Astaxanthin Mitigates ADHD Symptoms in Spontaneously Hypertensive Rats via Dopaminergic Modulation and Brain–Gut Axis Regulation
Abstract
1. Introduction
2. Results and Discussion
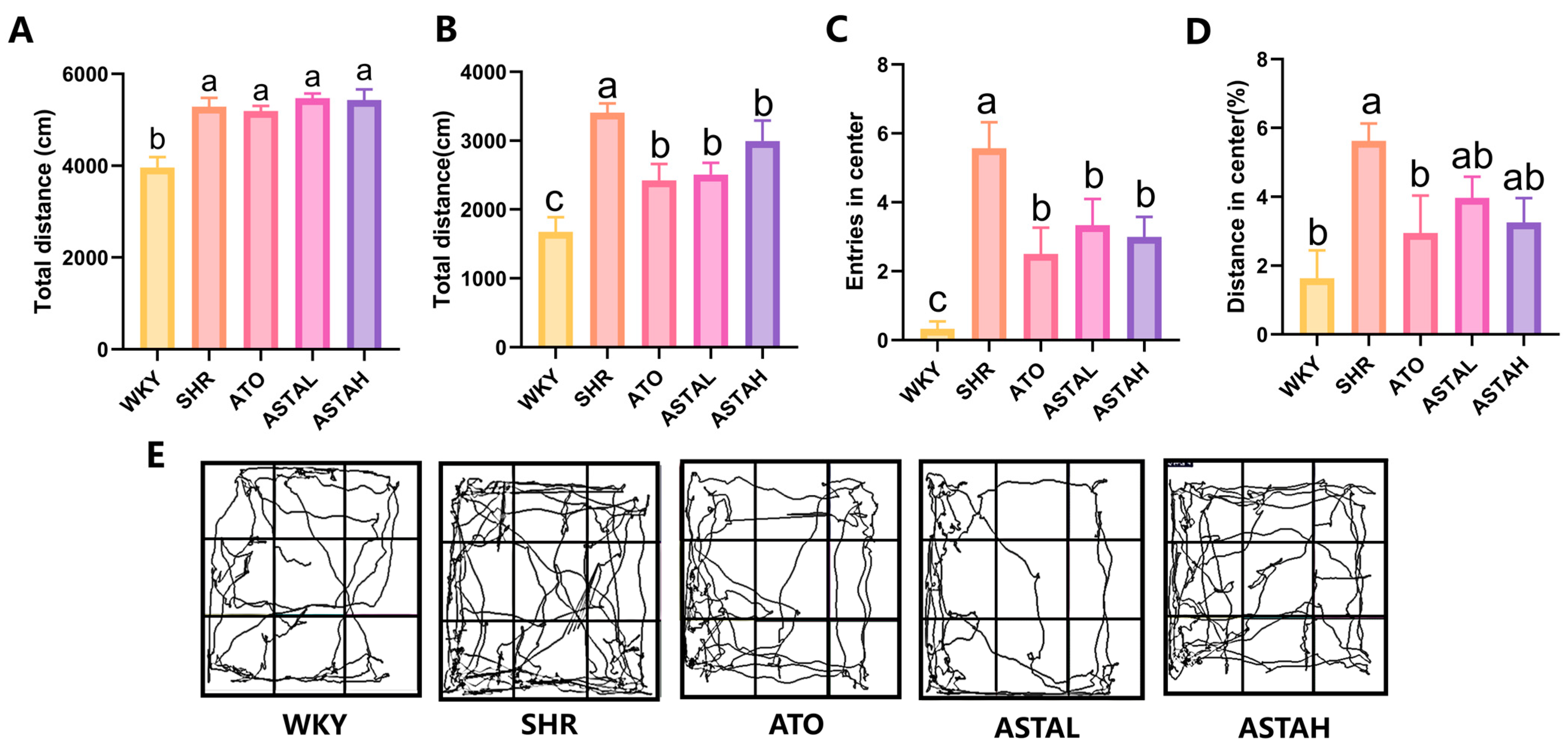
2.1. Alleviation of Hyperactivity and Anxiety Behavior by ASTA
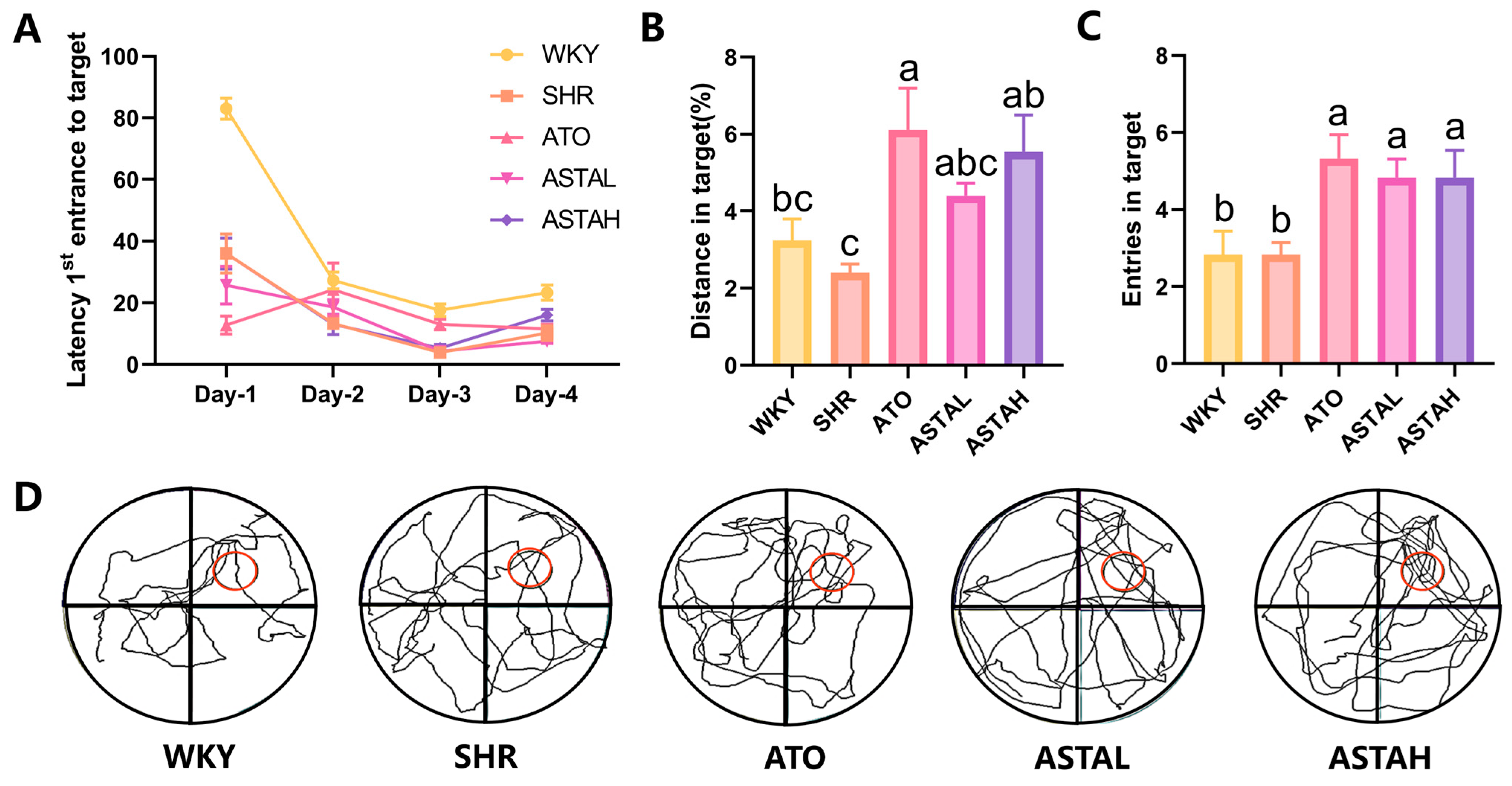
2.2. Effect of ASTA on Spatial Memory Ability
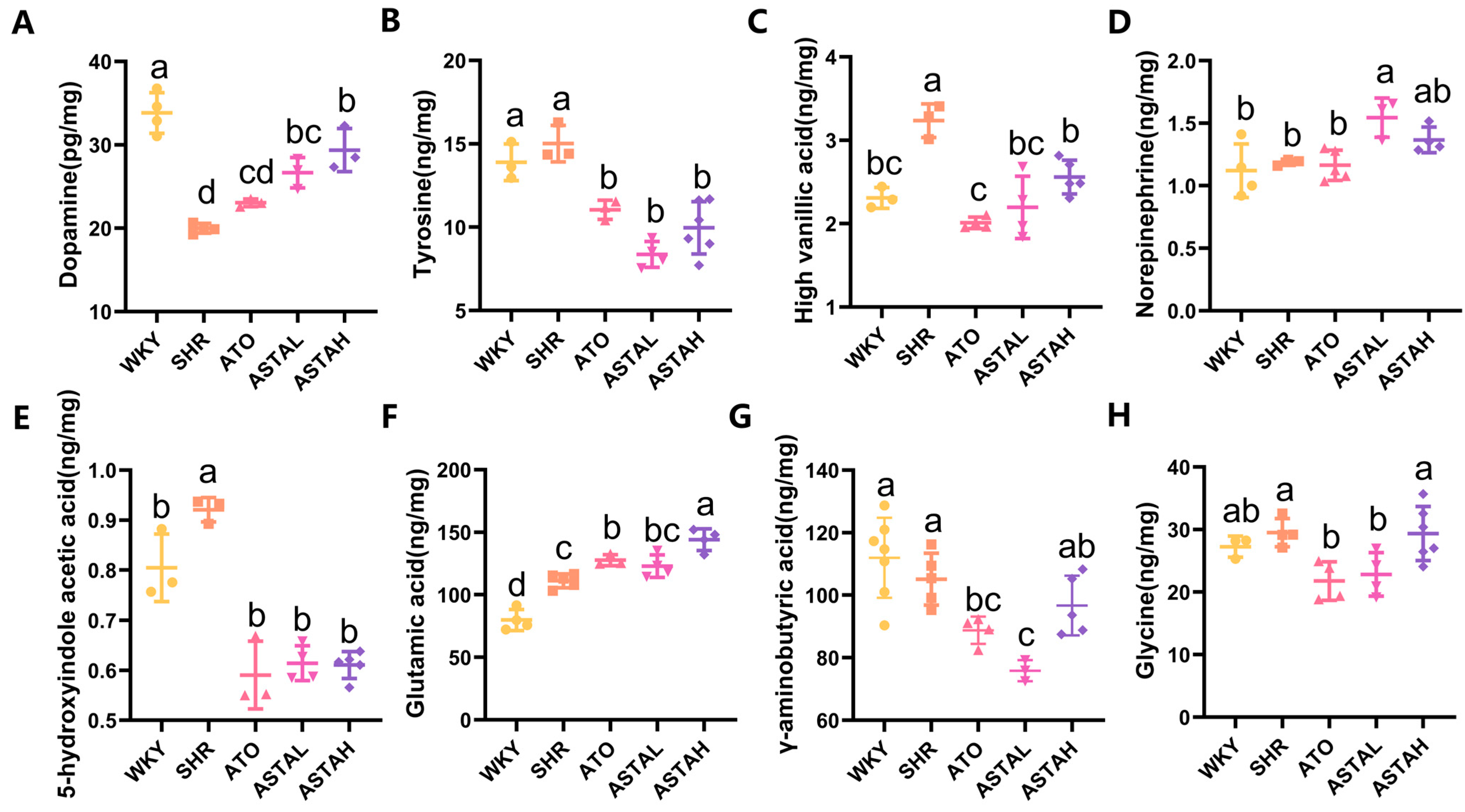
2.3. Regulation of Multiple Neurotransmitter Levels by ASTA
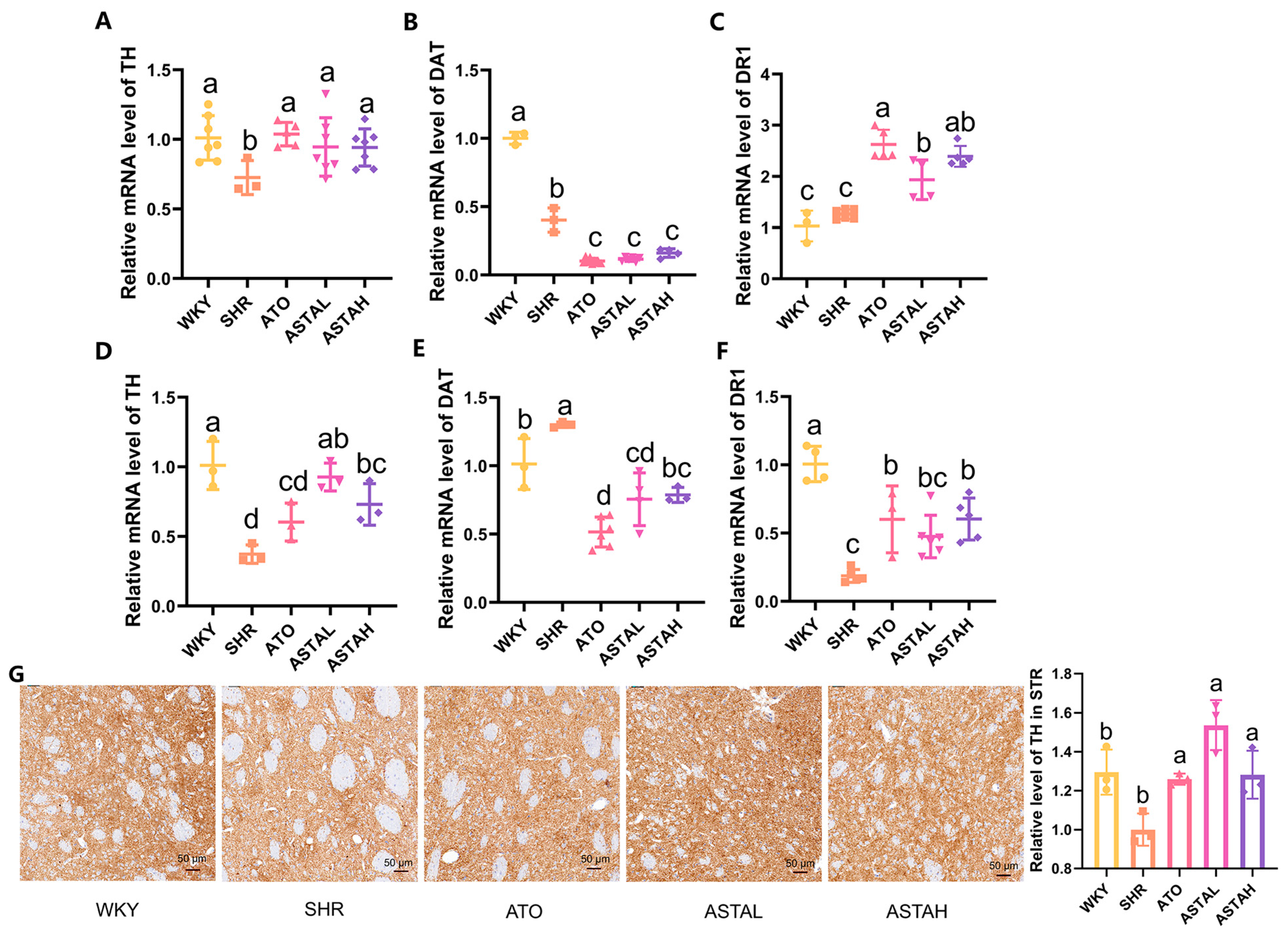
2.4. Regulation of the Dopamine System by ASTA
2.5. Regulation of Neural Pathway Factor Transcription Levels by ASTA
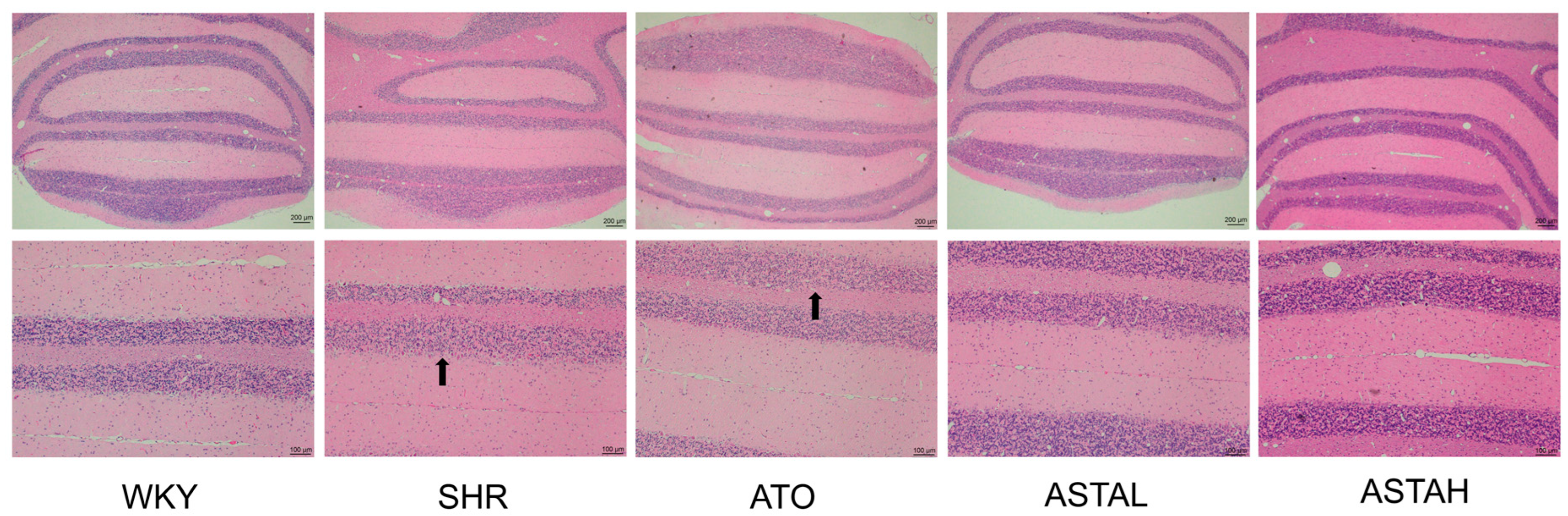
2.6. Histopathological Alterations in the Cerebellum Induced by ASTA
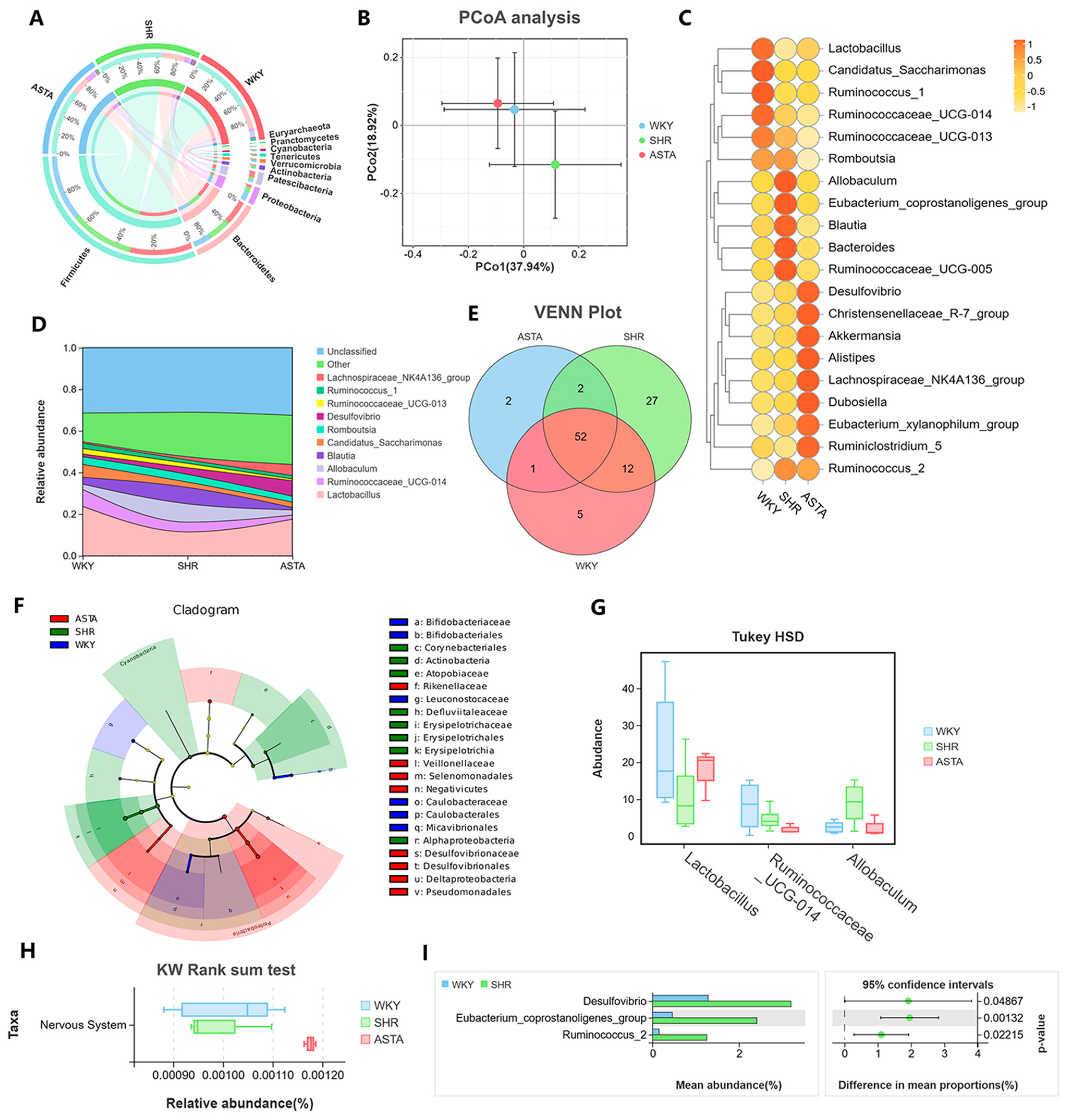
2.7. Regulation of Intestinal Microbiota by ASTA
3. Materials and Methods
3.1. Reagents
3.2. Animals and Drug Administration
3.3. Behavioral Tests
3.4. Liquid Chromatography–Mass Spectrometry (LC/MS) Analysis
3.5. Quantitative Real-Time PCR
3.6. Histological Staining and Immunohistochemical Staining
3.7. Analysis of Gut Microbiota
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention Deficit Hyperactivity Disorder |
| ASTA | astaxanthin |
| OFT | open field test |
| MWM | Morris water maze |
| DA | dopamine |
| DR1 | dopamine receptor 1 |
| DAT | dopamine transporter |
| TH | tyrosine hydroxylase |
| SNAP-25 | synaptic-associated protein 25 |
| SERT | serotonin transporter |
| GDNF | glial-cell-line-derived neurotrophic factor |
| 5-CSRTT | 5-choice serial reaction time test |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| ALS | Amyotrophic Lateral Sclerosis |
| HD | Huntington’s disease |
| SHR | spontaneously hypertensive rat |
| WKY | Wistar Kyoto |
| Tyr | tyrosine |
| HVA | homovanillic acid |
| NE | norepinephrine |
| 5-HIAA | 5-hydroxyindoleacetic acid |
| Ach | acetylcholine |
| Glu | glutamate |
| GABA | γ-aminobutyric acid |
