Abstract
A site-ordered quadruple perovskites, AA′3B4O12, can have 3d transition metals at A′ and B sites, and show complex magnetic interactions and behavior. Additional complexity appears when B site-ordered arrangements are realized in AA′3B2B′2O12. In this work, A site-ordered quadruple perovskites, RMn3Ni2Mn2O12 with R = Bi, Ce, and Ho, were prepared by a high-pressure, high-temperature method at about 6 GPa and about 1500 K. The R = Bi and Ce samples were found to crystallize in space group Im-3 with a disordered distribution of Ni2+ and Mn4+ cations in one B site. On the other hand, the R = Ho sample crystallized in space group Pn-3 and showed partial ordering of Ni2+ and Mn4+ cations between two B sites. The structural data (and bond valence sums) suggest that cerium has the oxidation state +3, which is unusual for such perovskites. Magnetic properties were investigated by magnetic susceptibility and specific heat measurements, which showed the presence of one magnetic transition near 36 K for R = Bi; there was evidence for the presence of two magnetic transitions near 27 K and 33 K for R = Ce, and near 10 K and 36 K for R = Ho. Curie–Weiss parameters were estimated for all samples from high-temperature magnetic measurements up to 750 K. The total effective magnetic moment for R = Ce also suggests the presence of Ce3+. A magnetic field of 90 kOe had the largest effect on the specific heat of the R = Ho sample, and almost no effects on the specific heat of the R = Bi sample.
1. Introduction
Simple perovskite oxides, ABO3, usually have non-magnetic A cations (such as, Ca2+, Sr2+, Ba2+, Pb2+, La3+, Y3+, and Bi3+) or magnetic rare-earth cations [1,2,3,4,5]. Therefore, the magnetism of ABO3 perovskites is primarily driven by B cations (if they are magnetic). Even if both A and B cations are magnetic, they behave separately at higher temperatures, and the interaction between A and B cations is weak. For example, RFeO3 perovskites have Fe3+ ordering at about 600–740 K, while R3+ ordering takes place below about 10 K [6].
On the other hand, A site-ordered quadruple perovskites, AA′3B4O12, have a small square-planar A′ site, which usually accommodates 3d transition metals [7,8,9,10,11]. Therefore, such perovskites can show complex magnetic interactions and behavior and much stronger interactions between cations in the A′ and B sites. Additional complexity can appear when different cations are located at the B sites. Depending on the size and charge difference [2], B site-ordered arrangements can be realized, leading to the general formula for such perovskites being AA′3B2B′2O12 [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. High degrees of B site cation orders are usually realized when the charge difference between B and B′ cations is higher than 3, or when there is a large difference in the electronic properties between B and B′ cations [2]. When the charge difference between B and B′ cations is equal to 2, synthesis conditions start to play a very important role in the degree of cation order [2].
Special attention has been given in the literature to perovskite-related compounds with B = Ni2+ and B′ = Mn4+ [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], because such a combination is expected to give ferromagnetic (FM) interactions according to the Goodenough–Kanamori rules [47]. The charge difference between Ni2+ and Mn4+ is 2; therefore, it is often challenging to obtain fully ordered samples.
Simple R2NiMnO6 perovskites can be prepared at ambient pressure from R = La to R = Lu. The degree of Ni2+ and Mn4+ ordering has significant effects on the FM properties of R2NiMnO6 [35,36,37,38] and on the saturation magnetization values. In nearly ordered samples, TC (TC is an FM Curie temperature) decreases almost linearly from 280 K for R = La [30,36] to 40 K for to R = Lu [12,44,45]. Such changes in TC correlate with changes in the Ni–O–Mn bond angles. Further reduction in the Ni–O–Mn bond angle results in dramatic changes in magnetic interactions and properties. For example, Sc2NiMnO6, even with full ordering of Ni2+ and Mn4+, shows two antiferromagnetic (AFM) transitions and a complex magnetodielectric response [41]. In2NiMnO6, also with full ordering of Ni2+ and Mn4+, demonstrates a complex incommensurate AFM structure below TN = 26 K (TN is an AFM Néel temperature) and spin-induced ferroelectric polarization at TN [39,40]. As Lu2NiMnO6 appears to be located near a phase boundary between FM and AFM ground states, a moderate pressure can change Ni–O–Mn bond angles enough to induce a transition from an FM ground state to an incommensurate AFM ground state [44]. It should be noted that Sc2NiMnO6 and In2NiMnO6 can only be prepared using a high-pressure, high-temperature method.
Peculiarities of both the R2NiMnO6 and AA′3B2B′2O12 perovskite families are combined in the case of LaMn3Ni2Mn2O12 [12], which can be prepared only using a high-pressure, high-temperature method. LaMn3Ni2Mn2O12 is located in a region of a phase diagram with unusual properties similar to those of Sc2NiMnO6 and In2NiMnO6. For example, two magnetic transitions were found in LaMn3Ni2Mn2O12 at 34 K and 46 K with original magnetic structures [12,13]. Such perovskites were recently extended to RMn3Ni2Mn2O12 with R = Nd, Sm, Gd, and Dy [48], which can also be prepared only using a high-pressure, high-temperature method.
In this work, we investigated RMn3Ni2Mn2O12 with R = Bi, Ce, and Ho. Bi-containing perovskites are exceptional members of any perovskite family [49,50,51,52,53] in the majority of cases, as a Bi3+ cation has the lone electron pair and promotes polar distortions. Ce usually has the oxidation state of +4; therefore, Ce-containing members of any perovskite family may differ from other members of the same series with R3+. Perovskites with different oxidation states of Ce are reported in the literature [54,55,56,57,58,59,60,61,62]. Ho3+ is the smallest cation for which RMn3Ni2Mn2O12 perovskites have been prepared so far.
2. Results and Discussion
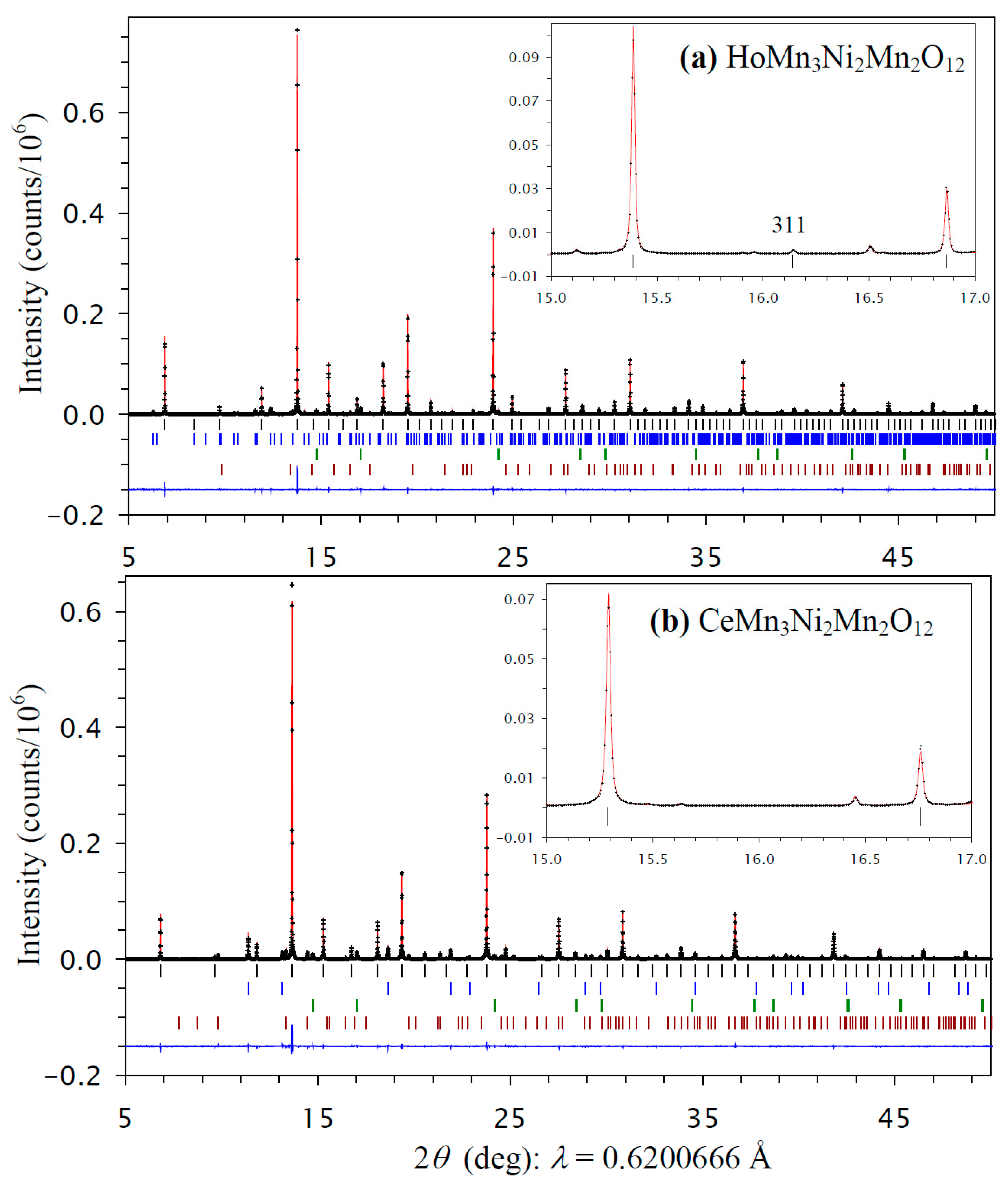
All prepared RMn3Ni2Mn2O12 samples with R = Bi, Ce, and Ho had a cubic crystal structure, as all reflections belonging to RMn3Ni2Mn2O12 could be indexed in a cubic system. The samples also contained different impurities depending on R: the R = Bi sample had Bi2O2CO3, NiO, NiMnO3, and GdFeO3-type impurities, the R = Ce had CeO2, NiO, and NiMnO3 impurities, and the R = Ho sample had HoMn2O5, NiO, and NiMnO3 impurities. All cubic reflections of the R = Bi and Ce samples could be indexed in space group Im-3, which is the parent (maximum) symmetry of the AA′3B4O12-type perovskites [7,8,9,10,11]. On the other hand, a (311) reflection was observed in the synchrotron XRPD data of the R = Ho sample (the inset of Figure 1a). This reflection is forbidden in space group Im-3, and suggests space group Pn-3, which is observed in cases of full or partial B site ordering of AA′3B2B′2O12-type perovskites [12,14,15,16,17,18,19].
