Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues
Abstract
:1. Introduction
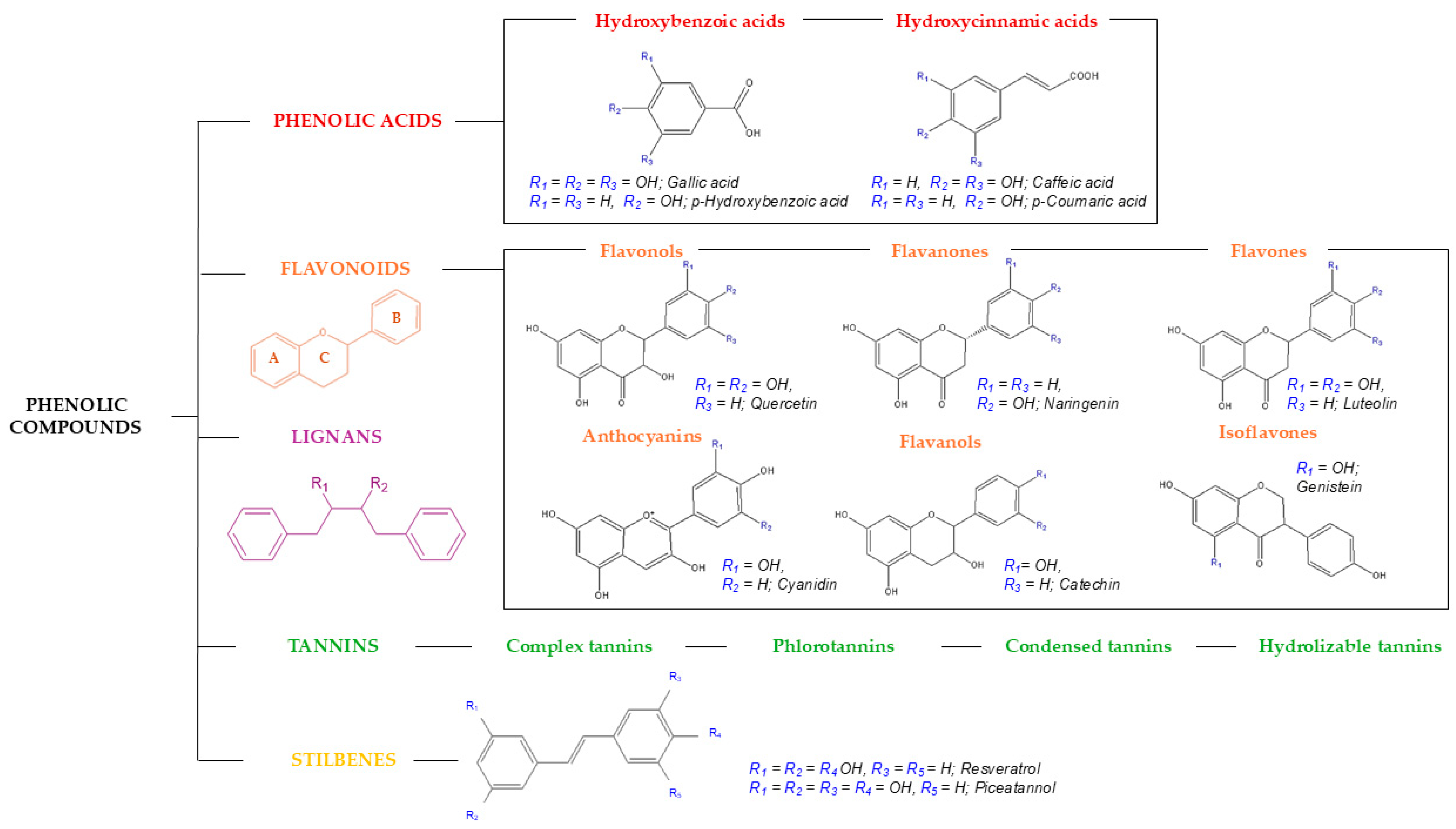
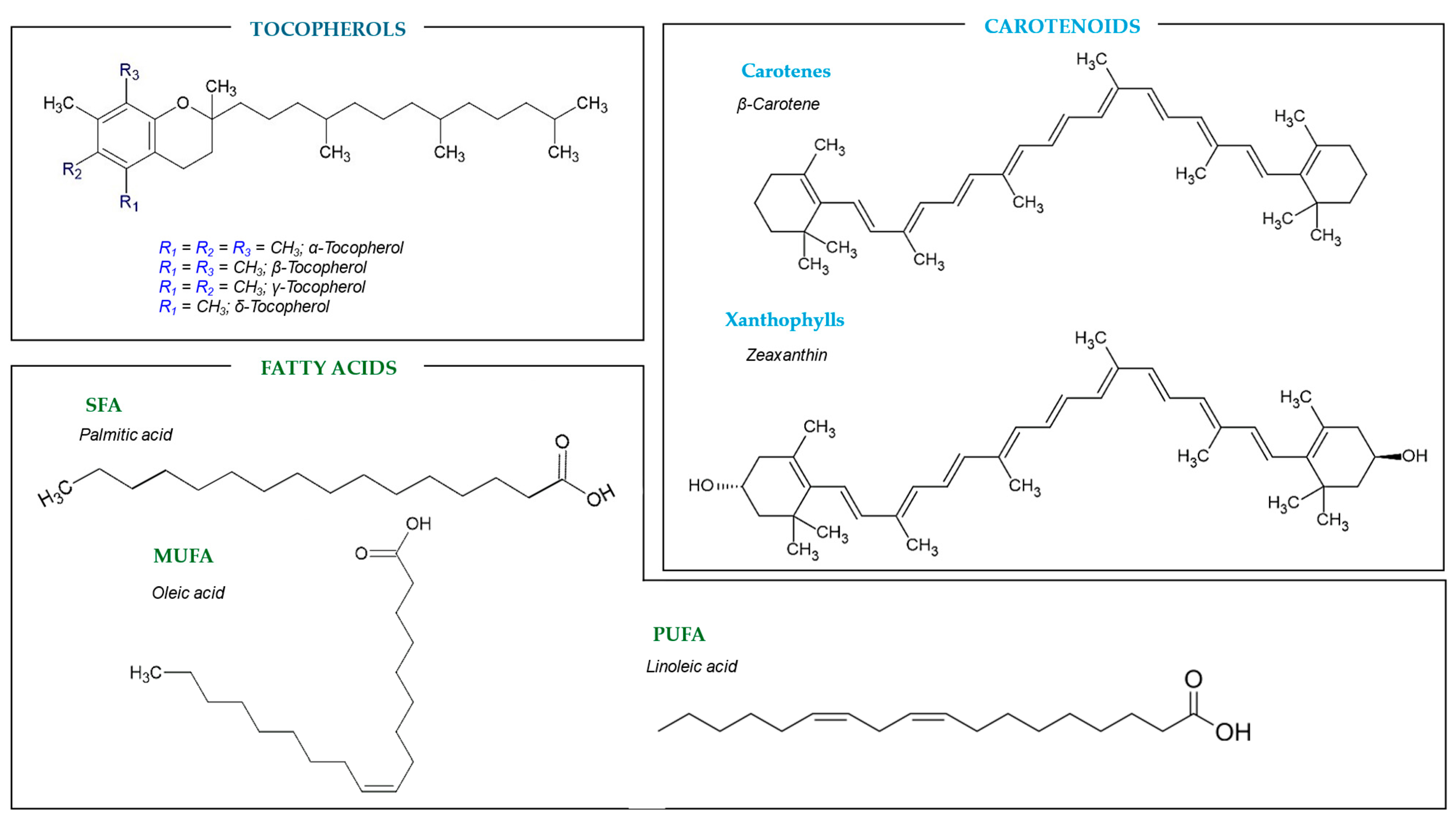
2. Bioactive Compounds Classification
2.1. Polyphenols
2.2. Tocopherols
2.3. Carotenoids
2.4. Fatty Acids
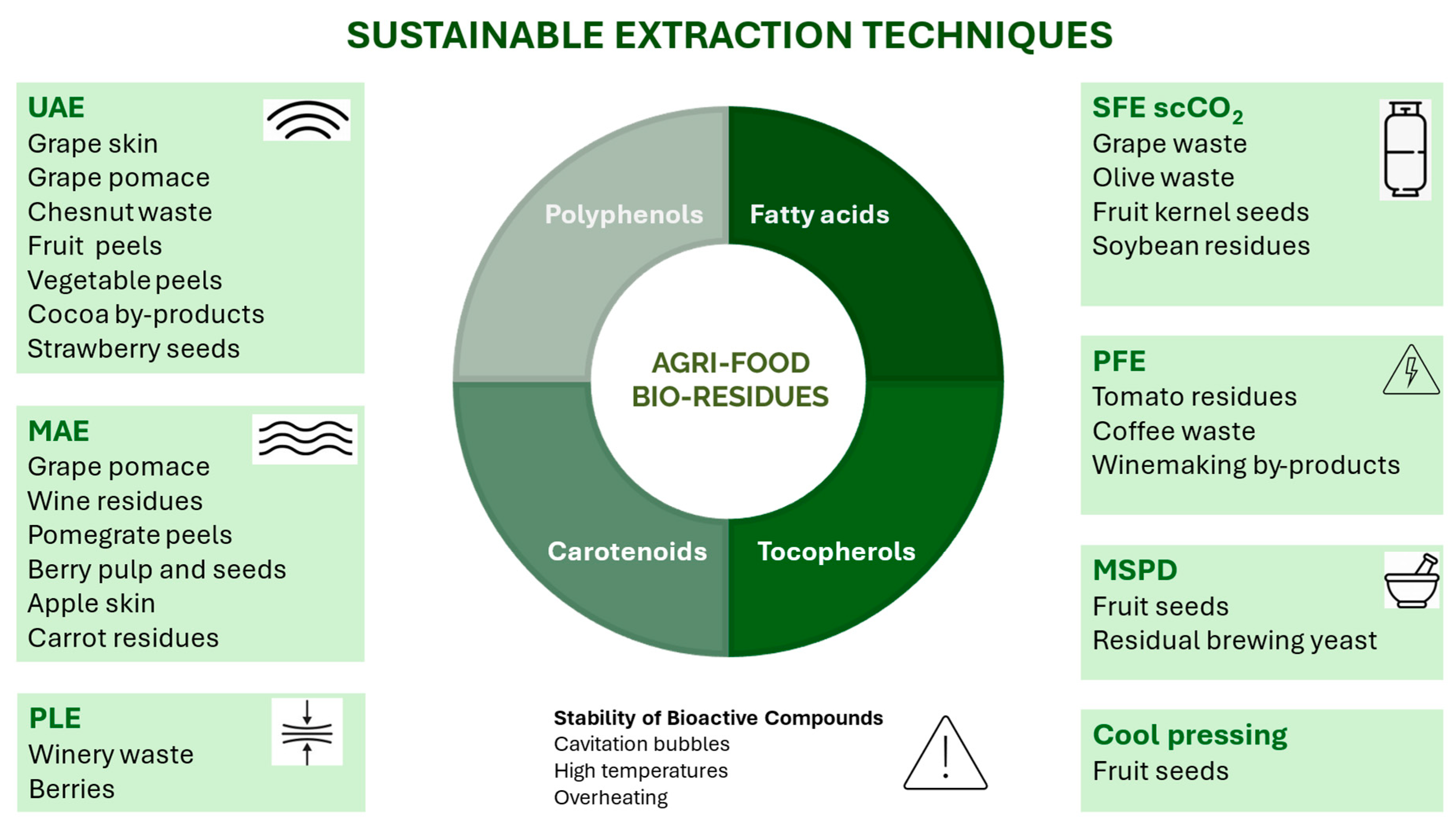
3. Sources of Bioactive Compounds: Agri-Food Bio-Residues
4. Sustainable Extraction Methods of Bioactive Compounds from Bio-Residues
5. Characterization of Bioactive Compounds in Bio-Residue Extracts
5.1. Determination of Polyphenols
5.1.1. Total Phenolic Content Determination
5.1.2. Individual Phenolic Content Determination
5.1.3. Polyphenol Content in Agri-Food Bio-Residue Extracts
5.2. Determination of Tocopherols
5.3. Determination of Carotenoids
5.4. Determination of Fatty Acids
6. Evaluation of Bioactive Properties of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids
6.1. Methods for Antioxidant Activity Assessment
6.2. Methods for Antimicrobial Activity Assessment
6.3. Methods for Anticarcinogenic, Neuroprotective, and Anti-Inflammatory Activity Assessment
6.4. Bioactive Properties of Agri-Food Bio-Residue Extracts
7. Potential Applications of Bioactive Compounds
8. Limitation in Agri-Food Bio-Residues Valorization: Potential Toxic Substances and Techno–Economic Considerations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Khanal, S.; Karimi, K.; Majumdar, S.; Kumar, V.; Verma, R.; Bhatia, S.K.; Kuca, K.; Esteban, J.; Kumar, D. Sustainable Utilization and Valorization of Potato Waste: State of the Art, Challenges, and Perspectives. Biomass Convers. Biorefinery 2024, 14, 23335–23360. [Google Scholar] [CrossRef]
- Ferreira, J.; Tkacz, K.; Turkiewicz, I.P.; Santos, I.; Camoesas e Silva, M.; Lima, A.; Sousa, I. Exploring the Bioactive Properties and Therapeutic Benefits of Pear Pomace. Antioxidants 2024, 13, 784. [Google Scholar] [CrossRef]
- Rodríguez-Blázquez, S.; Pedrera-Cajas, L.; Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Rodríguez-Bencomo, J.J.; Miranda, R. The Potential of Plum Seed Residue: Unraveling the Effect of Processing on Phytochemical Composition and Bioactive Properties. Int. J. Mol. Sci. 2024, 25, 1236. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Ramón Vidal, D.; Martorell, P.; Plaza, M.; Marina, M.L. Composition of Nonextractable Polyphenols from Sweet Cherry Pomace Determined by DART-Orbitrap-HRMS and Their In Vitro and In Vivo Potential Antioxidant, Antiaging, and Neuroprotective Activities. J. Agric. Food Chem. 2022, 70, 7993–8009. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Finimundy, T.C.; Pinela, J.; Pires, T.C.S.P.; Mandim, F.; Vaz, J.; Corrêa, R.C.G.; Oliveira, M.B.P.P.; Barros, L. Brazilian Berry Waste as a Source of Bioactive Compounds: Grumixama (Eugenia brasiliensis Lam.) as a Case Study. Food Funct. 2023, 14, 3994–4005. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.F.P.; Pereira, E.; Melgar, B.; Stojković, D.; Sokovic, M.; Calhelha, R.C.; Pereira, C.; Abreu, R.M.V.; Ferreira, I.C.F.R.; Barros, L. Eggplant Fruit (Solanum melongena L.) and Bio-Residues as a Source of Nutrients, Bioactive Compounds, and Food Colorants, Using Innovative Food Technologies. Appl. Sci. 2020, 11, 151. [Google Scholar] [CrossRef]
- Mittal, P.; Dhankhar, S.; Chauhan, S.; Garg, N.; Bhattacharya, T.; Ali, M.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, W.; et al. A Review on Natural Antioxidants for Their Role in the Treatment of Parkinson’s Disease. Pharmaceuticals 2023, 16, 908. [Google Scholar] [CrossRef]
- Capaldi, G.; Binello, A.; Aimone, C.; Mantegna, S.; Grillo, G.; Cravotto, G. New Trends in Extraction-Process Intensification: Hybrid and Sequential Green Technologies. Ind. Crops Prod. 2024, 209, 117906. [Google Scholar] [CrossRef]
- Castillo, A.; Celeiro, M.; Rubio, L.; Bañobre, A.; Otero-Otero, M.; Garcia-Jares, C.; Lores, M. Optimization of Bioactives Extraction from Grape Marc via a Medium Scale Ambient Temperature System and Stability Study. Front. Nutr. 2022, 9, 1008457. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Veríssimo, L.; Finimundy, T.; Rodrigues, J.; Oliveira, I.; Gonçalves, J.; Fernandes, I.P.; Barros, L.; Heleno, S.A.; Calhelha, R.C. Chemical and Bioactive Screening of Green Polyphenol-Rich Extracts from Chestnut By-Products: An Approach to Guide the Sustainable Production of High-Added Value Ingredients. Foods 2023, 12, 2596. [Google Scholar] [CrossRef]
- Soares, C.; Moreira, M.M.; Ramos, S.; Ramalhosa, M.J.; Correia, M.; Svarc-Gajić, J.; Delerue-Matos, C.; Barroso, M.F. A Critical Assessment of Extraction Methodologies for the Valorization of Agricultural Wastes: Polyphenolic Profile and Bioactivity. Processes 2023, 11, 1767. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; da Silva, L.P.; Penna, N.G. Green Extraction Methods and Microencapsulation Technologies of Phenolic Compounds From Grape Pomace: A Review. Food Bioprocess Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- de Andrade Maia, F.; Fasolin, L.H. Recovery of Bioactive Compounds from Pineapple Waste through High-Pressure Technologies. J. Supercrit. Fluids 2025, 218, 106455. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of Pulsed Electric Fields to Improve Product Yield and Waste Valorization in Industrial Tomato Processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Screening the Extraction Process of Phenolic Compounds from Pressed Grape Seed Residue: Towards an Integrated and Sustainable Management of Viticultural Waste. LWT 2022, 169, 113988. [Google Scholar] [CrossRef]
