Cloud Point Behavior of Poly(trifluoroethyl methacrylate) in Supercritical CO2–Toluene Mixtures
Abstract
1. Introduction
2. Results and Discussion
2.1. Cloud Point Pressures as a Function of Temperature
2.2. Effect of Toluene as a Cosolvent on Cloud Point Pressure
2.3. Density Trends of scCO2–Toluene Mixtures at Cloud Point Conditions
3. Materials and Methods
3.1. Materials
- Carbon Dioxide (CO2): High-purity () CO2 was purchased from Airgas (Philadelphia, PA, USA).
- Poly(Trifluoroethyl Methacrylate) [Poly(TFEMA)]: The fluorinated polymer was obtained from Specific Polymers (Castries, France). The polymer was used as received without further purification. This polymer exhibits a molecular weight (Mn) of 31,283 g/mol, a weight-average molecular weight (Mw) of 65,501 g/mol, and a polydispersity index (PDI) of 2.1, as specified by the supplier.
- Toluene: Toluene ( purity) was purchased from Sigma Aldrich (St. Louis, MO, USA).
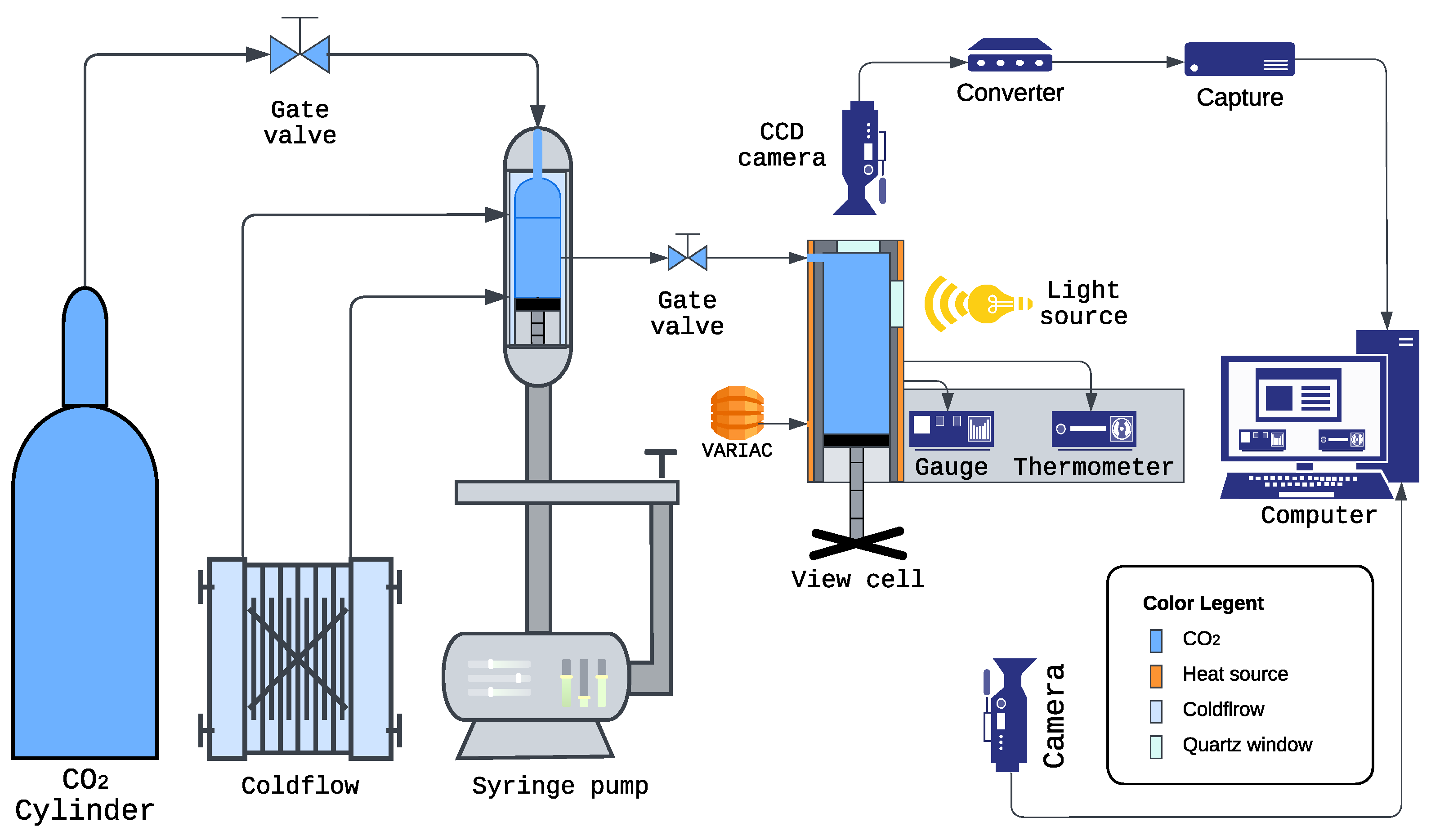
3.2. Experimental Setup
3.2.1. Carbon Dioxide Delivery and Pressurization
3.2.2. Phase Monitor (Variable-Volume View Cell)
3.2.3. Temperature Control
3.2.4. Pressure Measurement
3.2.5. Optical Imaging and Video Recording
- Vanxse CCTV Mini HD 1/3 CCD 960H Auto Iris Camera (Model BX2812, Shenzhen Kaixing Security Technology Co., Ltd., Shenzhen, China, NTSC): This camera was positioned to capture the optical appearance of the solution through the quartz window of the Phase Monitor. Changes in clarity, such as the onset of turbidity, indicated the cloud point.
