Modulation of Tumor Metabolism in Acute Leukemia by Plant-Derived Polymolecular Drugs and Their Effects on Mitochondrial Function
Abstract
1. Introduction
2. Results
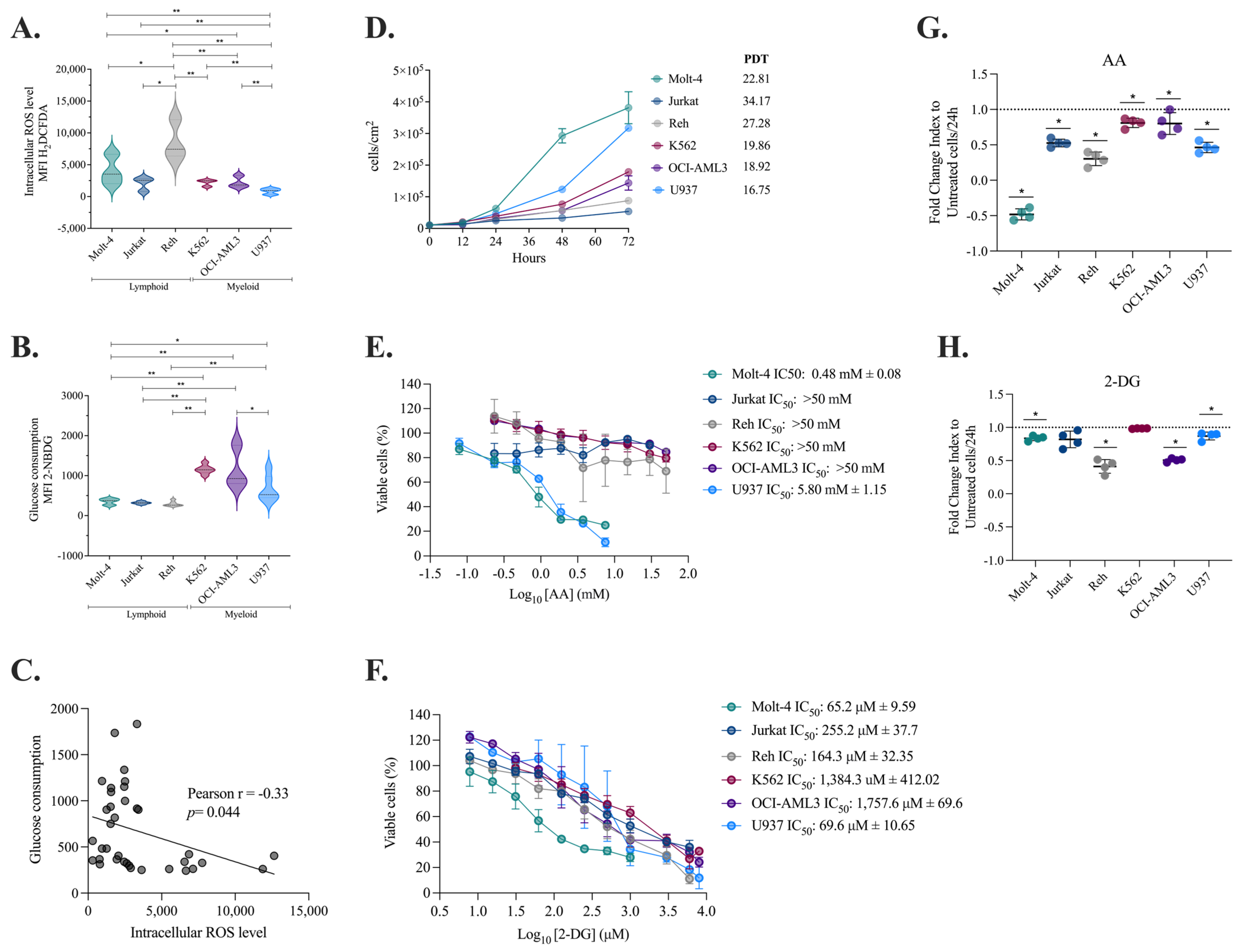
2.1. Myeloid Cells Produce Fewer ROS, Consume More Glucose, and Duplicate at a Faster Rate than Lymphoid Cells
2.2. Decreased Intracellular ROS Levels Affect the Viability and Proliferation of Myeloid and Lymphoid Leukemia Cells
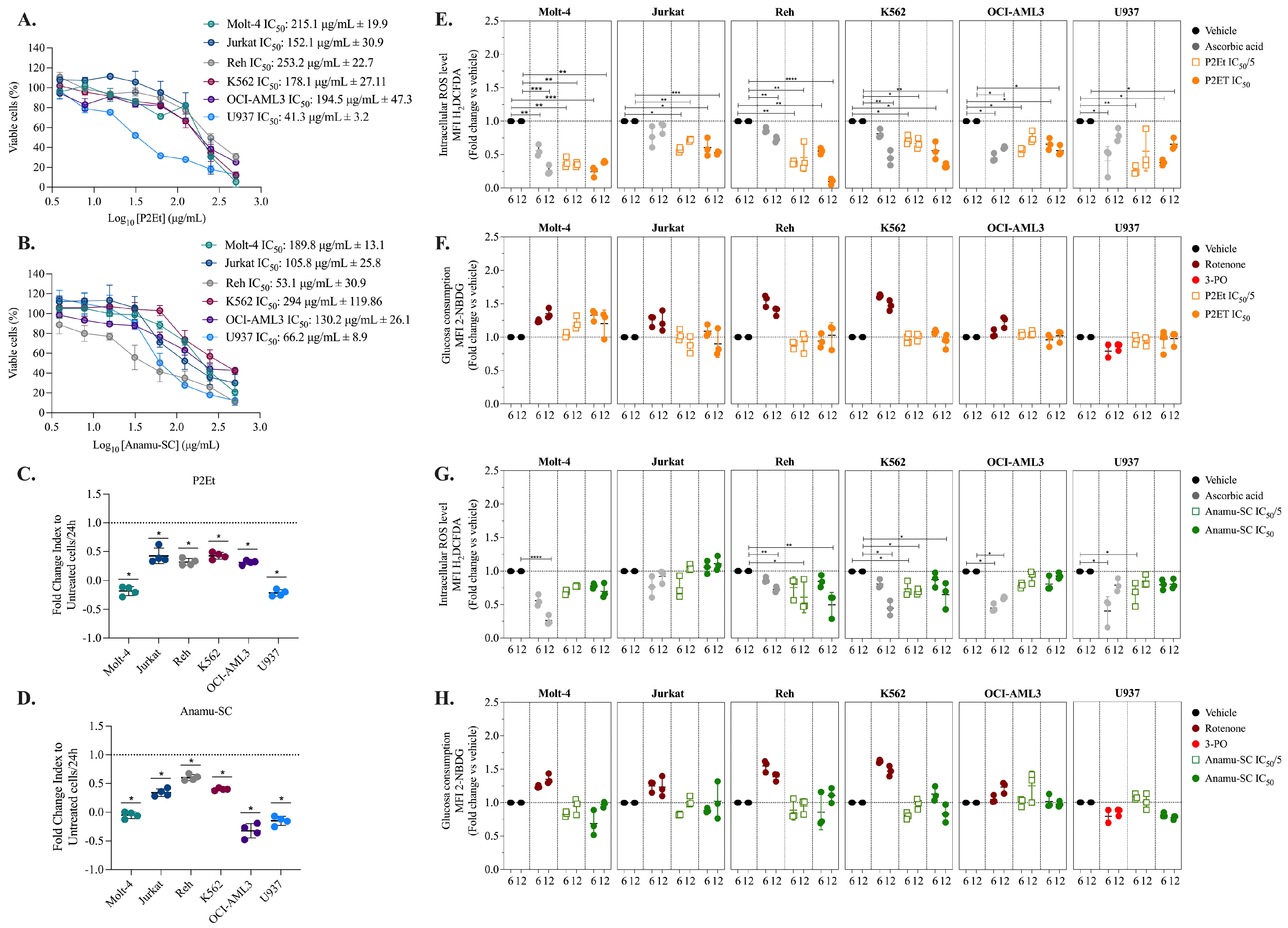
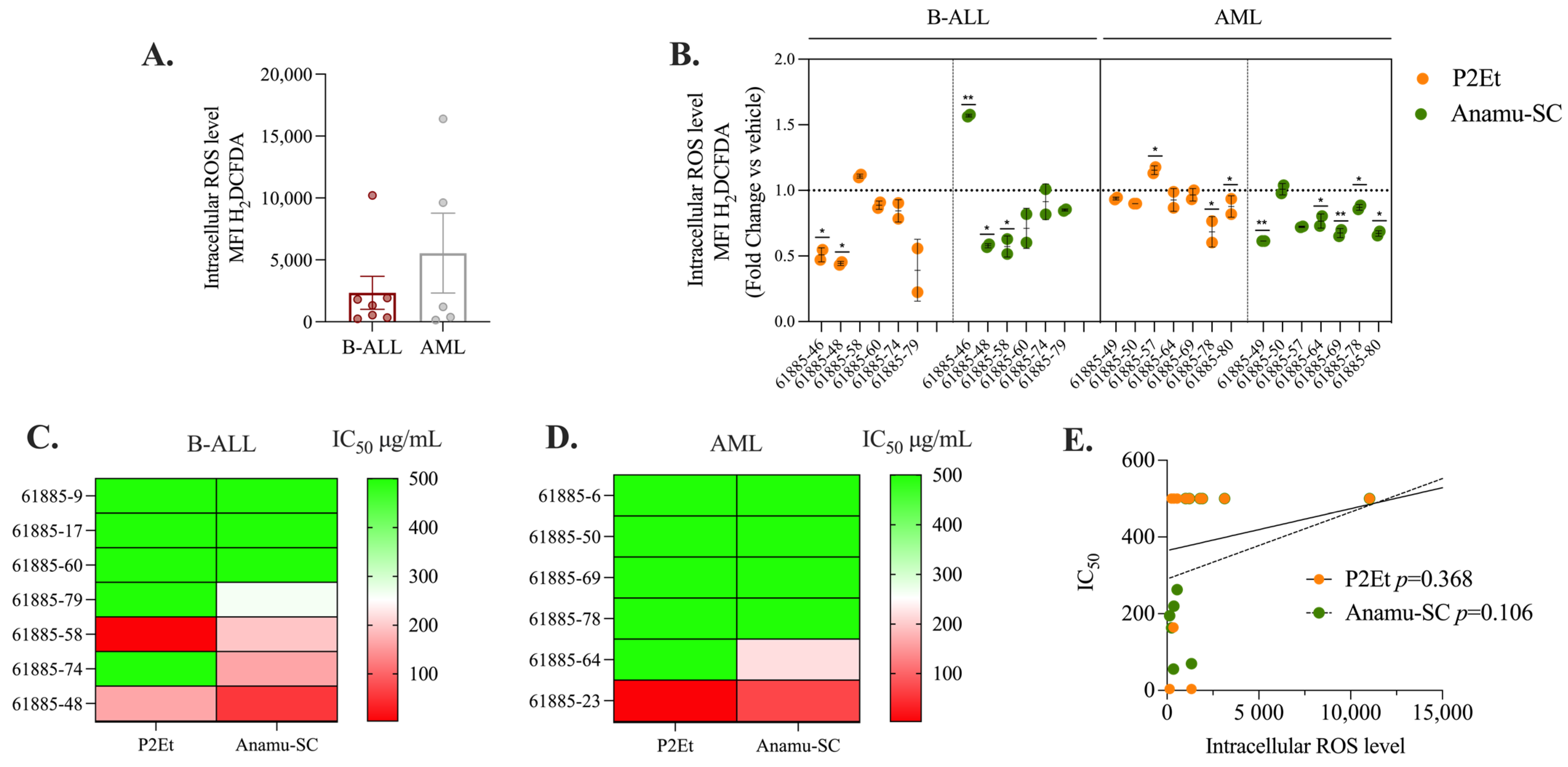
2.3. P2Et and Anamu-SC Are Intracellular Antioxidants in Patient-Derived Leukemia Blasts
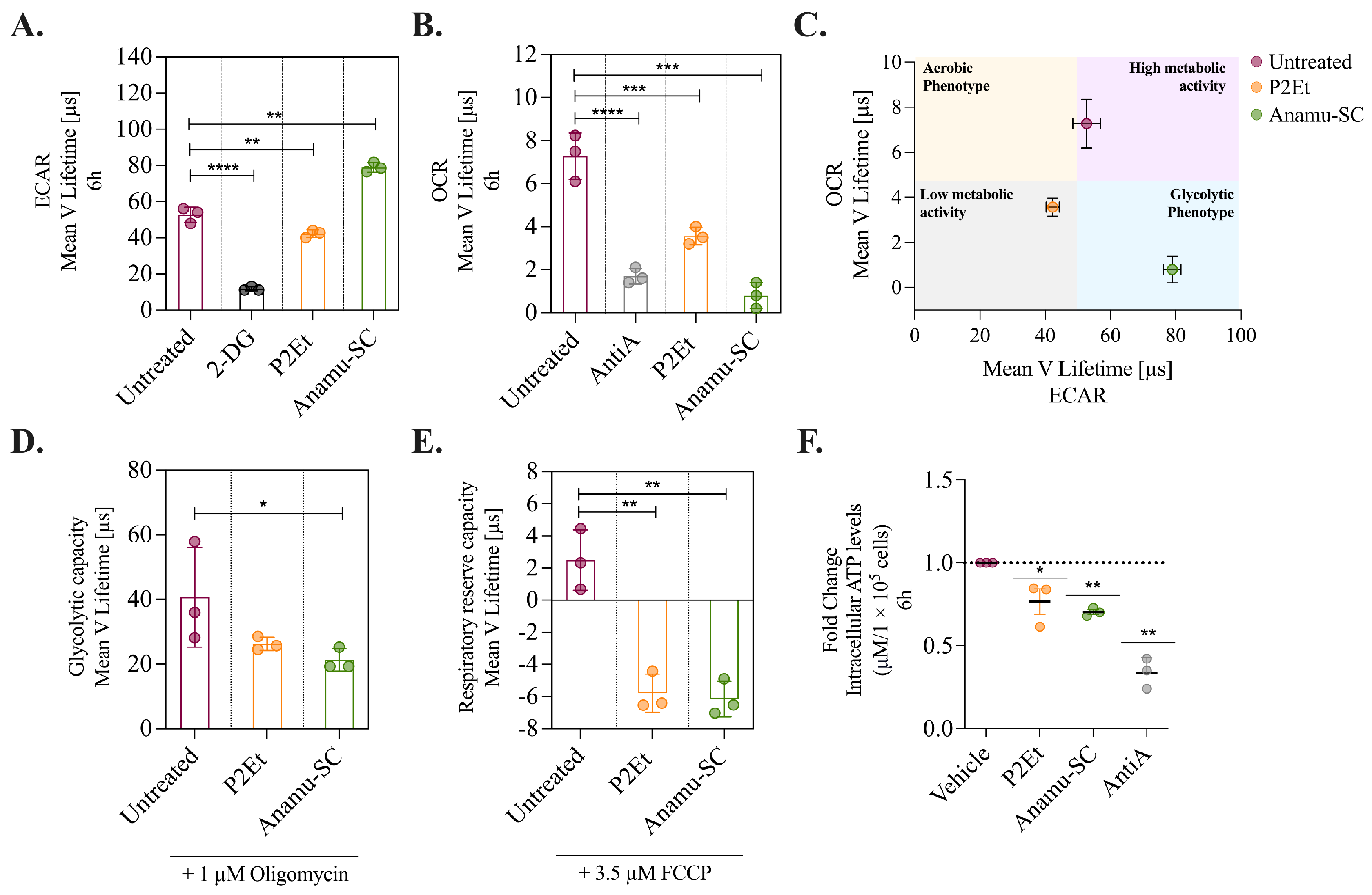
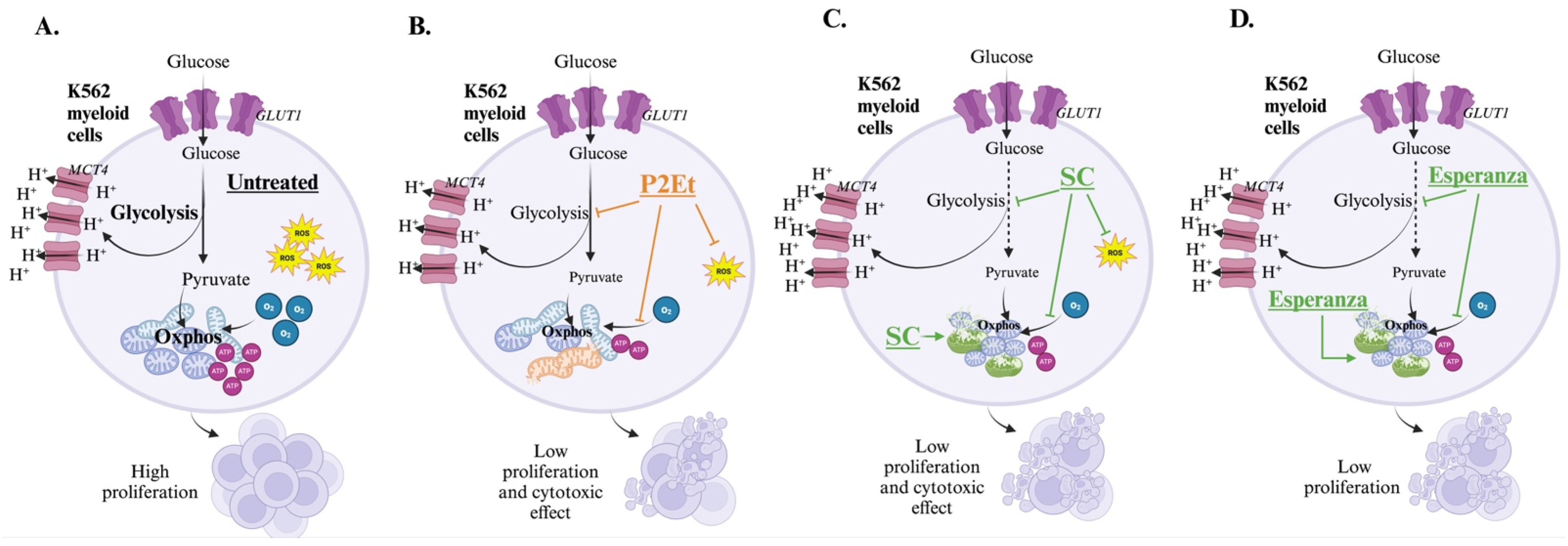
2.4. P2Et and Anamu-SC Modulate Energy Metabolism and Affect Mitochondrial Function
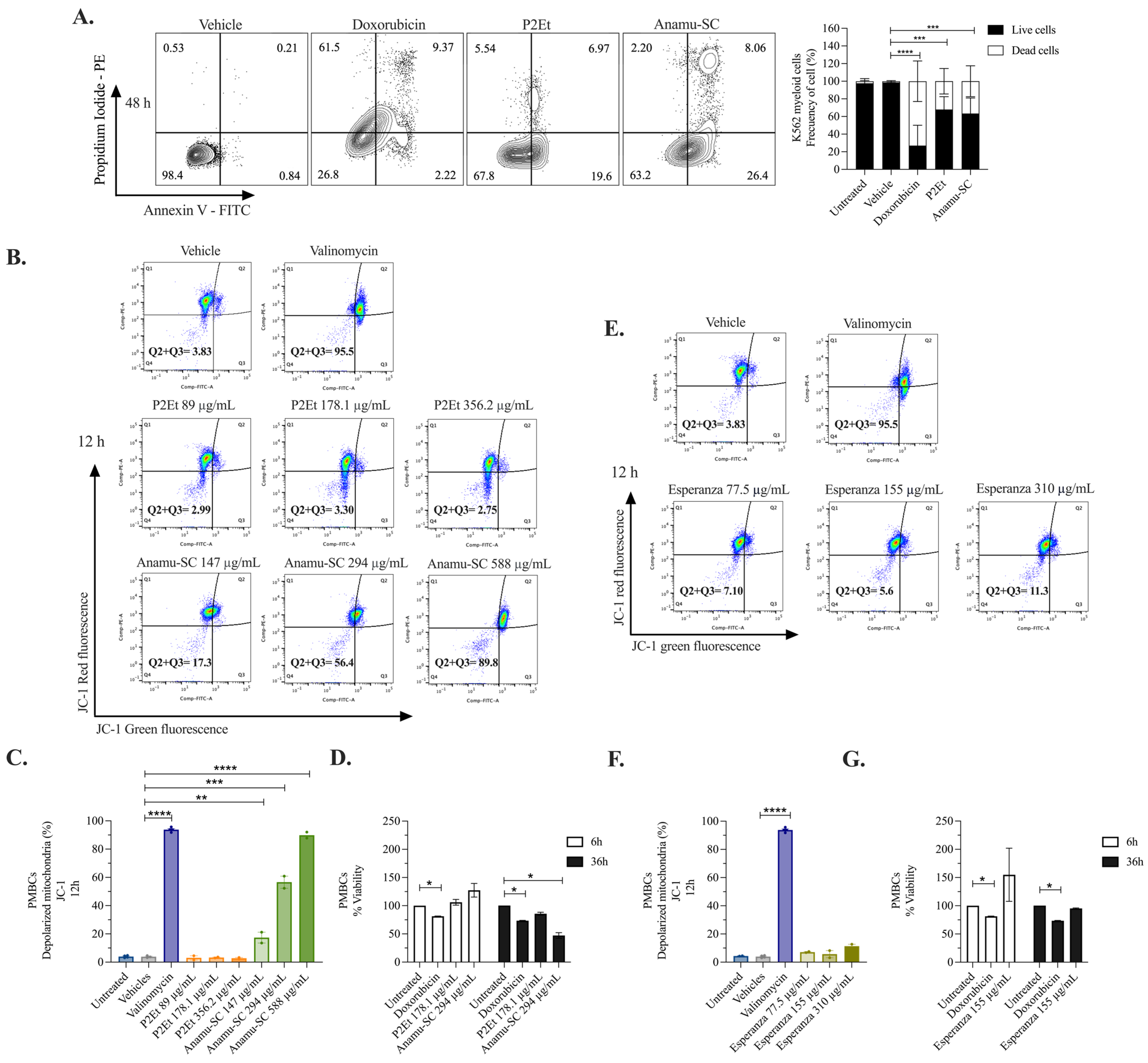
2.5. Anamu-SC but Not P2Et Increases Mitochondrial Fragmentation and Induces Changes in the Mitochondrial Membrane Potential (ΔΨm) of K562 Myeloid Cells
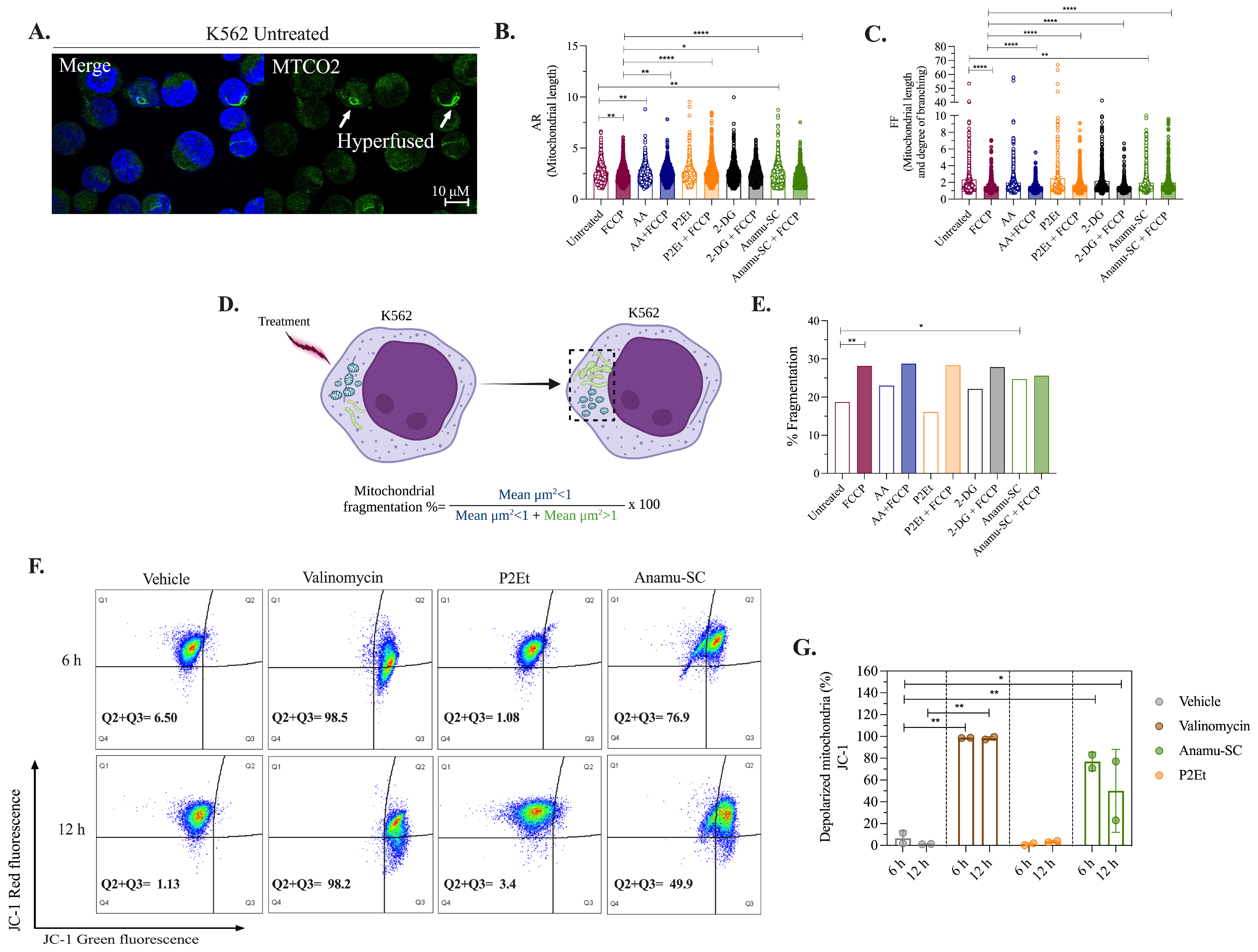
2.6. Induction of Apoptosis and Tumor Selectivity of P2Et and P. alliacea Extracts
2.7. Anamu-SC Induces Alterations in Glycerophospholipids, Fatty Acids, and Purine Nucleosides in K562 Cells
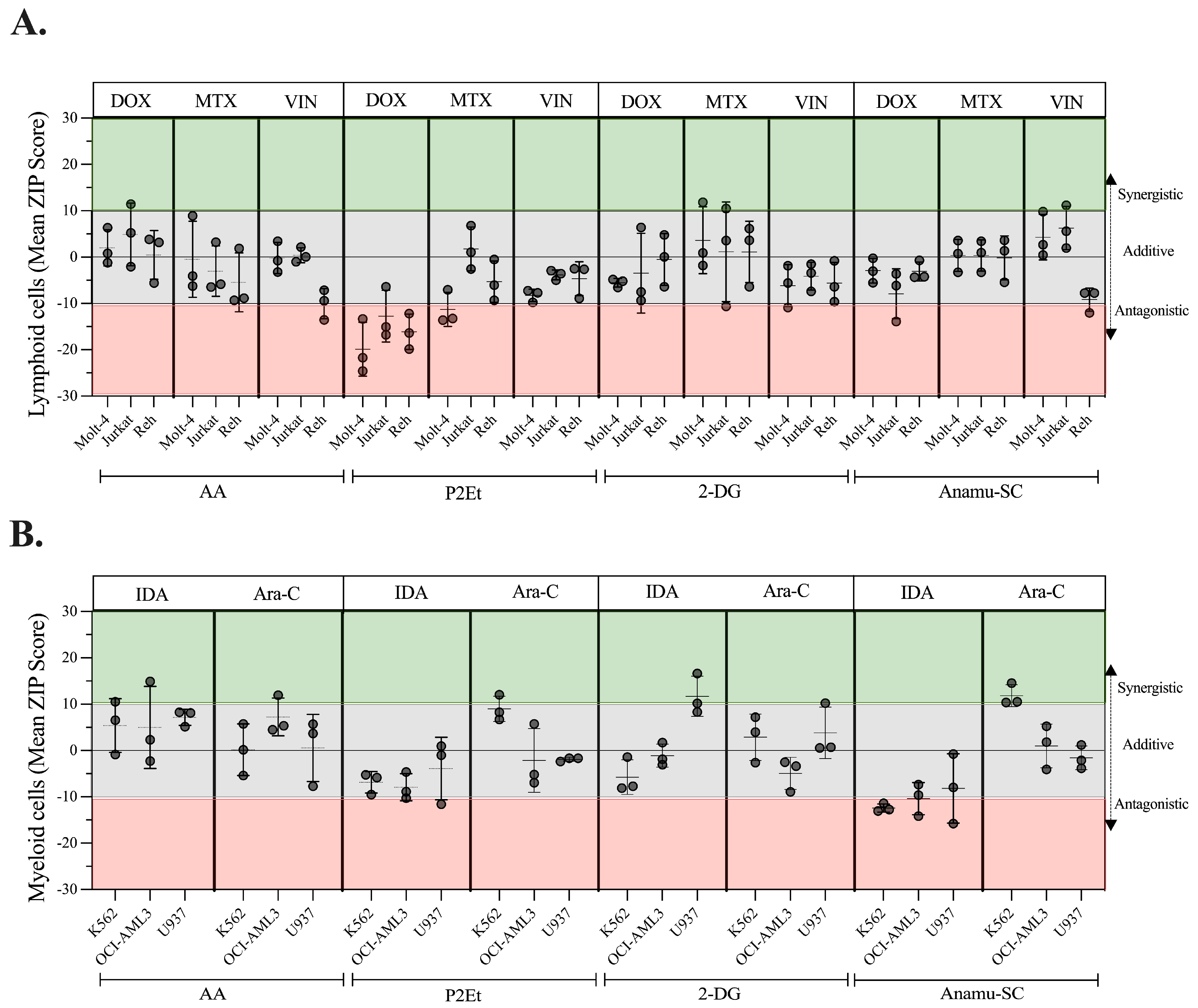
2.8. Regulation of Energy Metabolism Can Positively or Negatively Interfere with Chemotherapeutics
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. PDPDs Obtained from C. spinosa and P. alliacea
4.3. Patients and Peripheral Blood-Derived Leukemia Blasts
4.4. Intracellular ROS Measurement
4.5. Glucose Consumption Assay
4.6. In Vitro Cytotoxicity Assay
4.7. Proliferation Assay
4.8. Oxygen Consumption and Extracellular Acidification Rates
4.9. Mitochondrial Morphological Analysis
4.10. Cell Death Evaluation
4.11. Mitochondrial Membrane Potential (ΔΨm) Assay
4.12. Measurement of Intracellular ATP
4.13. Untargeted Metabolomics Analysis by LC-QTOF-MS
Data Processing, Analysis, and Annotation of Statistically Significant Molecular Features
4.14. Interaction Assays Between Antitumor Drugs and PDPDs
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, S.H.; Campoo, E.; Lee, N.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumors for Haematopoietic and Lymphoid Tissues; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Kreitz, J.; Schönfeld, C.; Seibert, M.; Stolp, V.; Alshamleh, I.; Oellerich, T.; Steffen, B.; Schwalbe, H.; Schnütgen, F.; Kurrle, N.; et al. Metabolic Plasticity of Acute Myeloid Leukemia. Cells 2019, 8, 805. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, L.; Tosello, V.; Peroni, E.; Piovan, E. Insights on Metabolic Reprogramming and Its Therapeutic Potential in Acute Leukemia. Int. J. Mol. Sci. 2021, 22, 8738. [Google Scholar] [CrossRef] [PubMed]
- Loew, A.; Köhnke, T.; Rehbeil, E.; Pietzner, A.; Weylandt, K.-H. A Role for Lipid Mediators in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2019, 20, 2425. [Google Scholar] [CrossRef] [PubMed]
- Sriskanthadevan, S.; Jeyaraju, D.V.; Chung, T.E.; Prabha, S.; Xu, W.; Skrtic, M.; Jhas, B.; Hurren, R.; Gronda, M.; Wang, X.; et al. AML cells have low spare reserve capacity in their respiratory chain that renders them susceptible to oxidative metabolic stress. Blood 2015, 125, 2120–2130. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Duran, M.I.; Ballesteros-Ramírez, R.; Tellez, A.; Torregrosa, L.; Olejua, P.A.; Galvis, S.; Urueña, C.; Fiorentino, S. Safety Evaluation in Healthy Colombian Volunteers of P2Et Extract Obtained from Caesalpinia spinosa: Design 3+3 Phase I Clinical Trial. Evid.-Based Complement. Altern. Med. 2022, 2022, 7943001. [Google Scholar] [CrossRef]
