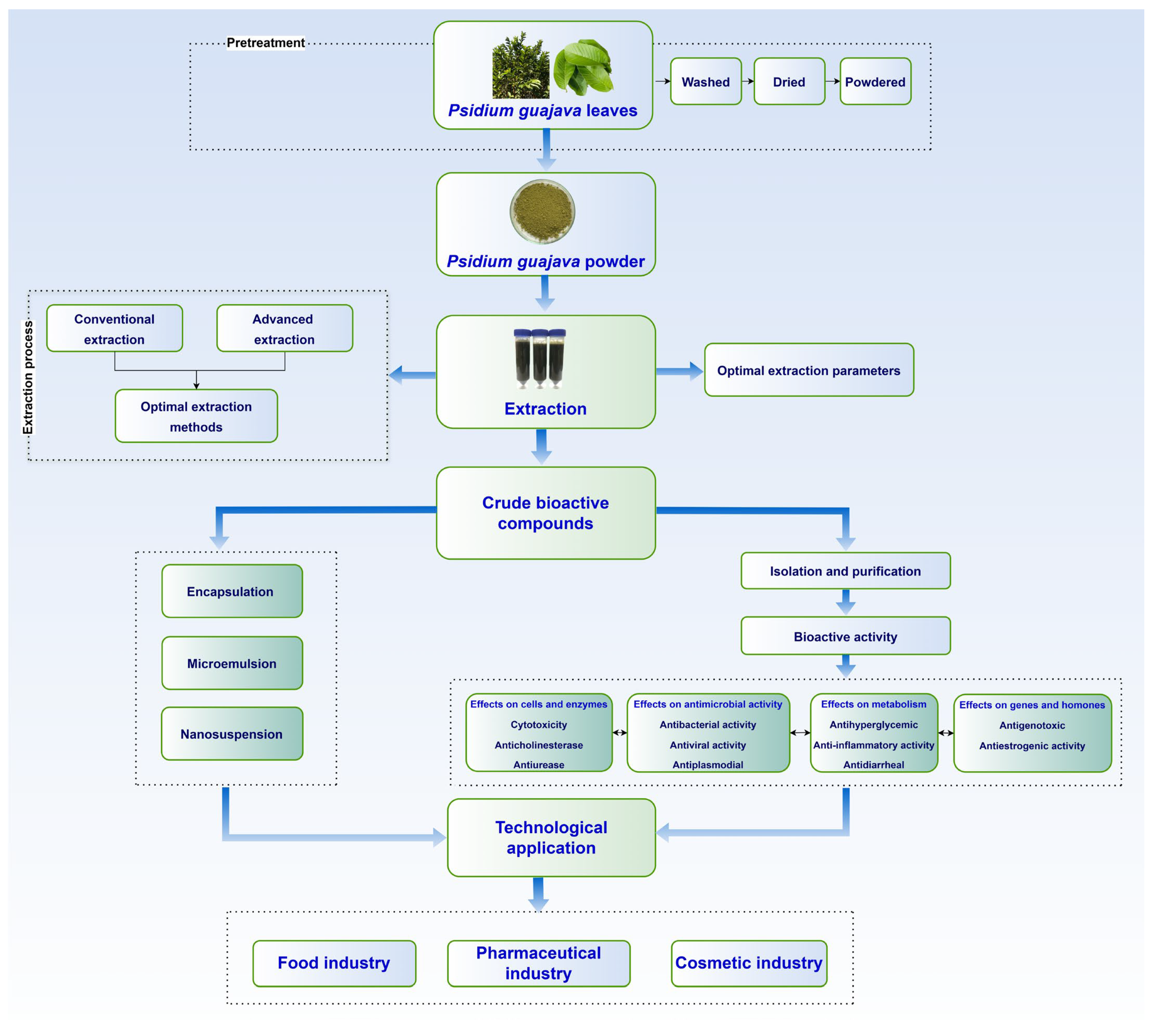
Bioactive Compounds from Guava Leaves (Psidium guajava L.): Characterization, Biological Activity, Synergistic Effects, and Technological Applications
Abstract
:1. Introduction
2. Bioactive Compounds in Psidium Guajava Leaf Extract
2.1. Nutritional Composition of Psidium Guajava Leaf
2.2. Phenolic Compounds
2.2.1. Phenolic Acids
2.2.2. Flavonoids
2.2.3. Tannins
2.2.4. Terpenes and Terpenoids
2.2.5. Alkaloids
3. Biological Properties and Synergy Effects
3.1. Cytotoxicity
3.2. Anticholinesterase Activity
3.3. Antiurease Activity
3.4. Antibacterial Activity
3.5. Antiviral Activity
3.6. Antiplasmodial Activity
3.7. Antihyperglycemic Activity
3.8. Anti-Inflammatory Activity
3.9. Synergy Effects
3.10. Other Activities
| No | Country | Biological Activity | Solvent Extraction | Research Methodology | Targeted Pathogens | Remarks on Antimicrobial Activities | Reference |
|---|---|---|---|---|---|---|---|
| Cytotoxicity | |||||||
| 1 | Brazil | High cytotoxic activity against cancer cells | Essential oil | Cytotoxicity assay |
| IC50: 5.8–12.4 µg/mL | [49] |
| 2 | India | Effective antifungal and cytotoxic properties in vitro | Methanol | Cytotoxicity assay |
| MIC: 0.78–1.25 mg/mL | [51] |
| 3 | Nigeria | Strong antibacterial activity, particularly against E. coli | Ethanol |
|
| MIC: 1.56–3.12 mg/mL | [52] |
| Anticholinesterase activity | |||||||
| 4 | Algeria | Correlation observed between phenolic content vs. antioxidant activity | Ethyl acetate | Antioxidant assays
| IC50: 4.26 µg/mL | [53] | |
| n-Butanol | IC50: 5.48 µg/mL | ||||||
| Chloroform | IC50 > 200 µg/mL | ||||||
| Antiurease activity | |||||||
| 5 | India | Strong urease inhibition (methanol extracts) and potential for treating urinary infections |
| Urease inhibition assay |
| IC50: 1.25–2.08 mg/mL (methanol is the most effective) | [55] |
| Antibacterial activity | |||||||
| 6 | Cameroon | Synergistic effect when combined with antibiotics | Distilled water |
|
|
| [14] |
| 7 | India | Synergistic effects when combined with antibiotics |
|
|
|
| [40] |
| 8 | Cameroon | Focused on multidrug- resistant bacteria | Methanol |
|
|
| [57] |
| 9 | Thailand | Effective in preventing dental caries | 95% Ethanol |
|
| MIC: 1.56 mg/mL no MBC, ↓ biofilm formation vs. acid production | [58] |
| 10 | India | Antimicrobial activity against skin pathogens | 70% Ethanol |
|
| MIC ranged from 480.20 to 621.09 µg/mL | [59] |
| 11 | Cameroon | Focused on biofilm eradication and reduced antibiotic resistance. |
|
|
| MIC: 64 μg/mL; strong activity when combined with doxycycline | [60] |
| 12 | Peru | Focused on periodontal bacteria | Methanol |
|
| MIC: 1.5 mg/mL, significant biofilm ↓ at sub-MIC levels | [61] |
| 13 | India | Focused on quorum sensing and virulence reduction | 95% Ethanol |
|
| MIC: 64–128 μg/mL dependent on extraction method (MAE performed best) | [62] |
| 14 | Japan | No impact on bacterial growth | 50% Ethanol |
|
| ↓ Secretion of T3SS proteins (EspB, SipB) and prevented bacterial adherence | [63] |
| 15 | Indonesia | Synergism with antibiotics such as tetracycline and ciprofloxacin | 70% Ethanol |
| Salmonella typhi | MIC not specified; ↓ AcrB expression from 11.48 to 7.39 μg/mL | [83] |
| Antiviral activity | |||||||
| 16 | India | Anti-chikungunya activity | Aqueous |
| Chikungunya virus (CHIKV) | ↑ Cell viability by 60%. Longifollen and quercetin had strong binding to nsP2 protease | [65] |
| 17 | Indonesia | Focused on supporting treatment for asymptomatic COVID-19 patients | Clinical | COVID-19 (markers of inflammation in patients) | ↓ NLR ratio and faster recovery rates in the Psidium guajava extract group | [66] | |
| 18 | India | Focused on HIV-1 inhibition and ROS scavenging | Methanol |
| HIV-1 (two different subtypes) | EC50: 0.070–0.085 mg/mL; more effective than Carica papaya | [67] |
| Antihyperglycemic activity | |||||||
| 19 | India | Antihyperglycemic, antioxidant | Aqueous | In vivo (rat model) | ↓ fasting blood glucose by 32% | [5] | |
| 20 | Spain | Inhibition of α-amylase and α-glucosidase | Methanol | In vitro | IC50 for α-glucosidase: 0.24–2.6 µM | [38] | |
| 21 | India | Improved insulin sensitivity | Methanol | In vivo (rat model) | ↓ HOMA-IR from 21.29 to 9.57 | [65] | |
| 22 | India | Reduction of lipid absorption | Methanol | In vivo (rat model) | ↓ Triglycerides by 19% | [71] | |
| 23 | Germany | Inhibition of glucose absorption | Ethanol |
| GLUT2 inhibition up to 74% | [72] | |
| 24 | Nigeria | Regulation of blood lipids, enhanced glycogen synthesis | Aqueous | In vivo (rat model) | LDL ↓ by 73%, HDL ↑ by 85% ↑ liver glycogen levels by 25% | [73] | |
| 25 | India | Reduction in non-alcoholic fatty liver disease (NAFLD) | Aqueous | In vivo (rat model) | ↓ Liver triglycerides by 5.7% | [74] | |
| 26 | India | Cardioprotective and antiglycative effects on diabetic myocardium | Ethyl acetate | In vivo (streptozotocin-induced diabetic rats) | ↑ Cardiac function and ↓ AGEs | [76] | |
| 27 | India | Reduction of oxidative stress | Aqueous | In vivo (rat model) | ↓ MDA levels from 41.27 to 34.21 nmol/mg | [80] | |
| 28 | India | Inhibition of advanced glycation end-product (AGE) formation | Aqueous | In vivo (rat model) | ↓ HbA1C from 9.67% to 5.64% | [91] | |
| 29 | China | Activation of antioxidant enzymes | Aqueous | In vivo (rat model) | ↑ SOD activity by 20% | [92] | |
| Anti-inflammatory activity | |||||||
| 30 | Thailand | Anti-inflammatory and antioxidant activities for potential anti-ulcer therapy | 70% Ethanol |
| DPPH IC50 = 11.62 µg/mL, ↓ NO, TNF-α, IL-6, IL-1β | [77] | |
| 31 | China | Anti-inflammatory effects by reducing resistin and TNF-α expression in knee osteoarthritis chondrocytes | 70% Ethanol |
| ↓ Resistin (56.59%), TNF-α (51.86%) | [79] | |
| 32 | China | Anti-inflammatory, antioxidant, and antihyperglycemic effects in diabetic rats | Aqueous |
| ↓ Blood glucose, NO, TNF-α, IL-6, lipid peroxidation | [80] | |
| 33 | Iran | Anti-inflammatory, antioxidant, and wound healing effects on oral mucositis | 70% Ethanol |
| Oral mucositis model | ↓ IL-6, ↑ TAC, fibroblast proliferation, thicker epithelium | [81] |
