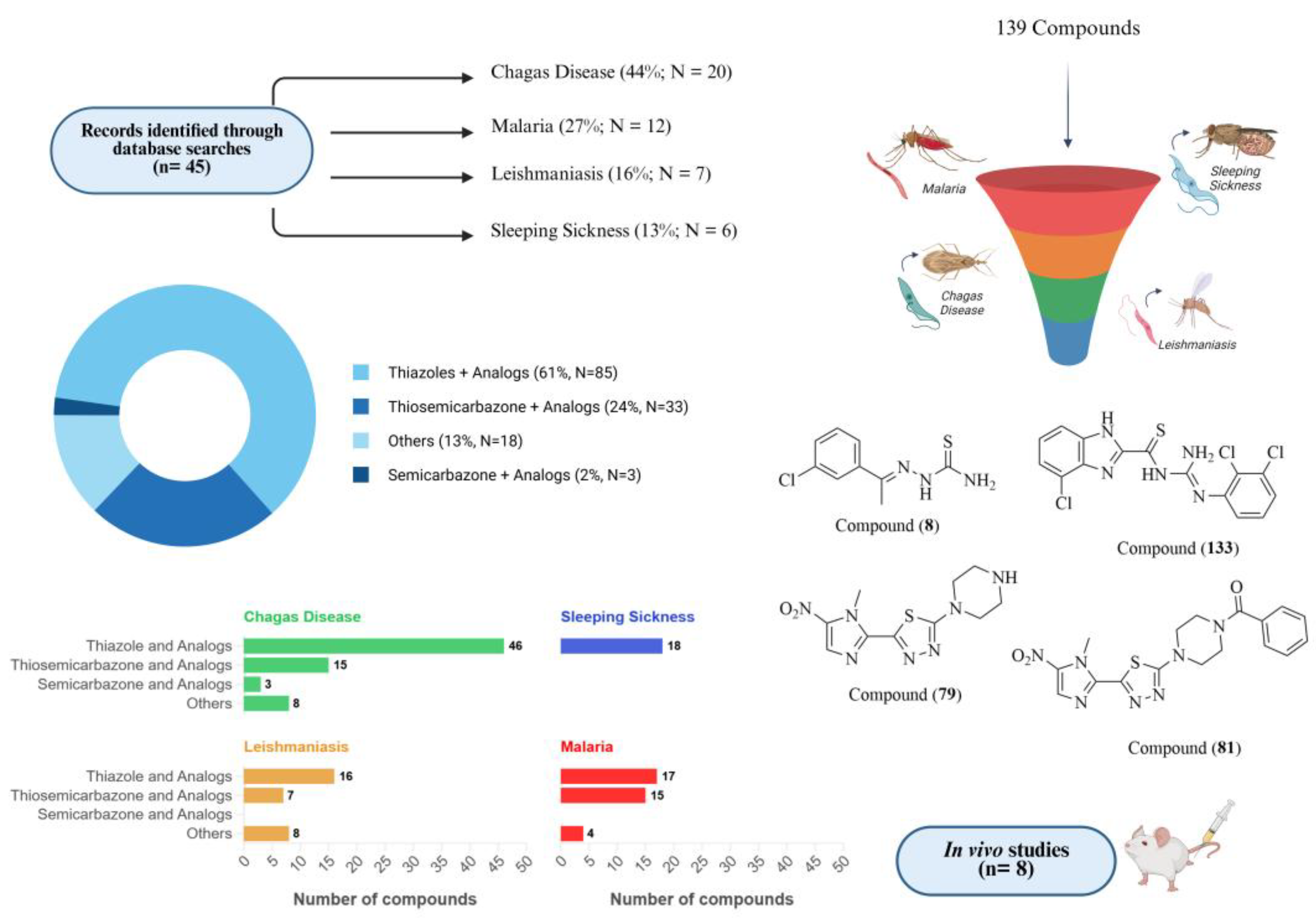
Recent Progress in Thiazole, Thiosemicarbazone, and Semicarbazone Derivatives as Antiparasitic Agents Against Trypanosomatids and Plasmodium spp.
Abstract
:1. Introduction
1.1. Chagas Disease
1.2. Sleeping Sickness
1.3. Leishmaniasis
1.4. Malaria
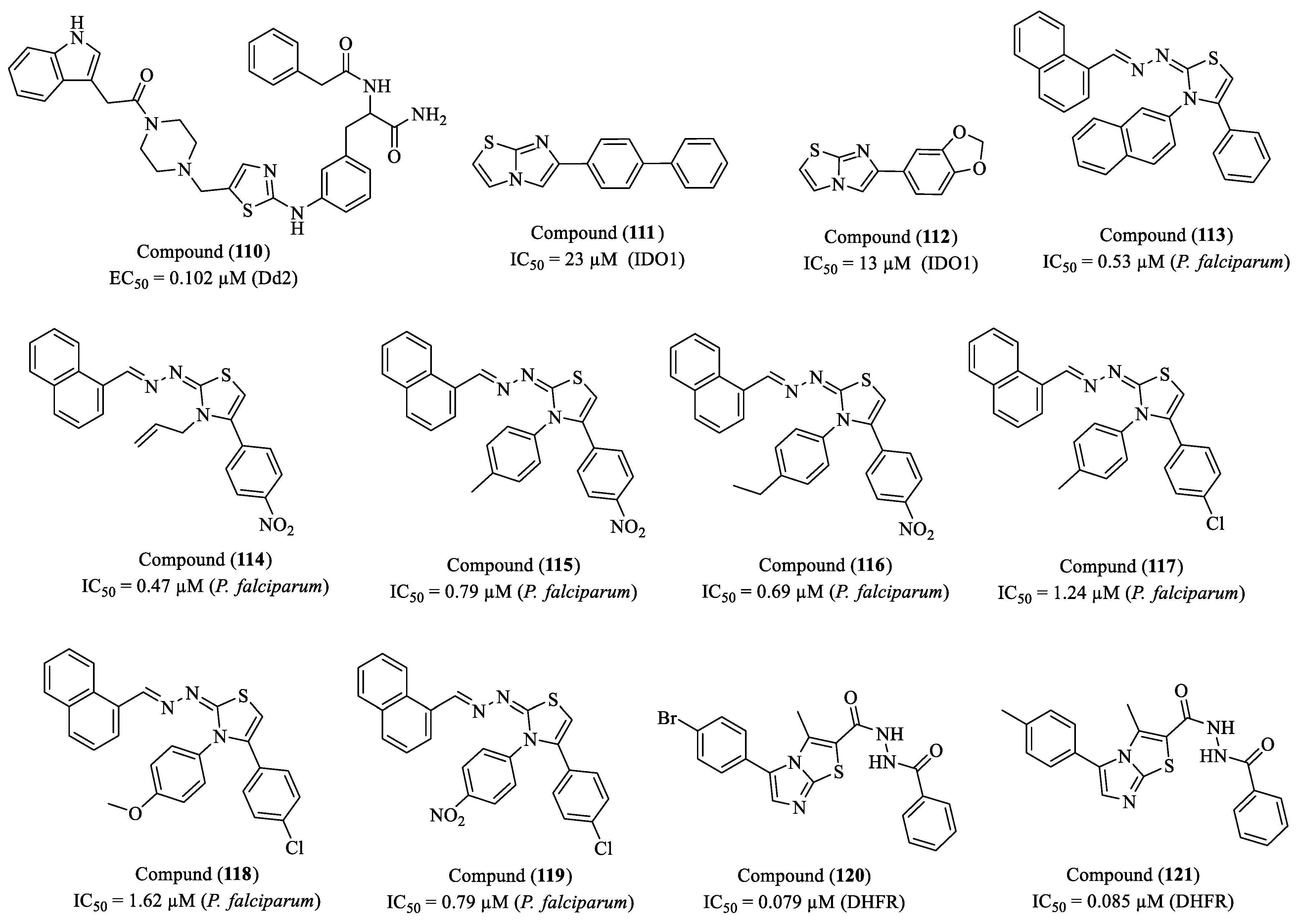
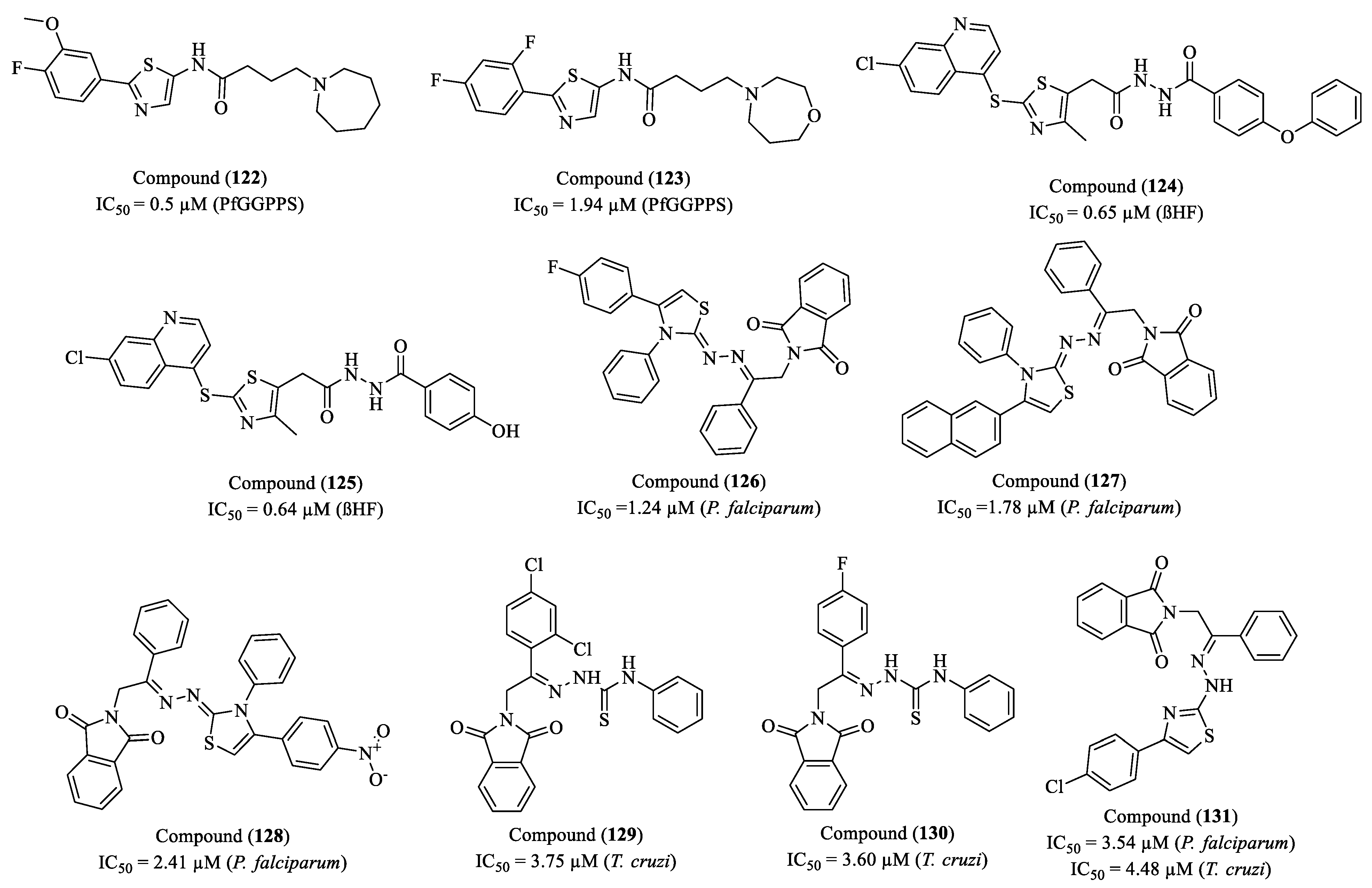
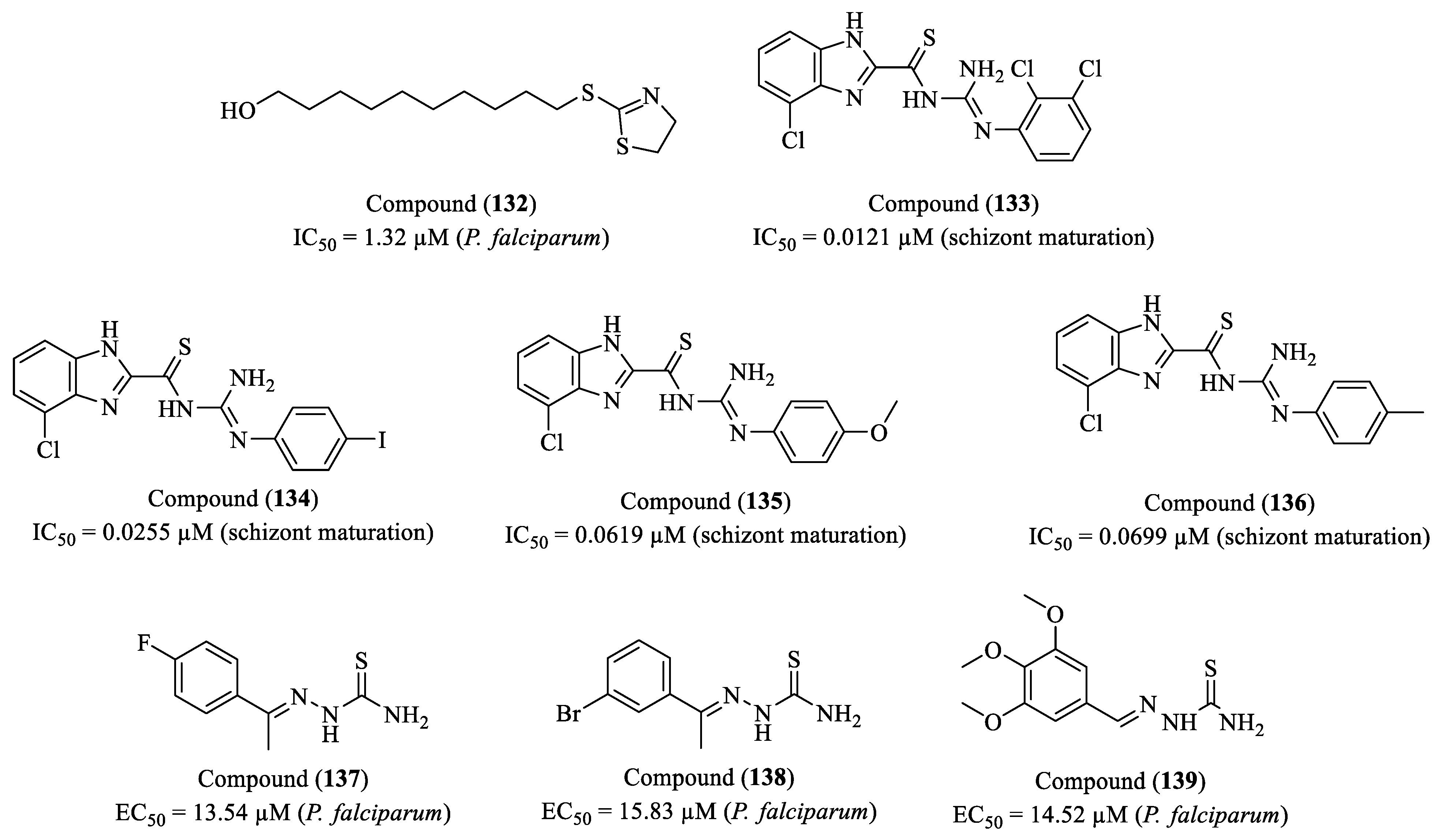
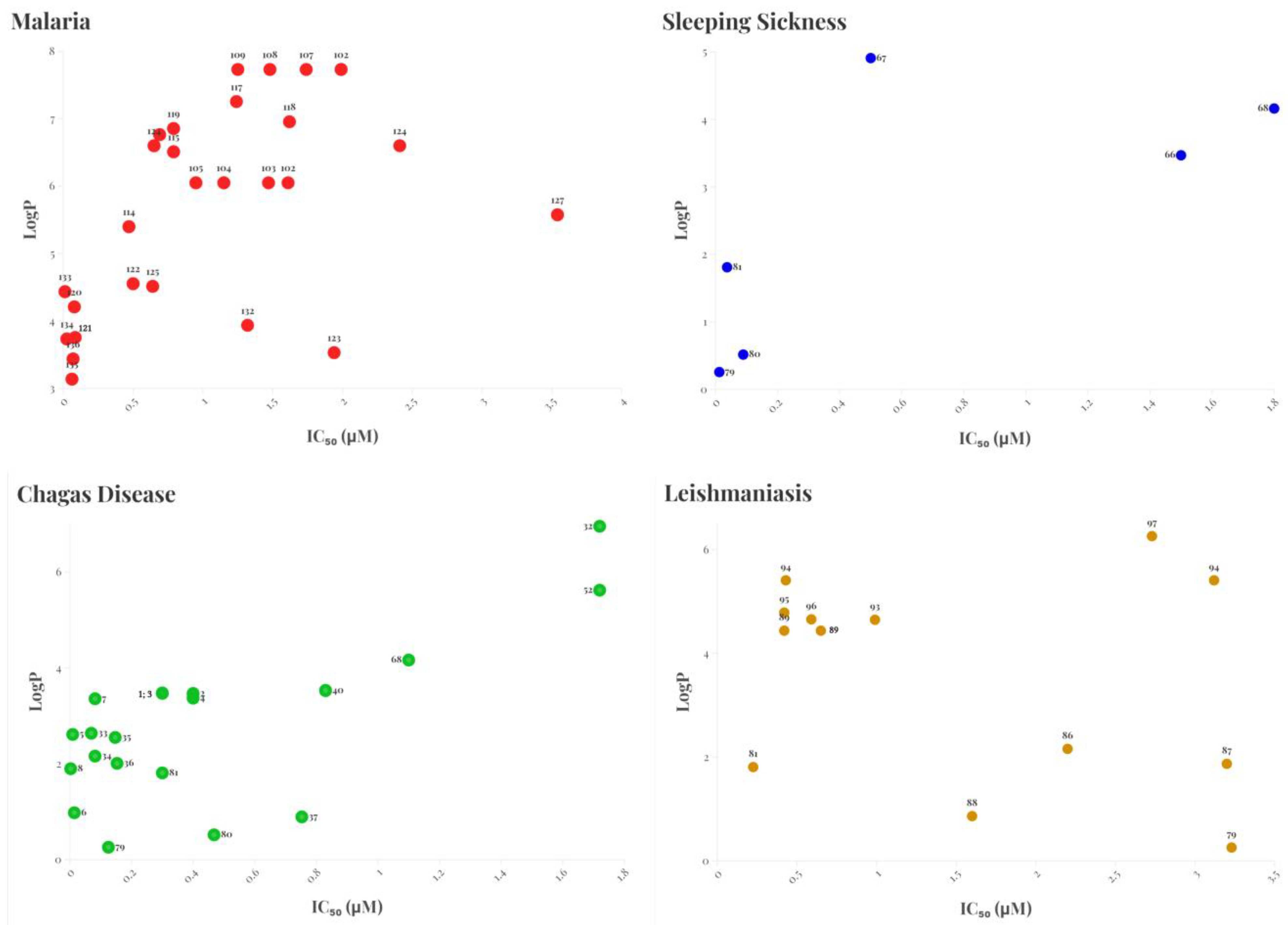
2. Perspectives
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMET | Absorption, distribution, metabolism, excretion, and toxicity |
| BBB | Blood–brain barrier |
| BZD | Benznidazole |
| CC50 | Concentration of cytotoxicity 50% |
| CPB | Cysteine protease B |
| DAC | Diminazene aceturate |
| DHFR | Dihydrofolate reductase |
| DNA | Deoxyribonucleic acid |
| DNDi | Drugs for Neglected Diseases Initiative |
| EC50 | Half maximal effective concentration |
| EpA | Oxidation potential |
| EpC | Reduction potential |
| FP2 | Falcipain-2 |
| HAT | Human African trypanosomiasis |
| IC50 | Half maximal inhibitory concentration |