- Angetube 1080P Webcam (Model XZC827, Angetube, Shenzhen, China, USB): This camera recorded the digital displays of the DP400TP panel meter (showing real-time pressure in psi) and the thermometer (showing temperature in °C).
3.2.6. Data Acquisition and Analysis
- The visual appearance of the polymer solution within the Phase Monitor.
- The pressure reading displayed on the DP400TP panel meter.
- The temperature reading from the system thermometer.
3.2.7. Overall System Integration
3.3. Experimental Procedure
3.3.1. Reference State and CO2 Loading
3.3.2. Compositions and Preparation
- (1)
- 0 wt% toluene + 3 wt% Poly(TFEMA) + 97 wt% CO2
- (2)
- 5 wt% toluene + 3 wt% Poly(TFEMA) + 92 wt% CO2
- (3)
- 10 wt% toluene + 3 wt% Poly(TFEMA) + 87 wt% CO2
- (4)
- 15 wt% toluene + 3 wt% Poly(TFEMA) + 82 wt% CO2
- (5)
- 20 wt% toluene + 3 wt% Poly(TFEMA) + 77 wt% CO2
- 0 wt% Toluene: 0.579 g of poly(TFEMA) + 18.723 g of scCO2.
- 5 wt% Toluene: 1.01753 g (1.18 mL) of toluene + 0.61052 g of poly(TFEMA) + 18.723 g of scCO2.
- 10 wt% Toluene: 2.15202 g (2.4957 mL) of toluene + 0.64561 g of poly(TFEMA) + 18.723 g of scCO2.
- 15 wt% Toluene: 3.42486 g (3.9718 mL) of toluene + 0.68497 g of poly(TFEMA) + 18.723 g of scCO2.
- 20 wt% Toluene: 4.86301 g (5.6396 mL) of toluene + 0.72945 g of poly(TFEMA) + 18.723 g of scCO2.
- (a)
- No toluene (0 wt%): A mass of 0.579 g of poly(TFEMA) alone was weighed on a Fisher Scientific (Waltham, MA, USA) Accu-124 analytical balance, then introduced into the cell.
- (b)
- Toluene-containing mixtures (5–20 wt%): The predetermined mass of poly(TFEMA) was first weighed and added to the cell. Afterward, the measured toluene (e.g., 1.18 mL for 5 wt%) was carefully pipetted into the cell.
- (c)
- Sealing and purging: After loading the cell with polymer and toluene (when applicable), the cell was sealed. CO2 was introduced gradually (at a flow rate of 10 mL/min) until the internal pressure reached roughly 600–700 psi (4.14–4.83 MPa). A brief purge was performed through the outlet valve to remove air; this was performed carefully so as to avoid loss of toluene or polymer. The outlet valve was then closed.
- (d)
- Pressurizing to reference state: The cell was wrapped with flexible heating tape and maintained at 31.2 °C using a Variac. The syringe pump was then used to increase the cell pressure to 1100 psi at a controlled flow (10–20 mL/min). Once the reference state (31.2 °C, 1100 psi) was stable, the inlet valve was closed to isolate the system.
