Anthraquinones and Aloe Vera Extracts as Potential Modulators of Inflammaging Mechanisms: A Translational Approach from Autoimmune to Onco-Hematological Diseases
Abstract
1. Introduction
2. Search Strategy
3. Inflammaging Mechanisms and Autoimmunity
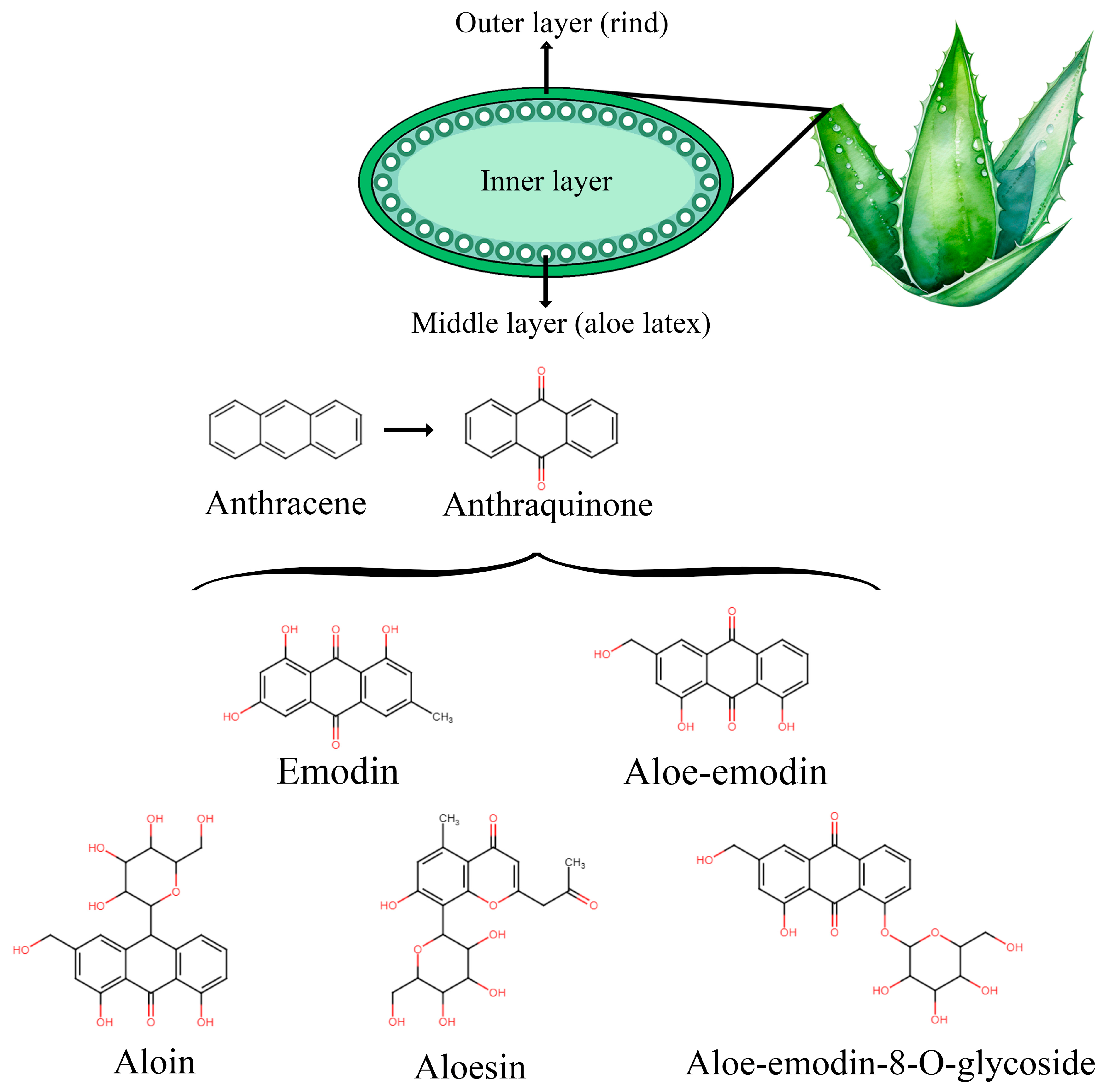
4. AV: Overview, Therapeutic Uses, and Safety Profile
4.1. Generalities and Bioactive Compounds
4.2. Main Clinical Applications and Adverse Effects of AV
5. Effects of AV in Autoimmune Diseases
5.1. Autoimmune Thyroiditis
5.2. Autoimmune Diabetes (AID)
5.3. Multiple Sclerosis (MS)
5.4. Rheumatoid Arthritis
5.5. Systemic Lupus Erythematosus
5.6. Other Autoimmune Diseases
5.7. Connecting the Dots: AV’s Influence on Inflammaging Mechanisms of Autoimmune Diseases
- Modulate inflammatory signals influencing cytokine, chemokine, prostaglandin, and adhesion molecule production (↓ IL-1β, IL-2, IL-6, IL-8, IL-12, IL-17A, IL-18, TNF-α, IFN-γ, TGF-β, CXCR4, CXCR5, CCL5, CCL20, PGE2, and ICAM-1; ↑ IL-4 and IL-10);
- Exert an inhibitory influence on inflammatory MAPKs (ERK1/2, JNK, and p38), PI3K/AKT1, and TLR4/MyD88 molecular pathways;
- Reduce pro-inflammatory enzyme activity or mRNA expression (↓ COX-2, MMP-1, and MMP-13);
- Modulate angiogenesis (↓ VEGF);
- Regulate transcriptional factor expression linked with inflammation (↑ GATA3 and Foxp3; ↓ NF-κB, RORγt, and T-bet);
- Influence autoimmune key mechanisms reducing antibody production;
- Regulate immune response influencing T-cells subset population balance (Th1–Th2);
- Positively modulate autophagy (↑ Beclin1, Atg12, and Atg5 expression);
- Negative influence on inflammasome NLRP3 and related molecule activity;
- Reduced oxidative stress-enhancing anti-oxidant effectors (↑ SOD, GPx, GSH, and CAT activity).
6. Future Perspectives: Autoimmune Cytopenias
6.1. Autoimmune Hemolytic Anemia
6.2. Immune Thrombocytopenia
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging. An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Garagnani, P.; Ostan, R.; Santoro, A.; Monti, D.; Salvioli, S. Inflammaging. In Handbook of Immunosenescence; Springer International Publishing: Cham, Switzerland, 2019; pp. 1599–1629. ISBN 9783319993737. [Google Scholar]
- Cannizzo, E.S.; Clement, C.C.; Sahu, R.; Follo, C.; Santambrogio, L. Oxidative Stress, Inflamm-Aging and Immunosenescence. J. Proteom. 2011, 74, 2313–2323. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and Anti-Inflammaging: A Systemic Perspective on Aging and Longevity Emerged from Studies in Humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Fuentes, F.; Vilahur, G.; Badimon, L.; Palomo, I. Mechanisms of Chronic State of Inflammation as Mediators That Link Obese Adipose Tissue and Metabolic Syndrome. Mediat. Inflamm. 2013, 2013, 136584. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging as Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Puspitasari, Y.M.; Ministrini, S.; Schwarz, L.; Karch, C.; Liberale, L.; Camici, G.G. Modern Concepts in Cardiovascular Disease: Inflamm-Aging. Front. Cell Dev. Biol. 2022, 10, 882211. [Google Scholar] [CrossRef] [PubMed]
- Moyse, E.; Krantic, S.; Djellouli, N.; Roger, S.; Angoulvant, D.; Debacq, C.; Leroy, V.; Fougere, B.; Aidoud, A. Neuroinflammation: A Possible Link between Chronic Vascular Disorders and Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 827263. [Google Scholar] [CrossRef]
- Olivieri, F.; Rippo, M.R.; Monsurrò, V.; Salvioli, S.; Capri, M.; Procopio, A.D.; Franceschi, C. MicroRNAs Linking Inflamm-Aging, Cellular Senescence and Cancer. Ageing Res. Rev. 2013, 12, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, Inflammation and Ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef]
- Goronzy, J.J.; Weyand, C.M. Immune Aging and Autoimmunity. Cell Mol. Life Sci. 2012, 69, 1615–1623. [Google Scholar] [CrossRef]
- Vadasz, Z.; Haj, T.; Kessel, A.; Toubi, E. Age-Related Autoimmunity. BMC Med. 2013, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.V.; Ghosh, R.; Coll, K.; Chun, C.; Yousefzadeh, M.J. The 3 I’s of Immunity and Aging: Immunosenescence, Inflammaging, and Immune Resilience. Front. Aging 2024, 5, 1490302. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Fulbright, J.W.; Goronzy, J.J. Immunosenescence, Autoimmunity, and Rheumatoid Arthritis. Exp. Gerontol. 2003, 38, 833–841. [Google Scholar] [CrossRef]
- Montoya-Ortiz, G. Immunosenescence, Aging, and Systemic Lupus Erythematous. Autoimmune Dis. 2013, 2013, 267078. [Google Scholar] [CrossRef]
- Fu, Y.; Feng, C.; Qin, S.; Xing, Z.; Liu, C.; Liu, Z.; Yu, H. Breaking Barriers: Advancing Cellular Therapies in Autoimmune Disease Management. Front. Immunol. 2024, 15, 1503099. [Google Scholar] [CrossRef]
- GBD 2019 IMID Collaborators. Global, Regional, and National Incidence of Six Major Immune-Mediated Inflammatory Diseases: Findings from the Global Burden of Disease Study 2019. EClinicalMedicine 2023, 64, 102193. [Google Scholar] [CrossRef]
- Low, C.E.; Loke, S.; Chew, N.S.M.; Lee, A.R.Y.B.; Tay, S.H. Vitamin, Antioxidant and Micronutrient Supplementation and the Risk of Developing Incident Autoimmune Diseases: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 15, 1453703. [Google Scholar] [CrossRef]
- Harirchian, M.H.; Mohammadpour, Z.; Fatehi, F.; Firoozeh, N.; Bitarafan, S. A Systematic Review and Meta-Analysis of Randomized Controlled Trials to Evaluating the Trend of Cytokines to Vitamin A Supplementation in Autoimmune Diseases. Clin. Nutr. 2019, 38, 2038–2044. [Google Scholar] [CrossRef]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.-A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, B.S.; Marimuthu, M.M.C.; Sundaram, V.A.; Saravanan, B.; Chandrababu, P.; Chopra, H.; Malik, T. Micro Nutrients as Immunomodulators in the Ageing Population: A Focus on Inflammation and Autoimmunity. Immun. Ageing 2024, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Kim, D.H.; Park, M.H.; Choi, Y.J.; Kim, N.D.; Lee, J.; Yu, B.P.; Chung, H.Y. Recent Advances in Calorie Restriction Research on Aging. Exp. Gerontol. 2013, 48, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Cipriano, C.; Muti, E.; Tesei, S.; Malavolta, M. Nutrient-Gene Interaction in Ageing and Successful Ageing. A Single Nutrient (Zinc) and Some Target Genes Related to Inflammatory/Immune Response. Mech. Ageing Dev. 2006, 127, 517–525. [Google Scholar] [CrossRef]
- Marchal, J.; Pifferi, F.; Aujard, F. Resveratrol in Mammals: Effects on Aging Biomarkers, Age-Related Diseases, and Life Span. Ann. N. Y. Acad. Sci. 2013, 1290, 67–73. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an Anti-Inflammatory and Anti-Aging Agent: Mechanisms and Clinical Implications. Mol. Nutr. Food Res. 2005, 49, 405–430. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Cîrîc, A.-I.; Begea, M. Anti-Aging Effects of Flavonoids from Plant Extracts. Foods 2024, 13, 2441. [Google Scholar] [CrossRef]