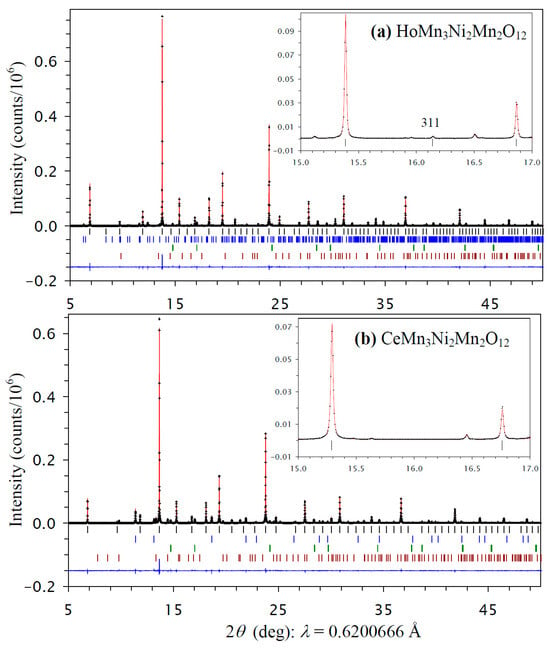
Figure 1.
Fragments of experimental (black crosses), calculated (red line), and difference (blue line at the bottom) room-temperature synchrotron X-ray powder diffraction patterns of (a) HoMn3Ni2Mn2O12 (the Pn-3 modification) and (b) CeMn3Ni2Mn2O12 (the Im-3 modification) in a 2θ range of 5° and 50°. The tick marks show possible Bragg reflection positions for the main phase (black) and impurities (from top to bottom for HoMn2O5 (blue), NiO (green), and NiMnO3 (brown) for R = Ho and for CeO2 (blue), NiO (green), and NiMnO3 (brown) for R = Ce). Insets show magnified parts in a 2θ range of 15° and 17°, and emphasize the presence of the (311) reflection for R = Ho from the B site ordering and the absence of such a reflection for R = Ce.
The distribution of Ni2+ and Mn4+ cations between the B and B′ sites in the R = Ho sample was refined with the following constraints: (1) the full site occupation, g(Mn) + g(Ni) = 1, where g is the occupation factor, should be kept, and (2) the total chemical composition should be kept. The obtained distribution of Ni2+ and Mn4+ cations was close to 0.8Ni2+ + 0.2Mn4+ for the B site and 0.2Ni2+ + 0.8Mn4+ for the B′ site. These values suggest a significant degree of Ni2+ and Mn4+ ordering. We note that similar distributions of Ni2+ and Mn4+ cations were found in samples with R = Nd, Sm, Gd, and Dy [48] prepared under the same conditions.
The refinement of the occupation factors of the R sites (together with atomic displacement parameters (ADPs) of the R sites and all other refined parameters) gave the following values: g(Bi) = 1.0001(13), g(Ce) = 0.9710(15), and g(Ho) = 0.9929(14). These values were close to 1 within the sensitivity of the method, as such deviations could be absorbed by reasonable ADPs. Therefore, we fixed the occupation factor of the R sites at 1 in the final reported models. We emphasize that the ADP for Bi was enhanced. However, all attempts to split (to reduce the ADP of Bi) the Bi site from the ideal (0, 0, 0) position to different positions failed. Enhanced ADPs for Bi are often observed in such perovskites with a cubic structure, even in neutron diffraction data [52]. Enhanced ADPs for Bi are caused by the effect of the lone electron pair of Bi3+ cations and random displacements of Bi3+ cations.
The refined structural parameters of HoMn3Ni2Mn2O12 are summarized in Table 1, and those of RMn3Ni2Mn2O12 with R = Bi and Ce in Table 2. Experimental, calculated, and difference synchrotron X-ray powder diffraction patterns are shown in Figure 1 for HoMn3Ni2Mn2O12 and CeMn3Ni2Mn2O12. Figure S1 gives experimental, calculated, and difference synchrotron X-ray powder diffraction patterns for BiMn3Ni2Mn2O12.

Table 1.
Structural parameters of HoMn3Ni2Mn2O12 (Pn-3; prepared at 1500 K) at room temperature from synchrotron powder X-ray diffraction data.

Table 2.
Structural parameters of RMn3Ni2Mn2O12 (Im-3; prepared at 1500 K) at room temperature from synchrotron powder X-ray diffraction data.
The bond valence sum (BVS) values [63,64] of the MnSQ site (where SQ stands for square-planar) were close to +3 for all three compounds (+2.94 for R = Bi, +2.97 for R = Ce, and +2.95 for R = Ho) suggesting that this site is occupied by Mn3+ cations. The BVS values of the Ni/Mn site (with R0 = 1.7035, which is the average value of R0(Ni2+) and R0(Mn4+) [63]) were +2.90 for R = Bi and +2.89 for R = Ce, again close to the average oxidation state of Ni2+ and Mn4+ cations. On the other hand, for R = Ho, the BVS value of the Ni1/Mn1 site (with the majority of Ni2+) was +2.28, and the BVS value of the Mn2/Ni2 site (with the majority of Mn4+) was +3.98, in agreement with the expected values. The BVS value of the Ni1/Mn1 site was somewhat higher than +2; however, similar values were observed in all other members of the RMn3Ni2Mn2O12 family with R = La [12], Nd, Sm, Gd, and Dy [48].
