Magnetically Modified Bentonite for Optimized Erythromycin Removal via RSM and DFT Analysis
Abstract
:1. Introduction
- (1)
- The development of a magnetically modified bentonite (MCB) for enhanced ERY adsorption: While bentonite has been studied as an adsorbent, its modification via calcination followed by magnetization is an innovative approach. This process not only enhances adsorption performance but also enables easy separation and recyclability, minimizing secondary environmental pollution;
- (2)
- The application of response surface methodology (RSM) for adsorption optimization: Unlike many adsorption studies that examine parameter effects individually, this work employs RSM to systematically optimize adsorption conditions, providing deeper insights into interactive effects and process efficiency;
- (3)
- Investigation of adsorption mechanisms using Density Functional Theory (DFT): Most studies on antibiotic adsorption focus on experimental data without in-depth molecular-level interaction analysis. This study employs DFT calculations to elucidate the binding mechanisms between ERY and MCB, providing a theoretical foundation for understanding adsorption behavior.
2. Discussion
2.1. Adsorbent Characterization
2.2. Influence of Parameters on ERY Adsorption
2.3. Adsorption Isotherms
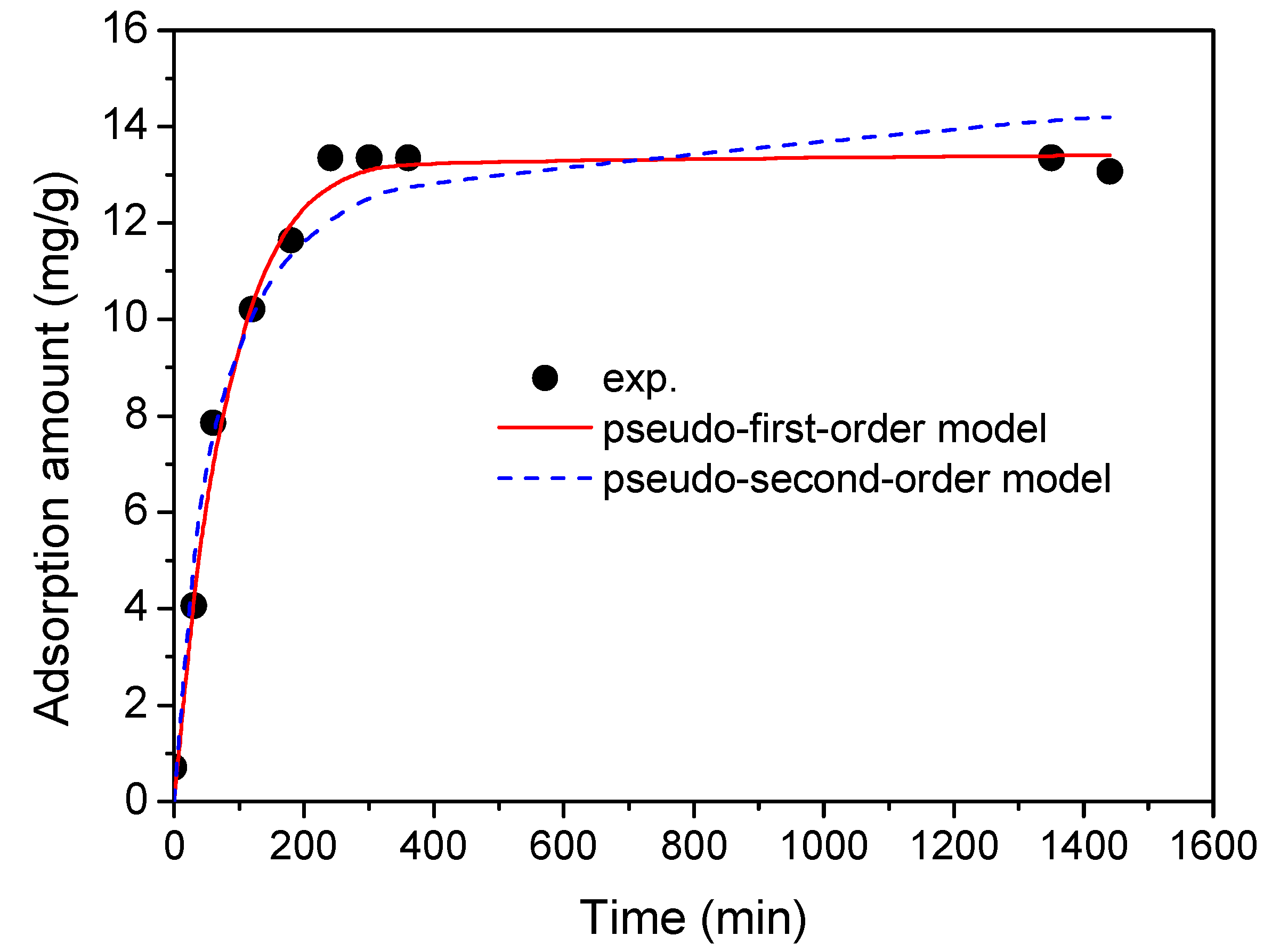
2.4. Adsorption Kinetics
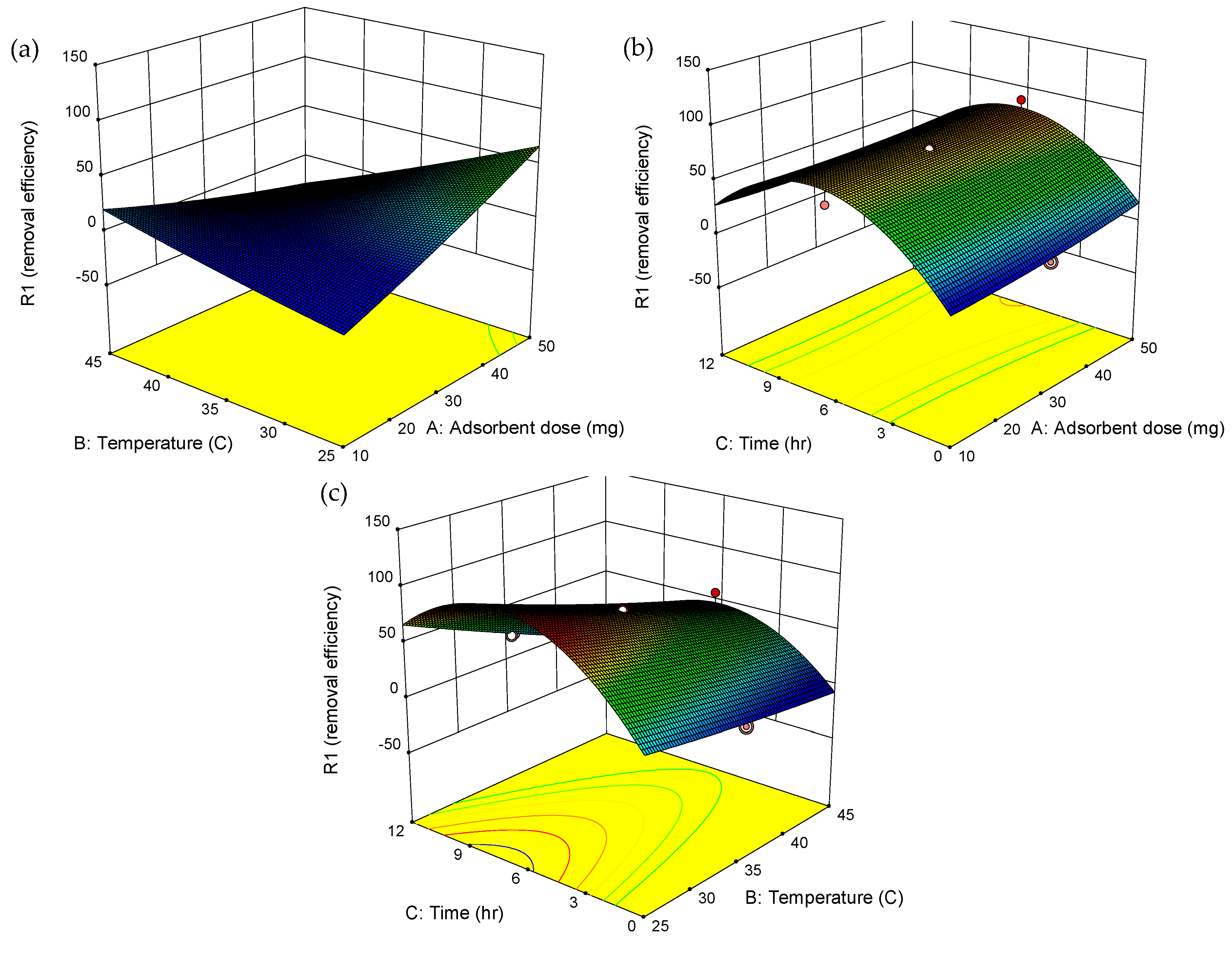
2.5. Adsorption Optimization
2.6. DFT Calculations
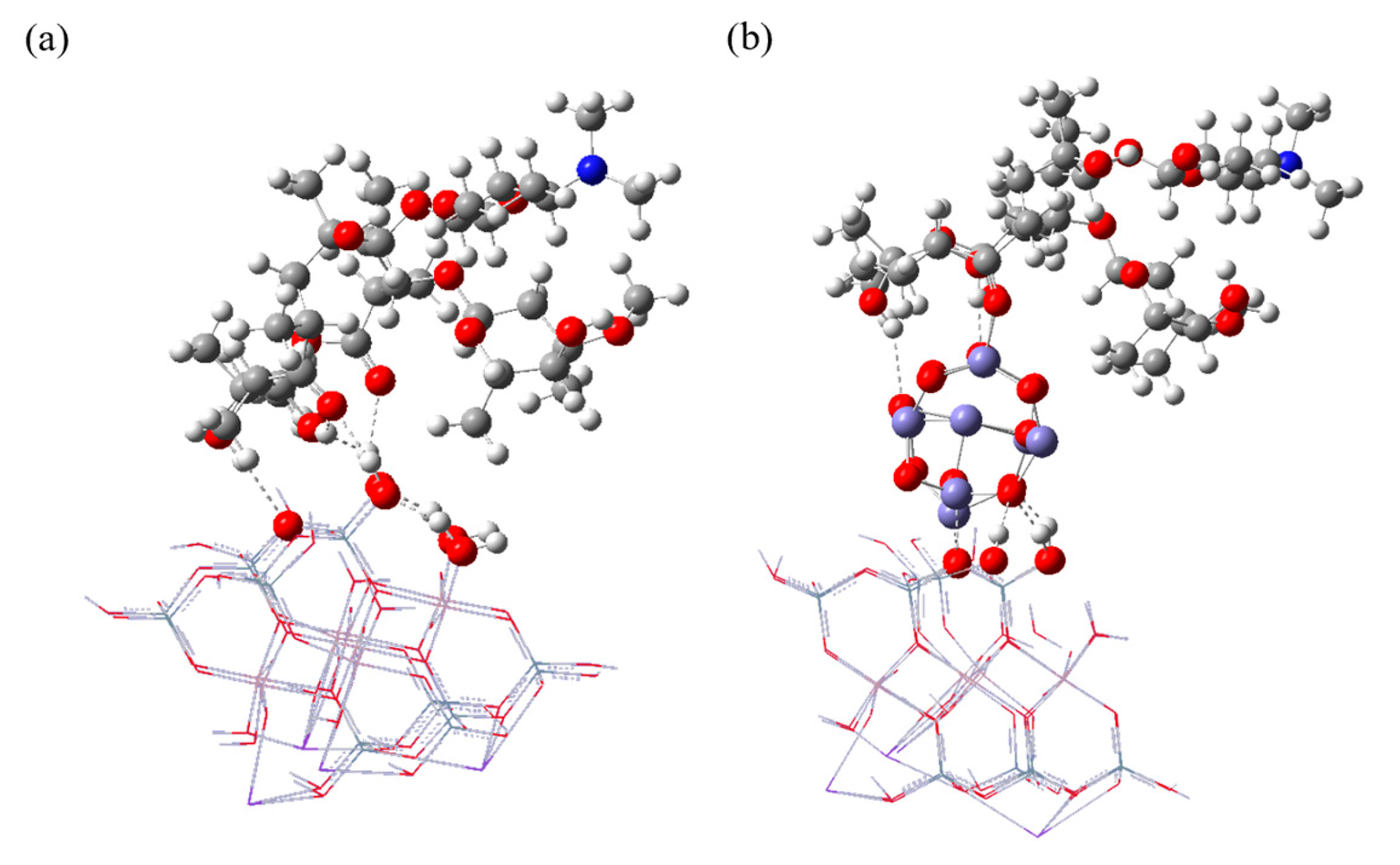
2.6.1. The Structure and Binding Behavior of ERY Adsorption on CB and MCB
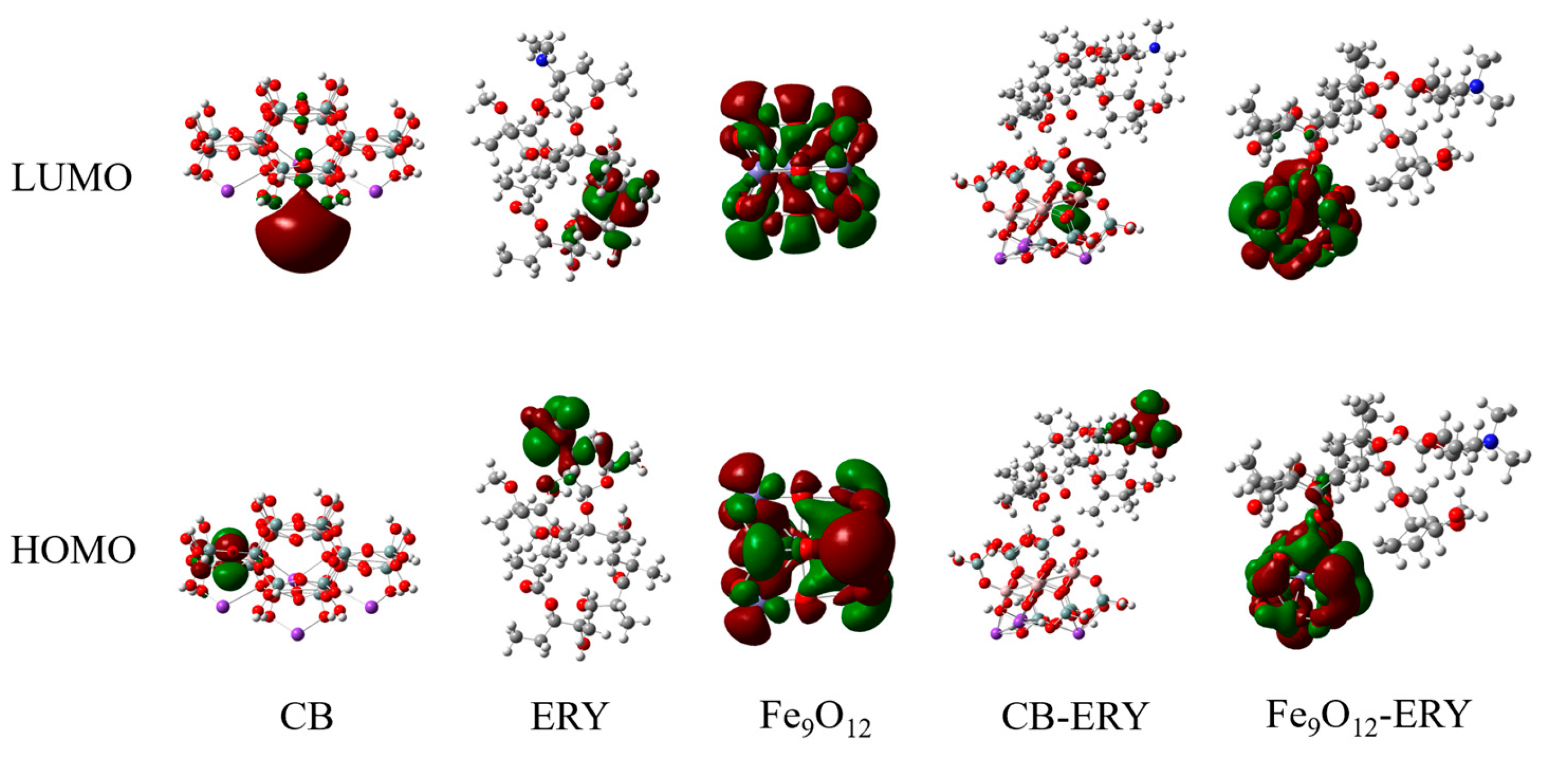