- Tozzi, F.; Núñez-Gómez, D.; Legua, P.; Del Bubba, M.; Giordani, E.; Melgarejo, P. Qualitative and Varietal Characterization of Pomegranate Peel: High-Value Co-Product or Waste of Production? Sci. Hortic. 2022, 291, 110601. [Google Scholar] [CrossRef]
- Benassai, E.; Del Bubba, M.; Ancillotti, C.; Colzi, I.; Gonnelli, C.; Calisi, N.; Salvatici, M.C.; Casalone, E.; Ristori, S. Green and Cost-Effective Synthesis of Copper Nanoparticles by Extracts of Non-Edible and Waste Plant Materials from Vaccinium Species: Characterization and Antimicrobial Activity. Mater. Sci. Eng. C 2021, 119, 111453. [Google Scholar] [CrossRef]
- Nancy Picot-Allain, M.C.; Amiri-Rigi, A.; Abdoun-Ouallouche, K.; Aberkane, L.; Djefal-Kerrar, A.; Mahomoodally, M.F.; Emmambux, M.N. Assessing the Bioactivity, Cytotoxicity, and Rheological Properties of Pectin Recovered from Citrus Peels. Food Biosci. 2022, 46, 101550. [Google Scholar] [CrossRef]
- Aussanasuwannakul, A.; Boonbumrung, S.; Pantoa, T. Valorization of Soybean Residue (Okara) by Supercritical Carbon Dioxide Extraction: Compositional, Physicochemical, and Functional Properties of Oil and Defatted Powder. Foods 2023, 12, 2698. [Google Scholar] [CrossRef]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What Is a Bioactive Compound? A Combined Definition for a Preliminary Consensus. Int. J. Nutr. Food Sci. 2014, 3, 174. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, B.R.; Corrêa, R.C.G.; de Lima Sampaio, S.; Barros, L. Bioactive Compounds from Food and Its By-Products: Current Applications and Future Perspectives. In Food Waste Conversion. Methods and Protocols in Food Science; Humana: New York, NY, USA, 2023; pp. 3–41. [Google Scholar]
- Casado, N.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Câmara, J.S.; Sierra, I. Two Novel Strategies in Food Sample Preparation for the Analysis of Dietary Polyphenols: Micro-Extraction Techniques and New Silica-Based Sorbent Materials. Trends Food Sci. Technol. 2020, 98, 167–180. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of Polyphenols and Their Properties. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–44. [Google Scholar]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Guerrero-Muñoz, N.; Villegas-Aguilar, M.d.C.; Pimentel-Moral, S.; Ramos-Escudero, F.; Segura-Carretero, A. LC-MS and Spectrophotometric Approaches for Evaluation of Bioactive Compounds from Peru Cocoa By-Products for Commercial Applications. Molecules 2020, 25, 3177. [Google Scholar] [CrossRef]
- Ferreyra, S.; Bottini, R.; Fontana, A. Background and Perspectives on the Utilization of Canes’ and Bunch Stems’ Residues from Wine Industry as Sources of Bioactive Phenolic Compounds. J. Agric. Food Chem. 2023, 71, 8699–8730. [Google Scholar] [CrossRef]
- Del Castillo-Llamosas, A.; Rodríguez-Martínez, B.; del Río, P.G.; Eibes, G.; Garrote, G.; Gullón, B. Hydrothermal Treatment of Avocado Peel Waste for the Simultaneous Recovery of Oligosaccharides and Antioxidant Phenolics. Bioresour. Technol. 2021, 342, 125981. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Zurdo, D.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E. A Comprehensive Analytical Review of Polyphenols: Evaluating Neuroprotection in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5906. [Google Scholar] [CrossRef]
- Ali, E.; Hussain, S.; Hussain, N.; Kakar, K.U.; Shah, J.M.; Zaidi, S.H.R.; Jan, M.; Zhang, K.; Khan, M.A.; Imtiaz, M. Tocopherol as Plant Protector: An Overview of Tocopherol Biosynthesis Enzymes and Their Role as Antioxidant and Signaling Molecules. Acta Physiol. Plant 2022, 44, 20. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (α-, β + γ- and δ-tocopherol) Levels in Plant Oils. Flavour Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Casquete, R.; Benito, M.J.; Martín, A.; Martínez, A.; Rivas, M.d.l.Á.; Córdoba, M.d.G. Influence of Different Extraction Methods on the Compound Profiles and Functional Properties of Extracts from Solid By-Products of the Wine Industry. LWT 2022, 170, 114097. [Google Scholar] [CrossRef]
- Hensley, K.; Benaksas, E.J.; Bolli, R.; Comp, P.; Grammas, P.; Hamdheydari, L.; Mou, S.; Pye, Q.N.; Stoddard, M.F.; Wallis, G.; et al. New Perspectives on Vitamin E: γ-Tocopherol and Carboxyethylhydroxychroman Metabolites in Biology and Medicine. Free Radic. Biol. Med. 2004, 36, 1–15. [Google Scholar] [CrossRef]
- Jūrienė, L.; Morkūnienė, V.; Venskutonis, P.R. Supercritical CO2 Extraction of Valuable Lipophilic Compounds from Pre-Fractionated Sour Cherry Pomace and Evaluation of Their Composition and Properties. J. CO2 Util. 2024, 85, 102890. [Google Scholar] [CrossRef]
- Dauber, C.; Carreras, T.; Fernández Fernández, A.; Irigaray, B.; Albores, S.; Gámbaro, A.; Ibáñez, E.; Vieitez, I. Response Surface Methodology for the Optimization of Biophenols Recovery from “Alperujo” Using Supercritical Fluid Extraction. Comparison between Arbequina and Coratina Cultivars. J. Supercrit. Fluids 2022, 180, 105460. [Google Scholar] [CrossRef]
- Sonawane, A.; Pathak, S.; Chandra Pradhan, R. Bioactive Compounds in Bael Fruit Pulp Waste: Ultrasound-Assisted Extraction, Characterization, Modeling, and Optimization Approaches. Biointerface Res. Appl. Chem. 2020, 11, 9318–9334. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Murray, M.T.; Capelli, B. Beta-Carotene and Other Carotenoids. In Textbook of Natural Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 443–450.e2. [Google Scholar]
- Ninčević Grassino, A.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of High Hydrostatic Pressure and Ultrasound-Assisted Extractions as a Novel Approach for Pectin and Polyphenols Recovery from Tomato Peel Waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, A.; Reglero, G.; Ibañez, E. Recent Trends in the Advanced Analysis of Bioactive Fatty Acids. J. Pharm. Biomed. Anal. 2010, 51, 305–326. [Google Scholar] [CrossRef]
