Redox Additive Electrolytes for Supercapacitors: A Mini-Review on Recent Developments and Future Directions
Abstract
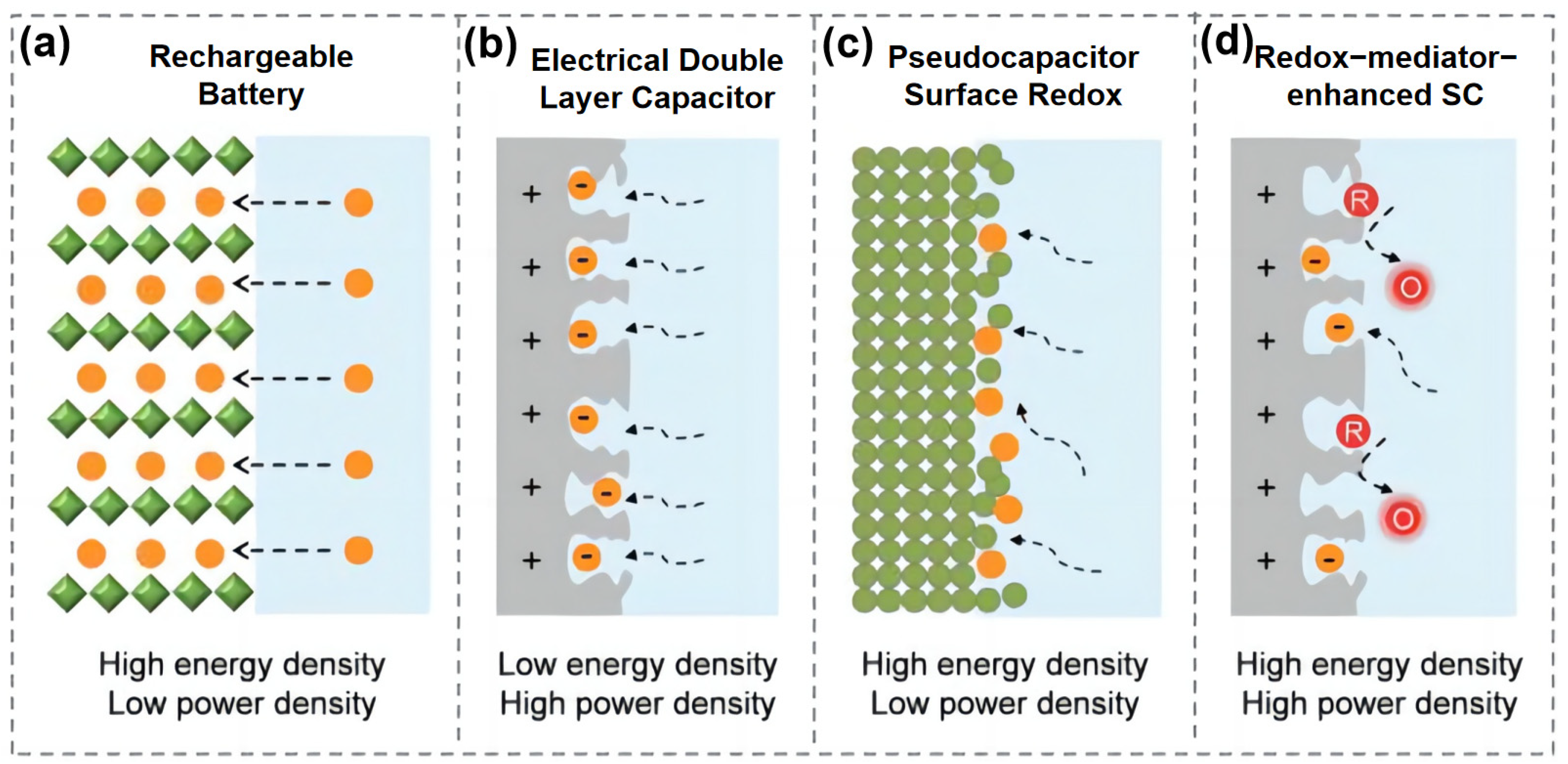
:1. Introduction
2. Redox-Mediated Aqueous Electrolytes
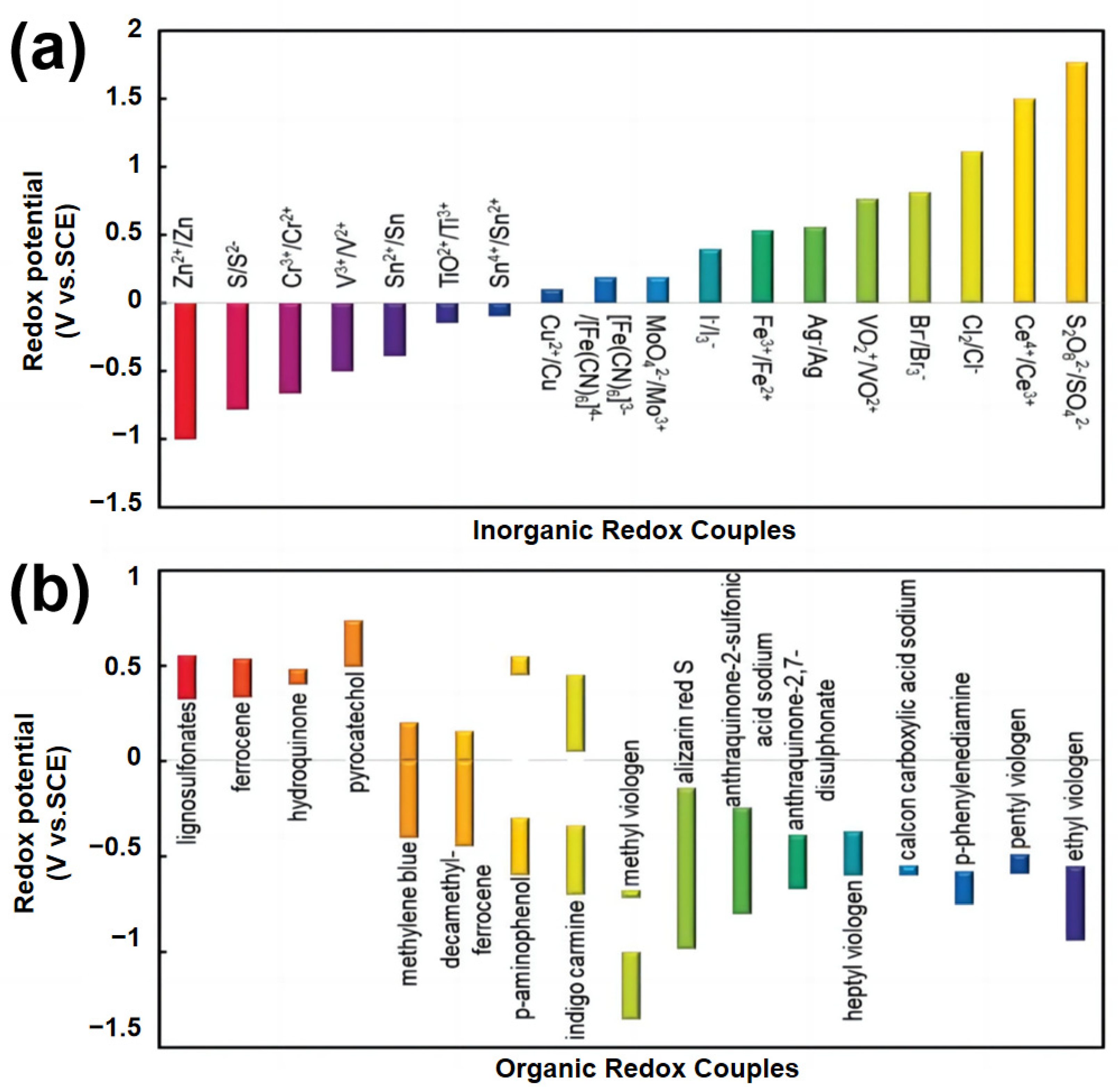
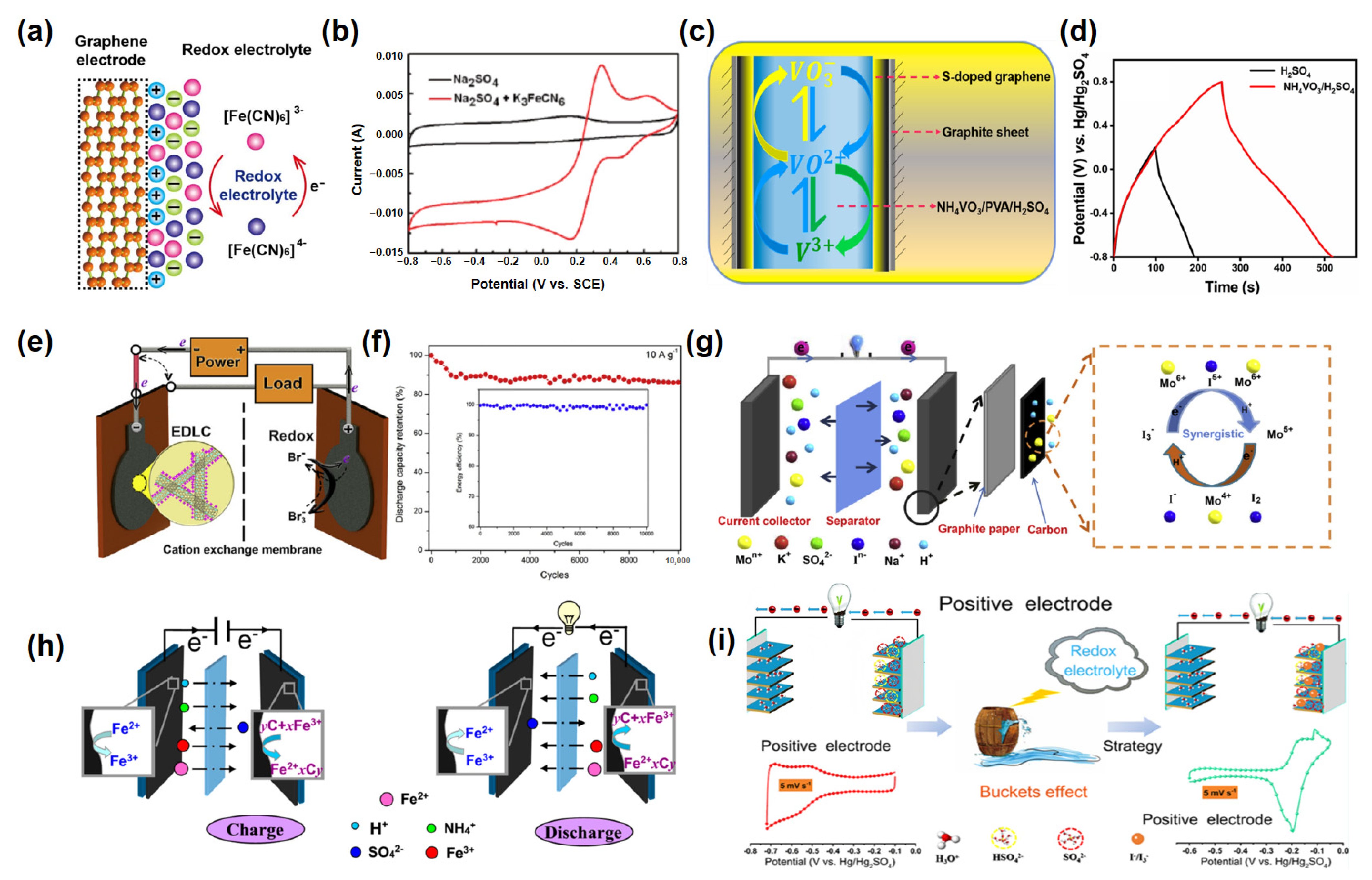
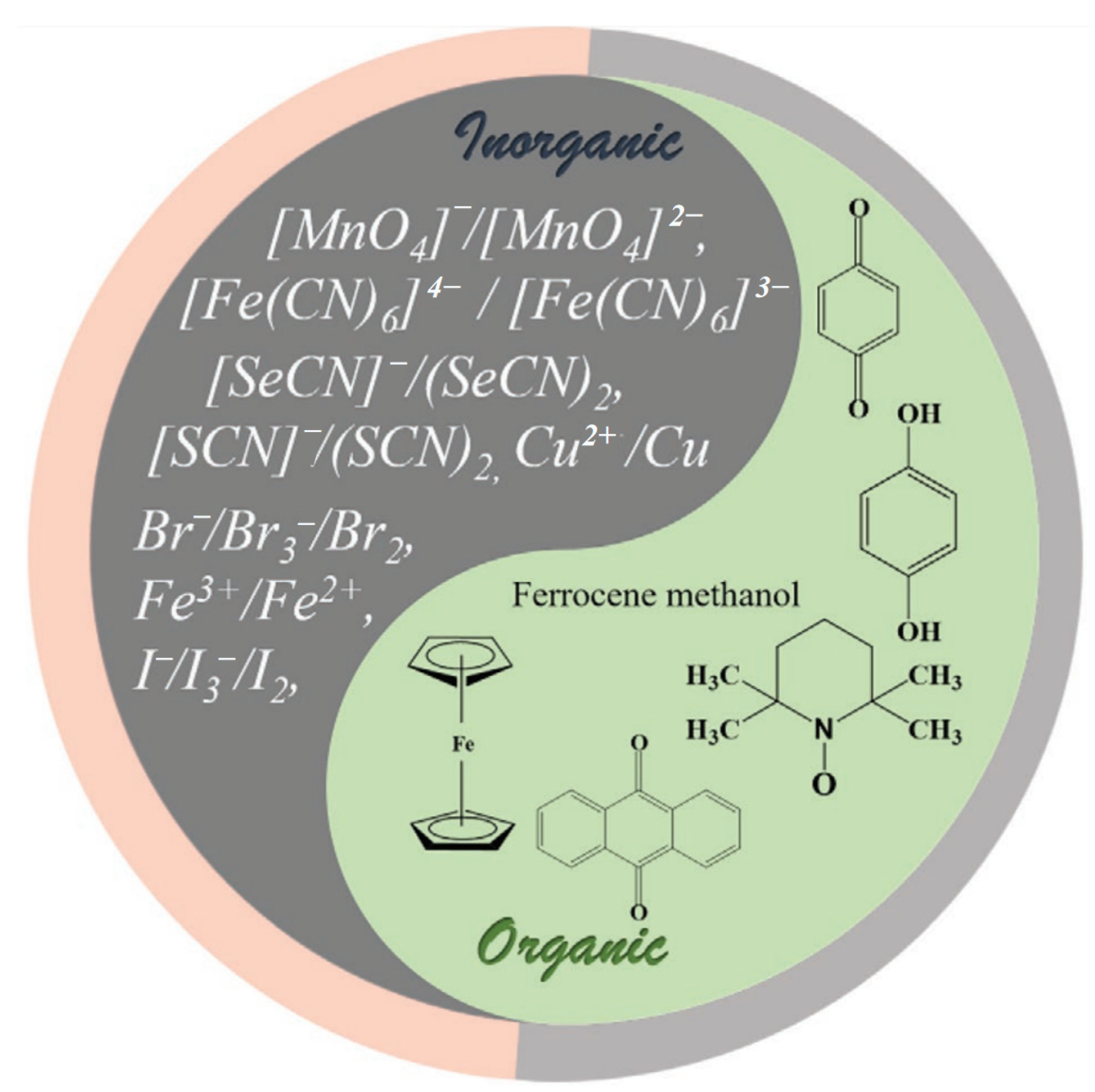
2.1. Inorganic Redox Additives in Aqueous Electrolytes
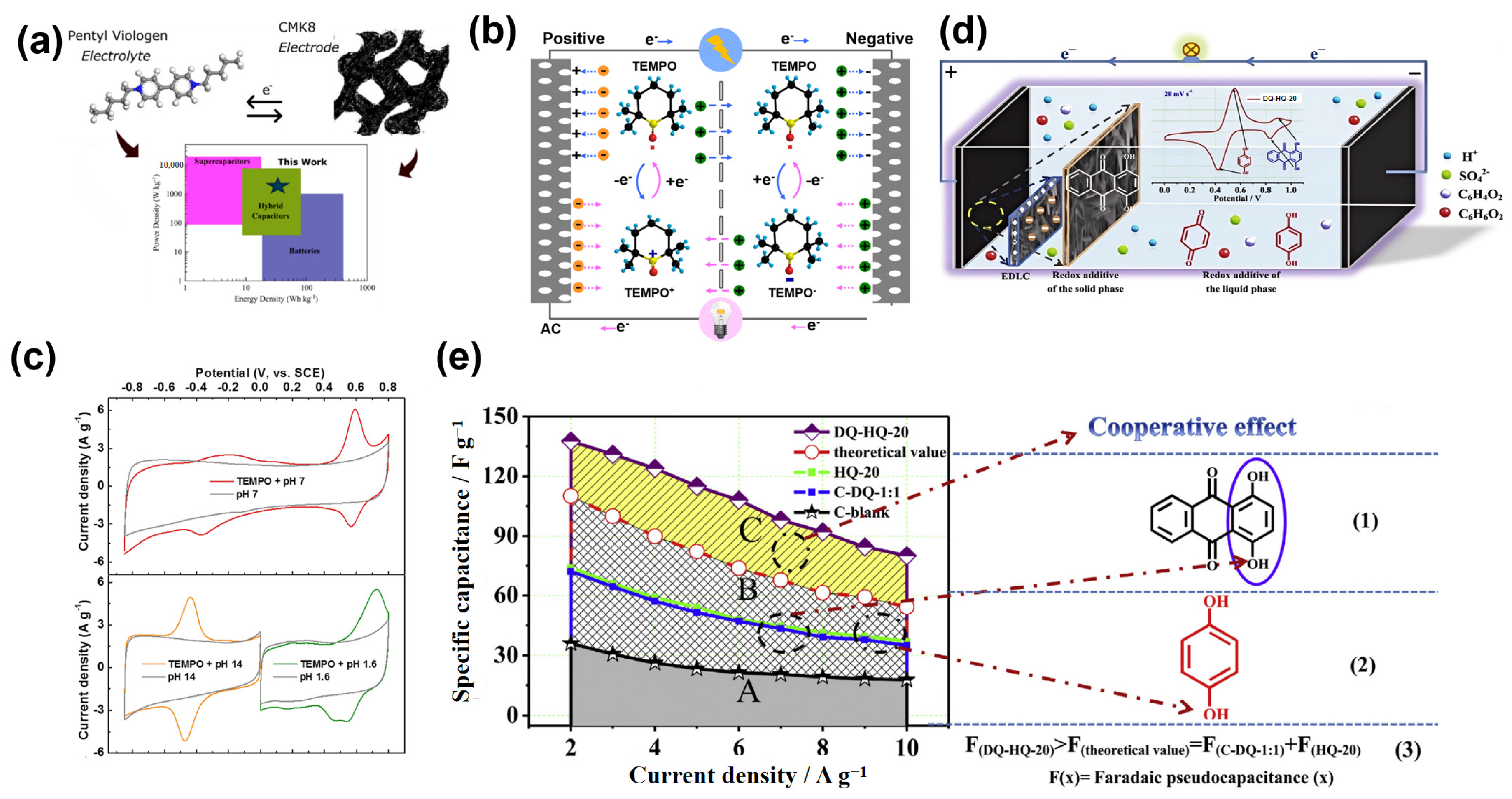
2.2. Organic Redox Additives in Aqueous Electrolytes
3. Redox-Mediated Non-Aqueous Electrolyte
3.1. Redox-Mediated Non-Aqueous Organic Electrolytes
3.2. Redox-Mediated Ionic Liquid Electrolytes
4. Redox-Mediated Gel Electrolytes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Xie, Y.; You, Y.; Yuan, J.; Xu, Q.; Xie, H.; Chen, Y. Rational Design of Electrode Materials for Advanced Supercapacitors: From Lab Research to Commercialization. Adv. Funct. Mater. 2023, 33, 2213095. [Google Scholar] [CrossRef]
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Bharti; Kumar, A.; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. J. Energy Storage 2022, 48, 103871. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, 8285. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kumar, H.; Kumar, G.; Sharma, S.; Aneja, R.; Sharma, A.K.; Kumar, R.; Kumar, P. Progress and challenges in electrochemical energy storage devices: Fabrication, electrode material, and economic aspects. Chem. Eng. J. 2023, 468, 143706. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Sun, H.; Zhou, J.; Yang, F.; Li, H.; Chen, H.; Chen, Y.; Liu, Z.; Qiu, Z.; et al. Progress and Perspective: MXene and MXene-Based Nanomaterials for High-Performance Energy Storage Devices. Adv. Electron. Mater. 2021, 7, 2000967. [Google Scholar] [CrossRef]
- Liu, X.; Lyu, D.; Merlet, C.; Leesmith, M.J.A.; Hua, X.; Xu, Z.; Grey, C.P.; Forse, A.C. Structural disorder determines capacitance in nanoporous carbons. Science 2024, 384, 321–325. [Google Scholar] [CrossRef]
- Chen, J.W.; Adit, G.; Li, L.; Zhang, Y.X.; Chua, D.H.C.; Lee, P.S. Optimization Strategies Toward Functional Sodium-Ion Batteries. Energy Environ. Mater. 2023, 6, e12633. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Kulova, T.L.; Fateev, V.N.; Seregina, E.A.; Grigoriev, A.S. A Brief Review of Post-Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 7242–7259. [Google Scholar] [CrossRef]
- Xu, J.J.; Cai, X.Y.; Cai, S.M.; Shao, Y.X.; Hu, C.; Lu, S.R.; Ding, S.J. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sust. Energ. Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Perdana, M.Y.; Johan, B.A.; Abdallah, M.; Hossain, M.E.; Aziz, M.A.; Baroud, T.N.; Drmosh, Q.A. Understanding the Behavior of Supercapacitor Materials via Electrochemical Impedance Spectroscopy: A Review. Chem. Rec. 2024, 24, e202400007. [Google Scholar] [CrossRef]
- Ghosh, S.; Jeong, S.M.; Polaki, S.R. A review on metal nitrides/oxynitrides as an emerging supercapacitor electrode beyond oxide. Korean J. Chem. Eng. 2018, 35, 1389–1408. [Google Scholar] [CrossRef]
- Huo, P.P.; Zhao, P.; Wang, Y.; Liu, B.; Yin, G.C.; Dong, M.D. A Roadmap for Achieving Sustainable Energy Conversion and Storage: Graphene-Based Composites Used Both as an Electrocatalyst for Oxygen Reduction Reactions and an Electrode Material for a Supercapacitor. Energies 2018, 11, 167. [Google Scholar] [CrossRef]
- Hu, L.T.; Zhai, T.Y.; Li, H.Q.; Wang, Y.G. Redox-Mediator-Enhanced Electrochemical Capacitors: Recent Advances and Future Perspectives. ChemSusChem 2019, 12, 1118–1132. [Google Scholar] [CrossRef]
- Guo, L.; Hu, P.; Wei, H. Development of supercapacitor hybrid electric vehicle. J. Energy Storage 2023, 65, 107269. [Google Scholar] [CrossRef]
- Reenu; Sonia; Phor, L.; Kumar, A.; Chahal, S. Electrode materials for supercapacitors: A comprehensive review of advancements and performance. J. Energy Storage 2024, 84, 110698. [Google Scholar] [CrossRef]