- Gao, Y.; Kuok, K.I.; Jin, Y.; Wang, R. Biomedical Applications of Aloe vera. Crit. Rev. Food Sci. Nutr. 2019, 59, S244–S256. [Google Scholar] [CrossRef]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic Inflammation and the Hallmarks of Aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef]
- Kornadt, A.E.; Kandler, C. Genetic and Environmental Sources of Individual Differences in Views on Aging. Psychol. Aging 2017, 32, 388–399. [Google Scholar] [CrossRef]
- Gill, R.; Tsung, A.; Billiar, T. Linking Oxidative Stress to Inflammation: Toll-like Receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F. Signaling by ROS Drives Inflammasome Activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef]
- O’Rourke, S.A.; Shanley, L.C.; Dunne, A. The Nrf2-HO-1 System and Inflammaging. Front. Immunol. 2024, 15, 1457010. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W. Redox Regulation of Transcriptional Activators. Free Radic. Biol. Med. 1996, 21, 335–348. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive Oxygen Species in Cell Signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 Inflammasome: Contributions to Inflammation-Related Diseases. Cell Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 Inflammasome in Autoimmune Diseases. Biomed. Pharmacother. 2020, 130, 110542. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Nordeng, J.; Schandiz, H.; Solheim, S.; Åkra, S.; Hoffman, P.; Roald, B.; Bendz, B.; Arnesen, H.; Helseth, R.; Seljeflot, I. The Inflammasome Signaling Pathway Is Actively Regulated and Related to Myocardial Damage in Coronary Thrombi from Patients with STEMI. Mediat. Inflamm. 2021, 2021, 5525917. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.A.; Smith Jr, M.F.; Sanders, M.K.; Ernst, P.B. Reactive Oxygen and Nitrogen Species Differentially Regulate Toll-like Receptor 4-Mediated Activation of NF-ΚB and Interleukin-8 Expression. Infect. Immun. 2004, 72, 2123–2130. [Google Scholar] [CrossRef]
- Costa, A.D.T.; Garlid, K.D. Intramitochondrial Signaling: Interactions among MitoKATP, PKCepsilon, ROS, and MPT. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H874–H882. [Google Scholar] [CrossRef]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive Oxygen and Nitrogen Species as Signaling Molecules Regulating Neutrophil Function. Free Radic. Biol. Med. 2007, 42, 153–164. [Google Scholar] [CrossRef]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive Oxygen Species (ROS) in Macrophage Activation and Function in Diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Gomez, C.R.; Nomellini, V.; Faunce, D.E.; Kovacs, E.J. Innate Immunity and Aging. Exp. Gerontol. 2008, 43, 718–728. [Google Scholar] [CrossRef]
- Feehan, J.; Tripodi, N.; Apostolopoulos, V. The Twilight of the Immune System: The Impact of Immunosenescence in Aging. Maturitas 2021, 147, 7–13. [Google Scholar] [CrossRef]
- Rodrigues, L.P.; Teixeira, V.R.; Alencar-Silva, T.; Simonassi-Paiva, B.; Pereira, R.W.; Pogue, R.; Carvalho, J.L. Hallmarks of Aging and Immunosenescence: Connecting the Dots. Cytokine Growth Factor Rev. 2021, 59, 9–21. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kouno, T.; Ikawa, T.; Hayatsu, N.; Miyajima, Y.; Yabukami, H.; Terooatea, T.; Sasaki, T.; Suzuki, T.; Valentine, M.; et al. Single-Cell Transcriptomics Reveals Expansion of Cytotoxic CD4 T Cells in Supercentenarians. Proc. Natl. Acad. Sci. USA 2019, 116, 24242–24251. [Google Scholar] [CrossRef]
- Song, S.; Tchkonia, T.; Jiang, J.; Kirkland, J.L.; Sun, Y. Targeting Senescent Cells for a Healthier Aging: Challenges and Opportunities. Adv. Sci. 2020, 7, 2002611. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; Ceprián, N.; Díaz-Del Cerro, E.; De la Fuente, M. The Role of Immune Cells in Oxi-Inflamm-Aging. Cells 2021, 10, 2974. [Google Scholar] [CrossRef]
- Yasmeen, F.; Pirzada, R.H.; Ahmad, B.; Choi, B.; Choi, S. Understanding Autoimmunity: Mechanisms, Predisposing Factors, and Cytokine Therapies. Int. J. Mol. Sci. 2024, 25, 7666. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Pathogenesis of Autoimmune Disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, R.; Zwergel, C.; Artico, M.; Taurone, S.; Ralli, M.; Greco, A.; Mai, A. The Emerging Role of Epigenetics in Human Autoimmune Disorders. Clin. Epigenet. 2019, 11, 34. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; McCabe, E.J.; Lieberman, S.M. Mechanisms Underlying Sex Differences in Autoimmunity. J. Clin. Investig. 2024, 134, e180076. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Wang, W.; Su, D.-M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing 2020, 17, 2. [Google Scholar] [CrossRef]
- Zhao, T.V.; Sato, Y.; Goronzy, J.J.; Weyand, C.M. T-Cell Aging-Associated Phenotypes in Autoimmune Disease. Front. Aging 2022, 3, 867950. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sanchez, P.; Teagle, A.R.; Prade, S.; Zamoyska, R. Modulation of TCR Signaling by Tyrosine Phosphatases: From Autoimmunity to Immunotherapy. Front. Cell Dev. Biol. 2020, 8, 608747. [Google Scholar] [CrossRef]
- Oh, J.; Wang, W.; Thomas, R.; Su, D.-M. Capacity of TTreg Generation Is Not Impaired in the Atrophied Thymus. PLoS Biol. 2017, 15, e2003352. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S. From Aging to Long COVID: Exploring the Convergence of Immunosenescence, Inflammaging, and Autoimmunity. Front. Immunol. 2023, 14, 1298004. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; Akbar, A.N. Convergence of Innate and Adaptive Immunity during Human Aging. Front. Immunol. 2016, 7, 445. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe vera and Its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef]
- Hossain, M.; MamunOrRashid, A.N.M.; Towfique, N.; Sen, M. A Review on Ethnopharmacological Potential of Aloe vera L. J. Intercult. Ethnopharmacol. 2013, 2, 113. [Google Scholar] [CrossRef]
- Kaur, S.; Bains, K. Aloe barbadensis Miller (Aloe vera). Int. J. Vitam. Nutr. Res. 2024, 94, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic Potential of Aloe vera—A Miracle Gift of Nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef]
- Rahman, S.; Carter, P.; Bhattarai, N. Aloe vera for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 6. [Google Scholar] [CrossRef]