| Gly | glycine |
| PFA | paraformaldehyde |
| PFC | prefrontal cortex |
| STR | striatum |
| LC/MS | liquid chromatography–mass spectrometry |
| IS | internal standard |
| ESI | electrospray ionization |
| VMAT-2 | vesicular monoamine transporter-2 |
| H&E | hematoxylin and eosin |
| MAO-B | Monoamine Oxidase B |
| LTP | long-term potentiation |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| IHC | immunohistochemistry |
| CE | cerebellum |
| DOAJ | Directory of Open Access Journals |
| TLA | three-letter acronym |
| LD | linear dichroism |
References
- Kohn, M.; Griffiths, K. Attention Deficit Hyperactivity Disorder (ADHD). In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021; p. B9780128188729000169. [Google Scholar]
- Posner, J.; Polanczyk, G.V.; Sonuga-Barke, E. Attention-Deficit Hyperactivity Disorder. Lancet 2020, 395, 450–462. [Google Scholar] [CrossRef]
- Santos, S.; Ferreira, H.; Martins, J.; Gonçalves, J.; Castelo-Branco, M. Male Sex Bias in Early and Late Onset Neurodevelopmental Disorders: Shared Aspects and Differences in Autism Spectrum Disorder, Attention Deficit/Hyperactivity Disorder, and Schizophrenia. Neurosci. Biobehav. Rev. 2022, 135, 104577. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, X.; Zhang, Z.; Yang, L. Composition of the Gut Microbiota in Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 838941. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, X.; Song, T.; Hou, X. Safety and Efficacy of Antioxidant Therapy in Children and Adolescents with Attention Deficit Hyperactivity Disorder: A Systematic Review and Network Meta-Analysis. PLoS ONE 2024, 19, e0296926. [Google Scholar] [CrossRef]
- Grosso, C.; Santos, M.; Barroso, M.F. From Plants to Psycho-Neurology: Unravelling the Therapeutic Benefits of Bioactive Compounds in Brain Disorders. Antioxidants 2023, 12, 1603. [Google Scholar] [CrossRef] [PubMed]