- Yang, Z.F.; Tian, J.R.; Yin, Z.F.; Cui, C.J.; Qian, W.Z.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Ishikawa, M.; Dokko, K.; Teng, H.S.; Lindberg, S.; Ajuria, J.; Balducci, A.; Frackowiak, E. Recent Progress in Electrolyte Systems for Supercapacitors. Electrochemistry 2024, 92, 074003. [Google Scholar] [CrossRef]
- Yu, L.P.; Chen, G.Z. Ionic Liquid-Based Electrolytes for Supercapacitor and Supercapattery. Front. Chem. 2019, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.Y.; Zhang, Y.K.; Ni, Y.H. Recent progress on development of electrolyte and aerogel electrodes applied in supercapacitors. J. Power Sources 2023, 560, 232698. [Google Scholar] [CrossRef]
- Dong, W.J.; Xie, M.; Zhao, S.W.; Qin, Q.L.; Huang, F.Q. Materials design and preparation for high energy density and high power density electrochemical supercapacitors. Mat. Sci. Eng. R 2023, 152, 100713. [Google Scholar] [CrossRef]
- Guo, T.Z.; Zhou, D.; Pang, L.X.; Sun, S.K.; Zhou, T.; Su, J.Z. Perspectives on Working Voltage of Aqueous Supercapacitors. Small 2022, 18, 2106360. [Google Scholar] [CrossRef]
- Qi, Z.; Ren, R.; Hu, J.; Chen, Y.; Guo, Y.; Huang, Y.; Wei, J.; Zhang, H.; Pang, Q.; Zhang, X.; et al. Flexible Supercapacitor with Wide Electrochemical Stable Window Based on Hydrogel Electrolyte. Small 2024, 20, 2400369. [Google Scholar] [CrossRef]
- Qorbani, M.; Chen, K.H.; Chen, L.C. Hybrid and Asymmetric Supercapacitors: Achieving Balanced Stored Charge across Electrode Materials. Small 2024, 20, 2400558. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, Q.; Xu, B. “Water-in-Salt” Electrolytes for Supercapacitors: A Review. ChemSusChem 2021, 14, 2501–2515. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J.; Wang, X.; Zhang, J.; Tian, Z.; Zhu, E.; Yang, L.; Guan, X.; Ren, H.; Wu, J.; et al. A review of advanced electrolytes for supercapacitors. J. Energy Storage 2024, 103, 114338. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Kim, W. Redox-Active Water-in-Salt Electrolyte for High-Energy-Density Supercapacitors. ACS Energy Lett. 2022, 7, 1266–1273. [Google Scholar] [CrossRef]
- Chen, Y.; Dou, Q.; Yang, J.; Huang, C.; Tang, P.; Xue, S.; Tang, A.; Yu, X.; Cao, Y.; Yan, X. CO2-regulated, ultrahigh-concentration aqueous electrolytes for high energy and power of supercapacitors. Chem. Eng. J. 2024, 496, 154007. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Li, P.; Du, X.; Ma, M.; Liang, Z.; Su, Y.; Xiong, L. Ultrahigh-voltage aqueous electrolyte for wide-temperature supercapacitors. J. Mater. Chem. A 2023, 11, 15532–15539. [Google Scholar] [CrossRef]
- Neto, C.; Pham, H.T.T.; Omnée, R.; Canizarès, A.; Slodczyk, A.; Deschamps, M.; Raymundo-Piñero, E. Exploring the Carbon/Electrolyte Interface in Supercapacitors Operating in Highly Concentrated Aqueous Electrolytes. ACS Appl. Mater. Interfaces 2022, 14, 44405–44418. [Google Scholar] [CrossRef] [PubMed]
- Frajnkovič, M.; Likitchatchawankun, A.; Douard, C.; Zhou, Y.; Baek, S.W.; Catton, I.; Crosnier, O.; Brousse, T.; Pilon, L. Calorimetry can detect the early onset of hydrolysis in hybrid supercapacitors with aqueous electrolytes. J. Power Sources 2022, 548, 232069. [Google Scholar] [CrossRef]
- Gong, X.; Xu, H.; Zhang, M.; Cheng, X.; Wu, Y.; Zhang, H.; Yan, H.; Dai, Y.; Zheng, J.-C. 2.4 V high performance supercapacitors enabled by polymer-strengthened 3 m aqueous electrolyte. J. Power Sources 2021, 505, 230078. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, X. Effects of current collectors on the self-discharge rates of supercapacitors with aqueous electrolyte. Mater. Sci. Semicond. Process. 2024, 182, 108691. [Google Scholar] [CrossRef]
- Apparla, N.; Manickavasakam, K.; Sharma, C.S. Augmenting the supercapacitive performance of candle soot-derived activated carbon electrodes in aqueous and non-aqueous electrolytes. J. Energy Storage 2023, 73, 109162. [Google Scholar] [CrossRef]
