Extraction of Cannabinoids and Terpenes from Hemp Flowers and Leaves (Cannabis sativa L., Futura 75): Chemical Profiling and Evaluation of Anticancer Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Plant Material and Extraction
2.2. Analysis of Cannabinoid Profiles of Extracts
2.3. Terpene Organic Compound Profiles of Extracts
2.4. Cytotoxicity of Extracts Toward Selected Cell Lines
3. Materials and Methods
3.1. Chemicals and Solvents
3.2. Plant Material and Sample Processing
3.2.1. Plant Material
3.2.2. Low-Pressure Extraction (LPE)
3.3. GC-MS Analysis of Cannabinoids in Prepared Extracts
3.4. Qualitative Analysis of Terpenoids in Prepared Extracts by HS-SPME-GC-MS
3.5. Biological Studies
3.5.1. Cell Lines and Cultured Mediums
3.5.2. Determination of Antiproliferative Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sejm Act of 24 March 2022 Amending the Act on Counteracting Drug Addiction. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20220000763/T/D20220763L.pdf (accessed on 22 January 2025).
- Kitdumrongthum, S.; Trachootham, D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 2023, 28, 2791. [Google Scholar] [CrossRef] [PubMed]
- Salamone, S.; Waltl, L.; Pompignan, A.; Grassi, G.; Chianese, G.; Koeberle, A.; Pollastro, F. Phytochemical Characterization of Cannabis sativa L. Chemotype V Reveals Three New Dihydrophenanthrenoids That Favorably Reprogram Lipid Mediator Biosynthesis in Macrophages. Plants 2022, 11, 2130. [Google Scholar] [CrossRef]
- European Commission Common Catalogue of Varieties of Agricultural Plant Species. Available online: https://food.ec.europa.eu/system/files/2023-08/plant-variety-catalogues_agricultural-plant-species.pdf (accessed on 22 January 2025).
- Frassinetti, S.; Moccia, E.; Caltavuturo, L.; Gabriele, M.; Longo, V.; Bellani, L.; Giorgi, G.; Giorgetti, L. Nutraceutical Potential of Hemp (Cannabis sativa L.) Seeds and Sprouts. Food Chem. 2018, 262, 56–66. [Google Scholar] [CrossRef]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial Cannabis Consumer Products Part 1: GC–MS Qualitative Analysis of Cannabis Cannabinoids. Forensic Sci. Int. 2018, 289, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kanabus, J.; Bryła, M.; Roszko, M.; Modrzewska, M.; Pierzgalski, A. Cannabinoids—Characteristics and Potential for Use in Food Production. Molecules 2021, 26, 6723. