Unveiling the Nutraceutical and Nutricosmetic Potential of Syzygium nervosum Flower Buds: A Focus on Phytochemicals and In Vitro Bioactivities
Abstract
:1. Introduction
2. Results and Discussion
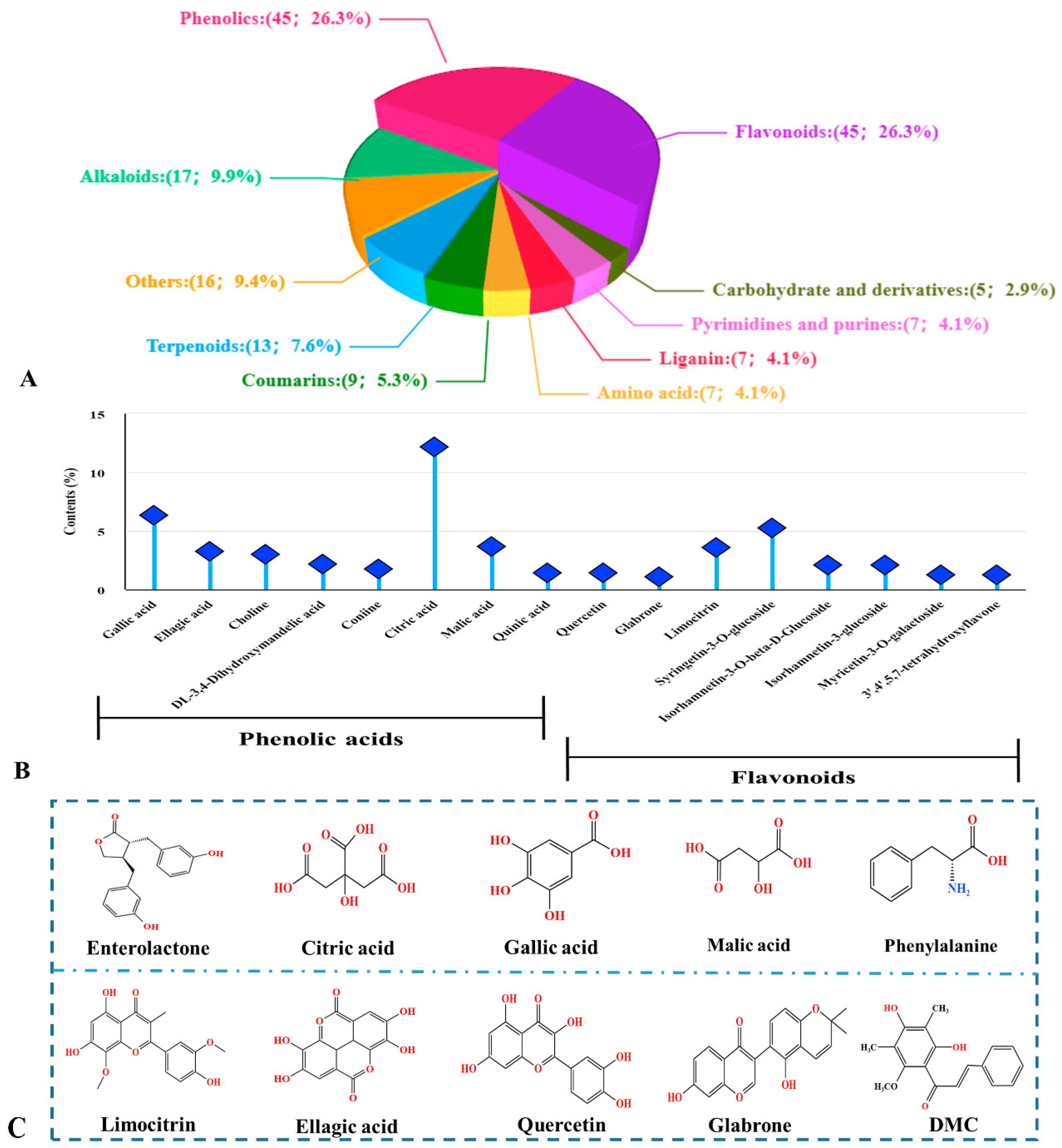
2.1. Phytochemical Analysis of S. nervosum Flower Buds
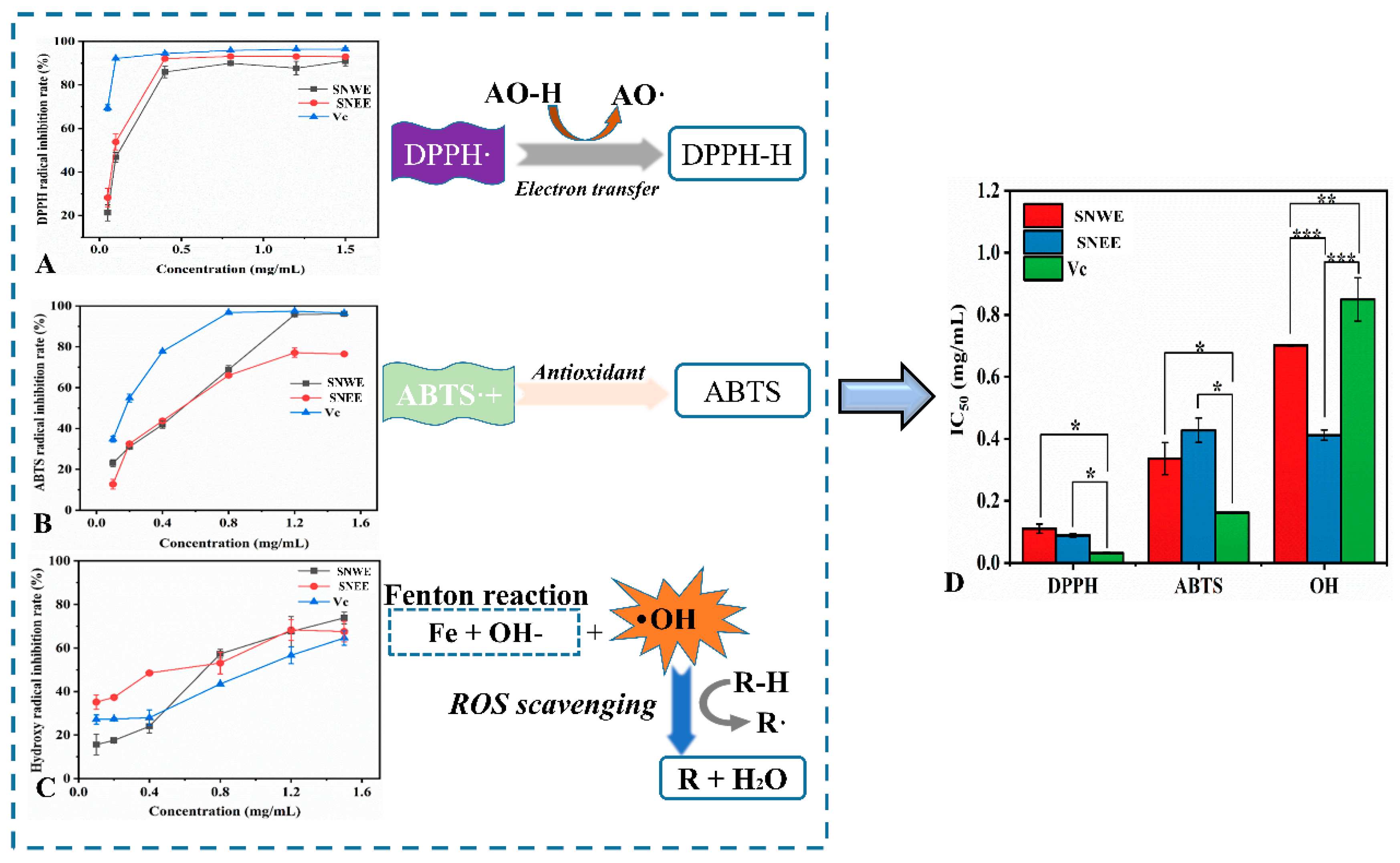
2.2. In Vitro Antioxidant Activity of the Flower Bud Extracts
2.3. Enzyme Inhibitory Activity of the Flower Bud Extracts
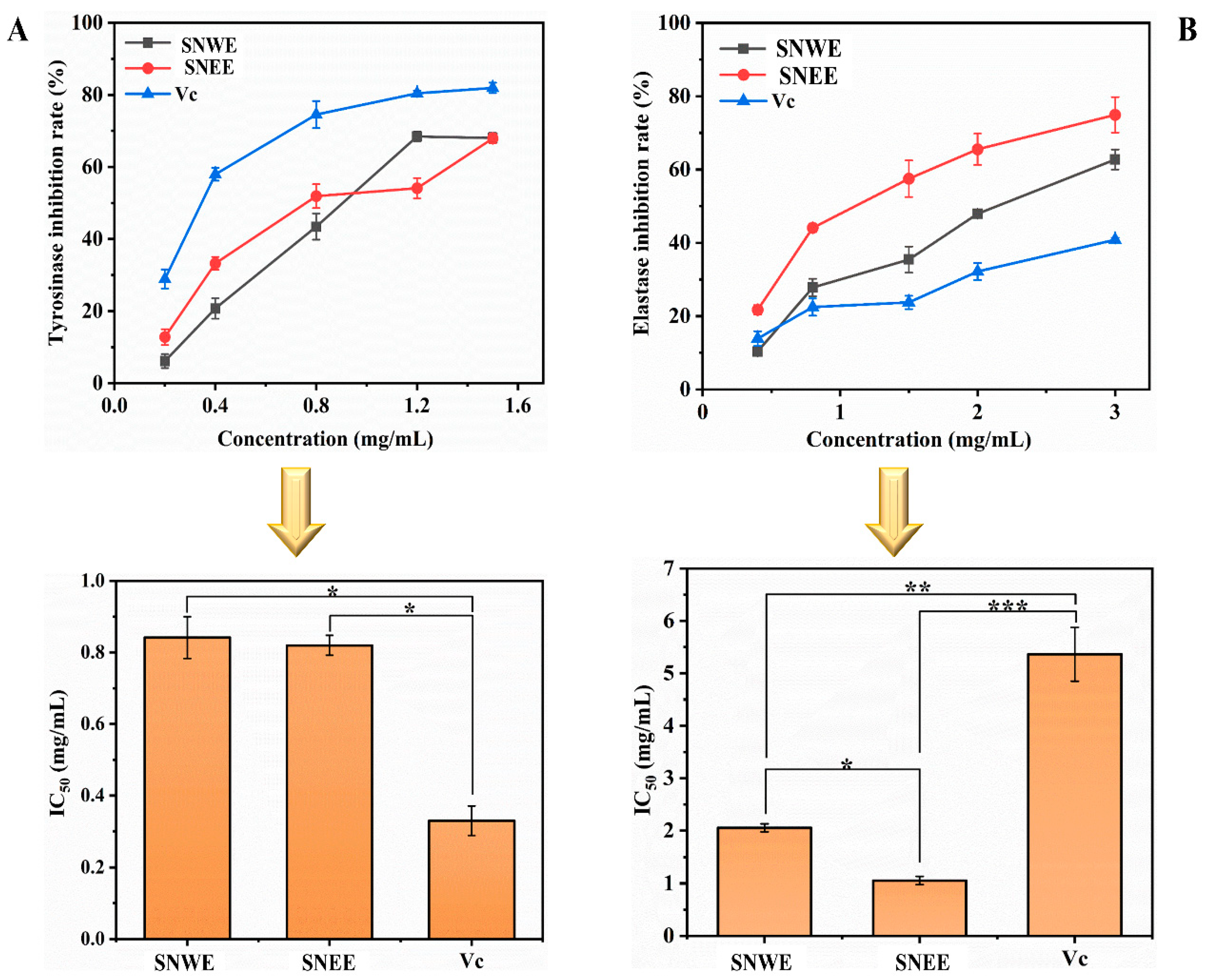
2.3.1. Tyrosinase and Elastase Inhibitory Assays
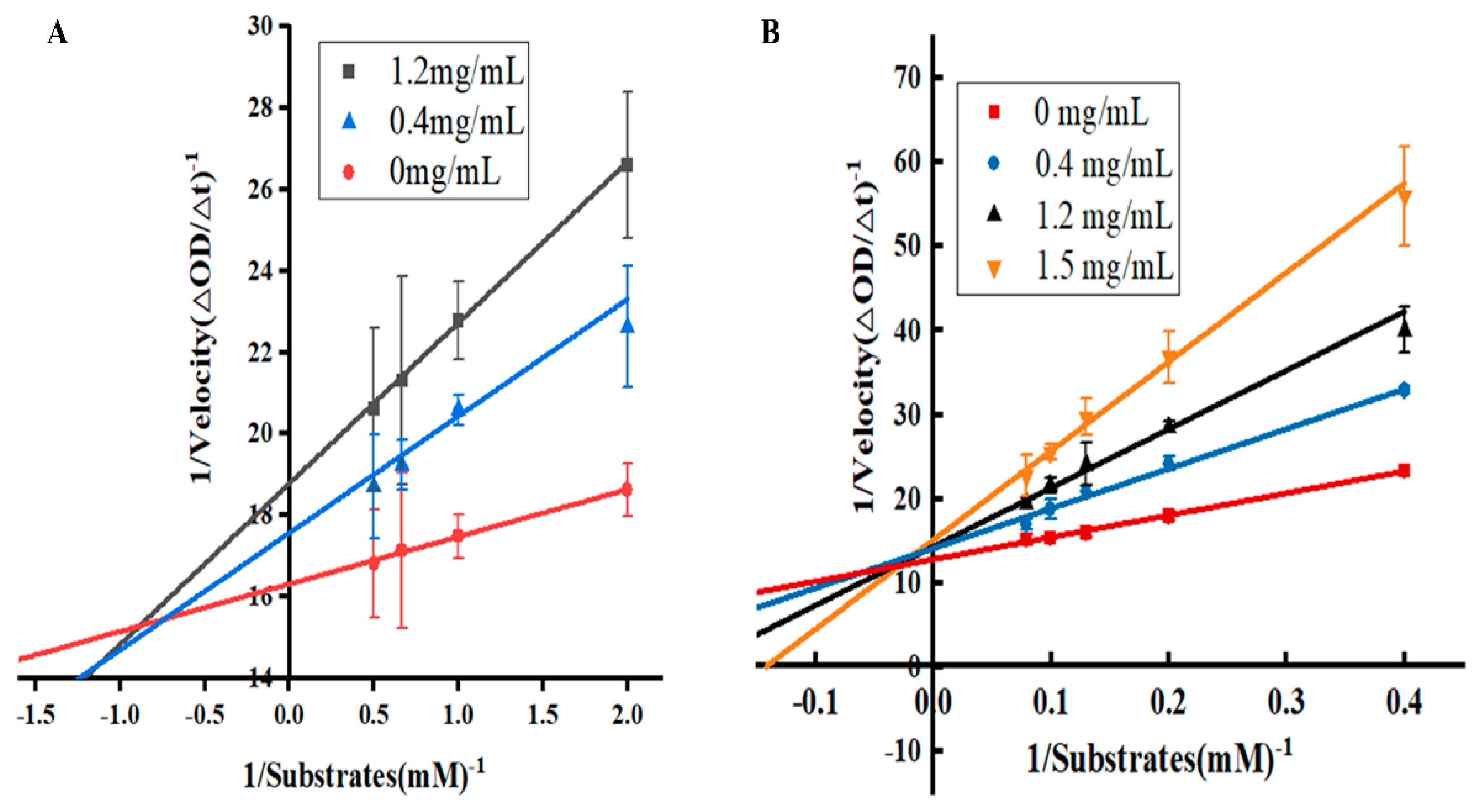
2.3.2. Enzyme Inhibition Kinetics Analysis
2.4. The Correlation Between Phytochemical Composition and Bioactivity
2.5. Molecular Docking Analysis
3. Future Perspectives
4. Materials and Methods
4.1. Plant Materials and Reagents
4.2. Preparation of the Flower Bud Extracts and Phytochemical Measurements
4.3. LC-QTOF-MS Analysis
4.4. Antioxidant Capacity Analysis of the Flower Bud Extracts
4.4.1. DPPH Radical Scavenging Capacity
4.4.2. Hydroxyl Radical Scavenging Capacity
4.4.3. ABTS Radical Scavenging Capacity
4.5. Tyrosinase and Elastase Inhibitory Activity of the S. nervosum Flower Bud Extracts
4.6. Enzyme Inhibition Kinetics of the Flower Bud Extracts
4.7. Molecular Docking
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products. Crit. Rev. Food Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, B.; Xu, B. An update on the health benefits promoted by edible flowers and involved mechanisms. Food Chem. 2021, 340, 127940. [Google Scholar] [CrossRef]