- Sahu, P.K.; Giri, D.D.; Singh, R.; Pandey, P.; Gupta, S.; Shrivastava, A.K.; Kumar, A.; Pandey, K.D. Therapeutic and Medicinal Uses of Aloe vera: A Review. Pharmacol. Pharm. 2013, 4, 599–610. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D.G. Aloe vera: A Short Review. Indian J. Dermatol. 2008, 53, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Molinari, G.P.; Colla, G. Phytochemical Constituents and in Vitro Radical Scavenging Activity of Different Aloe Species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The Therapeutic Properties and Applications of Aloe vera: A Review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Vogler, B.K.; Ernst, E. Aloe vera: A Systematic Review of Its Clinical Effectiveness. Br. J. Gen. Pract. 1999, 49, 823–828. [Google Scholar] [PubMed]
- Mandal, S.C.; Nayak, A.K.; Dhara, A.K. Herbal Biomolecules in Healthcare Applications; Academic Press: San Diego, CA, USA, 2021; ISBN 9780323858526. [Google Scholar]
- Fairbairn, J.W. Biological Assay and Its Relation to Chemical Structure. Pharmacology 1976, 14, 48–61. [Google Scholar] [CrossRef]
- Godding, E.W. Therapeutics of Laxative Agents with Special Reference to the Anthraquinones. Pharmacology 1976, 14, 78–101. [Google Scholar] [CrossRef]
- Malik, E.M.; Müller, C.E. Anthraquinones as Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, L. A Review on Bioactive Anthraquinone and Derivatives as the Regulators for ROS. Molecules 2023, 28, 8139. [Google Scholar] [CrossRef]
- Wu, X.; Ding, W.; Zhong, J.; Wan, J.; Xie, Z. Simultaneous Qualitative and Quantitative Determination of Phenolic Compounds in Aloe barbadensis Mill by Liquid Chromatography-Mass Spectrometry-Ion Trap-Time-of-Flight and High Performance Liquid Chromatography-Diode Array Detector. J. Pharm. Biomed. Anal. 2013, 80, 94–106. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of Biological Properties and Clinical Effectiveness of Aloe vera: A Systematic Review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef]
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Marra, M.; Conforti, F.; Lupi, F.R.; Gabriele, D.; Borges, F.; Sinicropi, M.S. Aloe vera—An Extensive Review Focused on Recent Studies. Foods 2024, 13, 2155. [Google Scholar] [CrossRef]
- Das, S.; Mishra, B.; Gill, K.; Ashraf, M.S.; Singh, A.K.; Sinha, M.; Sharma, S.; Xess, I.; Dalal, K.; Singh, T.P.; et al. Isolation and Characterization of Novel Protein with Anti-Fungal and Anti-Inflammatory Properties from Aloe vera Leaf Gel. Int. J. Biol. Macromol. 2011, 48, 38–43. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, T.; Sheng, L.; Wang, Z.; Tao, H.; Zhang, Q.; Zhang, Y.; Qi, Z. Aloin Suppresses Lipopolysaccharide-induced Inflammation by Inhibiting JAK1-STAT1/3 Activation and ROS Production in RAW264.7 Cells. Int. J. Mol. Med. 2018, 42, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Guo, S.; Yang, C.; Yang, J.; Chen, Y.; Shaukat, A.; Zhao, G.; Wu, H.; Deng, G. Barbaloin Protects against Lipopolysaccharide (LPS)-Induced Acute Lung Injury by Inhibiting the ROS-Mediated PI3K/AKT/NF-ΚB Pathway. Int. Immunopharmacol. 2018, 64, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Suzuki, K.; Hung, Y.-L.; Yang, M.-S.; Yu, C.-P.; Lin, S.-P.; Hou, Y.-C.; Fang, S.-H. Aloe Metabolites Prevent LPS-Induced Sepsis and Inflammatory Response by Inhibiting Mitogen-Activated Protein Kinase Activation. Am. J. Chin. Med. 2017, 45, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Budai, M.M.; Varga, A.; Milesz, S.; Tőzsér, J.; Benkő, S. Aloe vera Downregulates LPS-Induced Inflammatory Cytokine Production and Expression of NLRP3 Inflammasome in Human Macrophages. Mol. Immunol. 2013, 56, 471–479. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Magnusson, M.K.; Isaksson, S.; Larsson, F.; Öhman, L. Effects of Aloe barbadensis Mill. Extract (AVH200®) on Human Blood T Cell Activity in Vitro. J. Ethnopharmacol. 2016, 179, 301–309. [Google Scholar] [CrossRef]
- Debnath, T.; Ghosh, M.; Lee, Y.M.; Nath, N.C.D.; Lee, K.-G.; Lim, B.O. Identification of Phenolic Constituents and Antioxidant Activity of Aloe barbadensis Flower Extracts. Food Agric. Immunol. 2018, 29, 27–38. [Google Scholar] [CrossRef]
- Sun, Y.N.; Li, W.; Lee, S.H.; Jang, H.D.; Ma, J.Y.; Kim, Y.H. Antioxidant and Anti-Osteoporotic Effects of Anthraquinones and Related Constituents from the Aqueous Dissolved Aloe Exudates. Nat. Prod. Res. 2017, 31, 2810–2813. [Google Scholar] [CrossRef]
- Prueksrisakul, T.; Chantarangsu, S.; Thunyakitpisal, P. Effect of Daily Drinking of Aloe vera Gel Extract on Plasma Total Antioxidant Capacity and Oral Pathogenic Bacteria in Healthy Volunteer: A Short-Term Study. J. Complement. Integr. Med. 2015, 12, 159–164. [Google Scholar] [CrossRef]
- Choudhary, M.; Kochhar, A.; Sangha, J. Hypoglycemic and Hypolipidemic Effect of Aloe vera L. in Non-Insulin Dependent Diabetics. J. Food Sci. Technol. 2014, 51, 90–96. [Google Scholar] [CrossRef]
- Dick, W.R.; Fletcher, E.A.; Shah, S.A. Reduction of Fasting Blood Glucose and Hemoglobin A1c Using Oral Aloe vera: A Meta-Analysis. J. Altern. Complement. Med. 2016, 22, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.; Shim, K.-S.; Kong, H.; Lee, S.; Shin, S.; Kwon, J.; Jo, T.H.; Park, Y.-I.; Lee, C.-K.; Kim, K. Dietary Aloe Improves Insulin Sensitivity via the Suppression of Obesity-Induced Inflammation in Obese Mice. Immune Netw. 2011, 11, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, B.; Tomar, N.R.; Roy, P.; Gupta, A.K.; Kumar, A. In Vivo Evaluation of Hypoglycemic Activity of Aloe Spp. and Identification of Its Mode of Action on GLUT-4 Gene Expression in Vitro. Appl. Biochem. Biotechnol. 2011, 164, 1246–1256. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, J.W.; Ree, J.; Lim, J.S.; Lee, J.; Kim, J.I.; Thapa, S.B.; Sohng, J.K.; Park, Y.I. Aloe Emodin 3-O-Glucoside Inhibits Cell Growth and Migration and Induces Apoptosis of Non-Small-Cell Lung Cancer Cells via Suppressing MEK/ERK and Akt Signalling Pathways. Life Sci. 2022, 300, 120495. [Google Scholar] [CrossRef]
- Manirakiza, A.; Irakoze, L.; Manirakiza, S. Aloe and Its Effects on Cancer: A Narrative Literature Review. East Afr. Health Res. J. 2021, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chihara, T.; Shimpo, K.; Beppu, H.; Yamamoto, N.; Kaneko, T.; Wakamatsu, K.; Sonoda, S. Effects of Aloe-Emodin and Emodin on Proliferation of the MKN45 Human Gastric Cancer Cell Line. Asian Pac. J. Cancer Prev. 2015, 16, 3887–3891. [Google Scholar] [CrossRef]
- Suboj, P.; Babykutty, S.; Valiyaparambil Gopi, D.R.; Nair, R.S.; Srinivas, P.; Gopala, S. Aloe Emodin Inhibits Colon Cancer Cell Migration/Angiogenesis by Downregulating MMP-2/9, RhoB and VEGF via Reduced DNA Binding Activity of NF-κB. Eur. J. Pharm. Sci. 2012, 45, 581–591. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Mastova, A.V.; Polyakov, N.E. The Interaction of Anthracycline Based Quinone-Chelators with Model Lipid Membranes: 1H NMR and MD Study. Membranes 2023, 13, 61. [Google Scholar] [CrossRef]