The BVS values of the R site were +2.71 for R = Bi, +3.04 for R = Ce [64], and +2.63 for R = Ho. Reduced BVS values of Bi3+ cations are often observed in Bi-containing A site-ordered quadruple perovskites [49,52], and could be caused by the influence of the lone electron pair of Bi3+ cations. The BVS value for Ho3+ suggests that Ho3+ cations are highly underbonded inside the A site. The severe underbonding could be a reason why RMn3Ni2Mn2O12 compounds become unstable with smaller R3+ cations [65], such as with R = Er-Lu [48], under the synthesis conditions used. In RMn3Ni2Mn2O12, the BVS values of R3+ cations changed from +3.40 for R = La [12] to +2.72 for R = Dy [48]. Therefore, the BVS value for R = Ho agrees with the general tendency in such perovskites. These BVS values also suggest that cations at the A site can be highly overbonded or highly underbonded, but the structure can still tolerate such stress. Stabilization of the structure was realized through the high-pressure, high-temperature preparation method. The BVS value for Ce suggests that Ce has an oxidation state of +3. Therefore, the reduction of Ce4+ (added as CeO2) took place during the synthesis of CeMn3Ni2Mn2O12, and CeMn3Ni2Mn2O12 is not an exceptional member of the R3+Mn3Ni2Mn2O12 series. The reduction of Ce4+ occurred through the oxidation of a part of Mn3+, as the high-pressure synthesis was performed in sealed capsules in closed environment.
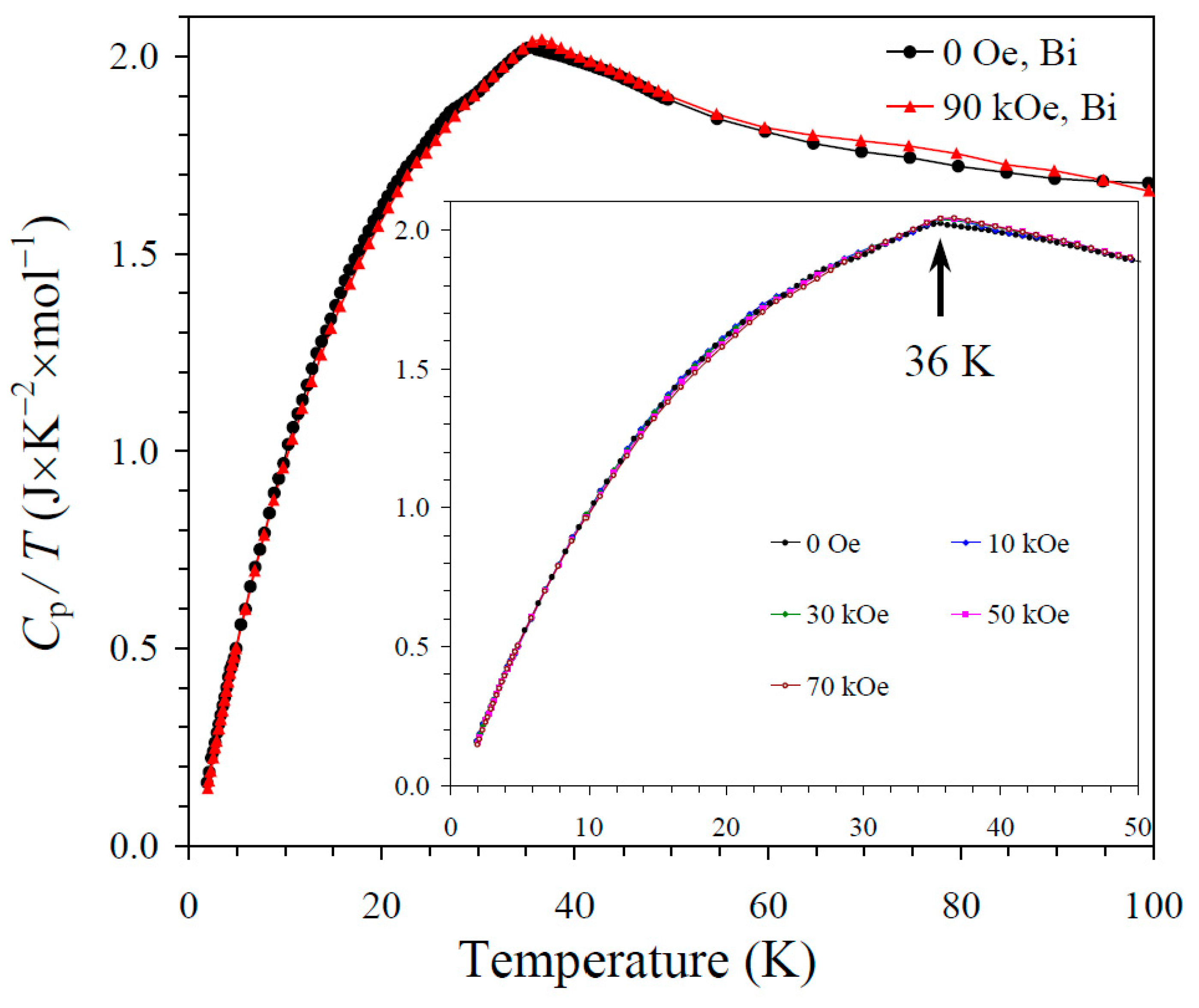
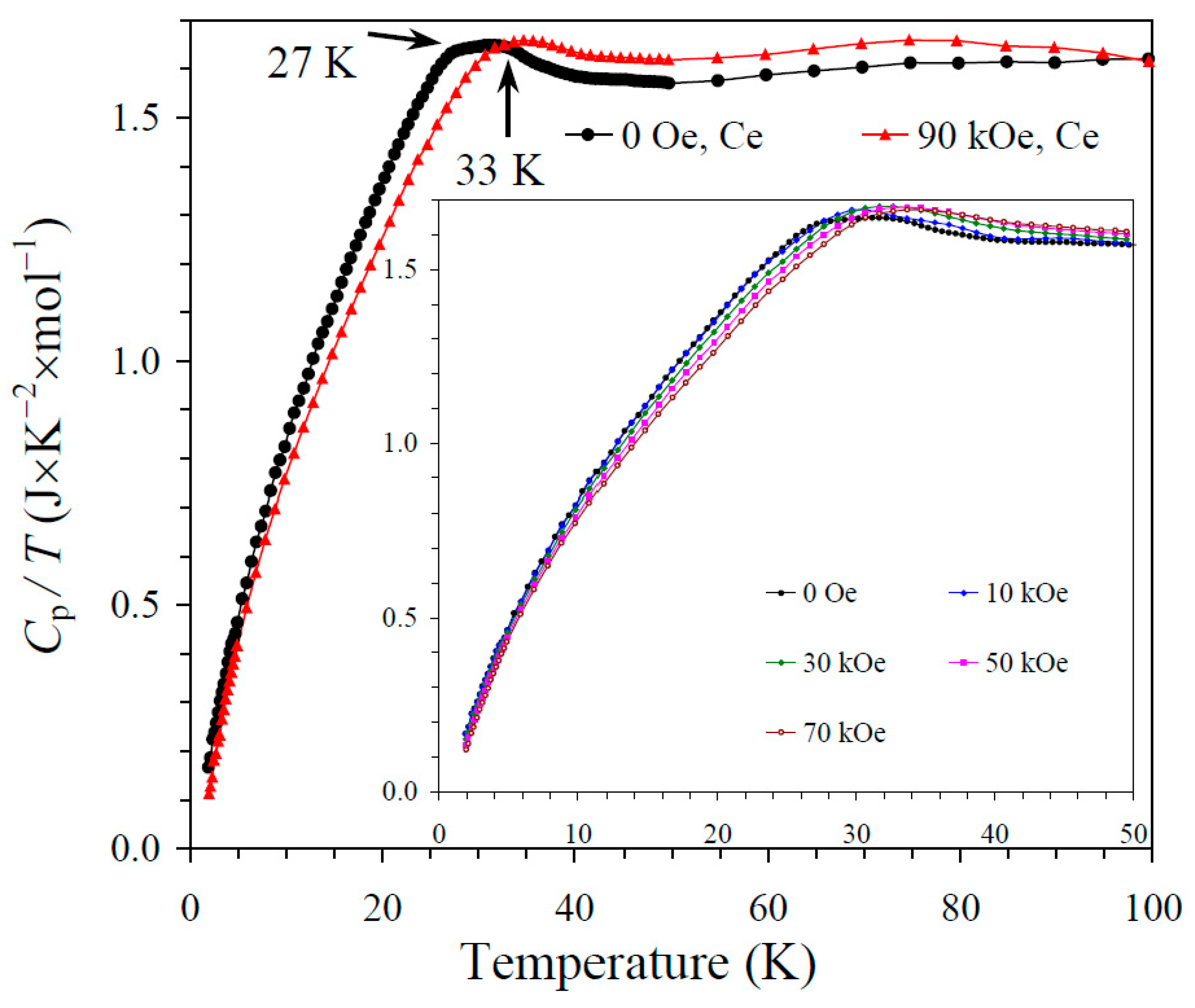
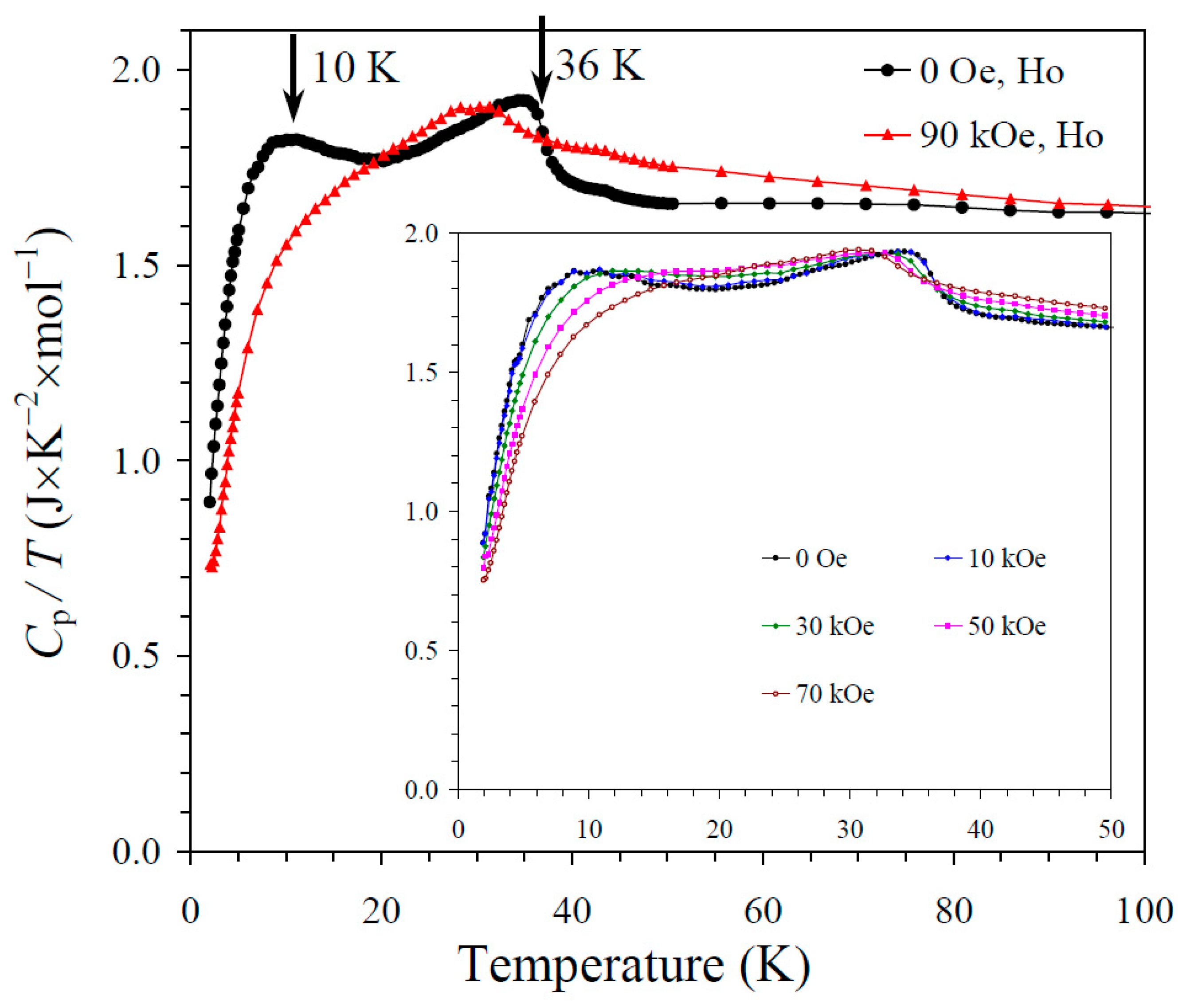
The results of the specific heat measurements are given in Figure 2, Figure 3 and Figure 4. None of the samples showed sharp peaks on specific heat curves. Nevertheless, clear anomalies were observed that could be assigned to long-range magnetic orderings. BiMn3Ni2Mn2O12 showed a clear kink at 36 K (Figure 2), below which the Cp/T values started decreasing sharply. High magnetic fields (up to 90 kOe) had almost no effects on the specific heat values of BiMn3Ni2Mn2O12, suggesting that the magnetic state was quite robust. CeMn3Ni2Mn2O12 showed a broadened kink at 33 K (Figure 3) and a second kink at 27 K, below which the Cp/T values started decreasing. High magnetic fields (up to 90 kOe) had weak effects on the specific heat values of CeMn3Ni2Mn2O12, and moved the positions of the broad anomaly to slightly higher temperatures. HoMn3Ni2Mn2O12 showed two broadened peaks near 36 K and 10 K (Figure 4). High magnetic fields (up to 90 kOe) noticeably smeared the anomaly near 10 K, and moved the positions of the second broad anomaly to slightly lower temperatures. The specific heat data of HoMn3Ni2Mn2O12 were very close to the specific heat curves of DyMn3Ni2Mn2O12 [48].
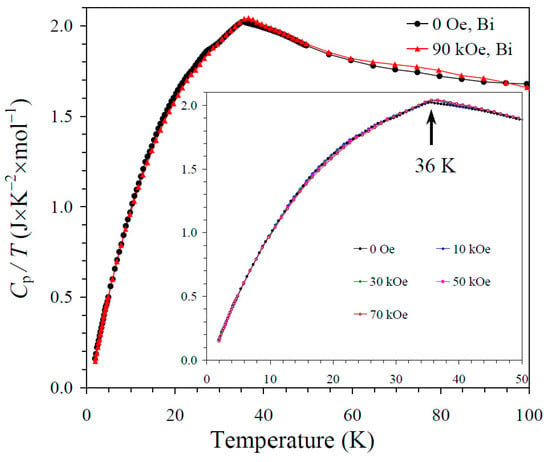
Figure 2.
Cp/T versus T curves of BiMn3Ni2Mn2O12 measured at H = 0 (black curves) and 90 kOe (red curves). The inset shows Cp/T versus T curves at H = 0, 10, 30, 50, and 70 kOe. The arrow shows the magnetic transition temperature.
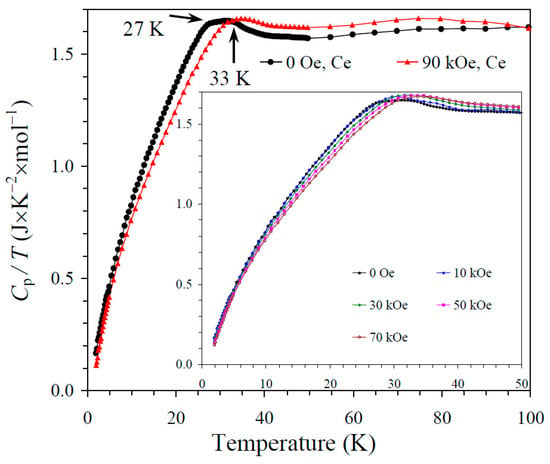
Figure 3.
Cp/T versus T curves of CeMn3Ni2Mn2O12 measured at H = 0 (black curves) and 90 kOe (red curves). The inset shows Cp/T versus T curves at H = 0, 10, 30, 50, and 70 kOe. The arrows show magnetic transition temperatures.
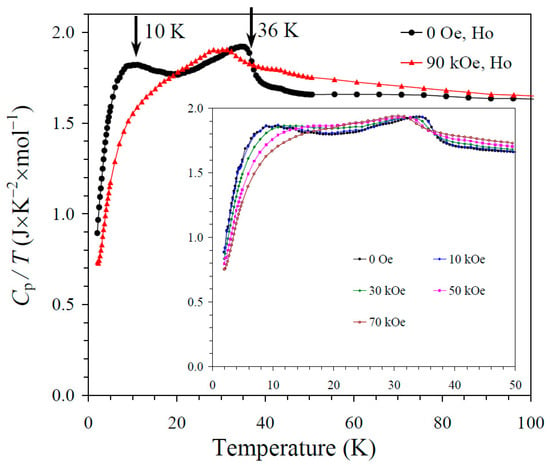
Figure 4.
Cp/T versus T curves of HoMn3Ni2Mn2O12 measured at H = 0 (black curves) and 90 kOe (red curves). The inset shows Cp/T versus T curves at H = 0, 10, 30, 50, and 70 kOe. The arrows show magnetic transition temperatures.
