Emerging Mycotoxins in Cheese: Simultaneous Analysis of Aflatoxin M1, Aflatoxicol, and Sterigmatocystin by LC-MS/MS
Abstract
:1. Introduction
2. Results and Discussion
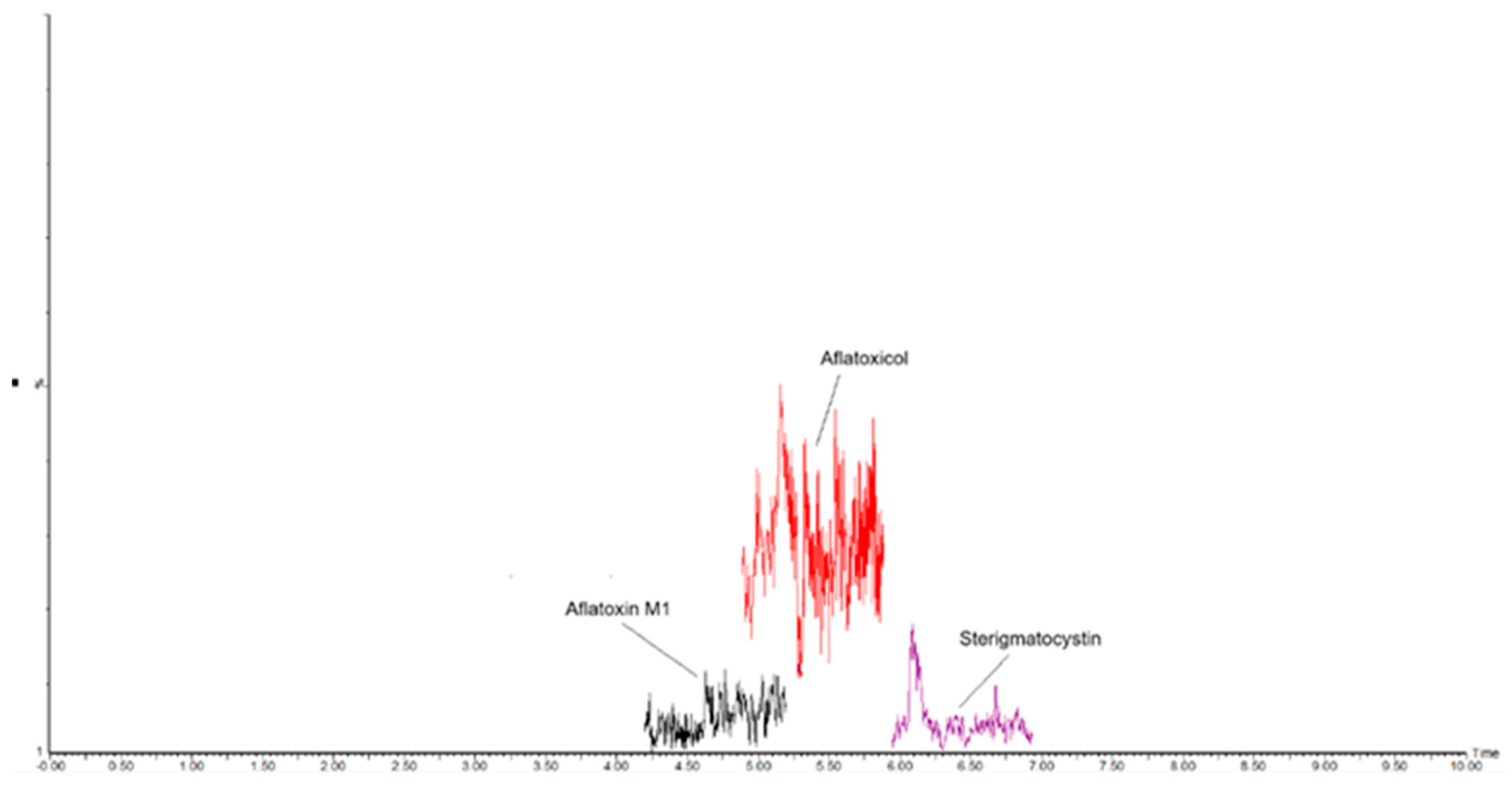
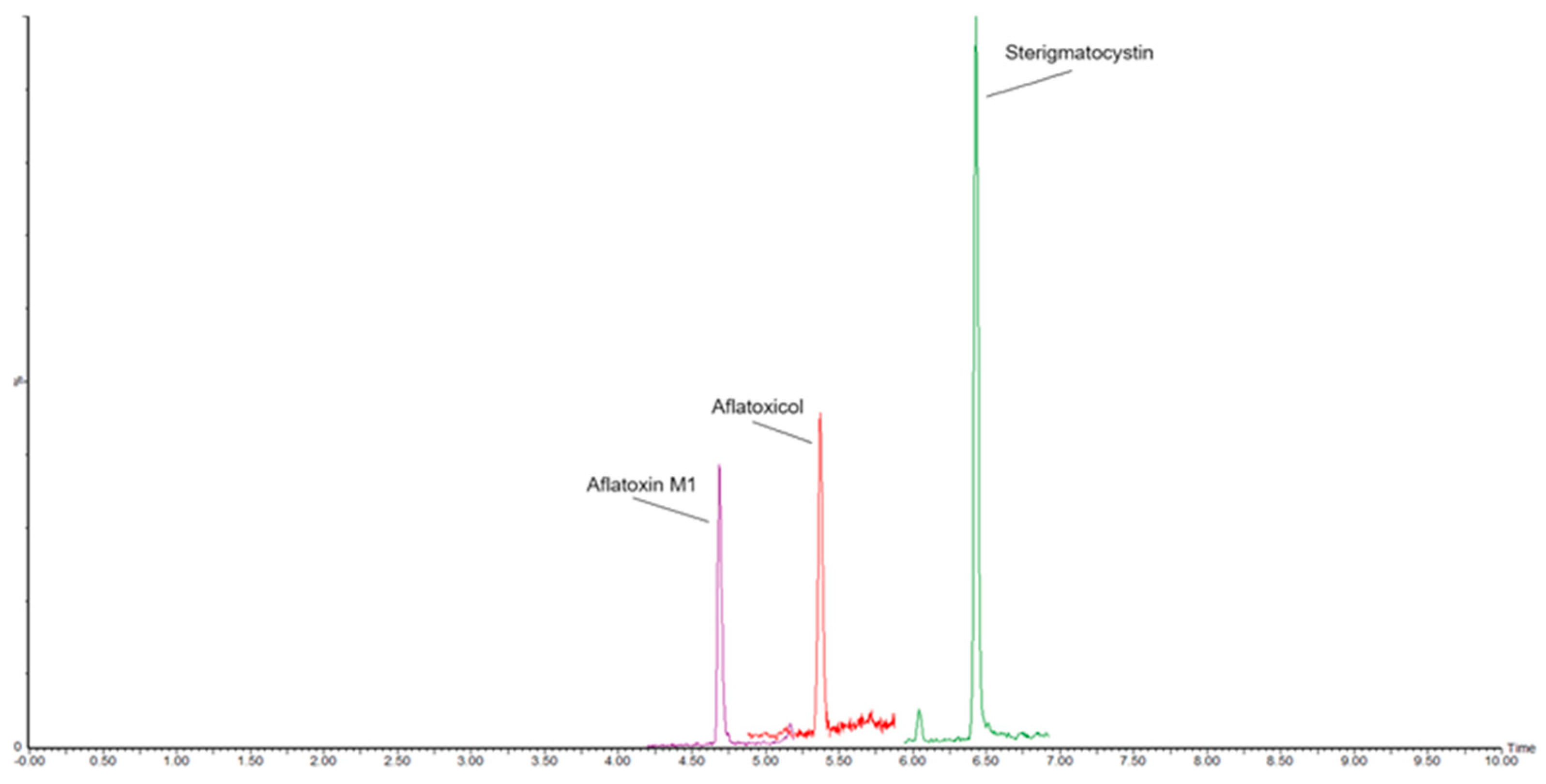
Preliminary Results of Cheese Samples Analyzed
3. Materials and Methods
3.1. Samples
3.2. Chemicals and Reagents
3.3. Working Standard Solutions
3.4. Sample Preparation and Analysis
3.5. Optimization of the Extraction and Clean-Up Steps
3.6. Instrumentation LC-MS/MS
3.7. Method Performance Parameters
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFL | Aflatoxicol |
| AFB1 | Aflatoxin B1 |
| AFM1 | Aflatoxin M1 |
| BCC | Back-calculated concentrations |
| CRM | Certified Reference Material |
| EFSA | European Food Safety Agency |
| EU | European Union |
| ILIS | Isotopically labeled internal standard |
| HPLC | High-performance liquid chromatography |
| IAC | Immunoaffinity column |
| IR | Ion ratio |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantitation |
| MIP | Molecularly Imprinted Polymers |
| MRM | Multiple reaction monitoring |
| PBS | Phosphate-Buffered Saline |
| RM | Reference Material |
| RSDr | Relative standard deviation under repeatability conditions |
| RT | Retention time |
| SPE | Solid-Phase Extraction |
| STC | Sterigmatocystin |
References