- Leffa, D.T.; Panzenhagen, A.C.; Salvi, A.A.; Bau, C.H.D.; Pires, G.N.; Torres, I.L.S.; Rohde, L.A.; Rovaris, D.L.; Grevet, E.H. Systematic Review and Meta-Analysis of the Behavioral Effects of Methylphenidate in the Spontaneously Hypertensive Rat Model of Attention-Deficit/Hyperactivity Disorder. Neurosci. Biobehav. Rev. 2019, 100, 166–179. [Google Scholar] [CrossRef]
- Quansah, E.; Ruiz-Rodado, V.; Grootveld, M.; Zetterström, T.S.C. Methylphenidate Alters Monoaminergic and Metabolic Pathways in the Cerebellum of Adolescent Rats. Eur. Neuropsychopharmacol. 2018, 28, 513–528. [Google Scholar] [CrossRef]
- Fu, D.; Wu, D.-D.; Guo, H.-L.; Hu, Y.-H.; Xia, Y.; Ji, X.; Fang, W.-R.; Li, Y.-M.; Xu, J.; Chen, F.; et al. The Mechanism, Clinical Efficacy, Safety, and Dosage Regimen of Atomoxetine for ADHD Therapy in Children: A Narrative Review. Front. Psychiatry 2022, 12, 780921. [Google Scholar] [CrossRef]
- Rosi, E.; Grazioli, S.; Villa, F.M.; Mauri, M.; Gazzola, E.; Pozzi, M.; Molteni, M.; Nobile, M. Use of Non-Pharmacological Supplementations in Children and Adolescents with Attention Deficit/Hyperactivity Disorder: A Critical Review. Nutrients 2020, 12, 1573. [Google Scholar] [CrossRef]
- Weyns, A.-S.; Ahannach, S.; Van Rillaer, T.; De Bruyne, T.; Lebeer, S.; Hermans, N. Enhancing Pediatric Attention-Deficit Hyperactivity Disorder Treatment: Exploring the Gut Microbiota Effects of French Maritime Pine Bark Extract and Methylphenidate Intervention. Front. Nutr. 2024, 11, 1422253. [Google Scholar] [CrossRef]
- Balendra, V.; Singh, S.K. Therapeutic Potential of Astaxanthin and Superoxide Dismutase in Alzheimer’s Disease. Open Biol. 2021, 11, 210013. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.; Park, S.J.; Park, S.M. Cholesterol Metabolism in the Brain and Its Association with Parkinson’s Disease. Exp. Neurobiol. 2019, 28, 554–567. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Kot, A.; Dufossé, L.; Gonçalves, C.N.D.P.; Pereira, J.F.B.; Santos-Ebinuma, V.C.; Raghavan, V.; Pessoa, A. Microbial Astaxanthin: From Bioprocessing to the Market Recognition. Appl. Microbiol. Biotechnol. 2023, 107, 4199–4215. [Google Scholar] [CrossRef] [PubMed]