| 34 | Vietnam | Immunomodulatory, antioxidant, and disease resistance effects in striped catfish | Ethanol |
| Edwardsiella ictaluri | ↓ Mortality (4.76% vs. 47.62%), ↑ lysozyme, Ig, NOS | [84] |
| 35 | India | Radioprotective effects, antioxidants, and anti-inflammatory activities against X-ray-induced damage in rats | 50% Methanol |
| ↓ COX-2, IL-6, micronucleus; ↑ IL-10, antioxidant enzymes | [91] | |
| Antidiarrheal activity | |||||||
| 36 | Indonesia | Acute toxicity evaluation of antidiarrheal herbal combination |
| No mortality; LD50 > 5 g/kg body weight in mice | [86] | ||
| Antigenotoxic activity | |||||||
| 37 | Brazil | Antigenotoxic, phospholipase and hemolytic activity inhibition |
|
|
| 75% inhibition of DXR-induced DNA damage; 63.16% inhibition of phospholipase activity | [88] |
| Antiestrogenic activity | |||||||
| 38 | Brazil | Antiestrogenic and antiproliferative activity |
|
|
| TGI = 2.27 µg/mL (MCF-7 BUS); inhibition of estradiol-induced proliferation | [90] |
4. Advances in Extraction Technology
5. Trends and Emerging Technologies
5.1. Encapsulation Technology
5.2. Microemulsion Technology
5.3. Nanosuspension Techniques
6. Technological Applications
6.1. Food Industry
6.2. Pharmaceutical Industry
6.3. Cosmetic Industry
7. Challenges in the Biological Activity of Psidium guajava L. Leaf Extract Compounds and Their Technological Applications
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| AcrB | Acriflavine Resistance Protein B |
| AGES | Advanced glycation end-products |
| AI | Artificial intelligence |
| AKT | Protein Kinase B |
| CB2 | Cannabinoid Receptor 2 |
| CEAE | Cell Extract Antioxidant Enzyme |
| COX-2 | Cyclooxygenase-2 |
| CUPRAC | Cupric Reducing Antioxidant Capacity |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DXR | Doxorubicin |
| EAE | Experimental Autoimmune Encephalomyelitis |
| EC50 | Half-maximal effective concentration |
| EHEC | Enterohemorrhagic Escherichia coli |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EPS | Extracellular Polysaccharide |
| EspB | Escherichia coli Secreted Protein B |
| FICI | Fractional Inhibitory Concentration Index |
| GAE | Gallic acid equivalent |
| GLUT2 | Glucose Transporter Type 2 |
| HDL | High-Density Lipoprotein |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| HRLC-HRMS/MS-QTOF | High-Resolution Liquid Chromatography-High-Resolution Tandem Mass Spectrometry and Quadrupole Time-Of-Flight |
| IC50 | Half-maximal inhibitory concentration |
| Ig | Immunoglobulin |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| LD50 | Lethal Dose 50% |
| LDL | Low-Density Lipoprotein |
| MAE | Microwave-Assisted Extraction |
| MBC | Minimum Bactericidal Concentration |
| MDA | Malondialdehyde |
| MIC | Minimum inhibitory concentration |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| PCR | Probe-based Polymerase Chain Reaction |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-Kinase |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RNA | Ribonucleic Acid |
| ROS | Reactive oxygen species |
| RSM | Response surface methodology |
| RT-PCR | Reverse-Transcription Polymerase Chain Reaction |
| SipB | Salmonella Invasion Protein B |
| SOD | Superoxide Dismutase |
| STZ | Streptozotocin |
| Sub-MIC | Sub-minimum inhibitory concentration |
| T3SS | Type 3 Secretion System |
| TAC | Total antioxidant capacity |
| TBARs | Thiobarbituric Acid Reactive Substances |
| TNF-α | Tumor Necrosis Factor-alpha |
| TPC | Total phenolic content |
| UAE | Ultrasound-assisted extraction |
| UV | Ultraviolet |
| SipB | Salmonella Invasion Protein B |
| SOD | Superoxide Dismutase |
| STZ | Streptozotocin |
| sub-MIC | Sub-minimum inhibitory concentration |
| T3SS | Type 3 Secretion System |
| TAC | Total antioxidant capacity |
| TBARs | Thiobarbituric Acid Reactive Substances |
| TNF-α | Tumor Necrosis Factor-alpha |
| TPC | Total phenolic content |
| UAE | Ultrasound-assisted extraction |
| UV | Ultraviolet |
References
- FAO. Major Tropical Fruits Market Review. In Prelims Results 2023; FAO: Rome, Italy, 2024. [Google Scholar]
- Liu, H.; Wei, S.; Shi, L.; Tan, H. Preparation, structural characterization, and bioactivities of polysaccharides from Psidium guajava: A review. Food Chem. 2023, 411, 135423. [Google Scholar] [CrossRef]
- Susanti, D.; Rahmawati, N.; Sholikhah, I.Y.M.; Mujahid, R.; Subositi, D.; Widodo, H.; Widiyastuti, Y.; Haryanti, S. Medicinal plants utilized for fitness disorders treatment by ethnic groups in Papua and West Papua Province, Indonesia. J. Appl. Pharm. Sci. 2023, 13, 149–160. [Google Scholar] [CrossRef]
- Sahu, M.C.; Dubey, D.; Rath, S.; Panda, T.; Padhy, R.N. Monograph: In vitro efficacy of 30 ethnomedicinal plants used by Indian aborigines against 6 multidrug resistant Gram-positive pathogenic bacteria. Asian Pac. J. Trop. Dis. 2015, 5, 136–150. [Google Scholar] [CrossRef]
- Mia, M.A.S.; Malitha, S.B.; Nur-E-alam, M.; Akter, M.; Rahman Ria, M.; Alam, M.Z. Ultrasound-Assisted Extraction, Characterization and Assessment of Vegetable Tannins from Guava (Psidium guajava) Leaves for Leather Tanning. Text. Leather Rev. 2024, 7, 872–891. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Dang, L.T.; Nguyen, H.T.; Hoang, H.H.; Lai, H.T.N.; Nguyen, H.T.T. Screening antibacterial effects of vietnamese plant extracts against pathogens caused acute hepatopancreatic necrosis disease in shrimps. Asian J. Pharm. Clin. Res. 2018, 11, 77–83. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Siska, S.; Sulayha, S. Comparative Study of Ethnomedicine Approach in Kutai Tribe at Loa Lepu Village and Muara Gusik Village, East Kalimantan, Indonesia. Trop. J. Nat. Prod. Res. 2023, 7, 3717–3725. [Google Scholar] [CrossRef]
- Altendorf, S. Minor tropical fruits: Mainstreaming a niche market. Food Outlook 2018, 1, 67–74. [Google Scholar]
- Murugan, G.; Nilsuwan, K.; Prodpran, T.; Ponnusamy, A.; Rhim, J.-W.; Kim, J.T.; Benjakul, S. Active Fish Gelatin/Chitosan Blend Film Incorporated with Guava Leaf Powder Carbon Dots: Properties, Release and Antioxidant Activity. Gels 2024, 10, 281. [Google Scholar] [CrossRef]
- Rajput, R.; Kumar, K. Protective effect of ethanolic extract of guava leaves (Psidium guajava L.) in alloxan-induced diabetic mice. Mater. Today Proc. 2021, 47, 437–439. [Google Scholar] [CrossRef]