- Hennessy, A.A.; Ross, P.R.; Fitzgerald, G.F.; Stanton, C. Sources and Bioactive Properties of Conjugated Dietary Fatty Acids. Lipids 2016, 51, 377–397. [Google Scholar] [CrossRef]
- Sanoja-López, K.A.; Guamán-Marquines, C.W.; Luque, R. Advanced Processes in Biomass/Waste Valorization: A Review. Sustain. Chem. Pharm. 2024, 41, 101704. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and Vegetable Waste Management: Conventional and Emerging Approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Portillo-López, R.; Morales-Contreras, B.E.; Lozano-Guzmán, E.; Basilio-Heredia, J.; Muy-Rangel, M.D.; Ochoa-Martínez, L.A.; Rosas-Flores, W.; Morales-Castro, J. Vegetable Oils as Green Solvents for Carotenoid Extraction from Pumpkin (Cucurbita argyrosperma Huber) Byproducts: Optimization of Extraction Parameters. J. Food Sci. 2021, 86, 3122–3136. [Google Scholar] [CrossRef] [PubMed]
- Macías-Garbett, R.; Sosa-Hernández, J.E.; Iqbal, H.M.N.; Contreras-Esquivel, J.C.; Chen, W.N.; Melchor-Martínez, E.M.; Parra-Saldívar, R. Combined Pulsed Electric Field and Microwave-Assisted Extraction as a Green Method for the Recovery of Antioxidant Compounds with Electroactive Potential from Coffee Agro-Waste. Plants 2022, 11, 2362. [Google Scholar] [CrossRef]
- Casazza, A.A.; Pettinato, M.; Perego, P. Polyphenols from Apple Skins: A Study on Microwave-Assisted Extraction Optimization and Exhausted Solid Characterization. Sep. Purif. Technol. 2020, 240, 116640. [Google Scholar] [CrossRef]
- Wójciak, W.; Żuk, M.; Sowa, I.; Mazurek, B.; Tyśkiewicz, K.; Wójciak, M. Recovery of Bioactive Components from Strawberry Seeds Residues Post Oil Extraction and Their Cosmetic Potential. Appl. Sci. 2024, 14, 783. [Google Scholar] [CrossRef]
- Kewlani, P.; Singh, L.; Singh, B.; Bhatt, I.D. Sustainable Extraction of Phenolics and Antioxidant Activities from Prinsepia Utilis Byproducts for Alleviating Aging and Oxidative Stress. Sustain. Chem. Pharm. 2022, 29, 100791. [Google Scholar] [CrossRef]
- Rodríguez-Blázquez, S.; Fernández-Ávila, L.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Miranda, R. Valorization of Defatted Cherry Seed Residues from Liquor Processing by Matrix Solid-Phase Dispersion Extraction: A Sustainable Strategy for Production of Phenolic-Rich Extracts with Antioxidant Potential. Antioxidants 2023, 12, 2041. [Google Scholar] [CrossRef]
- Cerón-Martínez, L.J.; Hurtado-Benavides, A.M.; Ayala-Aponte, A.; Serna-Cock, L.; Tirado, D.F. Bioactive Fractions Isolated from By-Products of the Guava (Psidium guajava) and Mango (Mangifera indica L.) Agri-Food Industry. Fluids 2023, 8, 256. [Google Scholar] [CrossRef]
- Evtuguin, D.; Aniceto, J.P.S.; Marques, R.; Portugal, I.; Silva, C.M.; Serafim, L.S.; Xavier, A.M.R.B. Obtaining Value from Wine Wastes: Paving the Way for Sustainable Development. Fermentation 2023, 10, 24. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive Mill and Winery Wastes as Viable Sources of Bioactive Compounds: A Study on Polyphenols Recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Aimone, C.; Grillo, G.; Boffa, L.; Giovando, S.; Cravotto, G. Tannin Extraction from Chestnut Wood Waste: From Lab Scale to Semi-Industrial Plant. Appl. Sci. 2023, 13, 2494. [Google Scholar] [CrossRef]
- Husanu, E.; Mero, A.; Rivera, J.G.; Mezzetta, A.; Ruiz, J.C.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Exploiting Deep Eutectic Solvents and Ionic Liquids for the Valorization of Chestnut Shell Waste. ACS Sustain. Chem. Eng. 2020, 8, 18386–18399. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Determination of Phenolic Compounds in Residual Brewing Yeast Using Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles. J. Chromatogr. A 2019, 1601, 255–265. [Google Scholar] [CrossRef]
- Brandão, A.S.; Caleja, C.; Dias, M.I.; ben Salha, A.; Rezouga, F.; Rodrigues, P.; Ferreira, I.C.F.R.; Barros, L.; Santos, J.M.R.C.A. Valorization of Pomace from Craft Cider: Nutritional Value, Chemical Composition, and Phenolic and Mineral Profiles. eFood 2023, 4, e85. [Google Scholar] [CrossRef]
- Lamine, M.; Hamdi, Z.; Zemni, H.; Rahali, F.Z.; Melki, I.; Mliki, A.; Gargouri, M. From Residue to Resource: The Recovery of High-Added Values Compounds through an Integral Green Valorization of Citrus Residual Biomass. Sustain. Chem. Pharm. 2024, 37, 101379. [Google Scholar] [CrossRef]
- Rodríguez-Blázquez, S.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; García-Sánchez, B.; Miranda, R. Valorization of Prunus Seed Oils: Fatty Acids Composition and Oxidative Stability. Molecules 2023, 28, 7045. [Google Scholar] [CrossRef]
- Vargas-Arana, G.; Merino-Zegarra, C.; Tang, M.; Pertino, M.W.; Simirgiotis, M.J. UHPLC–MS Characterization, and Antioxidant and Nutritional Analysis of Cocoa Waste Flours from the Peruvian Amazon. Antioxidants 2022, 11, 595. [Google Scholar] [CrossRef]
- Pathania, V.; Bora, B.; Kumar, R.; Sharma, K.; Neha, Y.; Kumar, A.; Singh, S.; Kumar, D.; Srivatsan, V. Valorization of German Chamomile Agri-Waste as a Source of High-Value Products: Characterization of Nutrients and Phytochemicals Towards Functional Food Development. Waste Biomass Valorization 2024, 1, 1. [Google Scholar] [CrossRef]
- Almeida, P.V.; Rodrigues, R.P.; Gaspar, M.C.; Braga, M.E.M.; Quina, M.J. Integrated Management of Residues from Tomato Production: Recovery of Value-Added Compounds and Biogas Production in the Biorefinery Context. J. Environ. Manag. 2021, 299, 113505. [Google Scholar] [CrossRef]
