Ultrasound-Assisted Extraction of Polysaccharides from Mulberry Leaves Using Response Surface Methodology: Purification and Component Identification of Extract
Abstract
1. Introduction
2. Results
2.1. Effect of Factors on Extraction
2.1.1. Effect of Liquid-to-Material Ratio on Yield of MLPs
2.1.2. Effect of Extraction Time on Yield of MLPs
2.1.3. Effect of Ultrasonic Power on Extraction Yield of MLPs
2.1.4. Effect of Extraction Temperature on Yield of MLPs
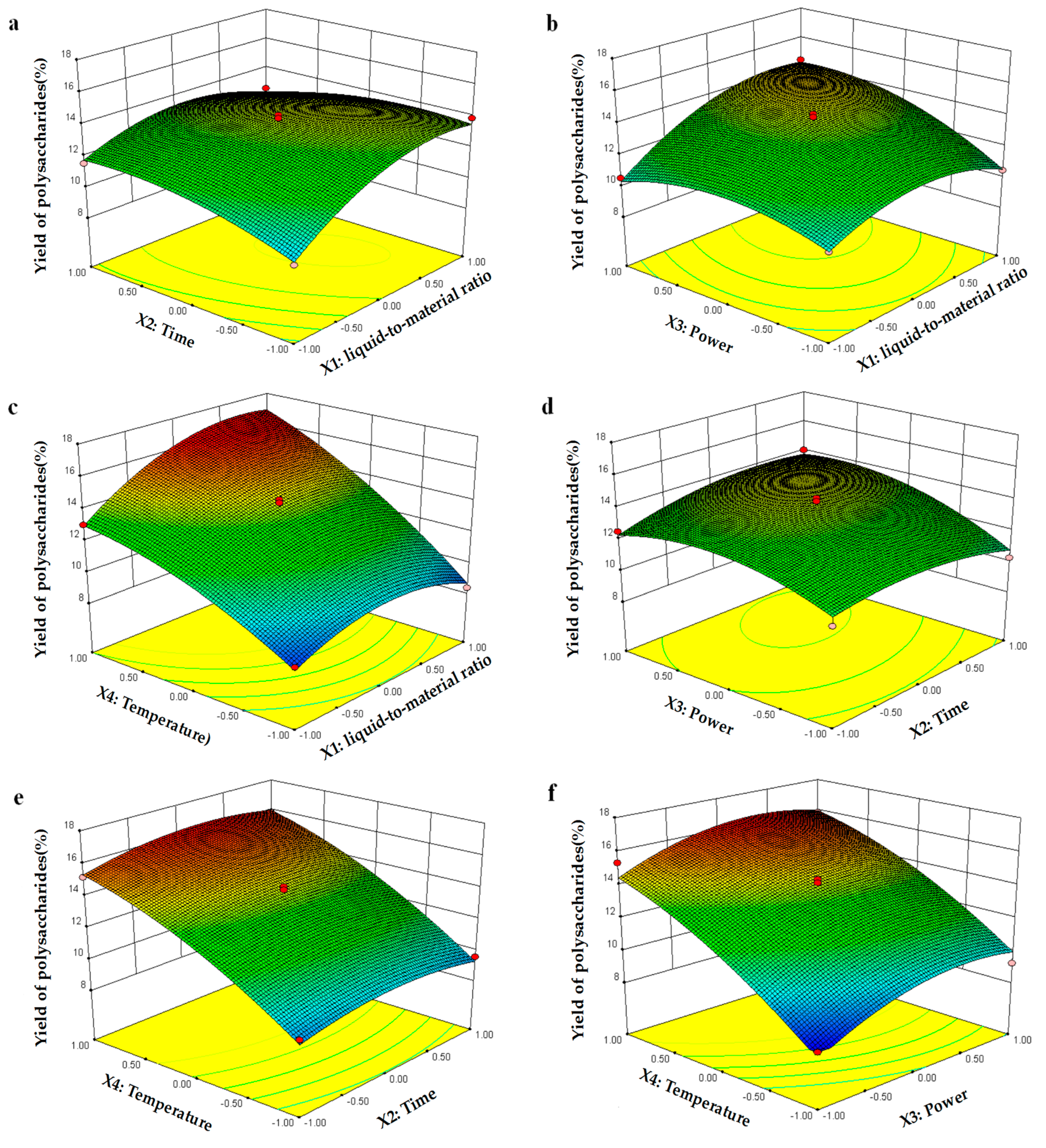
2.2. Box–Behnken Design
2.2.1. Model Building and Statistical Analysis
2.2.2. Optimization of the Procedure
2.2.3. Validation of the Model
2.3. Purification and Separation of Polysaccharides by Macroporous Resin
2.3.1. Resin Screening
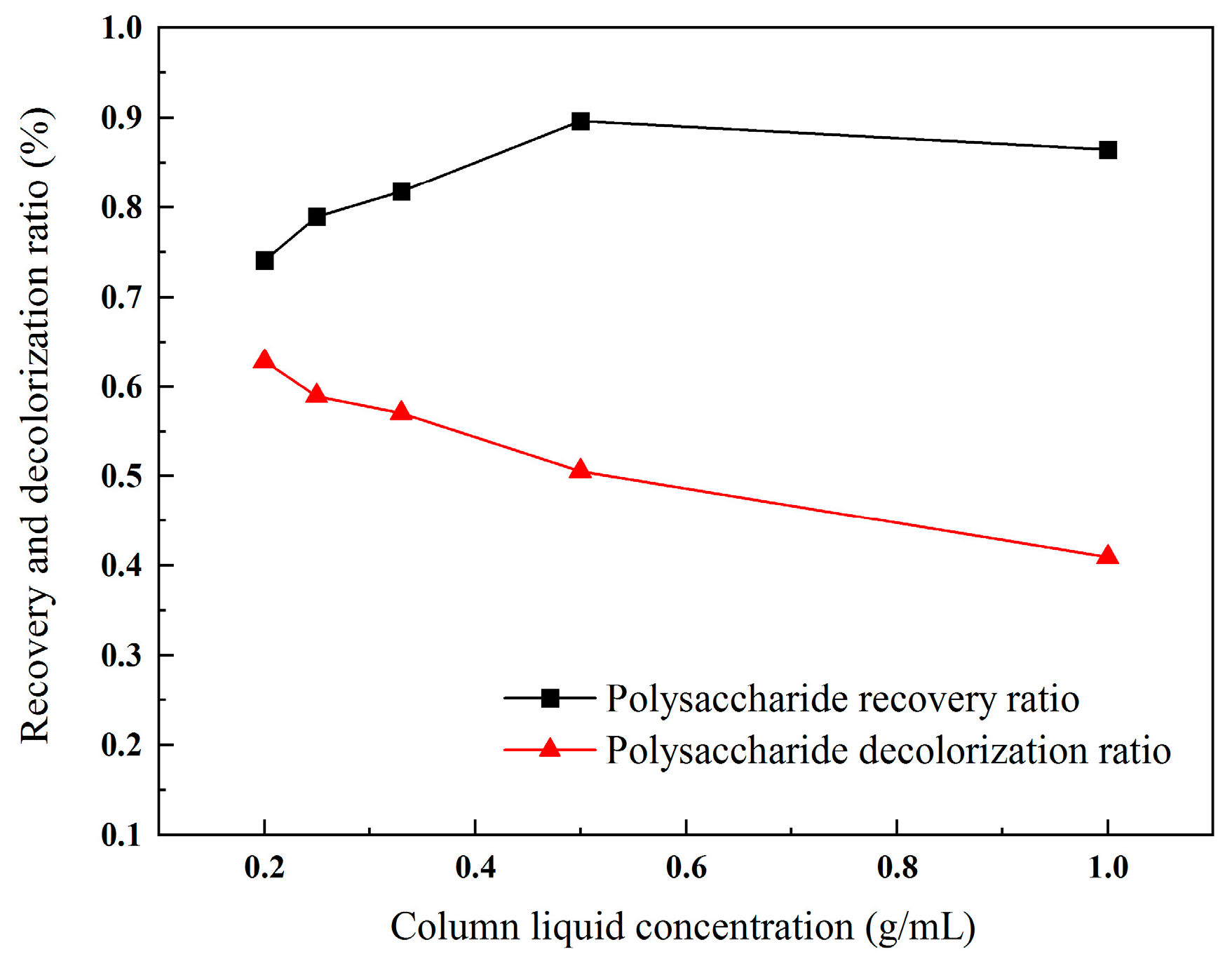
2.3.2. Influence of Column Liquid Concentration
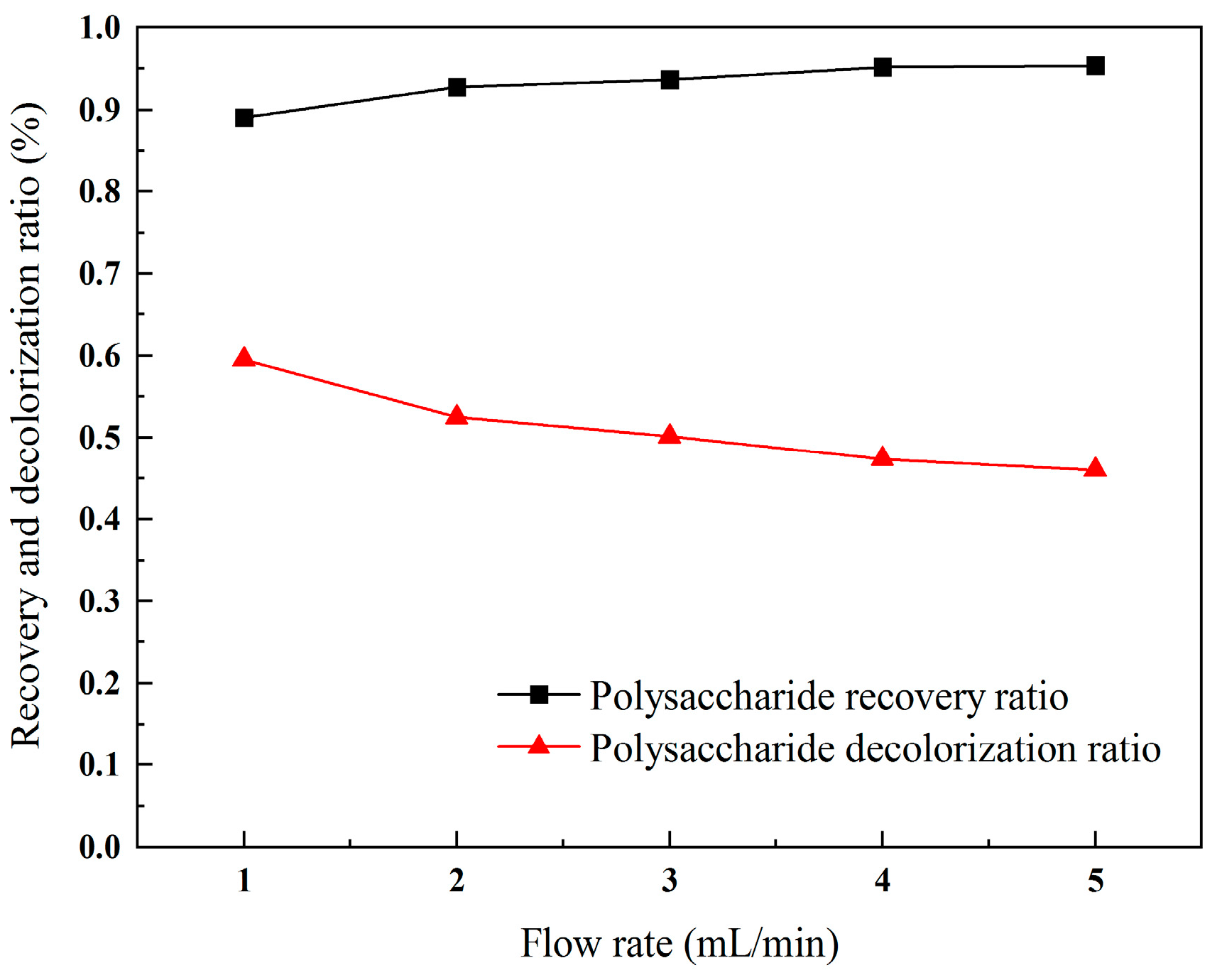
2.3.3. Influence of Flow Rate
2.4. Purification and Separation of Polysaccharides by Activated Carbon
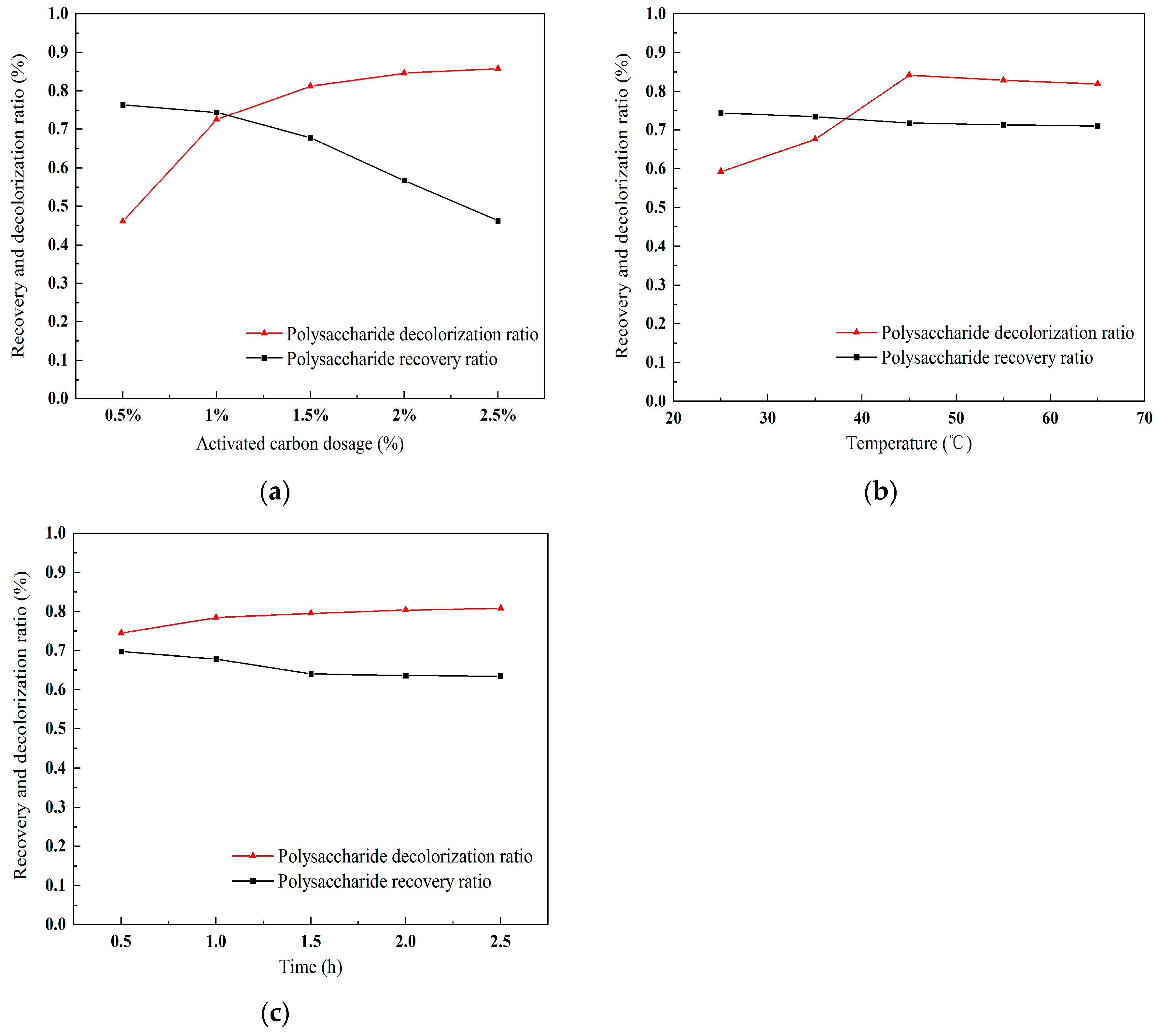
2.4.1. Effect of Activated Carbon Dose on Polysaccharide Purification
2.4.2. Effect of Temperature on Polysaccharide Purification
2.4.3. The Influence of Time on Polysaccharide Purification
2.5. Analysis of Polysaccharides Extracted by Ultrasonic-Assisted Extraction
2.5.1. Thin-Layer Chromatography Analysis
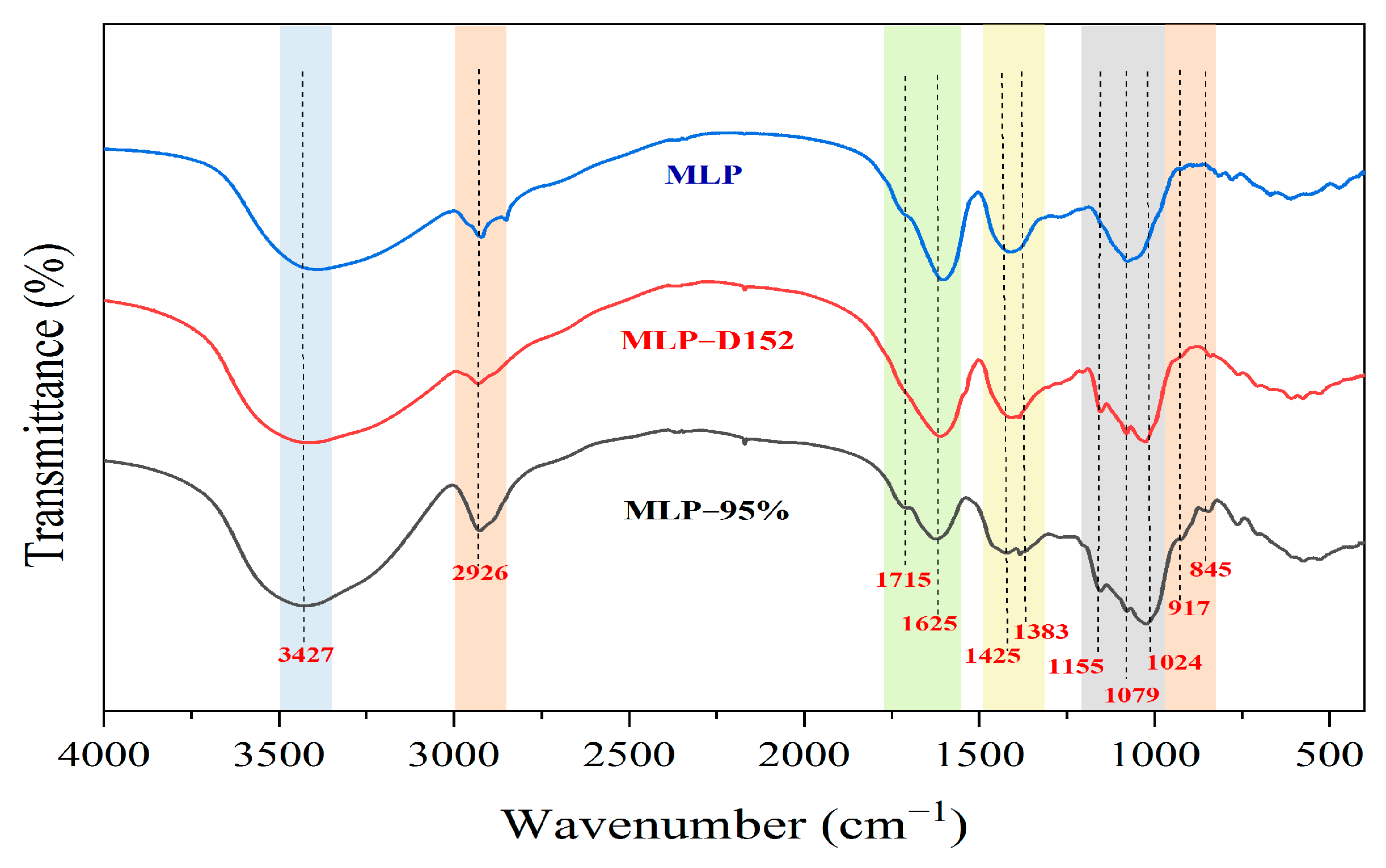
2.5.2. FT-IR Spectra Analysis
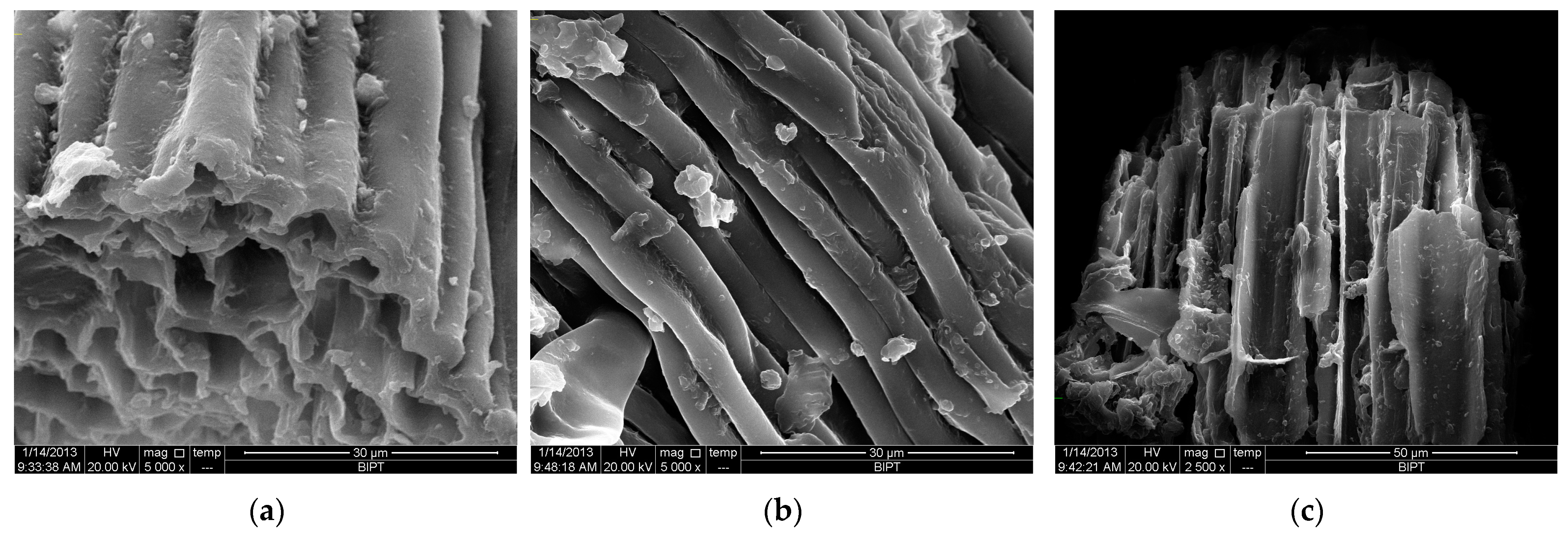
2.5.3. SEM Analysis
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Mulberry Leaves
3.3. Extraction of MLPs with CSE
3.4. Extraction of MLPs with UAE
3.5. Response Surface Methodology Experimental Designs
3.6. Purification of MLPs
3.6.1. Pretreatment of Macroporous Resins
3.6.2. Static Adsorption Experiment with Macroporous Resin
3.6.3. Dynamic Adsorption Experiment with Macroporous Resin
3.6.4. Purification of Polysaccharide by Activated Carbon
3.7. Analytical Methods
3.7.1. Quantitative Determination of Polysaccharides
3.7.2. Fourier-Transform Infrared (FT-IR) Spectra
3.7.3. Thin-Layer Chromatography