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Ocmín, P.G.; Marti, G.; Bonhomme, M.; Mathis, F.; Fournier, S.; Bertani, S.; Maciuk, A. Cannabinoids vs. Whole Metabolome: Relevance of Cannabinomics in Analyzing Cannabis Varieties. Anal. Chim. Acta 2021, 1184, 339020. [Google Scholar] [CrossRef]
- Jeong, G.H.; Kim, K.C.; Lee, J.H. Anti-Inflammatory Effects of Cannabigerol In Vitro and In Vivo Are Mediated Through the JAK/STAT/NFκB Signaling Pathway. Cells 2025, 14, 83. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Bacterial Properties of Cannabigerol Toward Streptococcus Mutans. Front. Microbiol. 2021, 12, 656471. [Google Scholar] [CrossRef]
- Luz-Veiga, M.; Amorim, M.; Pinto-Ribeiro, I.; Oliveira, A.L.S.; Silva, S.; Pimentel, L.L.; Rodríguez-Alcalá, L.M.; Madureira, R.; Pintado, M.; Azevedo-Silva, J.; et al. Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota. Int. J. Mol. Sci. 2023, 24, 2389. [Google Scholar] [CrossRef]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. The Origin and Biomedical Relevance of Cannabigerol. Int. J. Mol. Sci. 2022, 23, 7929. [Google Scholar] [CrossRef]
- Thorsen, T.S.; Kulkarni, Y.; Sykes, D.A.; Bøggild, A.; Drace, T.; Hompluem, P.; Iliopoulos-Tsoutsouvas, C.; Nikas, S.P.; Daver, H.; Makriyannis, A.; et al. Structural Basis of THC Analog Activity at the Cannabinoid 1 Receptor. Nat. Commun. 2025, 16, 486. [Google Scholar] [CrossRef] [PubMed]
- Anil, S.M.; Peeri, H.; Koltai, H. Medical Cannabis Activity Against Inflammation: Active Compounds and Modes of Action. Front. Pharmacol. 2022, 13, 908198. [Google Scholar] [CrossRef] [PubMed]
- Shehata, I.; Hashim, A.; Elsaeidy, A.; Nair, A.; Urits, I.; Viswanath, O.; Kaye, A.; Habib, M. Cannabinoids and Their Role in Chronic Pain Treatment: Current Concepts and a Comprehensive Review. Health Psychol. Res. 2022, 10, 35848. [Google Scholar] [CrossRef]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol—From Plant to Human Body: A Promising Bioactive Molecule with Multi-Target Effects in Cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Kim, J.H.; Han, J.H.; Ryu, B.R.; Lim, Y.S.; Lim, J.D.; Park, S.H.; Kim, C.H.; Lee, S.U.; Kwon, T.H. In Vitro and In Vivo Anti-Inflammatory Potential of Cannabichromene Isolated from Hemp. Plants 2023, 12, 3966. [Google Scholar] [CrossRef]