- Kumar, A.; Rawat, P.; Mahanty, B.N.; Mohanty, P. Nitrogen-enriched nanoporous polytriazine as efficient electrode material for high-performance supercapacitor application in non-aqueous medium with high cyclic stability. J. Energy Storage 2024, 96, 112650. [Google Scholar] [CrossRef]
- Lan, S.; Yu, C.; Yu, J.; Zhang, X.; Liu, Y.; Xie, Y.; Wang, J.; Qiu, J. Recent Advances in Low-Temperature Liquid Electrolyte for Supercapacitors. Small 2024, 2309286. [Google Scholar] [CrossRef]
- Pappu, S.; Anandan, S.; Rao, T.N.; Martha, S.K.; Bulusu, S.V. High-performance hybrid supercapacitor with electrochemically exfoliated graphene oxide incorporated NiCo2O4 in aqueous and non-aqueous electrolytes. J. Energy Storage 2022, 50, 104598. [Google Scholar] [CrossRef]
- Sadavar, S.; Wang, K.J.; Kang, T.; Hwang, M.; Saeed, G.; Yu, X.; Park, H.S. Anion storage for hybrid supercapacitor. Mater. Today Energy 2023, 37, 101388. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, M.; Liu, Y.; Fu, Y.; Gao, M.; Wang, G.; Wang, F.; Wang, Z.; Chen, G.; Yang, S.; et al. Largely Pseudocapacitive Two-Dimensional Conjugated Metal–Organic Framework Anodes with Lowest Unoccupied Molecular Orbital Localized in Nickel-bis(dithiolene) Linkages. J. Am. Chem. Soc. 2023, 145, 6247–6256. [Google Scholar] [CrossRef]
- Bagheri, A.; Bellani, S.; Beydaghi, H.; Eredia, M.; Najafi, L.; Bianca, G.; Zappia, M.I.; Safarpour, M.; Najafi, M.; Mantero, E.; et al. Functionalized Metallic 2D Transition Metal Dichalcogenide-Based Solid-State Electrolyte for Flexible All-Solid-State Supercapacitors. ACS Nano 2022, 16, 16426–16442. [Google Scholar] [CrossRef] [PubMed]
- Beknalkar, S.A.; Teli, A.M.; Satale, V.V.; Amate, R.U.; Morankar, P.J.; Yewale, M.A.; Shin, J.C. A critical review of recent advancements in high-temperature supercapacitors: Thermal kinetics, interfacial dynamics, employed strategies, and prospective trajectories. Energy Storage Mater. 2024, 66, 103217. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, S.; Kim, H.; Kim, Y. Lithium polymer supercapacitors with water-processable branched poly(ethylene imine)-based solid-state electrolytes. J. Energy Storage 2023, 57, 106010. [Google Scholar] [CrossRef]
- Liew, J.; Liu, L.; Loh, K.H.; Bashir, S.; Ramesh, K.; Ramesh, S. How viable are MXenes? Recent developments of MXene synthesis and its application in solid-state electrolytes. J. Energy Storage 2024, 84, 110868. [Google Scholar] [CrossRef]
- Murukadas, D.; Cho, Y.; Lee, W.; Lee, S.; Kim, H.; Kim, Y. Lithium supercapacitors with environmentally-friend water-processable solid-state hybrid electrolytes of zinc oxide/polymer/lithium hydroxide. Energy 2024, 290, 129984. [Google Scholar] [CrossRef]
- Samantaray, S.; Mohanty, D.; Hung, I.M.; Moniruzzaman, M.; Satpathy, S.K. Unleashing recent electrolyte materials for next-generation supercapacitor applications: A comprehensive review. J. Energy Storage 2023, 72, 108352. [Google Scholar] [CrossRef]
- Nandi, P.; Subramaniam, C. Employing Redox-Active Additives for Enhanced Charge Polarization and Twofold Higher Energy Density in Supercapacitor. Adv. Mater. Interfaces 2023, 10, 2202052. [Google Scholar] [CrossRef]
- Akinwolemiwa, B.; Peng, C.; Chen, G.Z. Redox electrolytes in supercapacitors. J. Electrochem. Soc. 2015, 162, A5054. [Google Scholar] [CrossRef]
- Dou, L.; Zhou, S.; Ma, J.; Zhao, C.; Cui, P.; Ye, S.; Feng, P.; Gu, X.; Huang, S.; Tao, X. Organic redox additive incorporated PANI hydrogel electrodes for flexible high-energy-density supercapacitors. J. Mater. Chem. 2024, 12, 521–532. [Google Scholar] [CrossRef]
- Rao, A.; Bhat, S.; De, S.; Cyriac, V.; Rag S, A. Study of [EMIM] [EtSO4] ionic liquid-based gel polymer electrolyte mediated with hydroquinone redox additive for flexible supercapacitors. J. Energy Storage 2023, 68, 107716. [Google Scholar] [CrossRef]