- Mathiazhagan, M.; Elangovan, D.; Chinnaiyan, V.; Shivashankara, K.S.; Sudhakar Rao, D.V.; Ravishankar, K.V. A high-density linkage map construction in guava (Psidium guajava L.) using genotyping by sequencing and identification of QTLs for leaf, peel, and pulp color in an intervarietal mapping population. Front. PLANT Sci. 2024, 15, 1335715. [Google Scholar] [CrossRef]
- Lekshmi, S.G.; Sethi, S.; Asrey, R.; Nagaraja, A.; Singh, K.P.; Kumar, R.; Anagha, P.K. Edible coating functionalized with ornamental plant extracts affect the postharvest quality of guava (Psidium guajava) during storage. Indian J. Anim. Sci. 2024, 94, 744–749. [Google Scholar] [CrossRef]
- Coelho, J.M.P.; Johann, G.; da Silva, E.A.; Palú, F.; Vieira, M.G.A. Extraction of natural antioxidants from strawberry guava leaf by conventional and non-conventional techniques. Chem. Eng. Commun. 2021, 208, 1131–1142. [Google Scholar] [CrossRef]
- Chouegouong, M.T.; Majoumouo, M.S.; Menkem, E.Z.; Yimgang, L.V.; Toghueo, R.M.K.; Etchu, K.A.; Boyom, F.F. Ethnopharmacological survey and antibacterial activity of medicinal plant extracts used against bacterial enteritis in rabbits. Adv. Tradit. Med. 2023, 23, 213–223. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Ton, N.M.N.; Nguyen, T.T.; Le, V.V.M.; Sajeev, D.; Schilling, M.W.; Dinh, T.T.N. Application of natural antioxidant extract from guava leaves (Psidium guajava L.) in fresh pork sausage. Meat Sci. 2020, 165, 108106. [Google Scholar] [CrossRef]
- Lee, H.; Eo, Y.; Kim, S.Y.; Lim, Y. Guava leaf extract attenuated muscle proteolysis in dexamethasone induced muscle atrophic mice via ubiquitin proteasome system, mTOR-autophagy, and apoptosis pathway. Nutr. Res. 2024, 127, 97–107. [Google Scholar] [CrossRef]
- Hirudkar, J.R.; Parmar, K.M.; Prasad, R.S.; Sinha, S.K.; Jogi, M.S.; Itankar, P.R.; Prasad, S.K. Quercetin a major biomarker of Psidium guajava L. inhibits SepA protease activity of Shigella flexneri in treatment of infectious diarrhoea. Microb. Pathog. 2020, 138, 103807. [Google Scholar] [CrossRef]
- Wongsanao, T.; Leemingsawat, W.; Panapisal, V.; Kritpet, T. Thermoregulatory effects of guava leaf extract-menthol toner application for post-exercise use. Pharm. Biol. 2021, 59, 854–859. [Google Scholar] [CrossRef]
- Berk, Z. Chapter 11—Extraction. In Food Process Engineering and Technology; Berk, Z., Ed.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2009; pp. 259–277. ISBN 978-0-12-373660-4. [Google Scholar]
- Bhagya Raj, G.V.S.; Dash, K.K. Ultrasound-assisted extraction of phytocompounds from dragon fruit peel: Optimization, kinetics and thermodynamic studies. Ultrason. Sonochem. 2020, 68, 105180. [Google Scholar] [CrossRef]
- Shabbir, H.; Kausar, T.; Noreen, S.; Rehman, H.U.; Hussain, A.; Huang, Q.; Gani, A.; Su, S.; Nawaz, A. In Vivo Screening and Antidiabetic Potential of Polyphenol Extracts from Guava Pulp, Seeds and Leaves. Animals 2020, 10, 1714. [Google Scholar] [CrossRef]
- Senbua, W.; Wichitwechkarn, J. Molecular identification of fungi colonizing art objects in Thailand and their growth inhibition by local plant extracts. 3 Biotech 2019, 9, 356. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Zeng, D.; Liu, Y.; Gao, Y.; Tsunoda, M.; Deng, S.; Xie, X.; Wang, R.; Li, L.-S.; et al. Amino Acid Profiling Study of Psidium guajava L. Leaves as an Effective Treatment for Type 2 Diabetic Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 9784382. [Google Scholar] [CrossRef] [PubMed]
- Gholkar, M.S.; Li, J.V.; Daswani, P.G.; Tetali, P.; Birdi, T.J. 1H nuclear magnetic resonance-based metabolite profiling of guava leaf extract: An attempt to develop a prototype for standardization of plant extracts. BMC Complement. Med. Ther. 2021, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Sarfraz, R.A.; Rashid, M.A.; Mahmood, A.; Shahid, M.; Noor, N. Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharm. Biol. 2016, 54, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Marinho, L.; Sganzerla, W.G.; Ferreira, V.C.; Jimenez Moreno, J.A.; Rostagno, M.A.; Forster-Carneiro, T. Advances in green extraction methods, biological properties, and applications of betanin and vitexin: An updated review and bibliometric analysis. Biocatal. Agric. Biotechnol. 2023, 51, 102744. [Google Scholar] [CrossRef]
- Kadhim, E.J.; Kareem, A.T. Isolation and identification of phenolic compounds in guava leaves and assessment of their cytotoxic effects against AMJ-13 and MCF-7 breast cancer cell lines. J. Fac. Med. Baghdad 2024, 66, 334–343. [Google Scholar]
- Manikandan, R.; Anand, A.V.; Kumar, S. Phytochemical and in vitro antidiabetic activity of Psidium guajava leaves. Pharmacogn. J. 2016, 8, 392–394. [Google Scholar] [CrossRef]
- Jayachandran Nair, C.V.; Ahamad, S.; Khan, W.; Anjum, V.; Mathur, R. Development and validation of high-performance thin-layer chromatography method for simultaneous determination of polyphenolic compounds in medicinal plants. Pharmacogn. Res. 2017, 9, S67–S73. [Google Scholar] [CrossRef]
- Chiari-Andréo, B.G.; Trovatti, E.; Marto, J.; De Almeida-Cincotto, M.G.J.; Melero, A.; Corrêa, M.A.; Chiavacci, L.A.; Ribeiro, H.; Garrigues, T.; Isaac, V.L.B. Guava: Phytochemical composition of a potential source of antioxidants for cosmetic and/or dermatological applications. Braz. J. Pharm. Sci. 2017, 53, e16141. [Google Scholar] [CrossRef]
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N.; et al. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Sam Arul Raj, M.; Amalraj, S.; Alarifi, S.; Kalaskar, M.G.; Chikhale, R.; Santhi, V.P.; Gurav, S.; Ayyanar, M. Nutritional Composition, Mineral Profiling, In Vitro Antioxidant, Antibacterial and Enzyme Inhibitory Properties of Selected Indian Guava Cultivars Leaf Extract. Pharmaceuticals 2023, 16, 1636. [Google Scholar] [CrossRef]
- Yumita, A.; Hikmawanti, N.P.E.; Hanani, E.; Saputri, C.W.; Hanana, P.H.; Ero, J.N.D.; Marcelena, M.; Baytisani, T.; Sofiana, F.A.; Shania, A.F.; et al. Exploring the Polyphenol Contents and Antioxidant Capacity of the Leaf Extracts of Selected Indonesian Syzygium Species. Trop. J. Nat. Prod. Res. 2023, 7, 3119–3124. [Google Scholar] [CrossRef]