- McPartland, J.M.; Russo, E.B. Cannabis and Cannabis Extracts. J. Cannabis Ther. 2001, 1, 103–132. [Google Scholar] [CrossRef]
- Sepulveda, D.E.; Vrana, K.E.; Kellogg, J.J.; Bisanz, J.E.; Desai, D.; Graziane, N.M.; Raup-Konsavage, W.M. The Potential of Cannabichromene (CBC) as a Therapeutic Agent. J. Pharmacol. Exp. Ther. 2024, 391, 206–213. [Google Scholar] [CrossRef]
- Chen, C.; Pan, Z. Cannabidiol and Terpenes from Hemp—Ingredients for Future Foods and Processing Technologies. J. Future Foods 2021, 1, 113–127. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From Plant Genome to Humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Chacon, F.T.; Raup-Konsavage, W.M.; Vrana, K.E.; Kellogg, J.J. Secondary Terpenes in Cannabis sativa L.: Synthesis and Synergy. Biomedicines 2022, 10, 3142. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef] [PubMed]
- LaVigne, J.E.; Hecksel, R.; Keresztes, A.; Streicher, J.M. Cannabis sativa Terpenes Are Cannabimimetic and Selectively Enhance Cannabinoid Activity. Sci. Rep. 2021, 11, 8232. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of Phenolic Compounds and Terpenes from Cannabis sativa L. By-Products: From Conventional to Intensified Processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef]
- Rožanc, J.; Kotnik, P.; Milojević, M.; Gradišnik, L.; Knez Hrnčič, M.; Knez, Ž.; Maver, U. Different Cannabis sativa Extraction Methods Result in Different Biological Activities against a Colon Cancer Cell Line and Healthy Colon Cells. Plants 2021, 10, 566. [Google Scholar] [CrossRef]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality Traits of “Cannabidiol Oils”: Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the Compositions of Cannabinoid and Terpenoids in Cannabis sativa Derived from Inflorescence Position along the Stem and Extraction Methods. Ind. Crops Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Farokhi, F.; Pierre, S.J.; Villeneuve, E. Cold Extraction Method for Cannabinoids and Terpenes from Cannabis by Polyunsaturated Lipid-Based Solvents. Patent WO 2020/028991 A1, 13 February 2020. [Google Scholar]