- Urueña, C.; Sandoval, T.A.; Lasso, P.; Tawil, M.; Barreto, A.; Torregrosa, L.; Fiorentino, S. Evaluation of chemotherapy and P2Et extract combination in ex-vivo derived tumor mammospheres from breast cancer patients. Sci. Rep. 2020, 10, 19639. [Google Scholar] [CrossRef]
- O’Brien, K.; Ried, K.; Binjemain, T.; Sali, A. Integrative Approaches to the Treatment of Cancer. Cancers 2022, 14, 5933. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liao, C.C.; Chen, H.J.; Hsieh, C.L.; Li, T.C. The Effectiveness of Traditional Chinese Medicine in Treating Patients with Leukemia. Evid.-Based Complement. Altern. Med. 2016, 2016, 8394850. [Google Scholar] [CrossRef]
- Guerra, A.R.; Duarte, M.F.; Duarte, I.F. Targeting Tumor Metabolism with Plant-Derived Natural Products: Emerging Trends in Cancer Therapy. J. Agric. Food Chem. 2018, 66, 10663–10685. [Google Scholar] [CrossRef]
- Gomez-Cadena, A.; Barreto, A.; Fioretino, S.; Jandus, C. Immune system activation by natural products and complex fractions: A network pharmacology approach in cancer treatment. Cell Stress 2020, 4, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.S.; Zhang, H.-J.; Tsang, S.W. Perspectives of Plant Natural Products in Inhibition of Cancer Invasion and Metastasis by Regulating Multiple Signaling Pathways. Curr. Med. Chem. 2018, 25, 5057–5087. [Google Scholar] [CrossRef] [PubMed]
- Luz, D.A.; Pinheiro, A.M.; Silva, M.L.; Monteiro, M.C.; Prediger, R.D.; Ferraz Maia, C.S.; Fontes-Júnior, E.A. Ethnobotany, phytochemistry and neuropharmacological effects of Petiveria alliacea L. (Phytolaccaceae): A review. J. Ethnopharmacol. 2016, 185, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Robledo, S.M.; Quintero, J.; Higuita, J.; Fernández, M.; Murillo, J.; Restrepo, A.; Arbeláez, N.; Montoya, A.; Ospina, V.; Pineda, T.; et al. Caesalpinia spinosa (Molina) Kuntze: Una nueva promesa para el tratamiento tópico de la leishmaniasis cutánea. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2020, 44, 915–936. [Google Scholar] [CrossRef]
- Ma, H.Y.; Wang, C.Q.; He, H.; Yu, Z.Y.; Tong, Y.; Liu, G.; Yang, Y.Q.; Li, L.; Pang, L.; Qi, H.Y. Ethyl acetate extract of Caesalpinia sappan L. inhibited acute myeloid leukemia via ROS-mediated apoptosis and differentiation. Phytomedicine 2020, 68, 153142. [Google Scholar] [CrossRef]
- Tran, M.H.; Nguyen, M.T.; Nguyen, H.D.; Nguyen, T.D.; Phuong, T.T. Cytotoxic constituents from the seeds of Vietnamese Caesalpinia sappan. Pharm. Biol. 2015, 53, 1549–1554. [Google Scholar] [CrossRef]
- Lopes-Martins, R.A.; Pegoraro, D.H.; Woisky, R.; Penna, S.C.; Sertié, J.A. The anti-inflammatory and analgesic effects of a crude extract of Petiveria alliacea L. (Phytolaccaceae). Phytomedicine 2002, 9, 245–248. [Google Scholar] [CrossRef]
- Christie, S.L.; Levy, A. Evaluation of the Hypoglycaemic Activity of Petiveria alliacea (Guinea Hen Weed) Extracts in Normoglycaemic and Diabetic Rat Models. West Indian Med. J. 2013, 62, 685–691. [Google Scholar] [CrossRef]
- Gunawan, V.; Soetjipto, H.; Mustika, A. Hypoglicemic and Antioxidant Activity of Petiveria alliacea in Diabetic Rat Models. Biomol. Health Sci. J. 2020, 31, 19–23. [Google Scholar] [CrossRef]
- dos Santos Júnior, H.M.; Oliveira, D.F.; de Carvalho, D.A.; Pinto, J.M.; Campos, V.A.; Mourão, A.R.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Evaluation of native and exotic Brazilian plants for anticancer activity. J. Nat. Med. 2010, 64, 231–238. [Google Scholar] [CrossRef]
- Murillo, N.; Lasso, P.; Urueña, C.; Pardo-Rodriguez, D.; Ballesteros-Ramírez, R.; Betancourt, G.; Rojas, L.; Cala, M.P.; Fiorentino, S. Petiveria alliacea Reduces Tumor Burden and Metastasis and Regulates the Peripheral Immune Response in a Murine Myeloid Leukemia Model. Int. J. Mol. Sci. 2023, 24, 12972. [Google Scholar] [CrossRef] [PubMed]