- Sengun, I.; Yaman, D.; Gonul, S. Mycotoxins and Mould Contamination in Cheese: A Review. World Mycotoxin J. 2008, 1, 291–298. [Google Scholar] [CrossRef]
- Hymery, N.; Vasseur, V.; Coton, M.; Mounier, J.; Jany, J.L.; Barbier, G.; Coton, E. Filamentous fungi and mycotoxins in cheese: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.J.; Dobson, D.W. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Lafont, P.; Siriwardana, M.G.; DeBoer, E. Contamination of dairy products by fungal metabolites. J. Environ. Pathol. Toxicol. 1990, 10, 99–102. [Google Scholar]
- Carvajal, M.; Rojo, F.; Méndez, I.; Bolaños, A. Aflatoxin B1 and its interconverting metabolite aflatoxicol in milk: The situation in Mexico. Food Addit. Contam. 2003, 20, 1077–1086. [Google Scholar] [CrossRef]
- Creppy, E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef]
- Sdogati, S.; Pacini, T.; Bibi, R.; Caporali, A.; Verdini, E.; Orsini, S.; Ortenzi, R.; Pecorelli, I. Co-Occurrence of Aflatoxin B1, Zearalenone and Ochratoxin A in Feed and Feed Materials in Central Italy from 2018 to 2022. Foods 2024, 13, 313. [Google Scholar] [CrossRef]
- Iha, M.H.; Barbosa, C.B.; Okada, I.A.; Trucksess, M.W. Aflatoxin M1 in milk and distribution and stability of aflatoxin M1 during production and storage of yoghurt and cheese. Food Control 2013, 29, 1–6. [Google Scholar] [CrossRef]