- Tiago, F.J.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Extraction of Bioactive Compounds From Cannabis sativa L. Flowers and/or Leaves Using Deep Eutectic Solvents. Front. Nutr. 2022, 9, 892314. [Google Scholar] [CrossRef]
- López-Olmos, C.; García-Valverde, M.T.; Hidalgo, J.; Ferrerio-Vera, C.; Sánchez de Medina, V. Comprehensive Comparison of Industrial Cannabinoid Extraction Techniques: Evaluation of the Most Relevant Patents and Studies at Pilot Scale. Front. Nat. Prod. 2022, 1, 1043147. [Google Scholar] [CrossRef]
- Kaur, J.; Sun, N.; Hill, J.E. Comprehensive Profiling of Terpenes and Terpenoids in Different Cannabis Strains Using GC × GC-TOFMS. Separations 2023, 10, 500. [Google Scholar] [CrossRef]
- Koltai, H.; Namdar, D. Cannabis Phytomolecule “Entourage”: From Domestication to Medical Use. Trends Plant Sci. 2020, 25, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Benito, S.; Moreno, E.; Seijo-Vila, M.; Tundidor, I.; Andradas, C.; Caffarel, M.M.; Caro-Villalobos, M.; Urigüen, L.; Diez-Alarcia, R.; Moreno-Bueno, G.; et al. Therapeutic Targeting of HER2–CB2R Heteromers in HER2-Positive Breast Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3863–3872. [Google Scholar] [CrossRef]
- Li, D.; Ilnytskyy, Y.; Ghasemi Gojani, E.; Kovalchuk, O.; Kovalchuk, I. Analysis of Anti-Cancer and Anti-Inflammatory Properties of 25 High-THC Cannabis Extracts. Molecules 2022, 27, 6057. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Mariano, A.; Gullì, M.; Fraschetti, C.; Vitalone, A.; Filippi, A.; Mannina, L.; Scotto d’Abusco, A.; Di Sotto, A. Role of Caryophyllane Sesquiterpenes in the Entourage Effect of Felina 32 Hemp Inflorescence Phytocomplex in Triple Negative MDA-MB-468 Breast Cancer Cells. Molecules 2021, 26, 6688. [Google Scholar] [CrossRef]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids Induce Cancer Cell Proliferation via Tumor Necrosis Factor α-Converting Enzyme (TACE/ADAM17)-Mediated Transactivation of the Epidermal Growth Factor Receptor. Cancer Res. 2004, 64, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Gironella, M.; Lorente, M.; Garcia, S.; Guzmán, M.; Velasco, G.; Iovanna, J.L. Cannabinoids Induce Apoptosis of Pancreatic Tumor Cells via Endoplasmic Reticulum Stress–Related Genes. Cancer Res. 2006, 66, 6748–6755. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cinquina, V.; Valenti, M.; Bolognini, D.; Gariboldi, M.; Monti, E.; Rubino, T.; Parolaro, D. Cannabidiol, a Non-Psychoactive Cannabinoid Compound, Inhibits Proliferation and Invasion in U87-MG and T98G Glioma Cells through a Multitarget Effect. PLoS ONE 2013, 8, e76918. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-Talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Świtalska, M.; Wietrzyk, J.; Wawrzenczyk, C. Synthesis of a Natural γ-Butyrolactone from Nerylacetone by Acremonium roseum and Fusarium oxysporum Cultures. Nat. Prod. Commun. 2011, 6, 367–370. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Gładkowski, W.; Świtalska, M.; Wietrzyk, J.; Szumny, A.; Gębarowska, E.; Wawrzeńczyk, C. Dehalogenation Activity of Selected Fungi Toward D-Iodo-c-Lactone Derived from trans,trans-Farnesol. Chem. Biodivers. 2016, 13, 477–482. [Google Scholar] [CrossRef]
- Nevozhay, D. Cheburator Software for Automatically Calculating Drug Inhibitory Concentrations from In Vitro Screening Assays. PLoS ONE 2014, 9, e106186. [Google Scholar] [CrossRef] [PubMed]
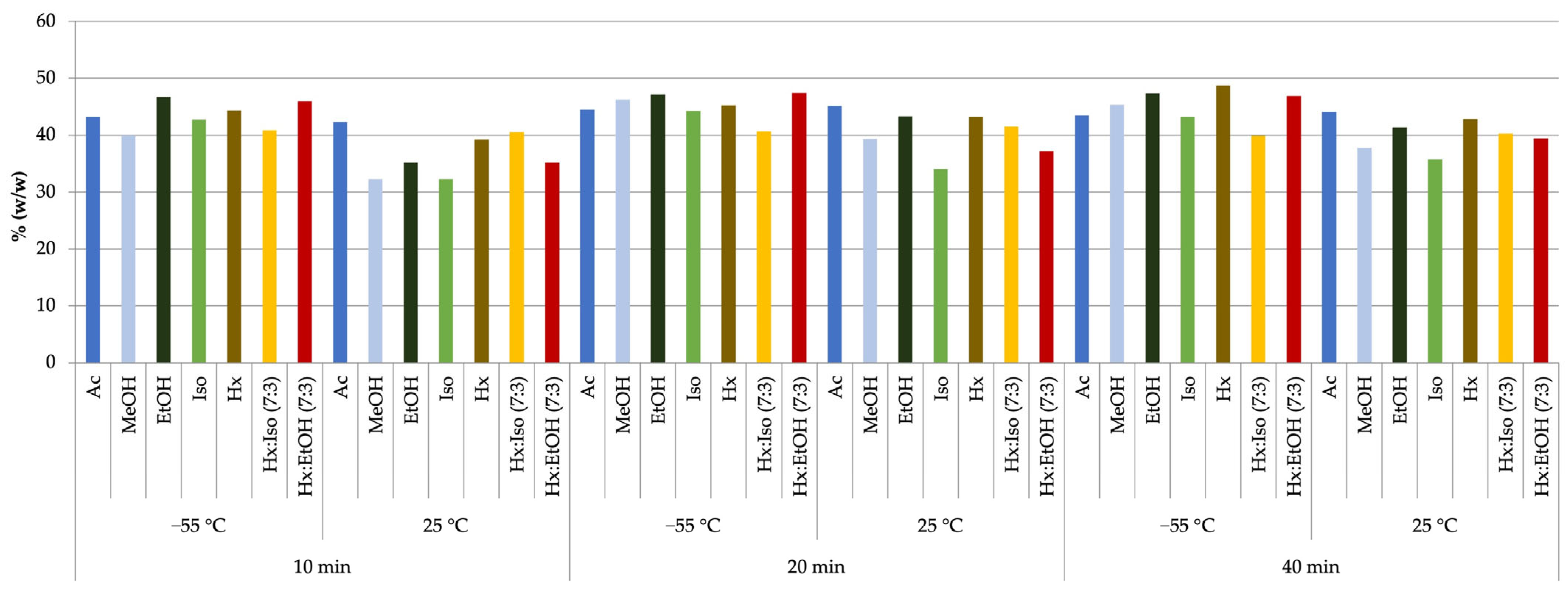

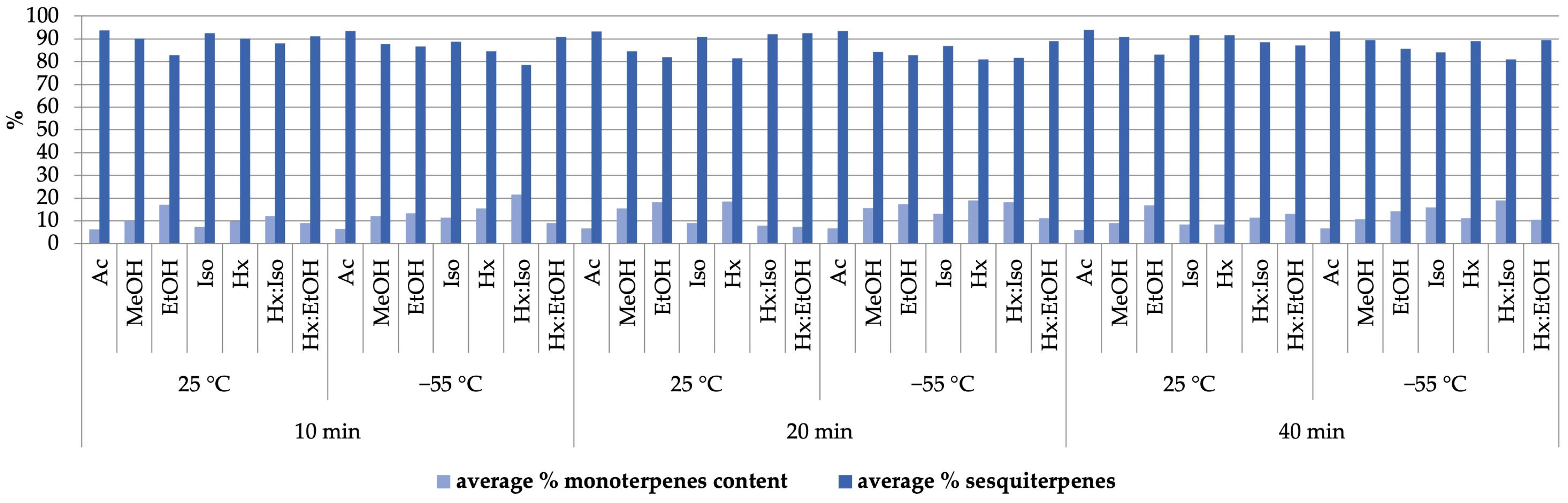
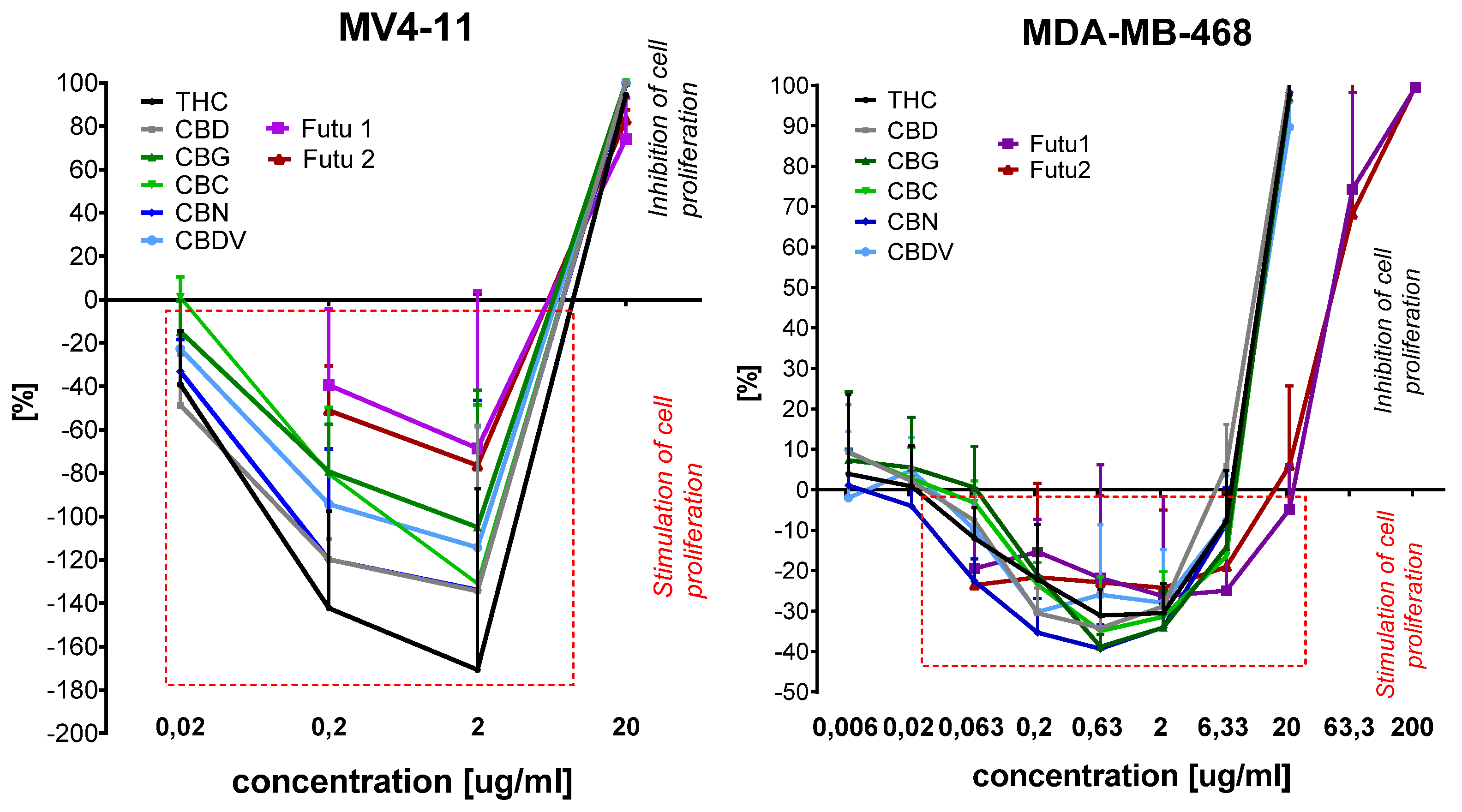
| Solvent | Temp. [°C] | Pressure [bar] | THC % Mean ± SD | CBD % Mean ± SD | CBG % Mean ± SD | CBC % Mean ± SD | CBN % Mean ± SD | % Total Cannabinoids |
|---|---|---|---|---|---|---|---|---|
| Ac | 25 | 1 | 1.10 ± 0.02 | 42.44 ± 0.51 | 1.28 ± 0.05 | 1.96 ± 0.03 | 1.09 ± 0.02 | 47.87 |
| 2 | 1.03 ± 0.02 | 39.02 ± 0.41 | 1.16 ± 0.05 | 1.80 ± 0.02 | 0.99 ± 0.01 | 44.00 | ||
| −55 | 1 | 1.07 ± 0.03 | 42.65 ± 0.15 | 1.36 ± 0.08 | 1.99 ± 0.03 | 1.11 ± 0.02 | 48.19 | |
| 2 | 1.01 ± 0.02 | 39.65 ± 0.39 | 1.12 ± 0.03 | 1.85 ± 0.02 | 1.03 ± 0.01 | 44.66 | ||
| MeOH | 25 | 1 | 0.90 ± 0.05 | 35.01 ± 0.10 | 1.35 ± 0.08 | 1.58 ± 0.12 | 1.01 ± 0.01 | 39.85 |
| 2 | 0.96 ± 0.09 | 36.84 ± 0.14 | 1.43 ± 0.12 | 1.68 ± 0.16 | 1.11 ± 0.02 | 42.01 | ||
| −55 | 1 | 0.79 ± 0.04 | 34.80 ± 0.03 | 0.99 ± 0.02 | 2.02 ± 0.21 | 1.35 ± 0.03 | 39.95 | |
| 2 | 0.82 ± 0.04 | 36.57 ± 0.07 | 1.03 ± 0.04 | 2.13 ± 0.28 | 1.47 ± 0.05 | 42.02 | ||
| EtOH | 25 | 1 | 1.05 ± 0.02 | 40.03 ± 0.42 | 1.45 ± 0.03 | 1.52 ± 0.03 | 1.11 ± 0.02 | 45.16 |
| 2 | 1.24 ± 0.00 | 42.56 ± 0.56 | 1.59 ± 0.09 | 1.63 ± 0.05 | 1.20 ± 0.00 | 48.22 | ||