- Kaur, P.; Subramanian, J.; Singh, A. Green Extraction of Bioactive Components from Carrot Industry Waste and Evaluation of Spent Residue as an Energy Source. Sci. Rep. 2022, 12, 16607. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, M.A.; Jamil, M.A.; Noreen, S.; Rehman, H.u.; Shabbir, H.; Ramzan, M.A. Determination of Total Phenolic, Flavonoid, Carotenoid, and Mineral Contents in Peel, Flesh, and Seeds of Pumpkin (Cucurbita maxima). J. Food Process Preserv. 2021, 45, e15542. [Google Scholar] [CrossRef]
- Pușcaș, A.; Tanislav, A.E.; Marc, R.A.; Mureșan, V.; Mureșan, A.E.; Pall, E.; Cerbu, C. Cytotoxicity Evaluation and Antioxidant Activity of a Novel Drink Based on Roasted Avocado Seed Powder. Plants 2022, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Bahaa Eldin, A.; Khalifa, I. Valorization and Extraction Optimization of Prunus Seeds for Food and Functional Food Applications: A Review with Further Perspectives. Food Chem. 2022, 388, 132955. [Google Scholar] [CrossRef]
- Espro, C.; Paone, E.; Mauriello, F.; Gotti, R.; Uliassi, E.; Bolognesi, M.L.; Rodríguez-Padrón, D.; Luque, R. Sustainable Production of Pharmaceutical, Nutraceutical and Bioactive Compounds from Biomass and Waste. Chem. Soc. Rev. 2021, 50, 11191–11207. [Google Scholar] [CrossRef]
- Bangar, S.P.; Dunno, K.; Dhull, S.B.; Kumar Siroha, A.; Changan, S.; Maqsood, S.; Rusu, A.V. Avocado Seed Discoveries: Chemical Composition, Biological Properties, and Industrial Food Applications. Food Chem. X 2022, 16, 100507. [Google Scholar] [CrossRef]
- Singh, P.; Krishnaswamy, K. Non-GMO-High Oleic Soybean Meal Value Addition and Studying the Functional and Reconstitution Behavior. Int. J. Food Prop. 2023, 26, 708–728. [Google Scholar] [CrossRef]
- Umego, E.C.; Barry-Ryan, C. Review of the Valorization Initiatives of Brewing and Distilling By-Products. Crit. Rev. Food Sci. Nutr. 2024, 64, 8231–8247. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, Identification and Quantification of Polyphenols from Spent Coffee Grounds by Chromatographic Methods and Chemometric Analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Ibarz, R.; Ferreira-Santos, P.; Teixeira, J.A.; Rocha, C.M.R.; Pérez-Fernández, M.; García-Juiz, S.; Osada, J.; Martín-Belloso, O.; et al. Valorization of Agro-Food by-Products and Their Potential Therapeutic Applications. Food Bioprod. Process. 2021, 128, 247–258. [Google Scholar] [CrossRef]
- Mellinas, C.; Solaberrieta, I.; Pelegrín, C.J.; Jiménez, A.; Garrigós, M.C. Valorization of Agro-Industrial Wastes by Ultrasound-Assisted Extraction as a Source of Proteins, Antioxidants and Cutin: A Cascade Approach. Antioxidants 2022, 11, 1739. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Enríquez, A.J.; Reyes-Ventura, E.; Villanueva-Rodríguez, S.J.; Moreno-Vilet, L. Effect of Ultrasound-Assisted Extraction Parameters on Total Polyphenols and Its Antioxidant Activity from Mango Residues (Mangifera indica L. Var. Manililla). Separations 2021, 8, 94. [Google Scholar] [CrossRef]
- Zahid, I.; Nazir, M.H.; Javed, M.A. Extraction of Bioactive Components from Date Palm Waste, Various Extraction Processes and Their Applications: A Review. Biomass Bioenergy 2024, 190, 107433. [Google Scholar] [CrossRef]
- Prelac, M.; Palčić, I.; Cvitan, D.; Anđelini, D.; Repajić, M.; Ćurko, J.; Kovačević, T.K.; Goreta Ban, S.; Užila, Z.; Ban, D.; et al. From Waste to Green: Water-Based Extraction of Polyphenols from Onion Peel and Their Adsorption on Biochar from Grapevine Pruning Residues. Antioxidants 2023, 12, 1697. [Google Scholar] [CrossRef]
- Stelluti, S.; Caser, M.; Demasi, S.; Scariot, V. Sustainable Processing of Floral Bio-Residues of Saffron (Crocus sativus L.) for Valuable Biorefinery Products. Plants 2021, 10, 523. [Google Scholar] [CrossRef]
- Buratto, R.T.; Cocero, M.J.; Martín, Á. Characterization of Industrial Açaí Pulp Residues and Valorization by Microwave-Assisted Extraction. Chem. Eng. Process.-Process Intensif. 2021, 160, 108269. [Google Scholar] [CrossRef]
- Caballero, A.S.; Romero-García, J.M.; Castro, E.; Cardona, C.A. Supercritical Fluid Extraction for Enhancing Polyphenolic Compounds Production from Olive Waste Extracts. J. Chem. Technol. Biotechnol. 2020, 95, 356–362. [Google Scholar] [CrossRef]
- Putra, N.R.; Wibobo, A.G.; Machmudah, S.; Winardi, S. Recovery of Valuable Compounds from Palm-Pressed Fiber by Using Supercritical CO2 Assisted by Ethanol: Modeling and Optimization. Sep. Sci. Technol. 2020, 55, 3126–3139. [Google Scholar] [CrossRef]
- Vladić, J.; Gavarić, A.; Jokić, S.; Pavlović, N.; Moslavac, T.; Popović, L.; Matias, A.; Agostinho, A.; Banožić, M.; Vidović, S. Alternative to Conventional Edible Oil Sources: Cold Pressing and Supercritical CO2 Extraction of Plum (Prunus domestica L.) Kernel Seed. Acta Chim. Slov. 2020, 67, 778–784. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Copa-Chipana, C.; Mamani-Apaza, L.O.; Luque-Vilca, O.M.; Campos-Quiróz, C.N.; Zirena-Vilca, F.; Mariotti-Celis, M.S. Selective Recovery of Polyphenols from Discarded Blueberries (Vaccinium corymbosum L.) Using Hot Pressurized Liquid Extraction Combined with Isopropanol as an Environmentally Friendly Solvent. Foods 2023, 12, 3694. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Mikkelsen, L.H.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. A Combined Approach Based on Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles and Liquid Chromatography to Determine Polyphenols from Grape Residues. J. Chromatogr. A 2021, 1644, 462128–462139. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E. Unlocking the in Vitro Neuroprotection of Sloe Residues Phenolic Extracts by Bioanalytical and Chemometric Strategies. Food Chem. 2025, 463, 141208. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Ordoñez-Torres, A.; Torres-León, C.; Hernández-Almanza, A.; Flores-Guía, T.; Luque-Contreras, D.; Aguilar, C.N.; Ascacio-Valdés, J. Ultrasound-microwave-assisted Extraction of Polyphenolic Compounds from Mexican “Ataulfo” Mango Peels: Antioxidant Potential and Identification by HPLC/ESI/MS. Phytochem. Anal. 2021, 32, 495–502. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Yilmaz, E.; Güneşer, B.A. Cold Pressed versus Solvent Extracted Lemon (Citrus limon L.) Seed Oils: Yield and Properties. J. Food Sci. Technol. 2017, 54, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Kazempour-Samak, M.; Rashidi, L.; Ghavami, M.; Sharifan, A.; Hosseini, F. Sour Cherry (Cerasus vulgaris Miller) Kernel Oil as the Novel Functional Edible Oil: Sensory Evaluation and Antioxidant and Physicochemical Properties. J. Food Qual. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, G.; Chen, L.; Yang, N.; Wu, Y.; Fang, W.; Zhang, R.; Wang, X.; Fu, C.; Zhang, P. Volatile Fatty Acid Production in Anaerobic Fermentation of Food Waste Saccharified Residue: Effect of Substrate Concentration. Waste Manag. 2023, 164, 29–36. [Google Scholar] [CrossRef]
- Oliveira, J.; Benvenutti, L.; Albuquerque, B.R.; Finimundy, T.C.; Mandim, F.; Pires, T.C.S.P.; Pereira, C.; Corrêa, R.C.G.; Barros, L.; Zielinski, A.A.F. Green Extraction of Anthocyanin from Red Cabbage Waste Using Acid Whey as a Promising Bio-Based Solvent. Innov. Food Sci. Emerg. Technol. 2025, 100, 103926. [Google Scholar] [CrossRef]
- Dabetic, N.; Todorovic, V.; Malenovic, A.; Sobajic, S.; Markovic, B. Optimization of Extraction and HPLC–MS/MS Profiling of Phenolic Compounds from Red Grape Seed Extracts Using Conventional and Deep Eutectic Solvents. Antioxidants 2022, 11, 1595. [Google Scholar] [CrossRef] [PubMed]
- Dabetić, N.; Todorović, V.; Panić, M.; Radojčić Redovniković, I.; Šobajić, S. Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Appl. Sci. 2020, 10, 4830. [Google Scholar] [CrossRef]
- Mattonai, M.; Massai, P.; Ribechini, E. Sustainable Microwave-Assisted Eutectic Solvent Extraction of Polyphenols from Vine Pruning Residues. Microchem. J. 2024, 197, 109816. [Google Scholar] [CrossRef]
- Gaharwar, S.S.; Kumar, A.; Rathod, K.S.; Shinde, S.V. Valorization of Malus domestica L. (Apple) Peels: A Case Study of Circular Bioeconomy. Sustain. Chem. Pharm. 2023, 36, 101301. [Google Scholar] [CrossRef]
- Gaharwar, S.S.; Kumar, A.; Mandavgane, S.A.; Rahagude, R.; Gokhale, S.; Yadav, K.; Borua, A.P. Valorization of Punica Granatum (Pomegranate) Peels: A Case Study of Circular Bioeconomy. Biomass Convers. Biorefinery 2024, 14, 7707–7724. [Google Scholar] [CrossRef]
- Krivošija, S.; Vidović, S.; Dueñas-Mas, M.J.; Ballesteros-Gómez, A. Supramolecular Biosolvent Extraction of Antioxidants from Orange Peel Residues from the Tea Industry. Microchem. J. 2024, 207, 112136. [Google Scholar] [CrossRef]
- Izcara, S.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Application of a Hybrid Large Pore Mesoporous Silica Functionalized with β-Cyclodextrin as Sorbent in Dispersive Solid-Phase Extraction. Toward Sustainable Sample Preparation Protocols to Determine Polyphenolic Compounds in Arbutus unedo L. Fruits by UHPLC-IT-MS/MS. J. Food Compos. Anal. 2023, 118, 105191. [Google Scholar] [CrossRef]
- Méndez, D.A.; Fabra, M.J.; Odriozola-Serrano, I.; Martín-Belloso, O.; Salvia-Trujillo, L.; López-Rubio, A.; Martínez-Abad, A. Influence of the Extraction Conditions on the Carbohydrate and Phenolic Composition of Functional Pectin from Persimmon Waste Streams. Food Hydrocoll. 2022, 123, 107066. [Google Scholar] [CrossRef]
- Bouloumpasi, E.; Hatzikamari, M.; Christaki, S.; Lazaridou, A.; Chatzopoulou, P.; Biliaderis, C.G.; Irakli, M. Assessment of Antioxidant and Antibacterial Potential of Phenolic Extracts from Post-Distillation Solid Residues of Oregano, Rosemary, Sage, Lemon Balm, and Spearmint. Processes 2024, 12, 140. [Google Scholar] [CrossRef]
- Denk, B.; Aydogan, B.; Bayram, İ.; Fidan, A.F. Investigation of the in Vitro Antioxidant Activity of Fermented Tomato Pruning Residues and Its Effects on Biochemical and Hematological Parameters of Rats. Turk. J. Vet. Anim. Sci. 2023, 47, 281–292. [Google Scholar] [CrossRef]
- dos Santos, O.V.; Vieira, E.L.S.; Soares, S.D.; da Conceiçao, L.R.V.; Nascimento, F.d.C.A.d.; Teixeira-Costa, B.E. Utilization of Agroindustrial Residue from Passion Fruit (Passiflora edulis) Seeds as a Source of Fatty Acids and Bioactive Substances. Food Sci. Technol. 2021, 41, 218–225. [Google Scholar] [CrossRef]
- da Nóbrega Santos, E.; de Santana Neto, D.C.; de Magalhães Cordeiro, Â.M.T.; de Albuquerque Meireles, B.R.L.; da Silva Ferreira, V.C.; da Silva, F.A.P. From Waste to Wonder: Unleashing the Antimicrobial and Antioxidant Potential of Acerola Residue Using a Central Composite Rotatable Design. J. Environ. Chem. Eng. 2023, 11, 111184. [Google Scholar] [CrossRef]
- Christou, A.; Parisis, N.A.; Venianakis, T.; Barbouti, A.; Tzakos, A.G.; Gerothanassis, I.P.; Goulas, V. Ultrasound-Assisted Extraction of Taro Leaf Antioxidants Using Natural Deep Eutectic Solvents: An Eco-Friendly Strategy for the Valorization of Crop Residues. Antioxidants 2023, 12, 1801. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Chen, F.; Xiao, J.; Liao, X.; Hu, X.; Ji, J.; Ma, L. Guideline for Measurement of Condensed Tannin. Food Front. 2023, 4, 533–541. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M.E.M. Tannins Characterization in Historic Leathers by Complementary Analytical Techniques ATR-FTIR, UV-Vis and Chemical Tests. J. Cult. Herit. 2013, 14, 499–508. [Google Scholar] [CrossRef]