3.7.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.K.; Yan, X.L.; Xu, H.Z.; Pan, L.C.; Zhai, X.L.; Xue, Y.; Chen, Y.J.; Liu, H.P.; Zhao, M.; Luo, L. Effects of mulberry leaf extract on growth, digestion, liver lipid metabolism and hypoglycaemic ability in mandarin fish (Siniperca chuatsi). Aquacult. Rep. 2024, 39, 102513–102525. [Google Scholar] [CrossRef]
- Singh, A.; Dar, M.Y.; Sharma, A.; Sharma, S.; Shrivastava, S.; Shukla, S. Therapeutic efficacy of Morus alba L. against N-nitrosodiethylamine induced subchronic hepatic ailment in rats. Toxicol. Environ. Health Sci. 2017, 9, 177–187. [Google Scholar] [CrossRef]
- Zhong, S.; Yang, Y.; Huo, J.X.; Sun, Y.Q.; Ren, N.; Lu, Q.H.; Li, D.; Zhan, P.F.; Wu, W.J.; Chen, H.Z.; et al. Dissection of gut microbiota and metabolites reveals the hypolipidemic effect of green mulberry leaf tea / black mulberry leaf tea in mice. J. Funct. Foods 2023, 111, 105906–105919. [Google Scholar] [CrossRef]
- Akbari Aghdam, M.; Pagan, A.; Garcia-Esta, J.; Atucha, N.M. Evaluation of the Effects of Mulberry Leaf Extracts Morus alba L. on Cardiovascular, Renal, and Platelet Function in Experimental Arterial Hypertension. Nutrients 2025, 17, 49. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, X.; Deng, Q.; Yang, M.; Li, S.; Zhang, Q.; Sun, Y.; Chen, H. Extraction, structural characterization and biological activities of polysaccharides from mulberry leaves: A review. Int. J. Biol. Macromol. 2024, 257, 128669–128682. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Huang, G.L.; Zhang, X.X. Analysis and properties of polysaccharides extracted from Brassica oleracea L. var. capitata L. by hot water extraction/ultrasonic-synergistic enzymatic method. Ultrason. Sonochem. 2025, 12, 107244–107258. [Google Scholar]
- Zhang, Y.H.; Song, H.Z.; Lu, J.; Wang, F.; Wang, L.F.; Xiong, L.; Shen, X.C. Ultrasound-assisted extraction, purification, structural characterization, and hypoglycemic activities of a polysaccharide from Momordica charantia L. Int. J. Biol. Macromol. 2025, 285, 138300. [Google Scholar] [CrossRef]
- Meng, L.; Chen, Y.; Zheng, Z.J.; Wang, L.; Xu, Y.H.; Li, X.J.; Xiao, Z.J.; Tang, Z.; Wang, Z.S. Ultrasound-Assisted Extraction of Paeonol from Moutan Cortex: Purification and Component Identification of Extract. Molecules 2024, 29, 622. [Google Scholar] [CrossRef]