- Sakita, A.M.P.; Ortega, P.F.R.; Silva, G.G.; Noce, R.D.; Lavall, R.L. Unveiling the performance metrics for supercapacitor electrodes with adsorbed redox additives. Electrochim. Acta 2021, 390, 138803. [Google Scholar] [CrossRef]
- Shanmugapriya, V.; Hariharan, G.; Arunpandiyan, S.; Babu, M.; Bharathi, S.; Selvakumar, B.; Arivarasan, A. Redox additives dependent supercapacitor performances of ZnO/SnO2@MWCNT nanocomposites. Diamond Relat. Mater. 2023, 140, 110482. [Google Scholar] [CrossRef]
- Surulinathan, A.; Gubendran, H.; Sambandam, B.; Ganapathy, S.; Ayyaswamy, A. Mixed metal oxide-based binder-free electrode and redox additive electrolyte combination for enhanced supercapacitor performance. J. Alloys Compd. 2024, 988, 174164. [Google Scholar] [CrossRef]
- Yang, N.; Yu, S.; Zhang, W.; Cheng, H.M.; Simon, P.; Jiang, X. Electrochemical Capacitors with Confined Redox Electrolytes and Porous Electrodes. Adv. Mater. 2022, 34, 2202380. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Mansi; Holdynski, M.; Deep, A.; Tiwari, U.K.; Nogala, W.; Shrivastav, V.; Sundriyal, S. Unravelling the electrochemistry of Ni-MOF derived nickel phosphide/carbon composite electrode and redox additive electrolyte for high performance supercapacitors. Mater. Today Chem. 2024, 39, 102165. [Google Scholar] [CrossRef]
- Khalafallah, D.; Zhang, Y.; Dai, H.; Liu, C.; Zhang, Q. Manipulating the overall capacitance of hierarchical porous carbons via structure- and pore-tailoring approach. Carbon 2024, 227, 119250. [Google Scholar] [CrossRef]
- Santos, M.C.G.; da Silva, D.R.; Pinto, P.S.; Ferlauto, A.S.; Lacerda, R.G.; Jesus, W.P.; da Cunha, T.H.R.; Ortega, P.F.R.; Lavall, R.L. Buckypapers of carbon nanotubes and cellulose nanofibrils: Foldable and flexible electrodes for redox supercapacitors. Electrochim. Acta 2020, 349, 136241. [Google Scholar] [CrossRef]
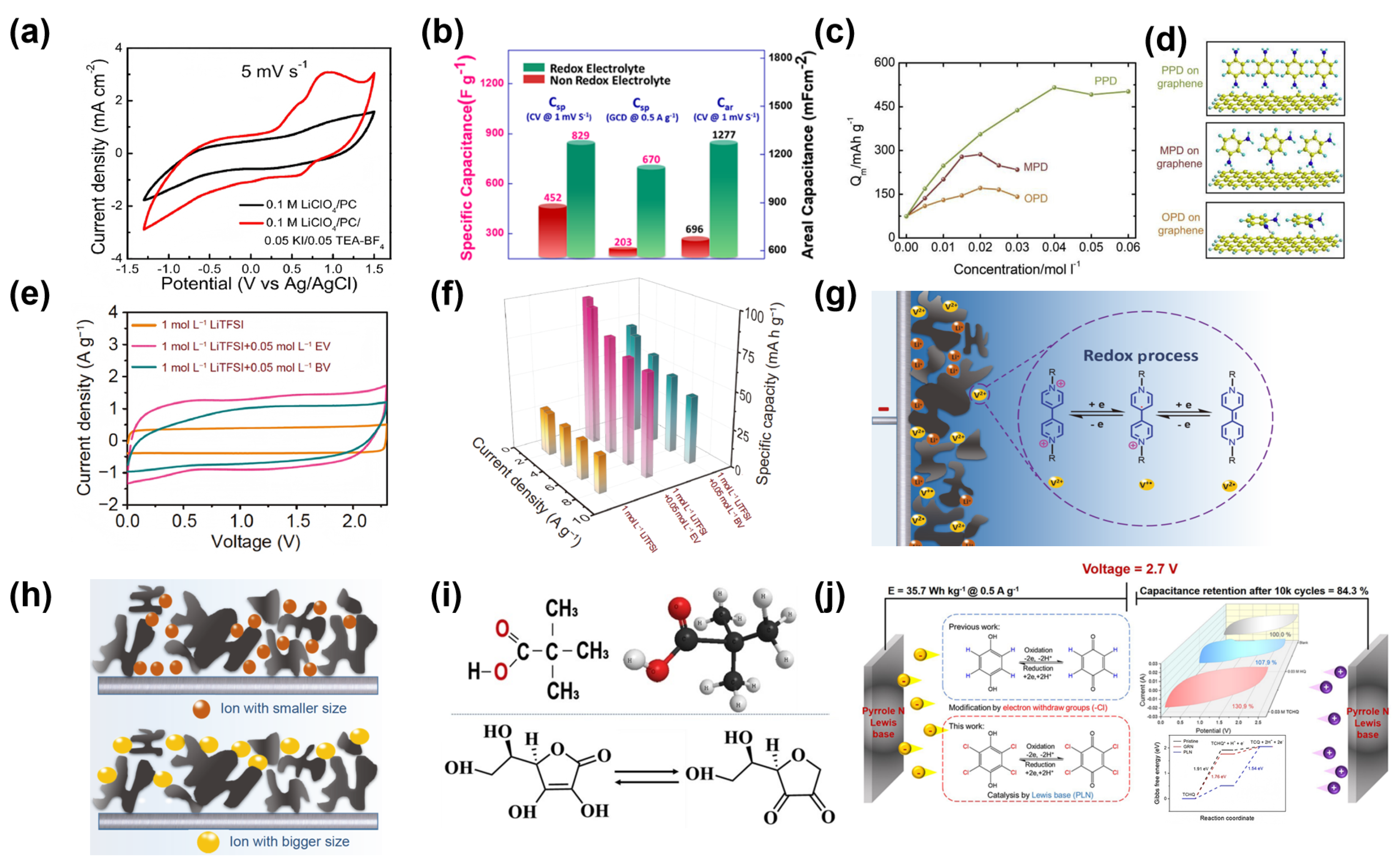
- Niu, Y.; Niu, J.; Ma, Y.; Zhi, L. Rational design of viologen redox additives for high-performance supercapacitors with organic electrolytes. Sci. China Mater. 2020, 64, 329–338. [Google Scholar] [CrossRef]
- Bhol, P.; Jagdale, P.B.; Barman, N.; Thapa, R.; Saxena, M.; Samal, A.K. Design and fabrication of nickel lanthanum telluride microfibers for redox additive electrolyte-based flexible solid-state hybrid supercapacitor. J. Energy Storage 2023, 65, 107286. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Li, X.; Wang, Q.; Liu, P. High performance flexible carbon cloth-based solid-state supercapacitors with redox-mediated gel electrolytes. Appl. Surf. Sci. 2022, 583, 152397. [Google Scholar] [CrossRef]
- Yadav, N.; Hashmi, S.A. Energy enhancement of quasi-solid-state supercapacitors based on a non-aqueous gel polymer electrolyteviaa synergistic effect of dual redox additives diphenylamine and potassium iodide. J. Mater. Chem. A 2020, 8, 18266–18279. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, M.I.; Cheng, F.; Lu, W. A review on selection criteria of aqueous electrolytes performance evaluation for advanced asymmetric supercapacitors. J. Energy Storage 2021, 40, 102729. [Google Scholar] [CrossRef]
- Chen, K.; Liu, F.; Xue, D.; Komarneni, S. Carbon with ultrahigh capacitance when graphene paper meets K3Fe(CN)6. Nanoscale 2015, 7, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Sandhiya, M.; Vivekanand; Suresh Balaji, S.; Sathish, M. Unrevealed Performance of NH4VO3 as a Redox-Additive for Augmenting the Energy Density of a Supercapacitor. J. Phys. Chem. C 2021, 125, 8068–8079. [Google Scholar] [CrossRef]
- Xiaohui, T.; Yu Hui, L.; Bolin, C.; Shan, H. Functionalized carbon nanotube based hybrid electrochemical capacitors using neutral bromide redox-active electrolyte for enhancing energy density. J. Power Sources 2017, 352, 118–126. [Google Scholar]
