Chemistry of 2-(2′-Aminophenyl)benzothiazole Derivatives: Syntheses, Photophysical Properties and Applications
Abstract
:1. Introduction
2. Overview of the Properties and Syntheses of 2-(2′-Aminophenyl)benzothiazole Derivatives
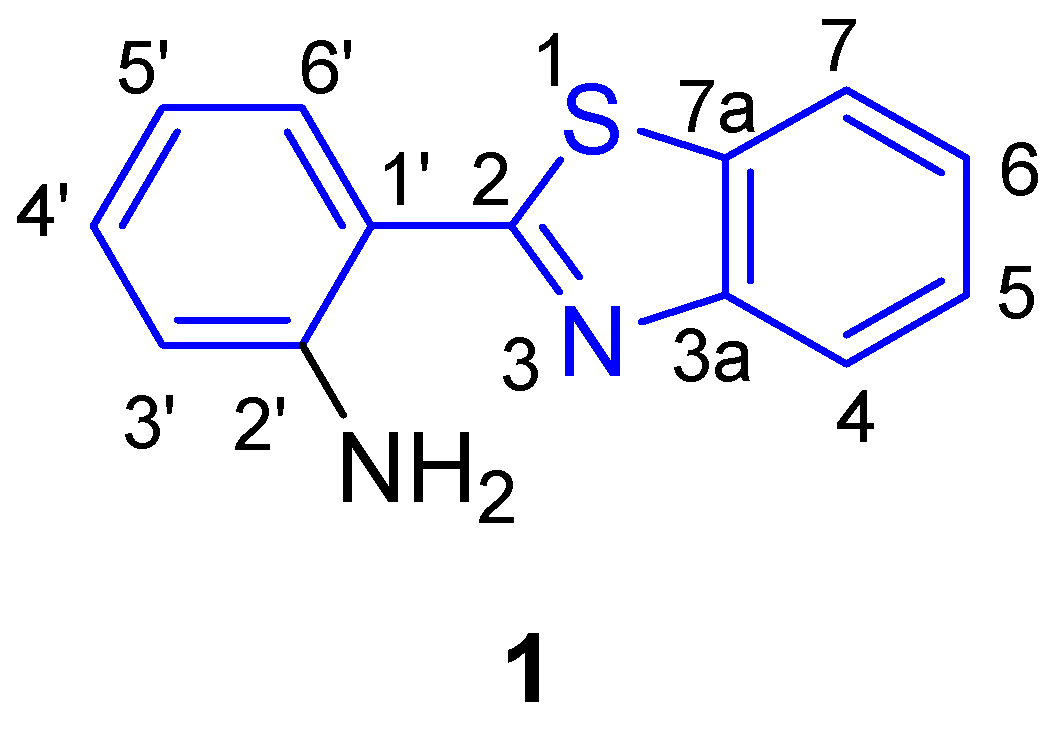
2.1. Structural Characterizations of 2-(2′-Aminophenyl)benzothiazole 1 and Its Derivatives
2.1.1. XRD Analysis
2.1.2. NMR Spectroscopy
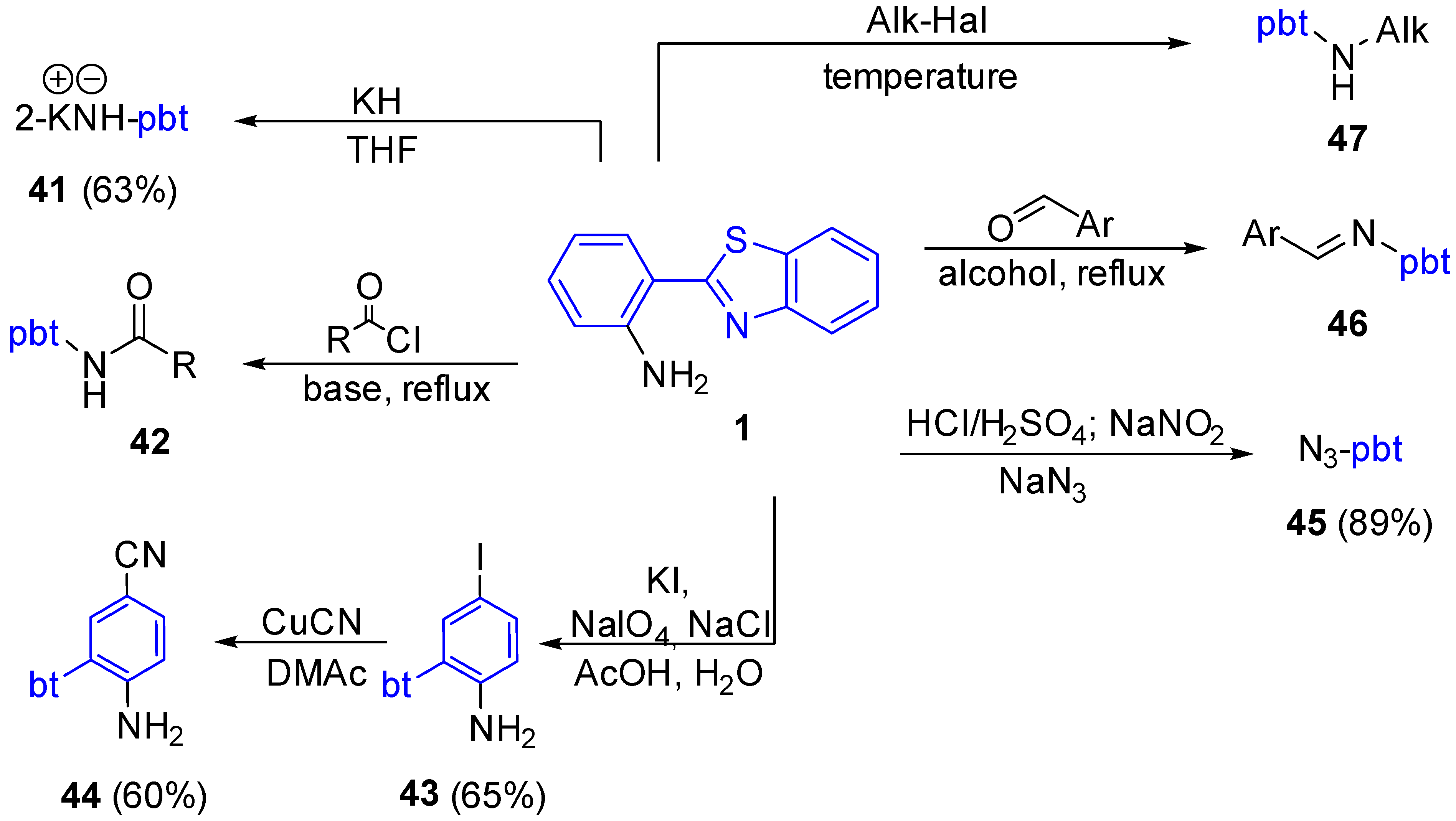
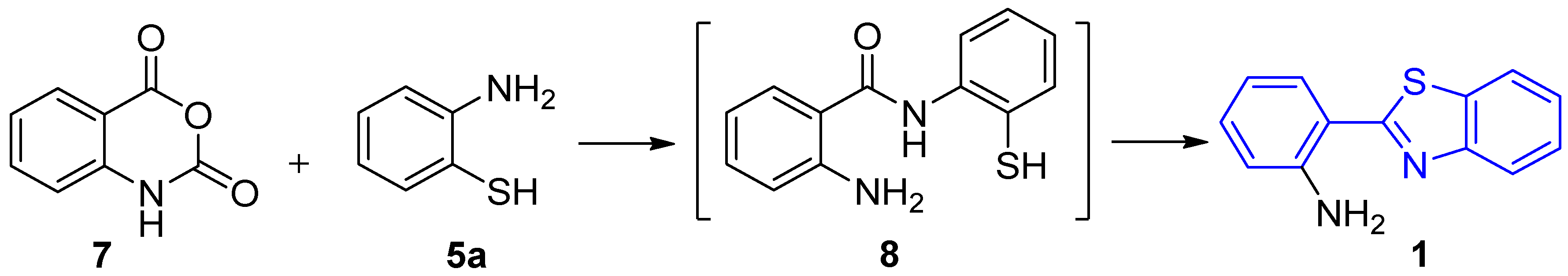
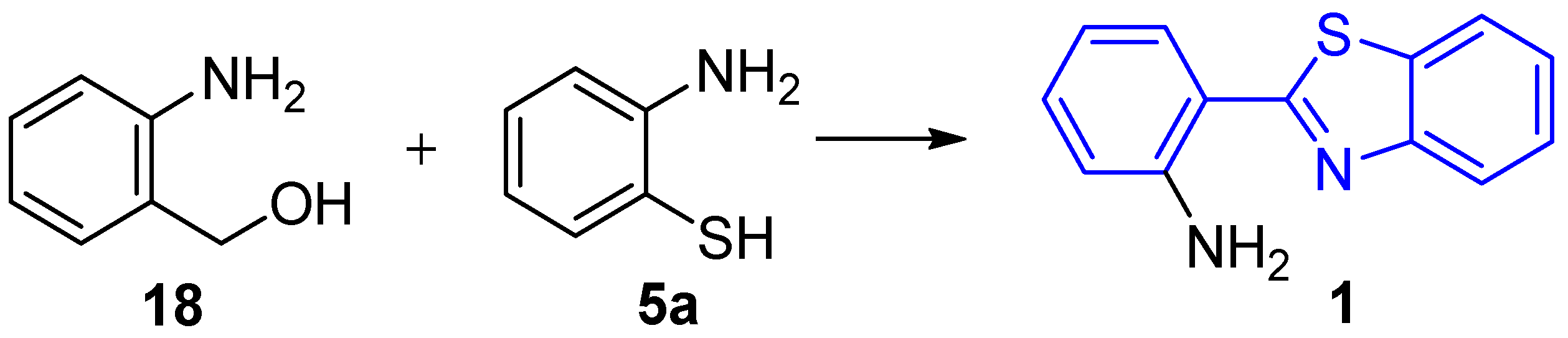
2.2. Synthesis of 2-(2′-Aminophenyl)benzothiazole and Derivatives
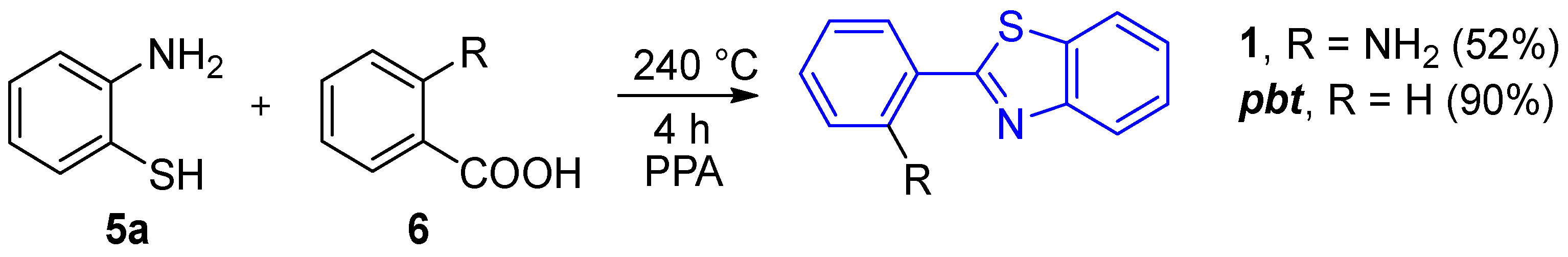
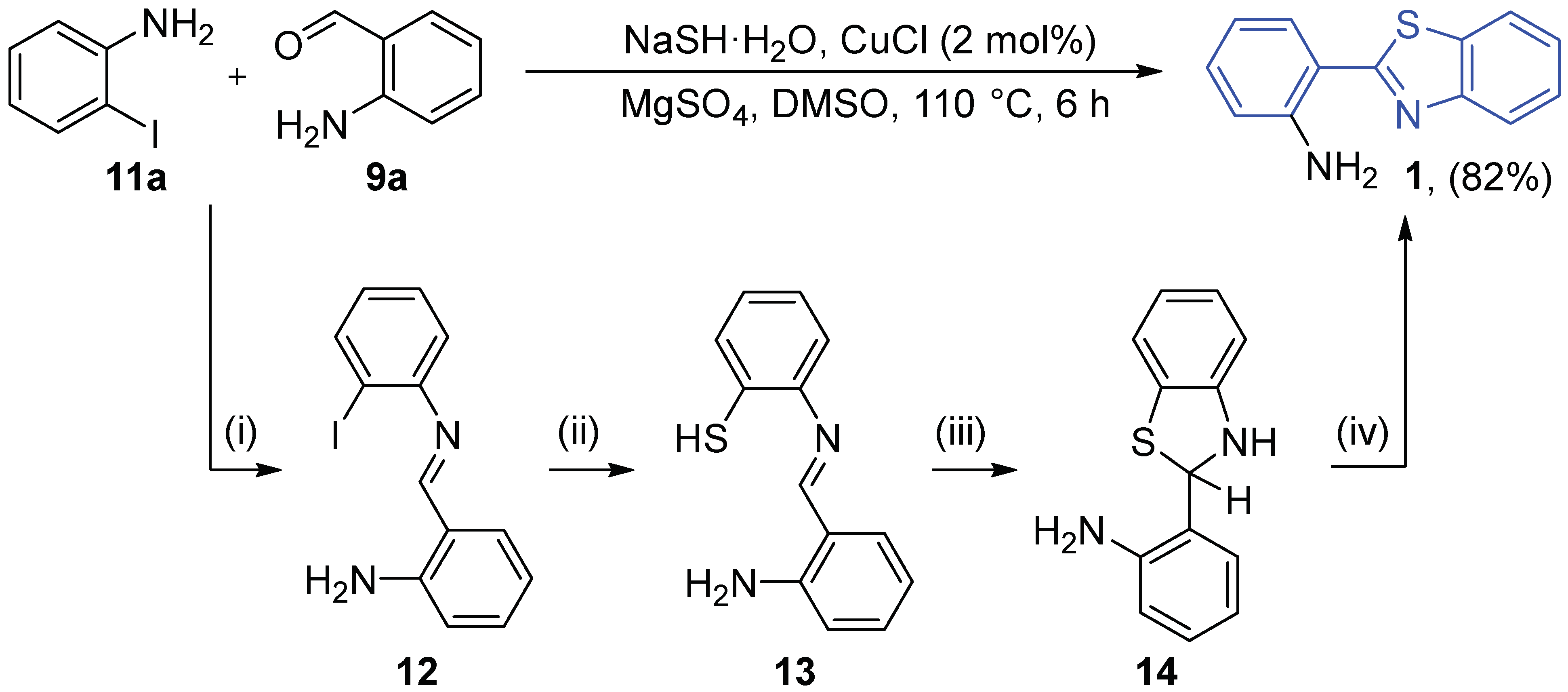
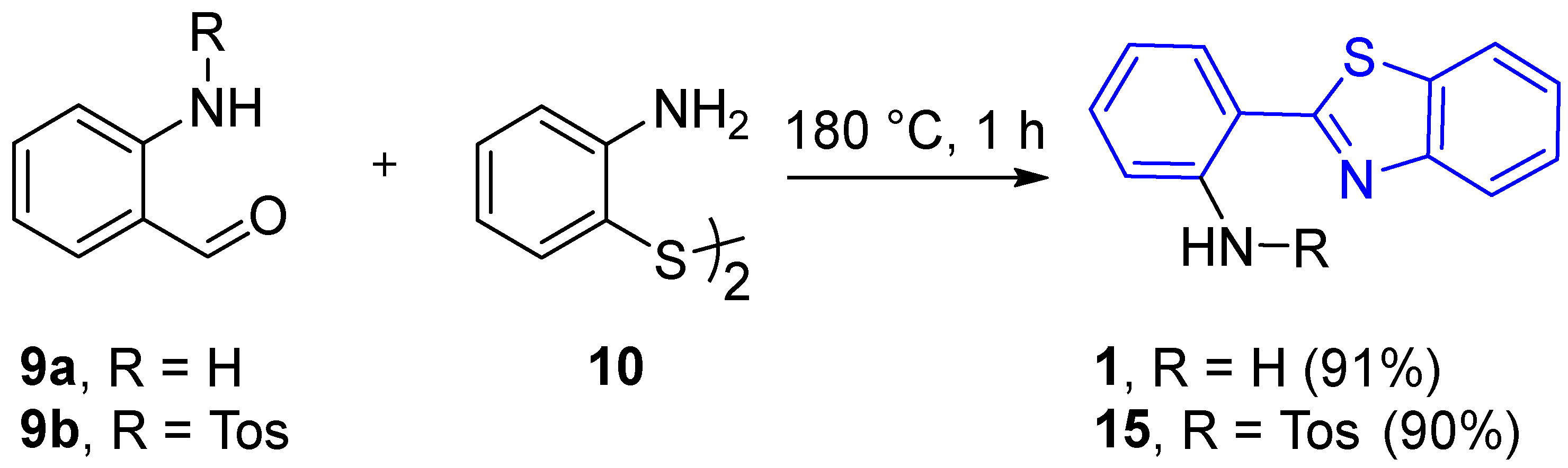
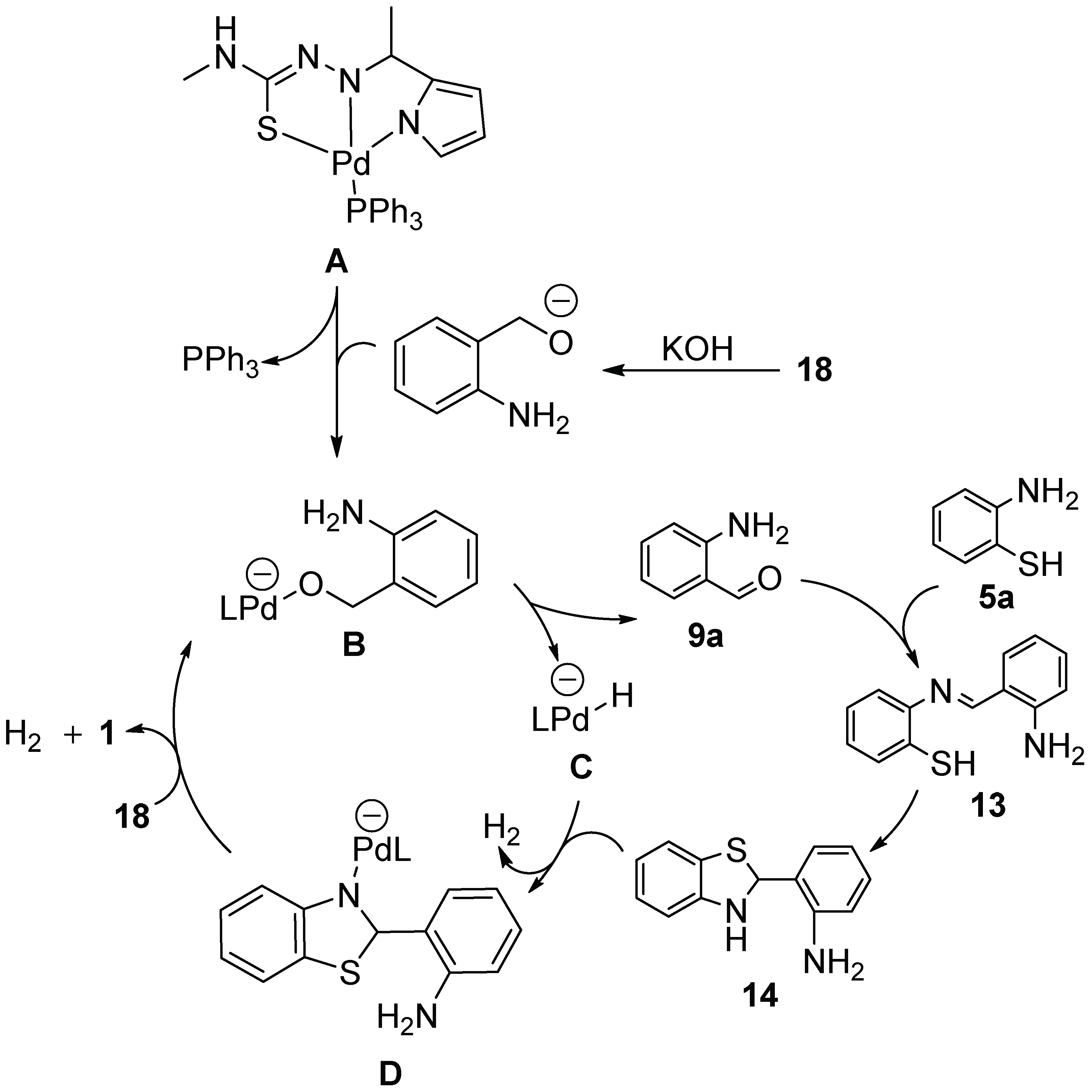
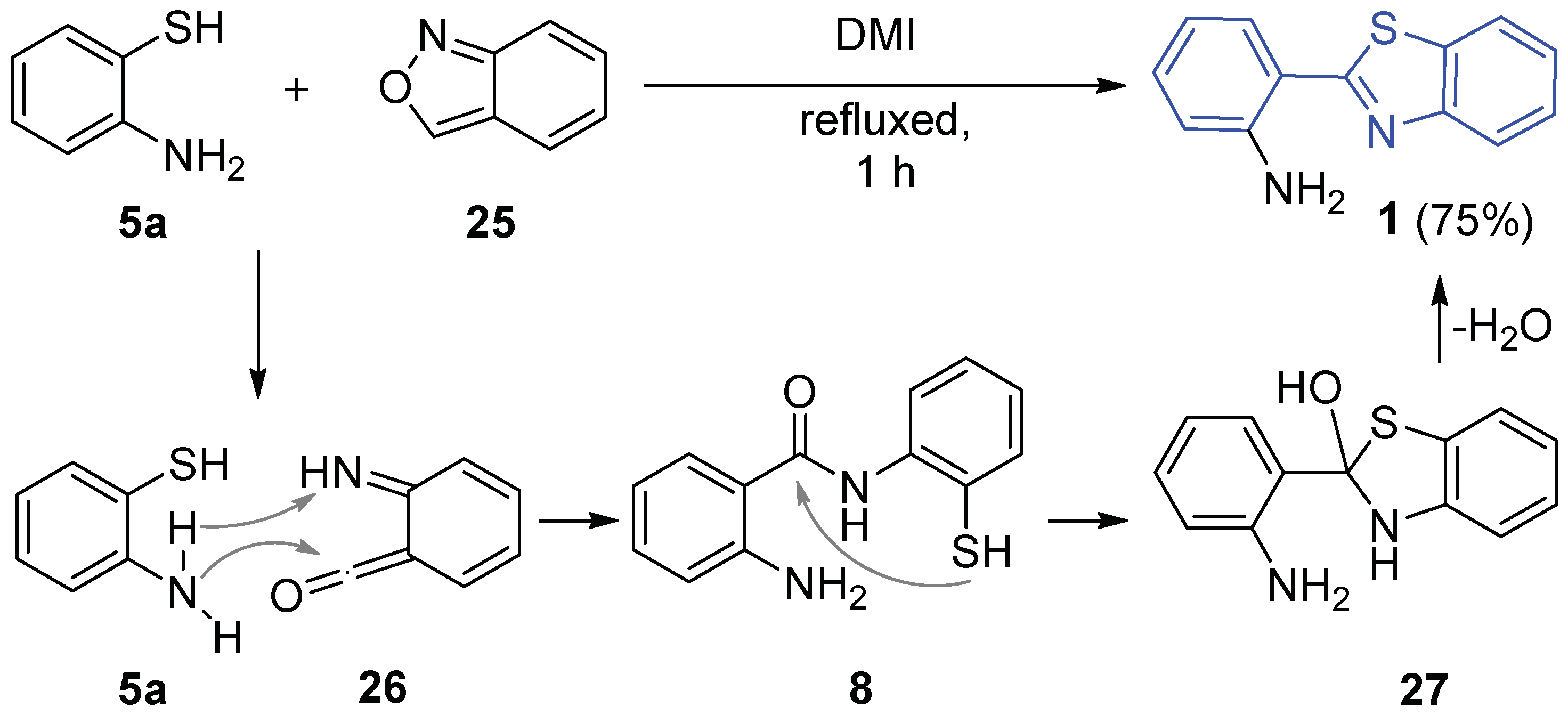
2.2.1. Parent 2-(2′-Aminophenyl)benzothiazole 1
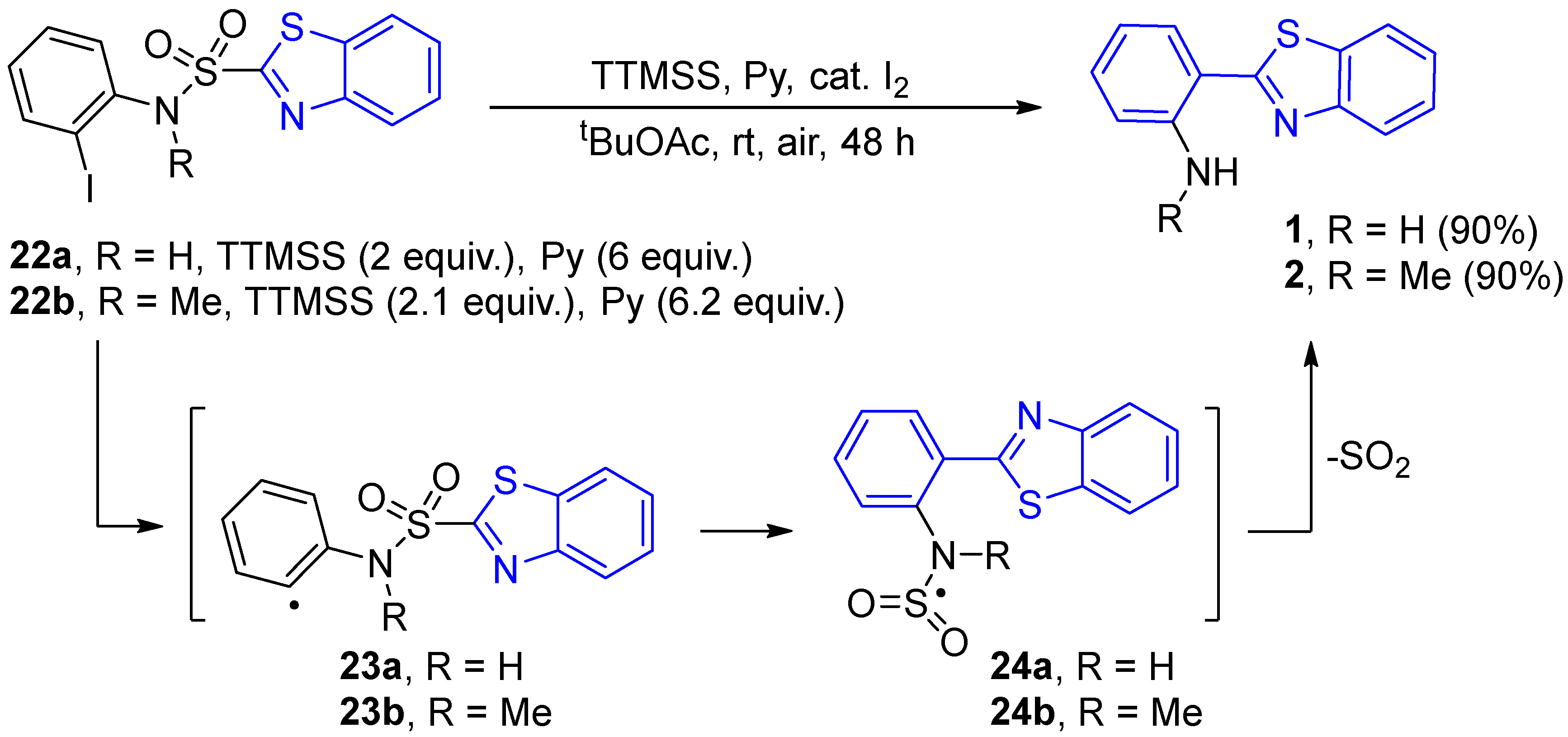
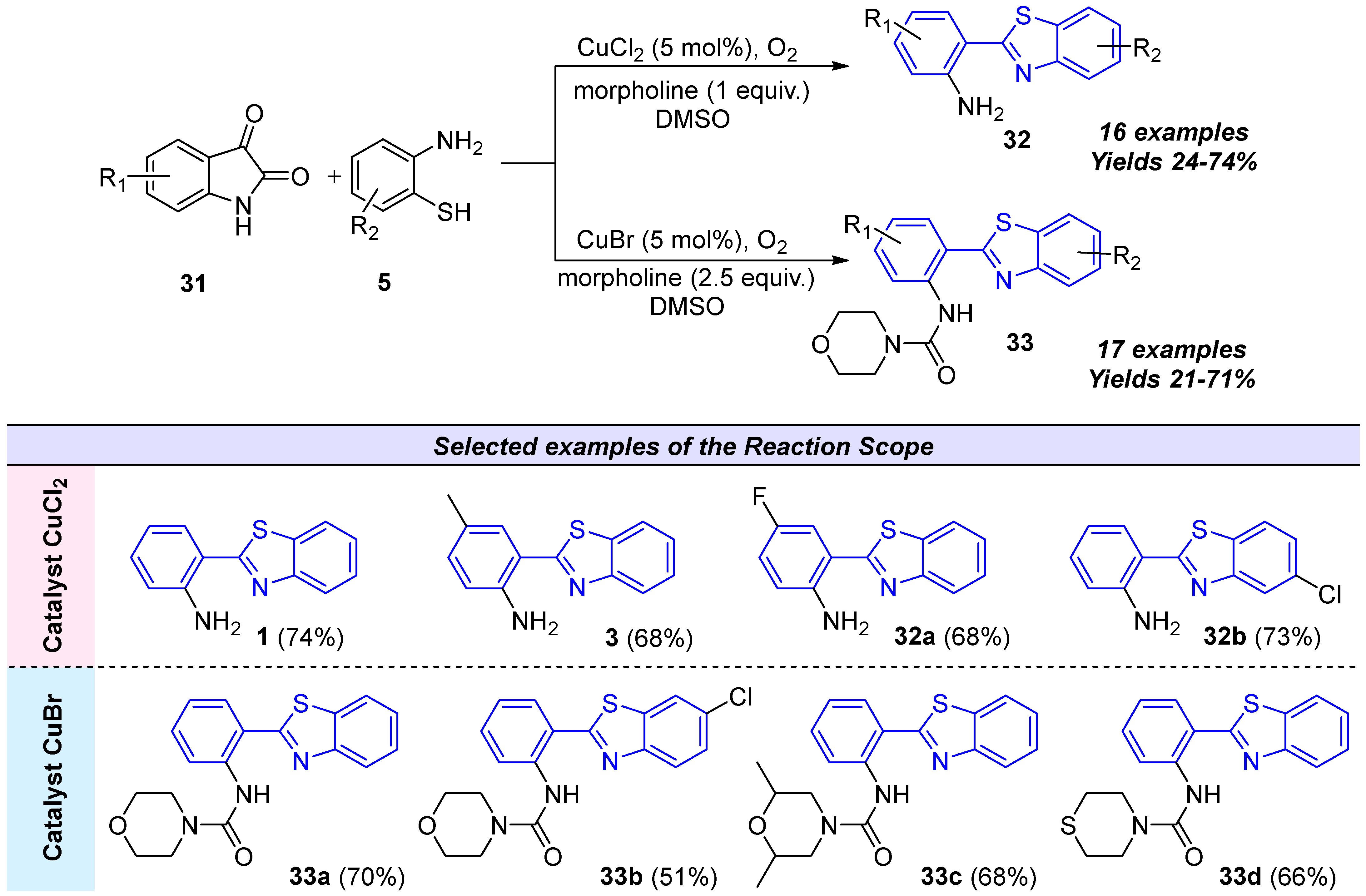
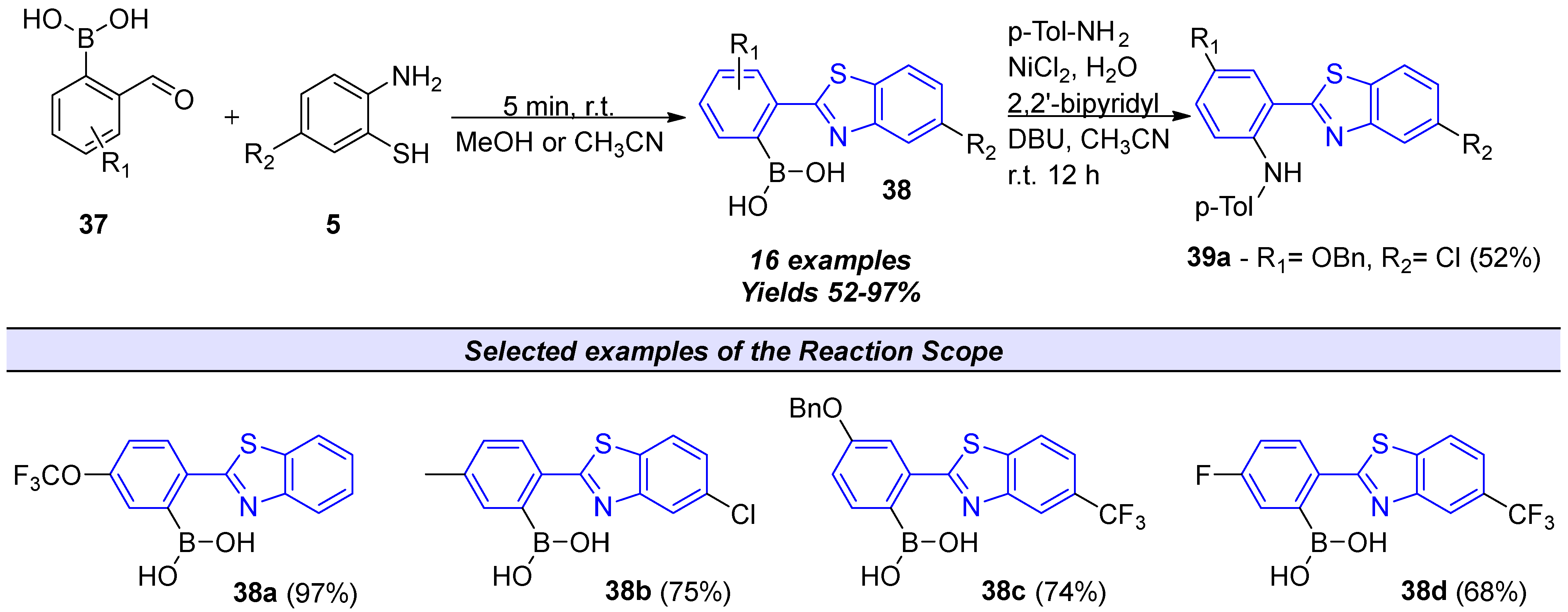
2.2.2. Derivatives of 2-(2′-Aminophenyl)benzothiazole
2.2.3. General Pathways Toward Functionalization of 2-(2′-Aminophenyl)benzothiazole
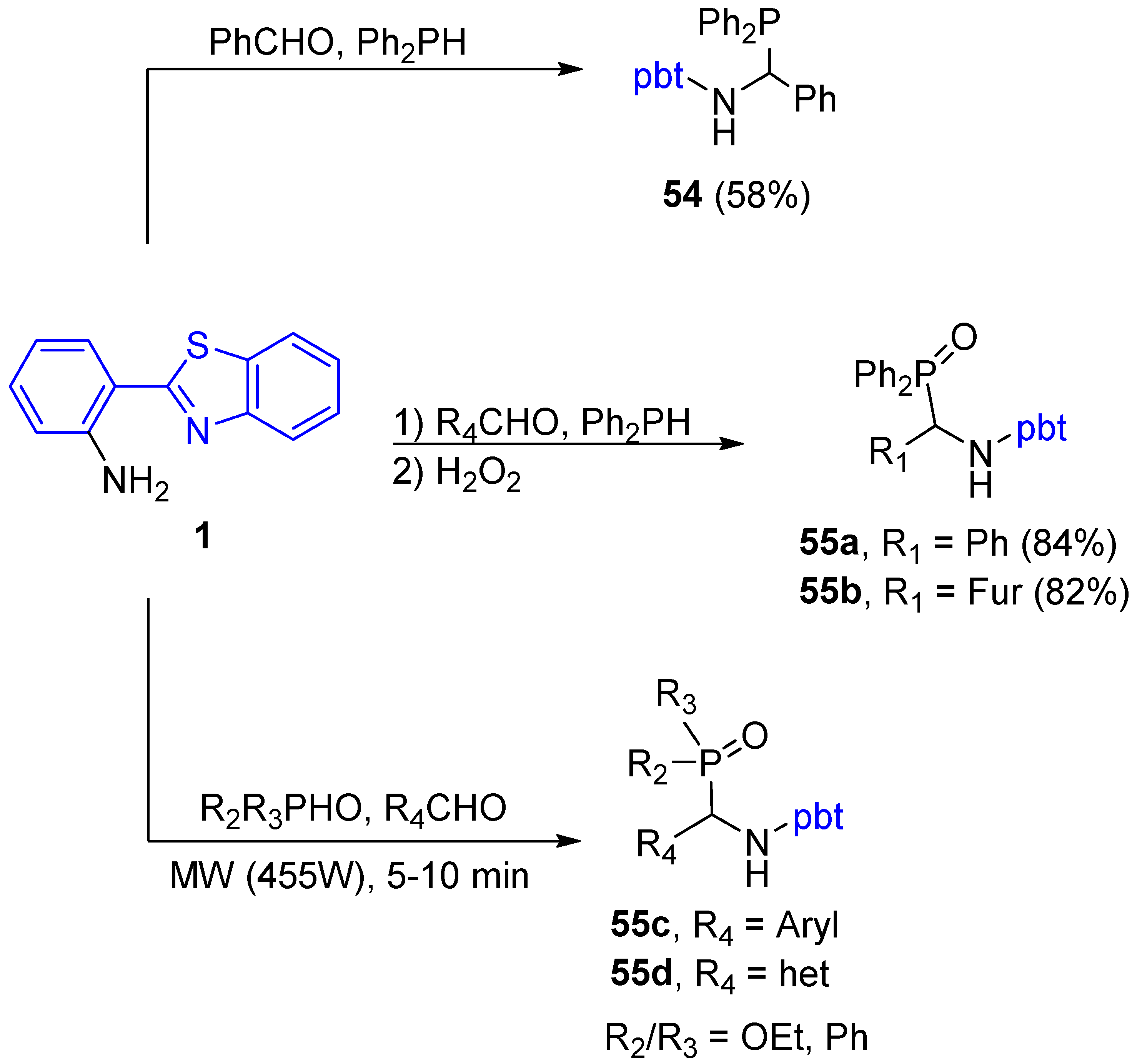
2.2.4. Functionalization of 2-(2′-Aminophenyl)benzothiazole with Phosphorous Groups
2.2.5. Functionalization of 2-(2′-Aminophenyl)benzothiazole with Silyl Group
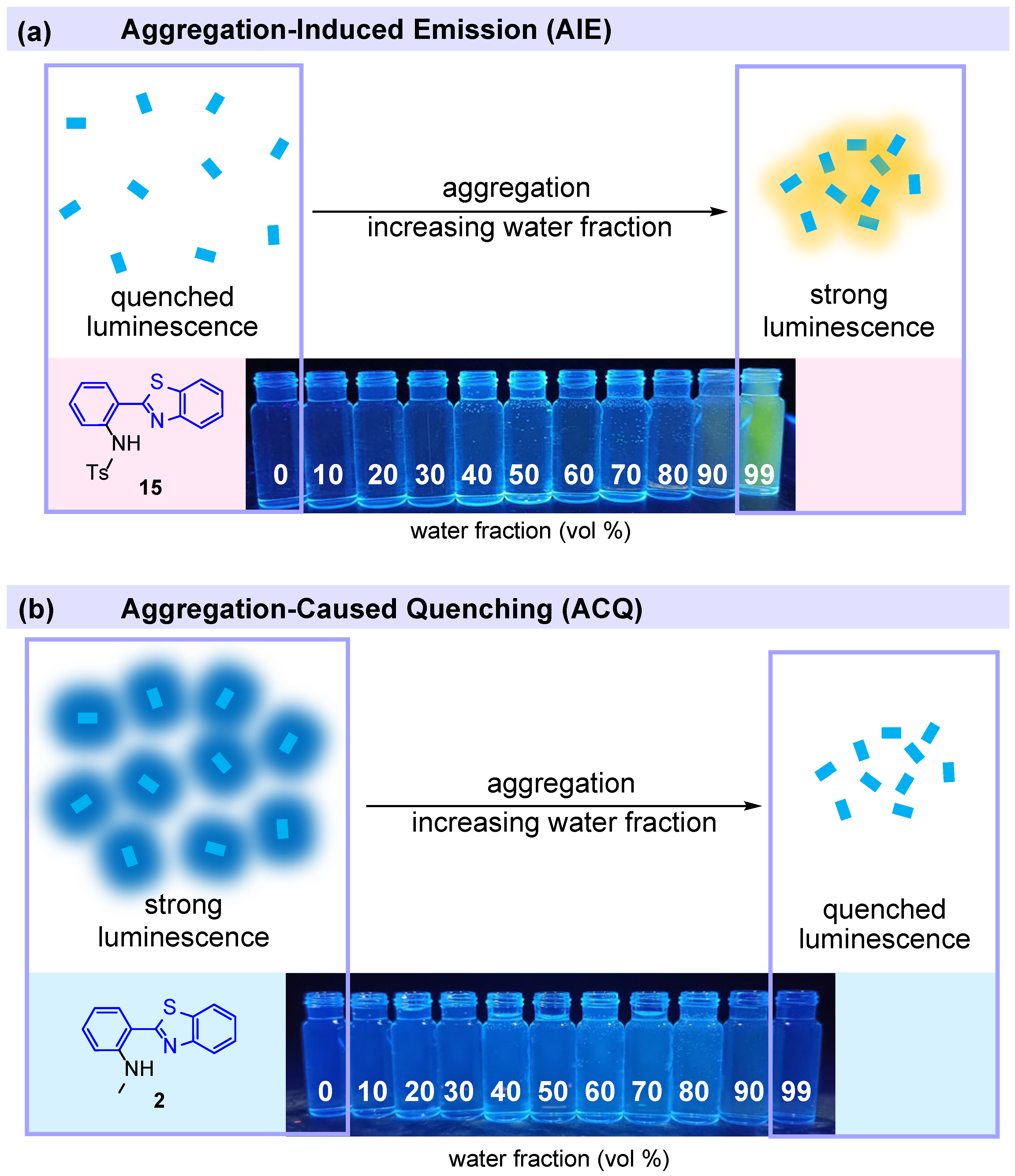
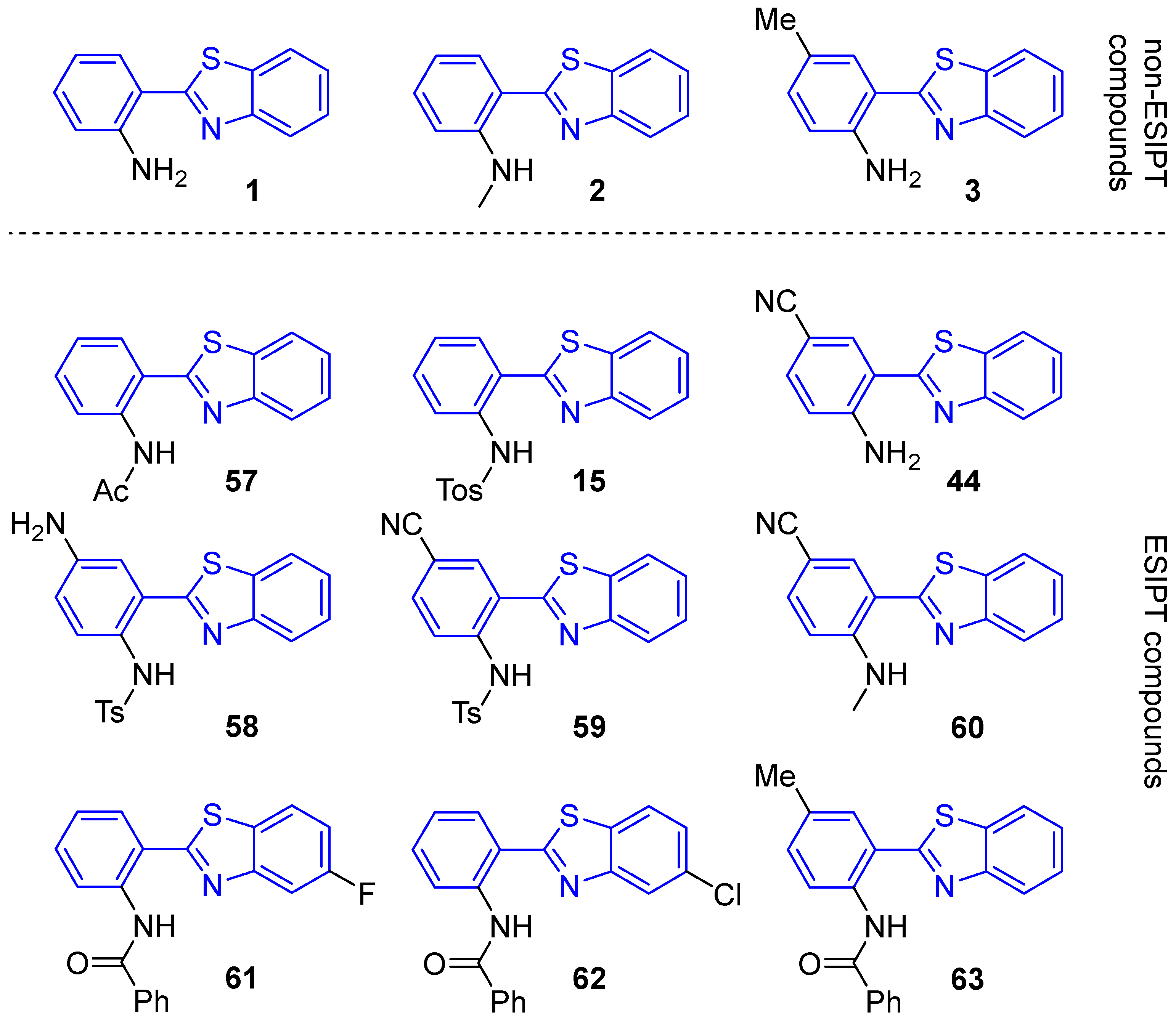
2.3. Luminescence Properties: ESIPT, AIE
2.3.1. ESIPT
2.3.2. AIE
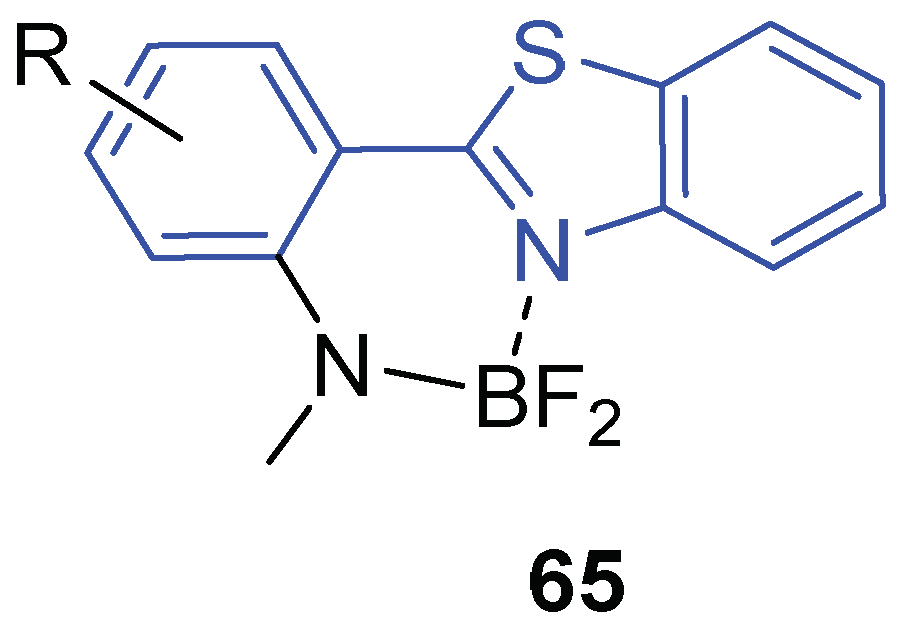
2.3.3. Miscellaneous Photophysical Properties
3. Overview of the Coordination Chemistry of 2-(2′-Aminophenyl)benzothiazole Derivatives
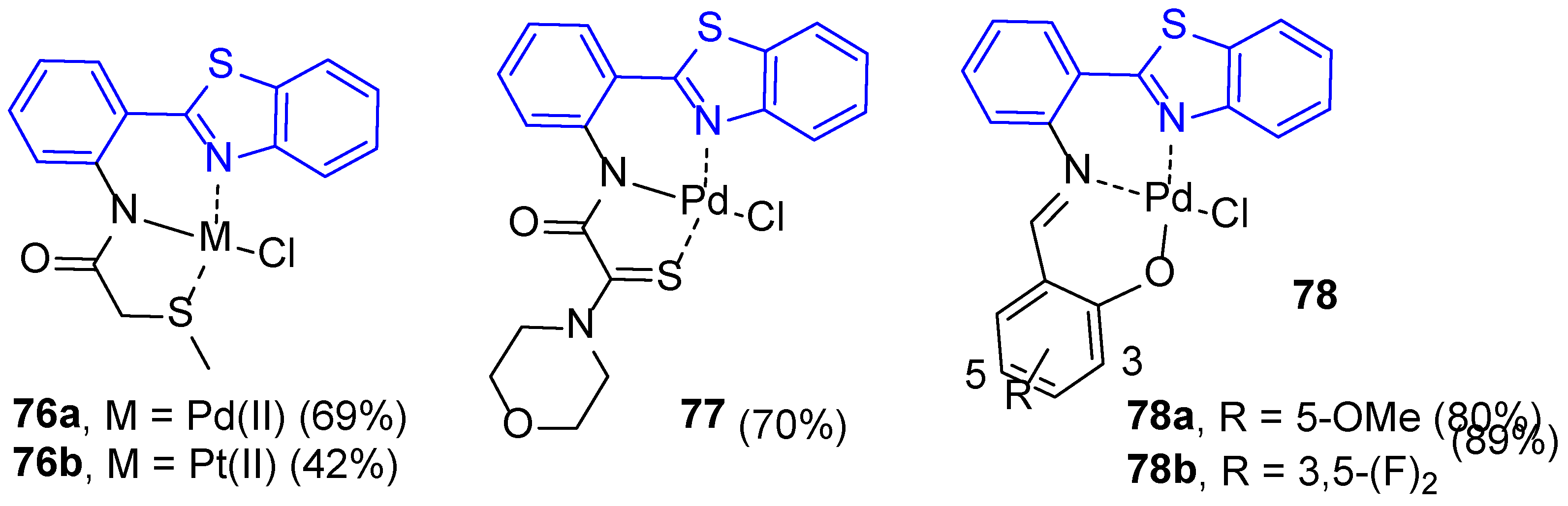
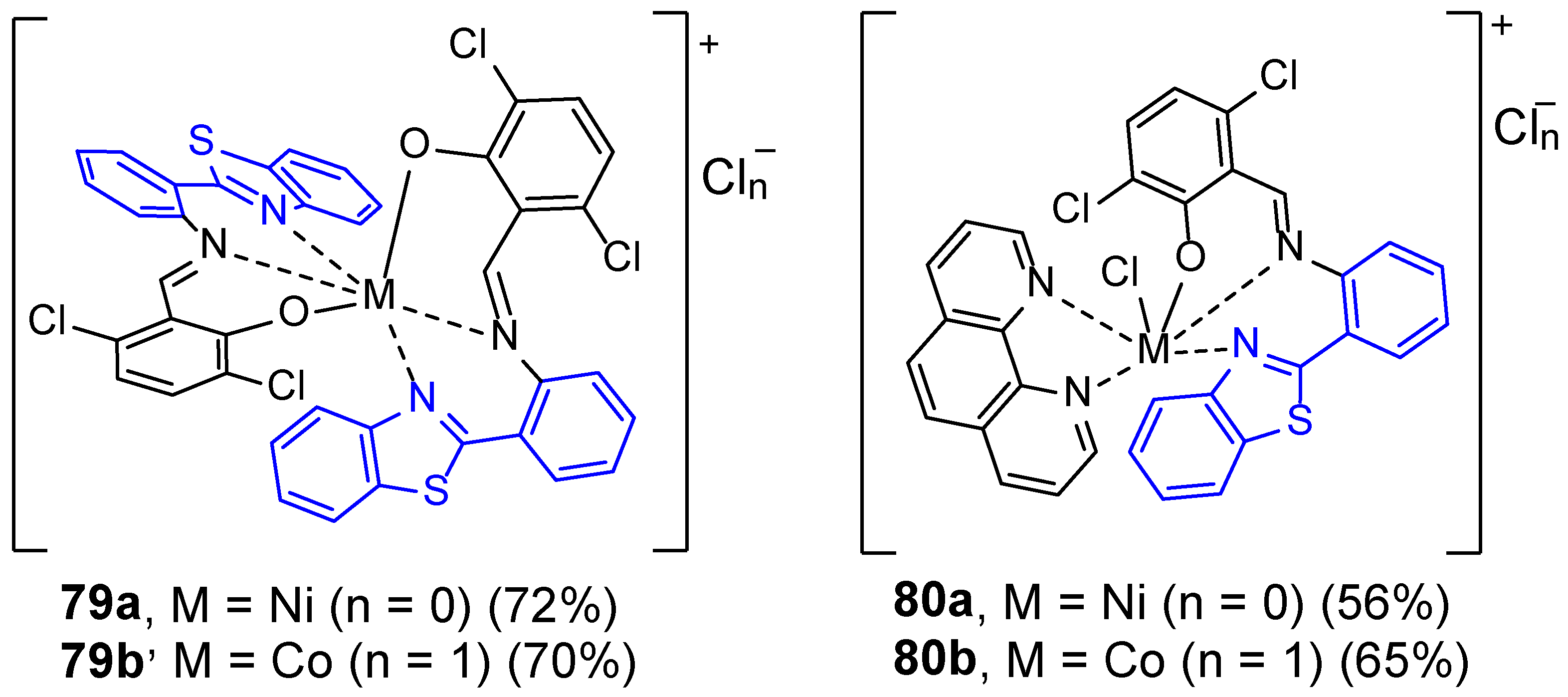
3.1. Coordination Complexes with Neutral Ligands Based on 2-(2′-Aminophenyl)benzothiazole
3.2. Coordination Complexes with Anionic Ligands Based on 2-(2′-Aminophenyl)benzothiazole
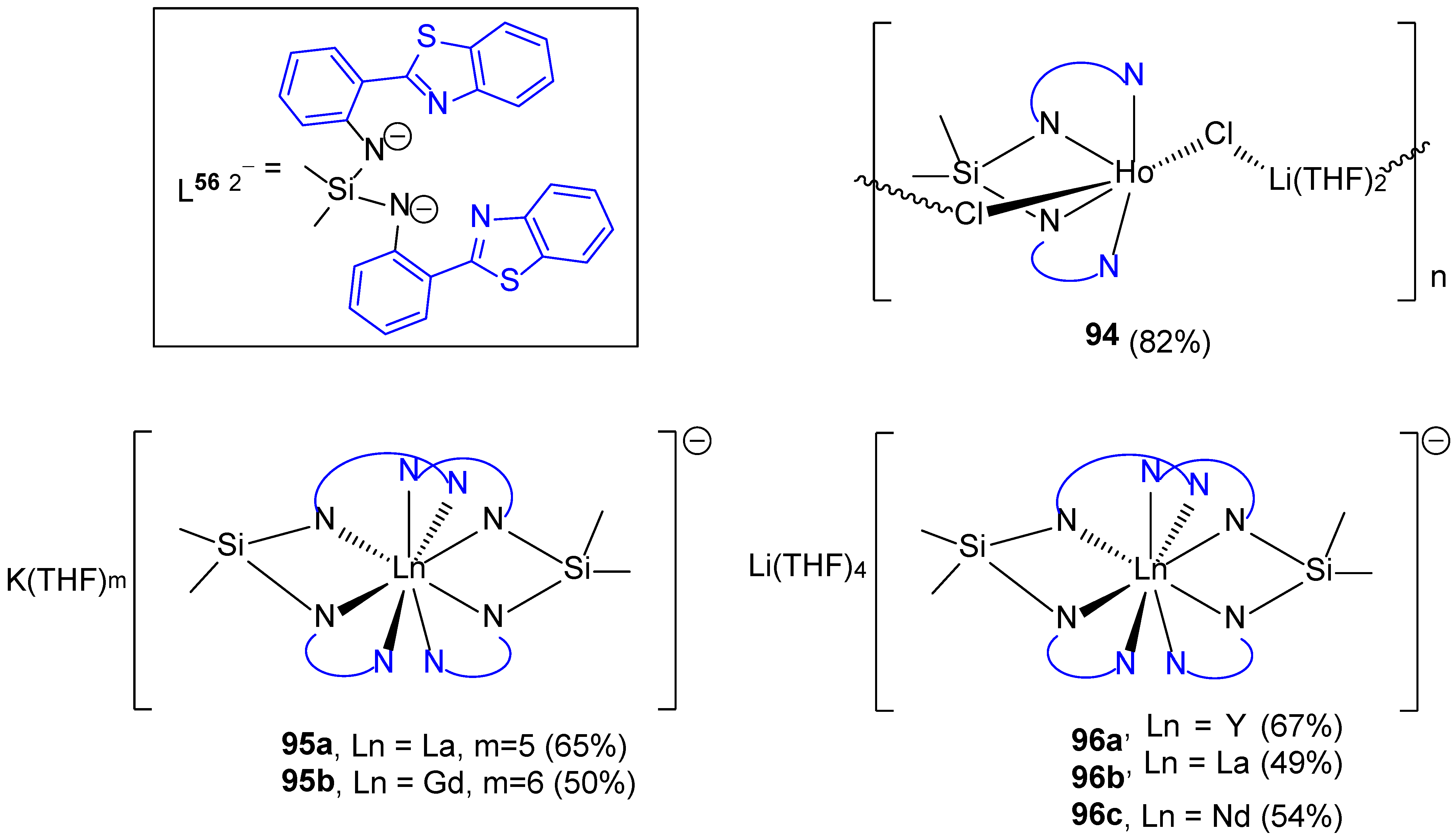
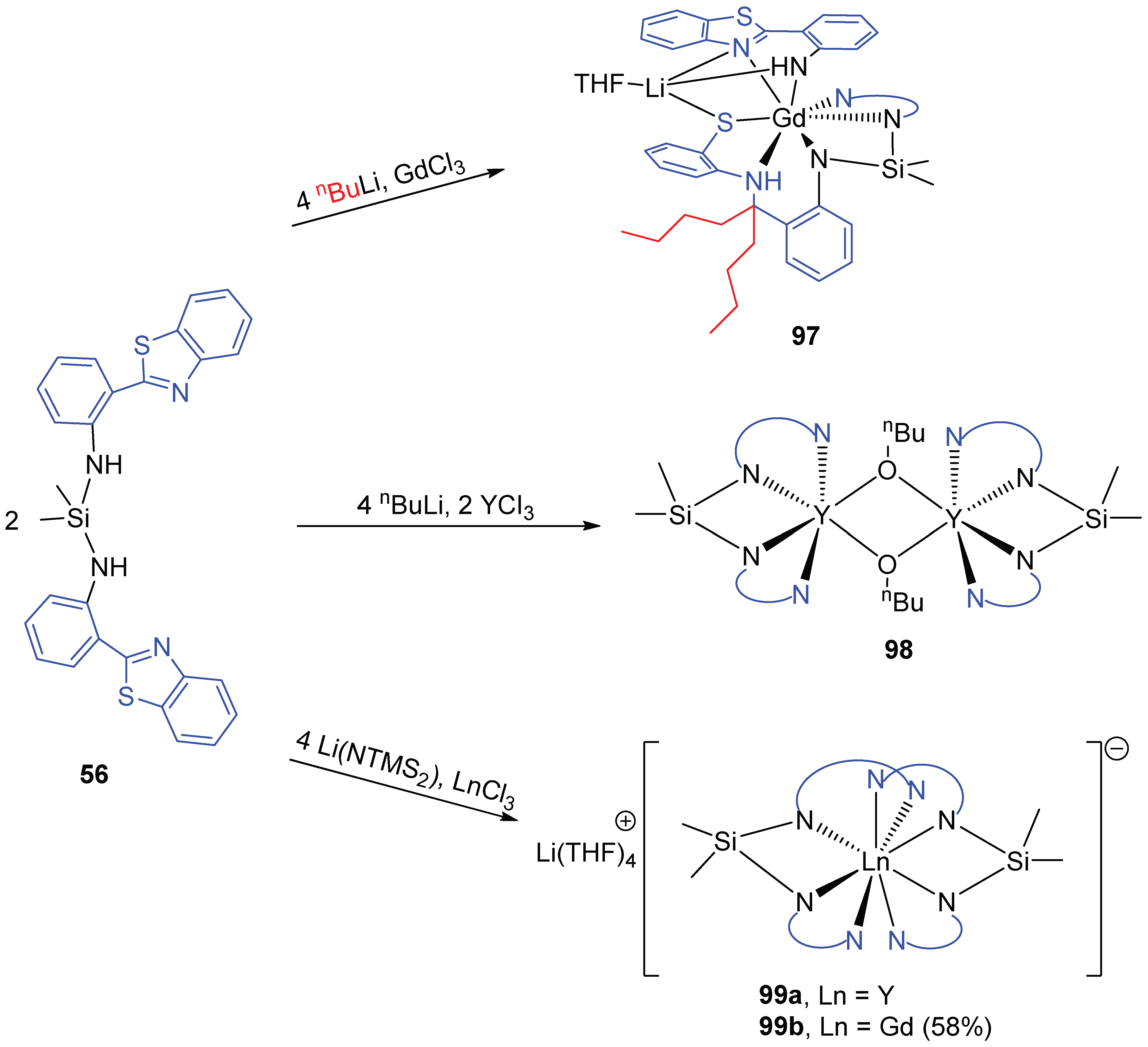
3.3. Coordination Complexes with Doubly Deprotonated NSiN Ligands Based on 2-(2′-Aminophenyl)benzothiazole
4. Overview of the Applications of 2-(2′-Aminophenyl)benzothiazole Derivatives
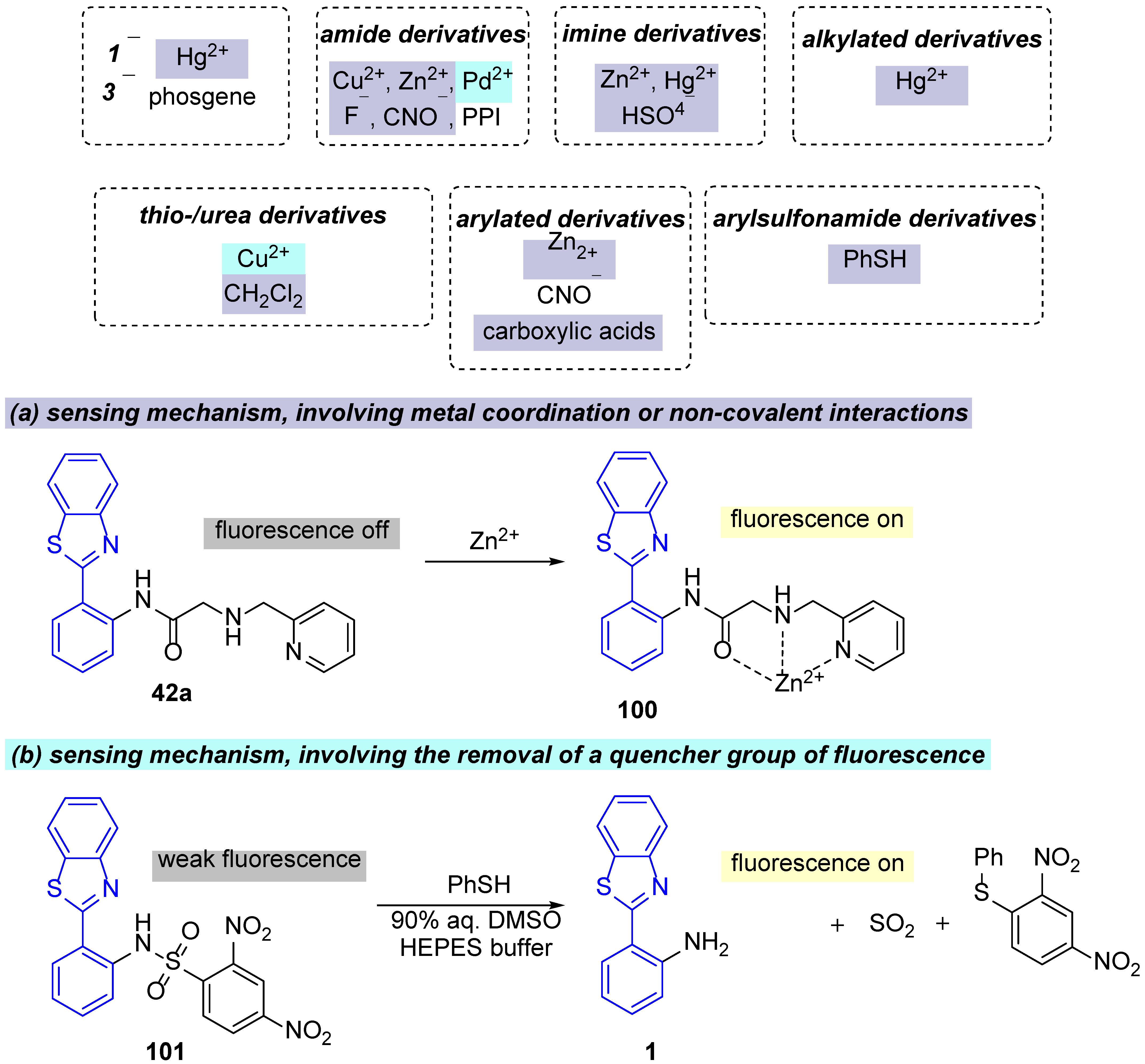
4.1. Applications as Small Molecule Sensors
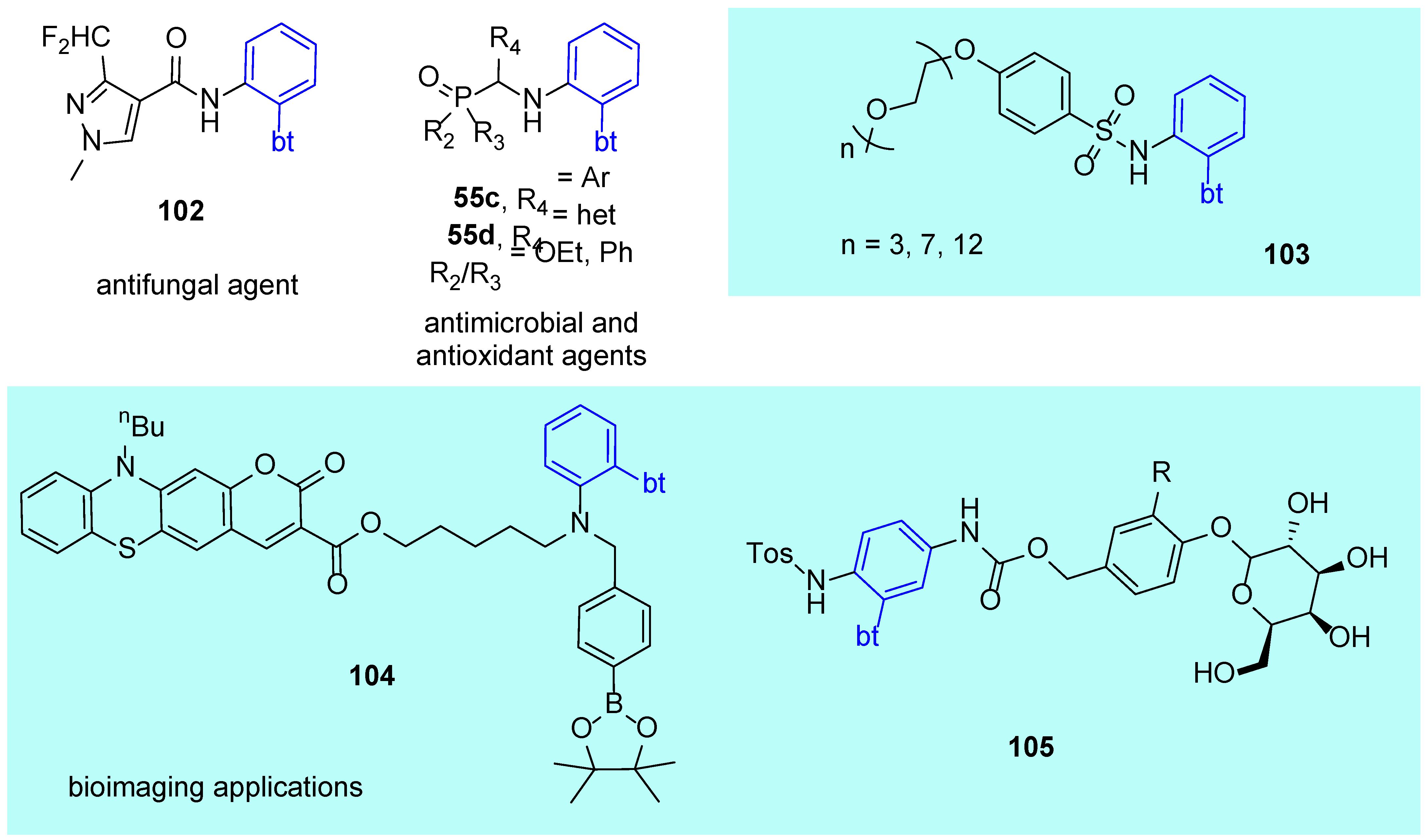
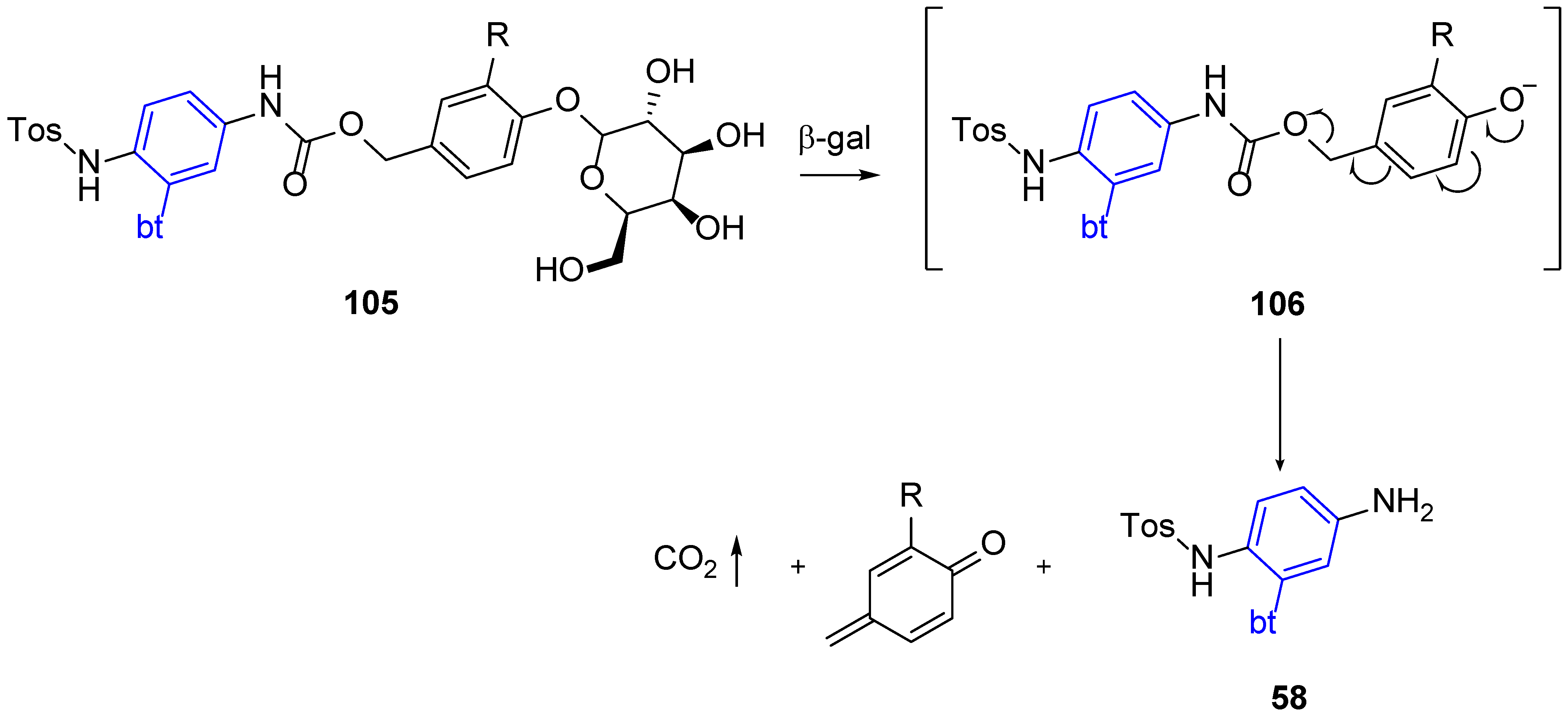
4.2. Applications in APIs and in Biosensing
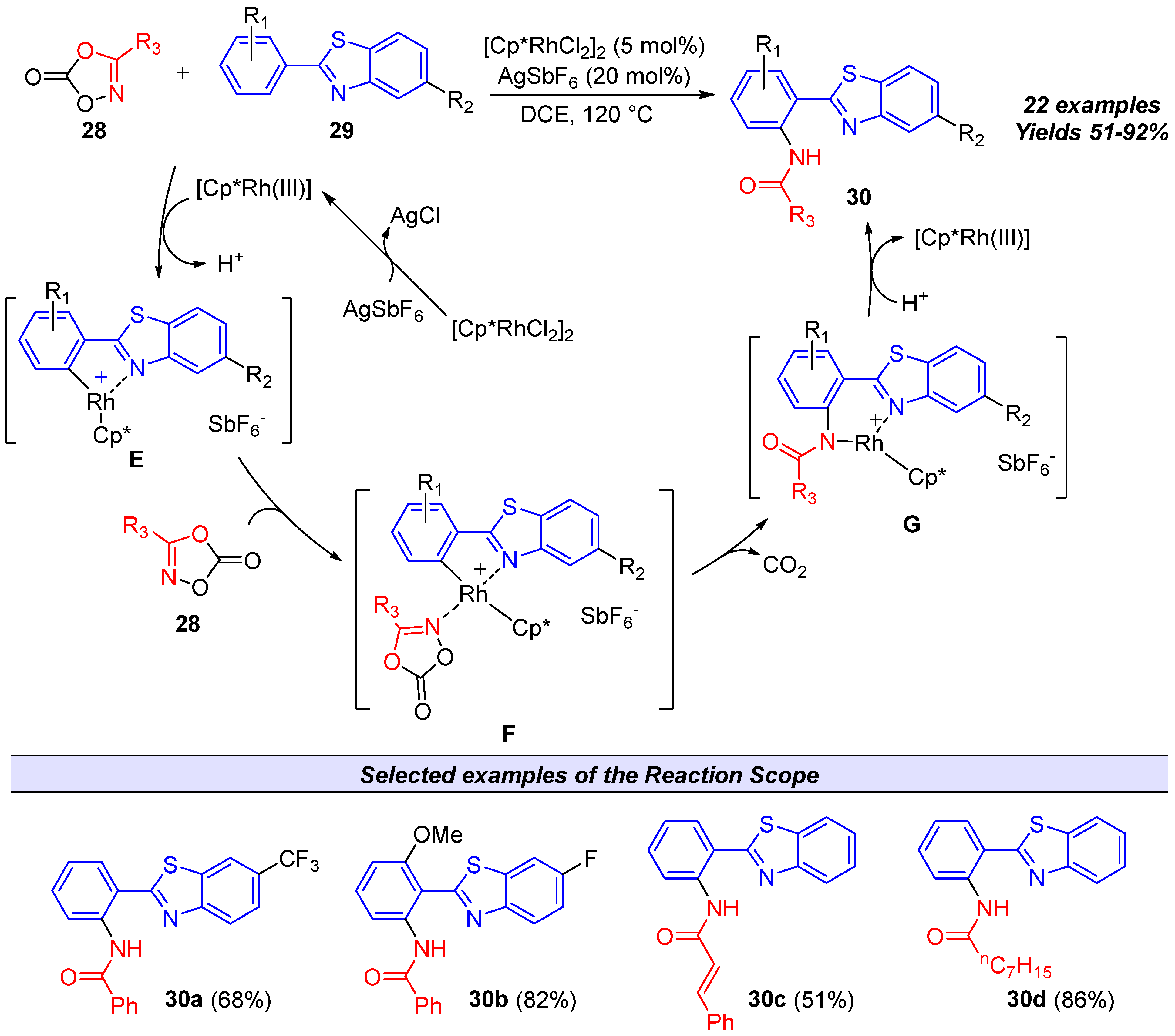
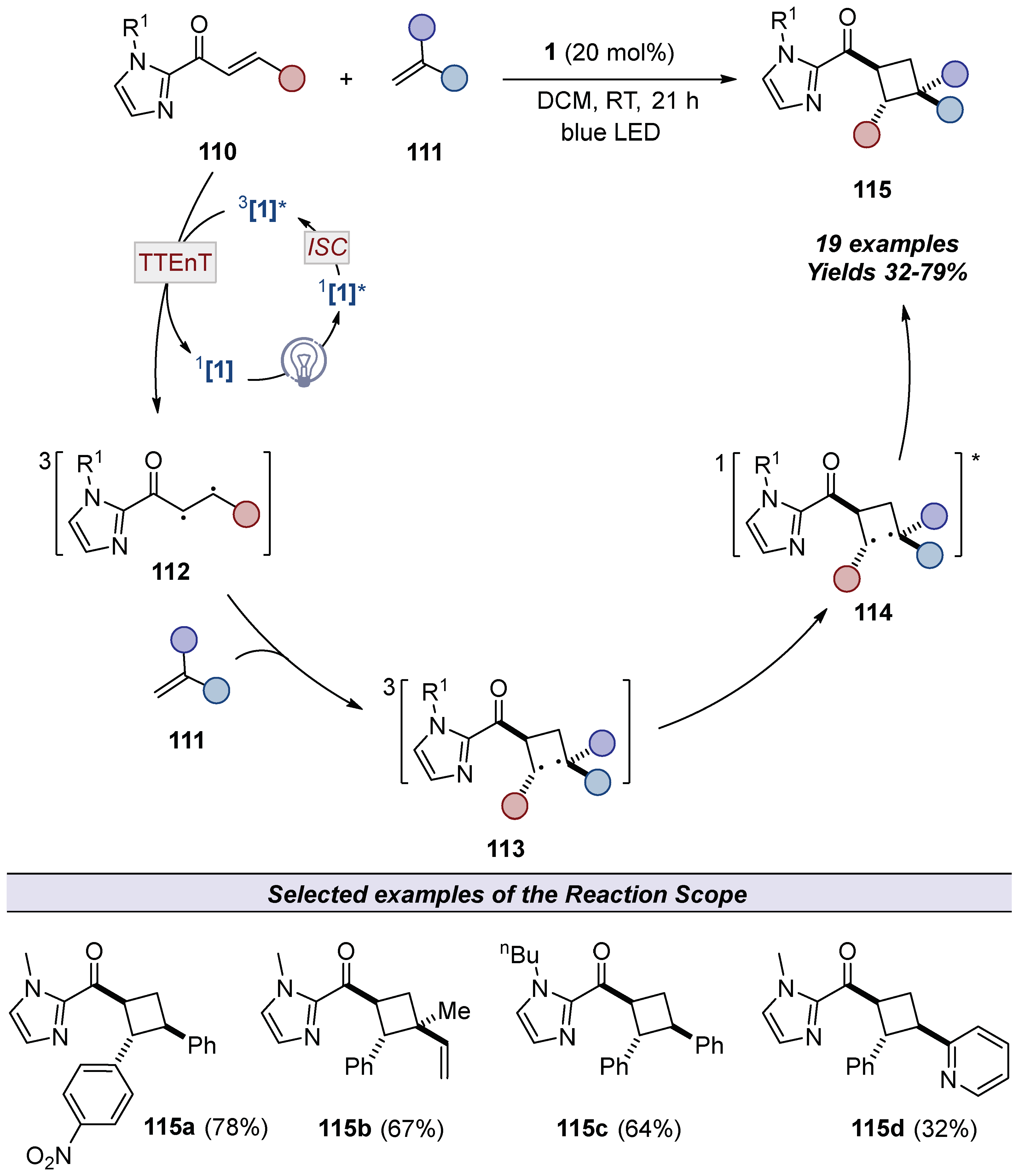
4.3. Applications in Catalysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-NH2-pbt | 2-(2′-aminophenyl)benzothiazole |
| abs | Absorption |
| ACQ | Aggregation-caused quenching |
| AIE | Aggregation-Induced Emission |
| Ala− | Alaninate |
| Alk | Alkyl substituent |
| Ar | Aryl substituent |
| bt | 2-benzothiazole |
| CBP | 4,4′-bis(N-carbozolyl)-1,1′-biphenyl |
| CCDC | Cambridge Crystallographic Data Center |
| COD | Cycloocta-1,5-diene |
| Cp* | 1,2,3,4,5-pentamethylcyclopentadienyl |
| DBU | 1,8-Diazabicyclo(5.4.0)undec-7-ene |
| DCE | 1,2-dichloroethane |
| DFT | Density-functional theory |
| DMI | Dimethyl-2-imidazolidinone |
| DMSO | Dimethylsulfoxide |
| DNA | Deoxyribonucleic acid |
| E | Element |
| em | Emission |
| equiv. | Equivalent |
| ESIPT | Excited State Intramolecular Proton Transfer |
| Fur | Furfuryl |
| Gly− | Glycinate |
| HEPES buffer | (4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid) |
| het | Heterocyclic fragment |
| IR | Infrared |
| ISC | Intersystem crossing |
| IUPAC | International Union of Pure and Applied Chemistry |
| L | Ligand |
| LED | Light emitting diode |
| LLCT | Ligand-to-ligand charge transfer |
| LMCT | Ligand-to-metal-charge-transfer |
| Ln | Rare-earth metal |
| Mes | Mesityl |
| MLCT | Metal-to-ligand charge transfer |
| MW | Microwave irradiation |
| NIR | near-infrared region |
| NMR | Nuclear magnetic resonance |
| NPD | N,N’-di(1-naphthyl)-N,N’-diphenyl-(1,1′-biphenyl)-4,4′-diamine |
| NS0 | Ground state of the normal form |
| NS1 | Excited state of the normal form |
| OFED | Organic field-effect transistors |
| OLED | Organic light-emitting device |
| p-Tol | Para-tolyl group |
| pbt | 2-phenylbenzothiazole |
| PET | Photoinduced electron transfer |
| PIC | Picrate ligand |
| PPA | Polyphosphoric acid |
| PPI | Pyrophosphate |
| PT | Proton transfer |
| PTA | Oligo(4,4′-(4″-methyl)triphenylamine) |
| RPT | Reverse proton transfer |
| S1 | First singlet excited state |
| SCXRD | Single crystal X-ray diffraction |
| T1 | First triplet excited state |
| TD-DFT | Time-dependent density-functional theory |
| TICT | Twisted intramolecular charge transfer |
| Tos | Tosyl |
| TS0 | Ground state of the tautomer |
| TS1 | Tautomer excited state |
| TTEnT | Triplet–triplet energy-transfer |
References
- Abdel-Wahab, B.F.; Mohamed, H.A. Pyrazolothiazoles: Synthesis and Applications. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1680–1693. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Carvalho, P.H.P.R.; Correa, J.R. Benzothiadiazole Derivatives as Fluorescence Imaging Probes: Beyond Classical Scaffolds. Acc. Chem. Res. 2015, 48, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Drakontaeidi, A.; Papanotas, I.; Pontiki, E. Multitarget Pharmacology of Sulfur–Nitrogen Heterocycles: Anticancer and Antioxidant Perspectives. Antioxidants 2024, 13, 898. [Google Scholar] [CrossRef] [PubMed]
- Zhylko, V.; Saliyeva, L.; Slyvka, N.; Grozav, A.; Yakovychuk, N.; Melnyk, D.; Melnyk, O.; Litvinchuk, M.; Vovk, M. Synthesis, Antimicrobial and Antioxidant Activity Evaluation, DFT-Calculation, and Docking Studies of 3-Aryl-5,6-Dihydroimidazo [2,1-b][1,3]Thiazoles. Curr. Chem. Lett. 2025, 14, 107–118. [Google Scholar]
- Leung-Toung, R.; Tam, T.F.; Wodzinska, J.M.; Zhao, Y.; Lowrie, J.; Simpson, C.D.; Karimian, K.; Spino, M. 3-Substituted Imidazo[1,2-d][1,2,4]-Thiadiazoles: A Novel Class of Factor XIIIa Inhibitors. J. Med. Chem. 2005, 48, 2266–2269. [Google Scholar]
- Dekker, F.J.; Ghizzoni, M.; van der Meer, N.; Wisastra, R.; Haisma, H.J. Inhibition of the PCAF Histone Acetyl Transferase and Cell Proliferation by Isothiazolones. Bioorg. Med. Chem. 2009, 17, 460–466. [Google Scholar]
- Bhatti, R.S.; Shah, S.; Suresh; Krishan, P.; Sandhu, J.S. Recent Pharmacological Developments on Rhodanines and 2,4-Thiazolidinediones. Int. J. Med. Chem. 2013, 2013, 793260. [Google Scholar]
- Kaur, N. Palladium-Catalyzed Approach to the Synthesis of S-Heterocycles. Catal. Rev.—Sci. Eng. 2015, 57, 478–564. [Google Scholar]
- Shao, Q.; Pan, W.; Liang, Q.; Li, C.; Zhang, F.; Yang, Y.; Liu, S.; Chen, G.; Fan, B. Design of a Dual-Function Prodrug Fluorescence Probes for Melanoma Detection and Anticancer Drug Release. Chin. J. Anal. Chem. 2025, 53, 100500. [Google Scholar]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.-E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly Phosphorescent Bis-Cyclometalated Iridium Complexes: Synthesis, Photophysical Characterization, and Use in Organic Light Emitting Diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% Internal Phosphorescence Efficiency in an Organic Light-Emitting Device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar]
- Fan, C.; Zhu, L.; Jiang, B.; Li, Y.; Zhao, F.; Ma, D.; Qin, J.; Yang, C. High Power Efficiency Yellow Phosphorescent OLEDs by Using New Iridium Complexes with Halogen-Substituted 2-Phenylbenzo[d]Thiazole Ligands. J. Phys. Chem. C 2013, 117, 19134–19141. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Lapis, A.A.M.; Da Silva Júnior, E.N.; Dupont, J. 2,1,3-Benzothiadiazole and Derivatives: Synthesis, Properties, Reactions, and Applications in Light Technology of Small Molecules. Eur. J. Org. Chem. 2012, 2013, 228–255. [Google Scholar]