- Prieto, K.; Cao, Y.; Mohamed, E.; Trillo-Tinoco, J.; Sierra, R.A.; Urueña, C.; Sandoval, T.A.; Fiorentino, S.; Rodriguez, P.C.; Barreto, A. Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK. Cell Death Discov. 2019, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, T.A.; Urueña, C.P.; Llano, M.; Gómez-Cadena, A.; Hernández, J.F.; Sequeda, L.G.; Loaiza, A.E.; Barreto, A.; Li, S.; Fiorentino, S. Standardized Extract from Caesalpinia spinosa is Cytotoxic over Cancer Stem Cells and Enhance Anticancer Activity of Doxorubicin. Am. J. Chin. Med. 2016, 44, 1693–1717. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cadena, A.; Uruenã, C.; Prieto, K.; Martinez-Usatorre, A.; Donda, A.; Barreto, A.; Romero, P.; Fiorentino, S. Immune-system-dependent anti-tumor activity of a plant-derived polyphenol rich fraction in a melanoma mouse model. Cell Death Dis. 2016, 7, e2243. [Google Scholar] [CrossRef]
- Lasso, P.; Gomez-Cadena, A.; Urueña, C.; Donda, A.; Martinez-Usatorre, A.; Barreto, A.; Romero, P.; Fiorentino, S. Prophylactic vs. therapeutic treatment with P2Et polyphenol-rich extract has opposite effects on tumor growth. Front. Oncol. 2018, 8, 356. [Google Scholar] [CrossRef]
- Arévalo-Ferrin, J.J.; García-Ortiz, J.A.; Arevalo-Olaya, C.M.; Quijano-Gómez, S.M.; Fiorentino-Gómez, S.; Rodríguez-Pardo, V.M. Plant-derived extracts P2Et and Anamu-SC affect NO and ROS levels in leukemic cells. Univ. Sci. 2023, 28, 201–216. [Google Scholar] [CrossRef]
- Hernández, J.F.; Urueña, C.P.; Cifuentes, M.C.; Sandoval, T.A.; Pombo, L.M.; Castañeda, D.; Asea, A.; Fiorentino, S. A Petiveria alliacea standardized fraction induces breast adenocarcinoma cell death by modulating glycolytic metabolism. J. Ethnopharmacol. 2014, 153, 641–649. [Google Scholar] [CrossRef]
- Ballesteros-Ramírez, R.; Aldana, E.; Herrera, M.V.; Urueña, C.; Rojas, L.Y.; Echeverri, L.F.; Costa, G.M.; Quijano, S.; Fiorentino, S. Preferential Activity of Petiveria alliacea Extract on Primary Myeloid Leukemic Blast. Evid.-Based Complement. Altern. Med. 2020, 2020, 4736206. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Patra, S.; Elahi, N.; Armorer, A.; Arunachalam, S.; Omala, J.; Hamid, I.; Ashton, A.W.; Joyce, D.; Jiao, X.; Pestell, R.G. Mechanisms Governing Metabolic Heterogeneity in Breast Cancer and Other Tumors. Front. Oncol. 2021, 11, 700629. [Google Scholar] [CrossRef]
- Saengboonmee, C.; Seubwai, W.; Pairojkul, C.; Wongkham, S. High glucose enhances progression of cholangiocarcinoma cells via STAT3 activation. Sci. Rep. 2016, 6, 18995. [Google Scholar] [CrossRef] [PubMed]
- Yucel, B.; Altundağ Kara, S.; Cekmen, M.B.; Ada, S.; Demircan Tan, B. STAT3 mediated regulation of glucose metabolism in leukemia cells. Gene 2022, 809, 146012. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Wang, D.; Wei, L.; Chen, J.; Li, H.; Liu, Y. Deferoxamine Inhibits Acute Lymphoblastic Leukemia Progression through Repression of ROS/HIF-1α, Wnt/β-Catenin, and p38MAPK/ERK Pathways. J. Oncol. 2022, 2022, 8281267. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhang, N.J.; Zhang, L.J. Oxidative stress in leukemia and antioxidant treatment. Chin. Med. J. 2021, 134, 1897–1907. [Google Scholar] [CrossRef]
- Mondet, J.; Lo Presti, C.; Chevalier, S.; Bertrand, A.; Tondeur, S.; Blanchet, S.; Mc Leer, A.; Pernet-Gallay, K.; Mossuz, P. Mitochondria in human acute myeloid leukemia cell lines have ultrastructural alterations linked to deregulation of their respiratory profiles. Exp. Hematol. 2021, 98, 53–62.e53. [Google Scholar] [CrossRef]