- Polaki, S.; Nayak, S.; Kumar, K.S.; B, R.P. High Resolution-Liquid Chromatograph Mass Spectrometer Characterization of Bioactive Compounds in Pineapple Wastes: Valorization of Antioxidant and Enzymatic Activity. J. Appl. Biol. Biotechnol. 2024, 12, 248–257. [Google Scholar] [CrossRef]
- Salem, Y.; Rajha, H.N.; Sunoqrot, S.; Hammad, A.M.; Castangia, I.; Manconi, M.; Manca, M.L.; Al Lababidi, D.; Touma, J.A.; Maroun, R.G.; et al. Exhausted Grape Seed Residues as a Valuable Source of Antioxidant Molecules for the Formulation of Biocompatible Cosmetic Scrubs. Molecules 2023, 28, 5049. [Google Scholar] [CrossRef]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valuation of Citrus Reticulata (Kinnow) Peel for the Extraction of Lutein Using Ultrasonication Technique. Biomass Convers. Biorefinery 2021, 11, 2157–2165. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Roriz, C.L.; Heleno, S.A.; Calhelha, R.; Dias, M.I.; Pinela, J.; Rosales-Conrado, N.; León-González, M.E.; Ferreira, I.C.F.R.; Barros, L. Valorisation of Black Mulberry and Grape Seeds: Chemical Characterization and Bioactive Potential. Food Chem. 2021, 337, 127998. [Google Scholar] [CrossRef]
- Marques, G.; Gutiérrez, A.; Barro, F.; del Río, J.C.; Rencoret, J. Seasonal Variability of Lipophilic Compounds in Oat (Avena sativa L.) Straw: A Comprehensive Chemical Study. J. Agric. Food Chem. 2024, 72, 19891–19903. [Google Scholar] [CrossRef]
- Anthon, G.; Barrett, D.M. Standardization of a Rapid Spectrophotometric Method for Lycopene Analysis. In Proceedings of the X International Symposium on the Processing Tomato, Beijing, China, 3–11 June 2007; pp. 111–128. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- de Rosso, V.V.; Mercadante, A.Z. Identification and Quantification of Carotenoids, By HPLC-PDA-MS/MS, from Amazonian Fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Sciacca, F.; Virzì, N.; Pecchioni, N.; Melilli, M.G.; Buzzanca, C.; Bonacci, S.; Di Stefano, V. Functional End-Use of Hemp Seed Waste: Technological, Qualitative, Nutritional, and Sensorial Characterization of Fortified Bread. Sustainability 2023, 15, 12899. [Google Scholar] [CrossRef]
- Quiceno Suarez, A.; Cadena-Chamorro, E.M.; Ciro-Velásquez, H.J.; Arango-Tobón, J.C. By-Products of the Cocoa Agribusiness: High Valueadded Materials Based on Their Bromatological and Chemical Characterization. Rev. Fac. Nac. Agron. Medellin 2024, 77, 10585–10599. [Google Scholar] [CrossRef]
- Rajapaksha, S.W.; Shimizu, N. Development and Characterization of Functional Starch-Based Films Incorporating Free or Microencapsulated Spent Black Tea Extract. Molecules 2021, 26, 3898. [Google Scholar] [CrossRef]
- Luntraru, C.M.; Apostol, L.; Oprea, O.B.; Neagu, M.; Popescu, A.F.; Tomescu, J.A.; Mulțescu, M.; Susman, I.E.; Gaceu, L. Reclaim and Valorization of Sea Buckthorn (Hippophae rhamnoides) By-Product: Antioxidant Activity and Chemical Characterization. Foods 2022, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Cushnie, B.; Echeverría, J.; Fowsantear, W.; Thammawat, S.; Dodgson, J.L.A.; Law, S.; Clow, S.M. Bioprospecting for Antibacterial Drugs: A Multidisciplinary Perspective on Natural Product Source Material, Bioassay Selection and Avoidable Pitfalls. Pharm. Res. 2020, 37, 125. [Google Scholar] [CrossRef]
- Romero, J.; Díez Méndez, A.; Castro-Alija, M.J.; Poveda, J.; Albertos, I. Quince (Cydonia Oblonga Mill.) Waste By-Product Characterization as a Potential Functional Ingredient. Sustainability 2024, 16, 8596. [Google Scholar] [CrossRef]
- Massironi, A.; Freire De Moura Pereira, P.; Verotta, L.; Jiménez-Quero, A.; Marzorati, S. Green Strategies for the Valorization of Industrial Medicinal Residues of Serenoa Repens Small (Saw palmetto) as Source of Bioactive Compounds. J. Environ. Manag. 2024, 370, 122843. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. The Progress and Application of Vitamin E Encapsulation—A Review. Food Hydrocoll. 2021, 121, 106998. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.-H.; Rajesh banu, J.; Kumar, G. Technological Advances in the Production of Carotenoids and Their Applications—A Critical Review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, G.; Guo, S.; Lin, Z.; Zeng, Y.; Wang, Q.; Li, J.; Yang, W. Antibacterial Activity of Camellia Oleifera Shells Polyphenols against Listeria Monocytogenes and Its Application for Preserving Sea Bass during Cold Storage. LWT 2025, 219, 117562. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Song, W.; Jiang, Y.; Dai, Y.; Qin, Z.; Liu, L.; Wei, S.; Chen, H. Structural Characterization of Complex Tannins from Euryale Ferox Fruit Peels and Their Inhibitory Mechanisms against Tyrosinase Activity and Melanogenesis. Int. J. Biol. Macromol. 2025, 298, 139909. [Google Scholar] [CrossRef]
- Végh, R.; Csóka, M.; Sörös, C.; Sipos, L. Food Safety Hazards of Bee Pollen—A Review. Trends Food Sci. Technol. 2021, 114, 490–509. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- González-Gómez, L.; Gañán, J.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Atropine and Scopolamine Occurrence in Spices and Fennel Infusions. Food Control 2023, 146, 109555. [Google Scholar] [CrossRef]
- Edgar, J.A.; Molyneux, R.J.; Colegate, S.M. Linking Dietary Exposure to 1,2-Dehydropyrrolizidine Alkaloids with Cancers and Chemotherapy-Induced Pulmonary and Hepatic Veno-Occlusive Diseases. J. Agric. Food Chem. 2020, 68, 5995–5997. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; Nielsen, E.; et al. Risk Assessment of Glycoalkaloids in Feed and Food, in Particular in Potatoes and Potato-derived Products. EFSA J. 2020, 18, e06222. [Google Scholar] [CrossRef]
- Eisenreich, A.; Sachse, B.; Gürtler, R.; Dusemund, B.; Lindtner, O.; Schäfer, B. What Do We Know about Health Risks Related to Thebaine in Food? Food Chem. 2020, 309, 125564. [Google Scholar] [CrossRef]