- Sukhikh, T.S.; Komarov, V.Y.; Konchenko, S.N.; Benassi, E. The Hows and Whys of Peculiar Coordination of 4-Amino-2,1,3-Benzothiadiazole. Polyhedron 2018, 139, 33–43. [Google Scholar]
- Li, T.-Y.; Wu, J.; Wu, Z.-G.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Rational Design of Phosphorescent Iridium(III) Complexes for Emission Color Tunability and Their Applications in OLEDs. Coord. Chem. Rev. 2018, 374, 55–92. [Google Scholar]
- Jang, J.-H.; Park, H.J.; Park, J.Y.; Kim, H.U.; Hwang, D.-H. Orange Phosphorescent Ir(III) Complexes Consisting of Substituted 2-Phenylbenzothiazole for Solution-Processed Organic Light-Emitting Diodes. Org. Electron. 2018, 60, 31–37. [Google Scholar]
- Hu, Y.-X.; Xia, X.; He, W.-Z.; Tang, Z.-J.; Lv, Y.-L.; Li, X.; Zhang, D.-Y. Recent Developments in Benzothiazole-Based Iridium(Ⅲ) Complexes for Application in OLEDs as Electrophosphorescent Emitters. Org. Electron. 2019, 66, 126–135. [Google Scholar]
- Sukhikh, T.S.; Ogienko, D.S.; Bashirov, D.A.; Konchenko, S.N. Luminescent Complexes of 2,1,3-Benzothiadiazole Derivatives. Russ. Chem. Bull. 2019, 68, 651–661. [Google Scholar]
- Prajapati, M.J.; Solanki, J.D.; Machhi, H.K.; Soni, S.S.; Sen, P.; Surati, K.R. Yellowish-Orange Phosphorescent Iridium(III) Complexes of Bis-Cyclometalated Ligand with Pyrazolone Derivatives: Synthesis, Characterization, Photophysical and Thermal Properties. J. Mater. Sci.: Mater. Electron. 2020, 31, 13778–13786. [Google Scholar]
- Li, Y.; Yao, J.; Wang, C.; Zhou, X.; Xu, Y.; Hanif, M.; Qiu, X.; Hu, D.; Ma, D.; Ma, Y. Highly Efficient Deep-Red/near-Infrared D-A Chromophores Based on Naphthothiadiazole for OLEDs Applications. Dye. Pigment. 2020, 173, 107960. [Google Scholar]
- Zhang, Y.; Song, J.; Qu, J.; Qian, P.-C.; Wong, W.-Y. Recent Progress of Electronic Materials Based on 2,1,3-Benzothiadiazole and Its Derivatives: Synthesis and Their Application in Organic Light-Emitting Diodes. Sci. China Chem. 2021, 64, 341–357. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Khisamov, R.M.; Konchenko, S.N. Unexpectedly Long Lifetime of the Excited State of Benzothiadiazole Derivative and Its Adducts with Lewis Acids. Molecules 2021, 26, 2030. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.G.; Souto, F.T.; Machado, V.G. 2,1,3-Benzothiadiazoles Are Versatile Fluorophore Building Blocks for the Design of Analyte-Sensing Optical Devices. Chemosensors 2024, 12, 156. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, W.; Zhou, Y.; Wei, C.; Huang, J.; Wang, Q.; Wang, L.; Yu, G. Chalcogenophene-Sensitive Charge Carrier Transport Properties in A–D–A′′–D Type NBDO-Based Copolymers for Flexible Field-Effect Transistors. Macromolecules 2018, 51, 8662–8671. [Google Scholar]
- Bulumulla, C.; Gunawardhana, R.; Kularatne, R.N.; Hill, M.E.; McCandless, G.T.; Biewer, M.C.; Stefan, M.C. Thieno[3,2-b]Pyrrole-Benzothiadiazole Banana-Shaped Small Molecules for Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2018, 10, 11818–11825. [Google Scholar]
- Kinik, F.P.; Ortega-Guerrero, A.; Ongari, D.; Ireland, C.P.; Smit, B. Pyrene-Based Metal Organic Frameworks: From Synthesis to Applications. Chem. Soc. Rev. 2021, 50, 3143–3177. [Google Scholar] [PubMed]
- Bhardwaj, V.K.; Saluja, P.; Hundal, G.; Hundal, M.S.; Singh, N.; Jang, D.O. Benzthiazole-Based Multifunctional Chemosensor: Fluorescent Recognition of Fe3+ and Chromogenic Recognition of HSO4−. Tetrahedron 2013, 69, 1606–1610. [Google Scholar] [CrossRef]
- Dhaka, G.; Singh, J.; Kaur, N. Benzothiazole Based Chemosensors Having Appended Amino Group(s): Selective Binding of Hg2+ Ions by Three Related Receptors. Inorganica Chim. Acta 2017, 462, 152–157. [Google Scholar] [CrossRef]
- Medeiros, G.A.; Correa, J.R.; de Andrade, L.P.; Lopes, T.O.; de Oliveira, H.C.B.; Diniz, A.B.; Menezes, G.B.; Rodrigues, M.O.; Neto, B.A.D. A Benzothiadiazole-Quinoline Hybrid Sensor for Specific Bioimaging and Surgery Procedures in Mice. Sens. Actuators B Chem. 2021, 328, 128998. [Google Scholar]
- Mao, P.; Song, Y.; Zhao, X.; Wu, W.; Wang, Y. A Ratiometric Benzimidazole-Based Fluorescent Probe for The Recognition of Phosgene in Solution and Gaseous Phases. J. Fluoresc. 2024, 1–9. [Google Scholar] [CrossRef]
- Velusamy, M.; Justin Thomas, K.R.; Lin, J.T.; Hsu, Y.-C.; Ho, K.-C. Organic Dyes Incorporating Low-Band-Gap Chromophores for Dye-Sensitized Solar Cells. Org. Lett. 2005, 7, 1899–1902. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bäuerle, P. Small Molecule Organic Semiconductors on the Move: Promises for Future Solar Energy Technology. Angew. Chem. Int. Ed. Engl. 2012, 51, 2020–2067. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bäuerle, P. Niedermolekulare Organische Halbleiter Auf Dem Vormarsch—Ausblick Auf Künftige Solartechniken. Angew. Chem. 2012, 124, 2060–2109. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Zhang, J.-Y.; Yu, Z.-T.; Feng, J.-Y.; Luo, W.-J.; Ye, J.-H.; Zou, Z.-G. Impact of Ligand Modification on Hydrogen Photogeneration and Light-Harvesting Applications Using Cyclometalated Iridium Complexes. Inorg. Chem. 2012, 51, 4123–4133. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Rakitin, O.A. Influence of structural factors on the photovoltaic properties of dye-sensitized solar cells. Russ. Chem. Rev. 2016, 85, 1146–1183. [Google Scholar] [CrossRef]
- Yang, C.; Mehmood, F.; Lam, T.L.; Chan, S.L.-F.; Wu, Y.; Yeung, C.-S.; Guan, X.; Li, K.; Chung, C.Y.-S.; Zhou, C.-Y.; et al. Stable Luminescent Iridium(Iii) Complexes with Bis(N-Heterocyclic Carbene) Ligands: Photo-Stability, Excited State Properties, Visible-Light-Driven Radical Cyclization and CO2 Reduction, and Cellular Imaging. Chem. Sci. 2016, 7, 3123–3136. [Google Scholar] [CrossRef]
- Neto, B.A.D.; Correa, J.R.; Spencer, J. Fluorescent Benzothiadiazole Derivatives as Fluorescence Imaging Dyes: A Decade of New Generation Probes. Chem. Eur. J. 2022, 28, e202103262. [Google Scholar] [CrossRef]
- Pozharskii, A.F.; Soldatenkov, A.T.; Katritzky, A.R. Heterocycles in Life and Society, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 209–246. ISBN 9781119998372. [Google Scholar]
- Shermolovich, Y.; Pazenok, S. Topics in Heterocyclic Chemistry, 1st ed.; Springer: Berlin, Germany, 2011; pp. 101–138. [Google Scholar]
- Leung-Toung, R.; Tam, T.F.; Zhao, Y.; Simpson, C.D.; Li, W.; Desilets, D.; Karimian, K. Synthesis of 3-Substituted Bicyclic Imidazo[1,2-d][1,2,4]Thiadiazoles and Tricyclic Benzo[4,5]Imidazo[1,2-d][1,2,4]Thiadiazoles. J. Org. Chem. 2005, 70, 6230–6241. [Google Scholar] [CrossRef]
- Mittal, S.; Samota, M.K.; Kaur, J.; Seth, G. Synthesis, Spectral, and Antifungal Evaluation of Phosphorylated and Thiophosphorylated Benzothiazole Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 2105–2113. [Google Scholar] [CrossRef]
- Carvalho, T.O.; Carvalho, P.H.P.R.; Correa, J.R.; Guido, B.C.; Medeiros, G.A.; Eberlin, M.N.; Coelho, S.E.; Domingos, J.B.; Neto, B.A.D. Palladium Catalyst with Task-Specific Ionic Liquid Ligands: Intracellular Reactions and Mitochondrial Imaging with Benzothiadiazole Derivatives. J. Org. Chem. 2019, 84, 5118–5128. [Google Scholar] [CrossRef]
- Kitamura, T.; Yamanishi, K.; Inoue, S.; Yan, Y.-N.; Yano, N.; Kataoka, Y.; Handa, M.; Kawamoto, T. Clamshell Palladium(II) Complexes: Suitable Precursors for Photocatalytic Hydrogen Production from Water. Eur. J. Inorg. Chem. 2022, 2022, e202200259. [Google Scholar]
- Rigotti, T.; Schwinger, D.P.; Graßl, R.; Jandl, C.; Bach, T. Enantioselective Crossed Intramolecular [2+2] Photocycloaddition Reactions Mediated by a Chiral Chelating Lewis Acid. Chem. Sci. 2022, 13, 2378–2384. [Google Scholar]
- Ma, J.; Zhang, X.; Huang, X.; Luo, S.; Meggers, E. Preparation of Chiral-at-Metal Catalysts and Their Use in Asymmetric Photoredox Chemistry. Nat. Protoc. 2018, 13, 605–632. [Google Scholar]