- Cai, R.; Tong, C.; Wang, H.; Yu, C.; Sun, M.; He, Z.; Feng, T.; Yao, L. Volatile profile of Astragalus sinicus L. flowers by GC×GC-TOF-MS and evaluation of nutraceutical and nutricosmetic potential of the flower extracts. Food Biosci. 2024, 61, 104988. [Google Scholar] [CrossRef]
- Nguyen, T.H. Assessing radical scavenging activity, potential antidiabetic, antigout and in vitro anti-inflammatory properties of Syzygium nervosum A. Cunn. ex DC. grown in Vietnam. J. Agric. Food Res. 2023, 12, 100614. [Google Scholar]
- Uddin, A.N.; Hossain, F.; Reza, A.A.; Nasrin, M.S.; Alam, A.K. Traditional uses, pharmacological activities, and phytochemical constituents of the genus Syzygium: A review. Food Sci. Nutr. 2022, 10, 1789–1819. [Google Scholar] [CrossRef]
- Mai, V.H.; Ponce-Zea, J.E.; Doan, T.P.; Vu, Q.H.; Ryu, B.; Lee, C.H.; Oh, W.K. Chalcone-monoterpene derivatives from the buds of Cleistocalyx operculatus and their potential as protein tyrosine phosphatase 1B inhibitors. J. Nat. Prod. 2024, 87, 1903–1913. [Google Scholar] [CrossRef]
- Wang, H.; Rao, P.; Qiu, Y.; Xiang, L. Interaction mechanism between hydroxychloroquine sulfate and collagen: Insights from multi-spectroscopy, molecular docking, and molecular dynamic simulation methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 303, 123155. [Google Scholar] [CrossRef]