- Blank-Landeshammer, B.; Ranetbauer, C.; Weghuber, J. Detection of Tropane Alkaloid Contaminations in Unprocessed Soybeans and Their Fate in Food and Feed Processing. Food Control 2025, 168, 110963. [Google Scholar] [CrossRef]
- Butovskaya, E.; Caprai, E.; Peloso, M.; Gasparini, M.; Borgia, M.; Abdul, M.E.; Candotti, P.; Menotta, S. Plant-Based Milk Alternatives: Assessing the Occurrence of Chemical and Microbiological Contaminants in Soy, Oat, Rice and Almond Beverages from Italian Market. Food Control 2025, 169, 111005. [Google Scholar] [CrossRef]
- Fernández-Pintor, B.; Paniagua, G.; Gañán, J.; Morante-Zarcero, S.; Garcinuño, R.M.; Fernández, P.; Sierra, I. Determination of Atropine and Scopolamine in Honey Using a Miniaturized Polymer-Based Solid-Phase Extraction Protocol Prior to the Analysis by HPLC-MS/MS. Polymer 2024, 298, 126904. [Google Scholar] [CrossRef]
- Makovi, C.M.; Parker, C.H.; Zhang, K. Determination of Amygdalin in Apricot Kernels and Almonds Using LC-MS/MS. J. AOAC Int. 2023, 106, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A.H. Amygdalin: A Review on Its Characteristics, Antioxidant Potential, Gastrointestinal Microbiota Intervention, Anticancer Therapeutic and Mechanisms, Toxicity, and Encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, A.S. Evaluating Economic Potential of Food Waste Valorization: Onward to a Diverse Feedstock Biorefinery. Curr. Opin. Green. Sustain. Chem. 2020, 26, 100385. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Fan, Z.; Liu, Z.; Xu, P.; Sawant, T.R.; Huang, G.; Deng, X.; Guo, J.; Wang, J.; et al. Valorization of Agricultural Residues: Challenges and Opportunities in the Production of Bio-Based Materials. BioResources 2025, 20(2), 1. [Google Scholar] [CrossRef]
- Kanaan, A.F.; dos Santos, K.C.; Menezes, M.A.H.; Hamerski, F.; Voll, F.A.P.; Corazza, M.L. Sequential Extraction of Industrial Spent Coffee Grounds Using Pressurized Fluids as Solvents. J. Supercrit. Fluids 2025, 218, 106503. [Google Scholar] [CrossRef]



| Processed Food | Food Waste | Food Waste Produced (%) | Bioactive Compounds | Ref. |
|---|---|---|---|---|
| Apple | Pomace Peels | 25–30 | Phenolic acids (hydroxycinnamic aids) Flavonoids (flavonols, flavanols) Tannins Tocopherols | [22,48,58] |
| Prunus fruits (apricot, cherry, peach, plum) | Pomace Stone (shell and seeds) | 30–60 | Phenolic acids (hydroxybenzoic and hydroxycinnamic acids) Flavonoids (flavonols, flavones, flavan-3-ols, anthocyanins) Tannins Tocopherols Carotenoids Fatty acids | [35,67] |
| Citrus fruits (lemon, lime, orange, tangerine, etc.) | Peels Seeds Leaves | 50 | Phenolic acids (hydroxybenzoic and hydroxycinnamic acids) Flavonoids (flavonols, flavones, flavan-3-ols, anthocyanins) Tocopherols | [59,68] |
| Avocado | Peels Seeds Leaves | 30 | Phenolic acids (hydroxybenzoic and hydroxycinnamic acids) Flavonoids (flavonols, flavanones, flavanols, flavones) Lignans Tannins Fatty acids | [29,69] |
| Tomato | Branches Pomace Peels | 8 | Phenolic acids (hydroxy-cinnamic acids) Flavonoids Carotenoids Fatty acids | [22,40,63] |
| Soybean | Soybean meal; soybean whey Okara | Up to 90% | Flavonoids (isoflavones) Fatty acids | [70] |
| Wine | Vine leaves Vine shoots Grape stalks Grape pomace Grape seeds Wine lees | 15–20 | Phenolic acids (hydroxybenzoic acids) Flavonoids (flavonols, flavanols, anthocyanins) Stilbenes Tannins (condensed tannins) Fatty acids | [53] |
| Beer | Spent grain Trub Spent yeast Spent kieselguhr | 15–85 | Phenolic acids (hydroxybenzoic and hydroxycinnamic aids) Flavonoids (flavanones, flavanols) Tannins Fatty acids | [71] |
| Coffee | Coffee pulp Spent coffee grounds | 50 | Phenolic acids (hydroxybenzoic and hydroxycinnamic aids) Lignans | [22,72] |
| Residues | TPC (mg GAE·g−1) | Total Subclass Content | Major Polyphenols or Families | Ref. |
|---|---|---|---|---|
| Plum seed (Prunus domestica L.) | 0.57–2.40 | TFC: 0.9–1.3 mg QE·g−1 | Hydroxybenzoic acids (5) Hydroxycinnamic acids (5) Flavanols (7) | [3] |
| Acerola (Malpighia emarginata) | 9 | TAC: 0.57 g·g−1 Flavones: 3.3 g·g−1 Tannins: 0.35 mg TAE·g−1 | Phenolic acids (10) Flavonoids (6) | [105] |
| Açaí (Euterpe oleracea Mart.) seeds, slurry, and pulp | 28.6 (pulp) 17.7 (seeds) 1.6 (slurry) | TFC: 0.82–2.53 mg GAE·g−1 TAC: 2.93 mg·g−1 (pulp) | Not reported | [80] |
| Taro (Colacasia esculenta L.) leaves | 5.0–24.0 | TFC: 2.5–6.0 mg CE·g−1 TF: 4.0–8.0 mg QE·g−1 THA: 2.0–4.5 mg CAE·g−1 | Flavones (8) Flavonols (3) Caffeic acid derivative (1) | [106] |
| Pomegranate peel (Punica granatum) | 3.5–10 g GAE·L−1 | Flavonoids and tannins (qualitative test) | Ellagic acid Gallic acid Ferulic acid p-Coumaric acid Protocatechuic acid Caffeic acid | [98] |
| Pomegranate peel (Punica granatum) | 115–249 | Not reported | Phenolic acids (2) Ellagitannins (8) | [16] |
| Peru cocoa (Theobroma cacao) | 4.9–31.3 | Flavan-3-ol: 19–130 mg CE·g−1 | Flavonoids (29) Phenolic acids (5) | [27] |
| Brazilian berry (Eugenia brasiliensis Lam.) seeds, peels, and whole residue | 118.5–134.0 mg·g−1 | Hydrolyzable tannins: 13–104 mg·g−1 Condensed tannins: 27 mg·g−1 Anthocyanins: 12.6–77 mg·g−1 | Hydrolyzable tannins (17) Condensed tannins (7) Anthocyanins (4) | [5] |
| Avocado (Persea americana) peel | 31.3–40.6 | TFC: 61.3–70.5 mg RE·g−1 | Flavonoids (22) Phenolic acids (21) Lignans (2) | [29] |
| Pineapple (Ananas comosus L.) fruit and peel | 14.4–54.8 | TFC: 26.9–49.7 mg·g−1 | Hydroxybenzoic acids Hydroxycinnamic acids | [109] |
| Residues | Bioactive Family | Target Compounds (mg·kg−1) | Instrumental Techniques | Ref. |
|---|---|---|---|---|