- Dong, X.; Wei, H.; Xiao Na, S.; Peng, C.; Xiang Ying, C. Redox additives of Na2MoO4 and KI: Synergistic effect and the improved capacitive performances for carbon-based supercapacitors. J. Power Sources 2016, 341, 448–456. [Google Scholar]
- Sun, X.N.; Hu, W.; Xu, D.; Chen, X.Y.; Cui, P. Integration of Redox Additive in H2SO4 Solution and the Adjustment of Potential Windows for Improving the Capacitive Performances of Supercapacitors. Ind. Eng. Chem. Res. 2017, 56, 2433–2443. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.; Luo, Y.; Zhao, H.; Du, Y.; Kong, L.B.; Que, W. Understanding MXene-Based “Symmetric” Supercapacitors and Redox Electrolyte Energy Storage. ACS Appl. Energy Mater. 2020, 3, 5006–5014. [Google Scholar] [CrossRef]
- Calcagno, G.; Evanko, B.; Stucky, G.D.; Ahlberg, E.; Yoo, S.J.; Palmqvist, A.E.C. Understanding the Operating Mechanism of Aqueous Pentyl Viologen/Bromide Redox-Enhanced Electrochemical Capacitors with Ordered Mesoporous Carbon Electrodes. ACS Appl. Mater. Interfaces 2021, 14, 20349–20357. [Google Scholar] [CrossRef]
- Hu, L.; Shi, C.; Guo, K.; Zhai, T.; Li, H.; Wang, Y. Electrochemical Double-Layer Capacitor Energized by Adding an Ambipolar Organic Redox Radical into the Electrolyte. Angew. Chem. Int. Ed. 2018, 57, 8214–8218. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Sun, X.N.; Hu, W.; Chen, X.Y. Carbon nanosheets-based supercapacitors: Design of dual redox additives of 1, 4-dihydroxyanthraquinone and hydroquinone for improved performance. J. Power Sources 2017, 357, 107–116. [Google Scholar] [CrossRef]
- Xiong, T.; Lee, W.S.V.; Chen, L.; Tan, T.L.; Huang, X.; Xue, J. Indole-based conjugated macromolecules as a redox-mediated electrolyte for an ultrahigh power supercapacitor. Energ. Environ. Sci. 2017, 10, 2441–2449. [Google Scholar] [CrossRef]
- Zhai, D.D.; Liu, H.; Wang, M.; Wu, D.; Chen, X.Y.; Zhang, Z.J. Integrating surface functionalization and redox additives to improve surface reactivity for high performance supercapacitors. Electrochim. Acta 2019, 323, 134810. [Google Scholar] [CrossRef]
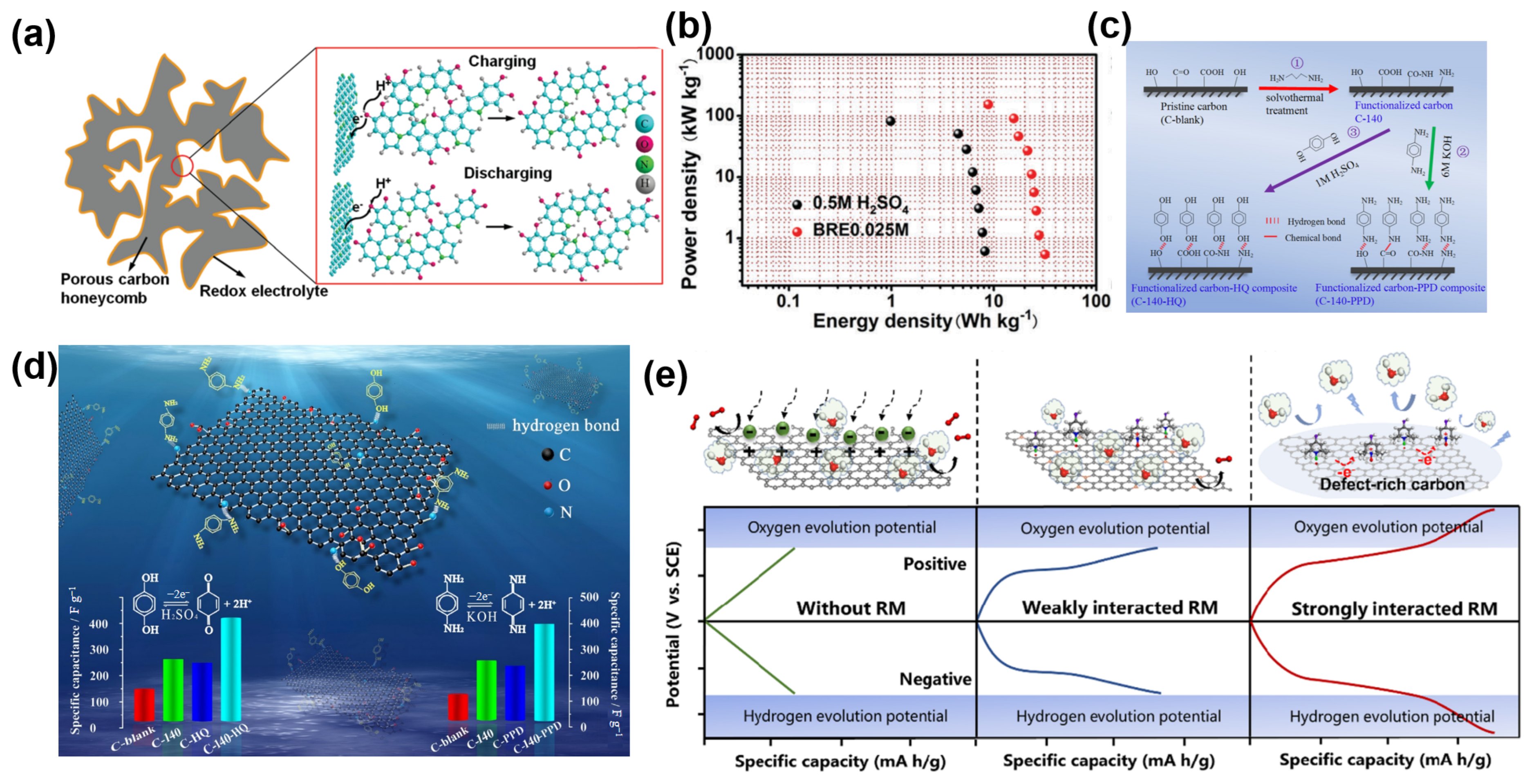
- Guan, L.; Zhu, Y.; Wan, Y.; Zhang, M.; Li, Q.; Teng, X.; Zhang, Y.; Yang, H.; Zhang, Y.; Hu, H.; et al. Strong Interaction Between Redox Mediators and Defect-Rich Carbons Enabling Simultaneously Boosted Voltage Windows and Capacitance for Aqueous Supercapacitors. Energy Environ. Mater. 2023, 7, e12658. [Google Scholar] [CrossRef]
- Wang, X.; Chandrabose, R.S.; Chun, S.; Zhang, T.; Evanko, B.; Jian, Z.; Boettcher, S.W.; Stucky, G.; Ji, X. High Energy Density Aqueous Electrochemical Capacitors with a KI-KOH Electrolyte. ACS Appl. Mater. Interfaces 2015, 7, 19978–19985. [Google Scholar] [CrossRef]
- Sandhiya, M.; Suresh Balaji, S.; Sathish, M. Boosting the Energy Density of Flexible Supercapacitors by Redox-Additive Hydrogels. Energy Fuels 2020, 34, 11536–11546. [Google Scholar] [CrossRef]
- Chan Hyun, J.; Kwak, J.H.; Yun, Y.S. Microporous waste charcoals for redox-mediated supercapacitors. J. Ind. Eng. Chem. 2019, 79, 204–209. [Google Scholar] [CrossRef]
- Lyu, D.; Märker, K.; Zhou, Y.; Zhao, E.W.; Gunnarsdóttir, A.B.; Niblett, S.P.; Forse, A.C.; Grey, C.P. Understanding Sorption of Aqueous Electrolytes in Porous Carbon by NMR Spectroscopy. J. Am. Chem. Soc. 2024, 146, 9897–9910. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P. Renewable Biopolymers Combined with Ionic Liquids for the Next Generation of Supercapacitor Materials. Int. J. Mol. Sci. 2023, 24, 7866. [Google Scholar] [CrossRef]
- Kumar, A.; Mahanty, B.N.; Rawat, A.; Muhammad, R.; Panigrahi, R.K.; Pradhan, D.; Mohanty, P. Transition-Metal-Substituted Nanoporous Manganese Ferrites Mn0.95M0.05Fe2O4 (M: Co, Cu, and Zn) as Electrode Materials for High-Performance Supercapacitors in Redox-Active Nonaqueous Electrolytes. Energy Fuels 2023, 37, 6810–6823. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, X.; Miao, X.; Liu, Y.; Chen, S.; Chen, Y.; Cheng, J.; Wang, W.; Zhang, Y. A phenylenediamine-mediated organic electrolyte for high performance graphene-hydrogel based supercapacitors. Electrochim. Acta 2018, 273, 495–501. [Google Scholar] [CrossRef]