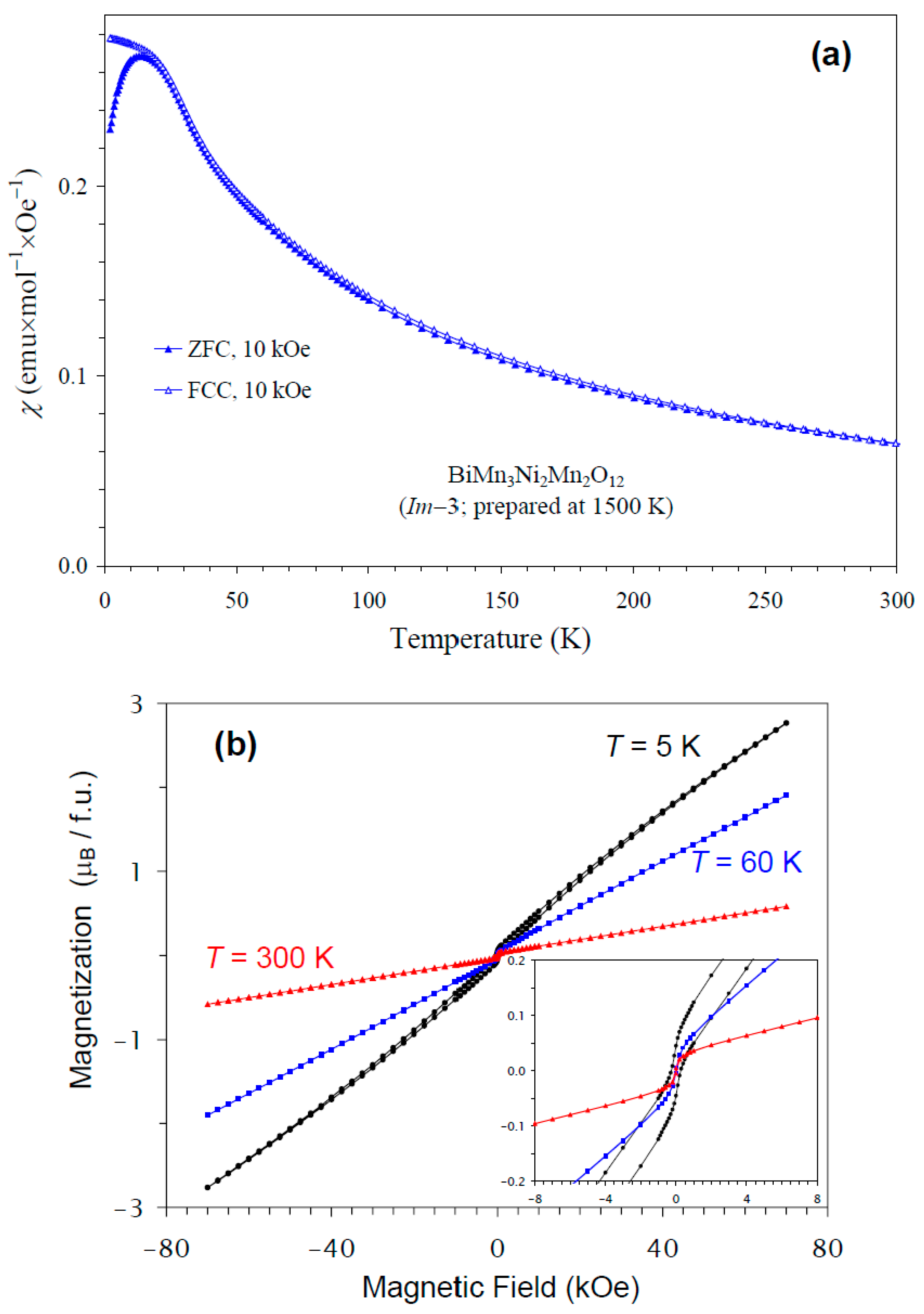
The temperature dependence of the magnetic susceptibility of BiMn3Ni2Mn2O12 is shown in Figure 5a, and Figure 5b gives an M versus H curve at 5 K, 60 K, and 300 K. The divergence between the ZFC and FCC curves was observed below about 15 K at H = 10 kOe; at the same temperature, a broad maximum was observed on the ZFC curve. However, no clear anomalies were detected near 36 K, even on differential curves. Therefore, only specific heat data could clearly identify a magnetic phase transition temperature in BiMn3Ni2Mn2O12. The M versus H curves showed a very weak step-like hysteresis near the origin. The hysteresis near the origin probably originated from a small amount of ferrimagnetic NiMnO3 impurity (with TC = 430 K [66]) with soft ferrimagnetic properties, as the hysteresis was observed even at 300 K. A slim extended hysteresis observed at 5 K (the inset of Figure 5b) originated from BiMn3Ni2Mn2O12.
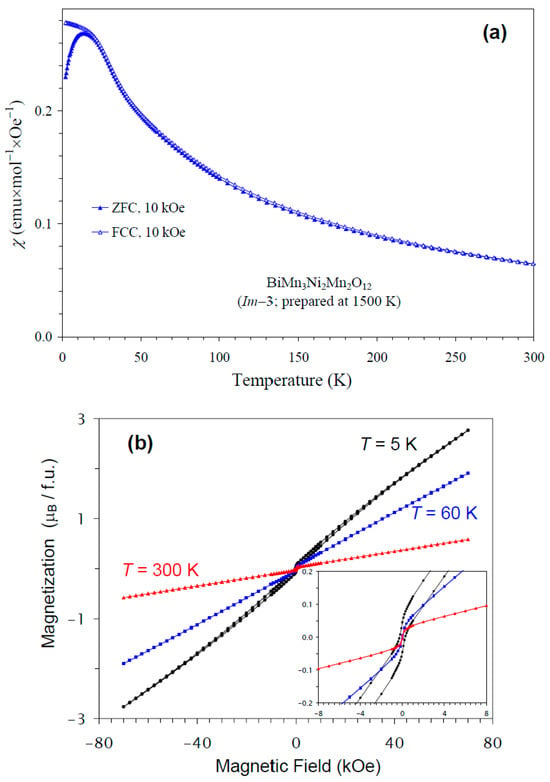
Figure 5.
(a) ZFC (filled symbols) and FCC (empty symbols) dc magnetic susceptibility curves (χ = M/H) of BiMn3Ni2Mn2O12 measured at H = 10 kOe. (b) M versus H curves of BiMn3Ni2Mn2O12 at T = 5 K, 60 K, and 300 K. The inset shows magnified parts near the origin.
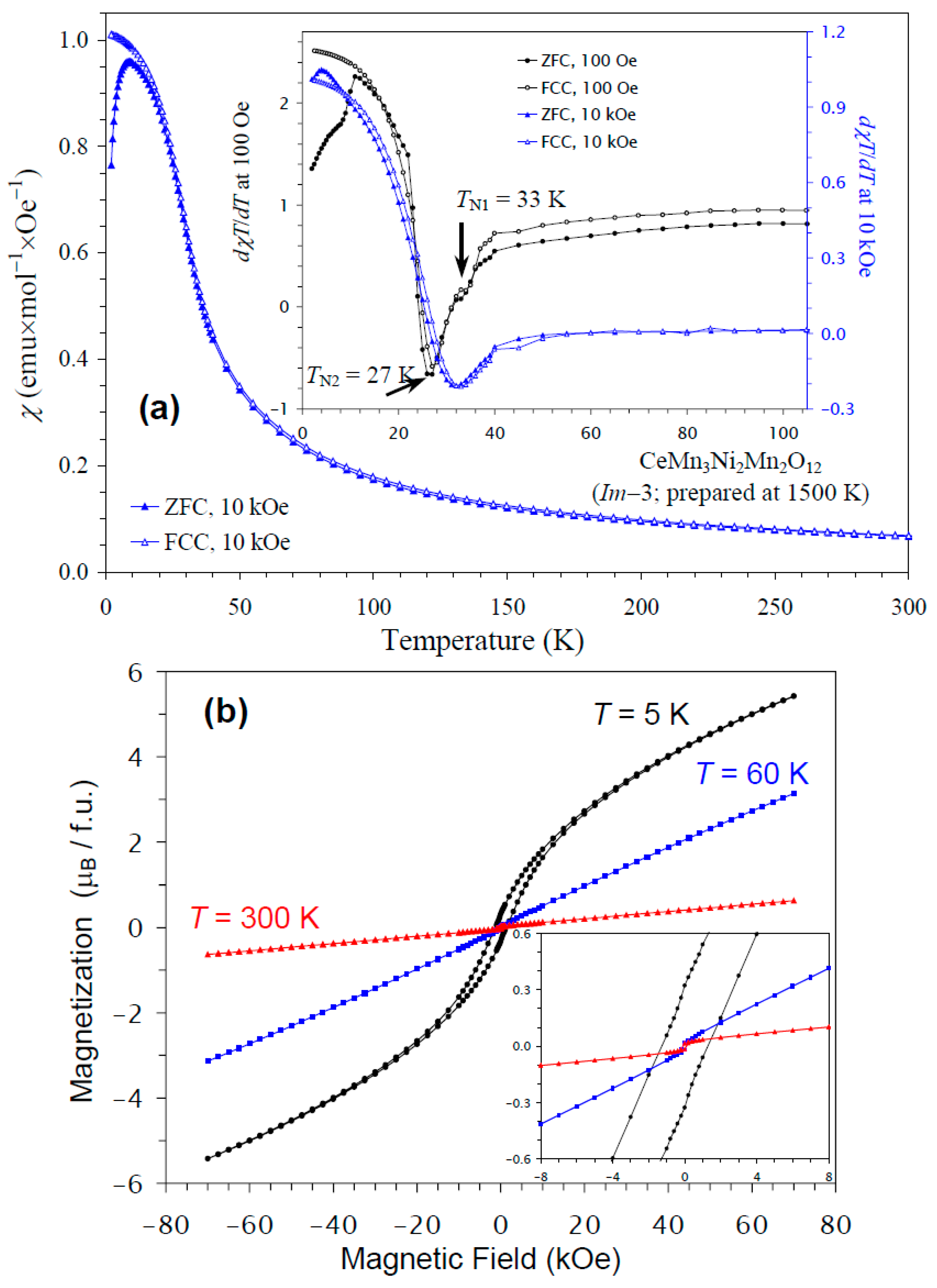
The temperature dependence of the magnetic susceptibility of CeMn3Ni2Mn2O12 is shown in Figure 6a, and Figure 6b gives an M versus H curve at 5 K, 60 K, and 300 K. The divergence between the ZFC and FCC curves was observed below about 10 K at H = 10 kOe; at nearly the same temperature, a broad maximum was observed on the ZFC curve. The differential curves showed some anomalies; for example, the dχT/dT versus T curves at H = 100 Oe showed peaks at 27 K with shoulders at 33 K, in agreement with the specific heat data; on the other hand, at H = 10 kOe, only one broad anomaly was seen at 33 K. The M versus H curve at 5 K demonstrated a slim extended hysteresis. Above TN (at 60 K and 300 K), a very weak, step-like hysteresis was observed near the origin, originating from ferrimagnetic NiMnO3 impurity [66]. Therefore, the extended hysteresis at 5 K should originate from the main phase.