2.6.2. Frontier Orbital and Binding Energy Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Adsorbent
3.3. Material Characterization
3.4. Batch Adsorption Study
3.5. Experimental Design and Statistical Analysis
3.6. Theoretical Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging environmental contaminants: A global perspective on policies and regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef] [PubMed]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechnol. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- Meng, F.; Ling, Y.; Li, Y.; Guo, M.; Wei, K.; Zhang, M.; Yang, C.; Shi, X.; Tang, B. Photoassisted synthesis of high-crystallinity TS-1 with triblock copolymer F127 and its application for photodegradation of erythromycin. Chem. Eng. J. 2023, 473, 145200. [Google Scholar] [CrossRef]
- Luiz, D.B.; Genena, A.K.; Virmond, E.; José, H.J.; Moreira, R.F.; Gebhardt, W.; Schröder, H.F. Identification of degradation products of erythromycin A arising from ozone and advanced oxidation process treatment. Water Environ. Res. 2010, 82, 797–805. [Google Scholar] [CrossRef]
- Hassan, A.F.; Elhassanein, A.; Khoj, M.A.; Shaltout, W.A. Fabrication of graphitic carbon nitride/gum Arabic/potassium carrageenan composite for efficient adsorption of erythromycin: Kinetic and thermodynamic studies. Int. J. Biol. Macromol. 2024, 276, 133999. [Google Scholar] [CrossRef]
- Ahmed, Y.; Rahman, M.M.; Alam, M.S.; Miah, M.I.; Choudhury, M.S.H.; Alharbi, O.A.; Akhtar, P.; Rahman, S.M. Harnessing neural network model with optimization for enhanced ciprofloxacin antibiotic adsorption from contaminated water: A transparent and objective framework. J. Water Process Eng. 2024, 65, 105724. [Google Scholar] [CrossRef]