- Chen, T.R. Modal karyotype of human leukemia cell line, K562 (ATCC CCL 243). Cancer Genet. Cytogenet. 1985, 17, 55–60. [Google Scholar] [CrossRef]
- Baykal-Köse, S.; Acikgoz, E.; Yavuz, A.S.; Gönül Geyik, Ö.; Ateş, H.; Sezerman, O.U.; Özsan, G.H.; Yüce, Z. Adaptive phenotypic modulations lead to therapy resistance in chronic myeloid leukemia cells. PLoS ONE 2020, 15, e0229104. [Google Scholar] [CrossRef]
- TeSlaa, T.; Teitell, M.A. Techniques to monitor glycolysis. Methods Enzymol. 2014, 542, 91–114. [Google Scholar] [CrossRef]
- Hernández, J.F.; Urueña, C.P.; Sandoval, T.A.; Cifuentes, M.C.; Formentini, L.; Cuezva, J.M.; Fiorentino, S. A cytotoxic Petiveria alliacea dry extract induces ATP depletion and decreases β-F1-ATPase expression in breast cancer cells and promotes survival in tumor-bearing mice. Braz. J. Pharmacogn. 2017, 27, 306–314. [Google Scholar] [CrossRef]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Raavicharla, S.; Shah, S.; Cox, D.; Jaiswal, J.K. Mitochondrial fragmentation enables localized signaling required for cell repair. J. Cell Biol. 2020, 219, e201909154. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.; Pardo-Rodriguez, D.; Urueña, C.; Lasso, P.; Arévalo, C.; Cala, M.P.; Fiorentino, S. Effect of Petiveria alliacea Extracts on Metabolism of K562 Myeloid Leukemia Cells. Int. J. Mol. Sci. 2023, 24, 17418. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.S.; Rapp, G.K. Herbal Interaction with Chemotherapeutic Drugs-A Focus on Clinically Significant Findings. Front. Oncol. 2019, 9, 1356. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re8. [Google Scholar] [CrossRef]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An interactive analysis and consensus interpretation of multi-drug synergies across multiple samples. Nucleic Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef]
- Egan, G.; Schimmer, A.D. Contribution of metabolic abnormalities to acute myeloid leukemia pathogenesis. Trends Cell Biol. 2023, 33, 455–462. [Google Scholar] [CrossRef]
- Chen, J.; Huang, C.; Zhu, Y.; Dong, L.; Cao, W.; Sun, L.; Sun, H.; Wan, D.; Liu, Y.; Zhang, Z.; et al. Identification of similarities and differences between myeloid and lymphoid acute leukemias using a gene-gene interaction network. Pathol.-Res. Pract. 2015, 211, 789–796. [Google Scholar] [CrossRef]
- Prieto-Bermejo, R.; Romo-González, M.; Pérez-Fernández, A.; Ijurko, C.; Hernández-Hernández, Á. Reactive oxygen species in haematopoiesis: Leukaemic cells take a walk on the wild side. J. Exp. Clin. Cancer Res. 2018, 37, 125. [Google Scholar] [CrossRef]
- Nielsen, I.; Groth-Pedersen, L.; Dicroce-Giacobini, J.; Jonassen, A.S.H.; Mortensen, M.; Bilgin, M.; Schmiegelow, K.; Jäättelä, M.; Maeda, K. Cationic amphiphilic drugs induce elevation in lysoglycerophospholipid levels and cell death in leukemia cells. Metabolomics 2020, 16, 91. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Siddiqui, A.J.; Shamsi, T.; Naz, A. SERUM metabolomics of acute lymphoblastic leukaemia and acute myeloid leukaemia for probing biomarker molecules. Hematol. Oncol. 2017, 35, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Morad, H.M.; Abou-Elzahab, M.M.; Aref, S.; El-Sokkary, A.M.A. Diagnostic Value of 1H NMR-Based Metabolomics in Acute Lymphoblastic Leukemia, Acute Myeloid Leukemia, and Breast Cancer. ACS Omega 2022, 7, 8128–8140. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Hui-Tao, Z.; Hong-Wen, X.; Chun-Lan, H.; Mei-Zhou, H. Serum Metabolomics Coupling with Clinical Laboratory Indicators Reveal Taxonomic Features of Leukemia. Front. Pharmacol. 2022, 13, 794042. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Pottosin, I.; Dobrovinskaya, O. Phenolic Compounds Cannabidiol, Curcumin and Quercetin Cause Mitochondrial Dysfunction and Suppress Acute Lymphoblastic Leukemia Cells. Int. J. Mol. Sci. 2020, 22, 204. [Google Scholar] [CrossRef]