- Salhab, A.S.; Edwards, G.S. Production of aflatoxicol from aflatoxin B1 by postmitochondrial liver fractions. J. Toxicol. Environ. Health 1977, 2, 583–587. [Google Scholar] [CrossRef]
- Theumer, M.G.; Henneb, Y.; Khoury, L.; Snini, S.P.; Tadrist, S.; Canlet, C.; Puel, O.; Oswald, I.P.; Audebert, M. Genotoxicity of aflatoxins and their precursors in human cells. Toxicol. Lett. 2018, 287, 100–107. [Google Scholar] [CrossRef]
- Carvajal-Moreno, M.; Vargas-Ortiz, M.; Hernández-Camarillo, E.; Ruiz-Velasco, S.; Rojo-Callejas, F. Presence of unreported carcinogens, aflatoxins and their hydroxylated metabolites, in industrialized Oaxaca cheese from Mexico City. Food Chem. Toxicol. 2019, 124, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.H.D.; Granero, A.M.; Zon, M.A.; Fernández, H. Sterigmatocystin: A Mycotoxin to Be Seriously Considered. Food Chem. Toxicol. 2018, 118, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Pietri, A.; Leni, G.; Mulazzi, A.; Bertuzzi, T. Ochratoxin A and sterigmatocystin in long-ripened Grana cheese: Occurrence, wheel rind contamination and effectiveness of cleaning techniques on grated products. Toxins 2022, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry of Health. Micotossine non Regolamentate: Metaboliti Dell’aflatossina B1 (Aflatossina M1 e Aflatossicolo) e Sterigmatocistina in Prodotti Lattiero-Caseari. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3065_allegato.pdf (accessed on 24 February 2025).
