Unveiling the Role of Metabolites from a Bacterial Endophyte in Mitigating Soil Salinity and Reducing Oxidative Stress
Abstract
1. Introduction
2. Results and Discussion
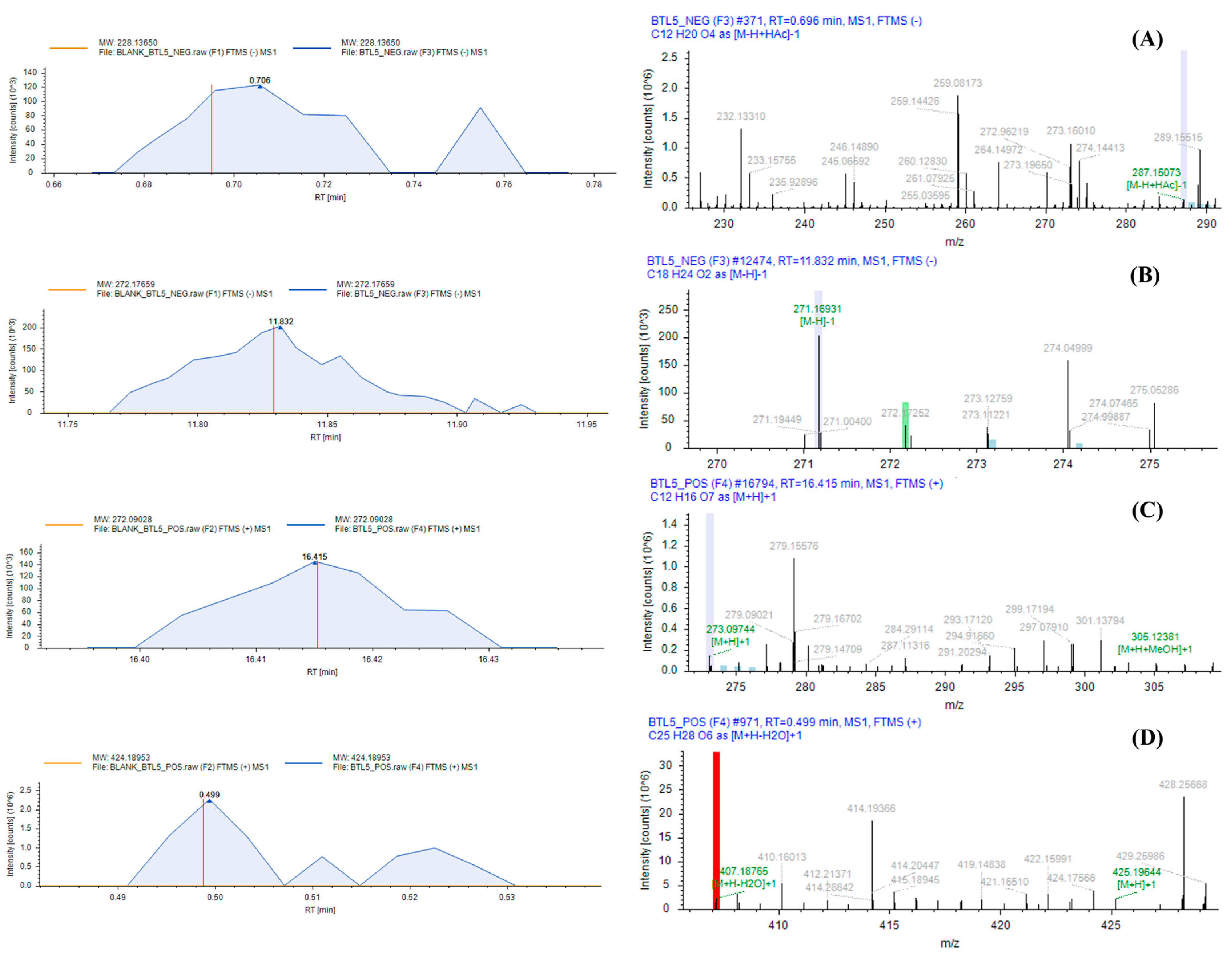
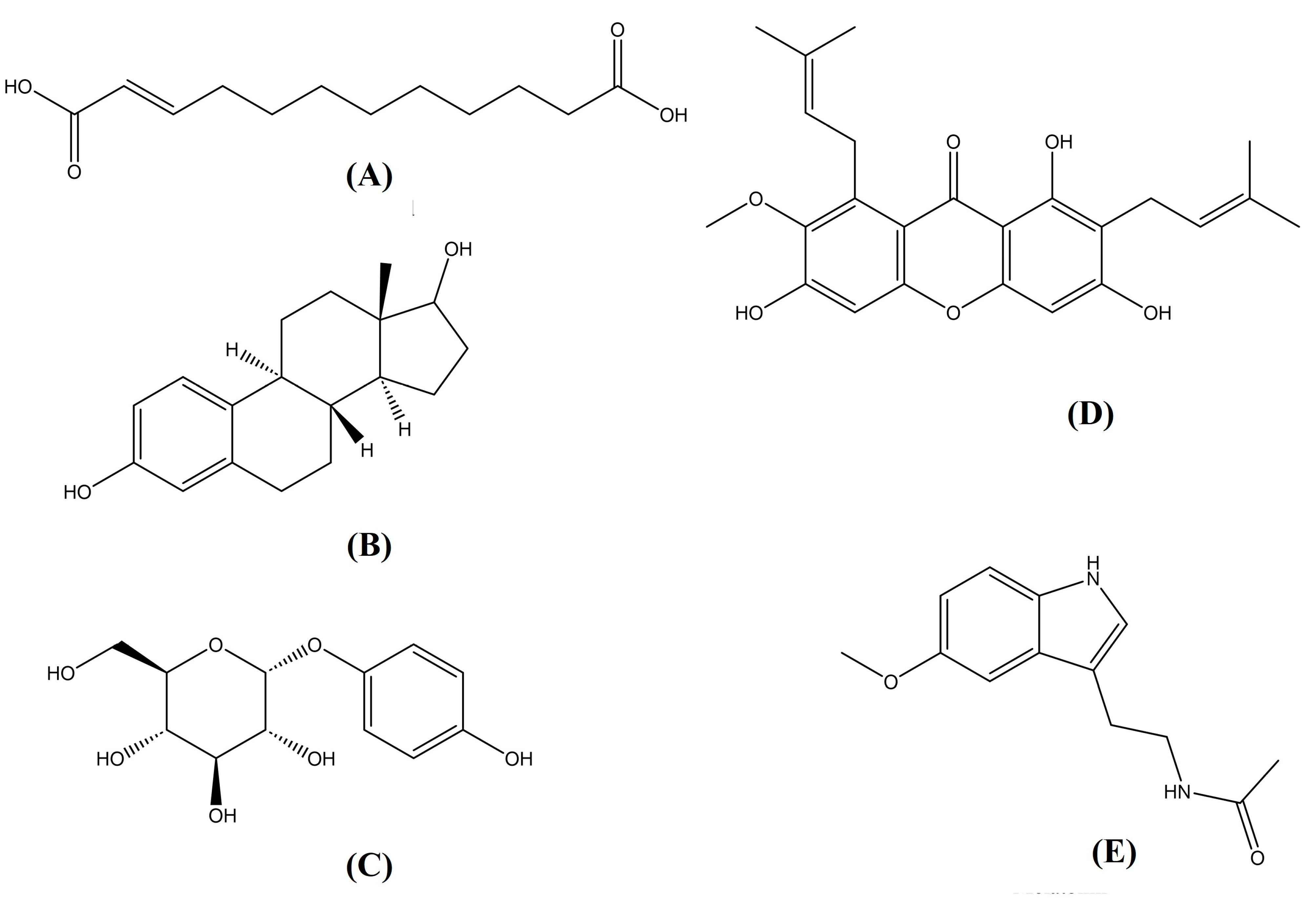
2.1. LC-HRMS Analysis
2.2. Preliminary Screening of Metabolites in Mung Bean Plants
2.3. Optimizing Dosage Through the Secondary Screening of Metabolites
2.3.1. Effect on Plant Growth
2.3.2. Effect on Antioxidant Enzymes
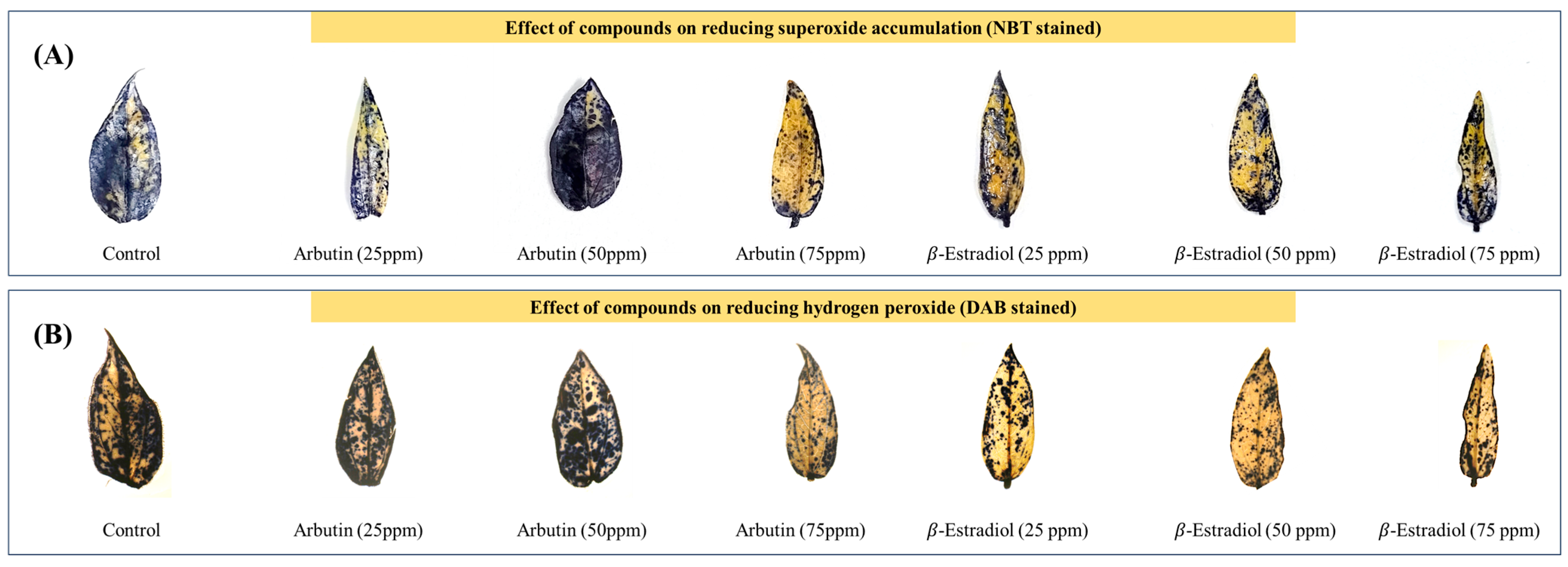
2.3.3. Level of ROS Accumulation in Leaf
2.4. Discussion
3. Materials and Methods
3.1. Extraction and Preparation of Crude Extracts from Bacterial Endophyte BTL5
3.2. Untargeted Metabolomics Using LC-HRMS Analysis
3.2.1. Sample Preparation for LC-HRMS
3.2.2. LC-HRMS Data Acquisition
3.2.3. Data Analysis
3.3. Preliminary Screening of Metabolites in Mung Bean Plants
3.4. Secondary Screening of Metabolites for Dose Optimization
3.4.1. Effect on Plant Growth
3.4.2. Effect on Antioxidant Enzyme Activity
3.4.3. Accumulation of Reactive Oxygen Species
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar]
- Sahu, P.K.; Singh, S.; Singh, U.B.; Chakdar, H.; Sharma, P.K.; Sarma, B.K.; Teli, B.; Bajpai, R.; Bhowmik, A.; Singh, H.V.; et al. Inter-genera colonization of Ocimum tenuiflorum endophytes in tomato and their complementary effects on Na⁺/K⁺ balance, oxidative stress regulation, and root architecture under elevated soil salinity. Front. Microbiol. 2021, 12, 744733. [Google Scholar]