- Quang-Vinh, N.; Bich, H.; Bui, T.; Minh-Dinh, T.; Minh-Trung, N.; Manh-Dung, D.; Anh-Dzung, N.; Tam, M.L.; Van-Cuong, T.; Tri-Nhut, P. Impact of Different Drying Temperatures on In Vitro Antioxidant and Antidiabetic Activities and Phenolic Compounds of Wild Guava Leaves Collected in the Central Highland of Vietnam. Nat. Prod. Commun. 2022, 17, 1934578X221095349. [Google Scholar] [CrossRef]
- Park, H.; Kim, B.; Kang, Y.; Kim, W. Study on Chemical Composition and Biological Activity of Psidium guajava Leaf Extracts. Curr. ISSUES Mol. Biol. 2024, 46, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Khonthun, C.; Khoothiam, K.; Chumphukam, O.; Thongboontho, R.; Oonlao, P.; Nuntaboon, P.; Phromnoi, K. Screening and characterization of antioxidant, anti-aging, and anti-microbial activity of herbal extracts. Funct. Foods Health Dis. 2023, 13, 52–68. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Liu, Y.; Wu, Z. Complex enzyme-assisted extraction releases antioxidative phenolic compositions from guava leaves. Molecules 2017, 22, 1648. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Girón, F.; Pérez-Garrido, A.; Pereira, A.S.P.; Gabaldón-Hernández, J.A.; Verardo, V.; Segura Carretero, A.; Pérez-Sánchez, H. Fishing the Targets of Bioactive Compounds from Psidium guajava L. Leaves in the Context of Diabetes. Int. J. Mol. Sci. 2023, 24, 5761. [Google Scholar] [CrossRef]
- Lima, A.M.B.; Siani, A.C.; Nakamura, M.J.; D’Avila, L.A. Selective and cost effective protocol to separate bioactive triterpene acids from plant matrices using alkalinized ethanol: Application to leaves of Myrtaceae species. Pharmacogn. Mag. 2015, 11, 470–476. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Rengarajan, R.L.; Radhakrishnan, R.; Mathew, S.; Qadri, I.; Vijaya Anand, A. Psidium guajava Leaf Extracts and Their Quercetin Protect HepG2 Cell Lines Against CCL4 Induced Cytotoxicity. Indian J. Clin. Biochem. 2019, 34, 324–329. [Google Scholar] [CrossRef]
- Kenneth, E.; Paul, T.; Istifanus, N.; Uba, U.; Rejoice, A.; Victor, O.; Mohammed, S.; Kenneth, E.; Paul, T.; Istifanus, N.; et al. Phytochemical analysis and antibacterial activity of Psidium guajava L. leaf extracts. GSC Biol. Pharm. Sci. 2017, 1, 13–19. [Google Scholar] [CrossRef]
- Farag, R.S.; Abdel-Latif, M.S.; Abd El Baky, H.H.; Tawfeek, L.S. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol. Rep. 2020, 28, e00536. [Google Scholar] [CrossRef]
- Arain, A.; Sherazi, S.T.H.; Mahesar, S.A. Essential Oil from Psidium guajava Leaves: An excellent source of β-caryophyllene. Nat. Prod. Commun. 2019, 14, 1934578X1984300. [Google Scholar] [CrossRef]
- Phonarknguen, R.; Nobsathian, S.; Assawasuparerk, K. Effect of Betulinic acid Extraction from Guava (Psidium guajava Linn.) Leaves Against Human Cholangiocarcinoma Cells. Asian Pac. J. Cancer Prev. 2022, 23, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Saber, F.R.; Ashour, R.M.; El-Halawany, A.M.; Mahomoodally, M.F.; Ak, G.; Zengin, G.; Mahrous, E.A. Phytochemical profile, enzyme inhibition activity and molecular docking analysis of Feijoa sellowiana O. Berg. J. Enzyme Inhib. Med. Chem. 2021, 36, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Vijayanand, S.; George, K. Bioactive potential and pharmacological activity of Psidium guajava. Curr. Trends Biotechnol. Pharm. 2021, 15, 524–539. [Google Scholar] [CrossRef]
- Lok, B.; Sandai, D.; Baharetha, H.M.; Nazari, V.M.; Asif, M.; Tan, C.S.; Majid, A.M.S.A. Anticancer effect of Psidium guajava (Guava) leaf extracts against colorectal cancer through inhibition of angiogenesis. Asian Pac. J. Trop. Biomed. 2020, 10, 293–307. [Google Scholar] [CrossRef]
- Saleh, A.J.; Othman, L.; Elchoueiry, M.; Ghanem, R.; Bazzi, S.; El-Sabban, M.; Abdel-Massih, R.M. Anti-proliferative activity of yerba mate (Ilex paraguariensis) aqueous extracts on human colorectal cancer cell lines. Funct. Foods Health Dis. 2021, 11, 499–511. [Google Scholar] [CrossRef]
- Jerônimo, L.B.; da Costa, J.S.; Pinto, L.C.; Montenegro, R.C.; Setzer, W.N.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Antioxidant and Cytotoxic Activities of Myrtaceae Essential Oils Rich in Terpenoids from Brazil. Nat. Prod. Commun. 2021, 16, 1934578X21996156. [Google Scholar] [CrossRef]
- Chandran, S.; Ponnusamy, T.; Bheeman, D.; Rajamani, R.K.; Bellan, C.S. Dextran sulfate stabilized silver nanoparticle: Next generation efficient therapy for cancer. Int. J. Appl. Pharm. 2020, 12, 59–63. [Google Scholar] [CrossRef]
- Bar, F.M.A.; Habib, M.M.A.; El-Senduny, F.F.; Badria, F.A. Cytotoxic activity of silver nanoparticles prepared from Psidium guajava L. (Myrtaceae) and Lawsonia inermis L. (Lythraceae) extracts. Trop. J. Pharm. Res. 2021, 20, 1791–1799. [Google Scholar] [CrossRef]
- Abd’quadri-Abojukoro, A.N.; Nkadimeng, S.M.; McGaw, L.J.; Nsahlai, I.V. Phytochemical composition and cytotoxicity of ethanolic extracts of some selected plants. J. Appl. Anim. Res. 2022, 50, 656–665. [Google Scholar] [CrossRef]
- Bouchoukh, I.; Hazmoune, T.; Boudelaa, M.; Bensouici, C.; Zellagui, A. Anticholinesterase and antioxidant activities of foliar extract from a tropical species: Psidium guajava L. (Myrtaceae) grown in Algeria. Curr. Issues Pharm. Med. Sci. 2019, 32, 160–167. [Google Scholar] [CrossRef]
- Boer, J.L.; Mulrooney, S.B.; Hausinger, R.P. Nickel-dependent metalloenzymes. Arch. Biochem. Biophys. 2014, 544, 142–152. [Google Scholar] [CrossRef]
- Bai, S.; Bharti, P.; Seasotiya, L.; Malik, A.; Dalal, S. In vitro screening and evaluation of some Indian medicinal plants for their potential to inhibit Jack bean and bacterial ureases causing urinary infections. Pharm. Biol. 2015, 53, 326–333. [Google Scholar] [CrossRef]
- Siriwatanametanon, N.; Dodgson, W.; Dodgson, J.L.A. Investigation of antimicrobial activity of 13 Thai medicinal plants against bacteria and fungi. J. Pure Appl. Microbiol. 2017, 11, 1351–1356. [Google Scholar] [CrossRef]
- Dzotam, J.K.; Kuete, V. Antibacterial and Antibiotic-Modifying Activity of Methanol Extracts from Six Cameroonian Food Plants against Multidrug-Resistant Enteric Bacteria. BioMed Res. Int. 2017, 2017, 1583510. [Google Scholar] [CrossRef]