- Banella, C.; Catalano, G.; Travaglini, S.; Pelosi, E.; Ottone, T.; Zaza, A.; Guerrera, G.; Angelini, D.F.; Niscola, P.; Divona, M.; et al. Ascorbate Plus Buformin in AML: A Metabolic Targeted Treatment. Cancers 2022, 14, 2565. [Google Scholar] [CrossRef]
- Hasanpourghadi, M.; Yeng Looi, C.; Kumar Pandurangan, A.; Sethi, G.; Fen Wong, W.; Rais Mustafa, M. Phytometabolites Targeting the Warburg Effect in Cancer Cells: A Mechanistic Review. Curr. Drug Targets 2017, 18, 1086–1094. [Google Scholar] [CrossRef]
- Hlozkova, K.; Pecinova, A.; Alquezar-Artieda, N.; Pajuelo-Reguera, D.; Simcikova, M.; Hovorkova, L.; Rejlova, K.; Zaliova, M.; Mracek, T.; Kolenova, A.; et al. Metabolic profile of leukemia cells influences treatment efficacy of L-asparaginase. BMC Cancer 2020, 20, 526. [Google Scholar] [CrossRef]
- Nishiura, T.; Suzuki, K.; Kawaguchi, T.; Nakao, H.; Kawamura, N.; Taniguchi, M.; Kanayama, Y.; Yonezawa, T.; Iizuka, S.; Taniguchi, N. Elevated serum manganese superoxide dismutase in acute leukemias. Cancer Lett. 1992, 62, 211–215. [Google Scholar] [CrossRef]
- Paydas, S.; Yuregir, G.T.; Sahin, B.; Seyrek, E.; Burgut, R. Intracellular glutathione content in leukemias. Oncology 1995, 52, 112–115. [Google Scholar] [CrossRef]
- Montaño, A.; Forero-Castro, M.; Marchena-Mendoza, D.; Benito, R.; Hernández-Rivas, J. New Challenges in Targeting Signaling Pathways in Acute Lymphoblastic Leukemia by NGS Approaches: An Update. Cancers 2018, 10, 110. [Google Scholar] [CrossRef]
- Rodrigues, A.; Costa, R.G.A.; Silva, S.L.R.; Dias, I.; Dias, R.B.; Bezerra, D.P. Cell signaling pathways as molecular targets to eliminate AML stem cells. Crit. Rev. Oncol./Hematol. 2021, 160, 103277. [Google Scholar] [CrossRef] [PubMed]
- Puente-Moncada, N.; Turos-Cabal, M.; Sánchez, A.M.; Antolín, I.; Herrera, F.; Rodriguez, J.; Duarte, C.; Rodriguez, C.; Martín, V. Role of glucose metabolism in the differential antileukemic effect of melatonin on wild-type and FLT3-ITD mutant cells. Oncol. Rep. 2020, 44, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.M.; Still, P.C.; Bueno Perez, L.; Grever, M.R.; Douglas Kinghorn, A. Potential of Plant-Derived Natural Products in the Treatment of Leukemia and Lymphoma. Curr. Drug Targets 2010, 11, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.; Ahmad Raus, R.; Daddiouaissa, D.; Ahmad, F.; Adzhar, N.S.; Latif, E.S.; Abdulhafiz, F.; Mohammed, A. Medicinal Plants with Anti-Leukemic Effects: A Review. Molecules 2021, 26, 2741. [Google Scholar] [CrossRef]
- Lapa, B.; Gonçalves, A.C.; Jorge, J.; Alves, R.; Pires, A.S.; Abrantes, A.M.; Coucelo, M.; Abrunhosa, A.; Botelho, M.F.; Nascimento-Costa, J.M.; et al. Acute myeloid leukemia sensitivity to metabolic inhibitors: Glycolysis showed to be a better therapeutic target. Med. Oncol. 2020, 37, 72. [Google Scholar] [CrossRef]
- Panina, S.B.; Baran, N.; Brasil da Costa, F.H.; Konopleva, M.; Kirienko, N.V. A mechanism for increased sensitivity of acute myeloid leukemia to mitotoxic drugs. Cell Death Dis. 2019, 10, 617. [Google Scholar] [CrossRef]
- Panina, S.B.; Pei, J.; Baran, N.; Konopleva, M.; Kirienko, N.V. Utilizing Synergistic Potential of Mitochondria-Targeting Drugs for Leukemia Therapy. Front. Oncol. 2020, 10, 435. [Google Scholar] [CrossRef]