- Yang, Z.; Li, J.L.; Liu, L.N.; Xie, Q.; Sui, N. Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front. Plant Sci. 2020, 10, 1722. [Google Scholar]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar]
- Gupta, A.; Tiwari, R.K.; Shukla, R.; Singh, A.N.; Sahu, P.K. Salinity alleviator bacteria in rice (Oryza sativa L.), their colonization efficacy, and synergism with Melatonin. Front. Plant Sci. 2023, 13, 1060287. [Google Scholar]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar]
- Gamalero, E.; Lingua, G.; Glick, B.R. Ethylene, ACC, and the plant growth-promoting enzyme ACC deaminase. Biology 2023, 12, 1043. [Google Scholar] [CrossRef]
- Al Hijab, L.Y.A.; Al-Hazmi, N.E.; Naguib, D.M. Rhizobacteria-priming improves common bean seeds germination under different abiotic stresses through improving hydrolysis and antioxidant enzymes kinetics parameters. Rhizosphere 2024, 29, 100842. [Google Scholar]
- Hameed, M.K.; Umar, W.; Razzaq, A.; Wei, S.; Niu, Q.; Huang, D.; Chang, L. Quantification of total polyphenols, antioxidants, anthocyanins, and secondary metabolites by UPLC VION IMS QTOF MS/MS analysis in green and red lettuce cultivars. Sci. Hortic. 2023, 315, 111994. [Google Scholar] [CrossRef]