- Wu, T.; He, Q.; Liu, Z.; Shao, B.; Liang, Q.; Pan, Y.; Huang, J.; Peng, Z.; Liu, Y.; Zhao, C.; et al. Tube wall delamination engineering induces photogenerated carrier separation to achieve photocatalytic performance improvement of tubular g-C3N4. J. Hazard. Mater. 2022, 424, 127177. [Google Scholar] [CrossRef]
- Erdem, N.G.; Tuna, O.; Simsek, E.B. Construction of scheelite CaWO4 with natural diatomite for boosted photocatalytic degradation of tetracycline and doxycycline antibiotics under natural conditions. J. Alloys Compd. 2024, 1005, 176117. [Google Scholar] [CrossRef]
- Yan, F.; An, L.; Xu, X.; Du, W.; Dai, R. A review of antibiotics in surface water and their removal by advanced electrocoagulation technologies. Sci. Total Environ. 2024, 906, 167737. [Google Scholar] [CrossRef]
- Tian, N.; Wu, B.; Zhang, W.H.; Jin, C.G.; Yin, M.J.; An, Q.F. Tailoring the microstructure of zwitterionic nanofiltration membranes via post-treatment for antibiotic desalination. Sep. Purif. Technol. 2024, 349, 127884. [Google Scholar] [CrossRef]
- Zegre, M.; Poljanska, E.; Caetano, L.A.; Goncalves, L.; Bettencourt, A. Research progress on biodegradable polymeric platforms for targeting antibiotics to the bone. Int. J. Pharm. 2023, 648, 123584. [Google Scholar] [CrossRef] [PubMed]
- Toor, M.; Jin, B.; Dai, S.; Vimonses, V. Activating natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater. J. Ind. Eng. Chem. 2015, 21, 653–661. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Sarwar, M.T.; Yang, H. Recent progress in radionuclides adsorption by bentonite-based materials as ideal adsorbents and buffer/backfill materials. Appl. Clay Sci. 2023, 232, 106796. [Google Scholar] [CrossRef]
