Structure Elucidation and Characterization of Novel Glycolipid Biosurfactant Produced by Rouxiella badensis DSM 100043T
Abstract
1. Introduction
2. Results and Discussion
2.1. Shake Flask and Bioreactor Cultivation
2.2. Purification and Structure Elucidation of Glucose–Lipid
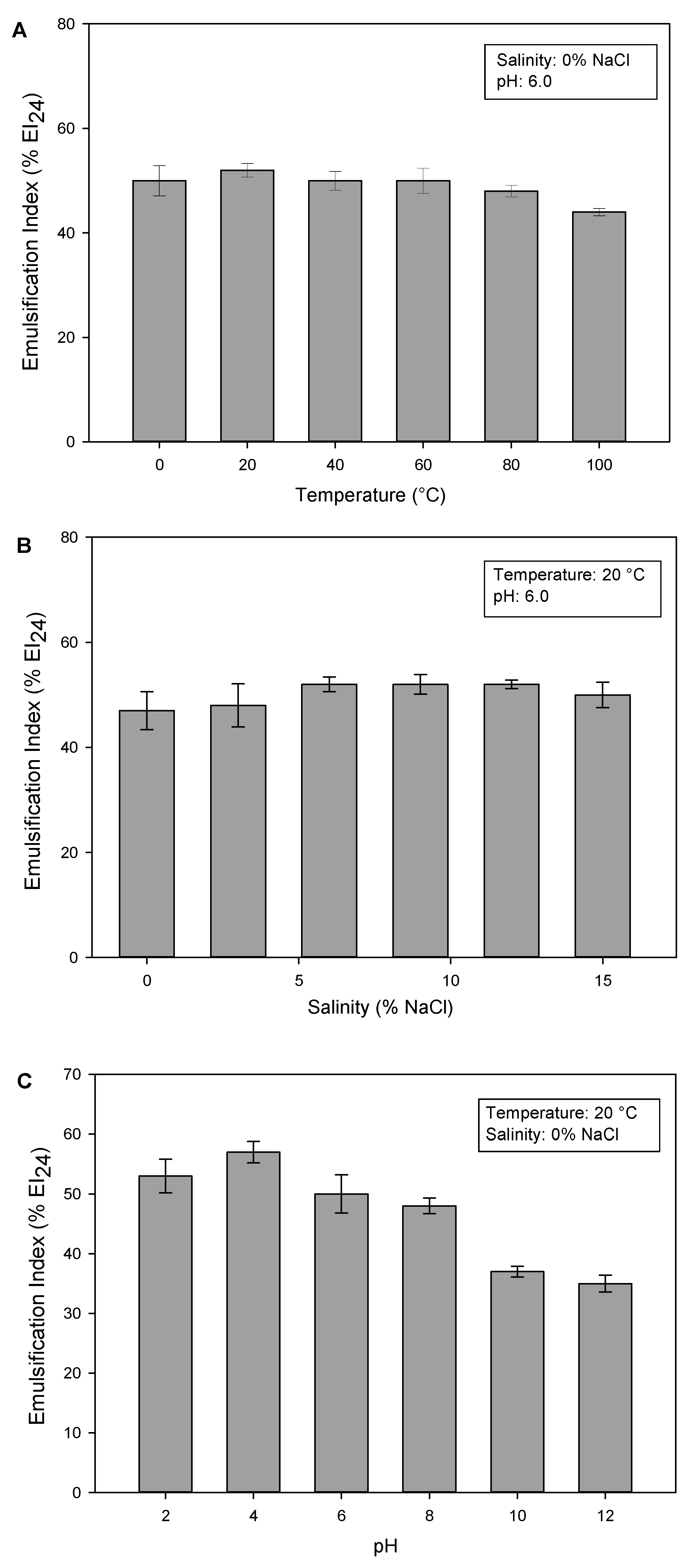
2.3. Characterization of Novel Glucose–Lipid Biosurfactant
3. Materials and Methods
3.1. Media and Cultivation Procedures
3.1.1. Shake Flask Cultivations
3.1.2. Bioreactor Cultivation
3.2. Sample Quantitative Analysis
3.2.1. Glycerol and Ammonia Quantification
3.2.2. Glucose–Lipid Quantification
3.2.3. Emulsification Assay
3.3. Structure Elucidation of Glycolipid
3.3.1. Isolation and Purification of Glucose–Lipid
3.3.2. Nuclear Magnetic Resonance (NMR) Analysis
3.3.3. Liquid Chromatography Electrospray Ionization Mass Spectrometry (LC-ESI/MS)
3.4. Characterization of Glucose–Lipid
3.4.1. Critical Micelle Concentration (CMC) Determination
3.4.2. Stability Test
3.5. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, Q.; Lou, H.; Wei, T.; Liu, X.; Chen, Q. Contributions of glycolipid biosurfactants and glycolipid-modified materials to antimicrobial strategy: A review. Pharmaceutics 2021, 13, 227. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid. Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. Glycolipid biosurfactants: Main properties and potential applications in agriculture and food industry. J. Sci. Food Agric. 2016, 96, 4310–4320. [Google Scholar] [CrossRef]
- Inès, M.; Dhouha, G. Glycolipid biosurfactants: Potential related biomedical and biotechnological applications. Carbohydr. Res. 2015, 416, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cedeño, L.R.; Santos-Villalobos, S.D.L.; Santoyo, G. Functional and genomic analysis of Rouxiella badensis SER3 as a novel biocontrol agent of fungal pathogens. Front. Microbiol. 2021, 12, 709855. [Google Scholar] [CrossRef] [PubMed]
- Novotny-Nuñez, I.; Perdigón, G.; Matar, C.; Martínez Monteros, M.J.; Yahfoufi, N.; Cazorla, S.I.; Maldonado-Galdeano, C. Evaluation of Rouxiella badensis Subsp Acadiensis (Canan SV-53) as a potential probiotic bacterium. Microorganisms 2023, 11, 1347. [Google Scholar] [CrossRef] [PubMed]
- Kügler, J.H.; Muhle-Goll, C.; Hansen, S.H.; Völp, A.R.; Kirschhöfer, F.; Kühl, B.; Brenner-Weiss, G.; Luy, B.; Syldatk, C.; Hausmann, R. Glycolipids produced by Rouxiella sp. DSM 100043 and isolation of the biosurfactants via foam-fractionation. AMB Exp. 2015, 5, 82. [Google Scholar] [CrossRef]
- Le Flèche-Matéos, A.; Kügler, J.H.; Hansen, S.H.; Syldatk, C.; Hausmann, R.; Lomprez, F.; Vandenbogaert, M.; Manuguerra, J.C.; Grimont, P.A.D. Rouxiella badensis sp. nov. and Rouxiella silvae sp. nov. isolated from peat bog soil and emendation description of the genus Rouxiella. Int. J. Syst. Evol. Microbiol. 2017, 67, 1255–1259. [Google Scholar] [CrossRef]
- Validation of Analytical Procedures: Methodology; US Food and Drug Administration: Silver Spring, MD, USA, 1999. Available online: http://webarchive.library.unt.edu/eot2008/20081003232148/http://www.fda.gov/cvm/Guidance/guida64.doc (accessed on 18 February 2024).