- Rao, M.J.; Feng, B.; Ahmad, M.H.; Tahir ul Qamar, M.; Aslam, M.Z.; Khalid, M.F.; Hussain, S.; Zhong, R.; Ali, Q.; Xu, Q.; et al. LC-MS/MS-based metabolomics approach identified novel antioxidant flavonoids associated with drought tolerance in citrus species. Front. Plant Sci. 2023, 14, 1150854. [Google Scholar]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Hamid, N.; Abideen, Z.; Zulfiqar, F.; Moosa, A.; Nafees, M.; El-Keblawy, A. Exogenous Melatonin application stimulates growth, photosynthetic pigments, and antioxidant potential of white beans under salinity stress. S. Afr. J. Bot. 2023, 160, 219–228. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant polyphenols: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar]
- Spaepen, S.; Vanderleyden, J.; Okon, Y. Plant growth-promoting actions of rhizobacteria. Adv. Bot. Res. 2007, 26, 1–38. [Google Scholar]
- Gagne-Bourque, F.; Mayer, B.F.; Charron, J.B.; Vali, H.; Bertrand, A.; Jabaji, S. Accelerated growth rate and increased drought stress resilience of wheat seedlings colonized by Bacillus subtilis B26. PLoS ONE 2018, 13, e0203490. [Google Scholar]
- Zhang, H.; Liu, Y.; Wang, J. Exogenous arbutin application enhances salt tolerance in wheat by modulating antioxidant enzyme activity. Environ. Stress Biol. 2020, 12, 102–115. [Google Scholar]
- Ahmed, M.; Ali, Q.; Iqbal, N. Role of β-estradiol in enhancing drought resistance in maize: Physiological and biochemical insights. Plant Sci. J. 2021, 178, 250–262. [Google Scholar]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C. The role of Melatonin in salt stress tolerance in plants: Molecular and physiological perspectives. Environ. Exp. Bot. 2022, 197, 104875. [Google Scholar]