- Choi, R.J.; Ngoc, T.M.; Bae, K.; Cho, H.-J.; Kim, D.-D.; Chun, J.; Khan, S.; Kim, Y.S. Anti-Inflammatory Properties of Anthraquinones and Their Relationship with the Regulation of P-Glycoprotein Function and Expression. Eur. J. Pharm. Sci. 2013, 48, 272–281. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal Activity of Anthraquinone Derivatives from Rheum Emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.L.; Fairbairn, D.W.; O’Neill, K.L.; Gage, T.L.; Sidwell, R.W. Anti-Human Cytomegalovirus Activity and Toxicity of Sulfonated Anthraquinones and Anthraquinone Derivatives. Antivir. Res. 1995, 28, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Xu, A.; Lee, R.; Nguyen, D.N.; Phan, T.A.; Hamada, S.M.; Panchel, R.; Tam, C.C.; Kim, J.H.; Cheng, L.W.; et al. The Inhibitory Activity of Anthraquinones against Pathogenic Protozoa, Bacteria, and Fungi and the Relationship to Structure. Molecules 2020, 25, 3101. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.A.; Faiesal, A.A.; Gamar, G.M.; El Dougdoug, N.K. Efficacy of Bacteriophages with Aloe vera Extract in Formulated Cosmetics to Combat Multidrug-Resistant Bacteria in Skin Diseases. Sci. Rep. 2025, 15, 4335. [Google Scholar] [CrossRef]
- Kouser, F.; Kumar, S.; Bhat, H.F.; Hassoun, A.; Bekhit, A.E.-D.A.; Bhat, Z.F. Aloe barbadensis Based Bioactive Edible Film Improved Lipid Stability and Microbial Quality of the Cheese. Foods 2023, 12, 229. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.; Alonso, J.L.; Pintado, M. In Vitro Assessment of the Prebiotic Potential of Aloe vera Mucilage and Its Impact on the Human Microbiota. Food Funct. 2015, 6, 525–531. [Google Scholar] [CrossRef]
- Quezada, M.P.; Salinas, C.; Gotteland, M.; Cardemil, L. Acemannan and Fructans from Aloe vera (Aloe barbadensis Miller) Plants as Novel Prebiotics. J. Agric. Food Chem. 2017, 65, 10029–10039. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Rokeya, B.; Ahmed, S.; Bhowmik, A.; Khalil, M.I.; Gan, S.H. In Vitro Antioxidant Effects of Aloe barbadensis Miller Extracts and the Potential Role of These Extracts as Antidiabetic and Antilipidemic Agents on Streptozotocin-Induced Type 2 Diabetic Model Rats. Molecules 2012, 17, 12851–12867. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.; Kothari, N. An Experimental Study Performed to Compare the Hepatoprotective Activity of Aloe vera and Silymarin in Carbon Tetra Chloride (Ccl4)-Induced Hepatotoxicity in Albino Rabbits. Asian J. Pharm. Clin. Res. 2022, 15, 90–94. [Google Scholar] [CrossRef]
- Cui, Y.; Ye, Q.; Wang, H.; Li, Y.; Yao, W.; Qian, H. Hepatoprotective Potential of Aloe vera Polysaccharides against Chronic Alcohol-Induced Hepatotoxicity in Mice. J. Sci. Food Agric. 2014, 94, 1764–1771. [Google Scholar] [CrossRef]
- Cui, Y.; Cheng, Y.; Guo, Y.; Xie, Y.; Yao, W.; Zhang, W.; Qian, H. Evaluating the Hepatoprotective Efficacy of Aloe vera Polysaccharides against Subchronic Exposure of Aflatoxins B1. J. Taiwan Inst. Chem. Eng. 2017, 76, 10–17. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Mohammadi, A.A.; Moshiri, A. Healing Potential of Injectable Aloe vera Hydrogel Loaded by Adipose-Derived Stem Cell in Skin Tissue-Engineering in a Rat Burn Wound Model. Cell Tissue Res. 2019, 377, 215–227. [Google Scholar] [CrossRef]
- Takzaree, N.; Hadjiakhondi, A.; Hassanzadeh, G.; Rouini, M.R.; Manayi, A.; Zolbin, M.M. Transforming Growth Factor-β (TGF-β) Activation in Cutaneous Wounds after Topical Application of Aloe vera Gel. Can. J. Physiol. Pharmacol. 2016, 94, 1285–1290. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Sendón, R.; Sanches-Silva, A. Aloe vera: Ancient Knowledge with New Frontiers. Trends Food Sci. Technol. 2017, 61, 94–102. [Google Scholar] [CrossRef]
- Tanaka, M.; Misawa, E.; Yamauchi, K.; Abe, F.; Ishizaki, C. Effects of Plant Sterols Derived from Aloe vera Gel on Human Dermal Fibroblasts in Vitro and on Skin Condition in Japanese Women. Clin. Cosmet. Investig. Dermatol. 2015, 8, 95–104. [Google Scholar] [CrossRef]
- Saito, M.; Tanaka, M.; Misawa, E.; Yao, R.; Nabeshima, K.; Yamauchi, K.; Abe, F.; Yamamoto, Y.; Furukawa, F. Oral Administration of Aloe vera Gel Powder Prevents UVB-Induced Decrease in Skin Elasticity via Suppression of Overexpression of MMPs in Hairless Mice. Biosci. Biotechnol. Biochem. 2016, 80, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Islam, R.; Rana, M.M.; Spitzhorn, L.-S.; Rahman, M.S.; Adjaye, J.; Asaduzzaman, S.M. Characterization of Burn Wound Healing Gel Prepared from Human Amniotic Membrane and Aloe vera Extract. BMC Complement. Altern. Med. 2019, 19, 115. [Google Scholar] [CrossRef]
- Teplicki, E.; Ma, Q.; Castillo, D.E.; Zarei, M.; Hustad, A.P.; Chen, J.; Li, J. The Effects of Aloe vera on Wound Healing in Cell Proliferation, Migration, and Viability. Wounds 2018, 30, 263–268. [Google Scholar] [PubMed]
- Misawa, E.; Tanaka, M.; Saito, M.; Nabeshima, K.; Yao, R.; Yamauchi, K.; Abe, F.; Yamamoto, Y.; Furukawa, F. Protective Effects of Aloe Sterols against UVB-Induced Photoaging in Hairless Mice. Photodermatol. Photoimmunol. Photomed. 2017, 33, 101–111. [Google Scholar] [CrossRef]
- Leng, H.; Pu, L.; Xu, L.; Shi, X.; Ji, J.; Chen, K. Effects of Aloe Polysaccharide, a Polysaccharide Extracted from Aloe vera, on TNF-α-induced HaCaT Cell Proliferation and the Underlying Mechanism in Psoriasis. Mol. Med. Rep. 2018, 18, 3537–3543. [Google Scholar] [CrossRef]
- Paulsen, E.; Korsholm, L.; Brandrup, F. A Double-Blind, Placebo-Controlled Study of a Commercial Aloe vera Gel in the Treatment of Slight to Moderate Psoriasis Vulgaris. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 326–331. [Google Scholar] [CrossRef]
- Pal, S.; Raj, M.; Singh, M.; Saurav, K.; Paliwal, C.; Saha, S.; Sharma, A.K.; Singh, M. The Effect of Aloe vera on Skin and Its Commensals: Contribution of Acemannan in Curing Acne Caused by Propionibacterium Acnes. Microorganisms 2024, 12, 2070. [Google Scholar] [CrossRef] [PubMed]
- Miroddi, M.; Navarra, M.; Calapai, F.; Mancari, F.; Giofrè, S.V.; Gangemi, S.; Calapai, G. Review of Clinical Pharmacology of Aloe vera L. in the Treatment of Psoriasis. Phytother. Res. 2015, 29, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Lagartoparra, A. Comparative Study of the Assay of and the Estimate of the Medium Lethal Dose (LD50 Value) in Mice, to Determine Oral Acute Toxicity of Plant Extracts. Phytomedicine 2001, 8, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Feng, Y.; Wang, H.; Yang, H. 90-Day Subchronic Toxicity Study of Aloe Whole-Leaf Powder. Wei Sheng Yan Jiu 2003, 32, 590–593. [Google Scholar]