- De Beauchamp, L.; Himonas, E.; Helgason, G.V. Mitochondrial metabolism as a potential therapeutic target in myeloid leukaemia. Leukemia 2022, 36, 1–12. [Google Scholar] [CrossRef]
- Pumiputavon, K.; Chaowasku, T.; Saenjum, C.; Osathanunkul, M.; Wungsintaweekul, B.; Chawansuntati, K.; Lithanatudom, P.; Wipasa, J. Cytotoxic and cytostatic effects of four Annonaceae plants on human cancer cell lines. In Vitr. Cell. Dev. Biol.-Anim. 2019, 55, 723–732. [Google Scholar] [CrossRef]
- Comin-Anduix, B.; Boros, L.G.; Marin, S.; Boren, J.; Callol-Massot, C.; Centelles, J.J.; Torres, J.L.; Agell, N.; Bassilian, S.; Cascante, M. Fermented Wheat Germ Extract Inhibits Glycolysis/Pentose Cycle Enzymes and Induces Apoptosis through Poly(ADP-ribose) Polymerase Activation in Jurkat T-cell Leukemia Tumor Cells. J. Biol. Chem. 2002, 277, 46408–46414. [Google Scholar] [CrossRef]
- Lee, E.A.; Angka, L.; Rota, S.G.; Hanlon, T.; Mitchell, A.; Hurren, R.; Wang, X.M.; Gronda, M.; Boyaci, E.; Bojko, B.; et al. Targeting mitochondria with avocatin B induces selective leukemia cell death. Cancer Res. 2015, 75, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, A.; Maitre, C.; Haywood-Small, S.; Mcdougall, G.; Cross, N.; Jordan-Mahy, N. Differential Effects of Polyphenols on Proliferation and Apoptosis in Human Myeloid and Lymphoid Leukemia Cell Lines. Anti-Cancer Agents Med. Chem. 2013, 13, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Mirzaei, S.; Hashemi, F.; Samarghandian, S.; Zabolian, A.; Hushmandi, K.; Ang, H.L.; Sethi, G.; Kumar, A.P.; et al. Gallic acid for cancer therapy: Molecular mechanisms and boosting efficacy by nanoscopical delivery. Food Chem. Toxicol. 2021, 157, 112576. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Zhang, M.; Meng, H.; Xu, D.; Xie, Y. Gallic acid targets acute myeloid leukemia via Akt/mTOR-dependent mitochondrial respiration inhibition. Biomed. Pharmacother. 2018, 105, 491–497. [Google Scholar] [CrossRef]
- Mamouni, K.; Kallifatidis, G.; Lokeshwar, B.L. Targeting Mitochondrial Metabolism in Prostate Cancer with Triterpenoids. Int. J. Mol. Sci. 2021, 22, 2466. [Google Scholar] [CrossRef]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models. Antioxid. Redox Signal. 2018, 29, 1589–1611. [Google Scholar] [CrossRef]
- Yang, J.H.; Chou, C.C.; Cheng, Y.W.; Sheen, L.Y.; Chou, M.C.; Yu, H.S.; Wei, Y.H.; Chung, J.G. Effects of glycolic acid on the induction of apoptosis via caspase-3 activation in human leukemia cell line (HL-60). Food Chem. Toxicol. 2004, 42, 1777–1784. [Google Scholar] [CrossRef]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of carbon quantum dots- quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Mookerjee, S.A.; Goncalves, R.L.S.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 2015, 1847, 171–181. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859 Pt B, 1558–1572. [Google Scholar] [CrossRef]
- Dong, J.; Ye, F.; Lin, J.; He, H.; Song, Z. The metabolism and function of phospholipids in Mitochondria. Mitochondrial Commun. 2023, 1, 2–12. [Google Scholar] [CrossRef]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Black, S.M. Carnitine Homeostasis, Mitochondrial Function, and Cardiovascular Disease. Drug Discov. Today Dis. Mech. 2009, 6, e31–e39. [Google Scholar] [CrossRef] [PubMed]
- Soler-Agesta, R.; Anel, A.; Galluzzi, L. Mitochondrial control of antigen presentation in cancer cells. Cancer Cell 2023, 41, 1849–1851. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, Y.; Luo, X.; Hu, Q. Oxidative resistance of leukemic stem cells and oxidative damage to hematopoietic stem cells under pro-oxidative therapy. Cell Death Dis. 2020, 11, 291. [Google Scholar] [CrossRef]