- Leichtweis, M.G.; Molina, A.K.; Petropoulos, S.A.; Carocho, M.; Pires, T.C.S.P.; Dias, M.I.; Calhelha, R.; Oliveira, M.B.P.P.; Pereira, C.; Barros, L. Valorization of Pumpkin Peel as a Source of Bioactive Compounds: Optimization of Heat- and Ultrasound-Assisted Extraction. Molecules 2023, 28, 3168. [Google Scholar] [CrossRef]
- Thajudeen, K.Y.; Asiri, Y.I.; Salam, S.; Thorakkattil, S.A.; Rahamathulla, M.; Uoorakkottil, I. A Box–Behnken Extraction Design and Hepatoprotective Effect of Isolated Eupalitin-3-O-β-D-Galactopyranoside from Boerhavia diffusa Linn. Molecules 2022, 27, 6444. [Google Scholar] [CrossRef]
- Chen, G.; Sun, M.; Chen, K.; Wang, L.; Sun, J. Ultrasonic-assisted decoloration of polysaccharides from seedless chestnut rose (rosa sterilis) fruit: Insight into the impact of different macroporous resins on its structural characterization and in vitro hypoglycemic activity. Foods 2024, 13, 1349. [Google Scholar] [CrossRef] [PubMed]
- Kirankumar, P.S.; Tian, L.L.; Li, H.; Johnston, C.T.; Boyd, S.A.; Teppen, B.J. Accelerated solvent extraction of dioxins sequestered in activated carbon: A response surface methodology-based optimization. Chemosphere 2025, 373, 144176–144187. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wu, S.S.; Wang, J.Y.; Yang, C.; Wang, Y.; Hu, Z.; Cai, W.; Liu, L.L. Optimization of Polysaccharide Extraction from Polygonatum cyrtonema Hua by Freeze–Thaw Method Using Response Surface Methodology. Molecules 2024, 29, 4879. [Google Scholar] [CrossRef]
- Solhi, L.; Sun, H.S.; Daswani, S.H.; Shojania, S.; Springate, C.M.K.; Brumer, H. Controlled sulfation of mixed-linkage glucan by Response Surface Methodology for the development of biologically applicable polysaccharides. Carbohydr. Polym. 2021, 269, 118275–118282. [Google Scholar] [CrossRef]
- Zhu, B.E.; Li, C.; Yao, Z.; Xu, H.; Ning, L.M. Efficient degradation of the polysaccharide extracted from Enteromorpha prolifera by using a novel polysaccharide lyase family 28 enzyme with high activity. Food Chem. 2025, 464, 141725–141734. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Q.; Wang, Z.; Borjigin, G.; Sun, J.; Zhao, Y.; Li, Q.; Shi, X.; Shah, S.F.A.; Wang, X.; et al. Ultrasound-assisted polysaccharide extraction from Fritillaria ussuriensis Maxim. and its structural characterization, antioxidant and immunological activity. Ultrason. Sonochem. 2024, 103, 105996–106006. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Zhou, P.; Li, X.Z.; Lu, Y.; Wang, Y.Q.; Yuan, H.; Yang, Y.H. Ultrasound-assisted deep eutectic solvent extraction of flavonol glycosides from Ginkgo biloba: Optimization of efficiency and mechanism. Ultrason. Sonochem. 2025, 114, 107254–107261. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Kimura, T.; Kondo, T.; Mitome, H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem 2003, 10, 149–156. [Google Scholar] [CrossRef]
- Zhao, S.; Kwok, K.C.; Liang, H. Investigation on ultrasound assisted extraction of saikosaponins from radix bupleuri. Sep. Purif. Technol. 2007, 55, 307–312. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; Wiley: New York, NY, USA, 1995; pp. 26–258. [Google Scholar]
- Zhao, H.; Yang, J.; Zeng, J.; Zhou, B.; Yang, M.; Yang, X.; Sun, R. Development of subcritical water extraction for areca alkaloids and its influence on the structure of areca nut husk. Molecules 2025, 30, 886. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, W.; Wang, Y.; Cai, W.; Ni, Q.; Jiang, C.; Li, J.; Shen, C. Genetic Algorithm-Back Propagation Neural Network Model- and Response Surface Methodology-Based Optimization of Polysaccharide Extraction from Cinnamomum cassia Presl, Isolation, Purification and Bioactivities. Foods 2025, 14, 686. [Google Scholar] [CrossRef]
- Samavati, V.; Yarmand, M.S. Statistical modeling of process parameters for the recovery of polysaccharide from morus alba leaf. Carbohydr. Polym. 2013, 98, 793–806. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Microwave-assisted extraction of polysaccharides from mulberry leaves. Int. J. Biol. Macromol. 2014, 72, 1–5. [Google Scholar] [CrossRef]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, C.; Wang, Z.; Dai, J.; Guan, C.; Sheng, J.; Tao, L.; Tian, Y. Separation, Purification, Structural Characterization, and In Vitro Hypoglycemic Activity of Polysaccharides from Panax notoginseng Leaves. Molecules 2025, 30, 830. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wang, Y.; Rong, M.; Xing, H.; Chi, R.-A.; Chen, C.; Liu, H.; Yang, L. Efficient Adsorption and Separation of Glyphosate in Aqueous Solution by Amino-Functionalized Poly(Glycidyl Methacrylate). Chem. Eng. J. 2024, 505, 158804–158817. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, G. Preparation, Structure and Properties of Litchi Pericarp Polysaccharide. Sci. Rep. 2025, 15, 6331–6346. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Ding, J.; Zhang, X.; Zhang, J.; Zhang, C.; Shang, G.; Zhu, L.; Zhang, Y.; Liu, Q.; Liu, Y. Multiple Fingerprinting and Chromatography-Immunoreactivity Relationship of Polysaccharides from Dioscorea Opposite Thunb. Food Chem. 2024, 464, 141891–141902. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Fujioka, J.; Imai, T.; Torigoe, K.; Tsukiyama, K. Mid-infrared free-electron laser tuned to the amide I band for converting insoluble amyloid-like protein fibrils into the soluble monomeric form. Lasers Med. Sci. 2014, 29, 1701–1707. [Google Scholar] [CrossRef]
- Zhuan, N.; Lulu, C.; Guangxia, L.; Hui, L.; Yanping, L.; Jianlong, M.; Jianbao, D.; Jin, Y. A Method for the Quantitative Analysis of Lycium Barbarum Polysaccharides (LBPs) Using Fourier-Transform Infrared Spectroscopy (FTIR): From Theoretical Computation to Experimental Application. Spectrochim. Acta Part A 2024, 326, 125204–125214. [Google Scholar]
- Yang, H.; Lei, C.; Li, D.; Zhang, N.; Lang, Y.; Wu, L.; Wang, M.; Tian, H.; Li, C. A Comparative Investigation on the Extraction-Function Relationship of Polysaccharides Derived from Moringa Oleifera Seeds in Terms of Antioxidant Capacity. Food Chem. 2025, 465, 142137–142149. [Google Scholar] [CrossRef]
- Kacuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Yang, C.X.; He, N.; Ling, X.P.; Zhang, C.X.; Yao, C.Y.; Wang, Z.Y.; Li, Q.B. The Isolation and Characterization of Polysaccharides from Longan Pulp. Sep. Purif. Technol. 2008, 63, 226–230. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, M.; Liu, M.; Bi, J. Physicochemical, rheological properties and in vitro hypoglycemic activities of polysaccharide fractions from peach gum. Carbohydr. Polym. 