- Grell, Y.; Demirel, N.; Harms, K.; Meggers, E. Chiral Bis(Oxazoline) Ligands as C2-Symmetric Chiral Auxiliaries for the Synthesis of Enantiomerically Pure Bis-Cyclometalated Rhodium(III) Complexes. Organometallics 2019, 38, 3852–3859. [Google Scholar]
- Ilichev, V.A.; Balashova, T.V.; Polyakova, S.K.; Rogozhin, A.F.; Kolybalov, D.S.; Bashirov, D.A.; Konchenko, S.N.; Yablonskiy, A.N.; Rumyantcev, R.V.; Fukin, G.K.; et al. Synthesis, Structure, and Luminescence Properties of Sodium and Ytterbium Complexes with 2-(Benzothiazol-2-Yl)Selenophenolate Ligands. Russ. Chem. Bull. 2022, 71, 298–305. [Google Scholar] [CrossRef]
- Mironova, O.A.; Ryadun, A.A.; Sukhikh, T.S.; Pushkarevsky, N.A.; Konchenko, S.N. Synthesis and Photophysical Properties of Rare Earth (La, Nd, Gd, Y, Ho) Complexes with Silanediamido Ligands Bearing a Chelating Phenylbenzothiazole Chromophore. New J. Chem. 2023, 47, 3406–3416. [Google Scholar] [CrossRef]
- Wang, M.-H.; Tsai, M.-Y.; Su, Y.-C.; Chiu, S.-T.; Lin, P.-H.; Long, J. Zero-Field Single-Molecule Magnet Behavior in a Series of Dinuclear Dysprosium(III) Complexes Based on Benzothiazolyl-Based Ligands and β-Diketonates. Cryst. Growth Des. 2024, 24, 422–431. [Google Scholar] [CrossRef]
- Afonin, M.Y.; Martynenko, P.A.; Kolybalov, D.S.; Khisamov, R.M.; Konchenko, S.N.; Sukhikh, T.S. Pd(II)- and Pt(II)-Assisted P–C Activation/Cyclization Reactions with a Luminescent α-Aminophosphine. Inorg. Chem. 2024, 63, 369–380. [Google Scholar] [CrossRef]
- Ibrahim, S.; Naik, N.; Shivamallu, C.; Raghavendra, H.L.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Amachawadi, R.G.; Kollur, S.P. Synthesis, Structure, and in Vitro Biological Studies of Benzothiazole Based Schiff Base Ligand and Its Binary and Ternary Co(III) and Ni(II) Complexes. Inorganica Chim. Acta 2024, 559, 121792. [Google Scholar]
- Ilichev, V.A.; Silantyeva, L.I.; Rogozhin, A.F.; Bochkarev, M.N. Lanthanide Coordination Compounds with Benzothiazolate Ligands: Synthesis, Structure and Luminescent Properties: Review. J. Struct. Chem. 2024, 65, 1825–1864. [Google Scholar]
- Mathis, C.A.; Wang, Y.; Holt, D.P.; Huang, G.-F.; Debnath, M.L.; Klunk, W.E. Synthesis and Evaluation of 11C-Labeled 6-Substituted 2-Arylbenzothiazoles as Amyloid Imaging Agents. J. Med. Chem. 2003, 46, 2740–2754. [Google Scholar] [PubMed]
- Djuidje, E.N.; Sciabica, S.; Buzzi, R.; Dissette, V.; Balzarini, J.; Liekens, S.; Serra, E.; Andreotti, E.; Manfredini, S.; Vertuani, S.; et al. Design, Synthesis and Evaluation of Benzothiazole Derivatives as Multifunctional Agents. Bioorg. Chem. 2020, 101, 103960. [Google Scholar]
- Liu, Y.; Feng, B.; Cao, X.; Tang, G.; Liu, H.; Chen, F.; Liu, M.; Chen, Q.; Yuan, K.; Gu, Y.; et al. A Novel “AIE + ESIPT” near-Infrared Nanoprobe for the Imaging of γ-Glutamyl Transpeptidase in Living Cells and the Application in Precision Medicine. Analyst 2019, 144, 5136–5142. [Google Scholar] [PubMed]
- Zhan, Z.; Su, Z.; Chai, L.; Li, C.; Liu, R.; Lv, Y. Multimodal Imaging Iridium(III) Complex for Hypochlorous Acid in Living Systems. Anal. Chem. 2020, 92, 8285–8829. [Google Scholar] [CrossRef]
- Xu, Z.-H.; Gao, H.; Zhang, N.; Zhao, W.; Cheng, Y.-X.; Xu, J.-J.; Chen, H.-Y. Ultrasensitive Nucleic Acid Assay Based on Cyclometalated Iridium(III) Complex with High Electrochemiluminescence Efficiency. Anal. Chem. 2021, 93, 1686–1692. [Google Scholar]
- Terpstra, K.; Huang, Y.; Na, H.; Sun, L.; Gutierrez, C.; Yu, Z.; Mirica, L.M. 2-Phenylbenzothiazolyl Iridium Complexes as Inhibitors and Probes of Amyloid β Aggregation. Dalton Trans. 2024, 53, 14258–14264. [Google Scholar]
- Sadek, O.; Galán, L.A.; Gendron, F.; Baguenard, B.; Guy, S.; Bensalah-Ledoux, A.; Le Guennic, B.; Maury, O.; Perrin, D.M.; Gras, E. Chiral Benzothiazole Monofluoroborate Featuring Chiroptical and Oxygen-Sensitizing Properties: Synthesis and Photophysical Studies. J. Org. Chem. 2021, 86, 11482–11491. [Google Scholar]
- He, P.; Chen, Y.; Li, X.-N.; Yan, Y.-Y.; Liu, C. AIPE-Active Cationic Ir(Iii) Complexes for Efficient Detection of 2,4,6-Trinitrophenol and Oxygen. Dalton Trans. 2023, 52, 128–135. [Google Scholar]
- Hu, Y.-X.; Xia, X.; He, W.-Z.; Chi, H.-J.; Dong, Y.; Xiao, G.-Y.; Lv, Y.-L.; Li, X.; Zhang, D.-Y. Novel Ir(III) Complexes Ligated with 2-(2,6-Difluoropyridin-3-Yl)Benzo[d]Thiazole for Highly Efficient OLEDs with Mild Efficiency Roll-Off. Dye. Pigment. 2019, 166, 254–259. [Google Scholar]
- Liu, D.; Ding, Q.; Fu, Y.; Song, Z.; Peng, Y. Rh-Catalyzed C–H Amidation of 2-Arylbenzo[d]Thiazoles: An Approach to Single Organic Molecule White Light Emitters in the Solid State. Org. Lett. 2019, 21, 2523–2527. [Google Scholar]
- Paul, R.; Chandra Shit, S.; Mandal, H.; Rabeah, J.; Kashyap, S.S.; Nailwal, Y.; Shinde, D.B.; Lai, Z.; Mondal, J. Benzothiazole-Linked Metal-Free Covalent Organic Framework Nanostructures for Visible-Light-Driven Photocatalytic Conversion of Phenylboronic Acids to Phenols. ACS Appl. Nano Mater. 2021, 4, 11732–11742. [Google Scholar] [CrossRef]
- Song, Y.; Hu, L.; Cheng, Q.; Chen, Z.; Su, H.; Liu, H.; Liu, R.; Zhu, S.; Zhu, H. Benzothiazole Derivatives with Varied π-Conjugation: Synthesis, Tunable Solid-State Emission, and Application in Single-Component LEDs. J. Mater. Chem. C Mater. 2022, 10, 6392–6401. [Google Scholar] [CrossRef]
- Song, Y.; He, Y.; Hu, L.; Cheng, Q.; Chen, Z.; Liu, R.; Zhu, S.; Zhu, H. Panchromatic Luminescent D–π–A Benzothiazoles with Different π-Bridging Modulation: Design, Synthesis and Application in WLED Devices. Mater. Chem. Front. 2023, 7, 2860–2870. [Google Scholar] [CrossRef]
- Chou, P.-T.; Studer, S.L.; Martinez, M.L. Studies of the Triplet State of 2-(2′-Hydroxyphenyl) Benzothiazole. Chem. Phys. Lett. 1991, 178, 393–398. [Google Scholar] [CrossRef]
- Yi, P.; Peng, H.; Wang, Z.; Yu, X.; Li, X.; Liang, Y. Theoretical Study of Structure, Energetic, Hydrogen Bonds Strength and Intramolecular Proton Transfer of 2-(2-R (ROH, NH2, SH) Phenyl (or Pyridyl)) Benzoxazoles (or Benzothiazoles). Chin. J. Chem. 2011, 29, 650–654. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, J.; Wu, W.; Yu, X.; Liu, Y. Accessing the Long-Lived Triplet Excited States in Bodipy-Conjugated 2-(2-Hydroxyphenyl) Benzothiazole/Benzoxazoles and Applications as Organic Triplet Photosensitizers for Photooxidations. J. Org. Chem. 2012, 77, 6166–6178. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, Y.-T.; Demchenko, A.P.; Chou, P.-T. Amino Proton Donors in Excited-State Intramolecular Proton-Transfer Reactions. Nat. Rev. Chem. 2018, 2, 131–143. [Google Scholar] [CrossRef]
- Katkova, M.A.; Pushkarev, A.P.; Balashova, T.V.; Konev, A.N.; Fukin, G.K.; Ketkov, S.Y.; Bochkarev, M.N. Near-Infrared Electroluminescent Lanthanide [Pr(Iii), Nd(Iii), Ho(Iii), Er(Iii), Tm(Iii), and Yb(Iii)] N,O-Chelated Complexes for Organic Light-Emitting Devices. J. Mater. Chem. 2011, 21, 16611–16620. [Google Scholar] [CrossRef]
- Balashova, T.V.; Burin, M.E.; Ilichev, V.A.; Starikova, A.A.; Marugin, A.V.; Rumyantcev, R.V.; Fukin, G.K.; Yablonskiy, A.N.; Andreev, B.A.; Bochkarev, M.N. Features of the Molecular Structure and Luminescence of Rare-Earth Metal Complexes with Perfluorinated (Benzothiazolyl)Phenolate Ligands. Molecules 2019, 24, 2376. [Google Scholar] [CrossRef]
- Wongsuwan, S.; Chatwichien, J.; Sirisaksoontorn, W.; Chainok, K.; Songsasen, A.; Chotima, R. Novel Pd(Ii) Pincer Complexes Bearing Salicylaldimine-Based Benzothiazole Derivatives: Synthesis, Structural Characterization, DNA/BSA Binding, and Biological Evaluation. New J. Chem. 2023, 47, 10624–10637. [Google Scholar] [CrossRef]