- Claret, C.; Bories, A.; Soucaille, P. Glycerol inhibition of growth and dihydroxyacetone production by Gluconobacter oxydans. Curr. Microbiol. 1992, 25, 149–155. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Stephanopoulos, G. Simple glycolipids of microbes: Chemistry, biological activity and metabolic engineering. Synth. Syst. Biotechnol. 2017, 3, 3–19. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Z.; Linhardt, R.J. Thin layer chromatography for the separation and analysis of acidic carbohydrates. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 1711–1732. [Google Scholar] [CrossRef]
- Foroozandeh, M.; Adams, R.W.; Nilsson, M.; Morris, G.A. Ultrahigh-resolution total correlation NMR spectroscopy. J. Am. Chem. Soc. 2014, 136, 11867–11869. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, M.; Adams, R.W.; Kiraly, P.; Nilsson, M.; Morris, G.A. Measuring couplings in crowded NMR spectra: Pure shift NMR with multiplet analysis. Chem. Commun. 2015, 51, 15410–15413. [Google Scholar] [CrossRef]
- Foroozandeh, M.; Adams, R.W.; Meharry, N.J.; Jeannerat, D.; Nilsson, M.; Morris, G.A. Ultrahigh-resolution NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 2014, 53, 6990–6992. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Conrad, J.; Hammerschick, T.; Vetter, W. Enrichment and structural assignment of geometric isomers of unsaturated furan fatty acids. Anal. Bioanal. Chem. 2023, 415, 6333–6343. [Google Scholar] [CrossRef]
- Abdel-Mohsen, H.T.; Conrad, J.; Beifuss, U. Laccase-catalyzed domino reaction between catechols and 6-substituted 1,2,3,4-tetrahydro-4-oxo-2-thioxo-5-pyrimidinecarbonitriles for the synthesis of pyrimidobenzothiazole derivatives. J. Org. Chem. 2013, 78, 7986–8003. [Google Scholar] [CrossRef]
- Furihata, K.; Seto, H. Decoupled HMBC (D-HMBC), an improved technique of HMBC. Tetrahedron Lett. 1995, 36, 2817–2820. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Exploring the world of rhamnolipids: A critical review of their production, interfacial properties, and potential application. Curr. Opin. Colloid. Interface Sci. 2023, 69, 101780. [Google Scholar] [CrossRef]
- Pal, S.; Chatterjee, N.; Das, A.K.; McClements, D.J.; Dhar, P. Sophorolipids: A comprehensive review on properties and applications. Adv. Colloid. Interface Sci. 2023, 313, 102856. [Google Scholar] [CrossRef]