- Jogaiah, S.; Abdelrahman, M.; Tran, L.S.P.; Ito, S. Different mechanisms of Melatonin-mediated stress tolerance in plants. Trends Plant Sci. 2020, 25, 333–345. [Google Scholar]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar]
- Lee, H.Y.; Back, K. 2-HydroxyMelatonin, rather than Melatonin, is responsible for RBOH-dependent reactive oxygen species production leading to premature senescence in plants. Antioxidants 2021, 10, 1782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wen, Z.; Zhang, J.; Chen, X.; Cui, J.; Xu, W.; Liu, H. Exogenous glutathione alleviates salt-induced oxidative stress in tomato seedlings by regulating glutathione metabolism, redox status, and the antioxidant system. Sci. Hortic. 2017, 220, 90–101. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Tabssum, F.; Zaman, Q.; Chen, Y.; Riaz, U.; Ashraf, W.; Aslam, A.; Ehsan, N.; Nawaz, R.; Aziz, H.; Shah, S. Exogenous application of proline improved salt tolerance in rice through modulation of antioxidant activities. Pak. J. Agric. Res. 2019, 32, 140–151. [Google Scholar]
- Kumar, R.; Sharma, P.; Verma, S. Regulation of stress-responsive genes by secondary metabolites: A potential strategy for plant stress management. J. Agric. Biotechnol. 2019, 15, 322–338. [Google Scholar]