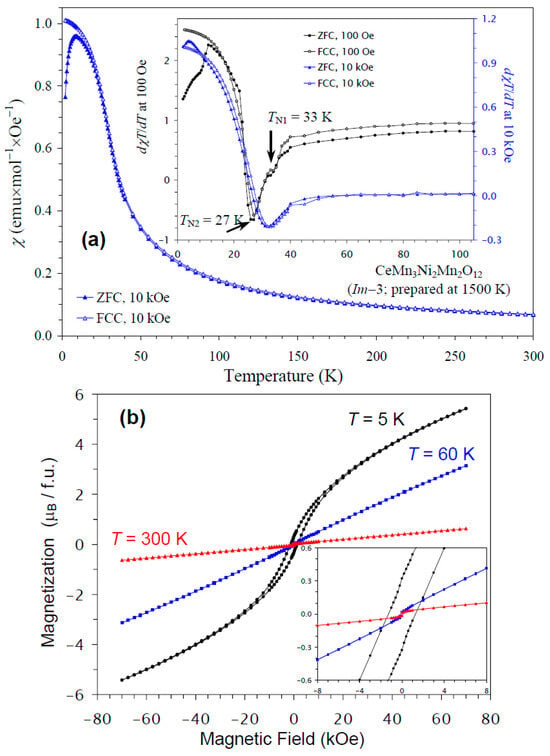
Figure 6.
(a) ZFC (filled symbols) and FCC (empty symbols) dc magnetic susceptibility curves (χ = M/H) of CeMn3Ni2Mn2O12 measured at H = 10 kOe. The inset shows ZFC and FCC dχT/dT versus T curves at H = 100 Oe (black curves) and 10 kOe (blue curves). (b) M versus H curves of CeMn3Ni2Mn2O12 at T = 5 K, 60 K, and 300 K. The inset shows magnified parts near the origin.
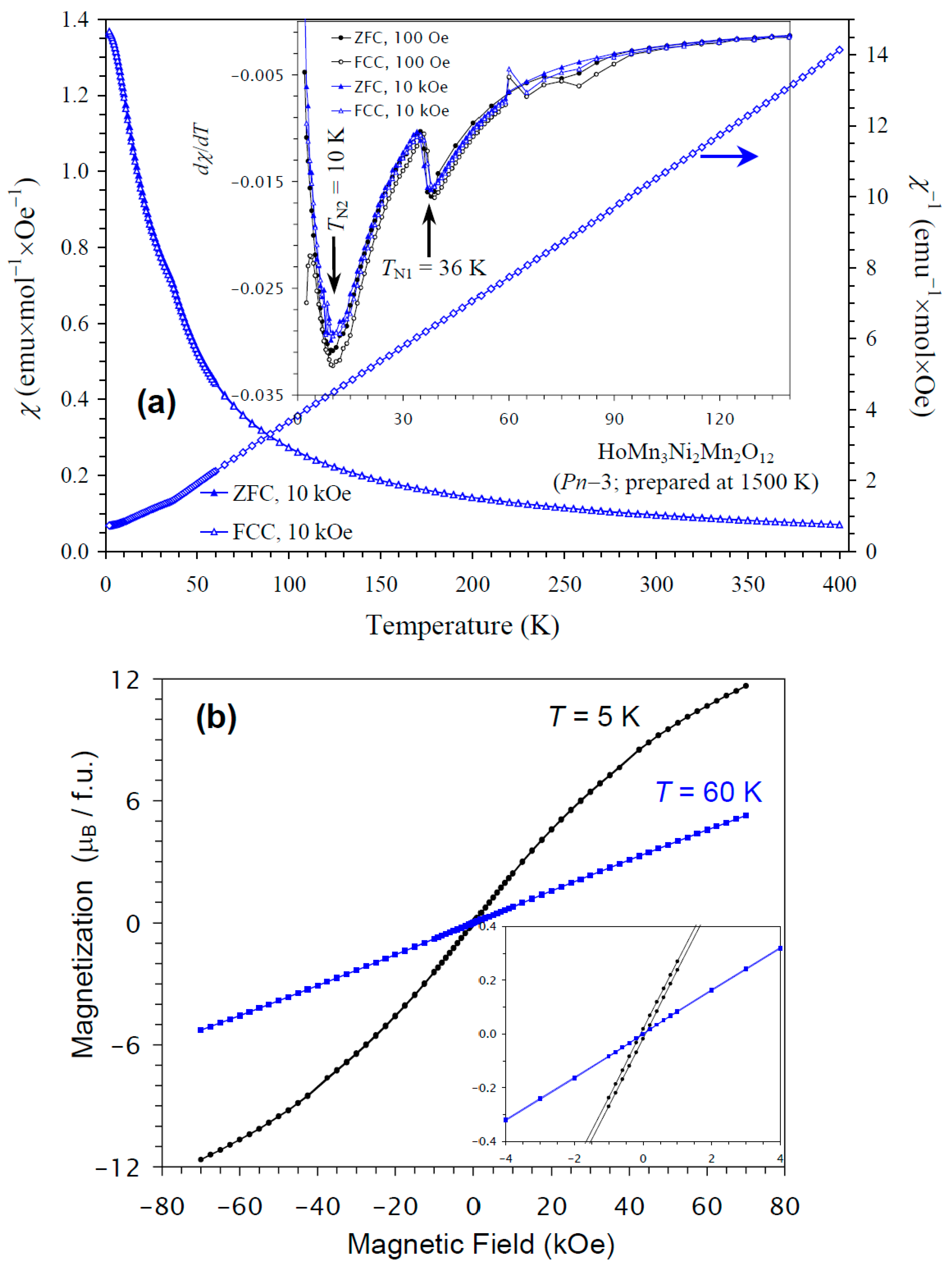
The temperature dependence of the magnetic susceptibility of HoMn3Ni2Mn2O12 is shown in Figure 7a, and Figure 7b gives M versus H curves at 5 K and 60 K. In this case, kinks on the χ versus T curves were clearly observed at 36 K. The differential dχ/dT versus T curves at H = 100 Oe and 10 kOe showed sharp anomalies at 36 K and broader anomalies near 10 K, in agreement with the specific heat data. The M versus H curves showed almost no hysteresis. No detectable step-like hysteresis was observed near the origin at 60 K (above TN of HoMn3Ni2Mn2O12) from NiMnO3 impurity, because of its very small amount. The χ versus T and M versus H curves of HoMn3Ni2Mn2O12 were very close to those of DyMn3Ni2Mn2O12 [48]. The ground states of Ho3+ and Dy3+ cations are, of course, different [67]. Nevertheless, these cations are quite close to each other, as they have close ionic radii (rVIII(Dy3+) = 1.027 Å versus rVIII(Ho3+) = 1.015 Å [65]), effective calculated magnetic moments (10.63μB for Dy3+ versus 10.60μB for Ho3+ [67]), and maximum ordered magnetic moments (of 10μB). Therefore, it could be expected that HoMn3Ni2Mn2O12 and DyMn3Ni2Mn2O12 would have similar magnetic properties and ground states.
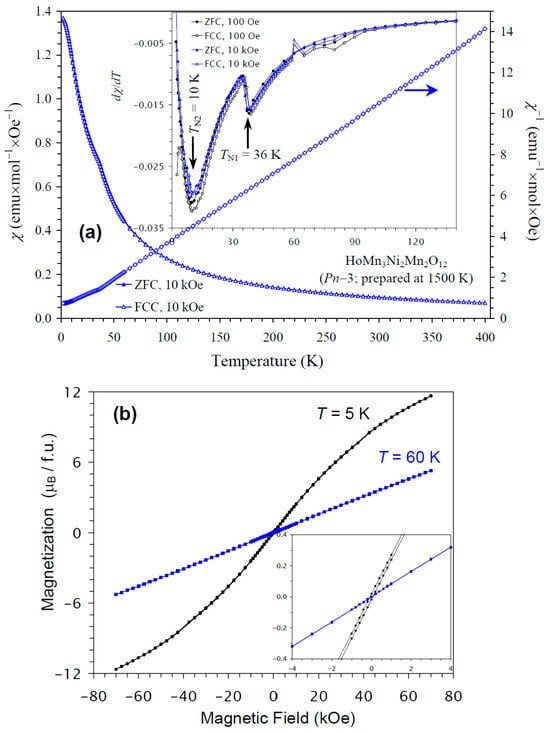
Figure 7.
(a) ZFC (filled symbols) and FCC (empty symbols) dc magnetic susceptibility curves (χ = M/H) of HoMn3Ni2Mn2O12 measured at H = 10 kOe. The inset shows ZFC and FCC dχ/dT versus T curves at H = 100 Oe (black curves) and 10 kOe (blue curves). The right-hand axis shows the FCC χ−1 versus T curve. The arrows show the magnetic transition temperatures. (b) M versus H curves of HoMn3Ni2Mn2O12 at T = 5 K and 60 K. The inset shows magnified parts near the origin.
