Phytochemical Characterization of Hibiscus tiliaceus L. Leaves and Evaluation of Their Antisickling, Antioxidant, and Anti-Inflammatory Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization
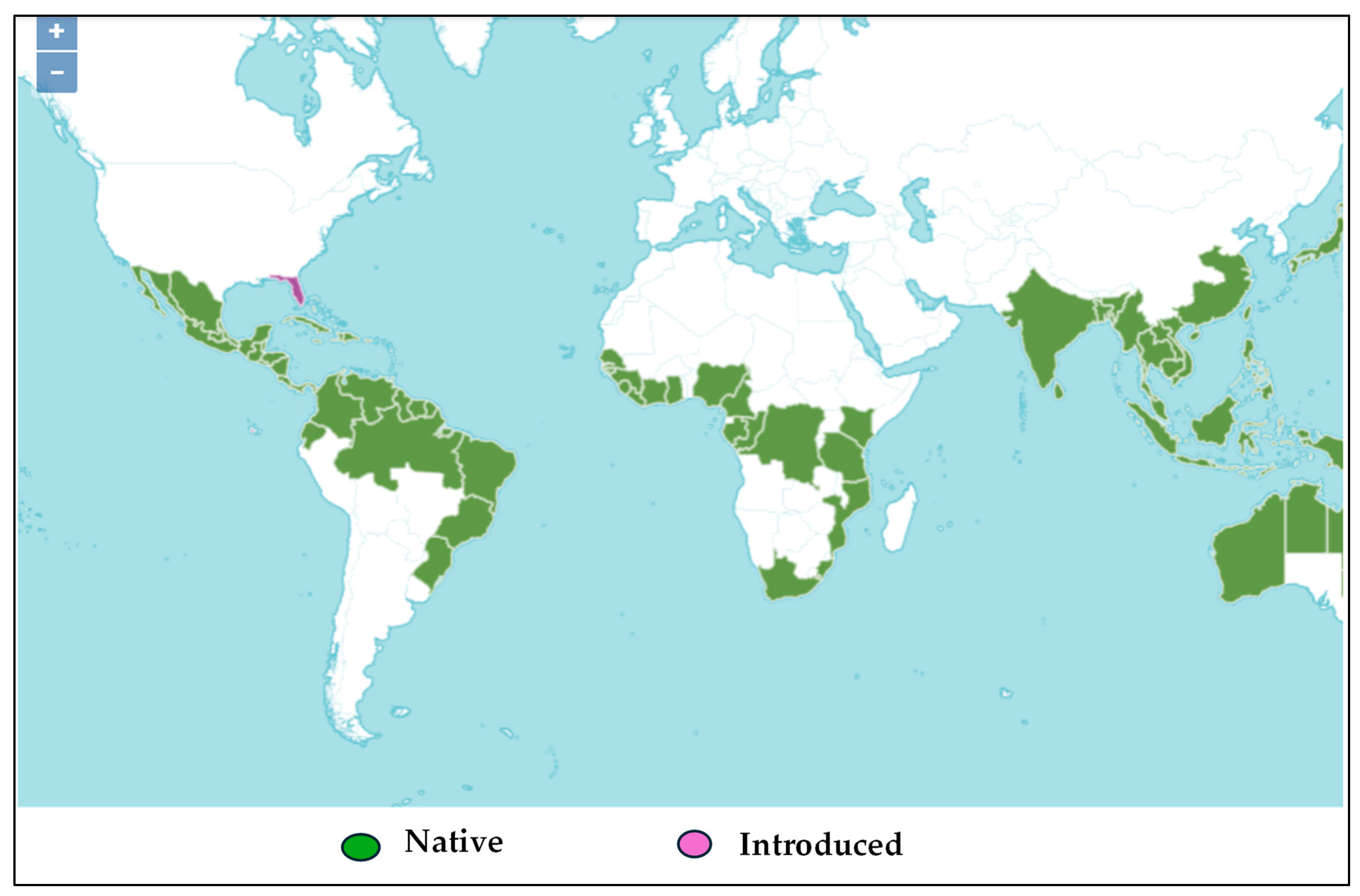
2.1.1. Samples and Chemicals Compositions
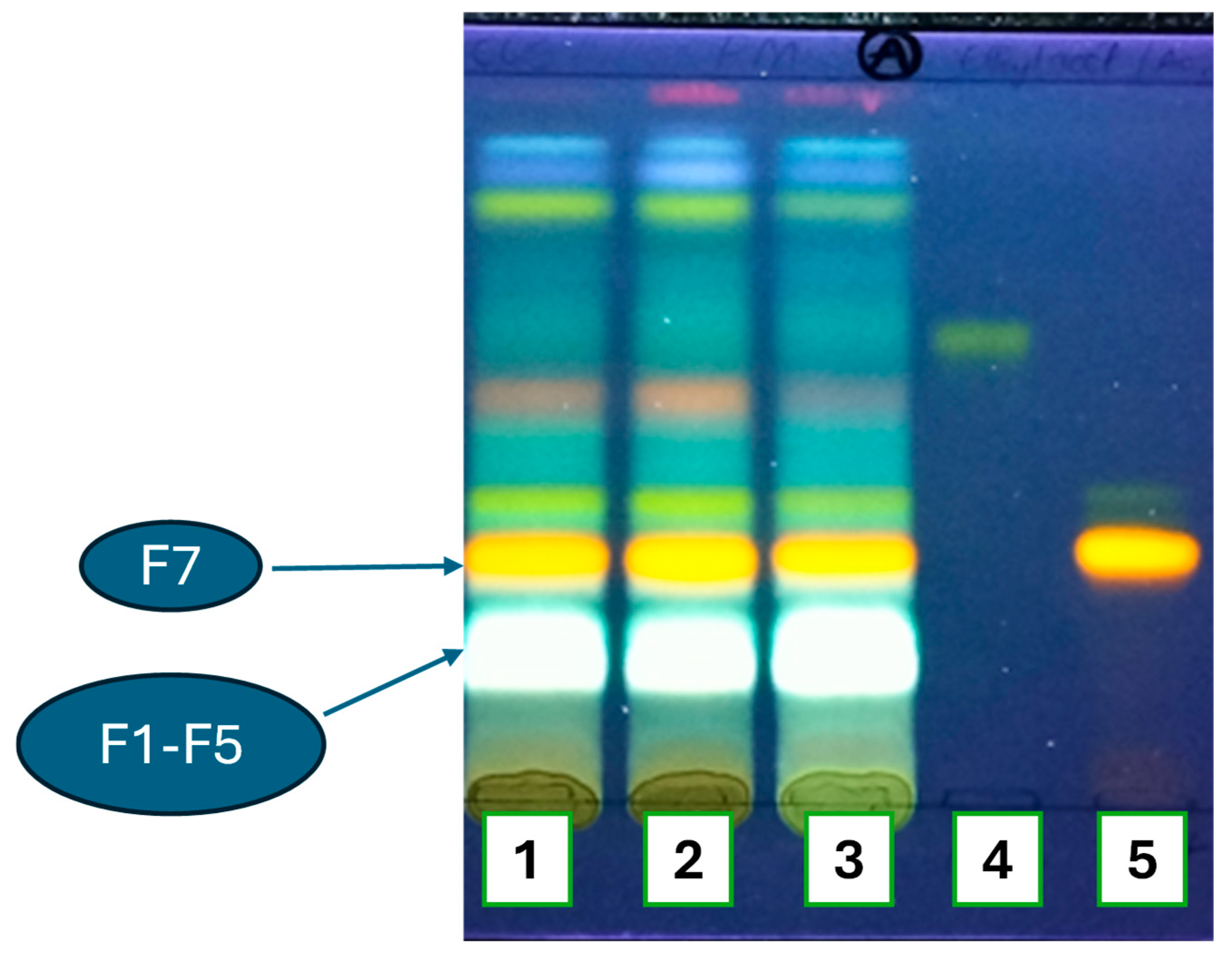
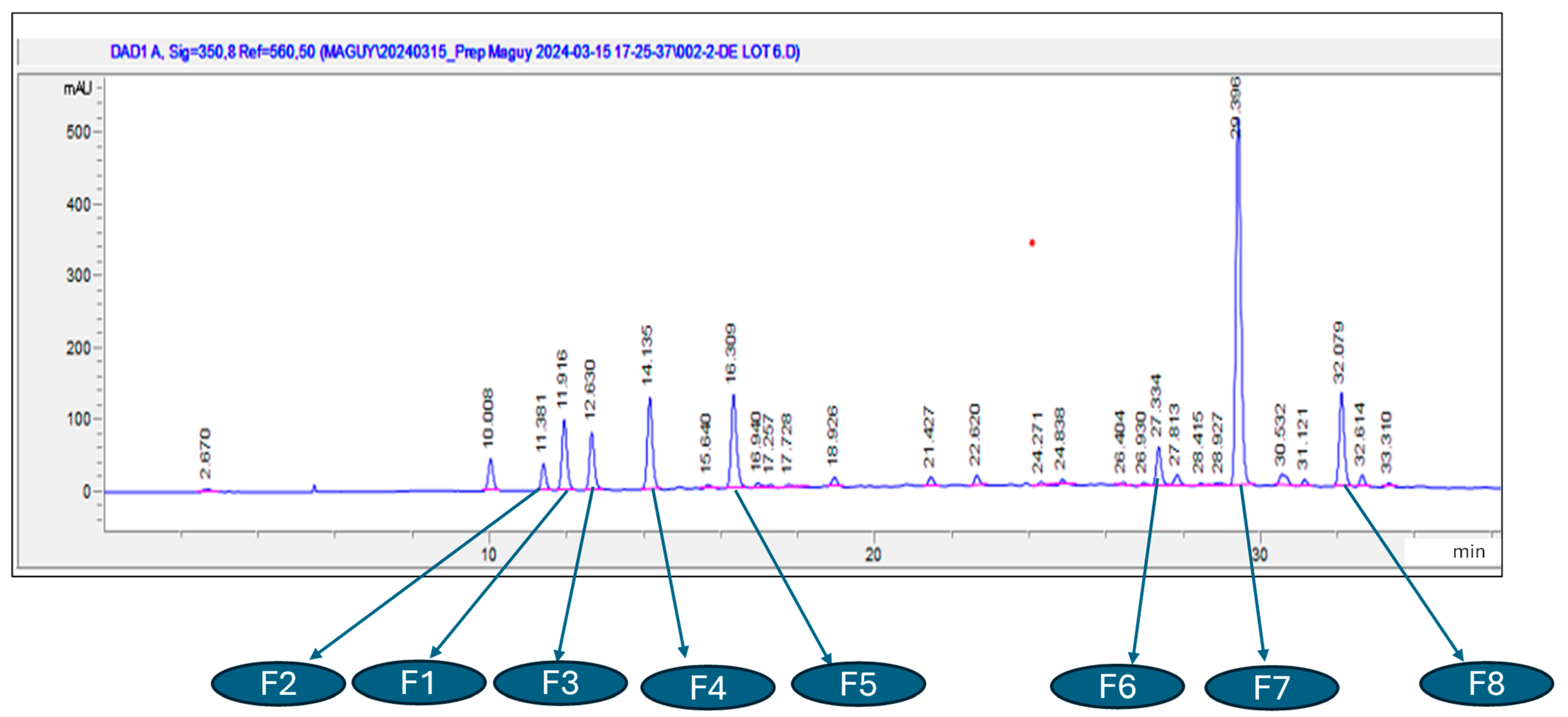
2.1.2. Chromatographic Profiles of Flavonoids and Phenol Acids
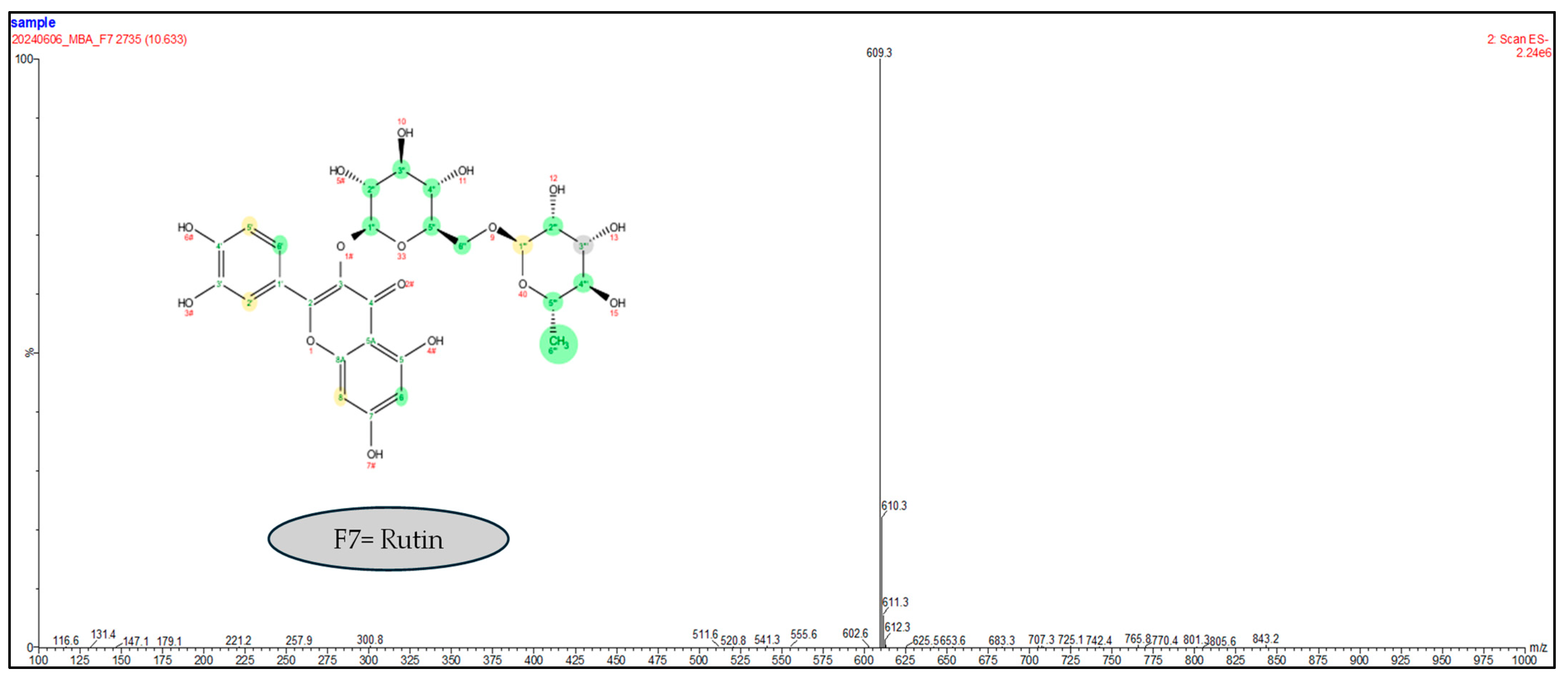
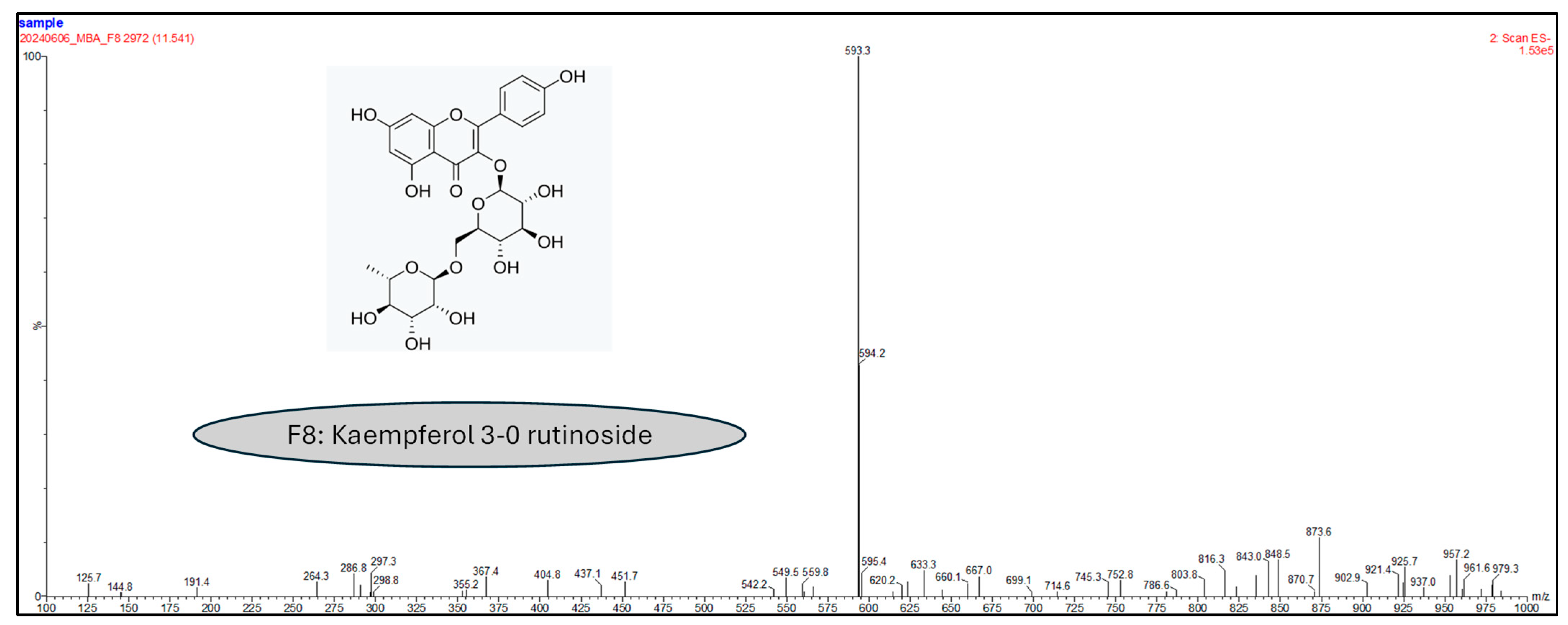