- Plyuta, N.; Barra, A.-L.; Novitchi, G.; Avarvari, N. Six-Coordinated Nickel(Ii) Complexes with Benzothiadiazole Schiff-Base Ligands: Synthesis, Crystal Structure, Magnetic and HFEPR Study. Dalton Trans. 2024, 53, 8835–8842. [Google Scholar] [PubMed]
- Palanisamy, S.; Lee, W.-Z.; Wang, Y.-M. CCDC 1483661: Experimental Crystal Structure Determination; Cambridge Crystallographic Data Centre: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Pierens, G.K.; Bernhardt, P.V.; Reutens, D.C. Heteronuclear NMR Spectroscopic Investigations of Hydrogen Bonding in 2-(Benzo[d]Thiazole-2′-Yl)-N-Alkylanilines. Magn. Reson. Chem. 2015, 53, 448–453. [Google Scholar] [PubMed]
- Chen, Y.; Fang, Y.; Gu, H.; Qiang, J.; Li, H.; Fan, J.; Cao, J.; Wang, F.; Lu, S.; Chen, X. Color-Tunable and ESIPT-Inspired Solid Fluorophores Based on Benzothiazole Derivatives: Aggregation-Induced Emission, Strong Solvatochromic Effect, and White Light Emission. ACS Appl. Mater. Interfaces 2020, 12, 55094–55106. [Google Scholar] [PubMed]
- Fang-Lu, F.; Jin-Qiu, J.; Xue-Mei, C. Synthesis, Crystal Structure and Fluorescent Properties of a Novel Benzothiazole-Derived Fluorescent Probe for Zn2+. J. Chem. Res. 2015, 39, 661–664. [Google Scholar]
- White, L.J.; Wells, N.J.; Blackholly, L.R.; Shepherd, H.J.; Wilson, B.; Bustone, G.P.; Runacres, T.J.; Hiscock, J.R. Towards Quantifying the Role of Hydrogen Bonding within Amphiphile Self-Association and Resultant Aggregate Formation. Chem. Sci. 2017, 8, 7620–7630. [Google Scholar]
- Wang, H.; Xu, Z.; Deng, G.-J.; Huang, H. Selective Formation of 2-(2-Aminophenyl)Benzothiazoles via Copper-Catalyzed Aerobic C−C Bond Cleavage of Isatins. Adv. Synth. Catal. 2020, 362, 1663–1668. [Google Scholar]
- Wang, G.; Ding, N.; Hao, H.; Jiang, Q.; Feng, Q.; Liu, K.; Hua, C.; Bian, H.; Fang, Y.; Liu, F. Controlling the Excited-State Relaxation for Tunable Single-Molecule Dual Fluorescence in Both the Solution and Film States. J. Mater. Chem. C Mater. 2022, 10, 1118–1126. [Google Scholar] [CrossRef]
- Kuz’mina, L.G.; Bezzubov, S.I.; Kulagin, S.V.; Bolotin, B.M. Structure and Physicochemical Properties of Three Structural Forms of Organic Luminophore 2-((2-Benzo[d]Thiazol-2-Yl)Phenyl)Carbamoyl)Benzoic Acid. Crystallogr. Rep. 2022, 67, 566–574. [Google Scholar] [CrossRef]
- Khisamov, R.M.; Ryadun, A.A.; Sukhikh, T.S.; Konchenko, S.N. Excitation Wavelength-Dependent Room-Temperature Phosphorescence: Unusual Properties of Novel Phosphinoamines. Mol. Syst. Des. Eng. 2021, 6, 1056–1065. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Kolybalov, D.S.; Khisamov, R.M.; Konchenko, S.N. Phenyl-2-benzothiazole-based α-aminophosphines: Synthesis, crystal structure, and photophysical properties. J. Struct. Chem. 2022, 63, 1446–1452. [Google Scholar]
- Sinitsa, D.K.; Pylova, E.K.; Mironova, O.A.; Bashirov, D.A.; Ryadun, A.A.; Sukhikh, T.S.; Konchenko, S.N. Lanthanide Complexes with a New Luminescent Iminophosphonamide Ligand Bearing Phenylbenzothiazole Substituents. Dalton Trans. 2024, 53, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Sinitsa, D.K.; Sukhikh, T.S.; Pushkarevsky, N.A.; Konchenko, S.N. New Asymmetric Iminophosphonamide Ligand and Its Yttrium Complex: Synthesis and Crystal Structure. J. Struct. Chem. 2024, 65, 666–675. [Google Scholar] [CrossRef]
- Billeau, S.; Chatel, F.; Robin, M.; Faure, R.; Galy, J.-P. 1H and 13C Chemical Shifts for 2-Aryl and 2-N-Arylamino Benzothiazole Derivatives. Magn. Reson. Chem. 2006, 44, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Pierens, G.K.; Venkatachalam, T.K.; Reutens, D. A Comparative Study between Para-Aminophenyl and Ortho-Aminophenyl Benzothiazoles Using NMR and DFT Calculations. Magn. Reson. Chem. 2014, 52, 453–459. [Google Scholar] [CrossRef]
- Meyer, M.; Molomut, N.; Nowak, M.; Ogur, M. Rearrangement of Aryl Benzothiazoles. Recl. Trav. Chim. Pays-Bas 1934, 53, 37–40. [Google Scholar] [CrossRef]
- Hein, D.W.; Alheim, R.J.; Leavitt, J.J. The Use of Polyphosphoric Acid in the Synthesis of 2-Aryl- and 2-Alkyl-Substituted Benzimidazoles, Benzoxazoles and Benzothiazoles1. J. Am. Chem. Soc. 1957, 79, 427–429. [Google Scholar] [CrossRef]
- Pylova, E.; Lasorne, B.; McClenaghan, N.; Jonusauskas, G.; Taillefer, M.; Konchenko, S.; Prieto, A.; Jaroschik, F. Visible-Light Organic Photosensitizers Based on 2-(2-Aminophenyl)Benzothiazoles for Photocycloaddition Reactions. Chem. Eur. J. 2024, 30, e202401851. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, W. A New Fluorescent Probe for Gasotransmitter H2S: High Sensitivity, Excellent Selectivity, and a Significant Fluorescence off–on Response. Chem. Commun. 2014, 50, 4214–4217. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Stimson, D.H.R.; Bhalla, R.; Pierens, G.K.; Reutens, D.C. Synthesis, Characterization and 11C-Radiolabeling of Aminophenyl Benzothiazoles: Structural Effects on the Alkylation of Amino Group. J. Label. Compd. Radiopharm. 2014, 57, 566–573. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, L.; Shang, Y.; Zhu, Z.; Jin, S.; Guo, Z.; Wang, X. Concurrent Suppression of Aβ Aggregation and NLRP3 Inflammasome Activation for Treating Alzheimer’s Disease. Chem. Sci. 2022, 13, 2971–2980. [Google Scholar] [CrossRef]
- Kim, J.K.; Bong, S.Y.; Park, R.; Park, J.; Jang, D.O. An ESIPT-Based Fluorescent Turn-on Probe with Isothiocyanate for Detecting Hydrogen Sulfide in Environmental and Biological Systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 278, 121333. [Google Scholar]
- Gajare, A.S.; Shaikh, N.S.; Jnaneshwara, G.K.; Deshpande, V.H.; Ravindranathan, T.; Bedekar, A. V Clay Catalyzed Conversion of Isatoic Anhydride to 2-(o-Aminophenyl)Oxazolines. J. Chem. Soc. Perkin Trans. 1 2000, 6, 999–1001. [Google Scholar]
- Fadda, A.A.; Refat, H.M.; Zaki, M.E.A.; Monir, E. Reactions of Isatoic Anhydride with Bifunctional Reagents: Synthesis of Some New Quinazolone Fused Heterocycles, 2-Substituted Anilinoheterocyclic Derivatives and Other Related Compounds. Synth. Commun. 2001, 31, 3537–3545. [Google Scholar] [CrossRef]
- Tseng, H.-W.; Liu, J.-Q.; Chen, Y.-A.; Chao, C.-M.; Liu, K.-M.; Chen, C.-L.; Lin, T.-C.; Hung, C.-H.; Chou, Y.-L.; Lin, T.-C.; et al. Harnessing Excited-State Intramolecular Proton-Transfer Reaction via a Series of Amino-Type Hydrogen-Bonding Molecules. J. Phys. Chem. Lett. 2015, 6, 1477–1486. [Google Scholar] [CrossRef]
- Huang, S.; Feng, B.; Cheng, X.; Huang, X.; Ding, J.; Yu, K.; Dong, J.; Zeng, W. Controlling ESIPT-Based AIE Effects for Designing Optical Materials with Single-Component White-Light Emission. Chem. Eng. J. 2023, 476, 146436. [Google Scholar]
- Feng, B.; Liu, Y.; Huang, S.; Huang, X.; Huang, L.; Liu, M.; Wu, J.; Du, T.; Wang, S.; Feng, X.; et al. Highly Selective Discrimination of Cysteine from Glutathione and Homo-Cysteine with a Novel AIE-ESIPT Fluorescent Probe. Sens. Actuators B Chem. 2020, 325, 128786. [Google Scholar] [CrossRef]
- Bi, A.; Liu, M.; Huang, S.; Zheng, F.; Ding, J.; Wu, J.; Tang, G.; Zeng, W. Construction and Theoretical Insights into the ESIPT Fluorescent Probe for Imaging Formaldehyde in Vitro and in Vivo. Chem. Commun. 2021, 57, 3496–3499. [Google Scholar]
- Feng, B.; Chu, F.; Huang, X.; Fang, Y.; Liu, M.; Liu, M.; Chen, F.; Zeng, W. Debut of a NIR ESIPT-Based Fluorescent Probe with Synergistic Effects for Boosting High-Contrast Imaging of β-Galactosidase in Ovarian Cancer. Sens. Actuators B Chem. 2023, 396, 134541. [Google Scholar]
- Ray, S.; Das, P.; Banerjee, B.; Bhaumik, A.; Mukhopadhyay, C. Piperazinylpyrimidine Modified MCM-41 for the Ecofriendly Synthesis of Benzothiazoles by the Simple Cleavage of Disulfide in the Presence of Molecular O2. RSC Adv. 2015, 5, 72745–72754. [Google Scholar]