- Gupta, N.; Thind, S. Improving photosynthetic performance of bread wheat under field drought stress by foliar applied glycine betaine. J. Agric. Sci. Technol. 2015, 17, 75–86. [Google Scholar]
- Singh, J.; Kaur, N.; Sharma, R. arbutin enhances osmotic adjustment and membrane stability in tomato seedlings under salinity stress. J. Plant Stress Adapt. 2022, 18, 290–305. [Google Scholar]
- Patel, A.; Desai, R.; Mehta, P. Β-Estradiol enhances drought tolerance in rice by regulating oxidative stress and photosynthetic efficiency. Agric. Sci. Rev. 2023, 20, 55–70. [Google Scholar]
- Li, X.; Zhao, Y.; Wang, L. Synergistic effects of arbutin and β-estradiol in improving soybean stress tolerance. Plant Physiol. Rep. 2021, 13, 145–159. [Google Scholar]
- Gonzalez, F.; Martinez, P.; Rios, D. Β-Estradiol treatment enhances antioxidant enzyme activity in cucumber plants under oxidative stress. J. Plant Stress Biol. 2020, 10, 189–198. [Google Scholar]
- Markwell, J.; Osterman, J.C.; Mitchell, J.L. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [PubMed]
- Kumari, N.; Mishra, G.P.; Dikshit, H.K.; Gupta, S.; Roy, A.; Sinha, S.K.; Mishra, D.C.; Das, S.; Kumar, R.R.; Nair, R.M.; et al. Identification of quantitative trait loci (QTLs) regulating leaf SPAD value and trichome density in mungbean (Vigna radiata L.) using genotyping-by-sequencing (GBS) approach. PeerJ 2024, 12, e16722. [Google Scholar] [PubMed]