- Iha, M.H.; Barbosa, C.B. Rosa Maria Duarte Favaro Chromatographic Method for the Determination of Aflatoxin M1 in Cheese, Yogurt, and Dairy Beverages. J. AOAC Int. 2011, 94, 1513–1518. [Google Scholar] [CrossRef]
- Hird, S.; Martin, K.; Collette Zabe, N.; Toth, D. Determination of Aflatoxins in a Wide Range of Food and Agricultural Commodities Using Immunoaffinity Chromatography Column Clean-Up Coupled with UPLC or HPLC with Fluorescence Detection; Application Notes Waters Corporation; VICAM: Rydalmere, NSW, Australia, 2021. [Google Scholar]
- Pietri, A.; Fortunati, P.; Mulazzi, A.; Bertuzzi, T. Enzyme-assisted extraction for the HPLC determination of aflatoxin M1 in cheese. Food Chem. 2016, 192, 235–241. [Google Scholar] [CrossRef]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef]
- Veršilovskis, A.; Van Peteghem, C.; de Saeger, S. Determination of sterigmatocystin in cheese by high-performance liquid chromatography-tandem mass spectrometry. Food Addit. Contam. A 2009, 26, 127–133. [Google Scholar] [CrossRef]
- Regal, P.; Díaz-Bao, M.; Barreiro, R.; Cepeda, A.; Fente, C. Application of molecularly imprinted polymers in food analysis: Clean-up and chromatographic improvements. Cent. Eur. J. Chem. 2012, 10, 766–784. [Google Scholar] [CrossRef]
- Ashley, J.; Shahbazi, M.-A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens. Bioelectron. 2017, 91, 606–615. [Google Scholar] [CrossRef]
- Vaz, A.; Cabral Silva, A.C.; Rodrigues, P.; Venâncio, A. Detection Methods for Aflatoxin M1 in Dairy Products. Microorganisms 2020, 8, 246. [Google Scholar] [CrossRef]
- González-jartín, J.M.; Rodríguez-Canás, I.; Alfonso, A.; Sainz, M.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Multianalyte Method for the Determination of Regulated, Emerging and Modified Mycotoxins in Milk: QuEChERS Extraction Followed by UHPLC—MS/MS Analysis Es Rodríguez-Ca N. Food Chem. 2021, 356, 129647. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.E.; Gill, B.D.; Indyk, H.E.; Rhemrev, R.; Pazdanska, M.; Mackay, N.; Marley, E. Determination of Aflatoxin M1 in Liquid Milk, Cheese, and Selected Milk Proteins by Automated Online Immunoaffinity Cleanup with Liquid Chromatography—Fluorescence Detection. J. AOAC Int. 2021, 104, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, J.; Huang, X.; Jin, Q.; Zhu, G. Determination of Sterigmatocystin in Infant Cereals from Hangzhou, China. J. AOAC Int. 2016, 99, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.D.W. Mycotoxins in cheese. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P., Everett, D.W., Eds.; Elsevier: New York, NY, USA, 2017; pp. 595–601. [Google Scholar]
- European Commission. Regulation (EC) 2023/915 of 25 April 2023. Official Journal of the European Union L 119/103 2023. pp. 103–157. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 17 January 2025).
- European Commission. Regulation No. 2023/2782 of 14 December 2023 Laying Down the Methods of Sampling and Analysis for the Control of the Levels of Mycotoxins in Food and Repealing EC Regulation No. 401/2006. Official Journal of the European Union. 2023, pp. 1–44. Available online: https://data.europa.eu/eli/reg_impl/2023/2782/oj (accessed on 15 January 2025).