- Dabaghi, S.; Ataei, S.A.; Taheri, A. Production of rhamnolipid biosurfactants in solid-state fermentation: Process optimization and characterization studies. BMC Biotechnol. 2023, 23, 2. [Google Scholar] [CrossRef]
- Ramnani, P.; Kumar, S.S.; Gupta, R. Concomitant production and downstream processing of alkaline protease and biosurfactant from Bacillus licheniformis RG1: Bioformulation as detergent additive. Process Biochem. 2005, 40, 3352–3359. [Google Scholar] [CrossRef]
- Nagarajan, R.; Ruckenstein, E. Critical micelle concentration: A transition point for micellar size distribution: A statistical thermodynamical approach. J. Colloid. Interface Sci. 1977, 60, 221–231. [Google Scholar] [CrossRef]
- Wu, B.; Xiu, J.; Yu, L.; Huang, L.; Yi, L.; Ma, Y. Biosurfactant production by Bacillus subtilis SL and its potential for enhanced oil recovery in low permeability reservoirs. Sci. Rep. 2022, 12, 7785. [Google Scholar] [CrossRef]
- Zhong, H.; Zeng, G.M.; Liu, J.X.; Xu, X.M.; Yuan, X.Z.; Fu, H.Y.; Huang, G.H.; Liu, Z.F.; Ding, Y. Adsorption of monorhamnolipid and dirhamnolipid on two Pseudomonas aeruginosa strains and the effect on cell surface hydrophobicity. Appl. Microbiol. Biotechnol. 2008, 79, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cao, W.; Jiang, M.; Saren, G.; Liu, J.; Cao, J.; Ali, I.; Yu, X.; Peng, C.; Naz, I. Isolation and characterization of biosurfactant-producing and diesel oil degrading Pseudomonas sp. CQ2 from Changqing oil field, China. RSC Adv. 2018, 8, 39710–39720. [Google Scholar] [CrossRef]
- Ambust, S.; Das, A.J.; Kumar, R. Bioremediation of petroleum contaminated soil through biosurfactant and Pseudomonas sp. SA3 amended design treatments. Curr. Res. Microb. Sci. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Braz, L.M.; Salazar-Bryam, A.M.; Andrade, G.S.S.; Tambourgi, E.B. Optimization and characterization of rhamnolipids produced by Pseudomonas aeruginosa ATCC 9027 using molasses as a substrate. World J. Microbiol. Biotechnol. 2022, 39, 51. [Google Scholar] [CrossRef]
- Safari, P.; Hosseini, M.; Lashkarbolooki, M.; Ghorbani, M.; Darzi, G.N. Evaluation of surface activity of rhamnolipid biosurfactants produced from rice bran oil through dynamic surface tension. J. Petrol. Explor. Prod. Technol. 2023, 13, 2139–2153. [Google Scholar] [CrossRef]