2.1.3. Liquid Chromatography Coupled to Mass Spectrometry Analyses
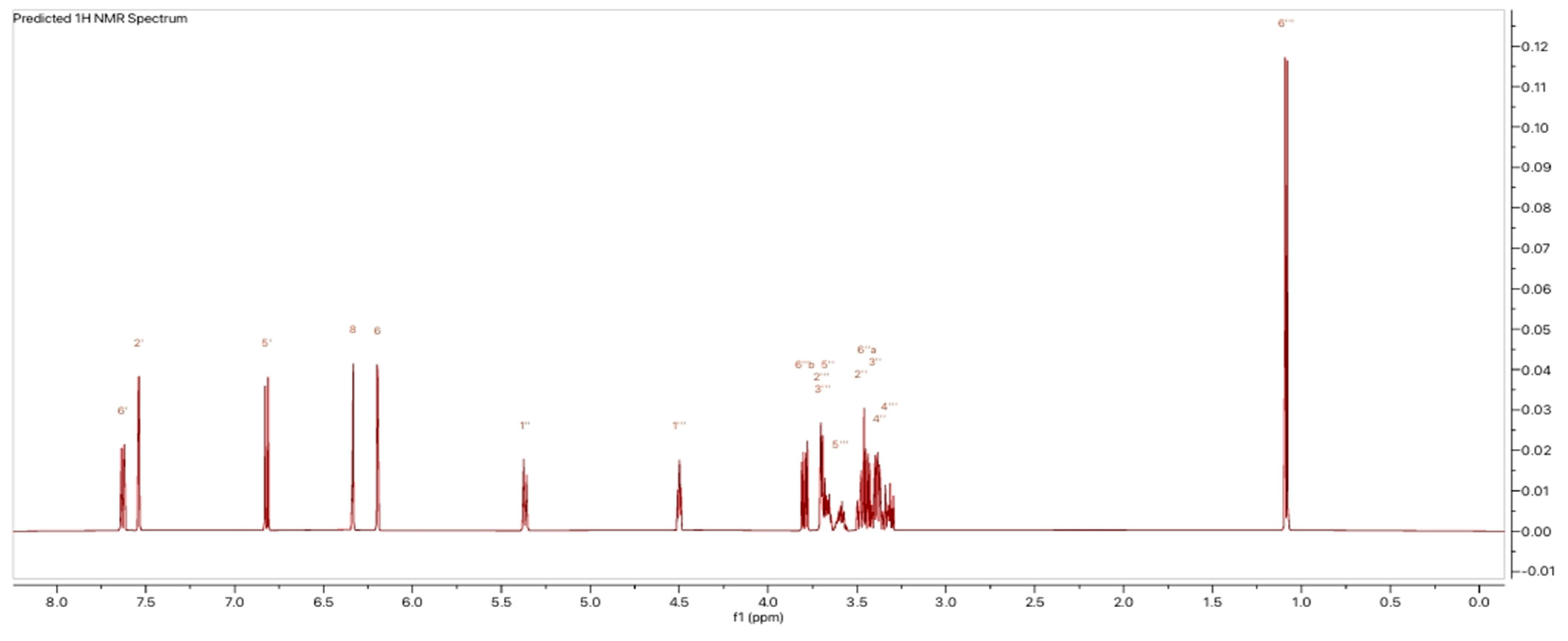
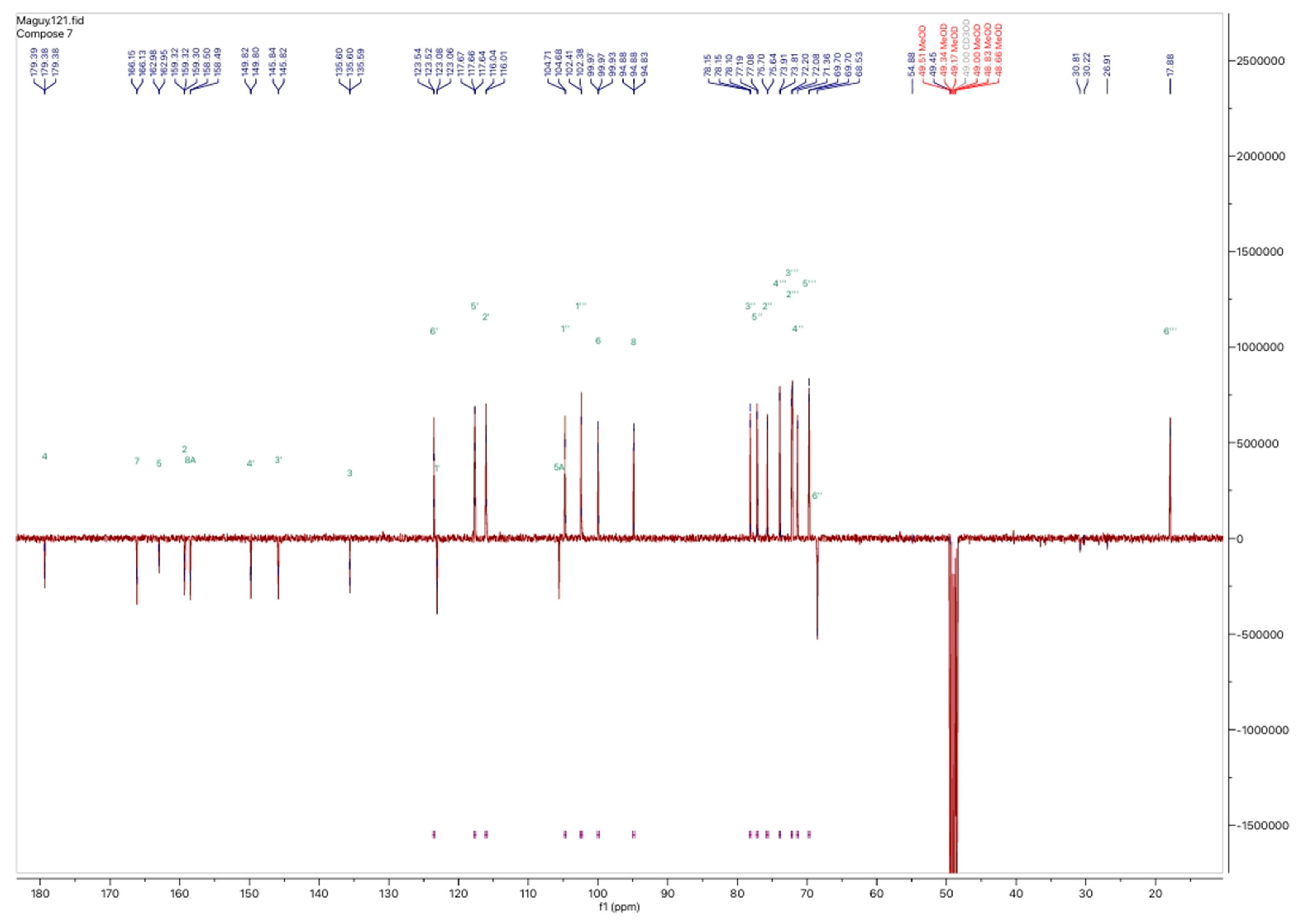
2.1.4. Nuclear Magnetic Resonance Analyses
2.2. Biological Activities Related to the SCD Clinical Consequences
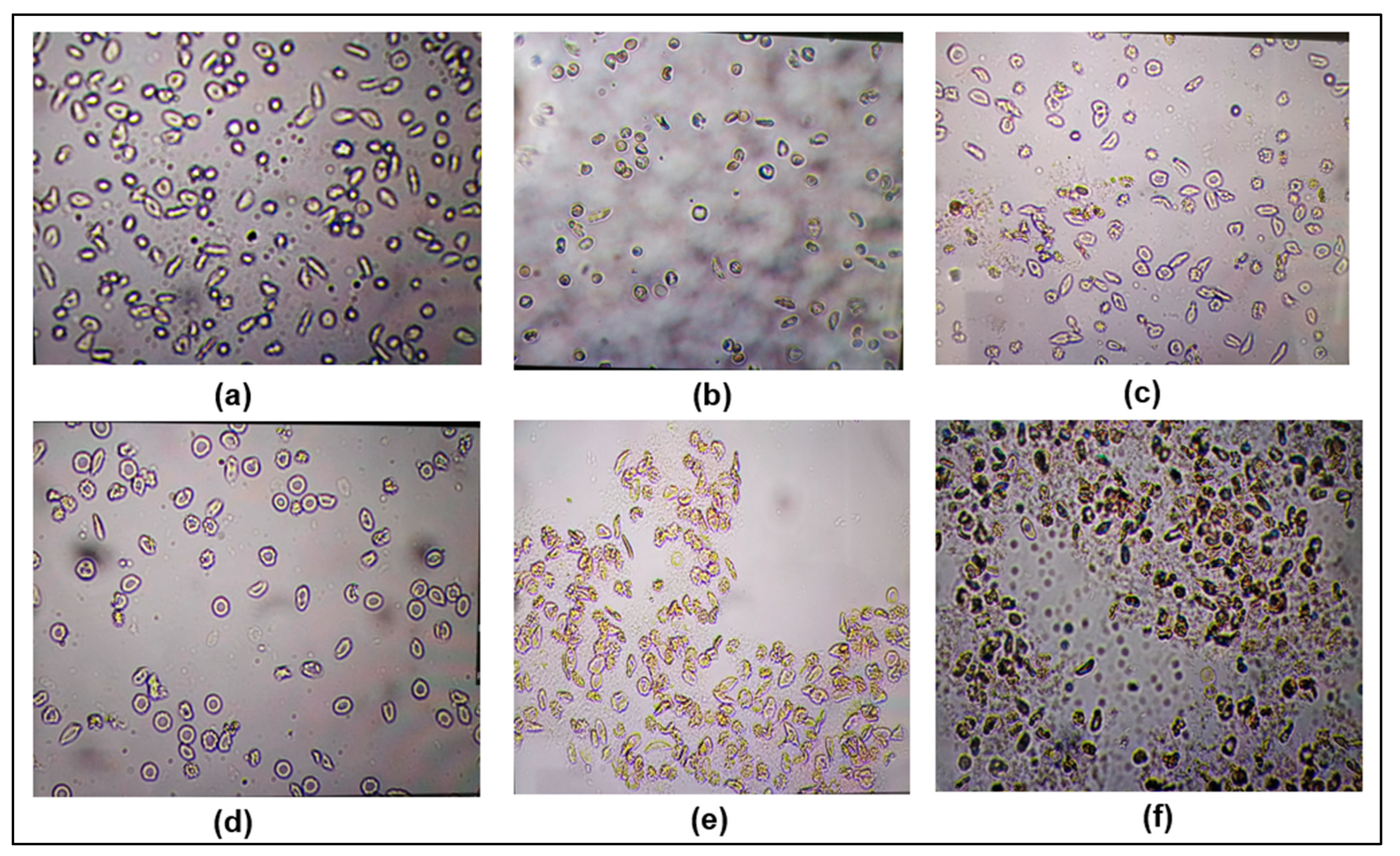
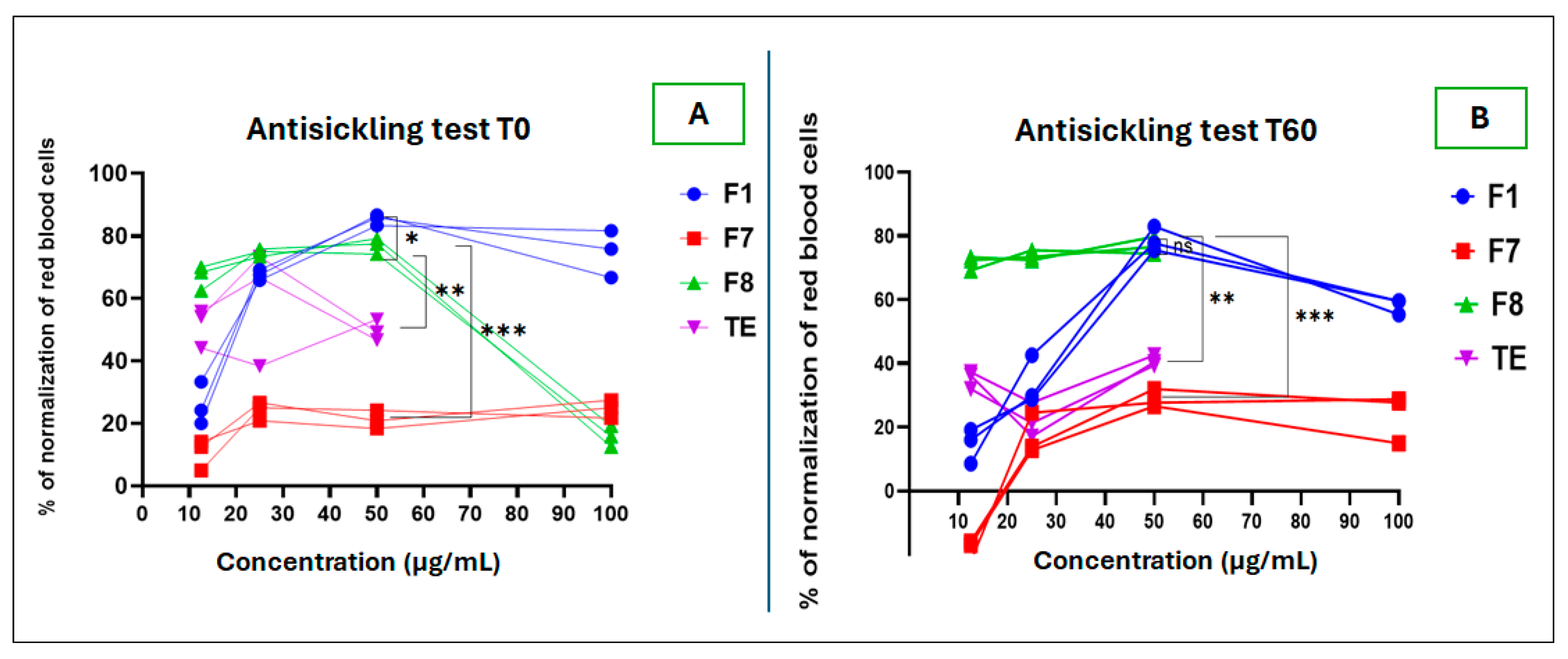
2.2.1. Antisickling Test
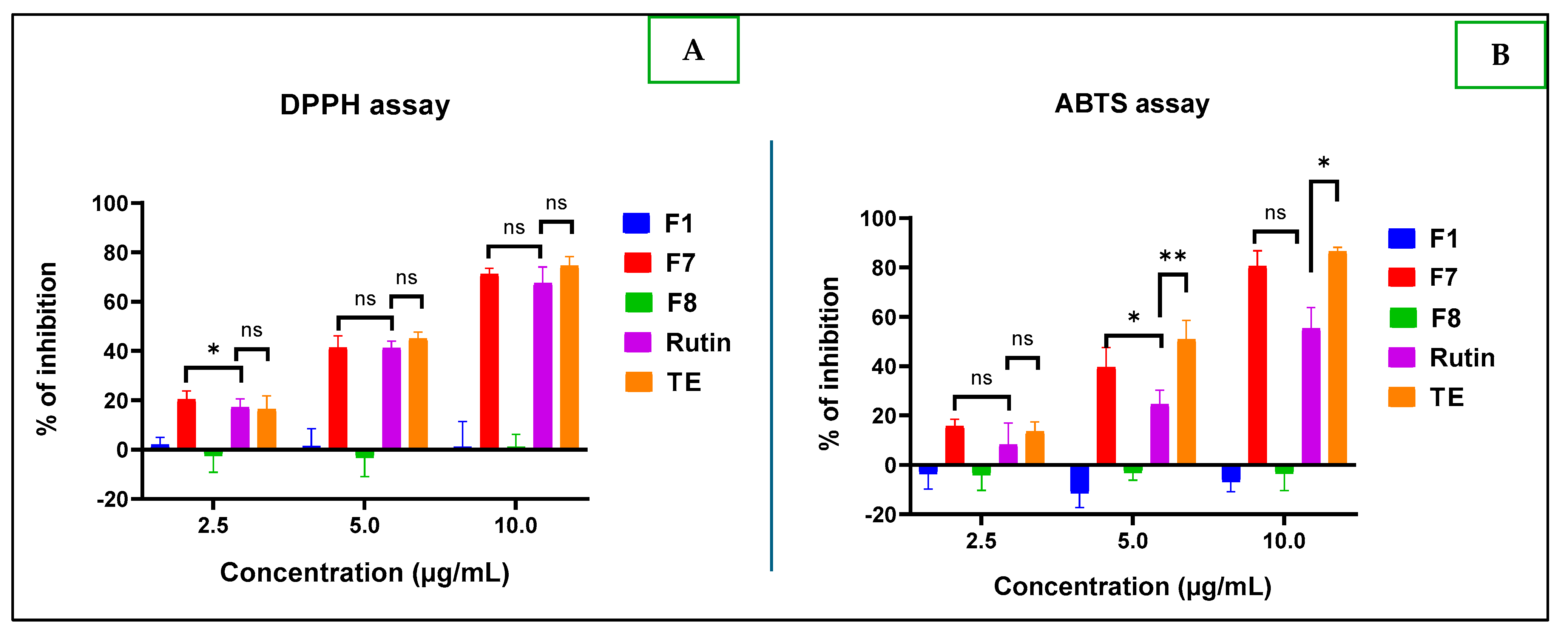
2.2.2. Antioxidant Tests
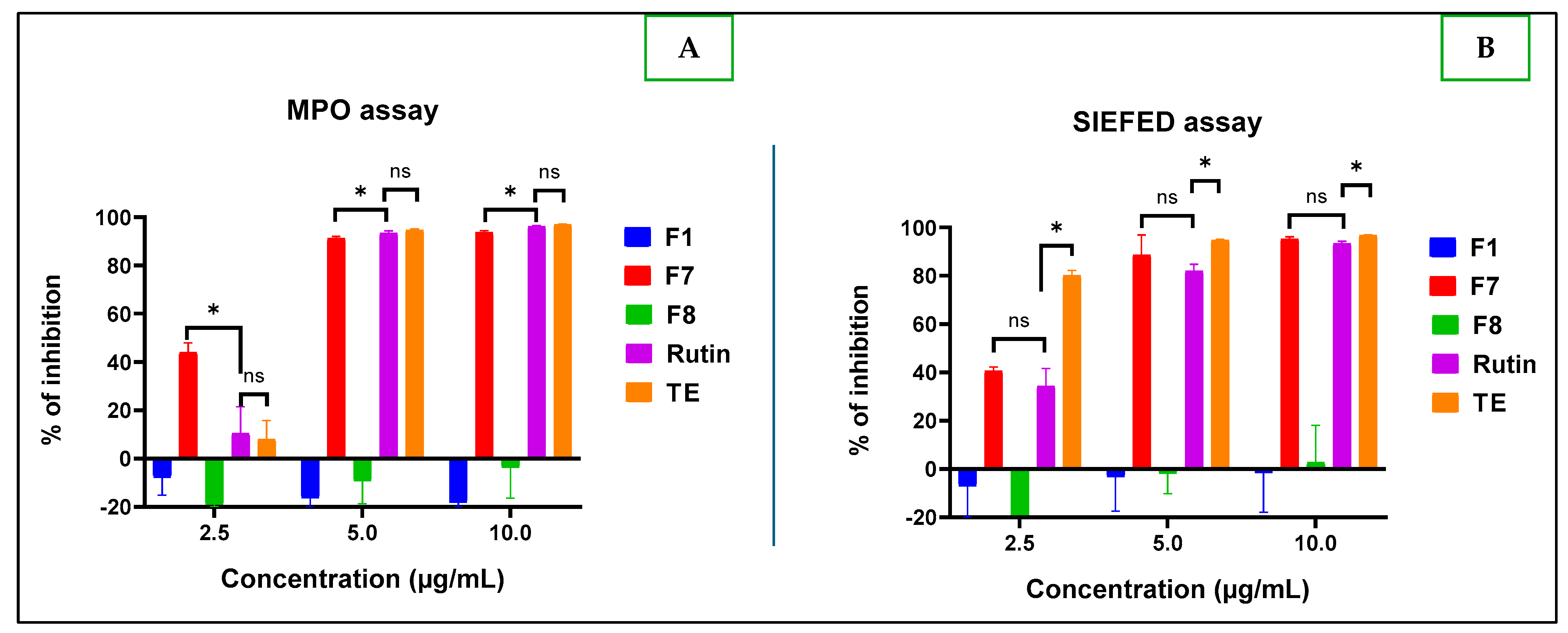
2.2.3. Anti-Inflammatory Test
3. Materials and Methods