- Regan, S.L.; Williams, M.T.; Vorhees, C.V. Review of Rodent Models of Attention Deficit Hyperactivity Disorder. Neurosci. Biobehav. Rev. 2022, 132, 621–637. [Google Scholar] [CrossRef]
- Sagvolden, T.; Metzger, M.A.; Schiorbeck, H.K.; Rugland, A.-L.; Spinnangr, I.; Sagvolden, G. The Spontaneously Hypertensive Rat (SHR) as an Animal Model of Childhood Hyperactivity (ADHD): Changed Reactivity to Reinforcers and to Psychomotor Stimulants. Behav. Neural Biol. 1992, 58, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Thanos, P.K.; Ivanov, I.; Robinson, J.K.; Michaelides, M.; Wang, G.-J.; Swanson, J.M.; Newcorn, J.H.; Volkow, N.D. Dissociation between Spontaneously Hypertensive (SHR) and Wistar–Kyoto (WKY) Rats in Baseline Performance and Methylphenidate Response on Measures of Attention, Impulsivity and Hyperactivity in a Visual Stimulus Position Discrimination Task. Pharmacol. Biochem. Behav. 2010, 94, 374–379. [Google Scholar] [CrossRef]
- Li, C.; Saliba, N.B.; Martin, H.; Losurdo, N.A.; Kolahdouzan, K.; Siddiqui, R.; Medeiros, D.; Li, W. Purkinje Cell Dopaminergic Inputs to Astrocytes Regulate Cerebellar-Dependent Behavior. Nat. Commun. 2023, 14, 1613. [Google Scholar] [CrossRef]
- Fu, J.; Li, J.; Sun, Y.; Liu, S.; Song, F.; Liu, Z. In-Depth Investigation of the Mechanisms of Schisandra Chinensis Polysaccharide Mitigating Alzheimer’s Disease Rat via Gut Microbiota and Feces Metabolomics. Int. J. Biol. Macromol. 2023, 232, 123488. [Google Scholar] [CrossRef]