- Ahmadi, M.; Niazi, F.; Jaafarzadeh, N.; Ghafari, S.; Jorfi, S. Characterization of the biosurfactant produced by Pesudomonas areuginosa strain R4 and its application for remediation pyrene-contaminated soils. J. Environ. Health Sci. Eng. 2021, 19, 445–456. [Google Scholar] [CrossRef]
- Daverey, A.; Pakshirajan, K. Production, characterization, and properties of sophorolipids from the yeast Candida bombicola using a low-cost fermentative medium. Appl. Biochem. Biotechnol. 2009, 158, 663–674. [Google Scholar] [CrossRef]
- Sen, S.; Borah, S.N.; Bora, A.; Deka, S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb. Cell Fact. 2017, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Lee, B.; Kim, E. Sophorolipid production by Candida bombicola ATCC 22214 from a corn-oil processing byproduct. J. Microbiol. Biotechnol. 2005, 15, 55–58. [Google Scholar]
- Alimadadi, N.; Soudi, M.R.; Talebpour, Z. Efficient production of tri-acetylated mono-acylated mannosylerythritol lipids by Sporisorium sp. aff. sorghi SAM20. J. Appl. Microbiol. 2018, 124, 457–468. [Google Scholar] [CrossRef]
- Niu, Y.; Wu, J.; Wang, W.; Chen, Q. Production and characterization of a new glycolipid, mannosylerythritol lipid, from waste cooking oil biotransformation by Pseudozyma aphidis ZJUDM34. Food Sci. Nutr. 2019, 7, 937–948. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeon, J.W.; Kim, S.B.; Oh, H.M.; Kwon, T.J.; Yoon, B.D. Surface and physico-chemical properties of a glycolipid biosurfactant, mannosylerythritol lipid, from Candida antarctica. Biotechnol. Lett. 2002, 24, 1637–1641. [Google Scholar] [CrossRef]
- White, D.; Hird, L.C.; Ali, S. Production and characterization of a trehalolipid biosurfactant produced by the novel marine bacterium Rhodococcus sp. , strain PML026. J. Appl. Microbiol. 2013, 115, 744–755. [Google Scholar] [CrossRef]
- Luong, T.M.; Ponamoreva, O.N.; Nechaeva, I.A.; Petrikov, K.V.; Delegan, Y.A.; Surin, A.K.; Linklater, D.; Filonov, A.E. Characterization of biosurfactants produced by the oil-degrading bacterium Rhodococcus erythropolis S67 at low temperature. World J. Microbiol. Biotechnol. 2018, 34, 20. [Google Scholar] [CrossRef] [PubMed]