- Shakil, R.; Shaikh, M.N.; Shah, S.S.; Reaz, A.H.; Roy, C.K.; Chowdhury, A.N.; Aziz, M.A. Development of a Novel Bio-based Redox Electrolyte using Pivalic Acid and Ascorbic Acid for the Activated Carbon-based Supercapacitor Fabrication. Asian J. Org. Chem. 2021, 10, 2220–2230. [Google Scholar] [CrossRef]
- Wang, Z.-F.; Yi, Z.; Yu, S.-C.; Fan, Y.-F.; Li, J.; Xie, L.; Zhang, S.-C.; Su, F.; Chen, C.-M. High-Voltage Redox Mediator of an Organic Electrolyte for Supercapacitors by Lewis Base Electrocatalysis. ACS Appl. Mater. Interfaces 2022, 14, 24497–24508. [Google Scholar] [CrossRef]
- Saha, M.; Kumar, A.; Kanaoujiya, R.; Behera, K.; Trivedi, S. A Comprehensive Review of Novel Emerging Electrolytes for Supercapacitors: Aqueous and Organic Electrolytes Versus Ionic Liquid-Based Electrolytes. Energy Fuels 2024, 38, 8528–8552. [Google Scholar] [CrossRef]
- Tang, X.; Xiao, D.; Xu, Z.; Liu, Q.; Ding, B.; Dou, H.; Zhang, X. A novel ionic liquid-based electrolyte assisting the high performance of low-temperature supercapacitors. J. Mater. Chem. A 2022, 10, 18374–18382. [Google Scholar] [CrossRef]
- Geng, C.; Fan, L.; Wang, C.; Wang, Y.; Sun, S.; Song, Z.; Liu, N.; Wu, J. High energy density and high working voltage of a quasi-solid-state supercapacitor with a redox-active ionic liquid added gel polymer electrolyte. New J. Chem. 2019, 43, 18935–18942. [Google Scholar] [CrossRef]
- Sun, L.; Zhuo, K.; Chen, Y.; Du, Q.; Zhang, S.; Wang, J. Ionic Liquid-Based Redox Active Electrolytes for Supercapacitors. Adv. Funct. Mater. 2022, 32, 2203611. [Google Scholar] [CrossRef]
- Navalpotro, P.; Palma, J.; Anderson, M.; Marcilla, R. High performance hybrid supercapacitors by using para-Benzoquinone ionic liquid redox electrolyte. J. Power Sources 2016, 306, 711–717. [Google Scholar] [CrossRef]
- Ma, N.; Kosasang, S.; Phattharasupakun, N.; Sawangphruk, M. Addition of Redox Additive to Ionic Liquid Electrolyte for High-Performance Electrochemical Capacitors of N-Doped Graphene Aerogel. J. Electrochem. Soc. 2019, 166, A695–A703. [Google Scholar] [CrossRef]
- Xie, H.J.; Gélinas, B.; Rochefort, D. Redox-active electrolyte supercapacitors using electroactive ionic liquids. Electrochem. Commun. 2016, 66, 42–45. [Google Scholar] [CrossRef]
- Poochai, C.; Sriprachuabwong, C.; Srisamrarn, N.; Chuminjak, Y.; Lomas, T.; Wisitsoraat, A.; Tuantranont, A. High performance coin-cell and pouch-cell supercapacitors based on nitrogen-doped reduced graphene oxide electrodes with phenylenediamine-mediated organic electrolyte. Appl. Surf. Sci. 2019, 489, 989–1001. [Google Scholar] [CrossRef]
- Cevher, D.; Cevher, S.C.; Cirpan, A. Gel electrolyte modification on D-A-D type conjugated polymer based supercapacitor. J. Energy Storage 2023, 62, 106962. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Chen, S.; Liu, X.; Liu, J.; He, W.; Liu, X. Highly Conductive Supramolecular Salt Gel Electrolyte for Flexible Supercapacitors. ACS Appl. Mater. Interfaces 2024, 16, 56170–56180. [Google Scholar] [CrossRef]
- Lee, H.; Gong, K.; Kang, H.; Jung, G.; Kim, J.Y.; Keum, K.; Kim, D.S.; Kim, S.; Kim, J.W.; Ha, J.S. A flexible, high-energy density, and temperature-tolerant asymmetric supercapacitor based on water-in-salt gel electrolyte. J. Alloys Compd. 2023, 960, 170714. [Google Scholar] [CrossRef]
- Mitta, S.B.; Harpalsinh, R.; Kim, J.; Park, H.S.; Um, S.H. Flexible Supercapacitor with a Pure DNA Gel Electrolyte. Adv. Mater. Interfaces 2022, 9, 2200133. [Google Scholar] [CrossRef]
- Shuaibu, A.D.; Shah, S.S.; Alzahrani, A.S.; Aziz, M.A. Development and assessment of an innovative gel electrolyte using polyvinyl alcohol, lithium sulfate, and 1-butyl-3-methylimidazolium trifluoromethanesulfonate for advanced supercapacitor performance. J. Energy Storage 2024, 92, 112040. [Google Scholar] [CrossRef]