- Núñez-Jaramillo, L.; Herrera-Solís, A.; Herrera-Morales, W. ADHD: Reviewing the Causes and Evaluating Solutions. J. Pers. Med. 2021, 11, 166. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Li, Z.; Liu, X.; He, J.; Zhao, Z.; He, M.; Nie, B.; Liu, Z.; Chen, Y.; et al. Overexpression of Sirt6 Ameliorates Sleep Deprivation Induced-Cognitive Impairment by Modulating Glutamatergic Neuron Function. Neural Regen. Res. 2023, 18, 2449–2458. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Hou, W.; Bi, D.; Yin, F.; Gao, Y.; Huang, D.; Li, Y.; Cao, Z.; Yan, Y.; et al. Fecal Microbiota Transplantation Improves VPA-Induced ASD Mice by Modulating the Serotonergic and Glutamatergic Synapse Signaling Pathways. Transl. Psychiatry 2023, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Y.; Cheng, Y.; Yao, W.; Qian, H. Neuroprotective Effects of Polysaccharide from Sparassis Crispa on Alzheimer’s Disease-like Mice: Involvement of Microbiota-Gut-Brain Axis. Int. J. Biol. Macromol. 2023, 225, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Coimbra, B.; Domingues, A.V.; Wezik, M.; Vieitas-Gaspar, N.; Gaspar, R.; Sousa, N.; Pinto, L.; Rodrigues, A.J.; Soares-Cunha, C. Involvement of Nucleus Accumbens D2-Medium Spiny Neurons Projecting to the Ventral Pallidum in Anxiety-like Behaviour. J. Psychiatry Neurosci. 2023, 48, E267–E284. [Google Scholar] [CrossRef]
- MacDonald, H.J.; Kleppe, R.; Szigetvari, P.D.; Haavik, J. The Dopamine Hypothesis for ADHD: An Evaluation of Evidence Accumulated from Human Studies and Animal Models. Front. Psychiatry 2024, 15, 1492126. [Google Scholar] [CrossRef]
- Kang, S.S.; Meng, L.; Zhang, X.; Wu, Z.; Mancieri, A.; Xie, B.; Liu, X.; Weinshenker, D.; Peng, J.; Zhang, Z.; et al. Tau Modification by the Norepinephrine Metabolite DOPEGAL Stimulates Its Pathology and Propagation. Nat. Struct. Mol. Biol. 2022, 29, 292–305. [Google Scholar] [CrossRef]
- Halperin, D.; Stavsky, A.; Kadir, R.; Drabkin, M.; Wormser, O.; Yogev, Y.; Dolgin, V.; Proskorovski-Ohayon, R.; Perez, Y.; Nudelman, H.; et al. CDH2 Mutation Affecting N-Cadherin Function Causes Attention-Deficit Hyperactivity Disorder in Humans and Mice. Nat. Commun. 2021, 12, 6187. [Google Scholar] [CrossRef]