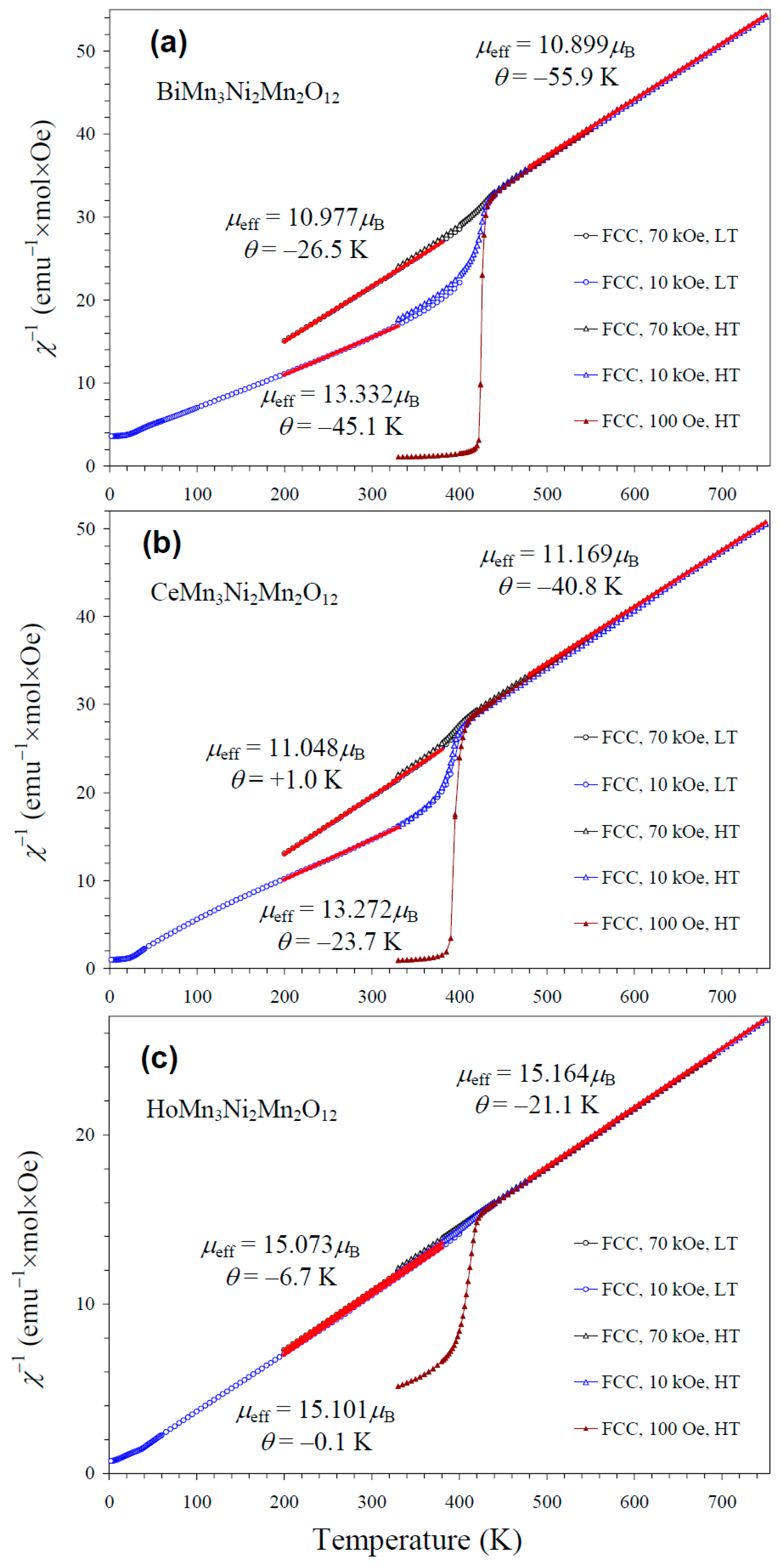
NiMnO3 is a ferrimagnetic material with a TC of about 430 K [66]. Therefore, it gives noticeable contributions to the measured magnetic properties in small magnetic fields (such as 100 Oe (Figure S2)) below 400 K (Figure 8). As NiMnO3 is a soft ferrimagnetic material, its magnetization saturates at high magnetic fields. Therefore, the larger the measurement magnetic field, the smaller the relative contribution of NiMnO3. However, we found that even at 70 kOe (the maximum available magnetic field), contributions from NiMnO3 affected parameters of the Curie–Weiss fits below 400 K (Figure 8). Therefore, we performed high-temperature magnetic measurements up to 750 K. Above about 440 K, magnetic susceptibilities were independent of the measurement magnetic field, and reliable, intrinsic Curie–Weiss parameters could be obtained. Inverse magnetic susceptibilities followed the Curie–Weiss law at high temperatures. The Curie–Weiss fits were performed between 480 and 750 K (for the 70 kOe FCC curves), and the resultant parameters are summarized in Table 3. The experimental effective magnetic moments (μeff) were very close to the calculated values (μcalc), supporting the charges of magnetic cations. In particular, the inclusion of the magnetic moment of Ce3+ [67] in the calculation gave a better agreement between μeff and μcalc; this fact gives indirect support for the +3 oxidation state of Ce, in addition to the structural data. We note that the Curie–Weiss temperatures (θ) were very small for the R = Ce and Ho samples when the fits were performed between 200 K and 380 K at 70 kOe (Figure 8).
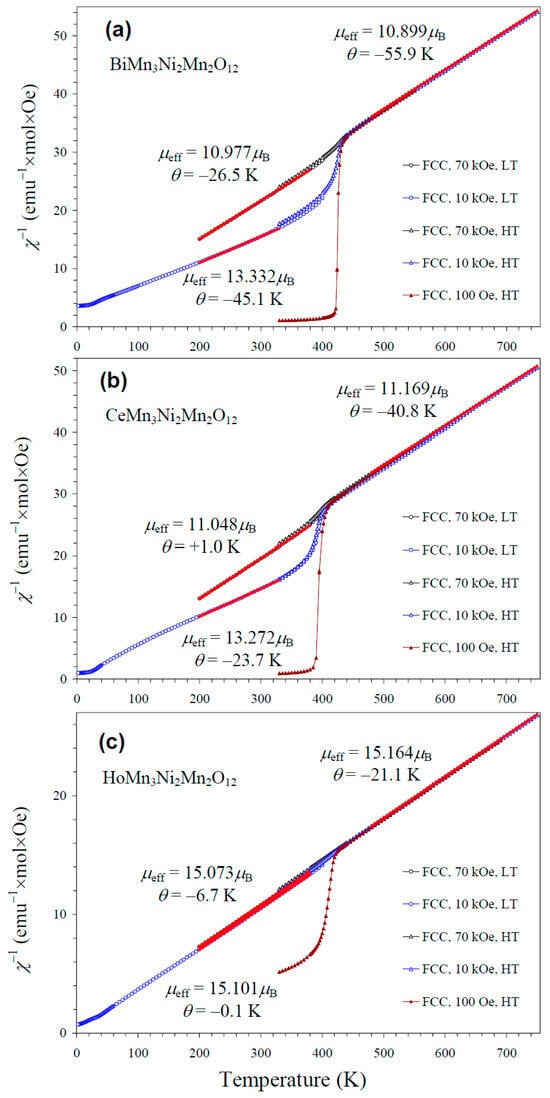
Figure 8.
Inverse dc magnetic susceptibility curves (χ−1 = H/M) of (a) BiMn3Ni2Mn2O12, (b) CeMn3Ni2Mn2O12, and (c) HoMn3Ni2Mn2O12 measured at H = 100 Oe (brown), 10 kOe (blue), and 70 kOe (black) on cooling (FCC curves) in the low-temperature (LT) region of 2–400 K (circles) and the high-temperature (HT) region of 330–750 K (triangles). The red lines show Curie–Weiss fits (for the 70 kOe data at HT and for the 10 kOe and 70 kOe data at LT); parameters of the fits are given in the figure.

Table 3.
Temperatures of magnetic anomalies and parameters of Curie–Weiss fits and M versus H curves at T = 5 K for RMn3Ni2Mn2O12.
RMn3Ni2Mn2O12 samples with R = Bi, Ce, and Ho were prepared under the same synthesis conditions (6 GPa and 1500 K). The same synthesis conditions were used for the preparation of RMn3Ni2Mn2O12 with R = Nd, Sm, Gd, and Dy [48]. These conditions produced partial ordering of Ni2+ and Mn4+ cations in the case of R = Nd-Ho. It was also found that an increase in the synthesis temperature to 1700 K produced samples with a disordered arrangement of Ni2+ and Mn4+ cations in the case of R = Nd and Sm [48]. Therefore, the disordered arrangement of Ni2+ and Mn4+ cations found in the R = Bi and Ce samples prepared at 1500 K could suggest that a (partial) ordering may be realized at lower synthesis temperatures. This possibility may be explored in the future. Note that larger amounts of impurities in the R = Ce sample (Table 2) could shift the real chemical composition of the main phase and contribute to the stabilization of the disordered structure.
3. Experimental
RMn3Ni2Mn2O12 samples with R = Bi, Ce, and Ho were prepared from stoichiometric mixtures of Bi2O3 (Rare Metallic Co., Tokyo, Japan, 99.9999%), CeO2 (Rare Metallic Co., Tokyo, Japan, 99.99%), Ho2O3 (Rare Metallic Co., Tokyo, Japan, 99.9%), Mn2O3 (Rare Metallic Co., Tokyo, Japan, 99.99%), MnO2 (Alfa Aesar, Ward Hill, MA, USA, 99.99%), and NiO (Rare Metallic Co., Tokyo, Japan, 99.9%). Single-phase Mn2O3 was prepared from a commercial MnO2 chemical (Rare Metallic Co., Tokyo, Japan, 99.99%) by annealing in air at 923 K for 24 h. The synthesis was performed at about 6 GPa and at about 1500 K for 2 h in sealed Au capsules, using a belt-type HP instrument. After annealing at 1500 K, the samples were cooled down to room temperature by turning off the heating current, and the pressure was slowly released.
X-ray powder diffraction (XRPD) data were collected at room temperature on a MiniFlex600 diffractometer (Rigaku, Tokyo, Japan) using CuKα radiation (2θ range of 8–100°, step width of 0.02°, and scan speed of 2°/min). Room-temperature synchrotron XRPD data were measured using the beamline BL02B2 [68,69] of SPring-8, Japan. Intensity data were collected between 1.00° and 83.02° at a 0.006° interval in 2θ with 100 s exposure time. A wavelength of λ = 0.4137875 Å was used for R = Bi (to reduce the absorption effect because of the presence of heavy Bi). A wavelength of λ = 0.6200666 Å was used for R = Ce and Ho. The samples were placed into open Lindemann glass capillary tubes (inner diameter: 0.2 mm), which were rotated during measurements. The Rietveld analysis of all XRPD data was performed using the RIETAN-2000 program [70]. Normalized (because of different measurement times) background (obtained through measurements of (different) empty capillary tubes) was subtracted from the raw synchrotron XRPD data. Background subtraction usually gives larger profile agreement factors (Rwp and Rp) because background intensities become very low. On the other hand, the background subtraction usually improves integrated agreement factors (RI and RF) because better structure descriptions are achieved. The remaining background was fitted by 12-order Legendre polynomials. We note that NiMnO3 impurity was fitted using the ilmenite-type model (space group R-3) in the R = Bi and Ce samples, and using the simple corundum-type model (space group R-3c) in the R = Ho sample, because of its very small amount.
Magnetic measurements were performed on SQUID magnetometers (Quantum Design MPMS3, San Diego, CA, USA) between 2 and 400 K in applied fields of 100 Oe, 10 kOe, and 70 kOe, under both zero-field-cooled (ZFC) and field-cooled on cooling (FCC) conditions. High-temperature magnetic measurements were performed between 330 K and 750 K with the same magnetic fields using a high-temperature MPMS3 option, where dense pellets of the samples with polished flat surfaces were attached to a high-temperature stick with Zircar cement. Isothermal magnetization was measured at temperatures of 5 K, 60 K, and 300 K, between −70 and 70 kOe.
The specific heat, Cp, was measured on cooling from 100 K to 2 K at different magnetic fields up to 90 kOe by a pulse relaxation method, using a commercial calorimeter (Quantum Design PPMS, San Diego, CA, USA). All magnetic and specific heat measurements were performed using pieces of dense pellets.
4. Conclusions
The synthesis of A site-ordered quadruple perovskites, RMn3Ni2Mn2O12 with R = Bi, Ce, and Ho, was performed by a high-pressure, high-temperature method at about 6 GPa and about 1500 K. Despite using the same synthesis conditions, the R = Bi and Ce samples crystallized in space group Im-3 with a disordered arrangement of Ni2+ and Mn4+ cations. On the other hand, the R = Ho sample crystallized in space group Pn-3 with partial ordering of Ni2+ and Mn4+ cations. By using a combination of magnetic susceptibility and specific heat measurements, one magnetic transition was detected in the R = Bi sample near 36 K. On the other hand, two magnetic transitions were found near 27 K and 33 K in the R = Ce sample, and near 10 K and 36 K in the R = Ho sample. Magnetic properties were affected by the presence of ferrimagnetic NiMnO3 impurity (with a TC of about 430 K). Therefore, Curie–Weiss parameters were estimated for all samples from high-temperature magnetic measurements up to 750 K. The total effective magnetic moment for R = Ce and structural information, obtained from the state-of-the-art synchrotron X-ray powder diffraction, suggested the presence of Ce3+.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30081749/s1: Figure S1: Fragments (between 3° and 25°) and magnified parts of experimental, calculated, and difference synchrotron X-ray powder diffraction patterns of BiMn3Ni2Mn2O12 at T = 297 K; Figure S2: ZFC and FCC magnetic susceptibility curves of RMn3Ni2Mn2O12 with R = Bi, Ce, and Ho at H = 100 Oe, between 330 K and 550 K.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the author upon request.