- Pham, T.L.; Doan, V.D.; Le Dang, Q.; Nguyen, T.A.; Nguyen, T.L.H.; Tran, T.D.T.; Nguyen, T.P.; Vo, T.K.; Nguyen, T.H.; Tran, D.L. Stable biogenic silver nanoparticles from Syzygium nervosum bud extract for enhanced catalytic, antibacterial and antifungal properties. RSC Adv. 2023, 13, 20994–21007. [Google Scholar] [CrossRef]
- Dung, N.T.; Kim, J.M.; Kang, S.C. Chemical composition, antimicrobial and antioxidant activities of the essential oil and the ethanol extract of Cleistocalyx operculatus (Roxb.) Merr and Perry buds. Food Chem. Toxicol. 2008, 46, 3632–3639. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.-L.; Kim, O.; Tran, H.N.K.; Tran, M.H.; Min, B.-S.; Hwangbo, C.; Lee, J.-H. Protective effects of extract of Cleistocalyx operculatus flower buds and its isolated major constituent against LPS-induced endotoxic shock by activating the Nrf2/HO-1 pathway. Food Chem. Toxicol. 2019, 129, 125–137. [Google Scholar] [CrossRef]
- Tran, P.T.; Ngo, T.Q.-M.; Lee, S.; Kim, O.; Tran, H.N.K.; Hwangbo, C.; Min, B.S.; Lee, J.-H. Identification of anti-osteoclastogenic compounds from Cleistocalyx operculatus flower buds and their effects on RANKL-induced osteoclastogenesis. J. Funct. Foods 2019, 60, 103388. [Google Scholar] [CrossRef]
- Pham, G.N.; Nguyen, T.T.T.; Nguyen-Ngoc, H. Ethnopharmacology, phytochemistry, and pharmacology of Syzygium nervosum. Evid.-Based Complement. Altern. Med. 2020, 2020, 8263670. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Marcílio Cândido, T.; Bueno Ariede, M.; Vieira Lima, F.; de Souza Guedes, L.; Robles Velasco, M.V.; Rolim Baby, A.; Rosado, C. Dietary supplements and the skin: Focus on photoprotection and antioxidant activity—A review. Nutrients 2022, 14, 1248. [Google Scholar] [CrossRef]