- Nganda, A.; Srivastava, P.; Lamba, B.Y.; Pandey, A.; Kumar, M. Advances in the fabrication, modification, and performance of biochar, red mud, calcium oxide, and bentonite catalysts in waste-to-fuel conversion. Environ. Res. 2023, 232, 116284. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, W.; Hou, R.; Yue, Y.; Feng, J.; Ishag, A.; Wang, X.; Qin, Y.; Sun, Y. Recent advances of application of bentonite-based composites in the environmental remediation. J. Environ. Manag. 2024, 362, 121341. [Google Scholar] [CrossRef]
- Achour, S.; Amokrane, S.; Chegrouche, S.; Nibou, D.; Baaloudj, O. Artificial neural network modeling of the hexavalent uranium sorption onto chemically activated bentonite. Res. Chem. Intermed. 2021, 47, 4837–4854. [Google Scholar] [CrossRef]
- Jara-Cobos, L.; Abad-Delgado, D.; Ponce-Montalvo, J.; Menendez, M.; Penafiel, M.E. Removal of ciprofloxacin from an aqueous medium by adsorption on natural and hydrolyzed bentonites. Front. Environ. Sci. 2023, 11, 1239754. [Google Scholar] [CrossRef]
- Maged, A.; Iqbal, J.; Kharbish, S.; Ismael, I.S.; Bhatnagar, A. Tuning tetracycline removal from aqueous solution onto activated 2:1 layered clay mineral: Characterization, sorption and mechanistic studies. J. Hazard Mater. 2020, 384, 121320. [Google Scholar] [CrossRef]
- Kale, R.C.; Ravi, K. A review on the impact of thermal history on compacted bentonite in the context of nuclear waste management. Environ. Technol. Innov. 2021, 23, 101728. [Google Scholar] [CrossRef]