2022, 296, 119954–119962. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Du, B.; Jeepipalli, S.P.K.; Xu, B. Critical review on alterations in physiochemical properties and molecular structure of natural polysaccharides upon ultrasonication. Ultrason. Sonochem. 2022, 90, 106170–106182. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, Y.H. Ultrasound-assisted polysaccharide extraction from Cercis chinensis and properites, antioxidant activity of polysaccharide. Ultrason. Sonochem. 2023, 96, 106422–106431. [Google Scholar] [CrossRef]
- Ng, Y.V.; Ali, M.K.M.; Wan Ishak, W.R. Optimization of aqueous extraction conditions for bioactive compounds and antioxidant properties of overripe banana (Musa acuminata) using response surface methodology. J. Agric. Food Res. 2025, 20, 101775–101786. [Google Scholar] [CrossRef]
- Atanacković Krstonošić, M.; Sazdanić, D.; Mikulić, M.; Ćirin, D.; Milutinov, J.; Krstonošić, V. Optimization of Surfactant-Mediated Green Extraction o f Phenolic Compounds from Grape Pomace Using Response Surface Methodology. Int. J. Mol. Sci. 2025, 26, 2072. [Google Scholar] [CrossRef]
- Robles-Apodaca, S.M.; González-Vega, R.I.; Ruíz-Cruz, S.; Estrada-Alvarado, M.I.; Cira-Chávez, L.A.; Márquez-Ríos, E.; Del-Toro-Sánchez, C.L.; Ornelas-Paz, J.d.J.; Suárez-Jiménez, G.M.; Ocaño-Higuera, V.M. Optimization of Extraction Process for Improving Polyphenols and Antioxidant Activity from Papaya Seeds (Carica papaya L.) Using Response Surface Methodology. Processes 2024, 12, 2729. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, H.Y.; Li, W.J.; Ye, J.Z. Chemical Constituents and Structural Characterization of Polysaccharides from Four Typical Bamboo Species Leaves. Molecules 2015, 20, 4162–4179. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Heng, Y.; Tian, J.; Zhang, S.; Shi, M. Structural characterization of polysaccharide from jujube (ziziphus jujuba mill.) fruit. Chem. Biol. Technol. Agric. 2021, 8, 54–61. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Tian, J.; Ma, K.; Liu, Y. Research progress on degradation methods and product properties of plant polysaccharides. J. Light. Ind. 2023, 38, 55–62. [Google Scholar]