- Phulpoto, I.A.; Yu, Z.; Hu, B.; Wang, Y.; Ndayisenga, F.; Li, J.; Liang, H.; Qazi, M.A. Production and characterization of surfactin-like biosurfactant produced by novel strain Bacillus nealsonii S2MT and it’s potential for oil contaminated soil remediation. Microb. Cell Fact. 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Schalchli, H.; Lamilla, C.; Rubilar, O.; Briceño, G.; Gallardo, F.; Durán, N.; Huenchupan, A.; Diez, M.C. Production and characterization of a biosurfactant produced by Bacillus amyloliquefaciens C11 for enhancing the solubility of pesticides. J. Environ. Chem. Eng. 2023, 11, 111572. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A. Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl. Biochem. Biotechnol. 2008, 150, 289–303. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Samykannu, M.; Achary, A. Utilization of agro-industry residue for rhamnolipid production by P. aeruginosa AMB AS7 and its application in chromium removal. Appl. Biochem. Biotechnol. 2017, 183, 70–90. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Biosurfactants and their applications in the oil and gas industry: Current state of knowledge and future perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef]
- Geissler, M.; Oellig, C.; Moss, K.; Schwack, W.; Henkel, M.; Hausmann, R. High-performance thin-layer chromatography (HPTLC) for the simultaneous quantification of the cyclic lipopeptides surfactin, iturin A and fengycin in culture samples of Bacillus species. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2017, 1044–1045, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.L.; Savage, R.I. Paper chromatography of some starch conversion products. Analyst 1952, 77, 401–406. [Google Scholar] [CrossRef]
- Harris, G.; Macwilliam, I. A Dipping Technique for Revealing Sugars on Paper Chromatograms; Chem Ind.: Jalan Samulun, Singapore, 1954. [Google Scholar]
- Anderson, K.; Li, S.C.; Li, Y.T. Diphenylamine-aniline-phosphoric acid reagent, a versatile spray reagent for revealing glycoconjugates on thin-layer chromatography plates. Anal. Biochem. 2000, 287, 337–339. [Google Scholar] [CrossRef]
- Svennerholm, L. Quantitive estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim. Biophys. Acta 1957, 24, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.R.; Chopade, B.A. Studies on bioemulsifier production by Acinetobacter strains isolated from healthy human skin. J. Appl. Microbiol. 2001, 91, 290–298. [Google Scholar] [CrossRef]