- Thaslim Basha, S.; Sudhamani, H.; Rasheed, S.; Venkateswarlu, N.; Vijaya, T.; Naga Raju, C. Microwave-Assisted Neat Synthesis of α-Aminophosphonate/Phosphinate Derivatives of 2-(2-Aminophenyl)Benzothiazole as Potent Antimicrobial and Antioxidant Agents. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 1339–1343. [Google Scholar]
- Park, N.; Heo, Y.; Kumar, M.R.; Kim, Y.; Song, K.H.; Lee, S. Synthesis of Benzothiazoles through Copper-Catalyzed One-Pot Three-Component Reactions with Use of Sodium Hydrosulfide as a Sulfur Surrogate. Eur. J. Org. Chem. 2012, 2012, 1984–1993. [Google Scholar] [CrossRef]
- Bakthadoss, M.; Selvakumar, R.; Srinivasan, J. An Efficient Protocol for the Synthesis of Benzoheterocyclic Compounds via Solid-State Melt Reaction (SSMR). Tetrahedron Lett. 2014, 55, 5808–5812. [Google Scholar] [CrossRef]
- Stevens, M.F.G.; Shi, D.-F.; Castro, A. Antitumour Benzothiazoles. Part 2. Formation of 2,2′-Diaminobiphenyls from the Decomposition of 2-(4-Azidophenyl)Benzazoles in Trifluoromethanesulfonic Acid. J. Chem. Soc. Perkin Trans. 1996, 1, 83–93. [Google Scholar] [CrossRef]
- Du, S.; Tian, Z.; Yang, D.; Li, X.; Li, H.; Jia, C.; Che, C.; Wang, M.; Qin, Z. Synthesis, Antifungal Activity and Structure-Activity Relationships of Novel 3-(Difluoromethyl)-1-Methyl-1H-Pyrazole-4-Carboxylic Acid Amides. Molecules 2015, 20, 8395–8408. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Yu, Y.; Zhao, H.; Guo, Y.; Yang, Y. A Highly Sensitive and Selective Fluorescent Probe Based on a Pd-Catalyzed Reaction for Detection of Pd2+. Anal. Methods 2019, 11, 6053–6061. [Google Scholar]
- Shi, X.; Guo, J.; Liu, J.; Ye, M.; Xu, Q. Unexpectedly Simple Synthesis of Benzazoles by TBuONa-Catalyzed Direct Aerobic Oxidative Cyclocondensation of o-Thio/Hydroxy/Aminoanilines with Alcohols under Air. Chem. Eur. J. 2015, 21, 9988–9993. [Google Scholar]
- Anandaraj, P.; Saranya, S.; Ramesh, R. Synthesis and Structural Characterization of Palladium Pincer Complexes: Sustainable Synthesis of Benzothiazoles. Appl. Organomet. Chem. 2023, 37, e7062. [Google Scholar]
- Arora, A.; Weaver, J.D. Photocatalytic Generation of 2-Azolyl Radicals: Intermediates for the Azolylation of Arenes and Heteroarenes via C–H Functionalization. Org. Lett. 2016, 18, 3996–3999. [Google Scholar]
- Nacsa, E.D.; Lambert, T.H. Cross-Coupling of Sulfonic Acid Derivatives via Aryl-Radical Transfer (ART) Using TTMSS or Photoredox. Org. Chem. Front. 2018, 5, 64–69. [Google Scholar]
- Jiang, J.; Cai, X.; Hu, Y.; Liu, X.; Chen, X.; Wang, S.-Y.; Zhang, Y.; Zhang, S. Thermo-Promoted Reactions of Anthranils with Carboxylic Acids, Amines, Phenols, and Malononitrile under Catalyst-Free Conditions. J. Org. Chem. 2019, 84, 2022–2031. [Google Scholar]
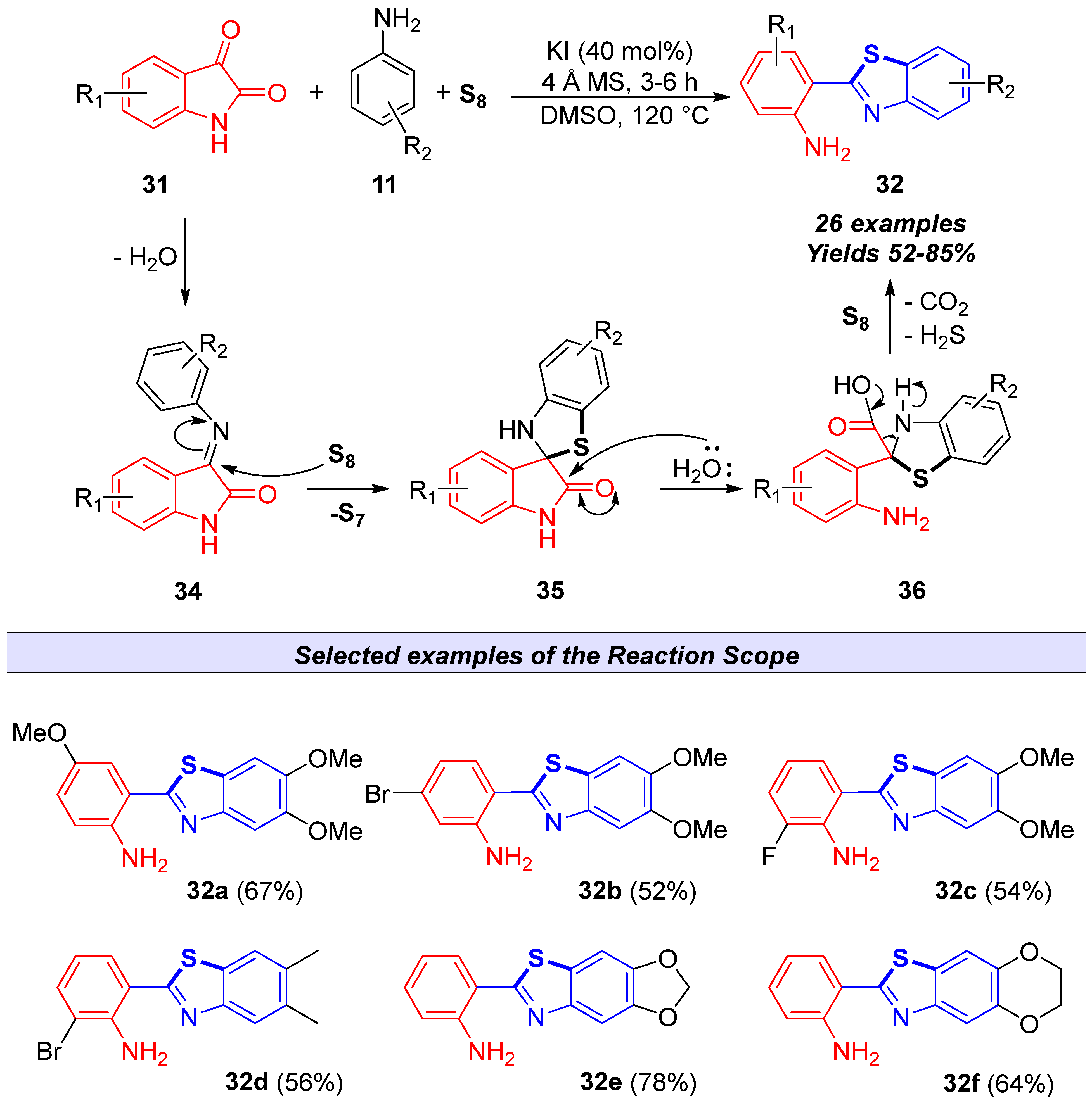
- Singh, M.; Vaishali; Paul, A.K.; Singh, V. Isatin as a 2-Aminobenzaldehyde Surrogate: Transition Metal-Free Efficient Synthesis of 2-(2′-Aminophenyl)Benzothiazole Derivatives. Org. Biomol. Chem. 2020, 18, 4459–4469. [Google Scholar] [CrossRef] [PubMed]
- Draganov, A.B.; Wang, K.; Holmes, J.; Damera, K.; Wang, D.; Dai, C.; Wang, B. Click with a Boronic Acid Handle: A Neighboring Group-Assisted Click Reaction That Allows Ready Secondary Functionalization. Chem. Commun. 2015, 51, 15180–15183. [Google Scholar] [CrossRef] [PubMed]
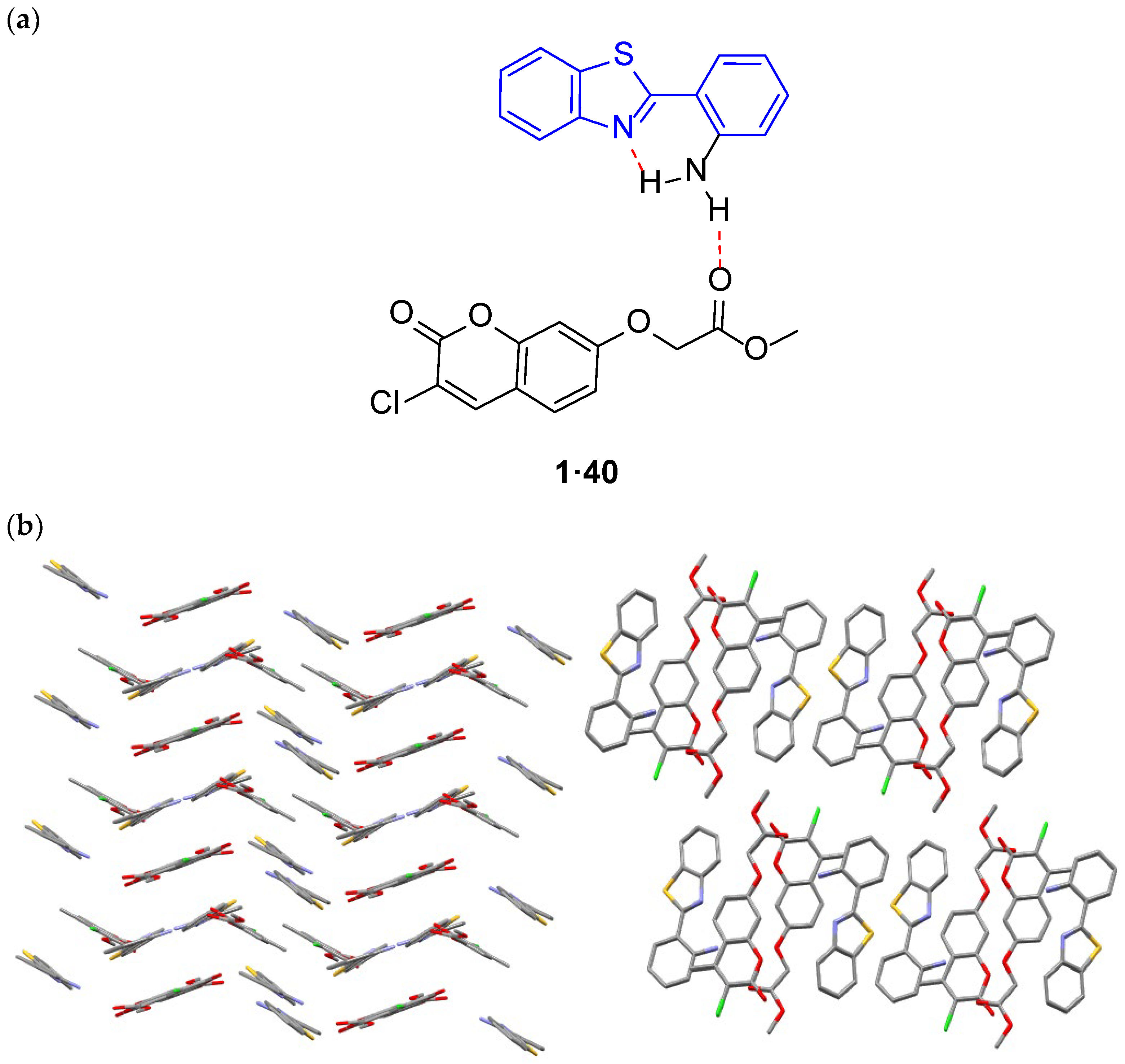
- Al-Amiery, A.A.; Al-Temimi, A.A.; Kadhum, A.A.H.; Al-Majedy, Y.K.; Al-Bayati, R.I.; Aday, H.A.; Mohamad, A.B. Co-Crystal Structure of Mixed Molecules of Methyl 2-(3-Chloro-4-Methyl-2-Oxo-2H-Chromen-7-Yloxy)Acetate and 2-(2-Aminophenyl)Benzothiazole. J. Struct. Chem. 2013, 54, 648–649. [Google Scholar] [CrossRef]
- Dhaka, G.; Singh, J.; Kaur, N. Benzothiazole Possessing Reversible and Reusable Selective Chemosensor for Fluoride Detection Based on Inhibition of Excited State Intramolecular Proton Transfer. Inorganica Chim. Acta 2016, 450, 380–385. [Google Scholar] [CrossRef]
- Ryu, H.; Choi, M.G.; Cho, E.J.; Chang, S.-K. Cu2+-Selective Fluorescent Probe Based on the Hydrolysis of Semicarbazide Derivative of 2-(2-Aminophenyl)Benzothiazole. Dye. Pigment. 2018, 149, 620–625. [Google Scholar] [CrossRef]
- Gülbaş, H.E.; Bozkurt, S.; Halay, E. Synthesis and Characterization of Dicarboxamide Derivative: Cu+2 Selective Turn-on Fluorometric and Colorimetric Chemosensor. J. Heterocycl. Chem. 2023, 60, 1223–1229. [Google Scholar] [CrossRef]
- Arafath, M.A.; Adam, F.; Al-Suede, F.S.R.; Razali, M.R.; Ahamed, M.B.K.; Abdul Majid, A.M.S.; Hassan, M.Z.; Osman, H.; Abubakar, S. Synthesis, Characterization, X-Ray Crystal Structures of Heterocyclic Schiff Base Compounds and in Vitro Cholinesterase Inhibition and Anticancer Activity. J. Mol. Struct. 2017, 1149, 216–228. [Google Scholar] [CrossRef]
- Palanisamy, S.; Wang, Y.-L.; Chen, Y.-J.; Chen, C.-Y.; Tsai, F.-T.; Liaw, W.-F.; Wang, Y.-M. In Vitro and in Vivo Imaging of Nitroxyl with Copper Fluorescent Probe in Living Cells and Zebrafish. Molecules 2018, 23, 2551. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, N.; Singh, N. Nitrogen and Sulfur Co-Doped Fluorescent Carbon Dots for the Trapping of Hg(Ii) Ions from Water. Mater. Adv. 2020, 1, 3009–3021. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Lu, D.-Y.; Lin, Y.-M.; Hwang, J.-K.; Yu, J.-S.K. Structural Transformations in Dinuclear Zinc Complexes Involving Zn–Zn Bonds. Chem. Commun. 2007, 40, 4125–4127. [Google Scholar] [CrossRef]
- Lu, D.; Yu, J.K.; Kuo, T.; Lee, G.; Wang, Y.; Tsai, Y. Theory-Guided Experiments on the Mechanistic Elucidation of the Reduction of Dinuclear Zinc, Manganese, and Cadmium Complexes. Angew. Chem. Int. Ed. Engl. 2011, 50, 7611–7615. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-L.; Chen, W.; Song, J. Lanthanide(II)−Alkali Sandwich Complexes with Cation−Arene π Interactions: Synthesis, Structure, and Solvent-Mediated Redox Transformations. Organometallics 2011, 30, 2252–2260. [Google Scholar]
- Pan, C.; Sheng, S.; Hou, C.; Pan, Y.; Wang, J.; Fan, Y. A New Type of Lanthanide Complex—Two Divalent Ytterbium Species Assembled from Cation–π Interactions. Eur. J. Inorg. Chem. 2012, 2012, 779–782. [Google Scholar]
- Zhou, L.; Yao, Y.; Li, C.; Zhang, Y.; Shen, Q. Synthesis and Characterization of a Series of New Lanthanide Derivatives Supported by Silylene-Bridged Diamide Ligands and Their Catalytic Activities for the Polymerization of Methyl Methacrylate. Organometallics 2006, 25, 2880–2885. [Google Scholar]
- Zhu, X.; Fan, J.; Wu, Y.; Wang, S.; Zhang, L.; Yang, G.; Wei, Y.; Yin, C.; Zhu, H.; Wu, S.; et al. Synthesis, Characterization, Selective Catalytic Activity, and Reactivity of Rare Earth Metal Amides with Different Metal−Nitrogen Bonds. Organometallics 2009, 28, 3882–3888. [Google Scholar] [CrossRef]
- Pan, C.-L.; Pan, Y.-S.; Wang, J.; Song, J.-F. A Heterometallic Sandwich Complex of Europium(Ii) for Luminescent Studies. Dalton Trans. 2011, 40, 6361. [Google Scholar]
- Mironova, O.A.; Lashchenko, D.I.; Ryadun, A.A.; Sukhikh, T.S.; Bashirov, D.A.; Pushkarevsky, N.A.; Konchenko, S.N. Synthesis and Photophysical Properties of Rare Earth Complexes Bearing Silanediamido Ligands Me2Si(NAryl)2 2− (Aryl = Dipp, Mes). New J. Chem. 2022, 46, 2351–2359. [Google Scholar]
- Mironova, O.A.; Ryadun, A.A.; Pushkarevskii, N.A.; Sukhikh, T.S.; Konchenko, S.N. Silandiamide Complexes of Lanthanides with N-Phenylbenzothiazole Substituents: Synthesis, Unexpected Products, Structure, and Luminescence. J. Struct. Chem. 2024, 65, 399–411. [Google Scholar]
- Chen, H.; Bartlett, R.A.; Dias, H.V.R.; Olmstead, M.M.; Power, P.P. Synthesis and Structural Characterization of Manganese(II) Derivatives of the Bulky, Chelating Bis(Amido) Ligands [(NMes)2SiMe2]2- and [DippNCH2CH2NDipp]2-, Their Neutral Amine Precursors, and Their Lithium Salts (Mes = 2,4,6-Me3C6H2; Dipp = 2,6-i-Pr2C6H3). Inorg. Chem. 1991, 30, 2487–2494. [Google Scholar]