- Rodríguez-Cañás, I.; González-Jartín, J.M.; Alvariño, R.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Detection of mycotoxins in cheese using an optimized analytical method based on a QuEChERS extraction and UHPLC-MS/MS quantification. Food Chem. 2023, 408, 135182. [Google Scholar] [CrossRef]
- Jakšić, S.; Živkov Baloš, M.; Popov, N.; Torović, L.; Krstović, S. Optimisation, validation and comparison of methods for aflatoxin M1 determination in cheese. Int. J. Dairy Technol. 2021, 74, 681–688. [Google Scholar] [CrossRef]
- Braun, D.; Eiser, M.; Puntscher, H.; Marko, D.; Warth, B. Natural contaminants in infant food: The case of regulated and emerging mycotoxins. Food Control 2021, 123, 107676. [Google Scholar] [CrossRef]
- Akinbami, L.J.; Chen, T.-C.; Davy, O.; Ogden, C.L.; Fink, S.; Clark, J.; Riddles, M.K.; Mohadjer, L.K. National Health and Nutrition Examination Survey, 2017–March 2020 Prepandemic File: Sample Design, Estimation, and Analytic Guidelines. 2(190). 2022. Available online: https://stacks.cdc.gov/view/cdc/115434 (accessed on 24 February 2025).
- CLAL. Per Capita Cheese Consumption—Year 2023. Available online: https://www.clal.it/en/?section=tabs_consumi_procapite#C (accessed on 24 February 2025).
- European Commission. Regulation (EU) 2022/2379 of the European Parliament and of the Council of 23 November 2022 on Statistics on Agricultural Input and Output, Amending Commission Regulation (EC) No 617/2008 and Repealing Regulations (EC) No 1165/2008, (EC) No 543/2009 and (EC) No 1185/2009 of the European Parliament and of the Council and Council Directive 96/16/EC. Available online: https://eur-lex.europa.eu/eli/reg/2022/2379/oj/eng (accessed on 7 April 2025).
- European Commission. Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on Official Controls and Other Official Activities Performed to Ensure the Application of Food and Feed Law, Rules on Animal Health and Welfare, Plant Health and Plant Protection Products, Amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and Repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation). Official Journal of the European Union. 2017. L95/1. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/?uri=CELEX%3A32017R0625 (accessed on 15 January 2025).
- European Commission. Directorate-General for Health and Food Safety (DG SANTE). Guidance Document SANTE 11312/2021 v2—Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Version 2 Implemented by 01/01/2024. Available online: https://food.ec.europa.eu/document/download/d4786faf-c574-4222-a5c6-45086b3920b8_en?filename=pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 24 February 2025).

| Recovery % | RSDr % | LOD (ng/kg) | LOQ (ng/kg) | Approximate RT (min) | ||
|---|---|---|---|---|---|---|
| Pietri et al., 2016 [17] | AFM1 | 87.1–96.3 | 1.4–2.3 | 5–20 | 15–60 | 4.9 |
| STC | - | - | - | - | - | |
| AFL | - | - | - | - | - | |
| Iha et al., 2011 [15] | AFM1 | 80 | 5.9 | 3 | 10 | 7.0 |
| STC | - | - | - | - | - | |
| AFL | - | - | - | - | - | |
| Rodríguez-Cañàs et al., 2023 [29] | AFM1 | 79.6–101.1 | 6.63–13.20 | 6–11 | 20–36 * | 5.9 |