- Gil, A.; Vicente, M.A. Progress and perspectives on pillared clays applied in energetic and environmental remediation processes. Curr. Opin. Green Sustain. Chem. 2020, 21, 56–63. [Google Scholar] [CrossRef]
- Cui, Q.; Chen, B. Review of polymer-amended bentonite: Categories, mechanism, modification processes and application in barriers for isolating contaminants. Appl. Clay Sci. 2023, 235, 106869. [Google Scholar] [CrossRef]
- Abdullahi, T.; Harun, Z.; Othman, M.H.D. A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Adv. Powder Technol. 2017, 28, 1827–1840. [Google Scholar] [CrossRef]
- Jannah, N.; Onggo, D. Synthesis of Fe3O4 nanoparticles for colour removal of printing ink solution. J. Phys. Conf. Ser. 2019, 1245, 012040. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y. Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. 2017, 202, 266–276. [Google Scholar] [CrossRef]
- Brahimi, S.; Boudjema, S.; Rekkab, I.; Choukchou-Braham, A.; Bachir, R. Synthesis and catalytic activity of vanadia-doped iron-pillared clays for cyclohexene epoxidation. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 63–76. [Google Scholar]
- Zaitan, H.; Bianchi, D.; Achak, O.; Chafik, T. A comparative study of the adsorption and desorption of o-xylene onto bentonite clay and alumina. J. Hazard. Mater. 2008, 153, 852–859. [Google Scholar] [CrossRef]
- Kumpulainen, S.; Kiviranta, L. Mineralogical and Chemical Characterization of Various Bentonite and Smectite-Rich Clay Materials Part A: Comparison and Development of Mineralogical Characterization Methods Part B: Mineralogical and Chemical Characterization of Clay Materials; Working Report of Posiva Oy, Version 1; Posiva Oy: Helsinki, Finland, 2010. [Google Scholar]