3.3.3. Cloud Point Determination
- (1)
- Single-phase formation: The internal volume of the cell was decreased (i.e., the piston moved inward) to increase pressure until the mixture appeared optically transparent in the cell’s quartz window. This corresponded to a single-phase region, as illustrated in Figure 7A.
- (2)
- Expansion to turbidity (cloud point): Next, the piston was slowly retracted, reducing the pressure at a controlled rate, until the first discernible turbidity was observed in the window (onset of clouding). This pressure was noted as . The visual appearance at this point is illustrated in Figure 7B, where the polymer-rich phase begins to form but the solution is only partially cloudy.
- (3)
- Further expansion: If the volume was expanded further, the system entered a fully two-phase region, exhibiting a milky or opaque appearance, as shown in Figure 7C. At this stage, the polymer is largely precipitated from the fluid phase.
- (4)
- Repetitions: Each temperature–composition combination was measured at least six times to ensure reproducibility. The mean value is reported, with typical standard deviations around .
3.4. Density Calculations
3.4.1. CO2 Density: AG–HGK Equation of State
3.4.2. Toluene Density: Tait Equation
3.4.3. Mixture Density: Mass-Weighted Mixing Law
4. Conclusions
- Cloud point trends: Cloud point pressures rise linearly with temperature, but fall with increasing toluene fraction, demonstrating how cosolvent addition effectively reduces the pressure required for polymer dissolution.
- Density: Toluene lowers the overall mixture density but boosts solvent–solute interactions, primarily due to the similarity in dispersion parameters between toluene and poly(TFEMA), leading to enhanced solvating capability for fluoropolymers such as poly(TFEMA).
- Process implications: At the highest cosolvent fraction of 20 wt%, we observed up to an approximately 40% reduction in cloud point pressure compared to neat scCO2, demonstrating a significant expansion of the processing window. These insights form a basis for designing supercritical fluid processes with lower pressure requirements, translating to potential energy savings and broader industrial applicability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AG | Altuin–Gadetskii |
| AG–HGK | Altuin–Gadetskii–Haar–Gallagher–Kell |
| CO2 | Carbon dioxide |
| HSPs | Hansen solubility parameters |
| HGK | Haar–Gallagher–Kell |
| PDI | Polydispersity index |
| scCO2 | Supercritical carbon dioxide |
| TFEMA | Trifluoroethyl methacrylate |
| Tol | Toluene |
| wt% | Weight percent |
| K | Kelvin |
| kg·m−3 | Kilograms per cubic meter (density unit) |
| MPa | Megapascal |
| psi | Pounds per square inch |
| T | Temperature |
| B(T) | Tait equation parameter dependent on temperature |
| C | Compressibility parameter in Tait equation |
| P0 | Reference pressure (0.1 MPa) |
| Pcloud | Cloud point pressure |
| Density | |
| Reference density at temperature T | |
| CCD | Charge-coupled device |
| HD | High definition |