| −55 | 1 | 0.85 ± 0.03 | 39.98 ± 0.35 | 1.05 ± 0.04 | 1.72 ± 0.06 | 0.94 ± 0.03 | 44.54 | |
| 2 | 0.97 ± 0.07 | 42.98 ± 0.42 | 1.16 ± 0.05 | 1.86 ± 0.08 | 1.03 ± 0.00 | 48.01 | ||
| Iso | 25 | 1 | 0.82 ± 0.02 | 37.35 ± 0.05 | 1.35 ± 0.02 | 1.82 ± 0.10 | 1.15 ± 0.03 | 42.49 |
| 2 | 0.94 ± 0.03 | 38.25 ± 0.08 | 1.50 ± 0.05 | 1.91 ± 0.19 | 1.33 ± 0.02 | 43.93 | ||
| −55 | 1 | 1.02 ± 0.07 | 36.72 ± 0.38 | 1.69 ± 0.06 | 1.82 ± 0.09 | 1.02 ± 0.01 | 42.27 | |
| 2 | 1.21 ± 0.20 | 37.81 ± 0.43 | 1.91 ± 0.08 | 1.95 ± 0.15 | 1.16 ± 0.02 | 44.03 | ||
| Hx | 25 | 1 | 1.35 ± 0.09 | 43.39 ± 0.09 | 1.07 ± 0.04 | 2.10 ± 0.06 | 1.14 ± 0.03 | 49.05 |
| 2 | 1.20 ± 0.07 | 37.03 ± 0.12 | 0.95 ± 0.03 | 1.95 ± 0.02 | 1.10 ± 0.05 | 42.23 | ||
| −55 | 1 | 1.34 ± 0.09 | 42.92 ± 0.45 | 0.93 ± 0.05 | 2.17 ± 0.21 | 1.25 ± 0.09 | 48.61 | |
| 2 | 1.15 ± 0.09 | 36.45 ± 0.28 | 0.87 ± 0.03 | 1.89 ± 0.17 | 1.01 ± 0.03 | 41.37 | ||
| Hx:Iso 7:3 | 25 | 1 | 1.21 ± 0.10 | 43.14 ± 0.75 | 1.65 ± 0.00 | 2.08 ± 0.07 | 1.37 ± 0.02 | 49.45 |
| 2 | 1.10 ± 0.05 | 41.01 ± 0.32 | 1.45 ± 0.05 | 1.82 ± 0.10 | 1.21 ± 0.04 | 46.59 | ||
| −55 | 1 | 1.25 ± 0.11 | 43.87 ± 0.32 | 1.63 ± 0.05 | 1.64 ± 0.02 | 1.10 ± 0.02 | 49.49 | |
| 2 | 1.05 ± 0.07 | 40.72 ± 0.21 | 1.50 ± 0.06 | 1.52 ± 0.03 | 1.07 ± 0.03 | 45.86 | ||
| Hx:EtOH 7:3 | 25 | 1 | 1.15 ± 0.00 | 45.96 ± 0.32 | 1.84 ± 0.09 | 1.61 ± 0.03 | 1.02 ± 0.02 | 51.57 |
| 2 | 0.95 ± 0.03 | 42.87 ± 0.39 | 1.79 ± 0.08 | 1.56 ± 0.05 | 0.99 ± 0.01 | 48.16 | ||
| −55 | 1 | 1.31 ± 0.32 | 44.90 ± 0.38 | 1.69 ± 0.03 | 1.78 ± 0.03 | 1.06 ± 0.02 | 50.74 | |
| 2 | 0.90 ± 0.21 | 42.01 ± 0.23 | 1.55 ± 0.02 | 1.64 ± 0.04 | 1.01 ± 0.01 | 47.11 |
| Compound | LRIexp1 | LRIlit2 | Content Range Min–Max [%] |
|---|---|---|---|
| α-Pinene | 933 | 933 | 0.01–1.16 |
| Benzaldehyde | 960 | 960 | 0.03–0.60 |
| Myrcene | 991 | 991 | 0.33–11.50 |
| α-Phellandrene | 1005 | 1007 | T3 |
| Unknown terpene | 1010 | 0.01–0.82 | |
| α-Terpinene | 1017 | 1018 | 0.03–0.87 |
| o-Cymene | 1024 | 1025 | 0.07–10.26 |
| Sylvestrene | 1028 | 1031 | T |
| Eucalyptol | 1031 | 1032 | 0.01–2.37 |
| γ-Terpinene | 1058 | 1058 | 0.01–6.59 |
| cis-Sabinene hydrate | 1067 | 1069 | 0.07–0.45 |
| Unknown terpene | 1072 | 0.03–0.43 | |
| p-Cymenene | 1090 | 1093 | 0.05–3.36 |
| trans-Sabinene hydrate | 1099 | 1099 | 0.03–1.42 |