- Gorini, S.; De Angelis, A.; Berrino, L.; Malara, N.; Rosano, G.; Ferraro, E. Chemotherapeutic drugs and mitochondrial dysfunction: Focus on doxorubicin, trastuzumab, and sunitinib. Oxidative Med. Cell. Longev. 2018, 2018, 7582730. [Google Scholar] [CrossRef]
- Urueña, C.; Cifuentes, C.; Castañeda, D.; Arango, A.; Kaur, P.; Asea, A.; Fiorentino, S. Petiveria alliacea extracts uses multiple mechanisms to inhibit growth of human and mouse tumoral cells. BMC Complement. Altern. Med. 2008, 8, 60. [Google Scholar] [CrossRef]
- Padilla-Arellanes, S.; Salgado-Garciglia, R.; Báez-Magaña, M.; Ochoa-Zarzosa, A.; López-Meza, J.E. Cytotoxicity of a Lipid-Rich Extract from Native Mexican Avocado Seed (Persea americana var. drymifolia) on Canine Osteosarcoma D-17 Cells and Synergistic Activity with Cytostatic Drugs. Molecules 2021, 26, 4178. [Google Scholar] [CrossRef]
- Mahbub, A.; Le Maitre, C.; Haywood-Small, S.; Cross, N.; Jordan-Mahy, N. Polyphenols act synergistically with doxorubicin and etoposide in leukaemia cell lines. Cell Death Discov. 2015, 1, 15043. [Google Scholar] [CrossRef]
- Yao, S.; Zhong, L.; Chen, M.; Zhao, Y.; Li, L.; Liu, L.; Xu, T.; Xiao, C.; Gan, L.; Shan, Z.; et al. Epigallocatechin-3-gallate promotes all-trans retinoic acid-induced maturation of acute promyelocytic leukemia cells via PTEN. Int. J. Oncol. 2021, 51, 899–906. [Google Scholar] [CrossRef]
- Dakik, H.; El Dor, M.; Bourgeais, J.; Kouzi, F.; Herault, O.; Gouilleux, F.; Zibara, K.; Mazurier, F. Diphenyleneiodonium Triggers Cell Death of Acute Myeloid Leukemia Cells by Blocking the Mitochondrial Respiratory Chain, and Synergizes with Cytarabine. Cancers 2022, 14, 2485. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Ramírez, R.; Lasso, P.; Urueña, C.; Saturno, J.; Fiorentino, S. Assessment of Acute and Chronic Toxicity in Wistar Rats (Rattus norvegicus) and New Zealand Rabbits (Oryctolagus cuniculus) of an Enriched Polyphenol Extract Obtained from Caesalpinia spinosa. J. Toxicol. 2024, 2024, 3769933. [Google Scholar] [CrossRef] [PubMed]
- Lasso, P.; Rojas, L.; Arévalo, C.; Urueña, C.; Murillo, N.; Barreto, A.; Costa, G.M.; Fiorentino, S. Tillandsia usneoides Extract Decreases the Primary Tumor in a Murine Breast Cancer Model but Not in Melanoma. Cancers 2022, 14, 5383. [Google Scholar] [CrossRef] [PubMed]
- Soares, T.; Rodrigues, D.; Sarraguça, M.; Rocha, S.; Lima, J.L.F.C.; Ribeiro, D.; Fernandes, E.; Freitas, M. Optimization of experimental settings for the assessment of reactive oxygen species production by human blood. Oxidative Med. Cell. Longev. 2019, 2019, 7198484. [Google Scholar] [CrossRef]
- Mao, Y.; Hoffman, T.; Wu, A.; Kohn, J. An Innovative Laboratory Procedure to Expand Chondrocytes with Reduced Dedifferentiation. Cartilage 2018, 9, 202–211. [Google Scholar] [CrossRef]
- Carlosama, C.; Arévalo, C.; Jimenez, M.C.; Lasso, P.; Urueña, C.; Fiorentino, S.; Barreto, A. Triple negative breast cancer migration is modified by mitochondrial metabolism alteration induced by natural extracts of C. spinosa and P. alliacea. Sci. Rep. 2024, 14, 20253. [Google Scholar] [CrossRef]
- Hamon, M.P.; Gergondey, R.; L’Honoré, A.; Friguet, B. Mitochondrial Lon protease-depleted HeLa cells exhibit proteome modifications related to protein quality control, stress response and energy metabolism. Free Radic. Biol. Med. 2020, 148, 83–95. [Google Scholar] [CrossRef]
- Koopman, W.J.H.; Visch, H.J.; Smeitink, J.A.M.; Willems, P.H.G.M. Simultaneous quantitative measurement and automated analysis of mitochondrial morphology, mass, potential, and motility in living human skin fibroblasts. Cytom. Part A J. Int. Soc. Anal. Cytol. 2006, 69, 1–12. [Google Scholar] [CrossRef]