| PID | Proportional–integral–derivative (controller) |
| RTD | Resistance temperature detector |
| EoS | Equation of state |
| OBS | Open Broadcaster Software |
References
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of supercritical carbon dioxide in materials processing and synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Leitner, W. Designed to dissolve. Nature 2000, 405, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Tutek, K.; Masek, A.; Kosmalska, A.; Cichosz, S. Application of Fluids in Supercritical Conditions in the Polymer Industry. Polymers 2021, 13, 729. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.M.; Long, D.P.; Cabañas, A.; Watkins, J.J. Deposition of Conformal Copper and Nickel Films from Supercritical Carbon Dioxide. Science 2001, 294, 141–145. [Google Scholar] [CrossRef]
- Cabañas, A.; Long, D.P.; Watkins, J.J. Deposition of Gold Films and Nanostructures from Supercritical Carbon Dioxide. Chem. Mater. 2004, 16, 2028–2033. [Google Scholar] [CrossRef]
- Sajfrtová, M.; Cerhová, M.; Jandová, V.; Dřínek, V.; Daniš, S.; Matějová, L. The effect of type and concentration of modifier in supercritical carbon dioxide on crystallization of nanocrystalline titania thin films. J. Supercrit. Fluids 2018, 133, 211–217. [Google Scholar] [CrossRef]
- Wei, M.; Wang, K.; Yanagida, M.; Sugihara, H.; Morris, M.A.; Holmes, J.D.; Zhou, H. Supercritical fluid processing of mesoporous crystalline TiO2 thin films for highly efficient dye-sensitized solar cells. J. Mater. Chem. 2007, 17, 3888–3893. [Google Scholar] [CrossRef]
- Sanli, D.; Bozbag, S.E.; Erkey, C. Synthesis of nanostructured materials using supercritical CO2: Part I. Physical transformations. J. Mater. Sci. 2012, 47, 2995–3025. [Google Scholar] [CrossRef]
- Kaleva, A.; Heinonen, S.; Nikkanen, J.P.; Levänen, E. Synthesis and Crystallization of Titanium Dioxide in Supercritical Carbon Dioxide (scCO2). IOP Conf. Ser. Mater. Sci. Eng. 2017, 175, 012034. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A Cost-Effective Supercapacitor Material of Ultrahigh Specific Capacitances: Spinel Nickel Cobaltite Aerogels from an Epoxide-Driven Sol-Gel Process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef]
- Naguib, H.E.; Park, C.B.; Song, S.W. Effect of Supercritical Gas on Crystallization of Linear and Branched Polypropylene Resins with Foaming Additives. Ind. Eng. Chem. Res. 2005, 44, 6685–6691. [Google Scholar] [CrossRef]
- Joshi, D.; Prausnitz, J. Supercritical Fluid Extraction with Mixed Solvents; Technical Report LBL-17154; Lawrence Berkeley National Laboratory, University of California: Berkeley, CA, USA, 1983. [Google Scholar]
- Khanyile, A.; Andrew, J.; Paul, V.; Sithole, B. A comparative study of supercritical fluid extraction and accelerated solvent extraction of lipophilic compounds from lignocellulosic biomass. Sustain. Chem. Pharm. 2022, 26, 100608. [Google Scholar] [CrossRef]
- Marco, I.D.; Riemma, S.; Iannone, R. Supercritical Carbon Dioxide Decaffeination Process: A Life Cycle Assessment Study. Chem. Eng. Trans. 2017, 57, 1699–1704. [Google Scholar] [CrossRef]