- Lee, J.; Lee, M.S.; Nam, K.W. Acute Toxic Hepatitis Caused by an Aloe vera Preparation in a Young Patient: A Case Report with a Literature Review. Taehan Sohwagi Hakhoe Chi [Korean J. Gastroenterol.] 2014, 64, 54–58. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Aloe vera: A Review of Toxicity and Adverse Clinical Effects. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 77–96. [Google Scholar] [CrossRef]
- Ferreira, M.; Teixeira, M.; Silva, E.; Selores, M. Allergic Contact Dermatitis to Aloe vera. Contact Dermat. 2007, 57, 278–279. [Google Scholar] [CrossRef]
- Avila, H.; Rivero, J.; Herrera, F.; Fraile, G. Cytotoxicity of a Low Molecular Weight Fraction from Aloe vera (Aloe barbadensis Miller) Gel. Toxicon 1997, 35, 1423–1430. [Google Scholar] [CrossRef]
- van Gorkom, B.A.; de Vries, E.G.; Karrenbeld, A.; Kleibeuker, J.H. Review Article: Anthranoid Laxatives and Their Potential Carcinogenic Effects. Aliment. Pharmacol. Ther. 1999, 13, 443–452. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, C.; Wu, F.; Fang, K.; Jiang, S.; Zhao, Y.; Chen, G.; Dong, R. Review on Melanosis Coli and Anthraquinone-Containing Traditional Chinese Herbs That Cause Melanosis Coli. Front. Pharmacol. 2023, 14, 1160480. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, A.; Völkner, W.; Mengs, U. Genotoxicity of Aloeemodin in Vitro and in Vivo. Mutat. Res.-Rev. Genet. Toxicol. 1996, 367, 123–133. [Google Scholar] [CrossRef]
- Hayes, A.W.; Pressman, P.; Clemens, R.; Singer, A.W.; Bauter, M.R. Evaluation of 90-Day Repeated Dose Oral Toxicity of an Aloe vera Inner Leaf Gel Beverage. Food Chem. Toxicol. 2024, 189, 114726. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, M.D.; Mellick, P.W.; Olson, G.R.; Felton, R.P.; Thorn, B.T.; Beland, F.A. Clear Evidence of Carcinogenic Activity by a Whole-Leaf Extract of Aloe barbadensis Miller (Aloe vera) in F344/N Rats. Toxicol. Sci. 2013, 131, 26–39. [Google Scholar] [CrossRef]
- Xia, Q.; Yin, J.J.; Fu, P.P.; Boudreau, M.D. Photo-Irradiation of Aloe vera by UVA--Formation of Free Radicals, Singlet Oxygen, Superoxide, and Induction of Lipid Peroxidation. Toxicol. Lett. 2007, 168, 165–175. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Dial, S.L.; Boudreau, M.D.; Xia, Q.; Fu, P.P.; Levy, D.D.; Moore, M.M.; Mei, N. In Vitro Investigation of the Mutagenic Potential of Aloe vera Extracts. Toxicol. Res. 2014, 3, 487–496. [Google Scholar] [CrossRef]
- Davis, R.H.; Parker, W.L.; Murdoch, D.P. Aloe vera as a Biologically Active Vehicle for Hydrocortisone Acetate. J. Am. Podiatr. Med. Assoc. 1991, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Chui, P.T.; Aun, C.S.T.; Gin, T.; Lau, A.S.C. Possible Interaction between Sevoflurane and Aloe vera. Ann. Pharmacother. 2004, 38, 1651–1654. [Google Scholar] [CrossRef]
- Abstracts of 48th ESCP Symposium on Clinical Pharmacy 23–25 October 2019, Ljubljana (Slovenia): The Digital Revolution Supporting Clinical Pharmacy through e-Health, Digital Support Systems, Big Data, and More. Int. J. Clin. Pharm. 2019, 42, 217–292. [CrossRef]
- Pressman, P.; Clemens, R.; Hayes, A.W. Aloe vera at the Frontier of Glycobiology and Integrative Medicine: Health Implications of an Ancient Plant. SAGE Open Med. 2019, 7, 2050312119875921. [Google Scholar] [CrossRef]
- Pressman, P.; Clemens, R.A.; Hayes, A.W. EFSA Strikes Again: A Commentary on Flawed Analysis. Eur. J. Food Sci. Technol. 2022, 10, 13–23. [Google Scholar] [CrossRef]
- Petranović Ovčariček, P.; Görges, R.; Giovanella, L. Autoimmune Thyroid Diseases. Semin. Nucl. Med. 2024, 54, 219–236. [Google Scholar] [CrossRef]
- Li, Q.; Wang, B.; Mu, K.; Zhang, J.-A. The Pathogenesis of Thyroid Autoimmune Diseases: New T Lymphocytes—Cytokines Circuits beyond the Th1-Th2 Paradigm. J. Cell Physiol. 2019, 234, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Ostan, R.; Mariotti, S.; Monti, D.; Vitale, G. The Aging Thyroid: A Reappraisal within the Geroscience Integrated Perspective. Endocr. Rev. 2019, 40, 1250–1270. [Google Scholar] [CrossRef] [PubMed]
- San Martín, A.; Griendling, K.K. Redox Control of Vascular Smooth Muscle Migration. Antioxid. Redox Signal 2010, 12, 625–640. [Google Scholar] [CrossRef]
- Magsino Jr, C.H.; Hamouda, W.; Ghanim, H.; Browne, R.; Aljada, A.; Dandona, P. Effect of Triiodothyronine on Reactive Oxygen Species Generation by Leukocytes, Indices of Oxidative Damage, and Antioxidant Reserve. Metabolism 2000, 49, 799–803. [Google Scholar] [CrossRef]
- Perrotta, C.; Buldorini, M.; Assi, E.; Cazzato, D.; De Palma, C.; Clementi, E.; Cervia, D. The Thyroid Hormone Triiodothyronine Controls Macrophage Maturation and Functions: Protective Role during Inflammation. Am. J. Pathol. 2014, 184, 230–247. [Google Scholar] [CrossRef]
- Rozing, M.P.; Westendorp, R.G.J.; Maier, A.B.; Wijsman, C.A.; Frölich, M.; de Craen, A.J.M.; van Heemst, D. Serum Triiodothyronine Levels and Inflammatory Cytokine Production Capacity. Age 2012, 34, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Bucci, L.; Capri, M.; Salvioli, S.; Scurti, M.; Pini, E.; Monti, D.; Franceschi, C. Immunosenescence and Immunogenetics of Human Longevity. Neuroimmunomodulation 2008, 15, 224–240. [Google Scholar] [CrossRef]
- Metro, D.; Cernaro, V.; Papa, M.; Benvenga, S. Marked Improvement of Thyroid Function and Autoimmunity by Aloe barbadensis Miller Juice in Patients with Subclinical Hypothyroidism. J. Clin. Transl. Endocrinol. 2018, 11, 18–25. [Google Scholar] [CrossRef]
- Sun, H.; Ye, Z.; Li, N.; Jin, F.; Yan, J.; Wu, K. Effect of Emodin on T Cell Subsets in NOD Mice with NaI-induced Experimental Autoimmune Thyroiditis. Mol. Med. Rep. 2018, 18, 4303–4312. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Sharma, R.; Khan, A.; Kar, A. Ameliorative Effect of Aloe Gel against L-T4-Induced Hyperthyroidism via Suppression of Thyrotropin Receptors, Inflammation and Oxidative Stress. Mol. Biol. Rep. 2020, 47, 2801–2810. [Google Scholar] [CrossRef] [PubMed]
- Akamizu, T.; Ikuyama, S.; Saji, M.; Kosugi, S.; Kozak, C.; McBride, O.W.; Kohn, L.D. Cloning, Chromosomal Assignment, and Regulation of the Rat Thyrotropin Receptor: Expression of the Gene Is Regulated by Thyrotropin, Agents That Increase CAMP Levels, and Thyroid Autoantibodies. Proc. Natl. Acad. Sci. USA 1990, 87, 5677–5681. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The Role of Inflammation in Insulitis and Beta-Cell Loss in Type 1 Diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- Chien, S.-C.; Wu, Y.-C.; Chen, Z.-W.; Yang, W.-C. Naturally Occurring Anthraquinones: Chemistry and Therapeutic Potential in Autoimmune Diabetes. Evid.-Based Complement. Alternat Med. 2015, 2015, 357357. [Google Scholar] [CrossRef]
- O’Reilly, L.A.; Hutchings, P.R.; Crocker, P.R.; Simpson, E.; Lund, T.; Kioussis, D.; Takei, F.; Baird, J.; Cooke, A. Characterization of Pancreatic Islet Cell Infiltrates in NOD Mice: Effect of Cell Transfer and Transgene Expression. Eur. J. Immunol. 1991, 21, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Wilson, S.B. Fatal Attraction: Chemokines and Type 1 Diabetes. J. Clin. Investig. 2002, 110, 1611–1613. [Google Scholar] [CrossRef]