- Phaiboon, N.; Pulbutr, P.; Sungthong, B.; Rattanakiat, S. Effects of the ethanolic extracts of guava leaves, licorice roots and cloves on the cariogenic properties of streptococcus mutans. Pharmacogn. J. 2019, 11, 1029–1036. [Google Scholar] [CrossRef]
- Shama, M.; Hridhya, K.V.; Kulandhaivel, M. Evaluation of antimicrobial activity and minimum inhibitory concentration of ethanolic extract of three medicinal plants against bacteria causing skin infection. J. Pure Appl. Microbiol. 2018, 12, 375–379. [Google Scholar] [CrossRef]
- Kenmeni, J.F.; Sifi, I.; Bisso, B.N.; Kayoka-Kabongo, P.N.; Tsopmene, U.J.; Dzoyem, J.P. Exploring Medicinal Plants for Antimicrobial Activity and Synergistic Effects with Doxycycline Against Bacterial Species. Sci. World J. 2024, 2024, 6238852. [Google Scholar] [CrossRef]
- Gómez, P.A.M.; Mendizábal, M.F.R.; Poma, R.D.C.; Cifuentes, T.V.R.; Quispe, F.M.M.; Torres, D.J.M.; Amaranto, R.E.B.; Medina, C.A.M.; Contreras, L.A.P. Antibacterial and Antiadhesion Effects of Psidium guajava Fractions on a Multispecies Biofilm Associated with Periodontitis. Pesqui. Bras. Em Odontopediatria E Clin. Integr. 2022, 22, e210080. [Google Scholar] [CrossRef]
- Patel, P.; Joshi, C.; Birdi, T.; Kothari, V. Anti-infective efficacy of Psidium guajava L. leaves against certain pathogenic bacteria. F1000Research 2019, 8, 12. [Google Scholar] [CrossRef]
- Nakasone, N.; Ogura, Y.; Higa, N.; Toma, C.; Koizumi, Y.; Takaesu, G.; Suzuki, T.; Yamashiro, T. Effects of Psidium guajava leaf extract on secretion systems of gram-negative enteropathogenic bacteria. Microbiol. Immunol. 2018, 62, 444–453. [Google Scholar] [CrossRef]
- Chandra Shekar, B.R.; Nagarajappa, R.; Jain, R.; Suma, S.; Mruthunjaya, K.; Thakur, R. Evaluating the antimicrobial efficacy of an innovative, novel herbal formulation on dental caries and plaque microorganisms—A clinical research. Biomed. Pharmacol. J. 2019, 12, 1633–1645. [Google Scholar] [CrossRef]
- Sharma, Y.; Kawatra, A.; Sharma, V.; Dhull, D.; Kaushik, S.; Yadav, J.P.; Kaushik, S. In-vitro and in-silico evaluation of the anti-chikungunya potential of Psidium guajava leaf extract and their synthesized silver nanoparticles. VirusDisease 2021, 32, 260–265. [Google Scholar] [CrossRef]
- Heppy, F.; Mulyana, R.; Afrainin Syah, N.; Tjandrawinata, R.R. The effect of Psidium guajava Leaves’ extract for mild and asymptomatic corona virus Disease-19. Saudi Pharm. J. 2023, 31, 592–596. [Google Scholar] [CrossRef]
- Jadaun, P.; Shah, P.; Harshithkumar, R.; Said, M.S.; Bhoite, S.P.; Bokuri, S.; Ravindran, S.; Mishra, N.; Mukherjee, A. Antiviral and ROS scavenging potential of Carica papaya Linn and Psidium guajava leaves extract against HIV-1 infection. BMC Complement. Med. Ther. 2023, 23, 82. [Google Scholar] [CrossRef]
- Adukpo, S.; Elewosi, D.; Asmah, R.H.; Nyarko, A.K.; Ekpe, P.K.; Edoh, D.A.; Ofori, M.F. Antiplasmodial and Genotoxic Study of Selected Ghanaian Medicinal Plants. Evid. Based Complement. Alternat. Med. 2020, 2020, 1582724. [Google Scholar] [CrossRef]
- Veerapandi, L.; Nivetha, T.; Karunyah Amirthadharshini, N.; Sinthiya, R. Evaluation and Comparative Study of Anti-diabetic Activity of Anthocyanins Derived from Various Natural Sources. J. Nat. Remedies 2022, 22, 105–111. [Google Scholar] [CrossRef]
- Rahman, S.; Saha, N.; Sarwar, S.; Shamim, A.A.; Shaheen, N. Can guava (Psidium guajava) leaf extracts develop an indigenous, simplified tool for a semi-quantitative assessment of iron in groundwater? J. Water Health 2022, 20, 1644–1653. [Google Scholar] [CrossRef]
- Mathur, R.; Dutta, S.; Velpandian, T.; Mathur, S.R. Psidium guajava Linn. leaf extract affects hepatic glucose transporter-2 to attenuate early onset of insulin resistance consequent to high fructose intake: An experimental study. Pharmacogn. Res. 2015, 7, 166–176. [Google Scholar] [CrossRef]
- Müller, U.; Stübl, F.; Schwarzinger, B.; Sandner, G.; Iken, M.; Himmelsbach, M.; Schwarzinger, C.; Ollinger, N.; Stadlbauer, V.; Höglinger, O.; et al. In Vitro and In Vivo Inhibition of Intestinal Glucose Transport by Guava (Psidium guajava) Extracts. Mol. Nutr. Food Res. 2018, 62, e1701012. [Google Scholar] [CrossRef]
- Tella, T.; Masola, B.; Mukaratirwa, S. The effect of Psidium guajava aqueous leaf extract on liver glycogen enzymes, hormone sensitive lipase and serum lipid profile in diabetic rats. Biomed. Pharmacother. 2019, 109, 2441–2446. [Google Scholar] [CrossRef]
- Sharma, P.; Nair, J.; Sinh, A.; Velpandian, T.; Tripathi, R.; Mathur, R. Guava Leaf Extract Suppresses Fructose Mediated Non-Alcoholic Fatty Liver Disease in Growing Rats. Diabetes Metab. Syndr. Obes. 2022, 15, 2827–2845. [Google Scholar] [CrossRef]
- Rahman, M.H.; Asrafuzzaman, M.; Tusher, M.M.H.; Mosihuzzaman, M.; Khan, M.S.H.; Shoeb, M.; Rokeya, B. Elucidation of anti-hyperglycemic activity of Psidium guajava L. leaves extract on streptozotocin induced neonatal diabetic Long-Evans rats. J. Ayurveda Integr. Med. 2023, 14, 100776. [Google Scholar] [CrossRef]
- Soman, S.; Rajamanickam, C.; Rauf, A.A.; Madambath, I. Molecular mechanisms of the antiglycative and cardioprotective activities of Psidium guajava leaves in the rat diabetic myocardium. Pharm. Biol. 2016, 54, 3078–3085. [Google Scholar] [CrossRef]
- Phromnoi, K.; Sinchaiyakij, P.; Khanaree, C.; Nuntaboon, P.; Chanwikrai, Y.; Chaiwangsri, T.; Suttajit, M. Anti-inflammatory and antioxidant activities of medicinal plants used by traditional healers for antiulcer treatment. Sci. Pharm. 2019, 87, 22. [Google Scholar] [CrossRef]
- Rotondo, R.; Cruz, P.S.; Masin, M.; Bürgi, M.; Girardini, J.; García, S.M.; Rodríguez, G.R.; Furlan, R.L.E.; Escalante, A.M. Artichoke extracts with potential application in chemoprevention and inflammatory processes. Braz. J. Pharm. Sci. 2022, 58, e19238. [Google Scholar] [CrossRef]
- Wu, T. The effect of Psidium guajava hydroalcoholic extract on reducing the expression of Resistin and TNF-α genes in articular chondrocytes of patients with knee osteoarthritis. Cell. Mol. Biol. 2021, 67, 74–81. [Google Scholar] [CrossRef]