3.1. Materials
3.1.1. Vegetable Materials
3.1.2. Chemical Materials
3.2. Methods
3.2.1. Preparation of Dry Aqueous Extracts
3.2.2. Separation by Column Chromatography
3.2.3. TLC Chromatography of Fractions
3.2.4. Analyses of Fractions Using LC-MS
3.2.5. Analyses of Fraction Using NMR
3.2.6. Biological Activities Tests
Antisickling Activity
- Treatment of sickle cell blood: Mix 500 µL of fresh blood with 500 µL of 2% sodium metabisulfite freshly prepared. Dilute the mixture obtained twice by adding 1000 µL of 0.9% NaCl.
- Preparation of the samples to be tested: Dissolve 50 mg of extract in 5 mL of methanol 18%. From the latter, make several dilutions with 0.9% NaCl to obtain four different concentrations to test: 12.5, 25, 50, and 100 µg/mL.
- By means of Eppendorf tubes, mix 100 µL of diluted treated blood with 100 µL of extract of the different concentrations.
- Place 5 µL of the mixture obtained in step 3 on a slide, cover with a coverslip, and observe under a 40-objective microscope for the morphological analysis of the erythrocytes on four different fields (n = 2). Methanol 18% was used as a negative control and zinc oxide as a positive control.
Antioxidant Activity
- ABTS Assay
- (a)
- Generation of ABTS•+ radicals: Separately prepare aqueous stock solutions of ABTS (7 mM) and sodium persulfate (2.45 mM), respectively. Mix and incubate in the dark for 12 to 16 h. Dilute the resulting solution in absolute methanol to adjust the absorbance to reach the value of 0.70 ± 0.02 at 734 nm, at 25 °C.