- Tronstad, K.; Nooteboom, M.; Nilsson, L.; Nikolaisen, J.; Sokolewicz, M.; Grefte, S.; Pettersen, I.; Dyrstad, S.; Hoel, F.; Willems, P.; et al. Regulation and Quantification of Cellular Mitochondrial Morphology and Content. Curr. Pharm. Des. 2014, 20, 5634–5652. [Google Scholar] [CrossRef]
- Hodneland Nilsson, L.I.; Nitschke Pettersen, I.K.; Nikolaisen, J.; Micklem, D.; Avsnes Dale, H.; Vatne Røsland, G.; Lorens, J.; Tronstad, K.J. A new live-cell reporter strategy to simultaneously monitor mitochondrial biogenesis and morphology. Sci. Rep. 2015, 5, 17217. [Google Scholar] [CrossRef]
- Castañeda, D.M.; Pombo, L.M.; Urueña, C.P.; Hernandez, J.F.; Fiorentino, S. A gallotannin-rich fraction from Caesalpinia spinosa (Molina) Kuntze displays cytotoxic activity and raises sensitivity to doxorubicin in a leukemia cell line. BMC Complement. Altern. Med. 2012, 12, 38. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef]
 metabolites present only in Anamu-SC extract or Esperanza-treated cell lysates.
metabolites present only in Anamu-SC extract or Esperanza-treated cell lysates.
 metabolites present only in Anamu-SC extract or Esperanza-treated cell lysates.
metabolites present only in Anamu-SC extract or Esperanza-treated cell lysates.| Metabolites Altered by P. alliacea Extracts | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Formula | Anamu-SC | p Value | Esperanza | p Value | ||
| FC | VIP | FC | VIP | ||||
| Indoles and derivatives | |||||||
| Tryptophanol | C10H11NO | 0.45 | 1.83 | 0.0021 * | 0.61 | - | 0.009 * |
| Indoleacrylic acid | C11H9NO2 | 1.42 | 1.75 | 0.0087 * | 1.36 | 1.34 | - |
| Glycerophospholipids | |||||||
| PC (18:3) | C26H48NO7P | 116.2 | 6.49 | 0.0022 * | 3.37 | - | 0.009 * |
| Fatty Acyls | |||||||
| Propionylcarnitine | C10H19NO4 | 0.68 | 1.44 | 0.0022 * | 0.59 | - | 0.010 * |
| Hydroxyoctadecatrienoylcarnitine | C25H43NO5 |  | 4.52 | 0.0028 * |  | - | 0.010 * |
| Carboxylic acids and derivatives | |||||||
| Glutathione | C10H17N3O6S | 0.87 | 2.71 | - | 1.31 | 3.55 | 0.019 * |
| Purine nucleosides | |||||||
| Adenosine monophosphate | C10H14N5O7P | 3.06 | 1.79 | 0.0022 * | 0.44 | 1.05 | 0.009 * |
| Organooxygen compounds | |||||||
| Pantothenate | C9H17NO5 | 0.72 | 1.52 | - | 0.99 | 1.31 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arévalo, C.; Carlosama, C.; Rojas, L.; Cala, M.P.; Hamon, M.-P.; Friguet, B.; Barreto, A.; Fiorentino, S. Modulation of Tumor Metabolism in Acute Leukemia by Plant-Derived Polymolecular Drugs and Their Effects on Mitochondrial Function. Molecules 2025, 30, 1783. https://doi.org/10.3390/molecules30081783
Arévalo C, Carlosama C, Rojas L, Cala MP, Hamon M-P, Friguet B, Barreto A, Fiorentino S. Modulation of Tumor Metabolism in Acute Leukemia by Plant-Derived Polymolecular Drugs and Their Effects on Mitochondrial Function. Molecules. 2025; 30(8):1783. https://doi.org/10.3390/molecules30081783
Chicago/Turabian StyleArévalo, Cindy, Carolina Carlosama, Laura Rojas, Mónica P. Cala, Marie-Paule Hamon, Bertrand Friguet, Alfonso Barreto, and Susana Fiorentino. 2025. "Modulation of Tumor Metabolism in Acute Leukemia by Plant-Derived Polymolecular Drugs and Their Effects on Mitochondrial Function" Molecules 30, no. 8: 1783. https://doi.org/10.3390/molecules30081783
APA StyleArévalo, C., Carlosama, C., Rojas, L., Cala, M. P., Hamon, M.-P., Friguet, B., Barreto, A., & Fiorentino, S. (2025). Modulation of Tumor Metabolism in Acute Leukemia by Plant-Derived Polymolecular Drugs and Their Effects on Mitochondrial Function. Molecules, 30(8), 1783. https://doi.org/10.3390/molecules30081783