- Seidi, A.; Benhamou, M.; Khalil, D.; Aalaoul, M.; Bennani, M.N. Elaboration and characterization of a natural bentonite-poly(ethylene glycol) composite: Development of an exact theoretical study in function of the polymer-density. Chem. Phys. Impact 2024, 9, 100673. [Google Scholar] [CrossRef]
- Jeeva, M.; Yaacob, W.Z.W. Comparison study on the adsorption of a synthetic textile dye using bentonite and surfactant modified bentonite. Bull. Geol. Soc. Malaysia 2018, 65, 107–117. [Google Scholar] [CrossRef]
- Deng, X.; Jiang, Y.; Nan, Z.; Liang, X.; Wang, G. Sorption properties and mechanisms of erythromycin and ampicillin in loess soil: Roles of pH, ionic strength, and temperature. Chem. Eng. J. 2022, 434, 134694. [Google Scholar] [CrossRef]
- Hailu, S.L.; Nair, B.U.; Redi-Abshiro, M.; Diaz, I.; Tessema, M. Preparation and characterization of cationic surfactant modified zeolite adsorbent material for adsorption of organic and inorganic industrial pollutants. J. Environ. Chem. Eng. 2017, 5, 3319–3329. [Google Scholar] [CrossRef]
- Mesdaghinia, A.; Azari, A.; Nodehi, R.N.; Yaghmaeian, K.; Bharti, A.K.; Agarwal, S.; Gupta, V.K.; Sharafi, K. Removal of phthalate esters (PAEs) by zeolite/Fe3O4: Investigation on the magnetic adsorption separation, catalytic degradation and toxicity bioassay. J. Mol. Liq. 2017, 233, 378–390. [Google Scholar] [CrossRef]
- Bentouhami, E.; Zaghouane-Boudiaf, H.; Touahria, Y.I.; Bellil, G.; Boublia, A.; Daas, N.; Dintzer, T.; Chafai, N.; Albrahim, M.; Elboughdiri, N. Efficient wastewater decontamination using magnetic bentonite-alginate beads: A comprehensive study of adsorption dynamics, regeneration, and molecular interactions. J. Environ. Chem. Eng. 2024, 12, 113000. [Google Scholar]