- Ngo, T.C.; Dao, D.Q.; Nguyen, M.T.; Nam, P.C. A DFT analysis on the radical scavenging activity of oxygenated terpenoids present in the extract of the buds of Cleistocalyx operculatus. RSC Adv. 2017, 7, 39686–39698. [Google Scholar] [CrossRef]
- Thanh, D.T.; Oanh, V.K.; Nguyen, H.C.; Ngan, L.T.M.; Hieu, T.T. Phytochemical composition, antioxidant, antibacterial, and enzyme inhibitory activities of organic extracts from flower buds of Cleistocalyx operculatus (Roxb.) Merr. et Perry. BioTechnologia 2024, 105, 137–147. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Blanco-Llamero, C.; Macário, H.F.; Guedes, B.N.; Fathi, F.; Oliveira, M.B.P.; Souto, E.B. Bioactives in Nutricosmetics: A Focus on Caffeine from Tea to Coffee. Cosmetics 2024, 11, 149. [Google Scholar] [CrossRef]
- Mai, T.T.; Fumie, N.; Van Chuyen, N. Antioxidant activities and hypolipidemic effects of an aqueous extract from flower buds of Cleistocalyx operculatus (Roxb.) Merr. and Perry. J. Food Biochem. 2009, 33, 790–807. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Shi, M.-J.; Wei, X.-Y.; Zhou, T. Enzymatic degradation of polysaccharide from Enteromorpha prolifera: An efficient way to enhance its antioxidant and tyrosinase inhibitory activities. J. Food Meas. Charact. 2021, 15, 4623–4634. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, G.; Zeng, Q.H.; Li, Y.; Liu, H.; Wang, J.J.; Zhao, Y. A systematic review of synthetic tyrosinase inhibitors and their structure-activity relationship. Crit. Rev. Food Sci. Nutr. 2022, 62, 4053–4094. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liang, Y.; Sun, M.; Song, S.; Wang, H.; Dong, Z.; Feng, T.; Yue, H. Characteristic volatile fingerprints of three edible marine green algae (Ulva spp.) in China by HS-GC-IMS and evaluation of the antioxidant bioactivities. Food Res. Int. 2022, 162, 112109. [Google Scholar] [CrossRef]
- Mechqoq, H.; Hourfane, S.; El Yaagoubi, M.; El Hamdaoui, A.; da Silva Almeida, J.R.G.; Rocha, J.M.; El Aouad, N. Molecular Docking, Tyrosinase, Collagenase, and Elastase Inhibition Activities of Argan By-Products. Cosmetics 2022, 9, 24. [Google Scholar] [CrossRef]
- Xue, D.; Jiang, S.; He, H.; Lametsch, R.; Zhang, M.; Li, C. Hemoglobin Hydrolyzate Promotes Iron Absorption in the Small Intestine through Iron Binding Peptides. J. Agric. Food Chem. 2024, 72, 15237–15247. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.G.; Zhong, D.L.; Duan, Z.Z.; Peng, Z.J.; Tang, W.; Song, Q.; Huang, X.; Hu, L.; Wang, Y.; et al. Biomimetic synthesis of an antiviral cinnamoylphloroglucinol collection from Cleistocalyx operculatus: A synthetic strategy based on biogenetic building blocks. Angew. Chem. Int. Ed. 2023, 62, e202312568. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Duan, Z. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food Chem. 2019, 297, 124910. [Google Scholar] [CrossRef]