- Heyd, M.; Kohnert, A.; Tan, T.H.; Nusser, M.; Kirschhöfer, F.; Brenner-Weiss, G.; Franzreb, M.; Berensmeier, S. Development and trends of biosurfactant analysis and purification using rhamnolipids as an example. Anal. Bioanal. Chem. 2008, 391, 1579–1590. [Google Scholar] [CrossRef]
- Eslami, P.; Hajfarajollah, H.; Bazsefidpar, S. Recent advancements in the production of rhamnolipid biosurfactants by Pseudomonas aeruginosa. RSC Adv. 2020, 10, 34014–34032. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, W.; Sun, L.; Wang, Y.; Liao, J.; Xu, D.; Liu, Y.; Ye, R.; Gooneratne, R. A sensitive method for simultaneous quantitative determination of surfactin and iturin by LC-MS/MS. Anal. Bioanal. Chem. 2017, 409, 179–191. [Google Scholar] [CrossRef]
- Bajpai, A.; Agnihotri, R.; Prakash, A.; Johri, B.N. Biosurfactant from Bacillus sp. A5F reduces disease incidence of Sclerotinia sclerotiorum in Soybean Crop. Curr. Microbiol. 2022, 79, 206. [Google Scholar] [CrossRef] [PubMed]
- Vahidinasab, M.; Adiek, I.; Hosseini, B.; Akintayo, S.O.; Abrishamchi, B.; Pfannstiel, J.; Henkel, M.; Lilge, L.; Voegele, R.T.; Hausmann, R. Characterization of Bacillus velezensis UTB96, demonstrating improved lipopeptide production compared to the strain B. velezensis FZB42. Microorganisms 2022, 10, 2225. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, A.F.; Al-Mossawy, M.I.; Al-Tamimi, W.H.; Al-Najm, F.M.; Hameed, Z.M. Enhancing the spontaneous imbibition process using biosurfactants produced from bacteria isolated from Al-Rafidiya oil field for improved oil recovery. J. Pet. Explor. Prod. Technol. 2020, 10, 3767–3777. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Hoffmann, M.; Fernandez Cano Luna, D.S.; Xiao, S.; Stegemüller, L.; Rief, K.; Heravi, K.M.; Lilge, L.; Henkel, M.; Hausmann, R. Towards the anaerobic production of surfactin using Bacillus subtilis. Front. Bioeng. Biotechnol. 2020, 26, 554903. [Google Scholar] [CrossRef]

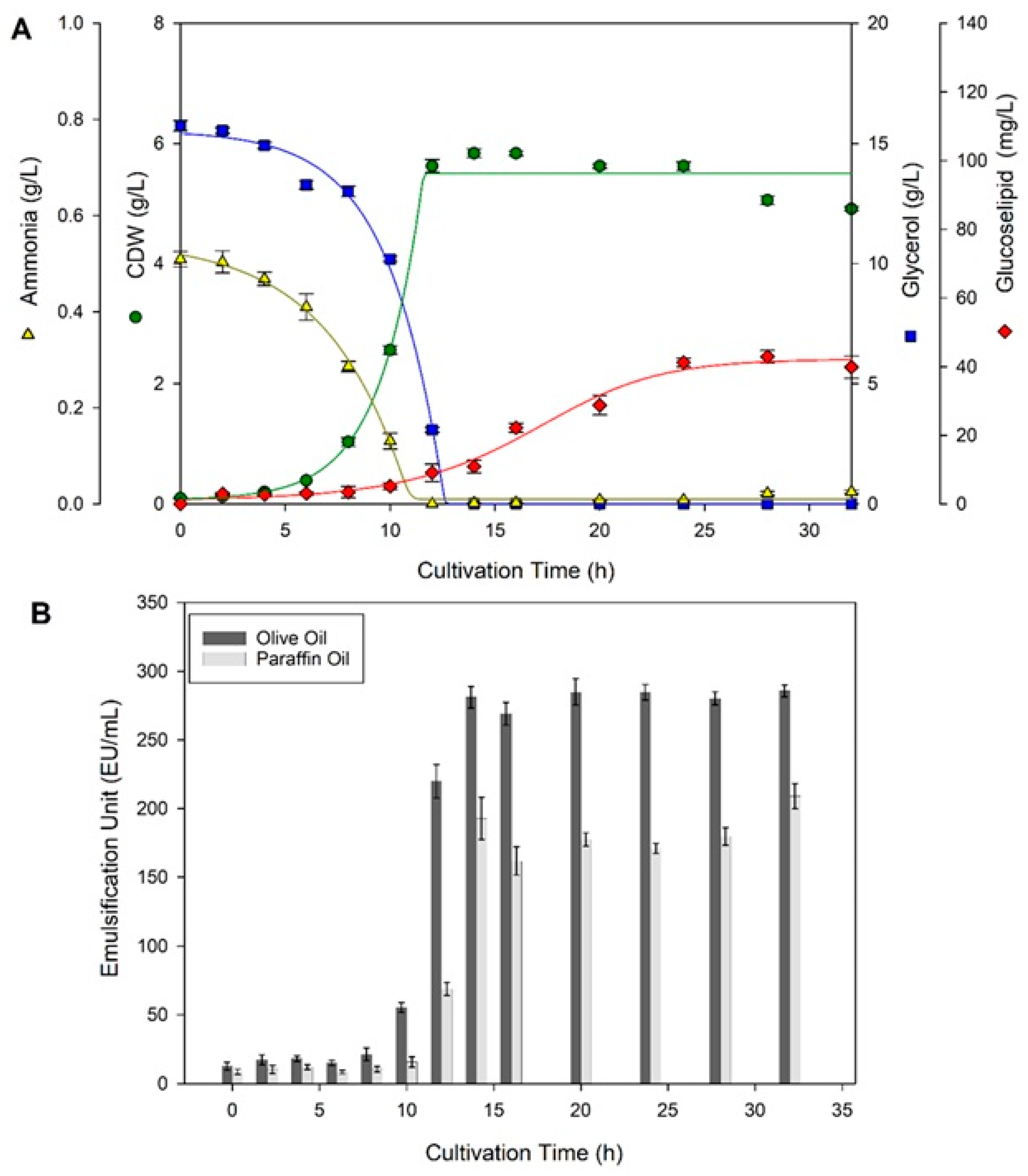
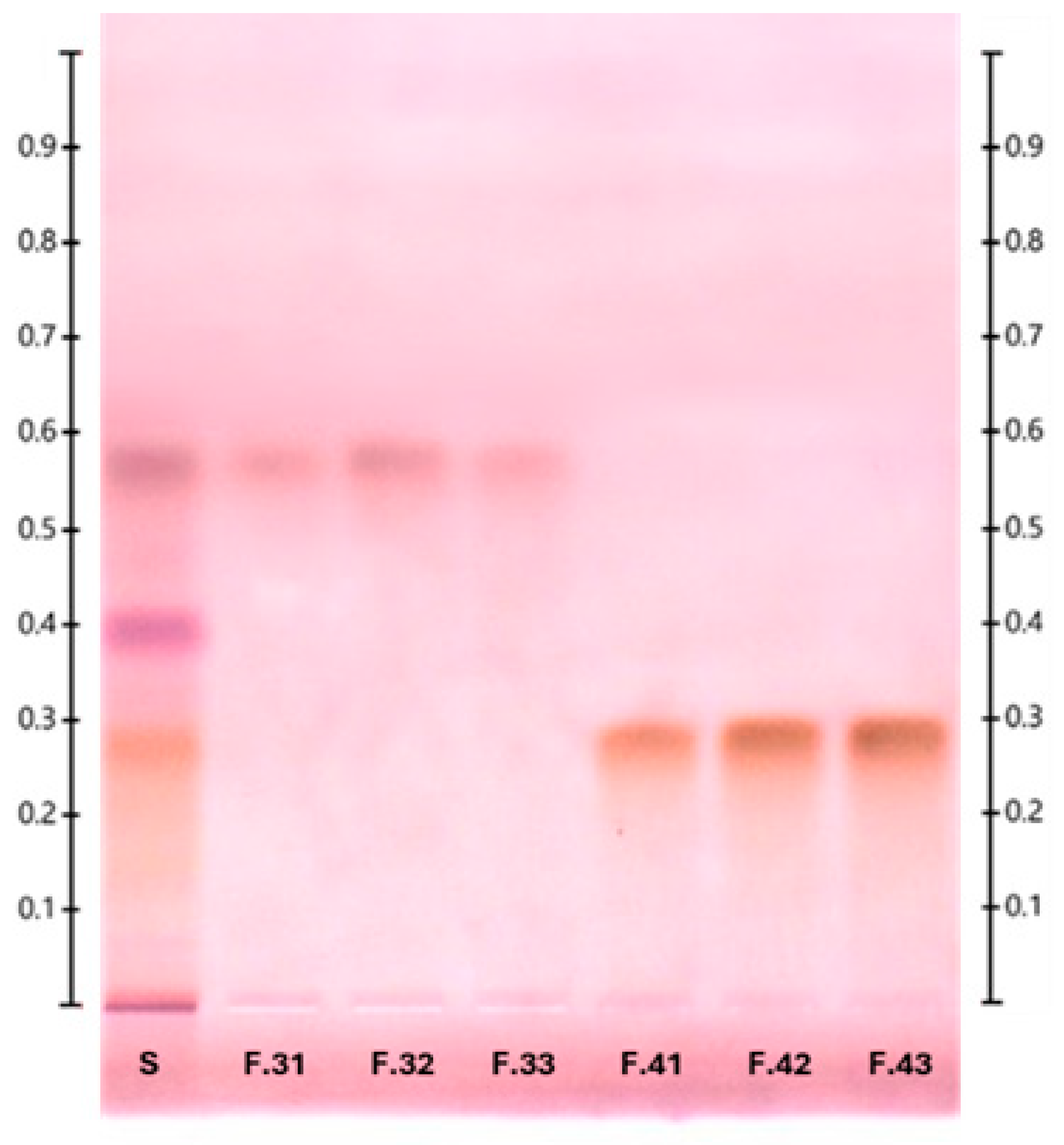

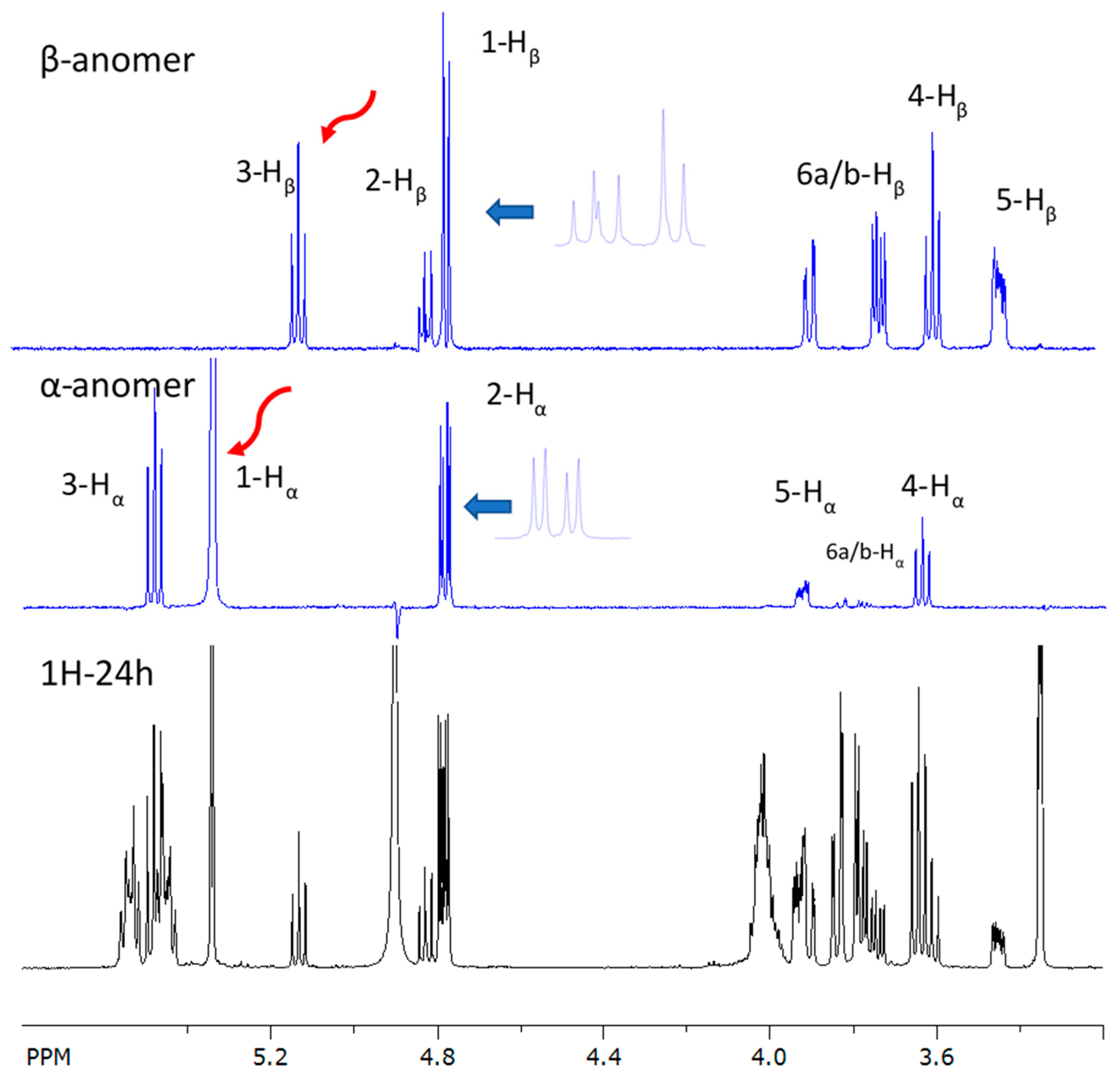
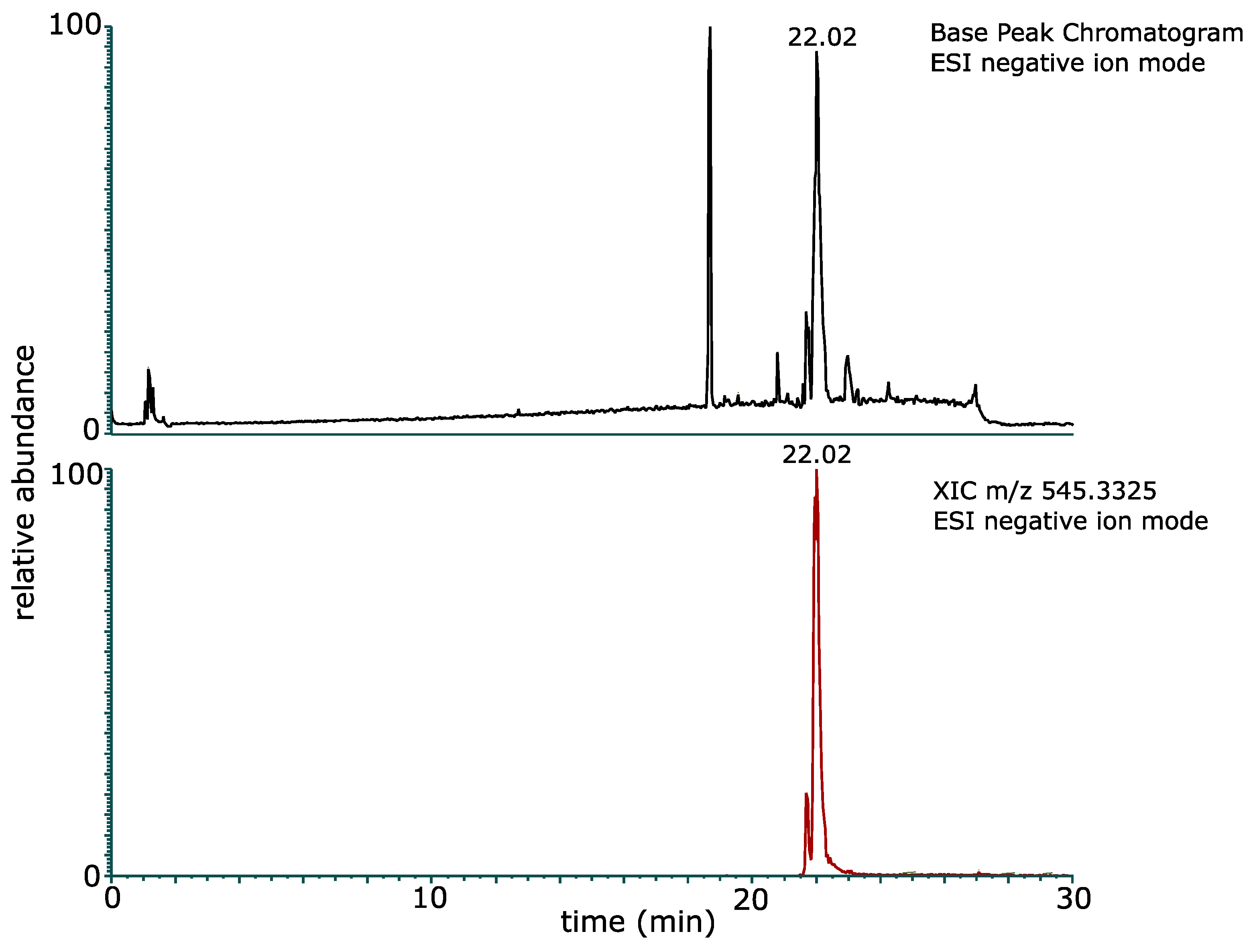

| Parameter | Shake Flasks (15 g/L Glycerol) | Shake Flasks (30 °C) | Bioreactor | ||||
|---|---|---|---|---|---|---|---|
| 20 °C | 25 °C | 30 °C | 10 g/L | 20 g/L | 30 g/L | ||
| CDWmax (g/L) | 6.26 | 6.55 | 5.41 | 4.98 | 6.12 | 5.48 | 5.84 |
| μ (1/h) | 0.25 | 0.32 | 0.34 | 0.34 | 0.31 | 0.29 | 0.34 |