- (b)
- Assay of antioxidant effect: Perform the analysis in triplicate using a microplate reader (Thermo Lab System, Vantaa, Finland). For each measurement, fill the well with 2 µL of the test compound solution and complete with 198 µL of ABTS•+ solution to reach a final volume of 200 µL; incubate for 30 min. Evaluate the absorbance of different solutions at 740 nm using a microplate reader (Thermo Lab system, Vantaa, Finland). For the controls, methanol and rutin were used as a negative control and a positive control, respectively. Determine the reducing capacity according to the following formula:
- DPPH Assay
- (a)
- Generation of DPPH• radicals: Dissolve 3.2 mg of DPPH radical by stirring for 60 min in 100 mL absolute methanol to obtain a stock solution. Dilute the stock solution in methanol until an absorbance of 0.650 ± 0.020 at 517 nm is obtained. Perform the assay in triplicate on a multi-well plate.
- (b)
- Assay of antioxidant effect: Fill each well with 2 µL of the tested compound and add 198 µL of the working DPPH• solution. Incubate the mixture for 30 min in a dark place. In the presence of an antioxidant, the DPPH•radical is reduced to DPPH, resulting in a color change from purple to yellow. To quantify the effect, the decrease in absorbance at 510 nm was measured using a Multiskan Ascent 96 plate reader (Thermo Lab System, Vantaa, Finland). Methanol and rutin were used as a negative control and a positive control, respectively. Calculate the inhibition percentage of the DPPH• radical using the same equation as the ABTS assay.
Anti-Inflammatory Activities
- (a)
- Preparation of the enzyme solution: Dilute 4 µL of human myeloperoxidase in 8 mL of phosphate-buffered saline (20 mM PBS, pH 7.4), containing 5 g/L of BSA and 0.1% Tween-20.
- (b)
- Preparation of the tested samples: Prepare the sample solution from the stock, dilute at final concentrations ranging from 2.5 to 10.0 µg/mL, and incubate for 10 min with the buffered solution of MPO (5 mU/mL).
- (c)
- Preparation of resultant solution (AR/H2O2/NO2-): This solution is composed of Amplex Red (AR), hydrogen peroxide as a peroxidase substrate (10 µM), and sodium nitrite (4.5 mM), prepared separately in a phosphate buffer at pH 7.4, except H2O2, for which distilled water was used.
- (d)
- MPO activity by classical assay: After incubation, measure the peroxidase activity by adding 10 µL of the sodium nitrite solution (4.5 mM, final concentration) and 100 µL of the reactional solution containing 10 µM hydrogen peroxide and 40 µM Amplex® Red (AR) in phosphate buffer (50 mM) at pH 7.4 to each well (100 µL of the mixture) of the tested compound or vehicle (ultrapure water) mixed with MPO into a transparent multi-well plate. Monitor the oxidation of AR into the fluorescent resorufin adduct (excitation = 544 nm; emission = 590 nm) for 30 min at 37 °C with a fluorescent plate reader (Fluoroskan Ascent, Fisher Scientific, Hampton, NH, USA).
- (e)
- MPO activity by SIEFED assay: Prepare samples with MPO and various concentrations of selected extract and incubate like in the classical assay. Load a mixture of 100 µL of each solution (MPO alone or MPO + extract) into the wells of a SIEFED microtiter plate (coated with rabbit polyclonal antibodies against human MPO and incubated for 2 h at 37 °C in darkness). Wash the wells four times with washing buffer, carefully dry the wells, and immediately measure the activity of the enzyme captured by the antibodies the same as for the classical assay, by adding 10 µL sodium nitrite solution (4.5 mM) and 100 µL of a reactional solution containing 10 µM hydrogen peroxide and 40 µM Amplex® in phosphate buffer (50 mM) at pH 7.4. Monitor the oxidation of AR into the fluorescent resorufin adduct (excitation = 544 nm; emission = 590 nm) for 30 min at 37 °C with a fluorescent plate reader (Fluoroskan Ascent, Fisher Scientific, Hampton, NH, USA). As for the MPO direct assay, a control assay set as the relative value of MPO activity was performed with purified MPO in the presence of PBS instead of the samples. For both MPO assays, calculate the inhibition percentage using the following formula:
3.2.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/questions-and-answers/item/neglected-tropical-diseases (accessed on 15 November 2024).
- Hotez, P.J.; Aksoy, S.; Brindley, P.J.; Kamhawi, S. What constitutes a neglected tropical disease? PLoS Negl. Trop. Dis. 2020, 14, e0008001. [Google Scholar] [CrossRef] [PubMed]
- Ware, R.E. Is Sickle Cell Anemia a Neglected Tropical Disease? PLoS Negl. Trop. Dis. 2013, 7, e2120. [Google Scholar] [CrossRef]
- Tshilolo, L.; Gonzalez, J.P. Stigmatization of sickle cell disease across the Democratic Republic of Congo: A presentation of two cases. Int. Health Trends Perspect. 2024, 4, 181–186. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.afro.who.int/health-topics/sickle-cell-disease (accessed on 8 April 2025).
- Williams, T.N. Sickle Cell Disease in Sub-Saharan Africa. Hematol. Oncol. Clin. N. Am. 2016, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Tshilolo, L.; Aissi, L.M.; Lukusa, D.; Kinsiama, C.; Wembonyama, S.; Gulbis, B.; Vertongen, F. Neonatal screening for sickle cell anaemia in the Democratic Republic of the Congo: Experience from a pioneer project on 31 204 newborns. J. Clin. Pathol. 2009, 62, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Katawandja, A.L.; Kingwengwe, A.A.; Shindano, E.M. Knowledge and Practices of Health Providers on the Diagnosis and Biological Monitoring of Sickle Cell Disease in the City of Kindu, in the East of the Democratic Republic of Congo. OALibrary 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Kasai, E.T.; Boemer, F.; Marini, R.D.; Kadima, J.N.; Batina, S.A.; Ngbonda, N.D.; Alworong’a, J.P.O. Systematic Screening of Neonatal Sickle Cell Disease with HemoType Sickle cell Kit-Test: Case Study and Literature Review. Open J. Blood Dis. 2020, 10, 12–21. [Google Scholar] [CrossRef]
- Shongo, Y.P.M. Sickle cell disease in the Democratic Republic of Congo/DRC: Burden of a heavier price. J. Med. Public Health 2022, 1, 30–33. [Google Scholar]
- Akinyemi, O.D.O.; Oguche, S.; Kehinde, A.; Edache, S.O.; Tolulope, O.A.; Ijeoma, N.D.; Ayuba, I.Z.; Atiene, S.S. Effectiveness and Safety of Hydroxyurea in the Treatment of Sickle Cell Anaemia Children in Jos, North Central Nigeria. J. Trop. Pediatr. 2020, 66, 290–298. [Google Scholar] [CrossRef]
- Wambebe, C.O.; Bamgboye, E.A.; Bidemi, O.B.; Hadiza, K.; Jafaru, A.M.; Ekpeyong, M.; Benedict, S.A.; Njoku, S.O.; Nasipuri, N.R.; Olubayo, O.K.; et al. Efficacy of Niprisan in the prophylactic management of patients with sickle cell disease. Curr. Ther. Res. 2001, 62, 26–34. [Google Scholar] [CrossRef]