- Jayachandran, M.; Vinayagam, R.; Ambati, R.R.; Xu, B.; Chung, S.S.M. Guava Leaf Extract Diminishes Hyperglycemia and Oxidative Stress, Prevents β-Cell Death, Inhibits Inflammation, and Regulates NF-kB Signaling Pathway in STZ Induced Diabetic Rats. BioMed Res. Int. 2018, 2018, 4601649. [Google Scholar] [CrossRef]
- Ghaderi, F.; Ebrahimi, E.; Sari Aslani, F.; Koohi-Hosseinabadi, O.; Koohpeyma, F.; Irajie, C.; Tanideh, N.; Iraji, A. The effect of hydroalcoholic extract of Psidium guajava L. on experimentally induced oral mucosal wound in rat. BMC Complement. Med. Ther. 2022, 22, 201. [Google Scholar] [CrossRef]
- Kumar, A.; Kumarchandra, R.; Rai, R.; Kumblekar, V. Radiation mitigating activities of Psidium guajava L. against whole-body X-ray-induced damages in albino Wistar rat model. 3 Biotech 2020, 10, 507. [Google Scholar] [CrossRef]
- Hendrarti, W.; Robiansyah, M.; Nur, S.; Amin, A.; Nisa, M.; Abidin, H.L. Potential of Ethanol Extract of Guava (Psidium guajava L.) Leaves as Adjuvant-Antibiotic on Salmonella Typhi Characterized. Bull. Pharm. Sci. Assiut 2023, 46, 1259–1268. [Google Scholar] [CrossRef]
- Nhu, T.Q.; Bich Hang, B.T.; Cornet, V.; Oger, M.; Bach, L.T.; Anh Dao, N.L.; Thanh Huong, D.T.; Quetin-Leclercq, J.; Scippo, M.-L.; Phuong, N.T.; et al. Single or Combined Dietary Supply of Psidium guajava and Phyllanthus amarus Extracts Differentially Modulate Immune Responses and Liver Proteome in Striped Catfish (Pangasianodon hyphophthalmus). Front. Immunol. 2020, 11, 797. [Google Scholar] [CrossRef]
- de Caldas Brandão Filho, J.O.; Filho, J.P.M.B.; Sá, R.D.; Randau, K.P. Extraction and quantification of oxalic acid in leaves of plant species used in the treatment of chronic non-communicable diseases. Rev. Colomb. Cienc. Quimico-Farm. 2022, 51, 7–25. [Google Scholar] [CrossRef]
- Iwo, M.I.; Arifin, P.F.; Mus, N.M.; Susilowidodo, R.A.; Wisastra, R. Acute toxicity study of anti-diarrheal herbal combination in mice. Toxicol. Int. 2021, 28, 177–183. [Google Scholar] [CrossRef]
- Lu, J.; Mao, D.; Li, X.; Ma, Y.; Luan, Y.; Cao, Y.; Luan, Y. Changes of intestinal microflora diversity in diarrhea model of KM mice and effects of Psidium guajava L. as the treatment agent for diarrhea. J. Infect. Public Health 2020, 13, 16–26. [Google Scholar] [CrossRef]
- Cesar, P.H.S.; Trento, M.V.C.; Oliveira, D.A.; Simão, A.A.; Vieira, L.F.A.; Marcussi, S. Prospection of effects of guava leaves infusion: Antigenotoxic action and enzymatic inhibition. Nat. Prod. Commun. 2017, 12, 957–960. [Google Scholar] [CrossRef]
- Ekaluo, U.B.; Ikpeme, E.V.; Uno, U.U.; Umeh, S.O.; Erem, F.A. Protective role of aqueous guava leaf extract against caffeine induced spermatotoxicity in albino rats. Res. J. Med. Plant 2016, 10, 98–105. [Google Scholar] [CrossRef]
- Bazioli, J.M.; Costa, J.H.; Shiozawa, L.; Ruiz, A.L.T.G.; Foglio, M.A.; de Carvalho, J.E. Anti-estrogenic activity of guajadial fraction, from guava leaves (Psidium guajava L.). Molecules 2020, 25, 1525. [Google Scholar] [CrossRef]
- Manikandan, R.; Anand, A.V.; Sampathkumar, P.; Manoharan, N. Protective effect of Psidium guajava leaf ethanolic extract against streptozotocin-induced diabetes and lipidosis in rats. Indian J. Anim. Res. 2018, 52, 1198–1205. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Wei, W.; Tian, X.; Wu, Z. Antioxidant and anti-diabetic activities of polysaccharides from guava leaves. Molecules 2019, 24, 1343. [Google Scholar] [CrossRef]
- Sampath Kumar, N.S.; Sarbon, N.M.; Rana, S.S.; Chintagunta, A.D.; Prathibha, S.; Ingilala, S.K.; Jeevan Kumar, S.P.; Sai Anvesh, B.; Dirisala, V.R. Extraction of bioactive compounds from Psidium guajava leaves and its utilization in preparation of jellies. AMB Express 2021, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Shaheena, S.; Chintagunta, A.D.; Dirisala, V.R.; Sampath Kumar, N.S. Extraction of bioactive compounds from Psidium guajava and their application in dentistry. AMB Express 2019, 9, 208. [Google Scholar] [CrossRef]
- Lorena, C.; Ressaissi, A.; Serralheiro, M.L. Bioactives from Psidium guajava leaf decoction: LC-HRMS-MS-Qtof identification, bioactivities and bioavailability evaluation. Food Chem. Adv. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Ayembilla, J.A.; Khalid, A.R.; Abubakari, S.B.; Adams, A.R.; Botchway, F.A.; Antwi, S.; Otu, P.N.Y.; Appiah, M.; Osei-Adjei, G.; Kottoh, K.O.; et al. Acute and Subchronic Toxicity Assessment of Conventional Soxhlet Cymbopogon citratus Leaves Extracts in Sprague–Dawley Rats. J. Toxicol. 2023, 2023, 8575741. [Google Scholar] [CrossRef]
- Kong, F.; Yu, S.; Feng, Z.; Wu, X. Optimization of ultrasonic-assisted extraction of antioxidant compounds from Guava (Psidium guajava L.) leaves using response surface methodology. Pharmacogn. Mag. 2015, 11, 463–469. [Google Scholar] [CrossRef]
- Kokilananthan, S.; Bulugahapitiya, V.P.; Manawadu, H.; Gangabadage, C.S. Isolation of saponins from the leaves of Psidium guajava and Garcinia quaesita and antioxidative capacities. Food Sci. Appl. Biotechnol. 2024, 7, 360–367. [Google Scholar] [CrossRef]
- Rezzadori, K.; Arend, G.D.; Jaster, H.; Díaz-de-Cerio, E.; Verardo, V.; Segura-Carretero, A.; Verruck, S.; Prudêncio, E.S.; Petrus, J.C.C. Bioavailability of bioactive compounds of guava leaves (Psidium guajava) aqueous extract concentrated by gravitational and microwave-assisted cryoconcentration. J. Food Process. Preserv. 2022, 46, 16241. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Zhang, L.; Zhu, H.; Niu, Y.; An, Y.; Liu, Z. An Efficient Approach for Separating Essential Oil and Polysaccharides Simultaneously from Fresh Leaves of Guajava by Microwave-Mediated Hydrodistillation with Lithium Salts and Antibacterial Activity of Essential Oil. Separations 2022, 9, 162. [Google Scholar] [CrossRef]
- Rehan, M.; Ahmed-Farid, O.A.; Ibrahim, S.R.; Hassan, A.A.; Abdelrazek, A.M.; Khafaga, N.I.M.; Khattab, T.A. Green and Sustainable Encapsulation of Guava Leaf Extracts (Psidium guajava L.) into Alginate/Starch Microcapsules for Multifunctional Finish over Cotton Gauze. ACS Sustain. Chem. Eng. 2019, 7, 18612–18623. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.N.; Do Amaral, C.P.; De Almeida, M.P.; Borges, T.K.S.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, J.S.C.; et al. Cytotoxic activity of poly-ɛ-caprolactone lipid-core nanocapsules loaded with lycopene-rich extract from red guava (Psidium guajava L.) on breast cancer cells. Food Res. Int. 2020, 136, 109548. [Google Scholar] [CrossRef]