| IDO1 | Indoleamine 2,3-dioxygenase 1 |
| ITPK1 | Inositol-tetrakisphosphate 1-kinase |
| Kd | Dissociation constant |
| Ki | Inhibition constant |
| L. spp. | Leishmania spp. |
| LC50 | Lethal concentration 50% |
| LD50 | Lethal dose 50% |
| LogP | Logarithm of the octanol–water partition coefficient |
| MTX | Methotrexate |
| NO | Nitric oxide |
| NTDs | Neglected tropical diseases |
| P. spp. | Plasmodium spp. |
| PfFPPS/GGPPS | Farnesyl/geranylgeranyl diphosphate synthase |
| PTR1 | Pteridine reductase 1 |
| QSAR | Quantitative structure–activity relationship |
| ROS | Reactive oxygen species |
| RR | Ribonucleotide reductase |
| SAR | Structure–activity relationship |
| SEM | Scanning electron microscopy |
| SI | Selectivity index |
| T. cruzi | Trypanosoma cruzi |
| T. b. spp. | Trypanosoma brucei spp. |
| TbCatL | Trypanosoma brucei cathepsin L |
| TcrPDEC | Phosphodiesterase C |
| TR | Trypanothione reductase |
References
- WHO. Neglected Tropical Diseases—GLOBAL, World Health Organization. 2025. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 10 February 2025).