| TPC | TFC | DPPH | ABTS | OH | TIAa | EIAb | |
|---|---|---|---|---|---|---|---|
| TPC | 1.00 | 0.92 ** | 0.89 * | 0.44 | 1.00 *** | 0.40 | 1.00 *** |
| TFC | 1.00 | 0.87 * | 0.39 | 1.00 *** | 0.39 | 0.99 *** | |
| DPPH | 1.00 | 0.70 | 0.90 * | 0.58 | 0.91 * | ||
| ABTS | 1.00 | 0.45 | 0.15 | 0.47 | |||
| OH | 1.00 | 0.38 | 1.00 *** | ||||
| TIA | 1.00 | 0.41 | |||||
| EIA | 1.00 |
| No. | Compounds | Binding Energy (kcal/mol) | |
|---|---|---|---|
| Tyrosinase | Elastase | ||
| 1 | Citric acid | −5.3 | −5.0 |
| 2 | Ellagic acid | −7.2 | −7.5 |
| 3 | Enterolactone | −7.6 | −6.6 |
| 4 | Gallic acid | −6.0 | −5.8 |
| 5 | Glabrone | −6.8 | −7.5 |
| 6 | Limocitrin | −6.7 | −7.0 |
| 7 | Malic acid | −4.9 | −4.5 |
| 8 | Phenylalanine | −5.8 | −4.9 |
| 9 | Quercetin | −7.9 | −7.5 |
| 10 | DMC | −6.4 | −6.9 |
| Hydrogen bonding | Docking Conformations | Residues | Amino Acid |
| Tyrosinase–ellagic acid complex | 85 (A chain) | His | |
| 244 (A chain) | His | ||
| Tyrosinase–enterolactone complex | 268 (A chain) | Arg | |
| Tyrosinase–quercetin complex | 85 (A chain) | His | |
| 263 (A chain) | His | ||
| 282 (A chain) | Ser | ||
| Elastase–ellagic complex | 57 (P chain) | His | |
| 191 (P chain) | Cys | ||
| 192 (P chain) | Asn | ||
| 195 (P chain) | Ser | ||
| Elastase–glabrone complex | 96 (P chain) | Ser | |
| 195 (P chain) | Ser | ||
| 216 (P chain) | Gly | ||