- Hill, M.S.; Hitchcock, P.B. [Me2Al(THF)2]+[{Me2Si(NDipp)2}2Zr2Cl5]- (Dipp = 2,6-Diisopropylphenyl), an Unusual Zirconium/Aluminum Ion Pair Containing a THF-Stabilized Dimethylaluminum Cation. Organometallics 2002, 21, 3258–3262. [Google Scholar] [CrossRef]
- Udhayakumari, D.; Jerome, P.; Vijay, N.; Hwan Oh, T. ESIPT: An Approach and Future Perspective for the Detection of Biologically Important Analytes. J. Lumin. 2024, 267, 120350. [Google Scholar] [CrossRef]
- Padalkar, V.S.; Seki, S. Excited-State Intramolecular Proton-Transfer (ESIPT)-Inspired Solid State Emitters. Chem. Soc. Rev. 2016, 45, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Wei, Y.-C.; Chou, P.-T. Correlation between Kinetics and Thermodynamics for Excited-State Intramolecular Proton Transfer Reactions. J. Phys. Chem. A 2021, 125, 6611–6620. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Padalkar, V.S.; Tathe, A.B.; Sekar, N. ESIPT-Inspired Benzothiazole Fluorescein: Photophysics of Microenvironment PH and Viscosity. Dye. Pigment. 2013, 98, 507–517. [Google Scholar] [CrossRef]
- Padalkar, V.; Ramasami, P.; Sekar, N. TD-DFT Study of Excited-State Intramolecular Proton Transfer (ESIPT) of 2-(1,3-Benzothiazol-2-Yl)-5-(N,N-Diethylamino)Phenol with Benzoxazole and Benzimidazole Analogues. Procedia. Comput. Sci. 2013, 18, 797–805. [Google Scholar] [CrossRef]
- Majumdar, P.; Zhao, J. 2-(2-Hydroxyphenyl)-Benzothiazole (HBT)-Rhodamine Dyad: Acid-Switchable Absorption and Fluorescence of Excited-State Intramolecular Proton Transfer (ESIPT). J. Phys. Chem. B 2015, 119, 2384–2394. [Google Scholar] [CrossRef]
- Wang, T.; Lv, M.; Zhang, Y.; Gao, Y.; Cai, Z.; Zhang, Y.; Song, J.; Liu, J.; Yin, H.; Shang, F. TDDFT Study on the ESIPT Properties of 2-(2′-Hydroxyphenyl)-Benzothiazole and Sensing Mechanism of a Derived Fluorescent Probe for Fluoride Ion. Molecules 2024, 29, 1541. [Google Scholar] [CrossRef]
- Peng, H.; Kong, S.; Deng, X.; Deng, Q.; Qi, F.; Liu, C.; Tang, R. Development of a Ratiometric Fluorescent Probe with Zero Cross-Talk for the Detection of SO2 Derivatives in Foods and Live Cells. J. Agric. Food Chem. 2023, 71, 14322–14329. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, S.; Abbas Abedi, S.A.; Jeong, M.; Li, H.; Hwa Kim, M.; Park, S.; Liu, X.; Yoon, J.; Chen, X. Janus-Type ESIPT Chromophores with Distinctive Intramolecular Hydrogen-bonding Selectivity. Angew. Chem. Int. Ed. Engl. 2023, 62, e202311543. [Google Scholar] [CrossRef]
- Jain, A.; De, S.; Haloi, P.; Barman, P. The Solvent-Regulated Excited State Reaction Mechanism of 2-(2′-Hydroxyphenyl)Benzothiazole Aggregates. Photochem. Photobiol. Sci. 2024, 23, 65–78. [Google Scholar] [CrossRef]
- Kanlayakan, N.; Kerdpol, K.; Prommin, C.; Salaeh, R.; Chansen, W.; Sattayanon, C.; Kungwan, N. Effects of Different Proton Donor and Acceptor Groups on Excited-State Intramolecular Proton Transfers of Amino-Type and Hydroxy-Type Hydrogen-Bonding Molecules: Theoretical Insights. New J. Chem. 2017, 41, 8761–8771. [Google Scholar] [CrossRef]
- Sukhikh, T.S.; Kolybalov, D.S.; Pylova, E.K.; Konchenko, S.N. Luminescent Zn Halide Complexes with 2-(2-Aminophenyl)Benzothiazole Derivatives. Inorganics 2022, 10, 138. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Tang, K.-C.; Chou, P.-T. Excited-State Proton Coupled Charge Transfer Modulated by Molecular Structure and Media Polarization. Chem. Soc. Rev. 2013, 42, 1379–1408. [Google Scholar] [PubMed]
- Ji, S.; Ding, Z.; Zhao, J.; Zheng, D. Substituent Control of Dynamical Process for Excited State Intramolecular Proton Transfer of Benzothiazole Derivatives. Chem. Phys. 2022, 560, 111568. [Google Scholar]
- Zhu, Q.; An, B.; Yuan, H.; Li, Y.; Guo, X.; Zhang, J. Computational Studies on Amino-Type Excited-State Intramolecular Proton Transfer and Subsequent Cis–Trans Isomerisation Reactions of Three 2-(2′-Aminophenyl)Benzothiazole Derivatives. Mol. Phys. 2017, 115, 288–296. [Google Scholar]
- An, B.; Yuan, H.; Zhu, Q.; Li, Y.; Guo, X.; Zhang, J. Theoretical Insight into the Excited-State Intramolecular Proton Transfer Mechanisms of Three Amino-Type Hydrogen-Bonding Molecules. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 175, 36–42. [Google Scholar]
- Yang, D.; Zhang, T.; Jia, M.; Cheng, S. Modulating NH-Based Excited-State Intramolecular Proton Transfer by Different Electron-Donating/Withdrawing Substituents in 2-(2′-Aminophenyl)Benzothiazole Compounds. Chem. Phys. Lett. 2019, 724, 57–66. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar]
- Peng, Q.; Shuai, Z. Molecular Mechanism of Aggregation-Induced Emission. Aggregate 2021, 2, e91. [Google Scholar]
- Liang, K.; Shao, Q.; Xia, G.; Wang, Y.; Jiang, L.; Hong, L.; Wang, H. Tuning Solid State Emission of Semisquaraines via Trimming Central-Ring Structures. Dye. Pigment. 2020, 173, 107926. [Google Scholar] [CrossRef]
- Yao, H.; Minami, H.; Funada, T. Organic Nanoparticles Based on Lewis-Pair Formation: Observation of Prototropically Controlled Dual Fluorescence. Photochem. Photobiol. Sci. 2018, 17, 1376–1385. [Google Scholar] [PubMed]
- Tseng, S.-M.; Chao, C.-M.; Chang, K.-H.; Wen, C.-S.; Chou, T.-C.; Tsai, T.-L.; Wu, T.-W.; Haung, X.-C.; Liu, J.-Q.; Hung, C.-H.; et al. Substituent Effects in Six(Anilido)-Five(Thiazole) Membered Ring Boron Difluoride Dyes. ChemPhotoChem 2022, 6, e202100188. [Google Scholar]
- Maurya, R.C.; Sharma, P. Synthesis, Magnetic and Spectral Studies of Co(II) Picrate Complexes with Heterocyclic Nitrogen Donors. Indian J. Chem. 1999, 38A, 509–513. [Google Scholar]
- Maurya, R.C.; Sharma, P.; Roy, S. Synthesis and Characterization of Some Mixed-Ligand Picrate Complexes of Nickel(II) Involving Heterocyclic Nitrogen Donors. Synth. React. Inorg. Met.-Org. Chem. 2003, 33, 683–698. [Google Scholar] [CrossRef]
- Pal, N.; Kumar, M.; Seth, G. Biological Important Ni(II) Ternary Complexes Derived from 2-Substituted Benzothiazoles and Amino Acids. J. Chemistry. 2011, 8, 612847. [Google Scholar]
- Booysen, I.N.; Gerber, T.I.A.; Mayer, P. Reactions of the Fac-[Re(CO)3]+ and [ReO]3+ Moieties with Substituted Benzothiazoles. Inorganica Chim. Acta 2010, 363, 1292–1296. [Google Scholar]
- Machura, B.; Wolff, M.; Gryca, I.; Palion, A.; Michalik, K. Novel Re(I) Tricarbonyl Complexes of Chelating Ligands with Aromatic N-Heterocycle Ring and Aliphatic Amine Donor—Synthesis, Spectroscopic Characterization, X-Ray Structure and DFT Calculations. Polyhedron 2011, 30, 2275–2285. [Google Scholar]
- Machura, B.; Wolff, M.; Gryca, I.; Kruszynski, R. Syntheses, Structures, Spectroscopic Properties and DFT Calculations of Re(V)-Benzothiazole and 2-(2-Aminophenyl)Benzothiazole Complexes. Polyhedron 2012, 40, 93–104. [Google Scholar] [CrossRef]
- Aleksanyan, D.V.; Churusova, S.G.; Rybalkina, E.Y.; Nelyubina, Y.V.; Kozlov, V.A. Pd(II) and Pt(II) Pincer Complexes of a Benzothiazole-Appended Methylthioacetamide Ligand: Synthesis and in Vitro Cytotoxicity. INEOS OPEN 2021, 6, 237–242. [Google Scholar]
- Aleksanyan, D.V.; Churusova, S.G.; Brunova, V.V.; Peregudov, A.S.; Shakhov, A.M.; Rybalkina, E.Y.; Klemenkova, Z.S.; Kononova, E.G.; Denisov, G.L.; Kozlov, V.A. Mechanochemistry for the Synthesis of Non-Classical N-Metalated Palladium(Ii) Pincer Complexes. Dalton Trans. 2021, 50, 16726–16738. [Google Scholar]
- Kaplunov, M.G.; Yakushchenko, I.K.; Krasnikova, S.S.; Pivovarov, A.P.; Balashova, I.O. Electroluminescent Materials Based on New Metal Complexes for Organic Light-Emitting Diodes. High Energ. Chem. 2008, 42, 563–565. [Google Scholar] [CrossRef]
- Krasnikova, S.S.; Shestakov, A.F.; Kaplunov, M.G. Experimental and Theoretical Study of Electroluminescence Spectra of the Light-Emitting Devices Based on Zinc Complexes of Amino-Substituted Ligands. Mol. Cryst. Liq. Cryst. 2014, 589, 56–66. [Google Scholar] [CrossRef]