- Bradley, L.M.; Asensio, V.C.; Schioetz, L.K.; Harbertson, J.; Krahl, T.; Patstone, G.; Woolf, N.; Campbell, I.L.; Sarvetnick, N. Islet-Specific Th1, but Not Th2, Cells Secrete Multiple Chemokines and Promote Rapid Induction of Autoimmune Diabetes. J. Immunol. 1999, 162, 2511–2520. [Google Scholar] [CrossRef]
- Meagher, C.; Sharif, S.; Hussain, S.; Cameron, M.J.; Arreaza, G.A.; Delovitch, T.L. Cytokines and Chemokines in the Pathogenesis of Murine Type 1 Diabetes. In Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2003; pp. 133–158. ISBN 9781461349525. [Google Scholar]
- Bromley, S.K.; Mempel, T.R.; Luster, A.D. Orchestrating the Orchestrators: Chemokines in Control of T Cell Traffic. Nat. Immunol. 2008, 9, 970–980. [Google Scholar] [CrossRef]
- Shen, M.-Y.; Liu, Y.-J.; Don, M.-J.; Liu, H.-Y.; Chen, Z.-W.; Mettling, C.; Corbeau, P.; Chiang, C.-K.; Jang, Y.-S.; Li, T.-H.; et al. Combined Phytochemistry and Chemotaxis Assays for Identification and Mechanistic Analysis of Anti-Inflammatory Phytochemicals in Fallopia Japonica. PLoS ONE 2011, 6, e27480. [Google Scholar] [CrossRef]
- Shen, M.-Y.; Lin, Y.-P.; Yang, B.-C.; Jang, Y.-S.; Chiang, C.-K.; Mettling, C.; Chen, Z.-W.; Sheu, J.-R.; Chang, C.L.; Lin, Y.-L.; et al. Catenarin Prevents Type 1 Diabetes in Nonobese Diabetic Mice via Inhibition of Leukocyte Migration Involving the MEK6/P38 and MEK7/JNK Pathways. Evid.-Based Complement. Alternat. Med. 2012, 2012, 982396. [Google Scholar] [CrossRef] [PubMed]
- Malaguti, C.; Vilella, C.A.; Vieira, K.P.; Souza, G.H.M.F.; Hyslop, S.; Zollner, R. de L. Diacerhein Downregulate Proinflammatory Cytokines Expression and Decrease the Autoimmune Diabetes Frequency in Nonobese Diabetic (NOD) Mice. Int. Immunopharmacol. 2008, 8, 782–791. [Google Scholar] [CrossRef]
- Rabinovitch, A. An Update on Cytokines in the Pathogenesis of Insulin-Dependent Diabetes Mellitus. Diabetes Metab. Rev. 1998, 14, 129–151. [Google Scholar] [CrossRef]
- Suk, K.; Kim, S.; Kim, Y.H.; Kim, K.A.; Chang, I.; Yagita, H.; Shong, M.; Lee, M.S. IFN-Gamma/TNF-Alpha Synergism as the Final Effector in Autoimmune Diabetes: A Key Role for STAT1/IFN Regulatory Factor-1 Pathway in Pancreatic Beta Cell Death. J. Immunol. 2001, 166, 4481–4489. [Google Scholar] [CrossRef] [PubMed]
- Pugliatti, M.; Sotgiu, S.; Rosati, G. The Worldwide Prevalence of Multiple Sclerosis. Clin. Neurol. Neurosurg. 2002, 104, 182–191. [Google Scholar] [CrossRef]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A Growing Global Problem with Widespread Inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, G.; Gordon-Dixon, A.; Emmanuel, A. Neurogenic Bowel Dysfunction in Patients with Multiple Sclerosis: Prevalence, Impact, and Management Strategies. Degener. Neurol. Neuromuscul. Dis. 2018, 8, 79–90. [Google Scholar] [CrossRef]
- Aharony, S.M.; Lam, O.; Corcos, J. Evaluation of Lower Urinary Tract Symptoms in Multiple Sclerosis Patients: Review of the Literature and Current Guidelines. J. Assoc. Urol. Can. [Can. Urol. Assoc. J.] 2017, 11, 61–64. [Google Scholar] [CrossRef]
- Magyari, M.; Sorensen, P.S. The Changing Course of Multiple Sclerosis: Rising Incidence, Change in Geographic Distribution, Disease Course, and Prognosis. Curr. Opin. Neurol. 2019, 32, 320–326. [Google Scholar] [CrossRef]
- Berglund, R.; Guerreiro-Cacais, A.O.; Adzemovic, M.Z.; Zeitelhofer, M.; Lund, H.; Ewing, E.; Ruhrmann, S.; Nutma, E.; Parsa, R.; Thessen-Hedreul, M.; et al. Microglial Autophagy-Associated Phagocytosis Is Essential for Recovery from Neuroinflammation. Sci. Immunol. 2020, 5, eabb5077. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J. The Molecular Basis of Neurodegeneration in Multiple Sclerosis. FEBS Lett. 2011, 585, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. A Brief History of T(H)17, the First Major Revision in the T(H)1/T(H)2 Hypothesis of T Cell-Mediated Tissue Damage. Nat. Med. 2007, 13, 139–145. [Google Scholar] [CrossRef]
- Kallaur, A.P.; Oliveira, S.R.; Simão, A.N.C.; Alfieri, D.F.; Flauzino, T.; Lopes, J.; de Carvalho Jennings Pereira, W.L.; de Meleck Proença, C.; Borelli, S.D.; Kaimen-Maciel, D.R.; et al. Cytokine Profile in Patients with Progressive Multiple Sclerosis and Its Association with Disease Progression and Disability. Mol. Neurobiol. 2017, 54, 2950–2960. [Google Scholar] [CrossRef] [PubMed]
- Oreja-Guevara, C.; Ramos-Cejudo, J.; Aroeira, L.S.; Chamorro, B.; Diez-Tejedor, E. TH1/TH2 Cytokine Profile in Relapsing-Remitting Multiple Sclerosis Patients Treated with Glatiramer Acetate or Natalizumab. BMC Neurol. 2012, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Moser, T.; Akgün, K.; Proschmann, U.; Sellner, J.; Ziemssen, T. The Role of TH17 Cells in Multiple Sclerosis: Therapeutic Implications. Autoimmun. Rev. 2020, 19, 102647. [Google Scholar] [CrossRef]
- Alves, C.C.S.; Castro, S.B.R.; Costa, C.F.; Dias, A.T.; Alves, C.J.; Rodrigues, M.F.; Teixeira, H.C.; Almeida, M.V.; Ferreira, A.P. Anthraquinone Derivative O,O′-Bis-(3′-Iodopropyl)-1,4-Dihydroxyanthraquinone Modulates Immune Response and Improves Experimental Autoimmune Encephalomyelitis. Int. Immunopharmacol. 2012, 14, 127–132. [Google Scholar] [CrossRef]
- Wei, M.; Yang, T.; Li, Q.; Zhou, D.; Du, Z.; Fan, Y. Protective Effects of Catalpol and Rhein in Murine Experimental Autoimmune Encephalomyelitis via Regulation of T Helper (Th) 1, Th2, Th17, and Regulatory T Cell Responses. Chung Chih Ying Wen Pan [J. Tradit. Chin. Med.] 2019, 39, 809–817. [Google Scholar]
- Zheng, K.; Lv, B.; Wu, L.; Wang, C.; Xu, H.; Li, X.; Wu, Z.; Zhao, Y.; Zheng, Z. Protecting Effect of Emodin in Experimental Autoimmune Encephalomyelitis Mice by Inhibiting Microglia Activation and Inflammation via Myd88/PI3K/Akt/NF-ΚB Signalling Pathway. Bioengineered 2022, 13, 9322–9344. [Google Scholar] [CrossRef]
- da Silva, L.C.; Lima, I.V.d.A.; da Silva, M.C.M.; Corrêa, T.A.; de Souza, V.P.; de Almeida, M.V.; de Oliveira, A.C.P.; Ferreira, A.P. A New Lipophilic Amino Alcohol, Chemically Similar to Compound FTY720, Attenuates the Pathogenesis of Experimental Autoimmune Encephalomyelitis by PI3K/Akt Pathway Inhibition. Int. Immunopharmacol. 2020, 88, 106919. [Google Scholar] [CrossRef]
- Cui, Y.-R.; Bu, Z.-Q.; Yu, H.-Y.; Yan, L.-L.; Feng, J. Emodin Attenuates Inflammation and Demyelination in Experimental Autoimmune Encephalomyelitis. Neural Regen. Res. 2023, 18, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.-K.; Song, Y.H.; Jeong, S.-J.; Lee, H.-J.; Jung, J.H.; Kim, B.; Song, H.S.; Huh, J.-E.; Kim, S.-H. Emodin Inhibits Proinflammatory Responses and Inactivates Histone Deacetylase 1 in Hypoxic Rheumatoid Synoviocytes. Biol. Pharm. Bull. 2011, 34, 1432–1437. [Google Scholar] [CrossRef]