- do Espirito Santo, A.T.; Siqueira, L.M.; Almeida, R.N.; Vargas, R.M.F.; do N Franceschini, G.; Kunde, M.A.; Cappellari, A.R.; Morrone, F.B.; Cassel, E. Decaffeination of yerba mate by supercritical fluid extraction: Improvement, mathematical modelling and infusion analysis. J. Supercrit. Fluids 2021, 168, 105096. [Google Scholar] [CrossRef]
- Pu, N.W.; Wang, C.A.; Sung, Y.; Liu, Y.M.; Ger, M.D. Production of few-layer graphene by supercritical CO2 exfoliation of graphite. Mater. Lett. 2009, 63, 1987–1989. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Wang, W.; Zhao, Y. Preparation of Two Dimensional Atomic Crystals-BN, WS2 and MoS2 by Supercritical CO2 Assisted with Ultrasonic. Ind. Eng. Chem. Res. 2013, 52, 4379–4382. [Google Scholar] [CrossRef]
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- von Schnitzler, J.; Eggers, R. Mass transfer in polymers in a supercritical CO2-atmosphere. J. Supercrit. Fluids 1999, 16, 81–92. [Google Scholar] [CrossRef]
- Ting, Y.S.; Hsieh, C.M. Prediction of solid solute solubility in supercritical carbon dioxide with organic cosolvents from the PR + COSMOSAC equation of state. Fluid Phase Equilibria 2016, 431, 48–57. [Google Scholar] [CrossRef]
- Ekart, M.P.; Bennett, K.L.; Ekart, S.M.; Gurdrial, G.S.; Liotta, C.L.; Eckert, C.A. Cosolvent Interactions in Supercritical Fluid Solutions. AIChE J. 1993, 39, 235–248. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Knutson, B.L.; Pouillot, F.; Liotta, C.L.; Eckert, C.A. Spectroscopic Study of Structure and Interactions in Cosolvent-Modified Supercritical Fluids. J. Phys. Chem. 1993, 97, 11823–11834. [Google Scholar] [CrossRef]
- Ren, H.; Song, J.; Xu, Q.; Yin, J. Solubility of the silver nitrate in supercritical carbon dioxide with ethanol and ethylene glycol as double cosolvents: Experimental determination and correlation. Chin. J. Chem. Eng. 2018, 27, 400–404. [Google Scholar] [CrossRef]
- Gurina, D.L.; Antipova, M.L.; Odintsova, E.G.; Petrenko, V.E. The study of peculiarities of parabens solvation in methanol- and acetone-modified supercritical carbon dioxide by computer simulation. J. Supercrit. Fluids 2017, 126, 47–54. [Google Scholar] [CrossRef]
- Yang, H.; Zhong, C. Modeling of the solubility of aromatic compounds in supercritical carbon dioxide–cosolvent systems using SAFT equation of state. J. Supercrit. Fluids 2005, 33, 99–106. [Google Scholar] [CrossRef]
- Ghoderao, P.N.; Lee, C.W.; Byun, H.S. Phase Behavior Investigation of the Vinyl Toluene and Poly(vinyl toluene) + Co-solvents in Supercritical CO2. J. Ind. Eng. Chem. 2023, 121, 92–99. [Google Scholar] [CrossRef]
- Wang, N.; Pei, C.; Zhong, Y.; Zhang, Y.; Liu, X.; Hou, J.; Yuan, Y.; Zhang, R. Molecular Dynamics Simulation of the Compatibility Between Supercritical Carbon Dioxide and Coating Resins Assisted by Co-Solvents. Materials 2024, 17, 6271. [Google Scholar] [CrossRef]