- Odod, A.V.; Nikonova, E.N.; Nikonov, S.Y.; Kopylova, T.N.; Kaplunov, M.G.; Krasnikova, S.S.; Nikitenko, S.L.; Yakushchenko, I.K. Electroluminescence of Zinc Complexes in Various OLED Structures. Russ. Phys. J. 2017, 60, 7–13. [Google Scholar] [CrossRef]
- Nikitenko, S.L.; Kaplunov, M.G.; Yakushchenko, I.K.; Echmaev, S.B. Electroluminescence and Photovoltaic Properties of Bis{N-[2-(Benzothiazol-2-Yl)Phenyl]-N-(4-Methylphenylsulfonyl)Amido}zinc. Russ. Chem. Bull. 2017, 66, 980–985. [Google Scholar] [CrossRef]
- Tang, L.; Xia, J.; Zhong, K.; Tang, Y.; Gao, X.; Li, J. A Simple AIE-Active Fluorogen for Relay Recognition of Cu2+ and Pyrophosphate through Aggregation-Switching Strategy. Dye. Pigment. 2020, 178, 108379. [Google Scholar] [CrossRef]
- Tang, L.; Dai, X.; Zhong, K.; Wen, X.; Wu, D. A Phenylbenzothiazole Derived Fluorescent Sensor for Zn(II) Recognition in Aqueous Solution Through “Turn-On” Excited-State Intramolecular Proton Transfer Emission. J. Fluoresc. 2014, 24, 1487–1493. [Google Scholar] [CrossRef]
- Janakipriya, S.; Tamilmani, S.; Thennarasu, S. A Novel 2-(2′-Aminophenyl)Benzothiazole Derivative Displays ESIPT and Permits Selective Detection of Zn2+ Ions: Experimental and Theoretical Studies. RSC Adv. 2016, 6, 71496–71500. [Google Scholar] [CrossRef]
- Halay, E. Cation Sensing by a Novel Triazine-Cored Intermediate as a Fluorescent Chemosensor Incorporating Benzothiazole Fluorophore. Res. Chem. Intermed. 2021, 47, 4281–4295. [Google Scholar] [CrossRef]
- Bozkurt, S.; Halay, E. Synthesis, Application and AIE Properties of Novel Fluorescent Tetraoxocalix[2]Arene[2]Triazine: The Detection of a Hazardous Anion, Cyanate. Tetrahedron 2020, 76, 131647. [Google Scholar] [CrossRef]
- Bozkurt, S.; Halay, E.; Durmaz, M.; Topkafa, M.; Ceylan, Ö. A Novel Turn-on Fluorometric “Reporter-Spacer-Receptor” Chemosensor Based on Calix[4]Arene Scaffold for Detection of Cyanate Anion. J. Heterocycl. Chem. 2021, 58, 1079–1088. [Google Scholar] [CrossRef]
- Choi, M.G.; Cho, M.J.; Ryu, H.; Hong, J.; Chang, S.-K. Fluorescence Signaling of Thiophenol by Hydrolysis of Dinitrobenzenesulfonamide of 2-(2-Aminophenyl)Benzothiazole. Dye. Pigment. 2017, 143, 123–128. [Google Scholar] [CrossRef]
- Halay, E.; Bozkurt, S. Enantioselective Recognition of Carboxylic Acids by Novel Fluorescent Triazine-Based Thiazoles. Chirality 2018, 30, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, M.; Acikbas, Y.; Bozkurt, S.; Capan, R.; Erdogan, M.; Ozkaya, C. A Novel Calix[4]Arene Thiourea Decorated with 2-(2-Aminophenyl)Benzothiazole Moiety as Highly Selective Chemical Gas Sensor for Dichloromethane Vapor. ChemistrySelect 2021, 6, 4670–4676. [Google Scholar]
- Chen, L.; Wu, D.; Kim, J.-M.; Yoon, J. An ESIPT-Based Fluorescence Probe for Colorimetric, Ratiometric, and Selective Detection of Phosgene in Solutions and the Gas Phase. Anal. Chem. 2017, 89, 12596–12601. [Google Scholar]
- Tang, Y.; Lee, D.; Wang, J.; Li, G.; Yu, J.; Lin, W.; Yoon, J. Development of Fluorescent Probes Based on Protection–Deprotection of the Key Functional Groups for Biological Imaging. Chem. Soc. Rev. 2015, 44, 5003–5015. [Google Scholar] [CrossRef]
- Liu, B.-Q.; Chen, Y.-T.; Chen, Y.-W.; Chung, K.-Y.; Tsai, Y.-H.; Li, Y.-J.; Chao, C.-M.; Liu, K.-M.; Tseng, H.-W.; Chou, P.-T. Ethylene Glycol Modified 2-(2′-Aminophenyl)Benzothiazoles at the Amino Site: The Excited-State N-H Proton Transfer Reactions in Aqueous Solution, Micelles and Potential Application in Live-Cell Imaging. Methods Appl. Fluoresc. 2016, 4, 014004. [Google Scholar] [CrossRef]
- Huang, T.; Yan, S.; Yu, Y.; Xue, Y.; Yu, Y.; Han, C. Dual-Responsive Ratiometric Fluorescent Probe for Hypochlorite and Peroxynitrite Detection and Imaging In Vitro and In Vivo. Anal. Chem. 2022, 94, 1415–1424. [Google Scholar]
- Leuma Yona, R.; Mazères, S.; Faller, P.; Gras, E. Thioflavin Derivatives as Markers for Amyloid-β Fibrils: Insights into Structural Features Important for High-Affinity Binding. ChemMedChem 2008, 3, 63–66. [Google Scholar]
- Li, X.; Tao, R.-R.; Hong, L.-J.; Cheng, J.; Jiang, Q.; Lu, Y.-M.; Liao, M.-H.; Ye, W.-F.; Lu, N.-N.; Han, F.; et al. Visualizing Peroxynitrite Fluxes in Endothelial Cells Reveals the Dynamic Progression of Brain Vascular Injury. J. Am. Chem. Soc. 2015, 137, 12296–12303. [Google Scholar] [CrossRef]
- Cellier, M.; Fabrega, O.J.; Fazackerley, E.; James, A.L.; Orenga, S.; Perry, J.D.; Salwatura, V.L.; Stanforth, S.P. 2-Arylbenzothiazole, Benzoxazole and Benzimidazole Derivatives as Fluorogenic Substrates for the Detection of Nitroreductase and Aminopeptidase Activity in Clinically Important Bacteria. Bioorg. Med. Chem. 2011, 19, 2903–2910. [Google Scholar]
- Sedgwick, A.C.; Sun, X.; Kim, G.; Yoon, J.; Bull, S.D.; James, T.D. Boronate Based Fluorescence (ESIPT) Probe for Peroxynitrite. Chem. Commun. 2016, 52, 12350–12352. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, K.; Li, J.; Xie, Y.; Li, Y.; Wang, X.; Xie, X.; Jiao, X.; Tang, B. Harnessing Se=N to Develop Novel Fluorescent Probes for Visualizing the Variation of Endogenous Hypobromous Acid (HOBr) during the Administration of an Immunotherapeutic Agent. Chem. Commun. 2021, 57, 12679–12682. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Liu, C.; Hu, Y.; Peng, T.; He, Y. A Novel Ratiometric Fluorescent Probe for Hypobromous Acid Detection in Living Cells. ChemistrySelect 2022, 7, e202203278. [Google Scholar] [CrossRef]
- Kanlayakan, N.; Kungwan, N. Theoretical Study of Heteroatom and Substituent Effects on Excited-State Intramolecular Proton Transfers and Electronic Properties of Amino-Type Hydrogen Bonding Molecules. J. Lumin. 2021, 238, 118260. [Google Scholar] [CrossRef]
- Kanlayakan, N.; Kungwan, N. Molecular Design of Amino-Type Hydrogen-Bonding Molecules for Excited-State Intramolecular Proton Transfer (ESIPT)-Based Fluorescent Probe Using the TD-DFT Approach. New J. Chem. 2021, 45, 12500–12508. [Google Scholar] [CrossRef]
- Huang, X.; Quinn, T.R.; Harms, K.; Webster, R.D.; Zhang, L.; Wiest, O.; Meggers, E. Direct Visible-Light-Excited Asymmetric Lewis Acid Catalysis of Intermolecular [2+2] Photocycloadditions. J. Am. Chem. Soc. 2017, 139, 9120–9123. [Google Scholar] [CrossRef]
- Daub, M.E.; Jung, H.; Lee, B.J.; Won, J.; Baik, M.-H.; Yoon, T.P. Enantioselective [2+2] Cycloadditions of Cinnamate Esters: Generalizing Lewis Acid Catalysis of Triplet Energy Transfer. J. Am. Chem. Soc. 2019, 141, 9543–9547. [Google Scholar] [CrossRef]
- Yao, Z.-J.; Lin, N.; Qiao, X.-C.; Zhu, J.-W.; Deng, W. Cyclometalated Half-Sandwich Iridium Complex for Catalytic Hydrogenation of Imines and Quinolines. Organometallics 2018, 37, 3883–3892. [Google Scholar] [CrossRef]
- Mdluli, V.; Diluzio, S.; Lewis, J.; Kowalewski, J.F.; Connell, T.U.; Yaron, D.; Kowalewski, T.; Bernhard, S. High-Throughput Synthesis and Screening of Iridium(III) Photocatalysts for the Fast and Chemoselective Dehalogenation of Aryl Bromides. ACS Catal. 2020, 10, 6977–6987. [Google Scholar] [CrossRef]
- Hu, N.; Jung, H.; Zheng, Y.; Lee, J.; Zhang, L.; Ullah, Z.; Xie, X.; Harms, K.; Baik, M.-H.; Meggers, E. Catalytic Asymmetric Dearomatization by Visible-Light-Activated [2+2] Photocycloaddition. Angew. Chem. Int. Ed. Engl. 2018, 57, 6242–6246. [Google Scholar]
- Zhang, L.; Meggers, E. Steering Asymmetric Lewis Acid Catalysis Exclusively with Octahedral Metal-Centered Chirality. Acc. Chem. Res. 2017, 50, 320–330. [Google Scholar]
- Geng, L.; Sun, R.; Zhang, D.-S.; Yu, M.-H.; Chang, Z.; Bu, X.-H. Harnessing Triplet Excitons: Advances in Luminescence Metal Coordination Compounds. Coord. Chem. Rev. 2024, 518, 216066. [Google Scholar]