- Feasey, N.; Wansbrough-Jones, M.; Mabey, D.C.W.; Solomon, A.W. Neglected tropical diseases. Br. Med. Bull. 2010, 93, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Malecela, M.N.; Ducker, C. A road map for neglected tropical diseases 2021–2030. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Branda, F.; Ali, A.Y.; Ceccarelli, G.; Albanese, M.; Binetti, E.; Giovanetti, M.; Ciccozzi, M.; Scarpa, F. Assessing the Burden of Neglected Tropical Diseases in Low-Income Communities: Challenges and Solutions. Viruses 2024, 17, 29. [Google Scholar] [CrossRef]
- Conteh, L.; Engels, T.; Molyneux, D.H. Socioeconomic aspects of neglected tropical diseases. Lancet 2010, 375, 239–247. [Google Scholar] [CrossRef]
- Abou-El-Naga, I.F. Demographic, socioeconomic and environmental changes affecting circulation of neglected tropical diseases in Egypt. Asian Pac. J. Trop. Med. 2015, 8, 881–888. [Google Scholar] [CrossRef]
- Houweling, T.A.J.; Karim-Kos, H.E.; Kulik, M.C.; Stolk, W.A.; Haagsma, J.A.; Lenk, E.J.; Richardus, J.H.; de Vlas, S.J. Socioeconomic Inequalities in Neglected Tropical Diseases: A Systematic Review. PLoS Negl. Trop. Dis. 2016, 10, e0004546. [Google Scholar] [CrossRef]
- DNDi, STRATEGIC PLAN 2021–2028-25 TREATMENTS IN 25 YEARS, Drugs for Neglected Diseases Initiative. 2021. Available online: https://dndi.org/wp-content/uploads/2021/03/DNDi-StrategicPlan-2021-2028.pdf (accessed on 2 February 2025).
- Mejía-Jaramillo, A.M.; Fernández, G.J.; Montilla, M.; Nicholls, R.S.; Triana-Chávez, O. Sensibilidad al benzonidazol de cepas de Trypanosoma cruzi sugiere la circulación de cepas naturalmente resistentes en Colombia. Biomédica 2012, 32, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R. Present situation and new strategies for Chagas disease chemotherapy: A proposal. Mem. Inst. Oswaldo Cruz 2009, 104, 549–554. [Google Scholar] [CrossRef]
- Greco, A.; Karki, R.; Gadiya, Y.; Deecke, C.; Zaliani, A.; Gul, S. Pharmacological profiles of neglected tropical disease drugs. Artif. Intell. Life Sci. 2024, 6, 100116. [Google Scholar] [CrossRef]
- Akinsolu, F.T.; Nemieboka, P.O.; Njuguna, D.W.; Ahadji, M.N.; Dezso, D.; Varga, O. Emerging resistance of neglected tropical diseases: A scoping review of the literature. Int. J. Environ. Res. Public Health 2019, 16, 1925. [Google Scholar] [CrossRef]
- Joshi, G.; Quadir, S.S.; Yadav, K.S. Road map to the treatment of neglected tropical diseases: Nanocarriers interventions. J. Control. Release 2021, 339, 51–74. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, K.K.; Bandyopadhyay, D. Heterocycles in the Treatment of Neglected Tropical Diseases. Curr. Med. Chem. 2021, 28, 472–495. [Google Scholar] [CrossRef] [PubMed]
- Shiran, J.A.; Kaboudin, B.; Panahi, N.; Razzaghi-Asl, N. Privileged small molecules against neglected tropical diseases: A perspective from structure activity relationships. Eur. J. Med. Chem. 2024, 271, 116396. [Google Scholar] [CrossRef] [PubMed]
- Goupil, L.S.; McKerrow, J.H. Introduction: Drug discovery and development for neglected diseases. Chem. Rev. 2014, 114, 11131–11137. [Google Scholar] [CrossRef]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole ring—A biologically active scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef]
- Silva, B.V.; Silva, B.N.M. Thio-and Semicarbazones: Hope in the Search for Treatment of Leishma-niasis and Chagas Disease. Med. Chem. 2017, 13, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, D.S.; Quach, P.; Richardson, D.R. Thiosemicarbazones: The new wave in cancer treatment. Future Med. Chem. 2009, 1, 1143–1151. [Google Scholar] [CrossRef]
- Jacob, Í.T.T.; Gomes, F.O.S.; de Miranda, M.D.S.; de Almeida, S.M.V.; da Cruz-Filho, I.J.; Peixoto, C.A.; da Silva, T.G.; Moreira, D.R.M.; de Melo, C.M.L.; de Oliveira, J.F.; et al. Anti-inflammatory activity of novel thiosemicarbazone compounds indole-based as COX inhibitors. Pharmacol. Rep. 2021, 73, 907–925. [Google Scholar] [CrossRef]
- Narasimhamurthy, K.H.; Swaroop, T.R.; Rangappa, K.S. A review on progress of thiazole derivatives as potential anti-inflammatory agents. Eur. J. Med. Chem. Rep. 2024, 12, 100225. [Google Scholar] [CrossRef]
- Koshelev, V.N.; Primerova, O.V.; Vorobyev, S.V.; Stupnikova, A.S.; Ivanova, L.V. Synthesis and Antioxidant Activity of Novel Thiazole and Thiazolidinone Derivatives with Phenolic Fragments. Appl. Sci. 2023, 13, 13112. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Le, T.H.; Bui, T.T.T. Antioxidant activities of thiosemicarbazones from substituted benzaldehydes and N-(tetra-O-acetyl-β-d-galactopyranosyl)thiosemicarbazide. Eur. J. Med. Chem. 2013, 60, 199–207. [Google Scholar] [CrossRef]
- Ali, S.M.M.; Jesmin, M.; Azad, M.A.K.; Islam, M.K.; Zahan, R. Anti-inflammatory and analgesic activities of acetophenone semicarbazone and benzophenone semicarbazone. Asian Pac. J. Trop. Biomed. 2012, 2, S1036–S1039. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, N.P. Synthesis, anti-inflammatory and analgesic evaluation of thiazole/oxazole substituted benzothiazole derivatives. Bioorg. Chem. 2021, 107, 104608. [Google Scholar] [CrossRef] [PubMed]
- Fabra, D.; Amariei, G.; Ruiz-Camino, D.; Matesanz, A.I.; Rosal, R.; Quiroga, A.G.; Horcajada, P.; Hidalgo, T. Proving the Antimicrobial Therapeutic Activity on a New Copper–Thiosemicarbazone Complex. Mol. Pharm. 2024, 21, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.T.; Elsharabasy, S.A.; Abdel-Aziem, A. Synthesis and antimicrobial activity of new series of thiazoles, pyridines and pyrazoles based on coumarin moiety. Sci. Rep. 2023, 13, 9912. [Google Scholar] [CrossRef]
- Sahil; Kaur, K.; Jaitak, V. Thiazole and Related Heterocyclic Systems as Anticancer Agents: A Review on Synthetic Strategies, Mechanisms of Action and SAR Studies. Curr. Med. Chem. 2022, 29, 4958–5009. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Q.; Wang, S.; Zeng, B.; Du, G.; Zhang, C.; Li, Y. Synthesis, cytotoxicity, and in vivo antitumor activity study of parthenolide semicarbazones and thiosemicarbazones. Bioorg. Med. Chem. 2020, 28, 115557. [Google Scholar] [CrossRef] [PubMed]
- Anthwal, T.; Nain, S. 1,3,4-Thiadiazole Scaffold: As Anti-Epileptic Agents. Front. Chem. 2022, 9, 671212. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Karpińska, M.; Matysiak, J.; Niewiadomy, A. Characterization and preliminary anticonvulsant assessment of some 1,3,4-thiadiazole derivatives. Pharmacol. Rep. 2015, 67, 588–592. [Google Scholar] [CrossRef]
- Marković, A.; Živković, A.; Atanasova, M.; Doytchinova, I.; Hofmann, B.; George, S.; Kretschmer, S.; Rödl, C.; Steinhilber, D.; Stark, H.; et al. Thiazole derivatives as dual inhibitors of deoxyribonuclease I and 5-lipoxygenase: A promising scaffold for the development of neuroprotective drugs. Chem. Biol. Interact 2023, 381, 110542. [Google Scholar] [CrossRef]
- Ahsan, M.; Stables, J. Psychomotor Seizure Test, Neurotoxicity and in vitro Neuroprotection Assay of some Semicarbazone Analogues. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 141–147. [Google Scholar] [CrossRef]
- Moreno-Rodríguez, A.; Salazar-Schettino, P.M.; Bautista, J.L.; Hernández-Luis, F.; Torrens, H.; Guevara-Gómez, Y.; Pina-Canseco, S.; Torres, M.B.; Cabrera-Bravo, M.; Martinez, C.M.; et al. In vitro antiparasitic activity of new thiosemicarbazones in strains of Trypanosoma cruzi. Eur. J. Med. Chem. 2014, 87, 23–29. [Google Scholar] [CrossRef]