- Lin, J.K.; Wu, S.S. Synthesis of dabsylhydrazine and its use in the chromatographic determination of monosaccharides by thin-layer and high-performance liquid chromatography. Anal. Chem. 1987, 59, 1320–1326. [Google Scholar] [CrossRef] [PubMed]


| X1 | X2 | X3 | X4 | Response (Y, Yield, %) | |
|---|---|---|---|---|---|
| 1 | 0 | −1 | −1 | 0 | 8.49 |
| 2 | −1 | 0 | −1 | 0 | 7.85 |
| 3 | 0 | 0 | −1 | −1 | 5.49 |
| 4 | 0 | 0 | −1 | 1 | 12.94 |
| 5 | 1 | 0 | −1 | 0 | 8.05 |
| 6 | 0 | 1 | −1 | 0 | 7.85 |
| 7 | 0 | −1 | 1 | 0 | 10.13 |
| 8 | −1 | 0 | 1 | 0 | 8.15 |
| 9 | 0 | 0 | 1 | −1 | 6.81 |
| 10 | 0 | 0 | 1 | 1 | 13.45 |
| 11 | 1 | 0 | 1 | 0 | 12.08 |
| 12 | 0 | 1 | 1 | 0 | 11.65 |
| 13 | −1 | −1 | 0 | 0 | 7.12 |
| 14 | 0 | −1 | 0 | −1 | 7.01 |
| 15 | 0 | −1 | 0 | 1 | 12.79 |
| 16 | 1 | −1 | 0 | 0 | 11.49 |
| 17 | −1 | 0 | 0 | −1 | 6.13 |
| 18 | −1 | 0 | 0 | 1 | 10.65 |
| 19 | 1 | 0 | 0 | −1 | 5.95 |
| 20 | 1 | 0 | 0 | 1 | 14.03 |
| 21 | −1 | 1 | 0 | 0 | 9.16 |
| 22 | 0 | 1 | 0 | −1 | 7.16 |
| 23 | 0 | 1 | 0 | 1 | 13.42 |
| 24 | 1 | 1 | 0 | 0 | 10.18 |
| 25 | 0 | 0 | 0 | 0 | 11.26 |
| 26 | 0 | 0 | 0 | 0 | 11.47 |
| 27 | 0 | 0 | 0 | 0 | 11.35 |
| 28 | 0 | 0 | 0 | 0 | 11.53 |
| 29 | 0 | 0 | 0 | 0 | 11.42 |
| Parameter | Estimate | Error | F-Value | p-Value | Significance |
|---|---|---|---|---|---|
| X1 | 1.06 | 0.14 | 59.69 | <0.0001 | *** |
| X2 | 0.2 | 0.14 | 2.11 | 0.1686 | -- |
| X3 | 0.97 | 0.14 | 49.64 | <0.0001 | *** |
| X4 | 3.23 | 0.14 | 553.38 | <0.0001 | *** |
| X1X2 | −0.84 | 0.24 | 12.42 | 0.0034 | ** |
| X1X3 | 0.93 | 0.24 | 15.40 | 0.0015 | ** |
| X1X4 | 0.89 | 0.24 | 14.03 | 0.0022 | ** |
| X2X3 | 0.54 | 0.24 | 5.16 | 0.0394 | * |
| X2X4 | 0.12 | 0.24 | 0.25 | 0.6214 | -- |
| X3X4 | −0.20 | 0.24 | 0.73 | 0.4085 | -- |
| X12 | −1.35 | 0.19 | 52.28 | <0.0001 | *** |
| X22 | −0.65 | 0.19 | 12.06 | 0.0037 | ** |
| X32 | −1.09 | 0.19 | 33.91 | <0.0001 | *** |
| X42 | −0.73 | 0.19 | 15.11 | 0.0016 | ** |
| Model | 11.41 | 0.21 | 56.47 | <0.0001 | *** |
| Mean | C.V.% | PRESS | R-Squared | Adj R-Squared | Pred R-Squared | Adeq Precision |
|---|---|---|---|---|---|---|
| 9.83 | 4.84 | 18.03 | 0.9862 | 0.9562 | 0.9008 | 27.242 |