- Subrahmanya, S.V.; Yethadka, S.N.; Nagaraja, G.K. Electronic Booster PEDOT:PSS-Enriched Guar Gum as Eco-Friendly Gel Electrolyte for Supercapacitor. ACS Omega 2024, 9, 24610–24615. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Liu, X.; Zhang, Q.; Liu, X. Post-tunable salt gel electrolytes toward flexible supercapacitor switches. J. Energy Chem. 2025, 100, 114–124. [Google Scholar] [CrossRef]
- Zhao, Z.; Rong, Y.; Cui, P.; Qin, G.; Wang, H.; Huang, X. High elongation, low hysteresis, fatigue resistant gel electrolyte for supercapacitor and strain sensor. J. Power Sources 2024, 622, 235307. [Google Scholar] [CrossRef]
- Park, Y.; Choi, H.; Lee, D.-G.; Kim, M.-C.; Tran, N.A.T.; Cho, Y.; Lee, Y.-W.; Sohn, J.I. Rational Design of Electrochemical Iodine-Based Redox Mediators for Water-Proofed Flexible Fiber Supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 2409–2415. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, N.; Hashmi, S.A. Ionic liquid incorporated, redox-active blend polymer electrolyte for high energy density quasi-solid-state carbon supercapacitor. J. Power Sources 2020, 451, 227771. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, N.; Hashmi, S.A. High-Energy-Density Carbon Supercapacitors Incorporating a Plastic-Crystal-Based Nonaqueous Redox-Active Gel Polymer Electrolyte. ACS Appl. Energy Mater. 2021, 4, 6635–6649. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, N.; Singh, M.K.; Hashmi, S.A. Nonaqueous, Redox-Active Gel Polymer Electrolyte for High-Performance Supercapacitor. Energy Technol. 2019, 7, 1900132. [Google Scholar] [CrossRef]
- Park, J.; Yoo, Y.-E.; Mai, L.; Kim, W. Rational Design of a Redox-Active Nonaqueous Electrolyte for a High-Energy-Density Supercapacitor Based on Carbon Nanotubes. ACS Sustain. Chem. Eng. 2019, 7, 7728–7735. [Google Scholar] [CrossRef]
- Tu, Q.-M.; Fan, L.-Q.; Pan, F.; Huang, J.-L.; Gu, Y.; Lin, J.-M.; Huang, M.-L.; Huang, Y.-F.; Wu, J.-H. Design of a novel redox-active gel polymer electrolyte with a dual-role ionic liquid for flexible supercapacitors. Electrochim. Acta 2018, 268, 562–568. [Google Scholar] [CrossRef]


| Electrode Materials | Electrolytes | Redox Additives | Voltage of Cell (V) | Specific Capacitance | Energy Density | Ref. |
|---|---|---|---|---|---|---|
| Carbon fibers | KOH | KI | 1.6 | 251 F g−1 | 7.1 W h kg−1 | [76] |
| Carbon nanotube | Na2SO4 | KBr | 1.5 | 92.12 F g−1 | 28.3 W h kg−1 | [66] |
| S doped graphene | H2SO4 | HQ | 1.6 | 300 F g−1 | 27 W h kg−1 | [77] |
| Nanoporous carbon | Li2SO4 | KI, Na2MoO4 | 1.0 | 470 F g−1 | 65.3 W h kg−1 | [67] |
| Porous carbon | H2SO4 | 5,6-dihydroxyindole /5,6-quinoneindole | 1.4 | 205 F g−1 | 8.8 W h kg−1 | [73] |
| Waste charcoal carbon | KOH | PPD | 1.0 | 512 F g−1 | -- | [78] |
| Additive Type | Advantages | Disadvantages |
|---|---|---|
| Inorganic Redox Additives | 1. High stability, maintaining stable performance over long periods. 2. Fast redox kinetics, enabling rapid charge and discharge. 3. Relatively low cost for some additives. | 1. Fixed redox potential, with poor adjustability. 2. Moderate solubility, and high concentrations may cause saturation and precipitation, affecting performance. 3. Potential poor compatibility with electrode materials, leading to parasitic reactions and reduced capacitor efficiency. |
| Organic Redox Additives | 1. Flexible molecular design, allowing for the adjustment of structures to achieve specific redox potentials. 2. High solubility, enabling better dispersion in aqueous electrolytes. 3. Good interaction with electrode materials, which can be further enhanced through surface functionalization. | 1. Relatively poor stability, prone to chemical degradation during long-term cycling. 2. High cost for some organic additives. 3. Parasitic side reactions may occur, reducing energy efficiency and cycle life. |