Acknowledgments
Synchrotron radiation experiments were conducted on the powder diffraction beamline BL02B2 at SPring-8, with the permission from the Japan Synchrotron Radiation Research Institute (Proposal Number: 2024B1825). The author thanks Y. Mori for his help with BL02B2 of SPring-8, and R. Liu for her preliminary studies of RMn3Ni2Mn2O12 compounds. MANA was supported by the World Premier International Research Center Initiative (WPI), MEXT, Japan.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Mitchell, R.H. Perovskites: Modern and Ancient; Almaz Press: Thunder Bay, ON, Canada, 2002. [Google Scholar]
- Abakumov, A.M.; Tsirlin, A.A.; Antipov, E.V. Transition-metal perovskites. In Comprehensive Inorganic Chemistry II (Second Edition): From Elements to Applications; Reedijk, J., Poeppelmeier, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 2, pp. 1–40. [Google Scholar]
- Knapp, M.C.; Woodward, P.M. A-site cation ordering in AA′BB′O6 perovskites. J. Solid State Chem. 2006, 179, 1076–1085. [Google Scholar] [CrossRef]
- King, G.; Woodward, P.M. Cation ordering in perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar] [CrossRef]
- Vasala, S.; Karppinen, M. A2B′B″O6 perovskites: A review. Prog. Solid State Chem. 2015, 43, 1–36. [Google Scholar] [CrossRef]
- Bousquet, E.; Cano, A. Non-collinear magnetism in multiferroic perovskites. J. Phys. Condens. Matter 2016, 28, 123001. [Google Scholar] [CrossRef]
- Vasil’ev, A.N.; Volkova, O.S. New functional materials AC3B4O12 (Review). Low Temp. Phys. 2007, 33, 895–914. [Google Scholar] [CrossRef]
- Long, Y. A-site ordered quadruple perovskite oxides AA′3B4O12. Chin. Phys. B 2016, 25, 078108. [Google Scholar] [CrossRef]
- Yamada, I. Novel catalytic properties of quadruple perovskites. Sci. Technol. Adv. Mater. 2017, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Belik, A.A.; Johnson, R.D.; Khalyavin, D.D. The rich physics of A-site-ordered quadruple perovskite manganites AMn7O12. Dalton Trans. 2021, 50, 15458–15472. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, X.H. Research progress on quadruple perovskite oxides. J. Mater. Chem. C 2024, 12, 9510–9561. [Google Scholar] [CrossRef]
- Yin, Y.Y.; Liu, M.; Dai, J.H.; Wang, X.; Zhou, L.; Cao, H.; Cruz, C.D.; Chen, C.T.; Xu, Y.; Shen, X.; et al. LaMn3Ni2Mn2O12: An A- and B-site ordered quadruple perovskite with A-site tuning orthogonal spin ordering. Chem. Mater. 2016, 28, 8988–8996. [Google Scholar] [CrossRef]
- Liu, M.; Hu, C.E.; Cheng, C.; Chen, X.R. A–B-intersite-dependent magnetic order and electronic structure of LaMn3Ni2Mn2O12: A first-principles study. J. Phys. Chem. C 2018, 122, 1946–1954. [Google Scholar] [CrossRef]
- Byeon, S.H.; Lufaso, M.W.; Parise, J.B.; Woodward, P.M.; Hansen, T. High-pressure synthesis and characterization of perovskites with simultaneous ordering of both the A- and B-site cations, CaCu3Ga2M2O12 (M = Sb, Ta). Chem. Mater. 2003, 15, 3798–3804. [Google Scholar] [CrossRef]
- Byeon, S.H.; Lee, S.S.; Parise, J.B.; Woodward, P.M.; Hur, N.H. High-pressure synthesis of metallic perovskite ruthenate Ca-Cu3Ga2Ru2O12. Chem. Mater. 2004, 16, 3697–3701. [Google Scholar] [CrossRef]
- Byeon, S.H.; Lee, S.S.; Parise, J.B.; Woodward, P.M.; Hur, N.H. New ferrimagnetic oxide CaCu3Cr2Sb2O12: High-pressure synthesis, structure, and magnetic properties. Chem. Mater. 2005, 17, 3552–3557. [Google Scholar] [CrossRef]
- Deng, H.S.; Liu, M.; Dai, J.H.; Hu, Z.W.; Kuo, C.Y.; Yin, Y.Y.; Yang, J.Y.; Wang, X.; Zhao, Q.; Xu, Y.; et al. Strong enhancement of spin ordering by A-site magnetic ions in the ferrimagnet CaCu3Fe2Os2O12. Phys. Rev. B 2016, 94, 024414. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Deng, H.; Agrestini, S.; Chen, K.; Lee, J.F.; Lin, H.J.; Chen, C.T.; Choueikani, F.; Ohresser, P.; et al. Comparative study on the magnetic and transport properties of B-site ordered and disordered CaCu3Fe2Os2O12. Inorg. Chem. 2022, 61, 16929–16935. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Shen, X.D.; Liu, Z.H.; Hu, Z.W.; Chen, K.; Ohresser, P.; Nataf, L.; Baudelet, F.; Lin, H.J.; et al. High-temperature ferrimagnetic half metallicity with wide spin-up energy gap in NaCu3Fe2Os2O12. Inorg. Chem. 2019, 58, 320–326. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Z.; Wang, W.; Hu, Z.; Lin, H.J.; Weng, S.C.; Chen, C.T.; Yu, R.; Tjeng, L.H.; Long, Y.W. High-pressure synthesis and spin glass behavior of a Mn/Ir disordered quadruple perovskite CaCu3Mn2Ir2O12. J. Phys. Condens. Matter 2020, 32, 075701. [Google Scholar] [CrossRef]
- Li, H.P.; Zhang, Q.; Zhu, Z.P.; Ge, Z.Z.; Li, C.S.; Meng, J.; Tian, Y. Unraveling the effect of B-site antisite defects on the electronic and magnetic properties of the quadruple perovskite CaCu3Fe2Nb2O12. Phys. Chem. Chem. Phys. 2019, 21, 3059–3065. [Google Scholar] [CrossRef]
- Guo, J.; Shen, X.D.; Liu, Z.H.; Qin, S.J.; Wang, W.P.; Ye, X.B.; Liu, G.X.; Yu, R.C.; Lin, H.J.; Chen, C.T.; et al. High-pressure synthesis of a B-site Co2+/Mn4+ disordered quadruple perovskite LaMn3Co2Mn2O12. Inorg. Chem. 2020, 59, 12445–12452. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Q.; Ye, X.; Wang, X.; Zhou, L.; Shen, X.; Chen, K.; Nataf, L.; Baudelet, F.; Agrestini, S.; et al. Quadruple perovskite oxide LaCu3Co2Re2O12: A ferrimagnetic half metal with nearly 100% B-site degree of order. Appl. Phys. Lett. 2020, 117, 152402. [Google Scholar] [CrossRef]
- Li, S.M.; Shu, M.F.; Wang, M.; Pan, C.B.; Zhao, G.C.; Yin, L.H.; Song, W.H.; Yang, J.; Zhu, X.B.; Sun, Y.P. Critical behavior at paramagnetic to ferrimagnetic phase transition in A-site ordered perovskite CaCu3Cr2Nb2O12. Phys. B Condens. Matter 2023, 648, 414376. [Google Scholar] [CrossRef]
- Morimura, A.; Kamiyama, S.; Hayashi, N.; Yamamoto, H.; Yamada, I. High-pressure syntheses, crystal structures, and magnetic properties of novel quadruple perovskite oxides LaMn3Ru2Mn2O12 and LaMn3Ru2Fe2O12. J. Alloys Compd. 2023, 968, 172263. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.H.; Ye, X.B.; Wang, X.; Lu, D.B.; Zhao, H.T.; Pi, M.C.; Chen, C.T.; Chen, J.L.; Kuo, C.Y.; et al. High-pressure synthesis of quadruple perovskite oxide CaCu3Cr2Re2O12 with a high ferrimagnetic Curie temperature. Inorg. Chem. 2024, 63, 3499–3505. [Google Scholar] [CrossRef]
- Kumar, L.; Datta, J.; Sen, S.; Ray, P.P.; Mandal, T.K. Ambient pressure synthesis and properties of LaCu3Fe2TiSbO12: New A-site ordered ferrimagnetic quadruple perovskite. J. Solid State Chem. 2021, 302, 122433. [Google Scholar] [CrossRef]
- Kumar, L.; Sen, S.; Mandal, T.K. Ambient pressure synthesis and structure and magnetic properties of a new A- and B-site ordered multinary quadruple perovskite. Dalton Trans. 2024, 53, 11060–11070. [Google Scholar] [CrossRef]
- Dass, R.I.; Yan, J.Q.; Goodenough, J.B. Oxygen stoichiometry, ferromagnetism, and transport properties of La2−xNiMnO6+δ. Phys. Rev. B 2003, 68, 064415. [Google Scholar] [CrossRef]
- Rogado, N.S.; Li, J.; Sleight, A.W.; Subramanian, M.A. Magnetocapacitance and magnetoresistance near room temperature in a ferromagnetic semiconductor: La2NiMnO6. Adv. Mater. 2005, 17, 2225–2227. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shiozawa, M.; Sato, K.; Abe, K.; Asai, K. Crystal structure, magnetism, and dielectric properties of La1−xBixNi0.5Mn0.5O3. J. Phys. Soc. Jpn. 2008, 77, 084701. [Google Scholar] [CrossRef]
- Choudhury, D.; Mandal, P.; Mathieu, R.; Hazarika, A.; Rajan, S.; Sundaresan, A.; Waghmare, U.V.; Knut, R.; Karis, O.; Nordblad, P.; et al. Near-room-temperature colossal magnetodielectricity and multiglass properties in partially disordered La2NiMnO6. Phys. Rev. Lett. 2012, 108, 127201. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Shi, L.; Zhou, S.M.; Zhao, J.I.; Liu, W.J. Near room-temperature magnetoresistance effect in double perovskite La2NiMnO6. Appl. Phys. Lett. 2013, 102, 222401. [Google Scholar] [CrossRef]
- Sánchez-Benítez, J.; Martínez-Lope, M.J.; Alonso, J.A.; García-Muñoz, J.L. Magnetic and structural features of the NdNi1−xMnxO3 perovskite series investigated by neutron diffraction. J. Phys. Condens. Matter 2011, 23, 226001. [Google Scholar] [CrossRef]