| Ratio of Water to Raw Material (mL/g) | Extraction Time (min) | Ultrasonic Power (W) | Extraction Temperature (°C) | Yield (%) | |
|---|---|---|---|---|---|
| Prediction | 16.1:1 | 58.2 | 484.5 | 65.1 | 15.17% |
| Actual | 16:1 | 58 | 500 | 65 | 14.47 ± 0.76% |
| Monosaccharides | Rhamnose | Xylose | Arabinose |
|---|---|---|---|
| Rf values | 0.63 | 0.59 | 0.66 |
| Variables | Symbol | Experimental Value | ||
|---|---|---|---|---|
| Low, −1 | Zero, 0 | High, 1 | ||
| The ratio of liquid to material (mL/g) | X1 | 12:1 | 14:1 | 16:1 |
| Time (min) | X2 | 40 | 60 | 80 |
| Power (W) | X3 | 400 | 450 | 500 |
| Temperature (°C) | X4 | 30 | 40 | 50 |
| Factors | Activated Carbon Dosage/% | Temperature/°C | Time/h |
|---|---|---|---|
| Values | 0.5, 1.0, 1.5, 2, 2.5 | 25, 35, 45, 55, 65 | 0.5, 1, 1.5, 2, 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zou, X.; Zhang, H.; Li, J.; Peng, X.; Ju, R.; Jia, Z.; Wen, Z.; Li, C. Ultrasound-Assisted Extraction of Polysaccharides from Mulberry Leaves Using Response Surface Methodology: Purification and Component Identification of Extract. Molecules 2025, 30, 1747. https://doi.org/10.3390/molecules30081747
Wang T, Zou X, Zhang H, Li J, Peng X, Ju R, Jia Z, Wen Z, Li C. Ultrasound-Assisted Extraction of Polysaccharides from Mulberry Leaves Using Response Surface Methodology: Purification and Component Identification of Extract. Molecules. 2025; 30(8):1747. https://doi.org/10.3390/molecules30081747
Chicago/Turabian StyleWang, Teng, Xiaolin Zou, Hong Zhang, Jiwen Li, Xiaoming Peng, Ruijun Ju, Zhaojun Jia, Zhenguo Wen, and Cuiqing Li. 2025. "Ultrasound-Assisted Extraction of Polysaccharides from Mulberry Leaves Using Response Surface Methodology: Purification and Component Identification of Extract" Molecules 30, no. 8: 1747. https://doi.org/10.3390/molecules30081747
APA StyleWang, T., Zou, X., Zhang, H., Li, J., Peng, X., Ju, R., Jia, Z., Wen, Z., & Li, C. (2025). Ultrasound-Assisted Extraction of Polysaccharides from Mulberry Leaves Using Response Surface Methodology: Purification and Component Identification of Extract. Molecules, 30(8), 1747. https://doi.org/10.3390/molecules30081747