- Cheng, D.-W.; Yue, Y.-F.; Chen, C.-X.; Hu, Y.-D.; Tang, Q.; Xie, M.; Liu, L.; Li, D.; Zhu, H.-L.; Cheng, M.-L. Emodin Alleviates Arthritis Pain through Reducing Spinal Inflammation and Oxidative Stress. Mol. Pain. 2022, 18, 17448069221146398. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-K.; Noh, E.-M.; Moon, S.-J.; Kim, J.-M.; Kwon, K.-B.; Park, B.-H.; You, Y.-O.; Hwang, B.-M.; Kim, H.-J.; Kim, B.-S.; et al. Emodin Suppresses Inflammatory Responses and Joint Destruction in Collagen-Induced Arthritic Mice. Rheumatology 2013, 52, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, A.D.; Panchal, P.V.; Harle, U.N.; Nanda, R.K.; Shaikh, H.M. Anti-Inflammatory and Antiarthritic Activity of Anthraquinone Derivatives in Rodents. Int. J. Inflam. 2014, 2014, 690596. [Google Scholar] [CrossRef]
- Yuan, X.; Dai, B.; Yang, L.; Lin, B.; Lin, E.; Pan, Y. Emodin Ameliorates Renal Injury in BXSB Mice by Modulating TNF-α/ICAM-1. Biosci. Rep. 2020, 40, BSR20202551. [Google Scholar] [CrossRef]
- Drory, Y.; Turetz, Y.; Hiss, Y.; Lev, B.; Fisman, E.Z.; Pines, A.; Kramer, M.R. Sudden Unexpected Death in Persons < 40 Years of Age. Am. J. Cardiol. 1991, 68, 1388–1392. [Google Scholar] [CrossRef]
- Cihakova, D.; Rose, N.R. Chapter 4 Pathogenesis of Myocarditis and Dilated Cardiomyopathy. In Advances in Immunology; Advances in immunology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 95–114. ISBN 9780123743251. [Google Scholar]
- Caforio, A.L.P.; Mahon, N.J.; Tona, F.; McKenna, W.J. Circulating Cardiac Autoantibodies in Dilated Cardiomyopathy and Myocarditis: Pathogenetic and Clinical Significance. Eur. J. Heart Fail. 2002, 4, 411–417. [Google Scholar] [CrossRef]
- Milenković, M.; Arsenović-Ranin, N.; Stojić-Vukanić, Z.; Bufan, B.; Vučićević, D.; Jančić, I. Quercetin Ameliorates Experimental Autoimmune Myocarditis in Rats. J. Pharm. Pharm. Sci. 2010, 13, 311–319. [Google Scholar] [CrossRef]
- Okura, Y.; Yamamoto, T.; Goto, S.; Inomata, T.; Hirono, S.; Hanawa, H.; Feng, L.; Wilson, C.B.; Kihara, I.; Izumi, T.; et al. Characterization of Cytokine and INOS MRNA Expression in Situ during the Course of Experimental Autoimmune Myocarditis in Rats. J. Mol. Cell Cardiol. 1997, 29, 491–502. [Google Scholar] [CrossRef]
- Song, Z.-C.; Wang, Z.-S.; Bai, J.-H.; Li, Z.; Hu, J. Emodin, a Naturally Occurring Anthraquinone, Ameliorates Experimental Autoimmune Myocarditis in Rats. Tohoku J. Exp. Med. 2012, 227, 225–230. [Google Scholar] [CrossRef]
- Zhu, W.; He, X.; Cheng, K.; Zhang, L.; Chen, D.; Wang, X.; Qiu, G.; Cao, X.; Weng, X. Ankylosing Spondylitis: Etiology, Pathogenesis, and Treatments. Bone Res. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Lindström, U.; Olofsson, T.; Wedrén, S.; Qirjazo, I.; Askling, J. Impact of Extra-Articular Spondyloarthritis Manifestations and Comorbidities on Drug Retention of a First TNF-Inhibitor in Ankylosing Spondylitis: A Population-Based Nationwide Study. RMD Open 2018, 4, e000762. [Google Scholar] [CrossRef]
- Ma, C.; Wen, B.; Zhang, Q.; Shao, P.-P.; Gu, W.; Qu, K.; Shi, Y.; Wang, B. Emodin Induces Apoptosis and Autophagy of Fibroblasts Obtained from Patient with Ankylosing Spondylitis. Drug Des. Dev. Ther. 2019, 13, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Pulido, T.; Campisi, J.; Alimirah, F.; Demaria, M. Cellular Senescence and the Senescence-Associated Secretory Phenotype as Drivers of Skin Photoaging. J. Investig. Dermatol. 2021, 141, 1119–1126. [Google Scholar] [CrossRef]
- Callender, L.A.; Carroll, E.C.; Beal, R.W.J.; Chambers, E.S.; Nourshargh, S.; Akbar, A.N.; Henson, S.M. Human CD8+ EMRA T Cells Display a Senescence-Associated Secretory Phenotype Regulated by P38 MAPK. Aging Cell 2018, 17, e12675. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of Innate Immunity System during Aging: NF-KB Signaling Is the Molecular Culprit of Inflamm-Aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-Mediated Regulation of NFkappaB and the Essentialness of NFkappaB for the Oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef]
- Kaushik, S.; Tasset, I.; Arias, E.; Pampliega, O.; Wong, E.; Martinez-Vicente, M.; Cuervo, A.M. Autophagy and the Hallmarks of Aging. Ageing Res. Rev. 2021, 72, 101468. [Google Scholar] [CrossRef]
- Zinecker, H.; Simon, A.K. Autophagy Takes It All—Autophagy Inducers Target Immune Aging. Dis. Model. Mech. 2022, 15, dmm049345. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed Interplay between Autophagy and Inflammasomes. Aging 2012, 4, 166–175. [Google Scholar] [CrossRef]
- Djavaheri-Mergny, M.; Amelotti, M.; Mathieu, J.; Besançon, F.; Bauvy, C.; Souquère, S.; Pierron, G.; Codogno, P. NF-KappaB Activation Represses Tumor Necrosis Factor-Alpha-Induced Autophagy. J. Biol. Chem. 2006, 281, 30373–30382. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, W.; Fattizzo, B. Autoimmune Hemolytic Anemias: Challenges in Diagnosis and Therapy. Transfus. Med. Hemotherapy 2024, 51, 321–331. [Google Scholar] [CrossRef]
- Jiang, H.; Mao, T.; Liu, Y.; Tan, X.; Sun, Z.; Cheng, Y.; Han, X.; Zhang, Y.; Wang, J.; Shi, L.; et al. Protective Effects and Mechanisms of Yinchen Linggui Zhugan Decoction in HFD-Induced Nonalcoholic Fatty Liver Disease Rats Based on Network Pharmacology and Experimental Verification. Front. Pharmacol. 2022, 13, 908128. [Google Scholar] [CrossRef]
- Caserta, S.; Zaccuri, A.M.; Innao, V.; Musolino, C.; Allegra, A. Immune Thrombocytopenia: Options and New Perspectives. Blood Coagul. Fibrinolysis 2021, 32, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kos, M.; Tomaka, P.; Mertowska, P.; Mertowski, S.; Wojnicka, J.; Błażewicz, A.; Grywalska, E.; Bojarski, K. The Many Faces of Immune Thrombocytopenia: Mechanisms, Therapies, and Clinical Challenges in Oncological Patients. J. Clin. Med. 2024, 13, 6738. [Google Scholar] [CrossRef]
- Naini, M.A.; Zargari-Samadnejad, A.; Mehrvarz, S.; Tanideh, R.; Ghorbani, M.; Dehghanian, A.; Hasanzarrini, M.; Banaee, F.; Koohi-Hosseinabadi, O.; Tanideh, N.; et al. Anti-Inflammatory, Antioxidant, and Healing-Promoting Effects of Aloe vera Extract in the Experimental Colitis in Rats. Evid.-Based Complement. Alternat. Med. 2021, 2021, 9945244. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Tang, X.-R.; Jia, G.-B.; Zhou, S.; Yue, G.-L.; He, C.-S. Effect of Emodin on Acute Lung Injury: A Meta-Analysis of Preclinical Trials. BMC Pulm. Med. 2024, 24, 596. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Ren, Y.; Liu, C. Aloe-Emodin Suppresses Oxidative Stress and Inflammation via a PI3K-Dependent Mechanism in a Murine Model of Sepsis. Evid.-Based Complement. Alternat Med. 2022, 2022, 9697887. [Google Scholar] [CrossRef]