- Cordeiro, N.J.V.; Oniyangi, O. Phytomedicines (medicines derived from plants) for sickle cell disease. Cochrane Database Syst. Rev. 2004, 3, CD004448. [Google Scholar] [CrossRef]
- Tshilolo, L.; Tomlinson, G.; Williams, T.N.; Brígida, S.; Olupot-Olupot, P.; Lane, A.; Banu, A.; Susan, E.S.; Latham, T.S.; Ware, R.E. Hydroxyurea for Children with Sickle Cell Anemia in Sub-Saharan Africa. N. Engl. J. Med. 2019, 380, 121–131. [Google Scholar] [CrossRef]
- Mukinayi, B.M.; Cibeyibeyi, G.K.; Disashi, G.T.; Gulbis, B. Sickle cell disease in the Democratic Republic of Congo: What are the obstacles to treatment with hydroxyurea? Pan. Afr. Med. J. 2021, 38, 41. [Google Scholar] [CrossRef] [PubMed]
- Bernaudin, F. Why, Who, When, and How? Rationale for Considering Allogeneic Stem Cell Transplantation in Children with Sickle Cell Disease. J. Clin. Med. 2019, 8, 1523. [Google Scholar] [CrossRef] [PubMed]
- Nurain, I.O.; Bewaji, C.O.; Jarrett, S.J.; Robertson, D.D.; Zhang, Y. Potential of Three Ethnomedicinal Plants as Antisickling Agents. Mol. Pharm. 2017, 14, 172–182. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ukwuani-Kwaja, A.N.; Maimuna, H. Ethnobotanical Survey and In vitro Antisickling Effect of Some Selected Medicinal Plants. Asian J. Res. Biochem. 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Yembeau, N.L.; Biapa Nya, P.N.; Pieme, C.A.; Tchouane, K.D.; Kengne Fotsing, C.B.; Nya Nkwikeu, K.J.; Feudjio, A.F.; Telefo, P.B. Ethnopharmacological Study of the Medicinal Plants Used in the Treatment of Sickle Cell Anemia in the West Region of Cameroon. Evid.-Based Complement. Altern. Med. 2022, 2022, 5098428. [Google Scholar] [CrossRef]
- Jeffery, T.D.; Matthew, L.R. A review of the effectiveness of hibiscus for treatment of metabolic syndrome. J. Ethnopharmacol. 2021, 270, 113762. [Google Scholar] [CrossRef]
- Abdul-Azeez, Z.M.; Shihab, H.M. Possible Protective Anticancer effect of Ethanol Fraction of Iraqi Hibiscus tiliaceus L. Leaves Extract on Diethylnitrosamine-induced Hepatocarcinogenesis in Male Rats. Iraqi J. Pharm. Sci. 2023, 32, 145–155. [Google Scholar] [CrossRef]
- Rosa, R.M.; Melecchi, M.I.; Halmenschlager, R.C.; Abad, F.C.; Simoni, C.R.; Carama, E.B.; Gashenriques, J.T.; Affi, J.S.; Ramos, A. Antioxidant and Antimutagenic Properties of Hibiscus tiliaceus L. Methanolic Extract. J. Agric Food Chem. 2006, 54, 7324–7330. [Google Scholar] [CrossRef]
- Vanzella, C.; Bianchetti, P.; Sbaraini, S.; Vanzin, S.I.; Melecchi, M.I.S.; Caramão, E.B.; Siqueira, I.R. Antidepressant-like effects of methanol extract of Hibiscus tiliaceus flowers in mice. BMC Complement. Altern. Med. 2012, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Andriani, Y.; Hanifah, W.; Kholieqoh, A.H.; Majid, F.A.A.; Hermansyah, H.; Amir, H.; Muhammad, T.S.T. Antibacterial activity of hexane and methanol fractions of some selected plants against Klebsiella pneumoniae. J. Adv. Pharm. Technol. Res. 2023, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Trung, V.T.; Linh, K.T.P.; Thu Trang, D.; Thanh Binh, P.; The Cuong, N.; Thanh, N.V.; Thao, N.P. Antimicrobial constituents from the leaves of Hibiscus tiliaceus L. Nat. Prod. Res. 2023, 1, 1050–1057. [Google Scholar] [CrossRef]
- Surana, A.R.; Kumbhare, M.R.; Gunjal, A.R.; Goswami, S.S.; Ghuge, D.M. Chemical characterization, thrombolytic and antioxidant activity of Hibiscus tiliaceus L. leaves. Nat. Prod. Res. 2022, 36, 6106–6110. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Awal, S.M.; Nazmir, S.; Nasrin, S.; Nurunnabi, S.R.; Uddin, S.J. Evaluation of pharmacological activity of Hibiscus tiliaceus. Springer Plus 2016, 5, 1209. [Google Scholar] [CrossRef]
- Vinh, L.B.; Nguyet, N.T.M.; Thanh, C.D.; Huong, T.T.; Tram, L.H.; Van Thong, N.; Kim, Y.H. Chemical constituents of Vietnamese mangrove Hibiscus tiliaceus with antioxidant and alpha-glucosidase inhibitory activity. Nat. Prod. Res. 2021, 35, 2899–2904. [Google Scholar] [CrossRef]
- Hidayati, D.N.; Maghfiroh, H.R.; Safitri, A. Antibacterial activity of ethanol extracts of Hibiscus tiliaceus L. leaves from different extraction methods against Escherichia coli and Staphylococcus aureus. Pharmaciana 2023, 13, 137. [Google Scholar] [CrossRef]
- Chen, J.J.; Huang, S.Y.; Duh, C.Y.; Chen, I.S.; Wang, T.C.; Fang, H.Y. A New Cytotoxic Amide from the Stem Wood of Hibiscus tiliaceus. Planta Med. 2006, 72, 935–938. [Google Scholar] [CrossRef]
- Bassant, M.M.I.; Marawan, A.E.; Doha, H.A.B.; Emad, N.Z.; Souad, E.G.; Mouchira, A.S. A pharmacological and toxicological biochemical study of cardiovascular regulatory effects of hibiscus, corn silk, marjoram, and chamomile. Heliyon 2024, 10, 22659. [Google Scholar] [CrossRef]
- Lê Huyền, T.; Trần, T.H.; Nguyễn, V.T.; Nguyễn, H.M. Flavonoid Glycoside Constituents from the Leaves of Hibiscus tiliaceus. JST Eng. Technol. Sustain. Dev. 2021, 31, 7–11. [Google Scholar] [CrossRef]
- Bamba Fall, A.; Vanhaelen-Fastré, R.; Vanhaelen, M.; Lo, I.; Toppet, M.; Ferster, A.; Fondu, P. In Vitro Antisickling Activity of a Rearranged Limonoid Isolated from Khaya senegalensis. Planta Med. 1999, 65, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, R.; Nic Lughadha, E.; Black, N. The World Checklist of Vascular Plants, a continuously updated resource for exploring global plant diversity. Sci. Data 2021, 8, 215. [Google Scholar] [CrossRef]
- Chaouqi, S.; Moratalla-López, N.; Alonso, G.L.; Lorenzo, C.; Zouahri, A.; Asserar, N.; Haidar, E.M.; Guedira, T. Effect of Soil Composition on Secondary Metabolites of Moroccan Saffron (Crocus sativus L.). Plants 2023, 12, 711. [Google Scholar] [CrossRef]
- Mudau, H.S.; Mokoboki, H.K.; Ravhuhali, K.E.; Mkhize, Z. Effect of Soil Type: Qualitative and Quantitative Analysis of Phytochemicals in Some Browse Species Leaves Found in Savannah Biome of South Africa. Molecules 2022, 27, 1462. [Google Scholar] [CrossRef] [PubMed]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, 2100345. [Google Scholar] [CrossRef]
- Ramani, S.; Basak, S.; Puneeth, S.; Karthick, M.; Malathi, R. Anti Hemolytic Activity of Rutin in case of Phenyl Hydrazine induced Hemolysis. Curr. Trends Biotechnol. Pharm. 2021, 15, 467–470. [Google Scholar] [CrossRef]
- Muhammad, A.; Waziri, A.D.; Forcados, G.E.; Sanusi, B.; Sani, H.; Malami, I.; Abubakar, I.B.; Oluwatoyin, H.Y.; Adinoyi, O.A.; Mohammed, H.A. Sickling-preventive effects of rutin is associated with modulation of deoxygenated haemoglobin, 2,3-bisphosphoglycerate mutase, redox status and alteration of functional chemistry in sickle erythrocytes. Heliyon 2019, 5, 01905. [Google Scholar] [CrossRef] [PubMed]