| μmax (1/h) | 0.32 (14 h) | 0.43 (14 h) | 0.53 (12 h) | 0.52 (12 h) | 0.54 (12 h) | 0.48 (12 h) | 0.48 (8 h) |
| Pmax (mg/L) | 69.9 (32 h) | 60.3 (28 h) | 89.3 (28 h) | 31.7 (32 h) | 55.9 (28 h) | 49.8 (32 h) | 42.9 (28 h) |
| qP/X (g/(g·h)) | 0.00043 | 0.00038 | 0.00066 | 0.00023 | 0.00036 | 0.00029 | 0.00031 |
| Atom Number | α-Anomer | β-Anomer | ||
|---|---|---|---|---|
| δH (ppm), Multiplicity, J (Hz) | δC (ppm) | δH (ppm), Multiplicity, J (Hz) | δC (ppm) | |
| 1 | 5.34, d, 3.6 | 91.19 | 4.76, d, 8.0 a | 96.15 |
| 2 | 4.78, dd, 3.6, 10.2 | 73.35 | 4.81, dd, 8.0, 9.6 | 74.53 |
| 3 | 5.48, dd, 9.4, 10.1 | 73.99 | 5.11, t-like, 9.4 | 77.00 |
| 4 | 3.64, t-like, 9.7 | 69.72 | 3.59, t-like, 9.6 a | 69.72 |
| 5 | 3.93, ddd, 2.3, 4.7, 10.0 | 72.78 | 3.43, ddd, 2.0, 5.3, 9.8 | 77.96 |
| 6a/b | 3.84, dd, 2.4, 12.0 3.78, dd, 4.8, 11.9 | 62.17 | 3.88, dd, 2.2, 12.0 a 3.72, dd, 5.5, 12.0 a | 62.40 |
| 1′ | - | 172.65 | - | 172.30 |
| 2′ | 2.57, dd, 8.8, 15.3 2.41, dd, 4.2, 15.3 | 42.66 | 2,59, dd, 4.2,15.2 2.39, ov | 42.73 |
| 3′ | 4.02, m | 69.35 | 4.02, ov | 69.22 b |
| 4′ | 2.28, br t, 6.9 | 35.93 | 2.29, ov | 35.83 |
| 5′ | 5.45, dtt, 1.5, 7.3, 10.9 | 125.95 | 5.45, ov | 126.00 |
| 6′ | 5.54, dtt, 1.5, 7.4, 10.9 | 133.61 | 5.54, ov | 133.59 |
| 7′ | 2.10, br q-like, 7.1 | 28.42 | 2.10, ov c | 28.42, ov c |
| 8′ | 1.39, quintet, 7.2 d | 30.73 | 1.39, ov c | 30.73, ov e |
| 9′ | 1.36, quintet, 7.9 d | 30.12 | 1.36, ov c | 30.12, ov c |
| 10′ | 1.31, quintet, 7.9 d | 33.04 f | 1.31, ov c | 33.04 f, ov c |
| 11′ | 1.34, sextet, 7.4 d | 23.72 g | 1.34, ov c | 23.72 g, ov c |
| 12′ | 0.93, t, 6.7 | 14.45 | 0.95, ov c | 14.45, ov c |
| 1″ | - | 173.06 | - | 172.99 |
| 2″ | 2.57, dd, 4.3, 15.1 2.47, dd, 8.4, 15.1 | 43.49 | 2.59, ov 2.47, ov | 43.42 |
| 3″ | 4.01, m | 69.26 | 4.01, ov c | 69.19 b |
| 4″ | 1.51, m d | 38.17 | 1.51, ov c | 38.17, ov c |
| 5″ | 1.47, m d 1.39, m d | 26.69 | 1.47, ov c 1.39, ov c | 26.67 |
| 6″ | 1.34, ov | 30.71 | 1.34, ov c | 30.69 e |
| 7″ | 1.34, quintet, 8.0 d | 30.48 | 1.34, ov c | 30.48, ov c |
| 8″ | 1.31, quintet, 7.9 d | 32.95 f | 1.31, ov c | 32.95 f, ov c |
| 9″ | 1.34, sextet, 7.4 d | 23.74 g | 1.34, ov c | 23.74 g, ov c |
| 10″ | 0.93, t, 6.7 | 14.45 | 0.95, ov c | 14.45, ov c |
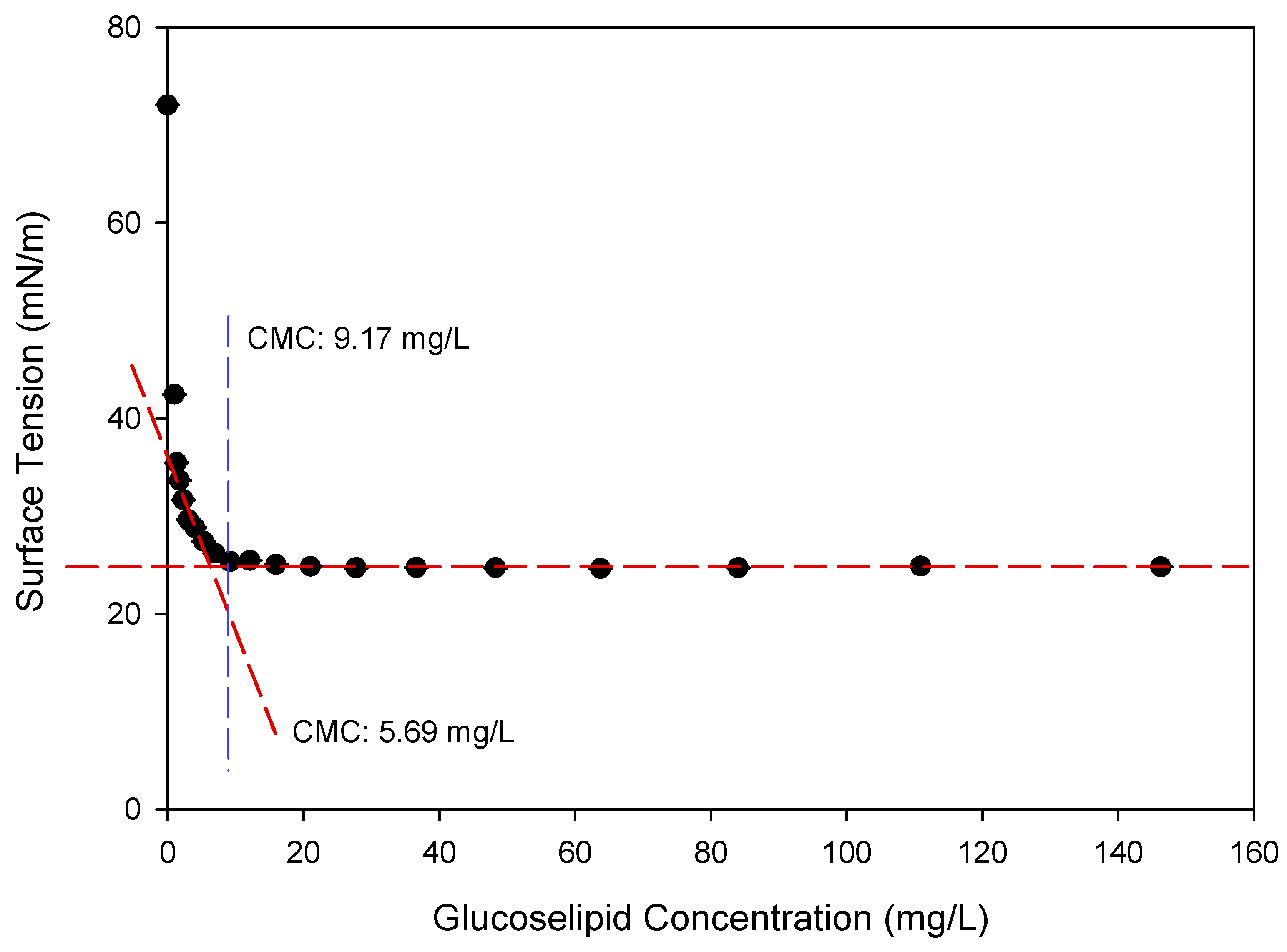
| Surfactant Type | Microorganism | Minimum Surface Tension (mN/m) | CMC (mg/L) | Reference |
|---|---|---|---|---|