- Behera, U.S.; Prasad, S.K.; Baskaran, D.; Byun, H.S. Phase Behavior of Biodegradable Poly(L-lactic acid) in Supercritical Solvents and Cosolvents. J. CO2 Util. 2024, 79, 102658. [Google Scholar] [CrossRef]
- Baskaran, D.; Behera, U.S.; Byun, H.S. Assessment of Solubility Behavior of a Copolymer in Supercritical CO2 and Organic Solvents: Neural Network Prediction and Statistical Analysis. ACS Omega 2024, 9, 40941–40955. [Google Scholar] [CrossRef]
- Wu, W.; Ke, J.; Poliakoff, M. Phase Boundaries of CO2 + Toluene, CO2 + Acetone, and CO2 + Ethanol at High Temperatures and High Pressures. J. Chem. Eng. Data 2006, 51, 1398–1403. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Q.; Rempel, G.L. Pressure-Density-Temperature Behavior of CO2/Acetone, CO2/Toluene, and CO2/Monochlorobenzene Mixtures in the Near-Critical Region. J. Chem. Eng. Data 2004, 49, 976–979. [Google Scholar] [CrossRef]
- Matsukawa, H.; Tsuji, T.; Otake, K. Measurement of the Density of Carbon Dioxide/Toluene Homogeneous Mixtures and Correlation with Equations of State. J. Chem. Thermodyn. 2022, 164, 106618. [Google Scholar] [CrossRef]
- Knez, Ž.; Škerget, M.; Ilič, L.; Lütge, C. Vapor–liquid equilibrium of binary CO2–organic solvent systems (ethanol, tetrahydrofuran, ortho-xylene, meta-xylene, para-xylene). J. Supercrit. Fluids 2008, 43, 383–389. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Han, B. Critical points and phase behavior of toluene-CO2 and toluene-H2-CO2 mixture in CO2-rich region. J. Supercrit. Fluids 2000, 18, 185–192. [Google Scholar] [CrossRef]
- Kwon, S.; Bae, W.; Lee, K.; Byun, H.S.; Kim, H. High Pressure Phase Behavior of Carbon Dioxide + 2,2,2-Trifluoroethyl Methacrylate and + Poly(2,2,2-trifluoroethyl methacrylate) Systems. J. Chem. Eng. Data 2007, 52, 89–92. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, T.; Li, J.; Pang, Q.; Yang, H.; Liu, G.; Huang, H.; Zhu, Y. Dynamics Simulation of the Effect of Cosolvent on the Solubility and Tackifying Behavior of PDMS Tackifier in Supercritical CO2 Fracturing Fluid. Colloids Surfaces Physicochem. Eng. Asp. 2023, 662, 130985. [Google Scholar] [CrossRef]
- Prasad, S.K.; Behera, U.S.; Lee, C.W.; Byun, H.S. Impact of Cosolvent Concentration of Ternary and Binary Solution for Biodegradable Poly(D,L-lactic acid) under Supercritical Solvents. J. Supercrit. Fluids 2024, 207, 106208. [Google Scholar] [CrossRef]
- Ciardelli, F.; Rubino, G.; Ranieri, G.; Licciulli, A.; Laviano, R. Fluorinated polymeric materials for the protection of monumental buildings. Macromol. Symp. 2000, 152, 211–222. [Google Scholar] [CrossRef]
- Kwon, S.; Bae, W.; Kim, H. The Effect of CO2 in Free-radical Polymerization of 2,2,2-Trifluoroethyl Methacrylate. Korean J. Chem. Eng. 2004, 21, 910–914. [Google Scholar] [CrossRef]
- Ratcharak, O.; Sane, A. Surface coating with poly(trifluoroethyl methacrylate) through rapid expansion of supercritical CO2 solutions. J. Supercrit. Fluids 2014, 89, 106–112. [Google Scholar] [CrossRef]