| Elastase–quercetin complex | 190 (P chain) | Ser | |
| 192 (P chain) | Asn | ||
| 195 (P chain) | Ser | ||
| 214 (P chain) | Ser |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Huang, L.; Sun, T.; Cao, Z.; Feng, T.; Wang, H.; Sun, M.; Yue, H.; Yu, C.; Tong, C.; et al. Unveiling the Nutraceutical and Nutricosmetic Potential of Syzygium nervosum Flower Buds: A Focus on Phytochemicals and In Vitro Bioactivities. Molecules 2025, 30, 1762. https://doi.org/10.3390/molecules30081762
Liu Y, Huang L, Sun T, Cao Z, Feng T, Wang H, Sun M, Yue H, Yu C, Tong C, et al. Unveiling the Nutraceutical and Nutricosmetic Potential of Syzygium nervosum Flower Buds: A Focus on Phytochemicals and In Vitro Bioactivities. Molecules. 2025; 30(8):1762. https://doi.org/10.3390/molecules30081762
Chicago/Turabian StyleLiu, Yan, Limei Huang, Tingting Sun, Zhen Cao, Tao Feng, Huatian Wang, Min Sun, Heng Yue, Chuang Yu, Chuanwang Tong, and et al. 2025. "Unveiling the Nutraceutical and Nutricosmetic Potential of Syzygium nervosum Flower Buds: A Focus on Phytochemicals and In Vitro Bioactivities" Molecules 30, no. 8: 1762. https://doi.org/10.3390/molecules30081762
APA StyleLiu, Y., Huang, L., Sun, T., Cao, Z., Feng, T., Wang, H., Sun, M., Yue, H., Yu, C., Tong, C., Yao, L., & Zhang, W. (2025). Unveiling the Nutraceutical and Nutricosmetic Potential of Syzygium nervosum Flower Buds: A Focus on Phytochemicals and In Vitro Bioactivities. Molecules, 30(8), 1762. https://doi.org/10.3390/molecules30081762