| Plum seed (Prunus domestica L.) | Tocopherols | α-tocopherol (2.0–2.5) β- and γ-tocopherol (5.7–11.2) δ-tocopherol (1.5–2.2) | HPLC–DAD | [3] |
| Alperujo Arbequina and Coratina | Tocopherols | α-tocopherol (173–335) β- and γ-tocopherol (27–95) δ-tocopherol (17–25) | HPLC–fluorescence | [36] |
| Pitted sour cherry (Prunus cerasus L.) pomace | Tocopherols | α-tocopherol (81.4–195.9) β- and γ-tocopherol (45.6–272.0) δ-tocopherol (11.4–27.9) | UHPLC–QTOF–MS/MS | [35] |
| Pear pomace (Pyrus communis L., var. Rocha) flours | Tocopherols | α-tocopherol (3.418–5.825) β-tocopherol (0.537–0.836) γ-tocopherol (0.378–0.606) δ-tocopherol (0.03–0.126) | UHPLC–fluorescence | [2] |
| Pineapple residue (Ananas comosus L. Merril) | Carotenoids | β-carotene (1.65–16.09) | UV–Vis spectroscopy | [13] |
| Tomato residue | Carotenoids | Lycopene (98.4–143.1) | HPLC–DAD | [14] |
| Pumpkin (Cucurbita Maxima) peel, flesh, and seeds | Carotenoids | β-carotene (9.9–61.8) | HPLC–DAD | [65] |
| Kinnow (Citrus reticulata) peel | Carotenoids | Lutein (29.7) | HPLC–DAD | [111] |
| Soybean residue (okara) | Fatty acids | Oleic acid (19.6–24.2%) Linoleic acid (49.4–55.1%) | GC–FID | [19] |
| Sour cherry (Cerasus Vulgaris Miller) kernel oil | Fatty acids | Linoleic acid (42.34%) Oleic acid (35.45%) Palmitic acid (6.54%) α-eleostearic acid (9.34%) | GC–FID | [91] |
| Eggplant fruit (Solanum melongena L.) pulp | Fatty acids | Palmitic acid (44.8%) Stearic acid (24.4%) | GC–FID | [6] |
| Cocoa (Theobroma cacao) waste flours | Fatty acids | Palmitic acid (26.8–34.3%) Stearic acid (3.1–15.8%) Oleic acid (7.6–36.8%) Linoleic acid (15.7–48.9%) Linolenic acid (2.2–3.6%) | GC–FID | [61] |
| Residue | Methods Applied | Bioactive Properties | Ref. |
|---|---|---|---|
| Pear pomace (Pyrus communis L.) flour | ABTS (2.3–3.0 mmol TE·100 g−1) ORAC (5.3–6.1 mmol TE·100 g−1) AChEi (15.10–23.43%) BChEi (9.61–16.04%) | Antioxidant Neuroprotective | [2] |
| Plum (Prunus domestica L.) seed | DPPH (10–36 mg·mL−1/0.9–1.9 mg·g−1) TBARS (1.3–5.0 mg·g−1) E. coli and S. aureus (MIC ≥ 20 mg·mL−1) Aβ aggregation inhibition (TEM) Aβ fibrils width reduction (30%) | Antioxidant Antibacterial Neuroprotective | [3] |
| Brazilian berry (Eugenia brasiliensis Lam.) | TBARS (0.90–1.34 µg·mL−1) Gram-positive bacteria (MIC = 0.078–2.5 mg·mL−1) Gram-negative bacteria (MIC = 0.078–20 mg·mL−1) A. fumigatus and A. brasiliensis (MIC = 0.31–1.25 mg·mL−1) LPS-NO (EC50 = 98–400 µg·mL−1) SRB (GI50 = 14.7–186.0 µg·mL−1) | Antioxidant Antibacterial Antifungal Anticarcinogenic Anti-inflammatory | [5] |
| Eggplant fruit (Solanun melongena L.) | TBARS (EC50 = 135–4300 µg·mL−1) Gram-positive and Gram-negative bacteria (MIC = 2–8 mg·mL−1; MBC = 4–8 mg·mL−1) P. ochrochloron (MIC = 1 mg·mL−1; MFC = 1 mg·mL−1) SRB (GI50 = 280–340 µg·mL−1) | Antioxidant Antibacterial Antifungal Anticarcinogenic | [6] |
| Chestnut (Castanea sativa Mill.) burs, shells, and leaves | DPPH (EC50 = 0.07–0.90 mg·mL−1) TBARS (EC50 = 0.002–2.0 mg·mL−1) FRAP (EC50 = 0.07–1.34 mg·mL−1) Gram-positive and Gram-negative bacteria (MIC ≤ 10 mg·mL−1; MBC = 5 mg·mL−1) A. fumigatus and A. brasiliensis (MIC = 10 mg·mL−1) | Antioxidant Antibacterial Antifungal | [10] |
| Quince (Cydonia oblonga Mill.) | DPPH (72% inhibition) ABTS (380 µmol TE·100 g−1) | Antioxidant | [122] |
| Pomegranate (Punica granatum) peel | ABTS (15–30 mM TE) FRAP (45–100 mmol iron eq.·L−1) Gram-positive and Gram-negative bacteria (agar diffusion method < 4 mm) | Antioxidant Antibacterial | [98] |
| Alperujo Arbequina and Coratina | ABTS (0.607–1.224 mg TE·g−1) ORAC (180–318 mg TE·g−1) Gram-positive and Gram-negative (MIC = 0.6–1.3 mg·mL−1) | Antioxidant Antibacterial | [36] |
| Saw palmetto (Serenoa repens) | ABTS (39–140 mg TE·g−1) DPPH (0.031–0.088 mg TE·g−1) Gram-positive and Gram-negative bacteria (MBC = 12.5–100 mg·mL−1) | Antioxidant Antibacterial | [123] |
| Acerola cherry (Malpighia emarginata) | DPPH (3.5 g TE·100 g−1) Gram-positive and Gram-negative bacteria (disk diffusion method = 7.9–8.2 mm) | Antioxidant Antibacterial | [105] |
| Oregano (Origanum vulgare) | DPPH–ABTS–FRAP (300–450 mg GAE·g−1) Gram-positive and Gram-negative bacteria (20% inhibition) | Antioxidant Antibacterial | [102] |
| Rosemary (Rosmarinus officinalis) | DPPH–ABTS–FRAP (250–300 mg GAE·g−1) Gram-positive and Gram-negative bacteria (100% inhibition) | ||
| Sage (Salvia fruticosa) | DPPH–ABTS–FRAP (300–350 mg GAE·g−1) Gram-positive and Gram-negative bacteria (100% inhibition) | ||
| Lemon balm (Melissa officinalis) | DPPH–ABTS–FRAP (500–700 mg GAE·g−1) Gram-positive and Gram-negative bacteria (20% inhibition) | ||
| Spearmint (Mentha spicata) | DPPH–ABTS–FRAP (350–500 mg GAE·g−1) Gram-positive and Gram-negative bacteria (100% inhibition) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Zurdo, D.; Gómez-Mejía, E.; Morante-Zarcero, S.; Rosales-Conrado, N.; Sierra, I. Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules 2025, 30, 1326. https://doi.org/10.3390/molecules30061326
Vicente-Zurdo D, Gómez-Mejía E, Morante-Zarcero S, Rosales-Conrado N, Sierra I. Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules. 2025; 30(6):1326. https://doi.org/10.3390/molecules30061326
Chicago/Turabian StyleVicente-Zurdo, David, Esther Gómez-Mejía, Sonia Morante-Zarcero, Noelia Rosales-Conrado, and Isabel Sierra. 2025. "Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues" Molecules 30, no. 6: 1326. https://doi.org/10.3390/molecules30061326
APA StyleVicente-Zurdo, D., Gómez-Mejía, E., Morante-Zarcero, S., Rosales-Conrado, N., & Sierra, I. (2025). Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules, 30(6), 1326. https://doi.org/10.3390/molecules30061326