- Cui, J.; Wang, S.; Bi, S.; Zhou, H.; Sun, L. Emodin-Based Regulation and Control of Serum Complement C5a, Oxidative Stress, and Inflammatory Responses in Rats with Urosepsis via AMPK/SIRT1. Iran. J. Allergy Asthma Immunol. 2024, 23, 550–562. [Google Scholar] [CrossRef]
| Authors | Study Type | Disease Models | AV Part(s) Used | Extraction Solvent(s) and Chromatographic Technique Used | Chemical Compound(s)/ Substance(s) Used (Dosage) | Main Biological or Chemical Marker Results | Cumulative Evidence |
|---|---|---|---|---|---|---|---|
| Metro et al. [152] | In vivo—women (30/15) | HT | AV leaf juice and pulp | - | Aloe barbadensis Miller juice (ABMJ) (50 mL/day) | ↓ TSH (−61%), TPOAb (−56%) ↑ FT4 (+23%), FT4:FT3 ratio (+49%) | Regulate hormonal balance and antibody production |
| Sun et al. [153] | In vivo— murine | NaI-induced experimental autoimmune thyroiditis | - | - | Emodin (75 mg/kg) | ↓ TgAb ↓ CD3+CD4+IL-4+/IFN-γ+ ↓ CD3CD8+ IL-4+/IFN-γ+ | Regulate immune responses (Th1–Th2) Role in ↓ HLA-II type expression |
| Panda et al. [154] | In vivo— murine | Hypertiroidism (Graves’ disease model) | AV leaves | Petroleum, chloroform, methanol, and ethanol; LC-ESI-MS | AV methanolic fraction (AVMF) (50 and 500 mg/kg/day) | ↓ IL-6, TNF-α ↑ SOD, CAT, GPx, GSH ↓ TSHR expression ↓ T3, T4 | Anti-inflammatory and anti-oxidative effects Influence gene expression Regulate hormonal balance |
| Shen et al. [163] | In vivo—murine; in vitro | AID | None (root of F. Japonica) | Methanol; HPLC | Emodin (4–40 mg/kg/day) | ↓ AID incidence >70% ↓ Leukocyte infiltration into pancreatic islets ↓ CXCR4-mediated migration via ERK1/2 and MEK1/2 inhibition | Influence lymphocyte activities and chemokine pathways |
| Shen et al. [164] | In vivo—murine; in vitro | AID | None (commercially available emodin product) | - | Emodin (IC50 = 0.3 µg/mL; 0.75 µg/mL) | ↓ CXCR4- and CXCR5-mediated migration via JNK and p38 inhibition | Influence chemokine pathways |
| Malaguti et al. [165] | In vivo— murine | AID | - | - | Diacerhein (5 and 10 mg/kg/day) | ↓ AID incidence ↓ IL-1β, TNF-α, and IFN-γ | Influence lymphocyte activities Anti-inflammatory effects |
| Alves et al. [180] | In vivo—murine; in vitro | EAE | None (commercially available mitoxantrone product) | Butanone; column chromatography | O,O′-bis-(3′-iodopropyl)-1,4-dihydroxyanthraquinone (1 mg/kg/day) | Improved clinical score ↓ Inflammatory cell infiltration ↓ Demyelination ↓ IL-17, IFN-γ, IL-12p40, IL-6, TGF-β, CCL5, and CCL20 | Positive clinical impact Immunoregulatory effect on lymphocyte populations Influence lymphocyte chemotaxis Reduce demyelination Anti-inflammatory effects |
| Wei et al. [181] | In vivo—murine | EAE | - | - | Rhein (5 mg/kg/day) and Catalpol (40 mg/kg/day) | Improved clinical score ↓ IL-2 and IL-17A ↑ IL-4 and IL-10 ↓ T-bet and RORγt ↑ GATA3 and Foxp3 | Positive clinical impact Anti-inflammatory effects Immunoregulatory effects on lymphocyte populations |
| Zheng et al. [182] | In vivo— murine | EAE | None (commercially available emodin product) | - | Emodin (30–60 mg/kg/day) | Improved clinical scores ↓ Inflammatory cell infiltration ↑ MBP and BDNF ↓ PI3K and AKT1 activation ↓ Mydd88 expression ↓ IL-6, TGF-β, IL-17A, and RORγt | Positive clinical impact Anti-inflammatory effects Improve remyelination |
| Cui et al. [184] | In vivo— murine; in vitro | EAE | None (commercially available emodin product) | - | Emodin (20 mg/kg/day) | ↓ IL-1β, IL-6, IL-18, and TNF-α and Iba-1 ↓ SIRT1/PGC-and NLRP3-related molecules | Anti-inflammatory effects Modulate inflammasome response |
| Ha et al. [185] | In vitro (LPS-stimulated synoviocytes) | RA | - | - | Emodin (0, 1, or 10 ng/mL) | ↓ IL-1β, IL-6, IL-18, and TNF-α ↓ PGE2, VEGF, MMP-1, and MMP-13 ↓ COX-2, MMP-1, and MMP-13 mRNA expression | Anti-inflammatory and anti-angiogenetic effects |
| Cheng et al. [186] | In vivo— mouse | RA | None (commercially available emodin product) | - | Emodin (10 mg/kg) | Relieves pain hypersensitivity of mice; ↓ spinal inflammation; inhibits spinal NLPR3 inflammasome activity; ↓ spinal AMPK expression | Positive clinical impact Anti-inflammatory effects |
| Kshirsagar et al. [188] | In vivo—mouse | RA | - | Dichloromethane | Aloe emodin (50 mg/kg); 4,5-dihydroxy-9,10-dioxo-9,10-dihydroanthracene-2 carboxylic acid (75 mg/kg) | ↓ Paw edema; ↓ arthritic score | Positive clinical impact Anti-inflammatory effects |
| Yuan et al. [189] | In vivo—mouse | SLE | None (commercially available emodin product) | - | Emodin (0, 5, 10 and 20 mg/kg.d) | Down-regulation of TNF-α; ↓ level of ICAM-1 | Theoretical base for a clinical application of emodin in the treatment of SLE |
| Song et al. [195] | In vivo—murine | EAM | None (commercially available Emodin product) | - | Emodin (50 mg/kg/day) | ↑ Echocardiography parameters (LVEDs, LVEDs, LVFS) ↑ Hw/Bw ratio ↓ Inflammatory infiltrate ↓ NF-κB p65 expression ↓ IL-1β and TNF-α | Anti-inflammatory effects Cardioprotective effects |
| Ma et al. [198] | In vitro | AS | None (commercially available Emodin product) | - | Emodin (0, 2, 5, 10, or 20 µM) | ↓ Viability ↑ apoptotic rate ↑ Bax, active caspase-9, and active caspase-3 ↓ Bcl-2 ↑ Beclin 1, Atg12, and Atg5 | Induce autophagy and apoptosis in fibroblasts Reduced viability of fibroblasts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordiano, R.; Caserta, S.; Minciullo, P.L.; Allegra, A.; Gangemi, S. Anthraquinones and Aloe Vera Extracts as Potential Modulators of Inflammaging Mechanisms: A Translational Approach from Autoimmune to Onco-Hematological Diseases. Molecules 2025, 30, 1251. https://doi.org/10.3390/molecules30061251
Cordiano R, Caserta S, Minciullo PL, Allegra A, Gangemi S. Anthraquinones and Aloe Vera Extracts as Potential Modulators of Inflammaging Mechanisms: A Translational Approach from Autoimmune to Onco-Hematological Diseases. Molecules. 2025; 30(6):1251. https://doi.org/10.3390/molecules30061251
Chicago/Turabian StyleCordiano, Raffaele, Santino Caserta, Paola Lucia Minciullo, Alessandro Allegra, and Sebastiano Gangemi. 2025. "Anthraquinones and Aloe Vera Extracts as Potential Modulators of Inflammaging Mechanisms: A Translational Approach from Autoimmune to Onco-Hematological Diseases" Molecules 30, no. 6: 1251. https://doi.org/10.3390/molecules30061251
APA StyleCordiano, R., Caserta, S., Minciullo, P. L., Allegra, A., & Gangemi, S. (2025). Anthraquinones and Aloe Vera Extracts as Potential Modulators of Inflammaging Mechanisms: A Translational Approach from Autoimmune to Onco-Hematological Diseases. Molecules, 30(6), 1251. https://doi.org/10.3390/molecules30061251