| Linalool | 1101 | 1101 | 0.02–1.76 |
| Fenchol | 1114 | 1119 | 0.01–3.16 |
| trans-Pinene hydrate | 1122 | 1121 | 0.02–0.88 |
| trans-p-Mentha-2,8-diene-1-ol | 1136 | 1140 | 0.03–0.40 |
| trans-Pinocarveol | 1139 | 1141 | 0.01–0.56 |
| Ipsdienol | 1147 | 1146 | 0.01–1.82 |
| Unknown terpene | 1158 | 0.03–3.69 | |
| Borneol | 1166 | 1173 | 0.02–2.37 |
| p-Cymen-8-ol | 1186 | 1189 | 2.30–15.23 |
| α-Terpineol | 1192 | 1195 | 0.12–5.57 |
| Unknown terpene | 1198 | 0.01–0.26 | |
| Citronellol | 1229 | 1232 | 0.01–0.21 |
| α-Ylangene | 1374 | 1371 | 0.01–2.04 |
| α-Copaene | 1378 | 1375 | 0.01–0.38 |
| β-Longipinene | 1410 | 1407 | T |
| α-cis-Bergamotene | 1419 | 1416 | 0.01–2.07 |
| E-Caryophyllene | 1425 | 1424 | 0.02–40.35 |
| β-cis-Farnesene | 1435 | 1440 | 0.01–0.23 |
| α-trans-Bergamotene | 1440 | 1432 | 0.03–13.78 |
| Azulene | 1448 | 1444 | 0.01–0.24 |
| Alloaromadendrene | 1455 | 1458 | T |
| α-Humulene | 1458 | 1454 | 4.21–33.50 |
| Sesquisabinene | 1461 | 1455 | 3.33–17.14 |
| 9-epi-(E)-Caryophyllene | 1466 | 1464 | 0.01–2.54 |
| γ-Gurjunene | 1481 | 1476 | 0.01–0.55 |
| α-Amorphene | 1484 | 1482 | 0.01–0.58 |
| β-Chamigrene | 1488 | 1479 | 0.01–1.16 |
| β-Selinene | 1490 | 1492 | 0.01–3.06 |
| Unknown sesquiterpene | 1494 | 0.03–5.24 | |
| α-Selinene | 1499 | 1501 | 0.01–3.36 |
| β-Bisabolene | 1512 | 1508 | 0.01–5.87 |
| β-Curcumene | 1515 | 1511 | 0.01–1.06 |
| γ-Cadinene | 1518 | 1512 | 0.01–0.90 |
| 7-epi-α-Selinene | 1523 | 1518 | T |
| β-Guaiene | 1526 | 1523 | T |
| Unknown sesquiterpene | 1528 | 0.03–3.32 | |
| Selina-4(15),7(11)-diene | 1541 | 1540 | 0.02–2.98 |
| Unknown sesquiterpene | 1543 | 0.01–1.29 | |
| Selina-3,7(11)-diene | 1548 | 1546 | 0.01–2.58 |
| (E)-Nerolidol | 1567 | 1561 | 0.01–0.78 |
| Caryophyllene oxide | 1589 | 1587 | 0.02–5.51 |
| Humulene epoxide I | 1592 | 1605 | 0.01–0.60 |
| Humulene epoxide II | 1615 | 1613 | 0.01–1.78 |
| Unknown sesquiterpene | 1657 | 0.01–0.49 |
| Lp | Compound | Cell Lines IC50 [μg/mL] | ||||
|---|---|---|---|---|---|---|
| A549 | HT-29 | AGS | MCF-7 | MCF-10A | ||
| 1 | THC | 3.94 ± 0.44 | 3.73 ± 0.59 | 6.35 ± 1.35 | 8.4 ± 1.26 | 11.13 ± 0.72 |
| 2 | CBD | 2.87 ± 0.24 | 3.04 ± 0.52 | 3.74 ± 0.28 | 3.67 ± 0.55 | 10.55 ± 0.63 |
| 3 | CBG | 3.71 ± 0.46 | 10.04 ± 1.14 | 6.11 ± 1.31 | 7.43 ± 1.43 | 10.74 ± 0.71 |