- Zhang, X.; Rovis, T. Photocatalyzed Triplet Sensitization of Oximes Using Visible Light Provides a Route to Nonclassical Beckmann Rearrangement Products. J. Am. Chem. Soc. 2021, 143, 21211–21217. [Google Scholar]
- Huang, M.; Zhang, L.; Pan, T.; Luo, S. Deracemization through Photochemical E/Z Isomerization of Enamines. Science 2022, 375, 869–874. [Google Scholar]
- Müller, C.; Bauer, A.; Maturi, M.M.; Cuquerella, M.C.; Miranda, M.A.; Bach, T. Enantioselective Intramolecular [2 + 2]-Photocycloaddition Reactions of 4-Substituted Quinolones Catalyzed by a Chiral Sensitizer with a Hydrogen-Bonding Motif. J. Am. Chem. Soc. 2011, 133, 16689–16697. [Google Scholar] [CrossRef]
- Tian, D.; Sun, X.; Cao, S.; Wang, E.-M.; Yin, Y.; Zhao, X.; Jiang, Z. Enantioselective Intermolecular [2 + 2] Photocycloadditions of Vinylazaarenes with Triplet-State Electron-Deficient Olefins. Chin. J. Catal. 2022, 43, 2732–2742. [Google Scholar]
- Miller, Z.D.; Lee, B.J.; Yoon, T.P. Enantioselective Crossed Photocycloadditions of Styrenic Olefins by Lewis Acid Catalyzed Triplet Sensitization. Angew Chem. Int. Ed. Engl. 2017, 56, 11891–11895. [Google Scholar]
- Großkopf, J.; Kratz, T.; Rigotti, T.; Bach, T. Enantioselective Photochemical Reactions Enabled by Triplet Energy Transfer. Chem. Rev. 2022, 122, 1626–1653. [Google Scholar]
- Patra, T.; Bellotti, P.; Strieth-Kalthoff, F.; Glorius, F. Photosensitized Intermolecular Carboimination of Alkenes through the Persistent Radical Effect. Angew Chem. Int. Ed. Engl. 2020, 59, 3172–3177. [Google Scholar]
- Poplata, S.; Tröster, A.; Zou, Y.-Q.; Bach, T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2+2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. [Google Scholar] [CrossRef]
- Dutta, S.; Erchinger, J.E.; Strieth-Kalthoff, F.; Kleinmans, R.; Glorius, F. Energy Transfer Photocatalysis: Exciting Modes of Reactivity. Chem. Soc. Rev. 2024, 53, 1068–1089. [Google Scholar] [PubMed]
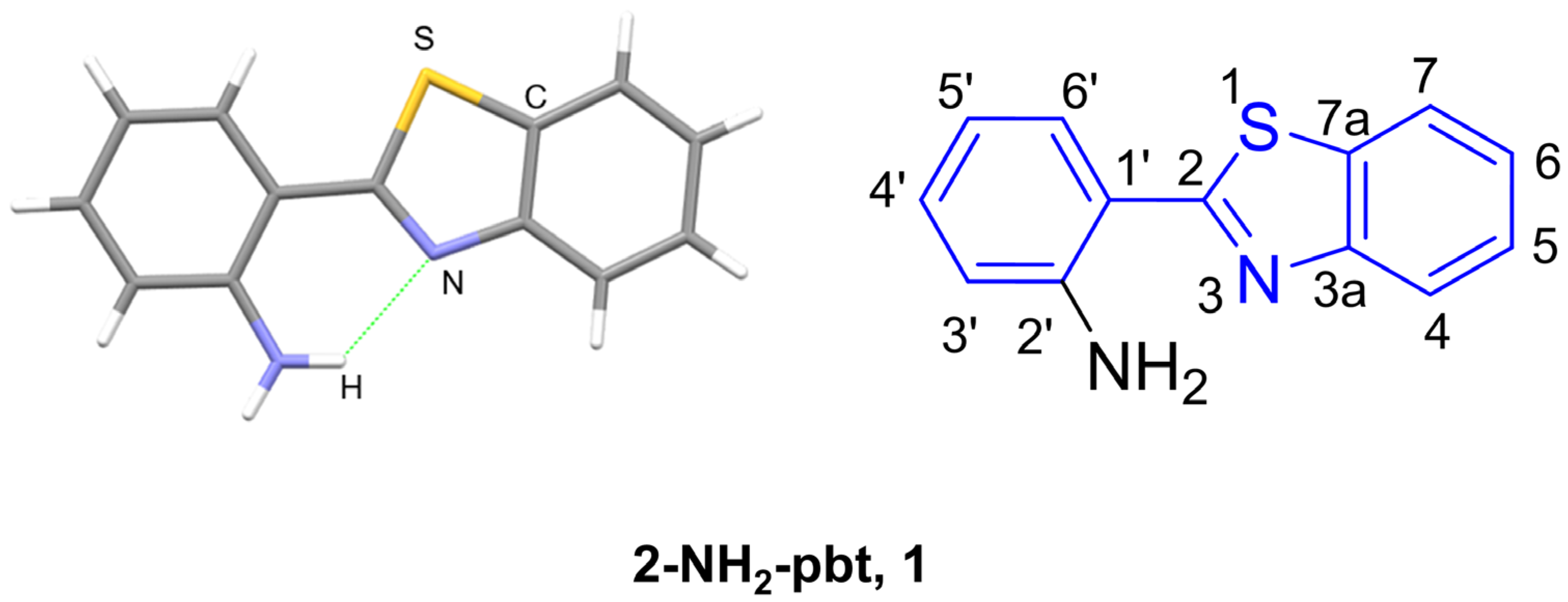






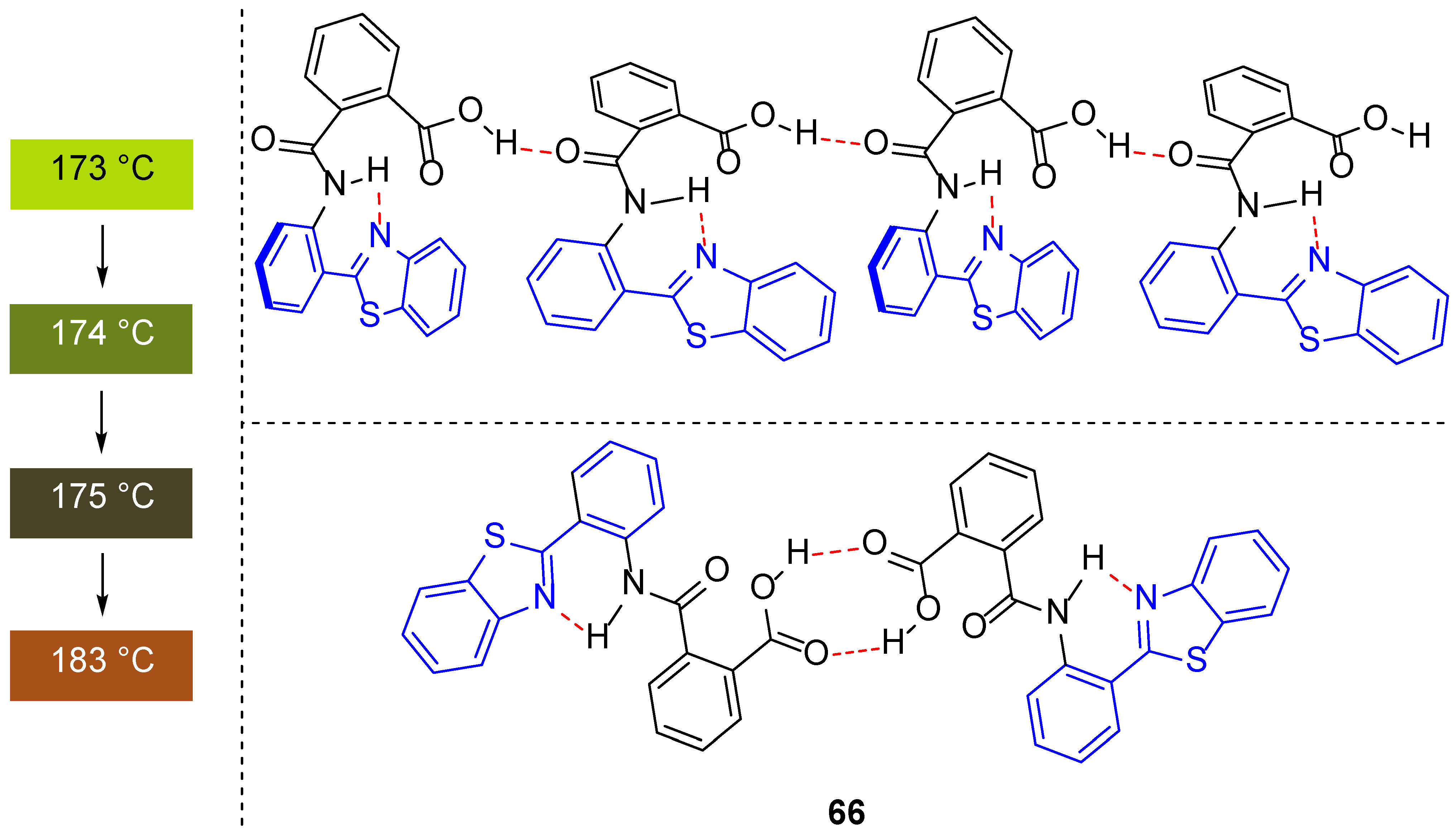
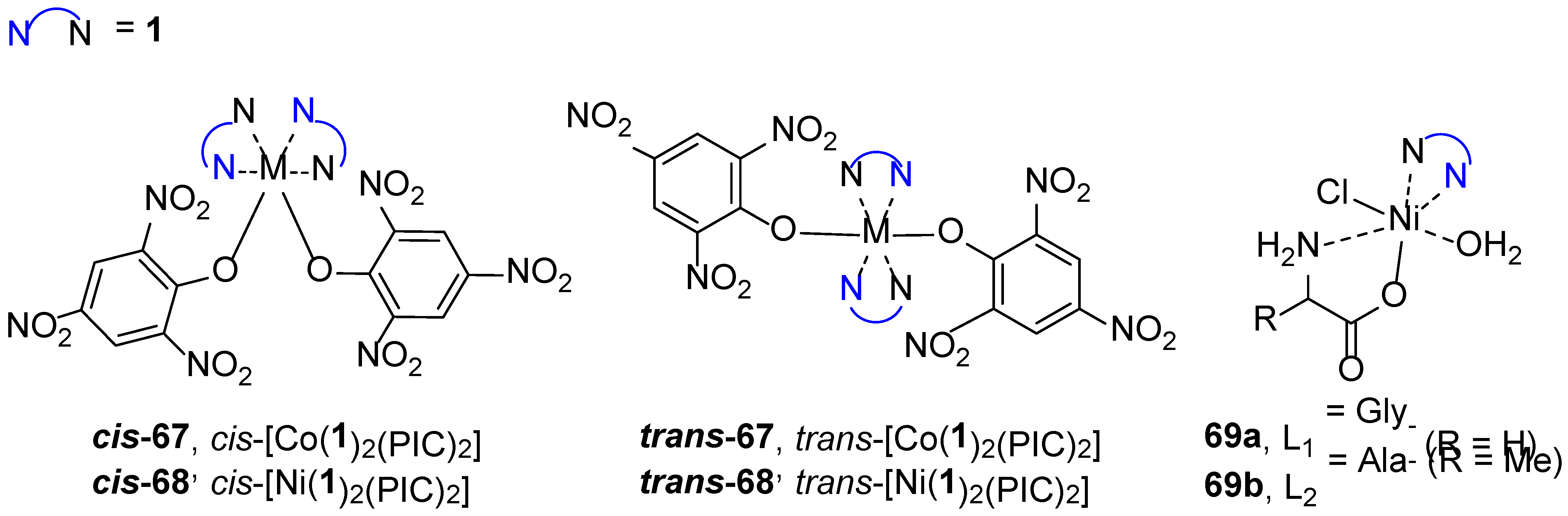
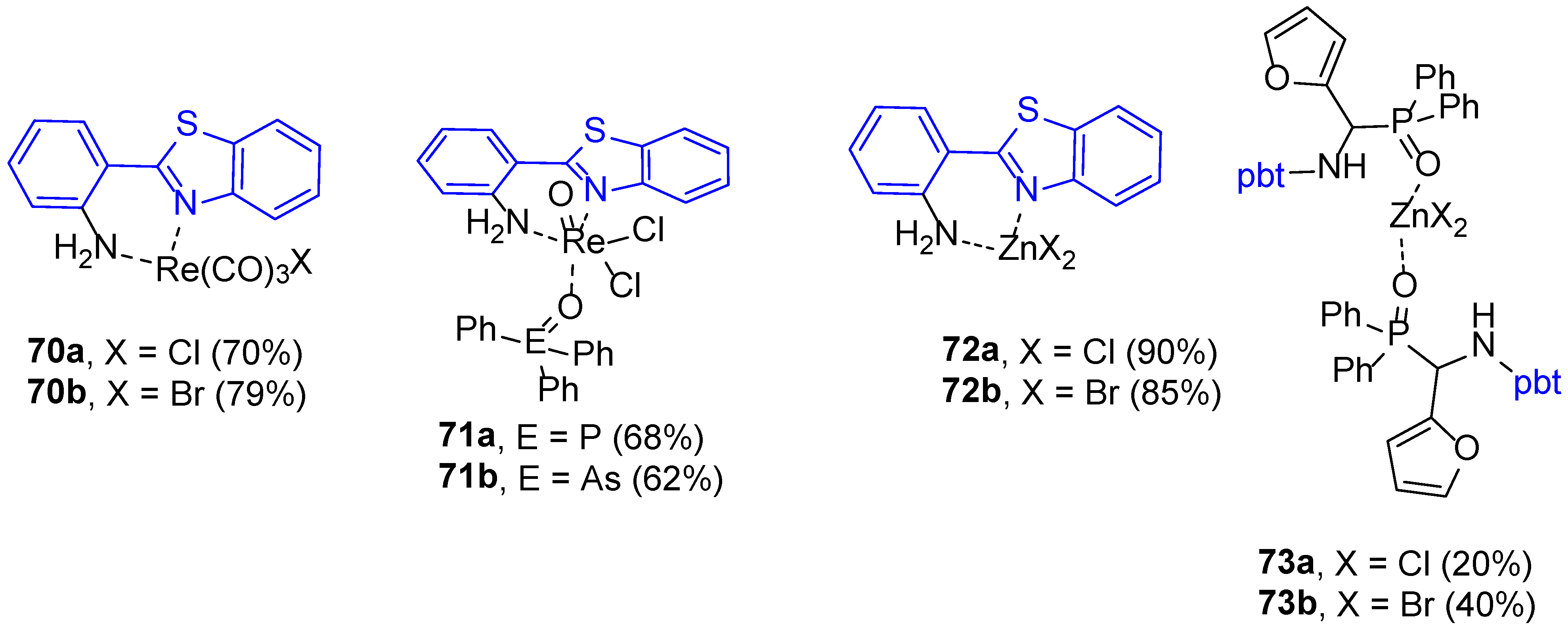
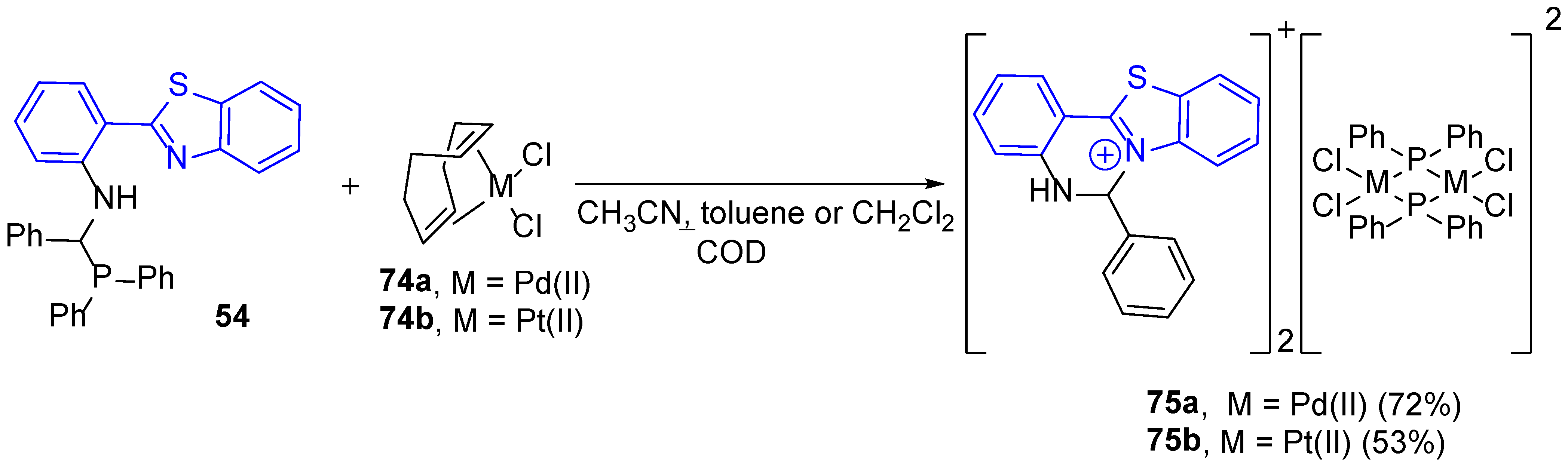

| 1H NMR Chemical Shifts, ppm | 13C NMR Chemical Shifts, ppm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1H | Benzene | Chloroform | Acetone | DMSO | 13C | Benzene | Chloroform | Acetone | DMSO |
| 3′ | 6.32 | 6.78 | 6.92 | 6.90 | 1′ | 115.7 | 115.2 | 115.0 | 113.1 |
| 4′ | 7.00 | 7.22 | 7.22 | 7.22 | 2′ | 147.8 | 146.7 | 148.9 | 147.6 |
| 5′ | 6.55 | 6.75 | 6.68 | 6.65 | 3′ | 117.3 | 116.7 | 117.6 | 116.5 |
| 6′ | 7.70 | 7.71 | 7.69 | 7.63 | 4′ | 132.1 | 131.5 | 132.8 | 131.7 |
| 4 | 7.88 | 7.97 | 7.99 | 8.00 | 5′ | 117.1 | 116.8 | 116.9 | 115.6 |
| 5 | 7.12 | 7.45 | 7.50 | 7.50 | 6′ | 131.0 | 130.3 | 130.9 | 129.9 |
| 6 | 6.97 | 7.35 | 7.41 | 7.41 | 2 | 170.2 | 169.2 | 170.2 | 168.8 |
| 7 | 7.40 | 7.86 | 8.02 | 8.07 | 3a | 154.7 | 154.7 | 154.8 | 153.2 |
| NH2 | 6.15 | 6.40 | 7.11 | 7.34 | 4 | 123.0 | 122.4 | 123.1 | 122.0 |
| 5 | 126.6 | 125.9 | 127.2 | 126.3 | |||||
| 6 | 125.3 | 124.8 | 125.4 | 125.0 | |||||
| 7 | 121.7 | 121.1 | 122.3 | 121.7 | |||||
| 7a | 134.0 | 133.2 | 133.9 | 132.4 | |||||
| Entry | Reaction Conditions | t, °C | Time, h | Yield, % | Ref. |
|---|---|---|---|---|---|
| 1 | acidic kaolinitic clay (20% w/w), dried PhCl, argon atmosphere | 120 | 20 | 55 | [95] |
| 2 | CH3COONa, glacial acetic acid | 120 | 3 | 80 | [96] |
| 3 | ZnCl2 (30 mol%), PhCl | 140 | 5 | 68 | [97] |

| Entry | Reaction Conditions | t, °C | Time, h | Yield, % | Ref. |
|---|---|---|---|---|---|
| (i) | modified silica dioxide catalyst (cat.), H2O, O2, I2 | 100 | 4 | 89 | [102] |
| (ii) | CH3COOH, MW (490 W) | - | 10 min | 99 | [103] |

| Entry | Starting Reagent | Step | Reaction Conditions | Time, h | Yield, % | Ref. |
|---|---|---|---|---|---|---|
| 1 | 16a | (i) | K3[Fe(CN)6], toluene, reflux | 10 | 72 | [107] |
| (ii) | NH2NH2·H2O, Pd-C, MeOH, reflux | 8 | 85 | |||
| 2 | 16a | (i) | 640 W | 10 min | 78 | [28] |
| (ii) | SnCl2·2H2O, abs. EtOH, 70 °C | 1 | 70 | |||
| 3 | 16a | (i) | EtOH, 80 °C | 3 | 71 | [108] |
| intermediate step | chloranil, CH2Cl2 | overnight | 86 | |||
| (ii) | Fe, HCl, NH4Cl, THF/H2O, 60 °C | 0.5 | 85 | |||
| 4 | 16b | (i) | pyridine, r.t. | 1 | 82 | [106] |
| (ii) | SnCl2·2H2O, EtOH, reflux | 4 | 85 |
| Entry | Reaction Conditions | t, °C | Time, h | Yield, % | Ref. |
|---|---|---|---|---|---|
| 1 | NaOtBu (50 mol %), air, toluene | 100 | 24 | 43 | [109] |
| 2 | Pd(II) catalyst (1 mol%), m-xylene, KOH | 120 | 12 | 71 | [110] |
| Compound | λ abs, nm | λ em, nm | TS1→TS0, nm | Ref. |
|---|---|---|---|---|
| 1 | 360 | 455 | - | [145] |
| 2 1 | 392 | 458 | - | [97] |
| 3 | 392 * | 469 | - | [76] |
| 57 1 | 335 | 395 | 565 | [97] |
| 15 1 | 330 | - | 555 | [97] |
| 44 1 | 363 | 419 | 588 | [97] |
| 58 1 | 362 | - | 649 | [97] |
| 59 1 | 336 | - | 540 | [97] |
| 60 1 | 393 | 436 | 623 | [97] |
| 30e | n.d. | 420 | 550 | [62] |
| 30f | n.d. | 435 | 550 | [62] |
| 30g | n.d. | 440 | 565 | [62] |

| Compound | NS1→TS1, eV | Twisted-TS0→TS0, eV |
|---|---|---|
| 1 | 0.38 | not calculated |
| 2 | 0.39 | not calculated |
| 57 | 0.30 | 0.33 |
| 15 | 0.12 | 0.27 |
| 44 | 0.34 | 0.34 |
| 59 | 0.12 | 0.83 |
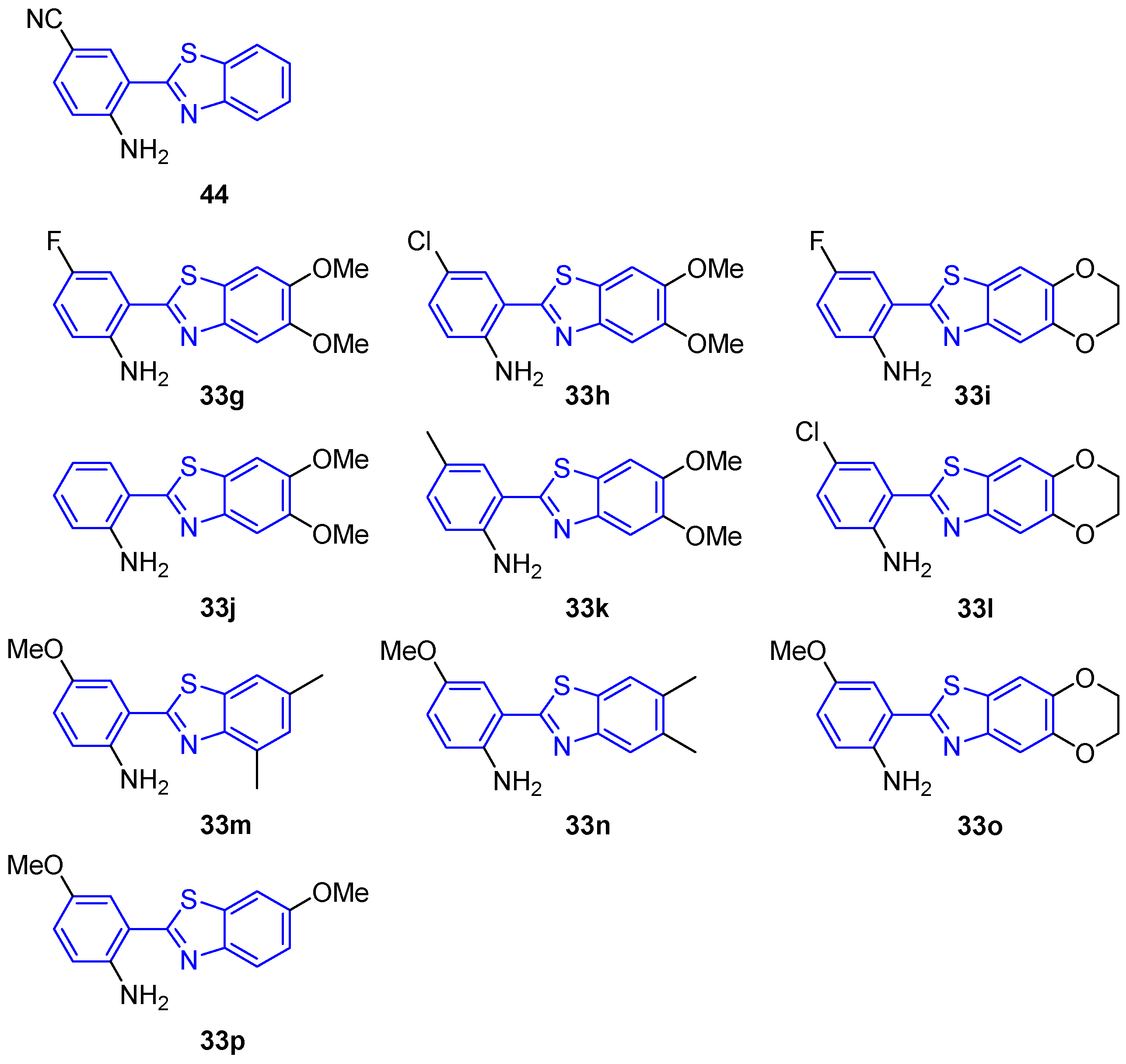
| Compound | λ abs, nm | λ em, nm | Ref. |
|---|---|---|---|
| 441 | 363 | 419, 588 | [97] |
| 33g | 265, 378 | 440 | [114] |
| 33h | 267, 379 | 440 | [114] |
| 33i | 270, 306, 378 | 440 | [114] |
| 33j | 265, 374 | 425 | [114] |
| 33k | 265, 314, 377 | 440 | [114] |
| 33l | 244, 270, 380 | 440 | [114] |
| 33m | 303, 385 | 470 | [114] |
| 33n | 300, 377 | 470 | [114] |
| 33o | 245, 336, 390 | 470 | [114] |
| 33p | 270, 322 | 465 | [114] |

| Compound | λabs, nm | λem, nm | τ, μs | Ref. |
|---|---|---|---|---|
| 50 | 255, 263, 291, 330–374 (br) | 455, 550 | 83 | [82] |
| 51 | 253, 262, 292, 307, 340–384 (br) | 450, 575 | 212 | [82] |
| 52 | 254, 261, 284, 296, 316–379 (br) | 455, 555 | 33, 700 | [82] |
| 54 | 260−320 (br.), 390 | 445, 600 | - | [50] |
| 55a | 300, 380 | 450, 610 | - | [83] |
| 55b | 300, 380 | 450, 610 | - | [83] |
| 53a | - | 475, 634 | - | [84] |
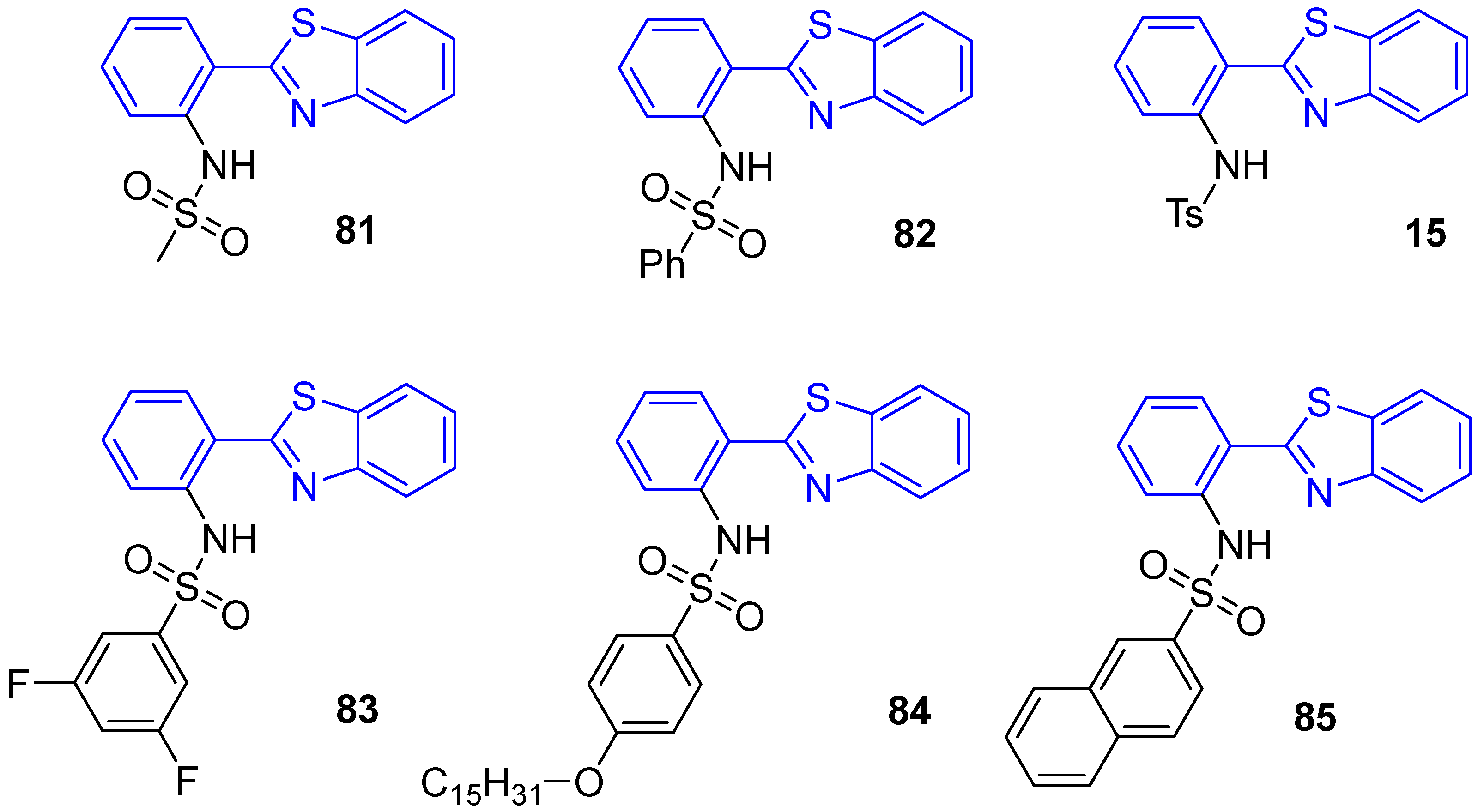
| Compound | λabs, nm | λem, nm | Brightness *, cd·m−2 (at 8 V) |
|---|---|---|---|
| Zn(L81)2 (86) | 385 | 463 | 100 |
| Zn(L82)2 (87) | 380 | 445 | 230 |
| Zn(L15)2 (88) | 390 | 440 | 140 |
| Zn(L83)2 (89) | 390 | 430 | - |
| Zn(L84)2 (90) | 380 | 445 | 270 |
| Zn(L85)2 (91) | 380 | 440 | - |
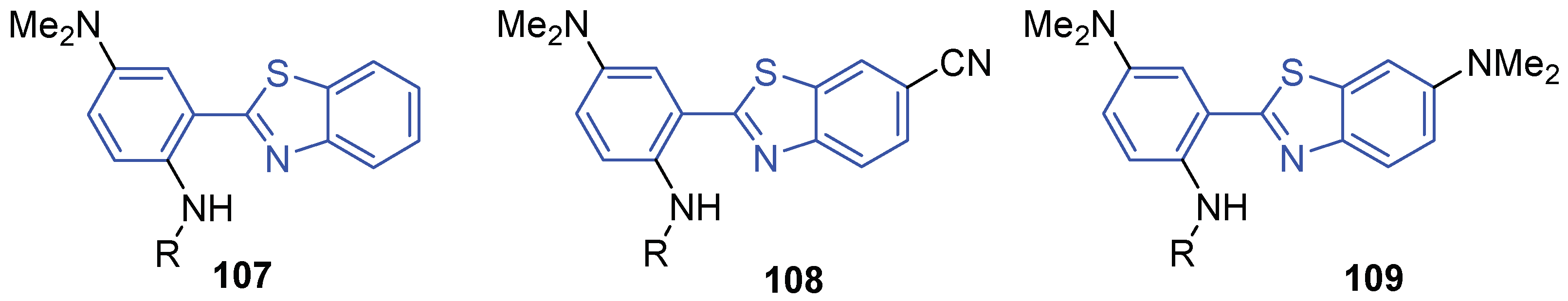
| Compound | R = Ac, ESIPT Barrier, eV | R = Ts, ESIPT Barrier, eV |
|---|---|---|
| 107 | 0.23 | 0.08 |
| 108 | 0.33 | 0.13 |
| 109 | 0.34 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pylova, E.K.; Sukhikh, T.S.; Prieto, A.; Jaroschik, F.; Konchenko, S.N. Chemistry of 2-(2′-Aminophenyl)benzothiazole Derivatives: Syntheses, Photophysical Properties and Applications. Molecules 2025, 30, 1659. https://doi.org/10.3390/molecules30081659
Pylova EK, Sukhikh TS, Prieto A, Jaroschik F, Konchenko SN. Chemistry of 2-(2′-Aminophenyl)benzothiazole Derivatives: Syntheses, Photophysical Properties and Applications. Molecules. 2025; 30(8):1659. https://doi.org/10.3390/molecules30081659
Chicago/Turabian StylePylova, Ekaterina K., Taisiya S. Sukhikh, Alexis Prieto, Florian Jaroschik, and Sergey N. Konchenko. 2025. "Chemistry of 2-(2′-Aminophenyl)benzothiazole Derivatives: Syntheses, Photophysical Properties and Applications" Molecules 30, no. 8: 1659. https://doi.org/10.3390/molecules30081659
APA StylePylova, E. K., Sukhikh, T. S., Prieto, A., Jaroschik, F., & Konchenko, S. N. (2025). Chemistry of 2-(2′-Aminophenyl)benzothiazole Derivatives: Syntheses, Photophysical Properties and Applications. Molecules, 30(8), 1659. https://doi.org/10.3390/molecules30081659











