 2009, 11, 2153–2156. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Wang, L.F.; Chen, M.Y. W2N/WC composite nanofibers as an efficient electrocatalyst for photoelectrochemical hydrogen evolution. RSC Adv. 2021, 11, 20285–20291. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.P.; Yeom, G.Y. Recent advances in doping of molybdenum disulfide: Industrial applications and future prospects. Adv. Mater. 2016, 28, 9024. [Google Scholar] [CrossRef]
- Xue, J.Y.; Li, F.L.; Zhao, Z.Y. Roadmap and direction towards high performance MoS2 hydrogenevolution catalysts. Inorg. Chem. 2019, 58, 11202–11209. [Google Scholar] [CrossRef]
- Zhao, M.X.; Yang, M.Y.; Huang, W.J. Synergism on electronic structures and active edges of metallic vanadium disulfide nanosheets via Co doping for efficient hydrogen evolution reaction in seawater. ChemCatChem 2021, 13, 2138. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S. Effect of Ni doping on electrocatalytic hydrogen evolution activity of MoS2. Int. J. Electrochem. Sci. 2019, 14, 11607–11615. [Google Scholar] [CrossRef]
- Li, M.; Cai, B.; Tian, R. Vanadium doped 1T MoS2 nanosheets for highly efficient electrocatalytic hydrogen evolution in both acidic and alkaline solutions. Chem. Eng. J. 2021, 409, 128158. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, M.W.; Sun, Z.M. In situ construction of a Mn2+-doped Ni3S2 electrode with highly enhanced urea oxidation reaction performance. ACS Sustain. Chem. Eng. 2020, 8, 8348. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, L.S.; Liu, Z.A. A Zn-doped Ni3S2 nanosheet array as a high-performance electrochemical water oxidation catalyst in alkaline solution. Chem. Commun. 2017, 53, 12446. [Google Scholar] [CrossRef]
- Bonde, J.; Moses, P.G.; Jaramillo, T.F. Hydrogen evolution on nano-particulate transition metal sulfides. Faraday Discuss. 2008, 140, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef]
- Merki, D.; Vrubel, H.; Rovelli, L. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 2012, 3, 15–25. [Google Scholar] [CrossRef]
- Wang, H.; Tsai, C.; Kong, D. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution. Nano Res. 2015, 8, 566–575. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, G.; Liu, X.; Shen, X.; Lv, J.; Hu, C.; Wang, Y.; Tan, W.; Sun, S.; Ma, Y.; et al. One step hydrothermal synthesis of Ni-MoS2-RGO bifunctional electrocatalysts for HER and OER. Int. J. Electrochem. Sci. 2021, 16, 210323. [Google Scholar] [CrossRef]
- Chen, L.X.; Chen, Z.W.; Wang, Y.; Yang, C.C.; Jiang, Q. Design of dual-modified MoS2 with nanoporous Ni and graphene as efficient catalysts for the hydrogen evolution reaction. ACS Catal. 2018, 8, 8107–8114. [Google Scholar] [CrossRef]
- Lin, C.; Gao, Z.; Jin, J. Boosting alkaline hydrogen evolution activity with Ni-doped MoS2/reduced graphene oxide hybrid aerogel. ChemSusChem 2019, 12, 457–466. [Google Scholar] [CrossRef]
- Yin, X.; Sun, G.; Song, A.; Wang, L.; Wang, Y.; Dong, H.; Shao, G. A novel structure of Ni-(MoS2/GO) composite coatings deposited on Ni foam under supergravity field as efficient hydrogen evolution reaction catalysts in alkaline solution. Electrochim. Acta 2017, 249, 52–63. [Google Scholar] [CrossRef]