- de Moraes Gomes, P.A.T.; de Oliveira Barbosa, M.; Santiago, E.F.; de Oliveira Cardoso, M.V.; Costa, N.T.C.; Hernandes, M.Z.; Moreira, D.R.M.; da Silva, A.C.; Santos, T.A.R.D.; Pereira, V.R.A.; et al. New 1,3-thiazole derivatives and their biological and ultrastructural effects on Trypanosoma cruzi. Eur. J. Med. Chem. 2016, 121, 387–398. [Google Scholar] [CrossRef]
- Nitin, M.; Pawar, S.; Chougle, M. A Review Article on Chemistry, Synthesis and Therapeutic Importance of Thiazole Derivatives. Int. J. Adv. Res. Sci. Commun. Technol. IJARSCT 2022, 2. [Google Scholar] [CrossRef]
- Patil, A.R.; Yallur, B.C.; Chinnam, S.; Ananthnag, G.S.; Santhosh, C.R.; Pant, G.; Kumar, S.G.P.; Hadagali, M.D. Pharmaceutical perspectives of thiazole analogues: An overview. Results Chem. 2024, 12, 101820. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Alfaifi, G.H.; Gomha, S.M. Recent Literature on the Synthesis of Thiazole Derivatives and their Biological Activities. Mini-Rev. Med. Chem. 2023, 24, 196–251. [Google Scholar] [CrossRef] [PubMed]
- Kempf, D.J.; Sham, H.L.; Marsh, K.C.; Flentge, C.A.; Betebenner, D.; Green, B.E.; McDonald, E.; Vasavanonda, S.; Saldivar, A.; Wideburg, N.E.; et al. Discovery of ritonavir, a potent inhibitor of HIV protease with high oral bioavailability and clinical efficacy. J. Med. Chem. 1998, 41, 602–617. [Google Scholar] [CrossRef]
- Pereira, M.; Vale, N. Ritonavir’s Evolving Role: A Journey from Antiretroviral Therapy to Broader Medical Applications. Curr. Oncol. 2024, 31, 6032–6049. [Google Scholar] [CrossRef] [PubMed]
- Bucourt, R.; Bormann, D.; Heymes, R.; Perronnet, M. Chemistry of cefotaxime. J. Antimicrob. Chemother. 1980, 6, 63–67. [Google Scholar] [CrossRef]
- Furst, D.E. Meloxicam: Selective COX-2 inhibition in clinical practice. Semin. Arthritis Rheum. 1997, 26, 21–27. [Google Scholar] [CrossRef]
- Distel, M.; Mueller, C.; Bluhmki, E.; Fries, J. Safety of Meloxicam: A Global Analysis of Clinical Trials. Rheumatology 1996, 35, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I.A.; Abramson, V.G.; Formisano, L.; Balko, J.M.; Estrada, M.V.; Sanders, M.E.; Juric, D.; Solit, D.; Berger, M.F.; Won, H.H.; et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kα-Specific Inhibitor, with Letrozole in ER+/HER2− Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 26–34. [Google Scholar] [CrossRef]
- Sharma, P.; Abramson, V.G.; O’Dea, A.; Nye, L.; Mayer, I.; Pathak, H.B.; Hoffmann, M.; Stecklein, S.R.; Elia, M.; Lewis, S.; et al. Clinical and Biomarker Results from Phase I/II Study of PI3K Inhibitor Alpelisib plus Nab-paclitaxel in HER2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2021, 27, 3896–3904. [Google Scholar] [CrossRef]
- Beraldo, H.; Gambino, D. The Wide Pharmacological Versatility of Semicarbazones, Thiosemicarba-zones and Their Metal Complexes. Mini Rev. Med. Chem. 2004, 4, 31–39. [Google Scholar]
- Mir, I.A.; Ain, Q.U.; Qadir, T.; Malik, A.Q.; Jan, S.; Shahverdi, S.; Nabi, S.A. A review of semicarbazone-derived metal complexes for application in biomedicine and related fields. J. Mol. Struct. 2024, 1295, 136216. [Google Scholar] [CrossRef]
- Lee, P.F.; Yang, C.-T.; Fan, D.; Vittal, J.J.; Ranford, J.D. Synthesis, characterization and physicochemical properties of copper(II) complexes containing salicylaldehyde semicarbazone. Polyhedron 2003, 22, 2781–2786. [Google Scholar] [CrossRef]
- Richardson, D.R.; Kalinowski, D.S.; Richardson, V.; Sharpe, P.C.; Lovejoy, D.B.; Islam, M.; Bernhardt, P.V. 2-Acetylpyridine Thiosemicarbazones are Potent Iron Chelators and Antiproliferative Agents: Redox Activity, Iron Complexation and Characterization of their Antitumor Activity. J. Med. Chem. 2009, 52, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Malarz, K.; Mrozek-Wilczkiewicz, A.; Serda, M.; Rejmund, M.; Polanski, J.; Musiol, R. The role of oxidative stress in activity of anticancer thiosemicarbazones. Oncotarget 2018, 9, 17689–17710. [Google Scholar] [CrossRef]
- Murren, J.; Modiano, M.; Clairmont, C.; Lambert, P.; Savaraj, N.; Doyle, T.; Sznol, M. Phase I and Pharmacokinetic Study of Triapine, a Potent Ribonucleotide Reductase Inhibitor, Administered Daily for Five Days in Patients with Advanced Solid Tumors 1, n.d. Available online: http://aacrjournals.org/clincancerres/article-pdf/9/11/4092/2083933/df1103004092.pdf (accessed on 4 February 2025).
- Nutting, C.M.; van Herpen, C.M.L.; Miah, A.B.; Bhide, S.A.; Machiels, J.-P.; Buter, J.; Kelly, C.; de Raucourt, D.; Harrington, K.J. Phase II study of 3-AP Triapine in patients with recurrent or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2009, 20, 1275–1279. [Google Scholar] [CrossRef]
- Okwera, A.; Byekwaso, F.; Mugerwa, R.; Okwera, A.; Byekwaso, F.; Ellner, J.; Vjecha, M.; Johnson, J.; Whalen, C.; Huebner, R. Randomised trial of thiacetazone and rifampicin-containing regimens for pulmonary tuberculosis in HIV-infected Ugandans. Lancet 1994, 344, 1323–1328. [Google Scholar] [CrossRef]
- Qian, L.; de Montellano, P.R.O. Oxidative Activation of Thiacetazone by the Mycobacterium tuberculosis Flavin Monooxygenase EtaA and Human FMO1 and FMO3. Chem. Res. Toxicol. 2006, 19, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Alkhatip, A.A.A.M.M.; Georgakis, M.; Valenzuela, L.R.M.; Hamza, M.; Farag, E.; Hodgkinson, J.; Hosny, H.; Kamal, A.M.; Wagih, M.; Naguib, A.; et al. Metal-Bound Methisazone; Novel Drugs Targeting Prophylaxis and Treatment of SARS-CoV-2, a Molecular Docking Study. Int. J. Mol. Sci. 2021, 22, 2977. [Google Scholar] [CrossRef]
- West, D.X.; El-Sawaf, A.K.; Bain, G.A. Metal complexes of N(4)-substituted analogues of the antiviral drug methisazone {1–methylisatin thiosemicarbazone}. Transit. Met. Chem. 1997, 23, 1–6. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Nitrofural (Nitrofurazone). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans/World Health Organization. Int. Agency Res. Cancer 1990, 50, 195–209. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526235/ (accessed on 11 February 2025).
- Fathy, U. Thiazole derivatives: Prospectives and biological applications. J. Sulfur Chem. 2024, 45, 786–828. [Google Scholar] [CrossRef]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef]
- Rocha-Roa, C.; Molina, D.; Cardona, N. A perspective on thiazolidinone scaffold development as a new therapeutic strategy for toxoplasmosis. Front. Cell Infect Microbiol. 2018, 8, 411332. [Google Scholar] [CrossRef]
- Martins, S.C.; Lazarin-Bidóia, D.; Desoti, V.C.; Falzirolli, H.; da Silva, C.C.; Ueda-Nakamura, T.; de O. Silva, S.; Nakamura, C.V. 1,3,4-Thiadiazole derivatives of R-(+)-limonene benzaldehyde-thiosemicarbazones cause death in Trypanosoma cruzi through oxidative stress. Microbes Infect 2016, 18, 787–797. [Google Scholar] [CrossRef]
- Tahghighi, A.; Babalouei, F. Thiadiazoles: The appropriate pharmacological scaffolds with leishmanicidal and antimalarial activities: A review. Iran. J. Basic Med. Sci. 2017, 20, 613–622. [Google Scholar] [CrossRef]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole—A Promising Structure in Medicinal Chemistry. ChemMedChem 2013, 8, 27–41. [Google Scholar] [CrossRef]
- Scarim, C.B.; Jornada, D.H.; Machado, M.G.M.; Ferreira, C.M.R.; Santos, J.L.D.; Chung, M.C. Thiazole, thio and semicarbazone derivatives against tropical infective diseases: Chagas disease, human African trypanosomiasis (HAT), leishmaniasis, and malaria. Eur. J. Med. Chem. 2019, 162, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The history of Chagas disease. Parasit. Vectors 2014, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- WHO. Chagas Disease (Also Known as American Trypanosomiasis), World Health Organization 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease- (accessed on day month year).