- Gbolo, B.Z.; Ngbolua, K.N.; Ciala, B.N.; Semay, I.; Mpiana, P.T.; Gerbaux, P.; Duez, P. LC-MS/MS Analysis of crude Flavonoid Compounds from Justicia secunda from Democratic Republic of the Congo and evaluation of their antisickling Activity. Nat. Resour. Hum. Health 2024, 4, 387–397. [Google Scholar] [CrossRef]
- Ekutsu, G.E.; Kitete, E.M.; Masengo, C.A.; Gbolo, B.Z.; Iteku, J.B.; Mavakala, B.K.; Mpiana, P.T.; Mudogo, V.; Ngbolua, K.N. Molecular Docking Simulation of the antisickling activity of Naringenin-7-O-glucoside and Kaempferol-3-O-glucoside from Uapaca heudelotii Baill. (Phyllanthaceae) and their ADMET profile. J. Oral Public Health 2024, 5, 2. [Google Scholar] [CrossRef]
- Igbinumwen, O.; Osaro, I.; Ebengho, M.I.; Edema, M.O.; Oviawe, A.P.; Momoh, S.M.; Eduwuirofo, L.O.; Iyekekpolor, R.; Oghomwen, T.M.; Oghomwen, R.O.; et al. Chemical characterizations and anti-sickling potential of methanol extract of Justicia carnea (flamingo plant). Zenodo 2024, 4, 65–75. [Google Scholar] [CrossRef]
- Hu, X.; Wang, M.; Pan, Y.; Xie, Y.; Han, J.; Zhang, X.; Niayale, N.; He, H.; Li, Q.; Zhao, T.; et al. Anti-inflammatory Effect of Astragalin and Chlorogenic Acid on Escherichia coli-Induced Inflammation of Sheep Endometrial Epithelium Cells. Front. Vet. Sci. 2020, 7, 201. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Lee, J.Y.; Kim, M.Y.; Kang, C.-H.; Hwang, H.S. Anti-Oxidative and Anti-Inflammatory Activities of Astragalus membranaceus Fermented by Lactiplantibacillus plantarum on LPS-Induced RAW 264.7 Cells. Fermentation 2021, 7, 252. [Google Scholar] [CrossRef]
- Choi, S.-S.; Park, H.-R.; Lee, K.-A. A Comparative Study of Rutin and Rutin Glycoside: Antioxidant Activity, Anti-Inflammatory Effect, Effect on Platelet Aggregation and Blood Coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef]
- Poret, M.; Tran, T.; Villotte, M.; Nüsse, O. Myeloperoxidase: An ingenious defense mechanism against pathogen infections in medicine and science. Med. Sci. 2017, 33, 741–743. [Google Scholar] [CrossRef][Green Version]
- Nur, E.; Biemond, B.J.; Otten, H.M.; Brandjes, D.P.; Schnog, J.J.B. Oxidative stress in sickle cell disease; pathophysiology and potential implications for disease management. Am. J. Hematol. 2011, 86, 484–489. [Google Scholar] [CrossRef]
- Queiroz, R.F.; Lima, E.S. Oxidative stress in sickle cell disease. Rev. Bras. Hematol. E Hemoter. 2013, 35, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.E.; Machado, R.P.; Laurentino, M.R.; Dos Santos, T.E.; Bandeira, I.C.; Maia Filho, P.A.; Figueiredo, M.F.; Martins, A.M.; Lemes, R.P. Clinical events and their relation to the tumor necrosis factor-alpha and interleukin-10 genotypes in Sickle-Cell-Anemia patients. Hematol. Oncol. Stem Cell Ther. 2016, 9, 14–19. [Google Scholar] [CrossRef]
- Domingos, I.F.; Pereira-Martins, D.A.; Sobreira, M.J.V.C. High levels of proinflammatory cytokines IL-6 and IL-8 are associated with a poor clinical outcome in sickle cell anemia. Ann. Hematol. 2020, 99, 947–953. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Weihrauch, D.; Jones, W.D.; Jing, X.; Shi, Y.; Gourlay, D. Inhibition of myeloperoxidase decreases vascular oxidative stress and increases vasodilatation in sickle cell disease mice. J. Lipid Res. 2013, 54, 3009–3015. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, H.; Wang, X.; Li, X.; Hu, J.; Zi, X.; Zhou, Y.; Pan, D.; Fu, Y. Kaempferol 3-O-Rutinoside, a Flavone Derived from Tetrastigma hemsleyanum Diels et Gilg, Reduces Body Temperature through Accelerating the Elimination of IL-6 and TNF-α in a Mouse Fever Model. Molecules 2024, 29, 1641. [Google Scholar] [CrossRef]
- Wadah, O.; Mona, S.M.; Hassan, S.K.; Abdelkhalig, M.; Shantier, S.W.; Bashier, O.; Abdoon, I. HPTLC Fingerprint Profile and Identification of Antidiabetic and Antioxidant Leads from Bauhinia rufescens L. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 3201463. [Google Scholar] [CrossRef]
- Hounkpe, B.W.; Chenou, F.; Domingos, I.F.; Cardoso, E.C.; Costa Sobreira, M.J.V.; Araujo, A.S.; Lucena-Araújo, A.R.; da Silva Neto, P.V.; Malheiro, A.; Fraiji, N.A.; et al. Neutrophil extracellular trap regulators in sickle cell disease: Modulation of gene expression of PADI4, neutrophil elastase, and myeloperoxidase during vaso-occlusive crisis. Res. Pract. Thromb. Haemost. 2021, 5, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Borive, A.M.; Mavar, M.H.; Memvanga, B.P.; Ndezu, A.R.; Nsasi, B.E.; Mouithys, M.A.; Batina, A.S.; Marini, D.R. Evidence-based Sickle Cell management: From Traditional remedies use to laboratory assessment. Phytomed. Plus, 2025; Under review. [Google Scholar]
- Souleymane, H.D.; Djibo, A.K.; Seyni, S.H.; Zakaria, O.; Botezatu, A.V.; Dinica, R.M.; Ibrahim Maman Laouali, A.; Kouakou, N.D.V. Phytochemical Characterization and In Vitro Evaluation of the Anti-Sickle Cell Activity of Aqueous and Ethanolic Extracts of Two Medicinal Plants from Niger: Flueggea virosa (Roxb. ex Willd.) Royle and Kigelia africana (Lam.) Benth. Plants 2023, 12, 3522. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nyssen, P.; Mouithys-Mickalad, A.; Minguet, G.; Sauvage, E.; Wouters, J.; Franck, T.; Hoebeke, M. Morphine, a potential inhibitor of myeloperoxidase activity. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2018, 1862, 2236–2244. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borive Amani, M.; Frederich, M.; Jansen, O.; Bonnet, O.; Ledoux, A.; Memvanga, P.B.; Batina Agasa, S.; Mouithys-Mickalad, A.; Djang’eing’a, R.M. Phytochemical Characterization of Hibiscus tiliaceus L. Leaves and Evaluation of Their Antisickling, Antioxidant, and Anti-Inflammatory Activities. Molecules 2025, 30, 1765. https://doi.org/10.3390/molecules30081765
Borive Amani M, Frederich M, Jansen O, Bonnet O, Ledoux A, Memvanga PB, Batina Agasa S, Mouithys-Mickalad A, Djang’eing’a RM. Phytochemical Characterization of Hibiscus tiliaceus L. Leaves and Evaluation of Their Antisickling, Antioxidant, and Anti-Inflammatory Activities. Molecules. 2025; 30(8):1765. https://doi.org/10.3390/molecules30081765
Chicago/Turabian StyleBorive Amani, Marguerite, Michel Frederich, Olivia Jansen, Olivier Bonnet, Allison Ledoux, Patrick B. Memvanga, Salomon Batina Agasa, Ange Mouithys-Mickalad, and Roland Marini Djang’eing’a. 2025. "Phytochemical Characterization of Hibiscus tiliaceus L. Leaves and Evaluation of Their Antisickling, Antioxidant, and Anti-Inflammatory Activities" Molecules 30, no. 8: 1765. https://doi.org/10.3390/molecules30081765
APA StyleBorive Amani, M., Frederich, M., Jansen, O., Bonnet, O., Ledoux, A., Memvanga, P. B., Batina Agasa, S., Mouithys-Mickalad, A., & Djang’eing’a, R. M. (2025). Phytochemical Characterization of Hibiscus tiliaceus L. Leaves and Evaluation of Their Antisickling, Antioxidant, and Anti-Inflammatory Activities. Molecules, 30(8), 1765. https://doi.org/10.3390/molecules30081765