- Fang, Z.; Shen, G.; Amin, N.; Lou, C.; Wang, C.; Fang, M. Effects of Neuroinflammation and Autophagy on the Structure of the Blood-Brain Barrier in ADHD Model. Neuroscience 2023, 530, 17–25. [Google Scholar] [CrossRef]
- Cabana-Domínguez, J.; Torrico, B.; Reif, A.; Fernàndez-Castillo, N.; Cormand, B. Comprehensive Exploration of the Genetic Contribution of the Dopaminergic and Serotonergic Pathways to Psychiatric Disorders. Transl. Psychiatry 2022, 12, 11. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Castells-Nobau, A.; Arnoriaga-Rodríguez, M.; Martin, M.; De La Vega-Correa, L.; Zapata, C.; Burokas, A.; Blasco, G.; Coll, C.; Escrichs, A.; et al. Microbiota Alterations in Proline Metabolism Impact Depression. Cell Metab. 2022, 34, 681–701.e10. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Wu, Q.; Fang, H.; Shao, X.; Ouyang, X.; He, Z.; Deng, Y.; Chen, C. Gut Microbiota Mediates the Susceptibility of Mice to Sepsis-Associated Encephalopathy by Butyric Acid. J. Inflamm. Res. 2022, 15, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, L.; Zhang, K.; Huang, Y.; Liu, Y.; Lu, X.; Liao, W.; Liu, X.; Zhang, Q.; Pan, W. Gut Microbiota Associated with Cryptococcal Meningitis and Dysbiosis Caused by Anti-Fungal Treatment. Front. Microbiol. 2022, 13, 1086239. [Google Scholar] [CrossRef]
- Koutzoumis, D.N.; Vergara, M.; Pino, J.; Buddendorff, J.; Khoshbouei, H.; Mandel, R.J.; Torres, G.E. Alterations of the Gut Microbiota with Antibiotics Protects Dopamine Neuron Loss and Improve Motor Deficits in a Pharmacological Rodent Model of Parkinson’s Disease. Exp. Neurol. 2020, 325, 113159. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yu, S.; Zhang, Y.; Yin, Y.; Zhou, J. Intestinal Dopamine Receptor D2 Is Required for Neuroprotection against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Dopaminergic Neurodegeneration. Neurosci. Bull. 2022, 38, 871–886. [Google Scholar] [CrossRef]