- Carod-Artal, F.J. American trypanosomiasis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 103–123. [Google Scholar] [CrossRef]
- Kalil-Filho, R. Globalization of Chagas Disease Burden and New Treatment Perspectives. J. Am. Coll. Cardiol. 2015, 66, 1190–1192. [Google Scholar] [CrossRef]
- Dias, J.C.P. Human Chagas Disease and Migration in the Context of Globalization: Some Particular Aspects. J. Trop. Med. 2013, 2013, 789758. [Google Scholar] [CrossRef]
- da Costa, A.C.; Rocha, E.A.; da Silva Filho, J.D.; de Barros Vasconcelos Fidalgo, A.S.O.; Nunes, F.M.M.; Viana, C.E.M.; Gomes, V.B.A.F.; de Fátima Oliveira, M. Prevalence of trypanosoma cruzi infection in blood donors. Arq. Bras. Cardiol. 2020, 115, 1082–1091. [Google Scholar] [CrossRef]
- Angheben, A.; Boix, L.; Buonfrate, D.; Gobbi, F.; Bisoffi, Z.; Pupella, S.; Gandini, G.; Aprili, G. Chagas disease and transfusion medicine: A perspective from non-endemic countries. Blood Transfus. 2015, 13, 540–550. [Google Scholar] [CrossRef]
- Robertson, L.J.; Havelaar, A.H.; Keddy, K.H.; Devleesschauwer, B.; Sripa, B.; Torgerson, P.R. The importance of estimating the burden of disease from foodborne transmission of Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2024, 18, e0011898. [Google Scholar] [CrossRef]
- Ferreira, R.T.B.; Cabral, M.L.; Martins, R.S.; Araujo, P.F.; da Silva, S.A.; Britto, C.; Branquinho, M.R.; Cardarelli-Leite, P.; Moreira, O.C. Detection and genotyping of Trypanosoma cruzi from açai products commercialized in Rio de Janeiro and Pará, Brazil. Parasit. Vectors 2018, 11, 233. [Google Scholar] [CrossRef]
- JHofflin, M.; Sadler, R.H.; Araujo, F.G.; Page, W.E.; Remington, J.S. Laboratory-acquired Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 437–440. [Google Scholar] [CrossRef]
- Kinoshita-Yanaga, A.T.; de Ornelas Toledo, M.J.; de Araújo, S.M.; Vier, B.P.; Gomes, M.L. Accidental infection by Trypanosoma cruzi follow-up by the polymerase chain reaction: Case report. Rev. Inst. Med. Trop. Sao Paulo 2009, 51, 295–298. [Google Scholar] [CrossRef]
- Cucunubá, Z.M.; Gutiérrez-Romero, S.A.; Ramírez, J.-D.; Velásquez-Ortiz, N.; Ceccarelli, S.; Parra-Henao, G.; Henao-Martínez, A.F.; Rabinovich, J.; Basáñez, M.-G.; Nouvellet, P.; et al. The epidemiology of Chagas disease in the Americas. Lancet Reg. Health-Am. 2024, 37, 100881. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.L.P.; Collaborators, R.A.S.S.; Machado, Í.; Cousin, E.; Perel, P.; Demacq, C.; Geissbühler, Y.; de Souza, A.; Liprandi, A.S.; Nascimento, B.R.; et al. The Burden of Chagas Disease in the Contemporary World: The RAISE Study. Glob. Heart 2024, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Medeiros, C.; de Araújo Silva, M.B.; de Oliveira, A.L.S.; Alves, S.M.M.; da Silveira Barros, M.D.N.D.; da Glória Aureliano de Melo Cavalcanti, M.; de Azevedo Oliveira, G.M.; de Fátima Velloso Carrazzone, C.; de Oliveira, W.A., Jr.; de Medeiros, Z.M. Mapping the morbidity and mortality of Chagas disease in an endemic area in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e5. [Google Scholar] [CrossRef]
- Andrade, M.V.; de Souza Noronha, K.V.M.; de Souza, A.; Motta-Santos, A.S.; Braga, P.E.F.; Bracarense, H.; de Miranda, M.C.C.; Nascimento, B.R.; Molina, I.; Martins-Melo, F.R.; et al. The economic burden of Chagas disease: A systematic review. PLoS Negl. Trop. Dis. 2023, 17, e0011757. [Google Scholar] [CrossRef]
- Crespillo-Andújar, C.; Comeche, B.; Hamer, D.H.; Arevalo-Rodriguez, I.; Alvarez-Díaz, N.; Zamora, J.; Pérez-Molina, J.A. Use of benznidazole to treat chronic Chagas disease: An updated systematic review with a meta-analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010386. [Google Scholar] [CrossRef]
- Bermudez, J.; Davies, C.; Simonazzi, A.; Real, J.P.; Palma, S. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 2016, 156, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.; Dias, N.; Paiva, T.; Hagström-Bex, L.; Nitz, N.; Pratesi, R.; Hecht, M. Current trends in the pharmacological management of Chagas disease. Int. J. Parasitol. Drugs Drug Resist. 2020, 12, 7–17. [Google Scholar] [CrossRef]
- Francisco, A.F.; Jayawardhana, S.; Olmo, F.; Lewis, M.D.; Wilkinson, S.R.; Taylor, M.C.; Kelly, J.M. Challenges in Chagas Disease Drug Development. Molecules 2020, 25, 2799. [Google Scholar] [CrossRef]
- PAHO/WHO. Less Than 10% of People with Chagas Receive a Diagnosis, Pan American Health Organization 2023. Available online: https://www.paho.org/en/news/13-4-2023-less-10-people-chagas-receive-diagnosis (accessed on 10 February 2025).
- Schijman, A.G.; Alonso-Padilla, J.; Britto, C.; Bernal, C.P.H. Retrospect, advances and challenges in Chagas disease diagnosis: A comprehensive review. Lancet Reg. Health-Am. 2024, 36, 100821. [Google Scholar] [CrossRef]
- Saraiva, R.M.; Mediano, M.F.F.; Mendes, F.S.N.S.; da Silva, G.M.S.; Veloso, H.H.; Sangenis, L.H.C.; da Silva, P.S.; Mazzoli-Rocha, F.; Sousa, A.S.; Holanda, M.T.; et al. Chagas heart disease: An overview of diagnosis, manifestations, treatment, and care. World J. Cardiol. 2021, 13, 654. [Google Scholar] [CrossRef]
- Britta, E.A.; Scariot, D.B.; Falzirolli, H.; Da Silva, C.C.; Ueda-Nakamura, T.; Filho, B.P.D.; Borsali, R.; Nakamura, C.V. 4-Nitrobenzaldehyde thiosemicarbazone: A new compound derived from S-(-)-limonene that induces mitochondrial alterations in epimastigotes and trypomastigotes of Trypanosoma cruzi. Parasitology 2015, 142, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Varela, J.; Cruces, E.; Fernández, M.; Gabay, M.; Leal, S.M.; Escobar, P.; Sanabria, L.; Serna, E.; Torres, S.; et al. Identification of a new amide-containing thiazole as a drug candidate for treatment of Chagas’ disease. Antimicrob. Agents Chemother. 2015, 59, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- de Melo Silva, V.G.; da Silva Sousa, L.M.; Junior, E.L.F.; Brondani, G.L.; de Albuquerque Oliveira, I.M.; Bedor, D.C.G.; Lopes, I.B.P.; Brayner, F.A.; Alves, L.C.; de Andrade Cavalcante, M.K.; et al. New series of 3-pyridyl-1,3-thiazoles: In vitro and in vivo anti-Trypanosomatidae profile, in vitro and in silico mechanism of action approach. Eur. J. Med. Chem. 2025, 284, 117191. [Google Scholar] [CrossRef]
- Rubio-Hernández, M.; Alcolea, V.; da Silva, E.B.; Giardini, M.A.; Fernandes, T.H.M.; Martínez-Sáez, N.; O’Donoghue, A.J.; Siqueira-Neto, J.L.; Pérez-Silanes, S. Synthesis and Biological Evaluation of New Chalcogen Semicarbazone (S, Se) and Their Azole Derivatives against Chagas Disease. J. Med. Chem. 2024, 67, 19038–19056. [Google Scholar] [CrossRef]
- Cristovão-Silva, A.C.; Brelaz-de-Castro, M.C.A.; da Silva, E.D.; Leite, A.C.L.; Santiago, L.B.A.A.; da Conceição, J.M.; da Silva Tiburcio, R.; de Santana, D.P.; Bedor, D.C.G.; de Carvalho, B.Í.V.; et al. Trypanosoma cruzi killing and immune response boosting by novel phenoxyhydrazine-thiazole against Chagas disease. Exp. Parasitol. 2024, 261, 108749. [Google Scholar] [CrossRef]
- de Souza, T.P.; Orlando, L.M.R.; da Silva Lara, L.; Paes, V.B.; Dutra, L.P.; Santos, M.S.D.; de Souza Pereira, M.C. Synthesis and Anti-Trypanosoma cruzi Activity of New Pyrazole-Thiadiazole Scaffolds. Molecules 2024, 29, 3544. [Google Scholar] [CrossRef]
- Haroon, M.; Akhtar, T.; Mehmood, H.; da Silva Santos, A.C.; da Conceição, J.M.; Brondani, G.L.; da Silva Tibúrcio, R.; Bedor, D.C.G.; da Silva, J.W.V.; Junior, P.A.S.; et al. Synthesis of hydrazinyl–thiazole ester derivatives, in vitro trypanocidal and leishmanicidal activities. Future Med. Chem. 2024, 16, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, D.T.; Oliveira, F.H.; Salles, J.P.; Bello, M.L.; Rodrigues, C.R.; Castro, H.C.; de Souza, H.; Reis, C.; Leme, R.; Mafra, J.; et al. Synthesis, biological evaluation and molecular modeling studies of new thiadiazole derivatives as potent P2X7 receptor inhibitors. Front. Chem. 2019, 7, 261. [Google Scholar] [CrossRef]
- Faria, A.F.M.; de Souza Ferreira Pereira, C.; Teixeira, G.P.; Galvão, R.M.D.S.; Pacheco, P.A.F.; Bello, M.L.; de Jesus, D.H.; Calabrese, K.; Gonzaga, D.T.G.; Boechat, N.; et al. In vitro evaluation of 2-(1H-pyrazol-1-yl)-1,3,4-thiadiazole derivatives against replicative and infective stages of Trypanosoma cruzi. J. Bioenerg. Biomembr. 2023, 55, 409–421. [Google Scholar] [CrossRef]
- Martins, L.C.; de Oliveira, R.B.; Lameira, J.; Ferreira, R.S. Experimental and Computational Study of Aryl-thiosemicarbazones Inhibiting Cruzain Reveals Reversible Inhibition and a Stepwise Mechanism. J. Chem. Inf. Model. 2023, 63, 1506–1520. [Google Scholar] [CrossRef]
- de Barros Dias, M.C.H.; Barbalho, M.S.; de Oliveira Filho, G.B.; de Oliveira Cardoso, M.V.; Leite, A.C.L.; da Silva Santos, A.C.; Silva, A.C.C.; de Castro, M.C.A.B.; Moura, D.M.N.; Ferreira, L.F.G.R.; et al. 1,3-Thiazole derivatives as privileged structures for anti-Trypanosoma cruzi activity: Rational design, synthesis, in silico and in vitro studies. Eur. J. Med. Chem. 2023, 257, 115508. [Google Scholar] [CrossRef]
- Rostán, S.; Porto, S.; Barbosa, C.L.N.; Assis, D.; Alvarez, N.; Machado, F.S.; Mahler, G.; Otero, L. A novel palladium complex with a coumarin-thiosemicarbazone hybrid ligand inhibits Trypanosoma cruzi release from host cells and lowers the parasitemia in vivo. J. Biol. Inorg. Chem. 2023, 28, 711–723. [Google Scholar] [CrossRef]