- Sun, X.; Dai, J.; Guo, Y. Semimetallic molybdenum disulfide ultrathin nanosheets as an efficient electrocatalyst for hydrogen evolution. Nanoscale 2014, 6, 8359. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, Y.; Sun, Z. Ga doped Ni3S2 ultrathin nanosheet arrays supported on Ti3C2-MXene/Ni foam: An efficient and stable 3D electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 2958. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Zhang, Y. Preparation of highly efficient Al-doped ZnO photocatalyst by combustion synthesis. Curr. Appl. Phys. 2013, 13, 697. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, W.; Ma, L. Dimeric [Mo2S12]2− cluster: A molecular analogue of MoS2 edges for superior hydrogen-evolution electrocatalysis. Angew. Chem. Int. Edit. 2015, 54, 1518. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, X.; Zhao, Y. Mn doped MoS2/reduced graphene oxide hybrid for enhanced hydrogen evolution. Appl. Surf. Sci. 2017, 425, 470–477. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Shen, Y. Ni-doped MoS2 nanoparticles as highly active hydrogen evolution electrocatalysts. RSC Adv. 2016, 6, 56–61. [Google Scholar] [CrossRef]
- Yin, Y.; Han, J.; Zhang, Y. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets. J. Am. Chem. Soc. 2016, 138, 7965–7972. [Google Scholar] [CrossRef]
- Ye, J.; Yu, Z.; Chen, W. Ionic-liquid mediated synthesis of molybdenum disulfide/grapheme composites: An enhanced electrochemical hydrogen evolution catalyst. Int. J. Hydrogen Energy 2016, 41, 49–61. [Google Scholar] [CrossRef]
- Leyral, G.; Brillouet, S.; Rousseau, J. Effect of the presence of ionic liquid during the NiMoS bulk preparation in the transformation of decanoic acid. Appl. Catal. A-Gen. 2017, 532, 120–132. [Google Scholar] [CrossRef]
- Sahoo, M.; Ramaprabhu, S. One-pot environment-friendly synthesis of boron doped graphene-SnO2 for anodic performance in Li ion battery. Carbon 2018, 127, 27–35. [Google Scholar] [CrossRef]
- Zheng, X.J.; Chen, L.Y.; Wang, J.W.; Zhu, H.W.; He, W.Y. One-step synthesis and enhanced electrocatalytic hydrogen evolution performance of interlayer-expanded molybdenum disulfide. J. Mater. Eng. 2023, 51, 84–92. [Google Scholar]
- Zhou, L.; He, W.Y.; Chen, L.Y.; Zhu, H.W.; Chen, L.J.; Ling, H.; Zheng, X.J. Preparation of Sn, P co-doped MoS2 nanoflowers and their electrocatalytic hydrogen evolution performance. Mater. Rep. 2023, 37, 20–26. [Google Scholar]
- Yang, C.G.; Huang, R.; Wang, D.E.; Tian, Z.J. Electrocatalytic hydrogen evolution performance of nitrogen-doped molybdenum disulfide nanocatalysts. Chem. Ind. Eng. Prog. 2024, 43, 465–472. [Google Scholar]
- Wang, D.Z.; Yang, L.Y.Y.; Liu, R.Q.; Guo, T.; Fei, H.; Wu, Z.Z. Preparation and electrocatalytic hydrogen evolution performance of spherical hollow MoS2/WS2 heterostructures. Trans. Nonferrous Metal. Soc. 2023, 33, 1540–1549. [Google Scholar] [CrossRef]
- Jia, F.H.; Guo, Y.C.; Zou, X.Y.; Wei, X.L.; Bao, W.W.; Li, Y. Highly efficient electrocatalytic hydrogen evolution behavior of MoS2/Ni3S2/NF in all-pH range. Fine Chem. 2023, 40, 1994–2002. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Li, Y.; Zhang, S.; Xing, C.; Wang, Q. Co-Improvement in Electrocatalytic Hydrogen Evolution Performance of MoS2 by Ni Doping and Graphene Oxide Compounding. Molecules 2025, 30, 963. https://doi.org/10.3390/molecules30040963
Guo G, Li Y, Zhang S, Xing C, Wang Q. Co-Improvement in Electrocatalytic Hydrogen Evolution Performance of MoS2 by Ni Doping and Graphene Oxide Compounding. Molecules. 2025; 30(4):963. https://doi.org/10.3390/molecules30040963
Chicago/Turabian StyleGuo, Guiquan, Yuqin Li, Shujiao Zhang, Cuijuan Xing, and Qi Wang. 2025. "Co-Improvement in Electrocatalytic Hydrogen Evolution Performance of MoS2 by Ni Doping and Graphene Oxide Compounding" Molecules 30, no. 4: 963. https://doi.org/10.3390/molecules30040963
APA StyleGuo, G., Li, Y., Zhang, S., Xing, C., & Wang, Q. (2025). Co-Improvement in Electrocatalytic Hydrogen Evolution Performance of MoS2 by Ni Doping and Graphene Oxide Compounding. Molecules, 30(4), 963. https://doi.org/10.3390/molecules30040963