| STC | 73.9–104.3 | 1.03–5.25 | 178–1005 | 588–3316 * | 8.4 | |
| AFL | - | - | - | - | - | |
| Carvajal-Moreno et al., 2019 [5] | AFM1 | 95 | - | 10 | - | 8.5–9.5 |
| STC | - | - | - | - | - | |
| AFL | 98 | - | 10 | - | 3.0–5.6 | |
| Jakšić et al., 2021 [30] | AFM1 | 84–112 | 8–18 | 10 | 30 | 5.7 |
| STC | - | - | - | - | - | |
| AFL | - | - | - | - | - | |
| Veršilovskis et al., 2009 [19] | AFM1 | - | - | - | - | - |
| STC | 96–104 | 17–27 | 30 | 100 | 7.7 | |
| AFL | - | - | - | - | - | |
| Current Study(This work) | AFM1 | 95 | 2.0–3.2 | 0.6 | 2.0 | 4.7 |
| STC | 98–103 | 2.1–2.7 | 0.2 | 1.0 | 6.4 | |
| AFL | 96–97 | 2.7–4.9 | 1.0 | 5.0 | 5.4 |
| Composition Parameter | Soft (MFFB% ≥ 62) | Semi-Hard (62 > MFFB% > 55) | Ripened-Hard (MFFB% ≤ 55) |
|---|---|---|---|
| Protein % | 15–19 | 29 | 29–34 |
| Fat % | 20–40 | 40 | 31–34 |
| Dry matter % | >30 | >70 | >75 |
| Mycotoxin | Linearity Range (ng/kg) | Calibration Curve Equation | R2 | LOD (ng/kg) | LOQ (ng/kg) |
|---|---|---|---|---|---|
| AFM1 | 2.0–200 | y = 2.05x − 0.38 | 0.9986 | 0.6 | 2.0 |
| STC | 1.0–100 | y = 1.26x − 0.15 | 0.9999 | 0.2 | 1.0 |
| AFL | 5.0–2000 | y = 136.4x − 135.9 * | 0.9982 | 1.0 | 5.0 |
| Mycotoxin | Replicates (n) | Concentration Range (ng/kg) | Performance Evaluation Parameters | |
|---|---|---|---|---|
| Recovery % | RSDr % | |||
| AFM1 | 6 | 25 | 95 | 2.0 |
| 5 | 50 | 95 | 3.2 | |
| STC | 6 | 25 | 103 | 2.1 |
| 5 | 50 | 98 | 2.7 | |
| AFL | 5 | 50 | 97 | 2.7 |
| 5 | 500 | 96 | 4.9 | |
| Certified Reference Material | AFM1 Concentration (ng/kg) | Replicates (n) | Recovery % | RSDr % |
|---|---|---|---|---|
| ERM BD 284—Milk powder | 440 | 10 | 101 | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cossu, M.; Sanna, A.; Mangano, G.; Ledda, G.; Chessa, G.; Gallo, P.; Vella, A.; Pecorelli, I.; Sdogati, S.; Gili, M.; et al. Emerging Mycotoxins in Cheese: Simultaneous Analysis of Aflatoxin M1, Aflatoxicol, and Sterigmatocystin by LC-MS/MS. Molecules 2025, 30, 1774. https://doi.org/10.3390/molecules30081774
Cossu M, Sanna A, Mangano G, Ledda G, Chessa G, Gallo P, Vella A, Pecorelli I, Sdogati S, Gili M, et al. Emerging Mycotoxins in Cheese: Simultaneous Analysis of Aflatoxin M1, Aflatoxicol, and Sterigmatocystin by LC-MS/MS. Molecules. 2025; 30(8):1774. https://doi.org/10.3390/molecules30081774
Chicago/Turabian StyleCossu, Maurizio, Andrea Sanna, Giuseppe Mangano, Giuseppe Ledda, Giannina Chessa, Pasquale Gallo, Antonio Vella, Ivan Pecorelli, Stefano Sdogati, Marilena Gili, and et al. 2025. "Emerging Mycotoxins in Cheese: Simultaneous Analysis of Aflatoxin M1, Aflatoxicol, and Sterigmatocystin by LC-MS/MS" Molecules 30, no. 8: 1774. https://doi.org/10.3390/molecules30081774
APA StyleCossu, M., Sanna, A., Mangano, G., Ledda, G., Chessa, G., Gallo, P., Vella, A., Pecorelli, I., Sdogati, S., Gili, M., & Boselli, C. (2025). Emerging Mycotoxins in Cheese: Simultaneous Analysis of Aflatoxin M1, Aflatoxicol, and Sterigmatocystin by LC-MS/MS. Molecules, 30(8), 1774. https://doi.org/10.3390/molecules30081774