- Da Cruz Filho, I.J.; De Oliveira, J.F.; Santos, A.C.S.; Pereira, V.R.A.; De Lima, M.C.A. Synthesis of 4-(4-chlorophenyl)thiazole compounds: In silico and in vitro evaluations as leishmanicidal and trypanocidal agents. An. Acad. Bras. Cienc. 2023, 95, e20220538. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, G.; Salas-Sarduy, E.; Vega, D.; Fabian, L.; Martini, M.F.; Moglioni, A.G. Thiosemicarbazone derivatives: Evaluation as cruzipain inhibitors and molecular modeling study of complexes with cruzain. Bioorg. Med. Chem. 2022, 61, 116708. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.F.P.; Santos, V.C.; Vieira, R.P.; da Silva, E.B.; Monti, L.; Krake, S.H.; Martinez, P.D.G.; Dias, L.C.; Caffrey, C.R.; Siqueira-Neto, J.L.; et al. From rational design to serendipity: Discovery of novel thiosemicarbazones as potent trypanocidal compounds. Eur. J. Med. Chem. 2022, 244, 114876. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; De Barros Dias, M.C.H.; da Silva Santos, A.C.; Pereira, V.R.A.; Freitas, L.A.B.; Balbinot, R.B.; Kaplum, V.; Nakamura, C.V.; Alves, L.C.; Brayner, F.A.; et al. The design, synthesis, and: In vitro trypanocidal and leishmanicidal activities of 1,3-thiazole and 4-thiazolidinone ester derivatives. RSC Adv. 2021, 11, 2487–2500. [Google Scholar] [CrossRef]
- de Oliveira Cardoso, M.V.; de Siqueira, L.R.P.; da Silva, E.B.; Costa, L.B.; Hernandes, M.Z.; Rabello, M.M.; Ferreira, R.S.; da Cruz, L.F.; Moreira, D.R.M.; Pereira, V.R.A.; et al. 2-Pyridyl thiazoles as novel anti-Trypanosoma cruzi agents: Structural design, synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2014, 86, 48–59. [Google Scholar] [CrossRef]
- de Oliveira Filho, G.B.; de Oliveira Cardoso, M.V.; Espíndola, J.W.P.; Silva, D.A.O.E.; Ferreira, R.S.; Coelho, P.L.; Anjos, P.S.D.; de Souza Santos, E.; Meira, C.S.; Moreira, D.R.M.; et al. Structural design, synthesis and pharmacological evaluation of thiazoles against Trypanosoma cruzi. Eur. J. Med. Chem. 2017, 141, 346–361. [Google Scholar] [CrossRef]
- González, D.L.N.; Castaño, J.A.G.; Núñez, W.E.R.; Duchowicz, P.R. Antiprotozoal QSAR modelling for trypanosomiasis (Chagas disease) based on thiosemicarbazone and thiazole derivatives. J. Mol. Graph Model 2021, 103, 107821. [Google Scholar] [CrossRef]
- Freitas, L.A.B.; da Silva Santos, A.C.; de Cássia Silva, G.; Albuquerque, F.N.D.N.; Silva, E.D.; de Simone, C.A.; Pereira, V.R.A.; Alves, L.C.; Brayner, F.A.; Leite, A.C.L.; et al. Structural improvement of new thiazolyl-isatin derivatives produces potent and selective trypanocidal and leishmanicidal compounds. Chem. Biol. Interact 2021, 345, 109561. [Google Scholar] [CrossRef]
- de Oliveira Filho, G.B.; de Oliveira Cardoso, M.V.; da Silva Santos, A.C.; Santos, T.A.R.D.; Cristovão-Silva, A.C.; Rubio, L.G.; da Silva Maia Neto, L.; Leite, P.G.; Machado, F.S.; Alves, L.C.; et al. Structural design, synthesis and anti-Trypanosoma cruzi profile of the second generation of 4-thiazolidinones chlorine derivatives. Chem. Biol. Interact 2021, 345, 109514. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cerón, S.; Gutiérrez-Nágera, N.A.; Mirzaeicheshmeh, E.; Cuevas-Hernández, R.I.; Trujillo-Ferrara, J.G. Phenylbenzothiazole derivatives: Effects against a Trypanosoma cruzi infection and toxicological profiles, (n.d.). Parasitol. Res. 2021, 120, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Hernández, R.I.; Girard, R.M.B.M.; Martínez-Cerón, S.; da Silva, M.S.; Elias, M.C.; Crispim, M.; Trujillo-Ferrara, J.G.; Silber, A.M. A Fluorinated Phenylbenzothiazole Arrests the Trypanosoma cruzi Cell Cycle and Diminishes the Infection of Mammalian Host Cells. Antimicrob. Agents Chemother. 2020, 64, 10–128. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.H.C.N.; Barbosa, J.M.C.; Bernardino, P.; Sueth-Santiago, V.; Wardell, S.M.S.V.; Wardell, J.L.; Decoté-Ricardo, D.; Melo, T.G.; da Silva, E.F.; Salomão, K.; et al. Synthesis and trypanocidal activity of novel pyridinyl-1,3,4-thiadiazole derivatives. Biomed. Pharmacother. 2020, 127, 110162. [Google Scholar] [CrossRef]
- Silva, B.N.M.; Junior, P.A.S.; Romanha, A.J.; Murta, S.M.F.; Lima, C.H.S.; Albuquerque, M.G.; D’Elia, E.; Rodrigues, J.G.A.; Ferreira, V.F.; Silva, F.C.; et al. Synthesis of New Thiosemicarbazones and Semicarbazones Containing the 1,2,3-1H-triazole-isatin Scaffold: Trypanocidal, Cytotoxicity, Electrochemical Assays, and Molecular Docking. Med. Chem. 2018, 15, 240–256. [Google Scholar] [CrossRef]
- WHO. Trypanosomiasis, Human African (Sleeping Sickness), World Health Organization 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 10 February 2025).
- Ortiz-Martínez, Y.; Kouamé, M.G.; Bongomin, F.; Lakoh, S.; Henao-Martínez, A.F. Human African Trypanosomiasis (Sleeping Sickness)—Epidemiology, Clinical Manifestations, Diagnosis, Treatment, and Prevention. Curr. Trop. Med. Rep. 2023, 10, 222. [Google Scholar] [CrossRef]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Kennedy, P.G.E. The evolving spectrum of human African trypanosomiasis. QJM Int. J. Med. 2024, 117, 391–395. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Barrett, M.P.; Blumberg, L.; Bukachi, S.A.; Chancey, R.J.; Edielu, A.; Matemba, L.; Mesha, T.; Mwanakasale, V.; et al. New WHO guidelines for treating rhodesiense human African trypanosomiasis: Expanded indications for fexinidazole and pentamidine. Lancet Infect Dis. 2025, 25, e77–e85. [Google Scholar] [CrossRef]
- NIH. Pivotal Study of Fexinidazole for Human African Trypanosomiasis in Stage 2 I ClinicalTrials. National Library of Medicine (2018). Available online: https://clinicaltrials.gov/study/NCT01685827?term=NCT01685827&rank=1 (accessed on 10 August 2024).
- Torreele, E.; Trunz, B.B.; Tweats, D.; Kaiser, M.; Brun, R.; Mazué, G.; Bray, M.A.; Pécoul, B. Fexinidazole—A New Oral Nitroimidazole Drug Candidate Entering Clinical Development for the Treatment of Sleeping Sickness. PLoS Negl. Trop. Dis. 2010, 4, e923. [Google Scholar] [CrossRef]
- Bernhard, S.; Kaiser, M.; Burri, C.; Mäser, P. Fexinidazole for Human African Trypanosomiasis, the Fruit of a Successful Public-Private Partnership. Diseases 2022, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Currier, R.B.; Ulrich, K.; Leroux, A.E.; Dirdjaja, N.; Deambrosi, M.; Bonilla, M.; Ahmed, Y.L.; Adrian, L.; Antelmann, H.; Jakob, U.; et al. An essential thioredoxin-type protein of Trypanosoma brucei acts as redox-regulated mitochondrial chaperone. PLoS Pathog. 2019, 15, e1008065. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.; Medeiros, A.; Benítez, D.; Perelmuter, K.; Serra, G.; Comini, M.A.; Scarone, L. In vitro activity and mode of action of distamycin analogues against African trypanosomes. Eur. J. Med. Chem. 2017, 126, 776–788. [Google Scholar] [CrossRef]
- Mallari, J.P.; Shelat, A.; Kosinski, A.; Caffrey, C.R.; Connelly, M.; Zhu, F.; McKerrow, J.H.; Guy, R.K. Discovery of trypanocidal thiosemicarbazone inhibitors of rhodesain and TbcatB. Bioorg. Med. Chem. Lett. 2008, 18, 2883–2885. [Google Scholar] [CrossRef]
- Ballesteros-Casallas, A.; Quiroga, C.; Ortiz, C.; Benítez, D.; Denis, P.A.; Figueroa, D.; Salas, C.O.; Bertrand, J.; Tapia, R.A.; Sánchez, P.; et al. Mode of action of p-quinone derivatives with trypanocidal activity studied by experimental and in silico models. Eur. J. Med. Chem. 2023, 246, 114926. [Google Scholar] [CrossRef] [PubMed]
- Racané, L.; Ptiček, L.; Kostrun, S.; Raić-Malić, S.; Taylor, M.C.; Delves, M.; Alsford, S.; Olmo, F.; Francisco, A.F.; Kelly, J.M. Bis-6-amidino-benzothiazole Derivative that Cures Experimental Stage 1 African Trypanosomiasis with a Single Dose. J. Med. Chem. 2023, 66, 13043–13057. [Google Scholar] [CrossRef]
- Cleghorn, L.A.T.; Wall, R.J.; Albrecht, S.; MacGowan, S.A.; Norval, S.; De Rycker, M.; Woodland, A.; Spinks, D.; Thompson, S.; Patterson, S.; et al. Development of a 2,4-Diaminothiazole Series for the Treatment of Human African Trypanosomiasis Highlights the Importance of Static-Cidal Screening of Analogues. J. Med. Chem. 2023, 66, 8896–8916. [Google Scholar] [CrossRef]
- Hendrickx, S.; Bulté, D.; Mabille, D.; Mols, R.; Claes, M.; Ilbeigi, K.; Ahmad, R.; Dirkx, L.; Van Acker, S.I.; Caljon, G. Comparison of Bioluminescent Substrates in Natural Infection Models of Neglected Parasitic Diseases. Int. J. Mol. Sci. 2022, 23, 16074. [Google Scholar] [CrossRef]
- Mousavi, A.; Foroumadi, P.; Emamgholipour, Z.; Mäser, P.; Kaiser, M.; Foroumadi, A. 2-(Nitroaryl)-5-Substituted-1,3,4-Thiadiazole Derivatives with Antiprotozoal Activities: In Vitro and In Vivo Study. Molecules 2022, 27, 5559. [Google Scholar] [CrossRef]
- Franco, J.; Scarone, L.; Comini, M.A. Novel distamycin analogues that block the cell cycle of African trypanosomes with high selectivity and potency. Eur. J. Med. Chem. 2020, 189, 112043. [Google Scholar] [CrossRef]
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef] [PubMed]
- WHO. Leishmaniasis, World Health Organization 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 10 February 2025).