| 4 | CBC | 3.26 ± 0.20 | 5.94 ± 1.86 | 5.53 ± 0.88 | 7.56 ± 1.52 | 10.53 ± 0.46 |
| 5 | CBN | 3.30 ± 0.15 | 6.07 ± 2.11 | 6.18 ± 0.98 | 5.79 ± 1.79 | 10.8 ± 0.68 |
| 6 | CBDV | 6.22 ± 2.26 | 3.6 ± 0.4 | 8.4 ± 1.98 | 8.43 ± 2.05 | 11.24 ± 0.26 |
| 7 | Futu1 | 10.04 ± 1.04 | 9.92 ± 1.79 | 15.41 ± 1.55 | 12.8 ± 2.72 | 58.0 ± 20.26 |
| 8 | Futu2 | 9.34 ± 3.36 | 7.31 ± 2.24 | 10.34 ± 2.99 | 9.01 ± 2.94 | 31.8 ± 11.25 |
| Lp | Compounds | Cell Line/Calculated Selectivity Index SI | |||
|---|---|---|---|---|---|
| A549 | HT-29 | AGS | MCF-7 | ||
| 1 | THC | 2.82 | 2.98 | 1.75 | 1.33 |
| 2 | CBD | 3.68 | 3.47 | 2.82 | 2.87 |
| 3 | CBG | 2.89 | 1.07 | 1.76 | 1.45 |
| 4 | CBC | 3.23 | 1.77 | 1.9 | 1.39 |
| 5 | CBN | 3.27 | 1.78 | 1.75 | 1.87 |
| 6 | CBDV | 1.81 | 3.12 | 1.34 | 1.33 |
| 7 | Futu1 | 5.8 | 5.85 | 3.76 | 4.53 |
| 8 | Futu2 | 3.4 | 4.45 | 3.08 | 3.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haczkiewicz, M.; Świtalska, M.; Łyczko, J.; Pluta, M.; Wietrzyk, J.; Gliszczyńska, A. Extraction of Cannabinoids and Terpenes from Hemp Flowers and Leaves (Cannabis sativa L., Futura 75): Chemical Profiling and Evaluation of Anticancer Properties. Molecules 2025, 30, 1325. https://doi.org/10.3390/molecules30061325
Haczkiewicz M, Świtalska M, Łyczko J, Pluta M, Wietrzyk J, Gliszczyńska A. Extraction of Cannabinoids and Terpenes from Hemp Flowers and Leaves (Cannabis sativa L., Futura 75): Chemical Profiling and Evaluation of Anticancer Properties. Molecules. 2025; 30(6):1325. https://doi.org/10.3390/molecules30061325
Chicago/Turabian StyleHaczkiewicz, Monika, Marta Świtalska, Jacek Łyczko, Magdalena Pluta, Joanna Wietrzyk, and Anna Gliszczyńska. 2025. "Extraction of Cannabinoids and Terpenes from Hemp Flowers and Leaves (Cannabis sativa L., Futura 75): Chemical Profiling and Evaluation of Anticancer Properties" Molecules 30, no. 6: 1325. https://doi.org/10.3390/molecules30061325
APA StyleHaczkiewicz, M., Świtalska, M., Łyczko, J., Pluta, M., Wietrzyk, J., & Gliszczyńska, A. (2025). Extraction of Cannabinoids and Terpenes from Hemp Flowers and Leaves (Cannabis sativa L., Futura 75): Chemical Profiling and Evaluation of Anticancer Properties. Molecules, 30(6), 1325. https://doi.org/10.3390/molecules30061325