- Pham, D.T.; Nguyen, D.X.T.; Lieu, R.; Huynh, Q.C.; Nguyen, N.Y.; Quyen, T.T.B.; Tran, V.D. Silk nanoparticles for the protection and delivery of guava leaf (Psidium guajava L.) extract for cosmetic industry, a new approach for an old herb. Drug Deliv. 2023, 30, 2168793. [Google Scholar] [CrossRef]
- Niamah, A.K.; Al-Sahlany, S.T.G.; Ibrahim, S.A.; Verma, D.K.; Thakur, M.; Singh, S.; Patel, A.R.; Aguilar, C.N.; Utama, G.L. Electro-hydrodynamic processing for encapsulation of probiotics: A review on recent trends, technological development, challenges and future prospect. FOOD Biosci. 2021, 44, 101458. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Simal-Gándara, J.; Torrado-Agrasar, A. Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2018, 111, 219–225. [Google Scholar] [CrossRef]
- Sutthisawatkul, P.; Piyanaetitham, P.; Chareonviriyaphap, T.; Leepasert, T.; Taengphan, W.; Karpkird, T. Microemulsion containing guava leaves essential oil: Enhanced anti-inflammatory, anti-oxidation, anti-tyrosinase activities and skin permeation. J. Drug Deliv. Sci. Technol. 2024, 95, 105536. [Google Scholar] [CrossRef]
- Saipriya, G.; Karuppaiah, A.; Syamala, G.; Venkatesh, G.; Siram, K.; Babu, D.; Sankar, V. Self-nanoemulsifying drug delivery system of aqueous leaf extracts of Justicia adhatoda and Psidium guajava to enhance platelet count. 3 Biotech 2022, 12, 124. [Google Scholar] [CrossRef]
- Nurdianti, L.; Yuliana, A.; Raras, E.; Setiawan, F.; Wulandari, W.T.; Firmansya, A. Antibacterial activity of guava leaf ethanolic extract (Psidium guajava L.) nanosuspension against Escherichia coli bacteria. Pharmaciana 2024, 14, 173. [Google Scholar] [CrossRef]
- Dinh, T.A.; Le, Y.N.; Pham, N.Q.; Ton-That, P.; Van-Xuan, T.; Ho, T.G.-T.; Nguyen, T.; Phuong, H.H.K. Fabrication of antimicrobial edible films from chitosan incorporated with guava leaf extract. Prog. Org. Coat. 2023, 183, 107772. [Google Scholar] [CrossRef]
- Katewaraphorn, J.; Aldred, A.K. A study of microcapsules containing Psidium guajava leaf extract for antibacterial agent on cotton fabric. Int. J. Chem. Eng. Appl. 2016, 7, 27. [Google Scholar] [CrossRef]
- Waresindo, W.X.; Luthfianti, H.R.; Edikresnha, D.; Suciati, T.; Noor, F.A.; Khairurrijal, K. A freeze–thaw PVA hydrogel loaded with guava leaf extract: Physical and antibacterial properties. RSC Adv. 2021, 11, 30156–30171. [Google Scholar] [CrossRef]
- Masood, F.; Muhammad, S.; Bokhari, H.; Yasin, T.; Hameed, A. Characterisation and evaluation of antibacterial potential of guava extract loaded poly-3-hydroxybutyrate-co-3-hydroxyvalerate nanoparticles against multidrug-resistant bacteria. Micro Nano Lett. 2017, 12, 352–357. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Spray-Drying Microencapsulation of Pink Guava (Psidium guajava) Carotenoids Using Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves as Encapsulating Materials. Polymers 2022, 14, 310. [Google Scholar] [CrossRef]
- Sukoco, A.; Yamamoto, Y.; Harada, H.; Hashimoto, A.; Yoshino, T. Fish oil-containing edible films with active film incorporated with extract of Psidium guajava leaves: Preparation and characterization of double-layered edible film. F1000Research 2024, 13, 816. [Google Scholar] [CrossRef]
- Jayani, N.I.E.; Salawane, B.L.; Pelopolin, H.Y.; Rani, K.C. Formulation and evaluation of two types of functional beverage granules made of extracts of guava leaves, purple sweet potato and cinnamon. Trop. J. Nat. Prod. Res. 2021, 5, 1024–1029. [Google Scholar] [CrossRef]
- Thombre, N.A.; Laddha, U.D.; Ahire, E.D.; Tajanpure, A.B.; Surse, S.N.; Kakad, S.P.; Kakad, A.V.; Anumone, D.; Sonawani, R. Formulation Development and Evaluation of Herbal Antifungal Cream by using Psidium guajava Leaf Extract. Int. J. Drug Deliv. Technol. 2023, 13, 1229–1234. [Google Scholar] [CrossRef]
- Okareh, O.T.; Alaiya, M.A.; Odeniyi, M.A. Formulation of antiseptic ointments from mangifera indica kernel, leaf and Psidium guajava leaf extracts. Trop. J. Nat. Prod. Res. 2019, 3, 307–313. [Google Scholar] [CrossRef]

| Substance | Chemical Structure | Group | Molecular Formula | Molecular Weight (Da) |
|---|---|---|---|---|
| 3-Sinapoylquinic acid | 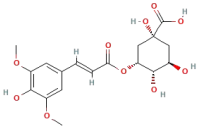 | Phenolic acids | C17H20O9 | 368.11 |
| (-)-Epicatechin 8-C-galactoside |  | Flavonoids | C15H14O7 | 306.07 |
| 3-Methoxysinensetin | 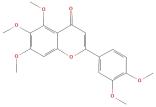 | Flavonoids | C18H14O8 | 358.07 |
| Quercetin 3-O-diglucoside and its derivaties | 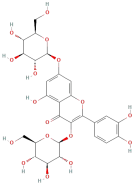 | Flavonoids | C21H20O12 | 464.09 |
| Kaempferol 3-O-xylosyl- rutinoside |  | Flavonoids | C33H40O20 | 756.66 |
| Schottenol ferulate |  | Terpenes | C39H60O5 | 608.44 |
| Sesamolinol 4′-O-β-D-glucosyl (1->6)-O-β-D-glucoside |  | Lignans | C26H36O13 | 540.56 |
| Esculin | 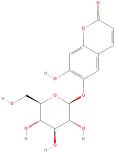 | Coumarins | C15H16O9 | 340.29 |
| Sources | TPC (mg GAE/g) | Extraction Solvent Used | Experimental Conditions | References |
|---|---|---|---|---|
| Pakistan | 83.34 | Methanol | Leaves were dried at 50 °C and then extracted in a rotary shaker at 350 rpm for 6 h at 65 °C (methanol), 70 °C (hexane), or 62 °C (chloroform) with a 1:10 (w/v) solid-to-solvent ratio. | [25] |
| India | 125.77 | Methanol | Leaves were extracted with methanol at room temperature, followed by filtration and concentration under reduced pressure. | [32] |
| Indonesia | 79.31 | 70% Ethanol | Leaves (8 g of dried powder) were extracted with 70% ethanol (1:10 w/v) via reflux at 70 °C for 30 min, followed by concentration under vacuum. | [33] |
| Vietnam | 145.38 | 50% Ethanol | Leaves were hot-air-dried at 50 °C for 9 h, extracted with 50% ethanol, and sonicated for 20 min. | [34] |
| Korea | 127.60 | 50% Ethanol | Leaves were extracted with ethanol (30%, 50%, 70%) for 24 h at 24 °C (1:20 w/v), followed by filtration and concentration under reduced pressure. | [35] |
| Thailand | 310.98 | 70% Ethanol | Leaves were extracted with 70% ethanol at room temperature for 4 h, followed by concentration under vacuum. | [36] |