- Pierpoint, W.S. The extraction of enzymes from plant tissues rich in phenolic compounds. Methods Mol. Biol. 2004, 244, 65–74. [Google Scholar] [PubMed]
- Brueske, C.H. Phenylalanine ammonia lyase activity in tomato roots infected and resistant to the root-knot nematode, Meloidogyne incognita. Physiol. Plant Pathol. 1980, 16, 409–414. [Google Scholar]
- Fridovich, I. Superoxide radical and superoxide dismutases. In Oxygen and Living Processes: An Interdisciplinary Approach; Springer: Berlin/Heidelberg, Germany, 1981; pp. 250–272. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuc, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Sahu, P.K.; Shafi, Z.; Singh, S.; Ojha, K.; Jayalakshmi, K.; Tilgam, J.; Manzar, N.; Sharma, P.K.; Srivastava, A.K. Colonization potential of endophytes from halophytic plants growing in the “Runn of Kutch” salt marshes and their contribution to mitigating salt stress in tomato cultivation. Front. Microbiol. 2023, 14, 1226149. [Google Scholar]

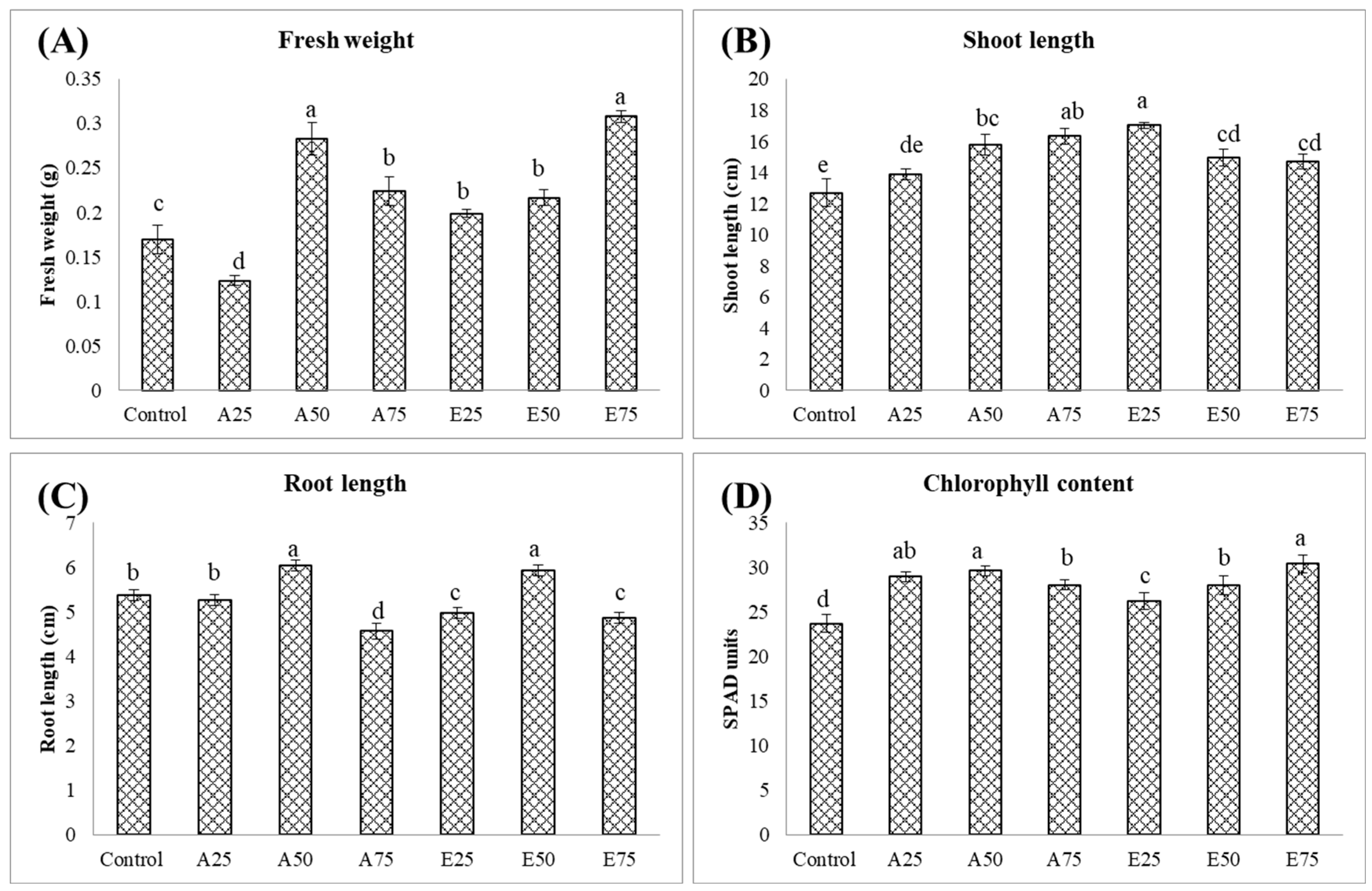
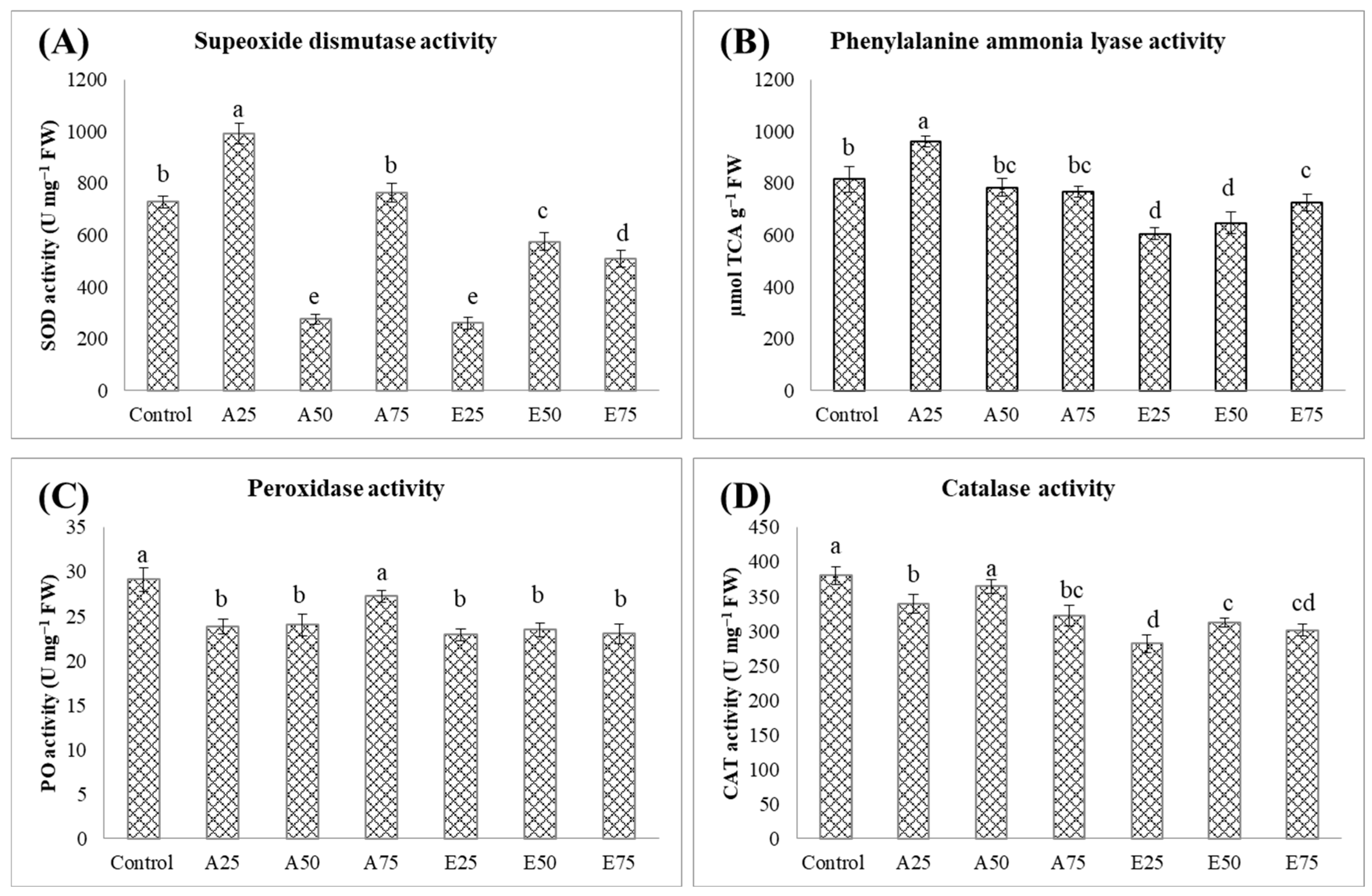
| Sl No. | Treatment | Fresh Weight (g) | Shoot Length (cm) | Chlorophyll Content (SPAD Units) |
|---|---|---|---|---|
| 1 | Negative Control | 0.17 de | 13.60 b | 27.33 b |
| 2 | Positive control | 0.15 f | 8.77 e | 22.77 cd |
| 3 | Traumatic acid | 0.16 ef | 9.70 d | 24.17 c |
| 4 | β-Estradiol | 0.24 a | 13.63 b | 26.87 b |
| 5 | Melatonin | 0.20 c | 11.73 c | 26.63 b |