- Wu, L.; Lyu, Y.; Srinivasagan, R.; Wu, J.; Ojo, B.; Tang, M.; El-Rassi, G.D.; Metzinger, K.; Smith, B.J.; Lucas, E.A.; et al. Astaxanthin-Shifted Gut Microbiota Is Associated with Inflammation and Metabolic Homeostasis in Mice. J. Nutr 2020, 150, 2687–2698. [Google Scholar] [CrossRef]
- Tuan, W.-J.; Babinski, D.E.; Rabago, D.P.; Zgierska, A.E. Treatment with Stimulants and the Risk of COVID-19 Complications in Adults with ADHD. Brain Res. Bull 2022, 187, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, B.; Kratochvil, C.J. Pharmacotherapy of Pediatric Attention-Deficit/Hyperactivity Disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2012, 21, 941–955. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Wu, J.; Zhu, Q.; Chen, C.; Li, Y. Implications of Gut Microbiota Dysbiosis and Fecal Metabolite Changes in Psychologically Stressed Mice. Front. Microbiol. 2023, 14, 1124454. [Google Scholar] [CrossRef]
- Nunez, N.; Martinez, C.; Saurina, J.; Nunez, O. High-Performanceliquid Chromatography with Fluorescence Detection Fingerprints as Chemical Descriptors to Authenticate the Origin, Variety and Roasting Degree of Coffee by Multivariate Chemometric Methods. J. Sci. Food Agric. 2021, 101, 65–73. [Google Scholar] [CrossRef]
- Wang, L.-J.; Kuo, H.-C.; Lee, S.-Y.; Huang, L.-H.; Lin, Y.; Lin, P.-H.; Li, S.-C. MicroRNAs Serve as Prediction and Treatment-Response Biomarkers of Attention-Deficit/Hyperactivity Disorder and Promote the Differentiation of Neuronal Cells by Repressing the Apoptosis Pathway. Transl. Psychiatry 2022, 12, 67. [Google Scholar] [CrossRef]
- Satoh, R.; Kawakami, K.; Nakadate, K. Effects of Smart Drugs on Cholinergic System and Non-Neuronal Acetylcholine in the Mouse Hippocampus: Histopathological Approach. J. Clin. Med. 2022, 11, 3310. [Google Scholar] [CrossRef] [PubMed]

| Analyte | Q1 Mass (m/z) | Q3 Mass (m/z) | DP (V) | CE (V) |
|---|---|---|---|---|