- Arenas, R.; Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- PAHO. Leishmaniasis: Epidemiological Report for the Americas. No.12 (December 2023), Pan American Health Organization (2023). Available online: https://iris.paho.org/handle/10665.2/59155 (accessed on 10 February 2025).
- Okwor, I.; Uzonna, J. Social and Economic Burden of Human Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489. [Google Scholar] [CrossRef]
- Knight, C.A.; Harris, D.R.; Alshammari, S.O.; Gugssa, A.; Young, T.; Lee, C.M. Leishmaniasis: Recent epidemiological studies in the Middle East. Front. Microbiol. 2023, 13, 1052478. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Azam, M.; Ramesh, V.; Singh, R. Unusual Observations in Leishmaniasis—An Overview. Pathogens 2023, 12, 297. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121. [Google Scholar] [CrossRef]
- de Vries, H.J.C.; Schallig, H.D. Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments. Am. J. Clin. Dermatol. 2022, 23, 823. [Google Scholar] [CrossRef]
- CDC. Clinical Care of Leishmaniasis|Leishmaniasis|CDC, Centers for Disease Control and Prevention 2024. Available online: https://www.cdc.gov/leishmaniasis/hcp/clinical-care/index.html (accessed on 10 February 2025).
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am. J. Trop. Med. Hyg. 2017, 96, 24–45. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Guideline for the Treatment of Leishmaniasis in the Americas, 2nd ed.; Pan American Health Organization: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Schröder, J.; Noack, S.; Marhöfer, R.J.; Mottram, J.C.; Coombs, G.H.; Selzer, P.M. Identification of Semicarbazones, Thiosemicarbazones and Triazine Nitriles as Inhibitors of Leishmania mexicana Cysteine Protease CPB. PLoS ONE 2013, 8, e77460. [Google Scholar] [CrossRef]
- Revuelto, A.; de Lucio, H.; García-Soriano, J.C.; Sánchez-Murcia, P.A.; Gago, F.; Jiménez-Ruiz, A.; Camarasa, M.-J.; Velázquez, S. Efficient Dimerization Disruption of Leishmania infantumTrypanothione Reductase by Triazole-phenyl-thiazoles. J. Med. Chem. 2021, 64, 6137–6160. [Google Scholar] [CrossRef]
- Battista, T.; Colotti, G.; Ilari, A.; Fiorillo, A. Targeting Trypanothione Reductase, a Key Enzyme in the Redox Trypanosomatid Metabolism, to Develop New Drugs against Leishmaniasis and Trypanosomiases. Molecules 2020, 25, 1924. [Google Scholar] [CrossRef] [PubMed]
- Gini, A.L.R.; João, E.E.; Lopes, J.R.; Da Cunha, P.S.T.; Velasquez, A.M.A.; Graminha, M.A.S.; Santos, J.L.D.; Scarim, C.B. Advances in Cysteine Protease B Inhibitors for Leishmaniasis Treatment. Curr. Drug Targets 2025, 26, 88–108. [Google Scholar] [CrossRef]
- Cohen, F.E.; Francisco, S.; Mckerrow, J.H. Thio semicarbazone and semicarbozone inhibitors of cysteine proteases and methods of their use 431. US7495023B2, 18 August 2005. [Google Scholar]
- Raghav, N.; Kaur, R. Chalcones, semicarbazones and pyrazolines as inhibitors of cathepsins B, H and L. Int. J. Biol. Macromol. 2015, 80, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Mijoba, A.; Parra-Giménez, N.; Fernandez-Moreira, E.; Ramírez, H.; Serrano, X.; Blanco, Z.; Espinosa, S.; Charris, J.E. Synthesis of Hybrid Molecules with Imidazole-1,3,4-thiadiazole Core and Evaluation of Biological Activity on Trypanosoma cruzi and Leishmania donovani. Molecules 2024, 29, 4125. [Google Scholar] [CrossRef]
- Coimbra, E.S.; Antinarelli, L.M.R.; de Oliveira Lemos, A.S.; da Silva Neto, A.F.; Pinheiro, A.C.; de Souza, M.V.N. Synthesis, biological evaluation and mechanism of action of benzothiazole derivatives with aromatic hydrazone moiety, a new class of antileishmanial compounds. Chem. Biol. Drug Des. 2024, 104, e14585. [Google Scholar] [CrossRef] [PubMed]
- de Aquino, T.M.; França, P.H.B.; Rodrigues, É.E.E.S.; Nascimento, I.J.S.; Santos-Júnior, P.F.S.; Aquino, P.G.V.; Santos, M.S.; Queiroz, A.C.; Araújo, M.V.; Alexandre-Moreira, M.S.; et al. Synthesis, Antileishmanial Activity and in silico Studies of Aminoguanidine Hydrazones (AGH) and Thiosemicarbazones (TSC) Against Leishmania chagasi Amastigotes. Med. Chem. 2021, 18, 151–169. [Google Scholar] [CrossRef]
- Gouveia, A.L.A.; Santos, F.A.B.; Alves, L.C.; Cruz-Filho, I.J.; Silva, P.R.; Jacob, I.T.T.; Soares, J.C.S.; Santos, D.K.D.N.; Souza, T.R.C.L.; Oliveira, J.F.; et al. Thiazolidine derivatives: In vitro toxicity assessment against promastigote and amastigote forms of Leishmania infantum and ultrastructural study. Exp. Parasitol. 2022, 236–237, 108253. [Google Scholar] [CrossRef]
- Neri, F.S.M.; Júnior, D.B.C.; Froes, T.Q.; da Silva, P.B.G.; Egito, M.S.D.; Moreira, P.O.L.; de Pilla Varotti, F.; Castilho, M.S.; Teixeira-Neto, R.G.; de Albuquerque, J.F.C.; et al. Antileishmanial activity evaluation of thiazolidine-2,4-dione against Leishmania infantum and Leishmania braziliensis. Parasitol. Res. 2020, 119, 2263–2274. [Google Scholar] [CrossRef]
- de Oliveira, V.V.G.; de Souza, M.A.A.; Cavalcanti, R.R.M.; de Oliveira Cardoso, M.V.; Leite, A.C.L.; da Silva Junior, V.A.; de Figueiredo, R.C.B.Q. Study of in vitro biological activity of thiazoles on Leishmania (Leishmania) infantum. J. Glob. Antimicrob. Resist. 2020, 22, 414–421. [Google Scholar] [CrossRef]
- Camargo, J.D.N.A.; Pianoski, K.E.; Santos, M.G.D.; Lazarin-Bidóia, D.; Volpato, H.; Moura, S.; Nakamura, C.V.; Rosa, F.A. Antiparasitic Behavior of Trifluoromethylated Pyrazole 2-Amino-1,3,4-thiadiazole Hybrids and Their Analogues: Synthesis and Structure-Activity Relationship. Front. Pharmacol. 2020, 11, 591570. [Google Scholar] [CrossRef]
- WHO. Malaria, World Health Organization 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 10 February 2025).