- Byun, H.S.; Kim, C.R.; Yoon, S.D. Cloud-point measurement of binary and ternary mixtures for the P(MMA-co-PnFPA) in supercritical fluoric solvents. J. Supercrit. Fluids 2017, 120, 226–239. [Google Scholar] [CrossRef]
- Annohene, G.; Tepper, G.C. Low temperature formation of CH3NH3PbI3 perovskite films in supercritical carbon dioxide. J. Supercrit. Fluids 2019, 154, 104604. [Google Scholar] [CrossRef]
- Annohene, G.; Pascucci, J.; Pestov, D.; Tepper, G.C. Supercritical fluid-assisted crystallization of CH2NH3PbI3 perovskite films. J. Supercrit. Fluids 2020, 156, 104684. [Google Scholar] [CrossRef]
- Annohene, G.; Tepper, G.C. Efficient perovskite solar cells processed in supercritical carbon dioxide. J. Supercrit. Fluids 2021, 171, 105203. [Google Scholar] [CrossRef]
- Annohene, G.; Tepper, G. Moisture Stability of Perovskite Solar Cells Processed in Supercritical Carbon Dioxide. Molecules 2021, 26, 7570. [Google Scholar] [CrossRef] [PubMed]
- Handy, K.; Tepper, G.C. Incorporation of Poly(TFEMA) in Perovskite Thin Films Using a Supercritical Fluid. Molecules 2023, 28, 5385. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Schreiber, D.R. Improving equation-of-state accuracy in the critical region; equations for carbon dioxide and neopentane as examples. Fluid Phase Equilibria 1988, 41, 1–17. [Google Scholar] [CrossRef]
- Rowane, A.J.; Mallepally, R.R.; Bamgbade, B.A.; Newkirk, M.S.; Baled, H.O.; Burgess, W.A.; Gamwo, I.K.; Tapriyal, D.; Enick, R.M.; McHugh, M.A. High-temperature, high-pressure viscosities and densities of toluene. J. Chem. Thermodyn. 2017, 115, 34–46. [Google Scholar] [CrossRef][Green Version]
- Franck, E.U.; Wiegand, S.K.G. The Density of Toluene at High Pressures to 673 K and 300 MPa. Berichte Bunsenges. Phys. Chem. 2010, 102, 1794–1797. [Google Scholar] [CrossRef]

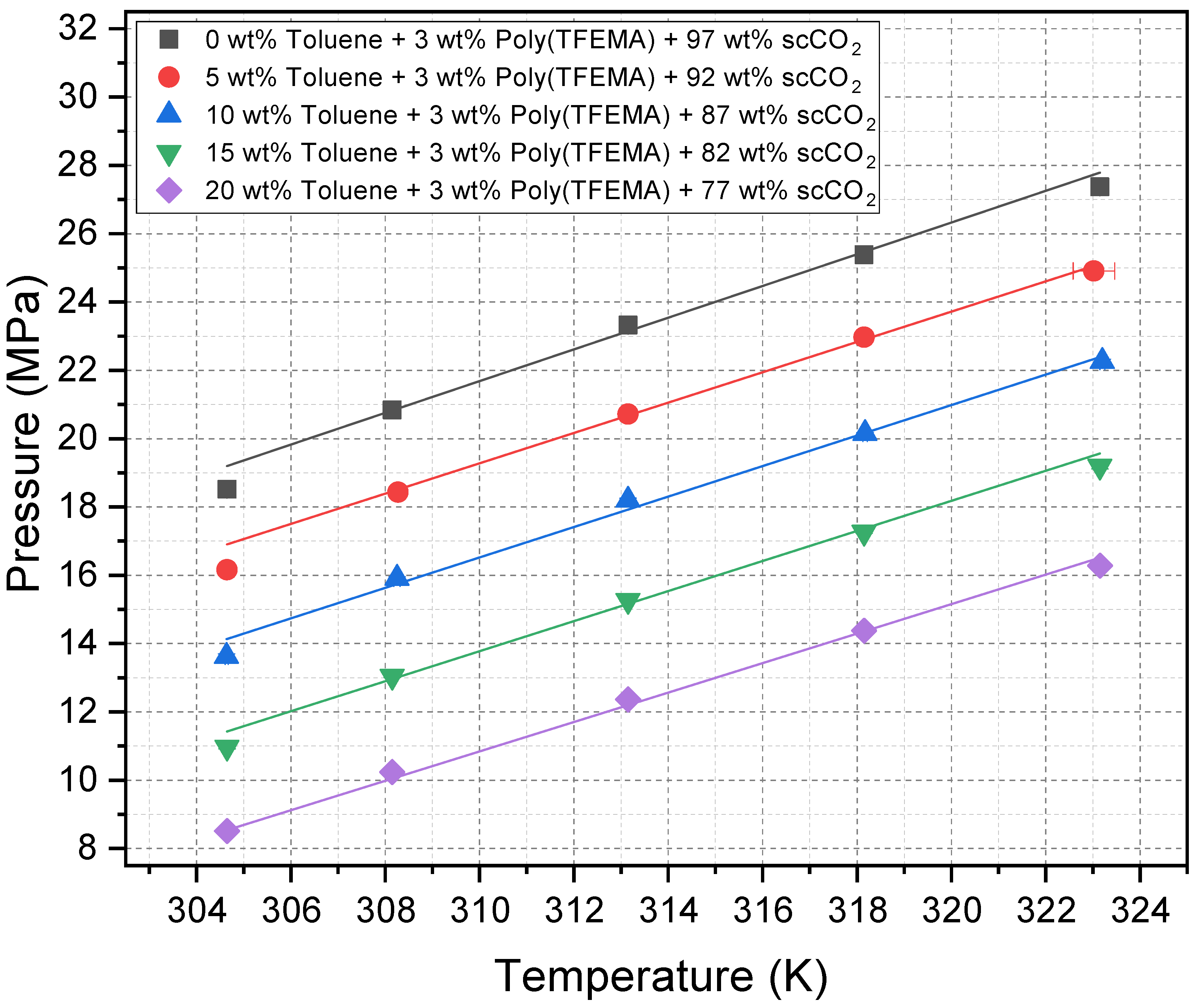



| Temp. | Cloud Point Pressure (MPa) | ||||
|---|---|---|---|---|---|
| (°C) | 0 wt% Tol | 5 wt% Tol | 10 wt% Tol | 15 wt% Tol | 20 wt% Tol |
| 31.5 | 18.52 | 16.16 | 13.63 | 10.97 | 8.51 |
| 35.0 | 20.84 | 18.43 | 15.91 | 13.04 | 10.24 |
| 40.0 | 23.32 | 20.72 | 18.20 | 15.26 | 12.37 |
| 45.0 | 25.38 | 22.97 | 20.15 | 17.26 | 14.38 |
| 50.0 | 27.37 | 24.91 | 22.26 | 19.19 | 16.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelaya, J.R.; Tepper, G.C. Cloud Point Behavior of Poly(trifluoroethyl methacrylate) in Supercritical CO2–Toluene Mixtures. Molecules 2025, 30, 1199. https://doi.org/10.3390/molecules30061199
Zelaya JR, Tepper GC. Cloud Point Behavior of Poly(trifluoroethyl methacrylate) in Supercritical CO2–Toluene Mixtures. Molecules. 2025; 30(6):1199. https://doi.org/10.3390/molecules30061199
Chicago/Turabian StyleZelaya, James R., and Gary C. Tepper. 2025. "Cloud Point Behavior of Poly(trifluoroethyl methacrylate) in Supercritical CO2–Toluene Mixtures" Molecules 30, no. 6: 1199. https://doi.org/10.3390/molecules30061199
APA StyleZelaya, J. R., & Tepper, G. C. (2025). Cloud Point Behavior of Poly(trifluoroethyl methacrylate) in Supercritical CO2–Toluene Mixtures. Molecules, 30(6), 1199. https://doi.org/10.3390/molecules30061199