- Nguyen, V.H.; Shim, J.J. Covalently bonded poly (acrylic acid)-Fe3O4 nanocomposite prepared in supercritical CO2 and its adsorption capacity for methylene blue. Synth. React. Inorg. Met. Org. Chem. 2012, 42, 449–454. [Google Scholar] [CrossRef]
- Oliveira, M.F.; da Silva, M.G.C.; Vieira, M.G.A. Equilibrium and kinetic studies of caffeine adsorption from aqueous solutions on thermally modified Verde-lodo bentonite. Appl. Clay Sci. 2019, 168, 366–373. [Google Scholar] [CrossRef]
- Hong, G.B.; Wang, Y.K. Synthesis of low-cost adsorbent from rice bran for the removal of reactive dye based on the response surface methodology. Appl. Surf. Sci. 2017, 423, 800–809. [Google Scholar] [CrossRef]
- Hong, G.B.; Yang, J.X. Dye removal using the solid residues from Glossogyne tenuifolia based on response surface methodology. J. Mol. Liq. 2017, 242, 82–90. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian. Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kumari, N.; Mohan, C. Basics of clay minerals and their characteristic properties. In Clay Clay Miner; IntechOpen: London, UK, 2021. [Google Scholar]
- Drits, V.A.; Zviagina, B.B.; McCarty, D.K.; Salyn, A.L. Factors responsible for crystal-chemical variations in the solid solutions from illite to aluminoceladonite and from glauconite to celadonite. Am. Mineral. 2010, 95, 348–361. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef]
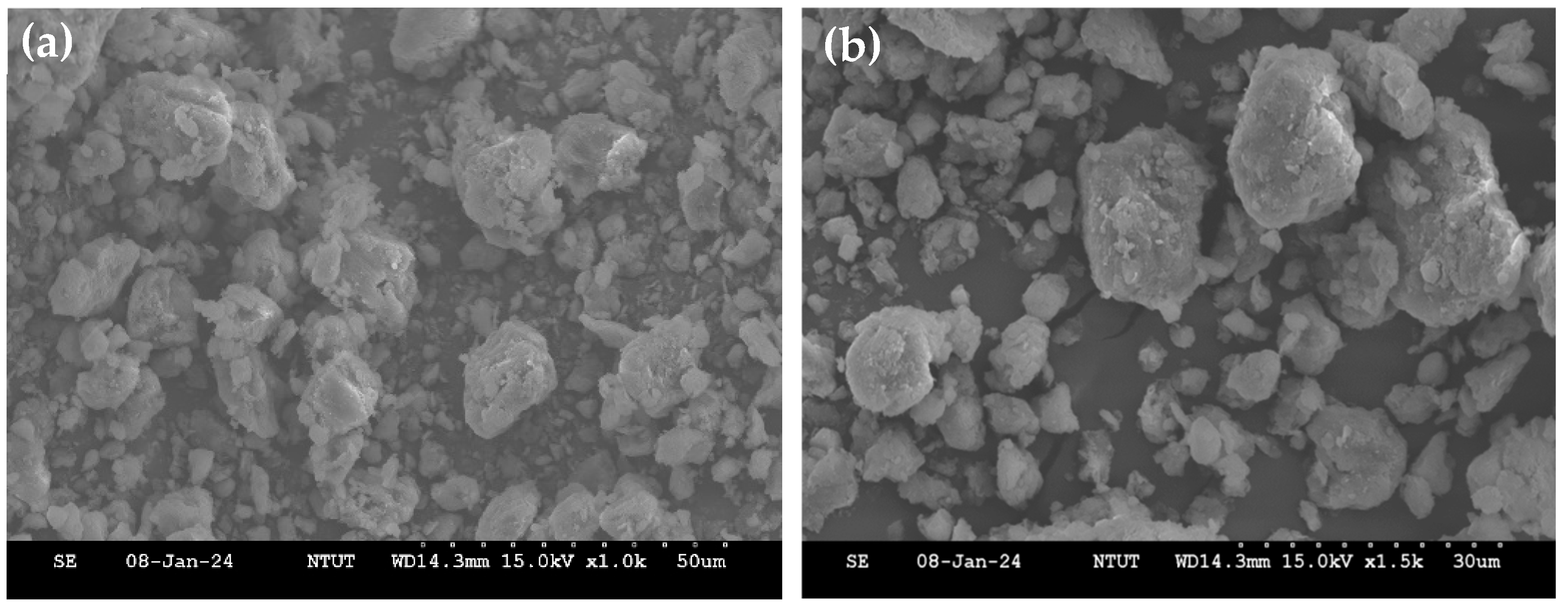
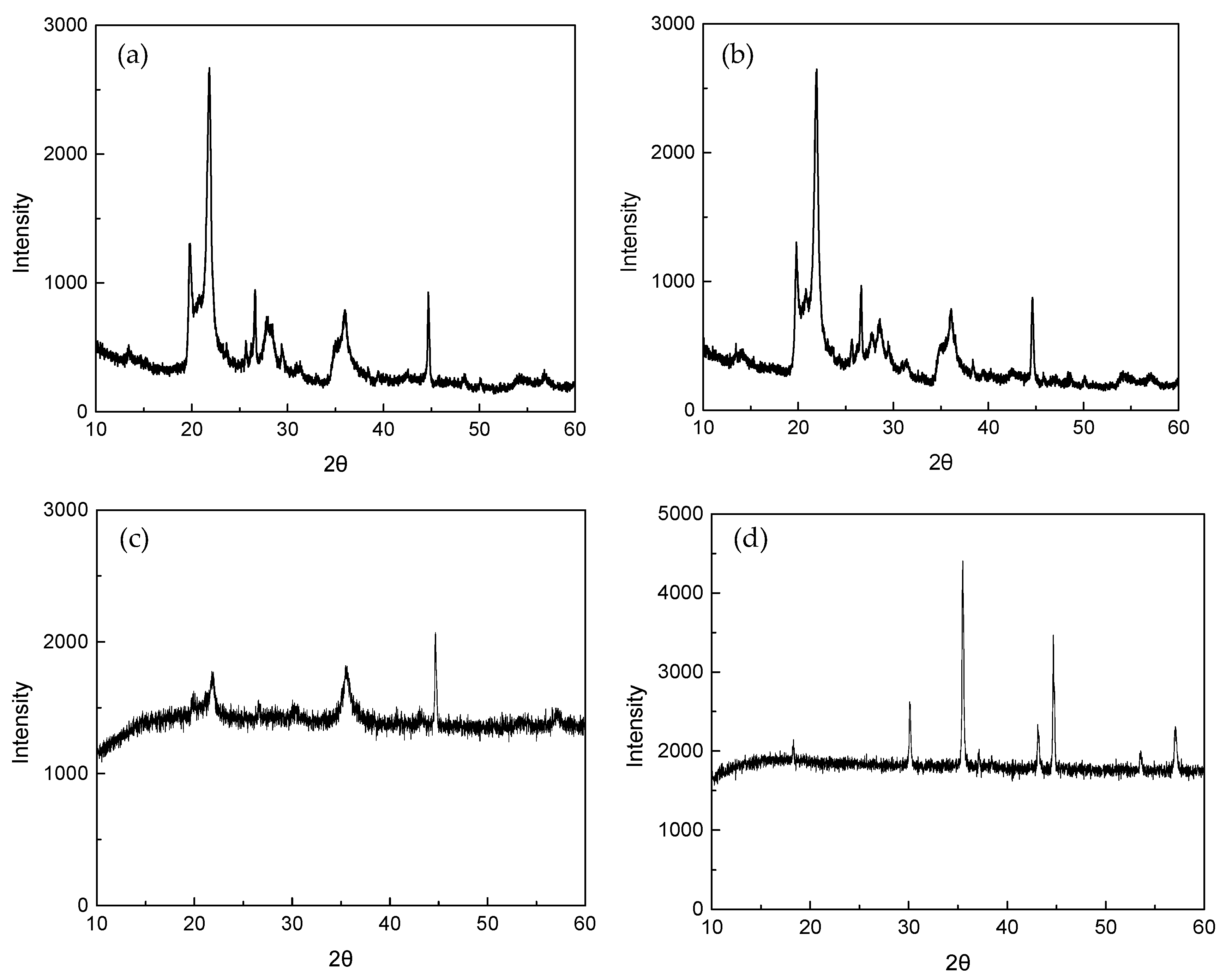
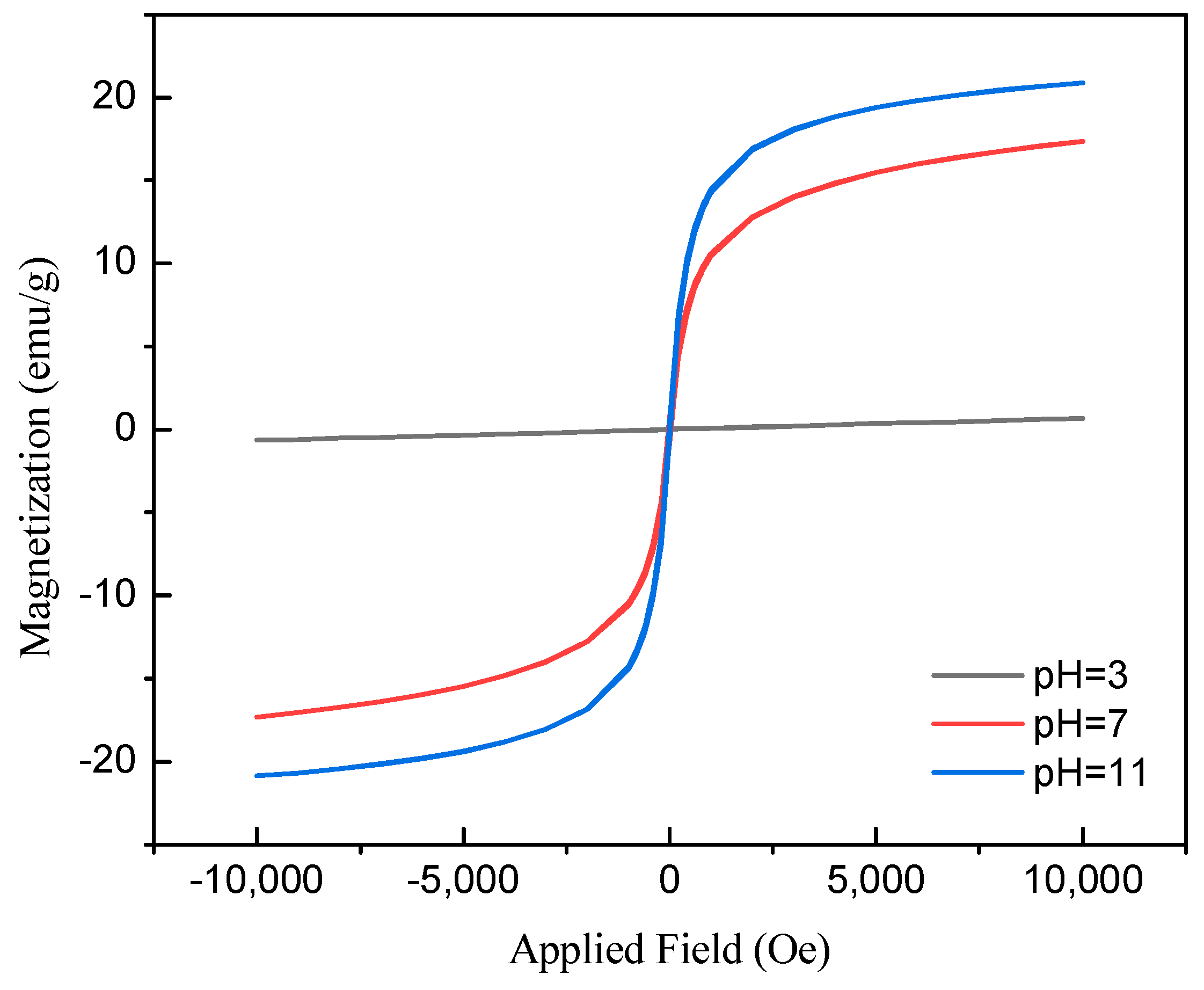

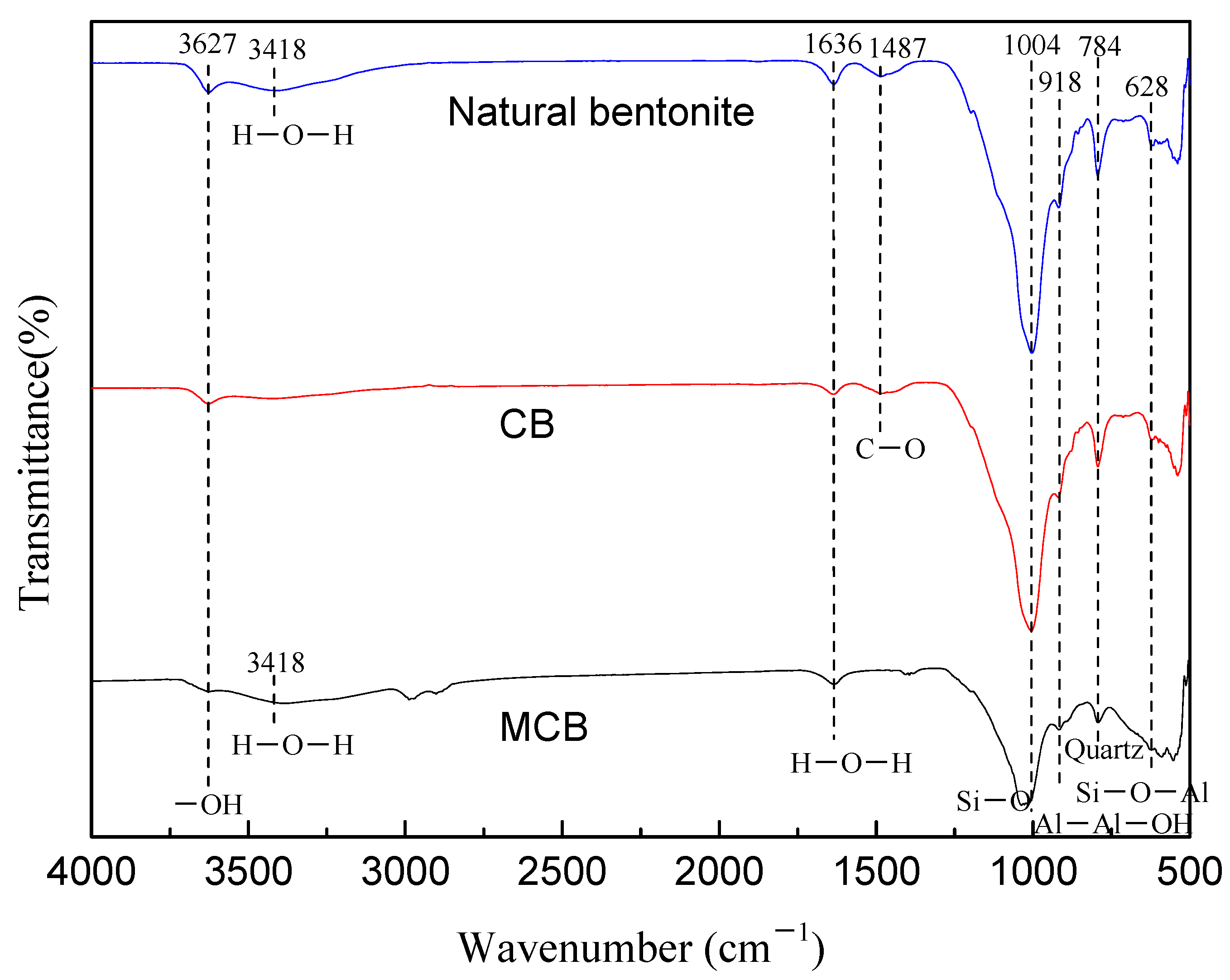