| 6 | Arbutin | 0.22 b | 15.60 a | 29.67 a |
| 7 | α-Mangostin | 0.18 d | 9.23 de | 18.10 e |
| 8 | Endophyte (BTL5) | 0.18 d | 11.53 c | 21.93 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahu, P.K.; Dhal, K.N.; Kale, N.; Kumar, V.; Rai, N.; Gupta, A.; Jaiswal, D.K.; Srivastava, A.K. Unveiling the Role of Metabolites from a Bacterial Endophyte in Mitigating Soil Salinity and Reducing Oxidative Stress. Molecules 2025, 30, 1787. https://doi.org/10.3390/molecules30081787
Sahu PK, Dhal KN, Kale N, Kumar V, Rai N, Gupta A, Jaiswal DK, Srivastava AK. Unveiling the Role of Metabolites from a Bacterial Endophyte in Mitigating Soil Salinity and Reducing Oxidative Stress. Molecules. 2025; 30(8):1787. https://doi.org/10.3390/molecules30081787
Chicago/Turabian StyleSahu, Pramod Kumar, Krishna Nanda Dhal, Nakul Kale, Vivek Kumar, Niharika Rai, Amrita Gupta, Durgesh Kumar Jaiswal, and Alok Kumar Srivastava. 2025. "Unveiling the Role of Metabolites from a Bacterial Endophyte in Mitigating Soil Salinity and Reducing Oxidative Stress" Molecules 30, no. 8: 1787. https://doi.org/10.3390/molecules30081787
APA StyleSahu, P. K., Dhal, K. N., Kale, N., Kumar, V., Rai, N., Gupta, A., Jaiswal, D. K., & Srivastava, A. K. (2025). Unveiling the Role of Metabolites from a Bacterial Endophyte in Mitigating Soil Salinity and Reducing Oxidative Stress. Molecules, 30(8), 1787. https://doi.org/10.3390/molecules30081787