- Retuerto, M.; Muñoz, Á.; Martínez-Lope, M.J.; Alonso, J.A.; Mompeán, F.J.; Fernández-Díaz, M.T.; Sánchez-Benítez, J. Magnetic interactions in the double perovskites R2NiMnO6 (R = Tb, Ho, Er, Tm) investigated by neutron diffraction. Inorg. Chem. 2015, 54, 10890–10900. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.J.; Fillman, R.; Whitaker, H.; Nag, A.; Tiwari, R.M.; Ramanujachary, K.V.; Gopalakrishnan, J.; Lofland, S.E. An investigation of structural, magnetic and dielectric properties of R2NiMnO6 (R = rare earth, Y). Mater. Res. Bull. 2009, 44, 1559–1564. [Google Scholar] [CrossRef]
- Nasir, M.; Kumar, S.; Patra, N.; Bhattacharya, D.; Jha, S.N.; Basaula, D.R.; Bhatt, S.; Khan, M.; Liu, S.W.; Biring, S.; et al. Role of antisite disorder, rare-earth size, and superexchange angle on band gap, Curie temperature, and magnetization of R2NiMnO6 double perovskites. ACS Appl. Electron. Mater. 2019, 1, 141–153. [Google Scholar] [CrossRef]
- Asai, K.; Fujiyoshi, K.; Nishimori, N.; Satoh, Y.; Kobayashi, Y.; Mizoguchi, M. Magnetic properties of REMe0.5Mn0.5O3 (RE = rare earth element, Me = Ni, Co). J. Phys. Soc. Jpn. 1998, 67, 4218–4228. [Google Scholar] [CrossRef]
- Yi, W.; Liang, Q.F.; Matsushita, Y.; Tanaka, M.; Belik, A.A. High-pressure synthesis, crystal structure, and properties of In2NiMnO6 with antiferromagnetic order and field-induced phase transition. Inorg. Chem. 2013, 52, 14108–14115. [Google Scholar] [CrossRef]
- Terada, N.; Khalyavin, D.D.; Manuel, P.; Yi, W.; Suzuki, H.S.; Tsujii, N.; Imanaka, Y.; Belik, A.A. Ferroelectricity induced by ferriaxial crystal rotation and spin helicity in a B-site-ordered double-perovskite multiferroic In2NiMnO6. Phys. Rev. B 2015, 91, 104413. [Google Scholar] [CrossRef]
- Yi, W.; Princep, A.J.; Guo, Y.F.; Johnson, R.D.; Khalyavin, D.D.; Manuel, P.; Senyshyn, A.; Presniakov, I.A.; Sobolev, A.V.; Matsushita, Y.; et al. Sc2NiMnO6: A double-perovskite with a magnetodielectric response driven by multiple magnetic orders. Inorg. Chem. 2015, 54, 8012–8021. [Google Scholar] [CrossRef]
- Ding, L.; Khalyavin, D.D.; Manuel, P.; Blake, J.; Orlandi, F.; Yi, W.; Belik, A.A. Colossal magnetoresistance in the insulating ferromagnetic double perovskites Tl2NiMnO6: A neutron diffraction study. Acta Mater. 2019, 173, 20–26. [Google Scholar] [CrossRef]
- Sobolev, A.V.; Glazkova, I.S.; Akulenko, A.A.; Sergueev, I.; Chumakov, A.I.; Yi, W.; Belik, A.A.; Presniakov, I.A. 61Ni nuclear forward scattering study of magnetic hyperfine interactions in double perovskites A2NiMnO6 (A = Sc, In, Tl). J. Phys. Chem. 2019, 123, 23628–23634. [Google Scholar] [CrossRef]
- Terada, N.; Colin, C.V.; Qureshi, N.; Hansen, T.; Matsubayashi, K.; Uwatoko, Y.; Belik, A.A. Pressure-induced incommensurate antiferromagnetic order in a ferromagnetic B-site ordered double-perovskite Lu2NiMnO6. Phys. Rev. B 2020, 102, 094412. [Google Scholar] [CrossRef]
- Manna, K.; Bera, A.K.; Jain, M.; Elizabeth, S.; Yusuf, S.M.; Anil Kumar, P.S. Structural-modulation-driven spin canting and reentrant glassy magnetic phase in ferromagnetic Lu2MnNiO6. Phys. Rev. B 2015, 91, 224420. [Google Scholar] [CrossRef]
- Dieguez, O.; Iniguez, J. Multiferroic Bi2NiMnO6 thin films: A computational prediction. Phys. Rev. B 2017, 95, 085129. [Google Scholar] [CrossRef]
- Weihe, H.; Gudel, H.U. Quantitative interpretation of the Goodenough-Kanamori rules: A critical analysis. Inorg. Chem. 1997, 36, 3632–3639. [Google Scholar] [CrossRef]
- Belik, A.A.; Liu, R.; Tanaka, M.; Yamaura, K. B-site-ordered and disordered structures in A-site-ordered quadruple perovskites RMn3Ni2Mn2O12 with R = Nd, Sm, Gd, and Dy. Molecules 2024, 29, 5488. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Dai, J.H.; Chai, Y.S.; Zhang, H.M.; Dong, S.; Cao, H.B.; Calder, S.; Yin, Y.Y.; Wang, X.; Shen, X.D.; et al. Realization of Large Electric Polarization and Strong Magnetoelectric Coupling in BiMn3Cr4O12. Adv. Mater. 2017, 29, 1703435. [Google Scholar] [CrossRef]
- Maia, A.; Kadlec, C.; Savinov, M.; Vilarinho, R.; Moreira, J.A.; Bovtun, V.; Kempa, M.; Míšek, M.; Kaštil, J.; Prokhorov, A.; et al. Can the Ferroelectric Soft Mode Trigger an Antiferromagnetic Phase Transition? J. Eur. Ceram. Soc. 2023, 43, 2479–2487. [Google Scholar] [CrossRef]
- Etter, M.; Isobe, M.; Sakurai, H.; Yaresko, A.; Dinnebier, R.E.; Takagi, H. Charge disproportionation of mixed-valent Cr triggered by Bi lone-pair effect in the A-site-ordered perovskite BiCu3Cr4O12. Phys. Rev. B 2018, 97, 195111. [Google Scholar] [CrossRef]
- Khalyavin, D.D.; Johnson, R.D.; Orlandi, F.; Radaelli, P.G.; Manuel, P.; Belik, A.A. Emergent helical texture of electric dipoles. Science 2020, 369, 680–684. [Google Scholar] [CrossRef]
- Belik, A.A.; Matsushita, Y.; Tanaka, M.; Johnson, R.D.; Khalyavin, D.D. A plethora of structural transitions, distortions and modulations in Cu-doped BiMn7O12 quadruple perovskites. J. Mater. Chem. C 2021, 9, 10232–10242. [Google Scholar] [CrossRef]
- Fu, W.T.; Ijdo, D.J.W. “Unusual” phase transitions in CeAlO3. J. Solid State Chem. 2006, 179, 2732–2738. [Google Scholar] [CrossRef]
- Vasylechko, L.; Senyshyna, A.; Trots, D.; Niewa, R.; Schnelle, W.; Knapp, M. CeAlO3 and Ce1−xRxAlO3 (R = La, Nd) solid solutions: Crystal structure, thermal expansion and phase transitions. J. Solid State Chem. 2007, 180, 1277–1290. [Google Scholar] [CrossRef]
- Errandonea, D.; Santamaria-Perez, D.; Martinez-Garcia, D.; Gomis, O.; Shukla, R.; Achary, S.N.; Tyagi, A.K.; Popescu, C. Pressure impact on the stability and distortion of the crystal structure of CeScO3. Inorg. Chem. 2017, 56, 8363–8371. [Google Scholar] [CrossRef]
- Cao, Y.M.; Cao, S.X.; Ren, W.; Feng, Z.J.; Yuan, S.J.; Kang, B.J.; Lu, B.; Zhang, J.C. Magnetization switching of rare earth orthochromite CeCrO3. Appl. Phys. Lett. 2014, 104, 232405. [Google Scholar] [CrossRef]
- Yuan, S.J.; Cao, Y.M.; Li, L.; Qi, T.F.; Cao, S.X.; Zhang, J.C.; DeLong, L.E.; Cao, G. First-order spin reorientation transition and specific-heat anomaly in CeFeO3. J. Appl. Phys. 2013, 114, 113909. [Google Scholar] [CrossRef]
- Yamada, I.; Etani, H.; Murakami, M.; Hayashi, N.; Kawakami, T.; Mizumaki, M.; Ueda, S.; Abe, H.; Liss, K.D.; Studer, A.J.; et al. Charge-order melting in charge-disproportionated perovskite CeCu3Fe4O12. Inorg. Chem. 2014, 53, 11794–11801. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Benítez, J.; Martínez-Lope, M.J.; Alonso, J.A. Preparation at moderate pressures, crystal and magnetic structure and magnetotransport of the ferrimagnetic CeCu3Mn4O12 perovskite. J. Appl. Phys. 2010, 107, 103904. [Google Scholar] [CrossRef]
- Kadyrova, N.I.; Zainulin, Y.G.; Tyutyunnik, A.P.; Kellerman, D.G.; Mel’nikova, N.V. Preparation specifics and properties of AMn3V4O12 (A = Ca, Ce, and Sm) high-pressure phases. Russ. J. Inorg. Chem. 2017, 62, 103–110. [Google Scholar] [CrossRef]
- Belik, A.A.; Katsuya, Y.; Tanaka, M.; Yamaura, K. Crystal structure and magnetic properties of A-site-ordered quadruple perovskite CeCu3Cr4O12. J. Alloys Compd. 2019, 793, 42–48. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Roulhac, P.L.; Palenik, G.J. Bond valence sums in coordination chemistry. The calculation of the oxidation state of cerium in complexes containing cerium bonded only to oxygen. Inorg. Chem. 2003, 42, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Pernet, M.; Joubert, J.C.; Ferrand, B. Etude par diffraction neutronique de l’ilmenite ferrimagnetique NiMnO3. Solid State Commun. 1975, 16, 503–508. [Google Scholar] [CrossRef]
- Kittel, C.; McEuen, P. Introduction to Solid State Physics; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Kawaguchi, S.; Takemoto, M.; Osaka, K.; Nishibori, E.; Moriyoshi, C.; Kubota, Y.; Kuroiwa, Y.; Sugimoto, K. High-throughput powder diffraction measurement system consisting of multiple MYTHEN detectors at beamline BL02B2 of SPring-8. Rev. Sci. Instrum. 2017, 88, 085111. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Takemoto, M.; Tanaka, H.; Hiraide, S.; Sugimoto, K.; Kubota, Y. Fast continuous measurement of synchrotron powder diffraction synchronized with controlling gas and vapour pressures at beamline BL02B2 of SPring-8. J. Synchrotron Rad. 2020, 27, 616–624. [Google Scholar] [CrossRef]
- Izumi, F.; Ikeda, T. A Rietveld-analysis programm RIETAN-98 and its applications to zeolites. Mater. Sci. Forum 2000, 321–324, 198–205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).