| China | 438.80 | Aqueous | Leaves processed through enzyme-assisted extraction using cellulase, xylanase, and β-glucosidase at 50 °C for 12 h, followed by enzyme inactivation at 80 °C for 20 min and drying at 60 °C. | [37] |
| Substance | Chemical Structure | Group | Molecular Formula | Molecular Weight (Da) |
|---|---|---|---|---|
| Malic acid | 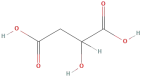 | Phenolic acids | C4H6O5 | 134.09 |
| 4-Hydroxybenzoic acid | 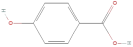 | Phenolic acids | C7H6O3 | 138.12 |
| 3,4-Dihydroxybenzoic acid | 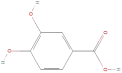 | Phenolic acids | C7H6O4 | 154.12 |
| Coumaric acid | 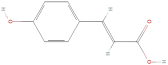 | Phenolic acids | C9H8O3 | 164.16 |
| Gallic acid |  | Phenolic acids | C7H5O6 | 170.12 |
| Caffeic acid |  | Phenolic acids | C9H8O4 | 180.16 |
| Ferulic acid | 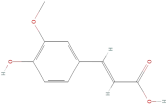 | Phenolic acids | C10H10O4 | 194.19 |
| Chlorogenic acid | 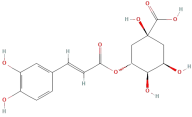 | Phenolic acids | C16H18O9 | 354.31 |
| Formononetin |  | Flavonoids | C16H12O4 | 268.26 |
| Genistein | 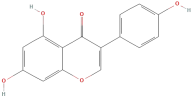 | Flavonoids | C15H10O5 | 270.24 |
| Kaempferol | 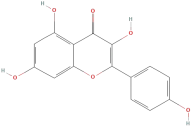 | Flavonoids | C15H10O6 | 286.24 |
| Epicatechin | 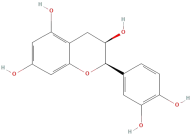 | Flavonoids | C15H14O6 | 290.27 |
| Catechin |  | Flavonoids | C15H14O6 | 290.27 |
| Quercetin | 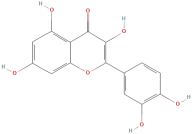 | Flavonoids | C15H10O7 | 302.24 |
| Morin | 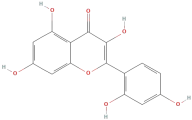 | Flavonoids | C15H10O7 | 302.24 |
| Gallocatechin | 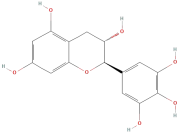 | Flavonoids | C15H14O7 | 306.27 |
| Tamarixetin | 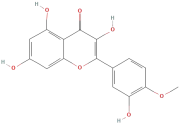 | Flavonoids | C16H12O7 | 316.26 |
| Myricetin | 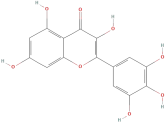 | Flavonoids | C15H10O8 | 318.24 |
| Avicularin | 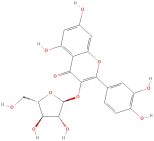 | Flavonoids | C20H18O11 | 434.35 |
| Gossypetin | 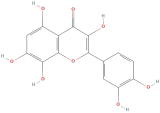 | Flavonoids | C21H20O13 | 464.37 |
| Isoquercitrin |  | Flavonoids | C21H20O12 | 464.38 |
| Quercetin 3-O-diglucoside | 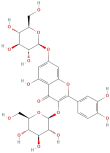 | Flavonoids | C21H20O12 | 464.38 |
| Kaempferol 3-O-glucoside | 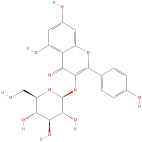 | Flavonoids | C21H20O11 | 448.37 |
| Rutin | 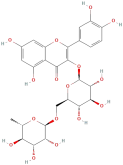 | Flavonoids | C27H30O16 | 610.52 |
| Ellagic acid |  | Tannins | C14H8O6 | 272.20 |
| Procyanidin B2 | 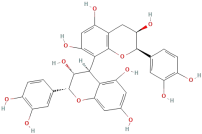 | Tannins | C30H26O12 | 578.53 |
| Method | Wall Materials | Key Findings | Applications | References |
|---|---|---|---|---|
| Coacervation | Calcium alginate | Produced a multi-functional cotton swab with antibacterial, antioxidant, and UV protection properties. | Textile and biomedical industries | [101] |
| Polymer–protein coating and self-feeding | Silk fibroin | Preserves the antioxidant activity of GLE and protects the extract from the effects of high temperature. | Food industry and cosmetic industry | [103] |
| Freeze-drying | Hydroxypropyl-beta-cyclodextrin (HPβCD) | ↑ Antioxidant stability by 26–38% when exposed to sunlight and ↑ antibacterial activity against Staphylococcus aureus and Escherichia coli by 4- and 2-fold, respectively. | Food and cosmetic industries | [106] |
| Emulsion system | Tween 80, Propylene Glycol | The particle size of the microemulsions ranged from 10 to 80 nm, with enhanced anti-inflammatory activities. | Food and cosmetic industries | [107] |
| Ionic gelation | Chitosan and sodium tripolyphosphate | The nanosuspension (245.7 nm) inhibited E. coli bacteria more effectively than GLE alone, even at low concentrations. | Food industry, pharmaceutical and cosmetic industries | [109] |
| Thin film hydration | Chitosan, glycerol | Chitosan films containing 2% GLE exhibit antioxidant, antibacterial, mechanical strength, and biodegradable properties. | Food packaging | [110] |
| In situ polymerization | Poly urea–formaldehyde shell | The treated fabrics exhibited antibacterial activity against Staphylococcus aureus but were ineffective against Escherichia coli. | Textile and pharmaceutical industries | [111] |
| Freeze–thaw | Polyvinyl alcohol (PVA) hydrogel | The hydrogel exhibited exudate absorption capacity and antibacterial activity. GLE imparted antibacterial properties to the hydrogel, while PVA is a biocompatible and nontoxic material. | Biomedical industry, particularly in wound care | [112] |
| Nanoprecipitation | Poly-3-hydroxybutyrate-co-3-hydroxyvalerate | Exhibited antibacterial effects against multidrug-resistant bacterial strains. | Pharmaceutical industry | [113] |
| Spray drying | Maltodextrin, gum arabic | Maltodextrin mixed with gum arabic was the most effective option for encapsulating the extract. | Food industry | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, H.D.; Nargotra, P.; Wang, H.-M.D.; Shieh, C.-J.; Liu, Y.-C.; Kuo, C.-H. Bioactive Compounds from Guava Leaves (Psidium guajava L.): Characterization, Biological Activity, Synergistic Effects, and Technological Applications. Molecules 2025, 30, 1278. https://doi.org/10.3390/molecules30061278
Huynh HD, Nargotra P, Wang H-MD, Shieh C-J, Liu Y-C, Kuo C-H. Bioactive Compounds from Guava Leaves (Psidium guajava L.): Characterization, Biological Activity, Synergistic Effects, and Technological Applications. Molecules. 2025; 30(6):1278. https://doi.org/10.3390/molecules30061278
Chicago/Turabian StyleHuynh, Hoang Duy, Parushi Nargotra, Hui-Min David Wang, Chwen-Jen Shieh, Yung-Chuan Liu, and Chia-Hung Kuo. 2025. "Bioactive Compounds from Guava Leaves (Psidium guajava L.): Characterization, Biological Activity, Synergistic Effects, and Technological Applications" Molecules 30, no. 6: 1278. https://doi.org/10.3390/molecules30061278
APA StyleHuynh, H. D., Nargotra, P., Wang, H.-M. D., Shieh, C.-J., Liu, Y.-C., & Kuo, C.-H. (2025). Bioactive Compounds from Guava Leaves (Psidium guajava L.): Characterization, Biological Activity, Synergistic Effects, and Technological Applications. Molecules, 30(6), 1278. https://doi.org/10.3390/molecules30061278