| Glucose–lipid | Rouxiella badensis DSM 100043T | 24.59 | 5.69 | This study |
| Rhamnolipid | Pseudomonas aeruginosa ATCC 9027 | 34.01 | 180 | [28] |
| Pseudomonas aeruginosa PTCC 1340 | 25.86 | 90 | [29] | |
| Pseudomonas aeruginosa R4 | 32.5 | 50 | [30] | |
| Sophorolipid | Starmerella bombicola NRRL Y-17069 | 34.15 | 59.43 | [31] |
| Rhodotorula babjevae YS3 | 32.6 | 130 | [32] | |
| Candida bombicola ATCC 22214 | 48 | 150 | [33] | |
| MELs * | Sporisorium sp. aff. sorghi SAM20 | 30 | 20 | [34] |
| Pseudozyma aphidis ZJUDM34 | 30.63 | 20 | [35] | |
| Candida antarctica SY16 | 29 | 10 | [36] | |
| Trehalolipid | Rhodococcus sp., PML026 | 29 | 250 | [37] |
| Rhodococcus erythropolis S67 | 27 | 32 | [38] | |
| Surfactin | Bacillus nealsonii S2MT | 34.5 | 40 | [39] |
| Bacillus amyloliquefaciens C11 | 28 | 51.5 | [40] | |
| Bacillus subtilis Isolate BS5 | 36 | 15.6 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harahap, A.F.P.; Conrad, J.; Wolf, M.; Pfannstiel, J.; Klaiber, I.; Grether, J.; Hiller, E.; Vahidinasab, M.; Salminen, H.; Treinen, C.; et al. Structure Elucidation and Characterization of Novel Glycolipid Biosurfactant Produced by Rouxiella badensis DSM 100043T. Molecules 2025, 30, 1798. https://doi.org/10.3390/molecules30081798
Harahap AFP, Conrad J, Wolf M, Pfannstiel J, Klaiber I, Grether J, Hiller E, Vahidinasab M, Salminen H, Treinen C, et al. Structure Elucidation and Characterization of Novel Glycolipid Biosurfactant Produced by Rouxiella badensis DSM 100043T. Molecules. 2025; 30(8):1798. https://doi.org/10.3390/molecules30081798
Chicago/Turabian StyleHarahap, Andre Fahriz Perdana, Jürgen Conrad, Mario Wolf, Jens Pfannstiel, Iris Klaiber, Jakob Grether, Eric Hiller, Maliheh Vahidinasab, Hanna Salminen, Chantal Treinen, and et al. 2025. "Structure Elucidation and Characterization of Novel Glycolipid Biosurfactant Produced by Rouxiella badensis DSM 100043T" Molecules 30, no. 8: 1798. https://doi.org/10.3390/molecules30081798
APA StyleHarahap, A. F. P., Conrad, J., Wolf, M., Pfannstiel, J., Klaiber, I., Grether, J., Hiller, E., Vahidinasab, M., Salminen, H., Treinen, C., Perino, E. H. B., & Hausmann, R. (2025). Structure Elucidation and Characterization of Novel Glycolipid Biosurfactant Produced by Rouxiella badensis DSM 100043T. Molecules, 30(8), 1798. https://doi.org/10.3390/molecules30081798