| Electrode Materials | Electrolytes | Redox Additives | Voltage of Cell (V) | Specific Capacitance | Energy Density | Ref. |
|---|---|---|---|---|---|---|
| Mn0.95Zn0.05Fe2O4 | LiClO4/PC | KI | 2.8 | 829 F g−1 | 77.5 W h kg−1 | [81] |
| N-doped activated carbon | SBPBF4/PC | TCHQ | 2.7 | 140 F g−1 | 35.7 W h kg−1 | [84] |
| Reduced graphene oxide | TEABF4/ACN | PPD | 3.0 | 340 F g−1 | 77.2 W h kg−1 | [92] |
| Pica carbon | PYR14TSFSI | p-BQ | 3.0 | 156 F g−1 | 30 W h kg−1 | [89] |
| Activated carbon | LiTFSI/ACN | EV | 2.3 | 73 mA h g−1 | 34 W h kg−1 | [59] |
| Activated carbon | [FcEIm][NT]/ACN | Ferrocene | 2.5 | -- | 13.2 W h kg−1 | [91] |
| N-doped reduced graphene oxide aerogel | [BMP][DCA] | Ferrocene methanol | 3.0 | 112.1 F g−1 | 34.2 W h kg−1 | [90] |
| Electrode Materials | Electrolytes | Redox Additives | Voltage of Cell (V) | Capacitance | Energy Density | Ref. |
|---|---|---|---|---|---|---|
| Carbon fiber | H3PO4/KI/PVA | KI | 1.0 | 461.8 F L−1 | 64.14 mW h L−1 | [101] |
| Activated carbon | PVA/PVP/EMIHSO4 | HQ | 1.2 | 485 F g−1 | 24.7 W h kg−1 | [102] |
| Activated carbon | PVDF-HFP/BMITFSI | NaI | 1.5 | 334 F g−1 | 26.1 W h kg−1 | [104] |
| Activated carbon | PVDF-HFP/SN/BMPTFSI | HQ | 2.0 | 289 F g−1 | 40 W h kg−1 | [103] |
| Carbon nanotubes | EMImTFSI/ADN | DmCc/DmCcPF6 | 3.1 | 57.1 F g−1 | 75.6 W h kg−1 | [105] |
| Activated carbon | PVA/Li2SO4 | BMIMI | 1.5 | 384.1 F g−1 | 29.3 W h kg−1 | [106] |
| Electrolyte System | Characteristics | Ionic Conductivity | Voltage Window | Cost | Safety | Application Scenarios |
|---|---|---|---|---|---|---|
| Aqueous Electrolytes | High ionic conductivity; Environmentally friendly; Low cost | High | Narrow | Low | High | Large-scale energy storage, cost-sensitive applications |
| Non-aqueous Electrolytes | Wide voltage window; High energy density; High-voltage operation | Moderate | Wide | High | Medium | High-performance energy storage devices |
| Solid-state/gel Electrolytes | High safety; Good mechanical stability; Flexibility | Low | Wide | Medium | High | Wearable devices, flexible electronics |
| Additive Type | Characteristics | Redox Potential | Stability | Solubility | Common Additives |
|---|---|---|---|---|---|
| Inorganic Additives | High stability; Fast redox kinetics | Fixed potential | High | Moderate | K3Fe(CN)6, NH4VO3, Na2MoO4, KI, FeSO4•(NH4)2SO4•6H2O, TiO2, etc. |
| Organic Additives | Flexible molecular design; High solubility | Tunable potential | Moderate | High | HQ, TEMPO, TCHQ, OPD, MPD, PPD, etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, L.; Guo, L.; Yao, H.; Cai, J.; Dong, X.; Wang, R.; Zhai, Z.; Chen, X.; Wei, X.; Li, D.; et al. Redox Additive Electrolytes for Supercapacitors: A Mini-Review on Recent Developments and Future Directions. Molecules 2025, 30, 1764. https://doi.org/10.3390/molecules30081764
Guan L, Guo L, Yao H, Cai J, Dong X, Wang R, Zhai Z, Chen X, Wei X, Li D, et al. Redox Additive Electrolytes for Supercapacitors: A Mini-Review on Recent Developments and Future Directions. Molecules. 2025; 30(8):1764. https://doi.org/10.3390/molecules30081764
Chicago/Turabian StyleGuan, Lu, Liangliang Guo, Haiyuan Yao, Jun Cai, Xuewei Dong, Ruonan Wang, Zhihua Zhai, Xuan Chen, Xiuzhi Wei, Dajin Li, and et al. 2025. "Redox Additive Electrolytes for Supercapacitors: A Mini-Review on Recent Developments and Future Directions" Molecules 30, no. 8: 1764. https://doi.org/10.3390/molecules30081764
APA StyleGuan, L., Guo, L., Yao, H., Cai, J., Dong, X., Wang, R., Zhai, Z., Chen, X., Wei, X., Li, D., Liu, X., Ji, S., & Meng, F. (2025). Redox Additive Electrolytes for Supercapacitors: A Mini-Review on Recent Developments and Future Directions. Molecules, 30(8), 1764. https://doi.org/10.3390/molecules30081764