| DA | 154.1 | 137.2 | 50 | 15 |
| NE | 170.1 | 152.2 | 90 | 10 |
| 5-HIAA | 192.1 | 146.1 | 60 | 20 |
| HVA | 183.1 | 137.1 | 90 | 25 |
| ACh | 146.1 | 87.1 | 80 | 20 |
| GABA | 104 | 87.1 | 30 | 15 |
| Tyr | 182 | 123 | 50 | 40 |
| Glu | 148.1 | 84.1 | 80 | 15 |
| Gly | 76 | 30 | 40 | 20 |
| IS | 137.9 | 120.1 | 25 | 25 |
| Forward Primer | Reverse Primer | |
|---|---|---|
| GAPDH | TTCACCACCATGGAGAAGGC | CTCGTGGTTCACACCCATCA |
| TH | TCCCAGGACATTGGACTTGC | AAGCCTTCAGCTCCCCATTC |
| DAT | GTCACCAACGGTGGCATCTA | AATTGCTGGACGCCGTAGAA |
| DR1 | CACCTGAGGTCCAAGGTGAC | AAGGACCCAAAGGGCCAAAA |
| SNAP-25 | AGTCACACAAGGCTACCAGC | GTGGCTTTGGAGGCAAACAG |
| SERT | CCGTCATCTGCATCCCTACC | ATGTCCCCACACGGGATTTC |
| GDNF | CGCTGACCAGTGACTCCAAT | CGCCGCTTGTTTATCTGGTG |
| VMAT-2 | CCCTGCACCTCCTCCAAATC | GATAGCTTCTGGTCCCGCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, Y.; Wu, N.; Wang, J.; Geng, L.; Yue, Y.; Zhang, Q. Astaxanthin Mitigates ADHD Symptoms in Spontaneously Hypertensive Rats via Dopaminergic Modulation and Brain–Gut Axis Regulation. Molecules 2025, 30, 1637. https://doi.org/10.3390/molecules30071637
Leng Y, Wu N, Wang J, Geng L, Yue Y, Zhang Q. Astaxanthin Mitigates ADHD Symptoms in Spontaneously Hypertensive Rats via Dopaminergic Modulation and Brain–Gut Axis Regulation. Molecules. 2025; 30(7):1637. https://doi.org/10.3390/molecules30071637
Chicago/Turabian StyleLeng, Yueyang, Ning Wu, Jing Wang, Lihua Geng, Yang Yue, and Quanbin Zhang. 2025. "Astaxanthin Mitigates ADHD Symptoms in Spontaneously Hypertensive Rats via Dopaminergic Modulation and Brain–Gut Axis Regulation" Molecules 30, no. 7: 1637. https://doi.org/10.3390/molecules30071637
APA StyleLeng, Y., Wu, N., Wang, J., Geng, L., Yue, Y., & Zhang, Q. (2025). Astaxanthin Mitigates ADHD Symptoms in Spontaneously Hypertensive Rats via Dopaminergic Modulation and Brain–Gut Axis Regulation. Molecules, 30(7), 1637. https://doi.org/10.3390/molecules30071637