- Liu, Q.; Jing, W.; Kang, L.; Liu, J.; Liu, M. Trends of the global, regional and national incidence of malaria in 204 countries from 1990 to 2019 and implications for malaria prevention. J. Travel Med. 2021, 28, taab046. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. The 2023 WHO World malaria report. Lancet Microbe 2024, 5, e214. [Google Scholar] [CrossRef] [PubMed]
- Meibalan, E.; Marti, M. Biology of Malaria Transmission. Cold Spring Harb. Perspect. Med. 2017, 7, a025452. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.N.; Lisa, R.C.; Ferguson, H.M. The transmission potential of malaria-infected mosquitoes (An.gambiae-Keele, An.arabiensis-Ifakara) is altered by the vertebrate blood type they consume during parasite development. Sci. Rep. 2017, 7, 591570. [Google Scholar] [CrossRef]
- de Castro Duarte, A.M.R.; Fernandes, L.N.; Silva, F.S.; Sicchi, I.L.; Mucci, L.F.; Curado, I.; Fernandes, A.; Medeiros-Sousa, A.R.; Ceretti-Junior, W.; Marrelli, M.T.; et al. Complexity of malaria transmission dynamics in the Brazilian Atlantic Forest. Curr. Res. Parasitol. Vector-Borne Dis. 2021, 1, 100032. [Google Scholar] [CrossRef]
- Haileselassie, W.; Parker, D.M.; Taye, B.; David, R.E.; Zemene, E.; Lee, M.-C.; Zhong, D.; Zhou, G.; Alemu, T.; Tadele, G.; et al. Burden of malaria, impact of interventions and climate variability in Western Ethiopia: An area with large irrigation based farming. BMC Public Health 2022, 22, 196. [Google Scholar] [CrossRef]
- Commons, R.J.; Simpson, J.A.; Thriemer, K.; Humphreys, G.S.; Abreha, T.; Alemu, S.G.; Añez, A.; Anstey, N.M.; Awab, G.R.; Baird, J.K.; et al. The effect of chloroquine dose and primaquine on Plasmodium vivax recurrence: A WorldWide Antimalarial Resistance Network systematic review and individual patient pooled meta-analysis. Lancet Infect Dis. 2018, 18, 1025–1034. [Google Scholar] [CrossRef]
- Tibon, N.S.; Ng, C.H.; Cheong, S.L. Current progress in antimalarial pharmacotherapy and multi-target drug discovery. Eur. J. Med. Chem. 2020, 188, 111983. [Google Scholar] [CrossRef]
- Pedro, R.S.; Brasil, P.; Pina-Costa, A.; Machado, C.R.; Damasceno, L.S.; Daniel-Ribeiro, C.T.; Guaraldo, L. Pharmacotherapy follow-up: Role in active malaria surveillance in a travel medicine centre outside the transmission area in Brazil. J. Clin. Pharm. Ther. 2017, 42, 750–757. [Google Scholar] [CrossRef]
- Pucca, M.B.; de Sousa, T.N.; de Melo, G.C.; Viana, G.M.R. Editorial: Challenges for diagnosis, treatment, and elimination of malaria. Front. Trop. Dis. 2024, 5, 1394693. [Google Scholar] [CrossRef]
- Li, J.; Docile, H.J.; Fisher, D.; Pronyuk, K.; Zhao, L. Current Status of Malaria Control and Elimination in Africa: Epidemiology, Diagnosis, Treatment, Progress and Challenges. J. Epidemiol. Glob. Health 2024, 14, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, P.J. Malaria in 2022: Challenges and Progress. Am. J. Trop. Med. Hyg. 2022, 106, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.B.; de Souza-Fagundes, E.M.; Soares, R.P.P.; Andrade, A.A.; Krettli, A.U.; Zani, C.L. Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives. Eur. J. Med. Chem. 2008, 43, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.K. Thiazole Containing Heterocycles with Antimalarial Activity. Curr. Drug Discov. Technol. 2018, 15, 196–200. [Google Scholar] [CrossRef]
- Kalita, T.; Choudhury, A.; Shakya, A.; Ghosh, S.K.; Singh, U.P.; Bhat, H.R. A Review on Synthetic Thiazole Derivatives as an Antimalarial Agent. Curr. Drug Discov. Technol. 2024, 21, 10–42. [Google Scholar] [CrossRef]
- Kumar, B.; Devi, J.; Dubey, A.; Tufail, A.; Sharma, S. Exploring the antimalarial, antioxidant, anti-inflammatory activities of newly synthesized transition metal(II) complexes bearing thiosemicarbazone ligands: Insights from molecular docking, DFT, MESP and ADMET studies. Inorg. Chem. Commun. 2024, 159, 111674. [Google Scholar] [CrossRef]
- Rayala, R.; Chaudhari, P.; Bunnell, A.; Roberts, B.; Chakrabarti, D.; Nefzi, A. Parallel Synthesis of Piperazine Tethered Thiazole Compounds with Antiplasmodial Activity. Int. J. Mol. Sci. 2023, 24, 17414. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Roy, A.; Behera, P.M.; Atri, A.K.; Kumar, K.; Manna, D.; Dixit, A.; Patil, M.T.; Kumari, R.M.; et al. Imidazo [2,1-b]thiazole based indoleamine-2,3-dioxygenase 1 (IDO1) inhibitor: Structure based design, synthesis, bio-evaluation and docking studies. Bioorg. Med. Chem. Lett. 2023, 96, 129532. [Google Scholar] [CrossRef]
- Santos, N.D.; Junior, N.D.; de Oliveira, J.F.; Duarte, D.M.; dos Santos Soares, J.C.; Marques, D.S.; da Silva Santos, A.C.; Nogueira, F.; Pereira, V.R.; de Lima, M.C.; et al. Synthesis, characterization, antioxidant and antiparasitic activities new naphthyl-thiazole derivatives. Exp. Parasitol. 2023, 248, 108498. [Google Scholar] [CrossRef]
- Da Silva, B.R.M.G.; Júnior, N.D.S.B.; De Oliveira, J.F.; Duarte, D.M.F.A.; Marques, D.S.C.; Nogueira, F.; De Lima, M.C.A.; Da Cruz Filho, I.J. In silico ADMET prediction, evaluation of cytotoxicity in mouse splenocytes and preliminary evaluation of in vitro antimalarial activity of 4-(4-chlorophenyl)thiazole compounds. An. Acad. Bras. Cienc. 2023, 95, e20230566. [Google Scholar] [CrossRef]
- Ewida, M.A.; Ewida, H.A.; Ahmed, M.S.; Allam, H.A.; ElBagary, R.I.; George, R.F.; Georgey, H.H.; El-Subbagh, H.I. 3-Methyl-imidazo [2,1-b]thiazole derivatives as a new class of antifolates: Synthesis, in vitro/in vivo bio-evaluation and molecular modeling simulations. Bioorg. Chem. 2021, 115, 105205. [Google Scholar] [CrossRef] [PubMed]
- Kabeche, S.; Aida, J.; Akther, T.; Ichikawa, T.; Ochida, A.; Pulkoski-Gross, M.J.; Smith, M.; Humphries, P.S.; Yeh, E. Nonbisphosphonate inhibitors of Plasmodium falciparum FPPS/GGPPS. Bioorg. Med. Chem. Lett. 2021, 41, 127978. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, H.; Fernandez, E.; Rodrigues, J.; Mayora, S.; Martínez, G.; Celis, C.; De Sanctis, J.B.; Mijares, M.; Charris, J. Synthesis and antimalarial and anticancer evaluation of 7-chlorquinoline-4-thiazoleacetic derivatives containing aryl hydrazide moieties. Arch. Pharm. 2021, 354, 2100002. [Google Scholar] [CrossRef] [PubMed]
- de Moraes Gomes, P.A.T.; de Oliveira Cardoso, M.V.; Santos, I.R.D.; de Sousa, F.A.; da Conceição, J.M.; de Melo Silva, V.G.; Duarte, D.; Pereira, R.; Oliveira, R.; Nogueira, F.; et al. Dual Parasiticidal Activities of Phthalimides: Synthesis and Biological Profile against Trypanosoma cruzi and Plasmodium falciparum. ChemMedChem 2020, 15, 2164–2175. [Google Scholar] [CrossRef]
- Da Silva, M.G.; Cardoso, J.F.; Perasoli, F.B.; Branquinho, R.T.; Mourão, R.S.; Tavares, H.D.S.; Xocaira, M.L.C.T.; Guimarães, D.S.M.; Viana, G.H.R.; Varotti, F.D.P.; et al. Nanoemulsion composed of 10-(4,5-dihydrothiazol-2-yl)thio)decan-1-ol), a synthetic analog of 3-alkylpiridine marine alkaloid: Development, characterization, and antimalarial activity. Eur. J. Pharm. Sci. 2020, 151, 105382. [Google Scholar] [CrossRef]
- Divatia, S.M.; Rajani, D.P.; Rajani, S.D.; Patel, H.D. Novel thiosemicarbazone derivatives containing benzimidazole moiety: Green synthesis and anti-malarial activity. Arab. J. Chem. 2019, 12, 1641–1651. [Google Scholar] [CrossRef]
- Nkungli, N.K.; Fouegue, A.D.T.; Tasheh, S.N.; Bine, F.K.; Hassan, A.U.; Ghogomu, J.N. In silico investigation of falcipain-2 inhibition by hybrid benzimidazole-thiosemicarbazone antiplasmodial agents: A molecular docking, molecular dynamics simulation, and kinetics study. Mol. Divers. 2024, 28, 475–496. [Google Scholar] [CrossRef]
- Matsa, R.; Makam, P.; Kaushik, M.; Hoti, S.L.; Kannan, T. Thiosemicarbazone derivatives: Design, synthesis and in vitro antimalarial activity studies. Eur. J. Pharm. Sci. 2019, 137, 104986. [Google Scholar] [CrossRef]
- NIH. ClinicalTrials.gov, National Institutes of Health 2025. Available online: https://clinicaltrials.gov/ (accessed on 15 February 2025).
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza Tada da Cunha, P.; Rodriguez Gini, A.L.; Man Chin, C.; dos Santos, J.L.; Benito Scarim, C. Recent Progress in Thiazole, Thiosemicarbazone, and Semicarbazone Derivatives as Antiparasitic Agents Against Trypanosomatids and Plasmodium spp. Molecules 2025, 30, 1788. https://doi.org/10.3390/molecules30081788
Souza Tada da Cunha P, Rodriguez Gini AL, Man Chin C, dos Santos JL, Benito Scarim C. Recent Progress in Thiazole, Thiosemicarbazone, and Semicarbazone Derivatives as Antiparasitic Agents Against Trypanosomatids and Plasmodium spp. Molecules. 2025; 30(8):1788. https://doi.org/10.3390/molecules30081788
Chicago/Turabian StyleSouza Tada da Cunha, Pamela, Ana Luísa Rodriguez Gini, Chung Man Chin, Jean Leandro dos Santos, and Cauê Benito Scarim. 2025. "Recent Progress in Thiazole, Thiosemicarbazone, and Semicarbazone Derivatives as Antiparasitic Agents Against Trypanosomatids and Plasmodium spp." Molecules 30, no. 8: 1788. https://doi.org/10.3390/molecules30081788
APA StyleSouza Tada da Cunha, P., Rodriguez Gini, A. L., Man Chin, C., dos Santos, J. L., & Benito Scarim, C. (2025). Recent Progress in Thiazole, Thiosemicarbazone, and Semicarbazone Derivatives as Antiparasitic Agents Against Trypanosomatids and Plasmodium spp. Molecules, 30(8), 1788. https://doi.org/10.3390/molecules30081788