| Samples | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| CB | 41.4 | 0.003 | 11.9 |
| MCB | 61.6 | 0.008 | 7.6 |
| Parameters | Equations | 25 °C | 30 °C | 35 °C | 40 °C |
|---|---|---|---|---|---|
| Langmuir | |||||
| qm (mg/g) | 9.93 | 11.53 | 46.73 | 1.31 | |
| KL (L/g) | 0.708 | 1.051 | 0.077 | 0.050 | |
| RL | 0.124 | 0.087 | 0.564 | 0.665 | |
| R2 | 0.994 | 0.981 | 0.914 | 0.622 | |
| Freundlich | |||||
| KF (mg/g)(L/mg)1/n | 4.51 | 4.98 | 3.55 | 0.001 | |
| n | 9.337 | 3.334 | 1.270 | 0.287 | |
| R2 | 0.831 | 0.712 | 0.974 | 0.896 | |
| D–R model | |||||
| qm (mg/g) | 9.24 | 11.66 | 17.49 | 29.40 | |
| E (kJ/mol) | 1.061 | 0.945 | 0.798 | 0.105 | |
| R2 | 0.972 | 0.971 | 0.936 | 0.960 |
| Model | Kinetic Parameters | ERY |
|---|---|---|
| qexp (mg/g) | 13.36 | |
| Pseudo-first-order | k1 (1/min) | 0.013 |
| qe (mg/g) | 13.4 | |
| R2 | 0.989 | |
| Pseudo-second-order | k2 (g/mg·min) | 1.3 × 10−3 |
| qe (mg/g) | 14.7 | |
| R2 | 0.959 |
| Run | Independent Variables | Responses | ||
|---|---|---|---|---|
| A: MBC Dosage (mg) | B: Temperature (°C) | C: Time (h) | ERY Removal Efficiency (%) | |
| 1 | 30 | 25 | 6 | 88.1 |
| 2 | 18.1 | 40.9 | 9.6 | 45.2 |
| 3 | 50 | 35 | 6 | 88.1 |
| 4 | 18.1 | 40.9 | 2.4 | 52.4 |
| 5 | 30 | 35 | 0 | 2.4 |
| 6 | 41.9 | 29.1 | 9.6 | 90.5 |
| 7 | 30 | 35 | 6 | 73.8 |
| 8 | 41.9 | 29.1 | 2.4 | 85.7 |
| 9 | 18.1 | 29.1 | 2.4 | 50.0 |
| 10 | 30 | 35 | 6 | 73.8 |
| 11 | 30 | 35 | 6 | 73.8 |
| 12 | 30 | 35 | 6 | 73.8 |
| 13 | 10 | 35 | 6 | 59.5 |
| 14 | 41.9 | 40.9 | 2.4 | 16.7 |
| 15 | 30 | 35 | 12 | 21.4 |
| 16 | 41.9 | 40.9 | 9.6 | 26.2 |
| 17 | 30 | 45 | 6 | 57.1 |
| 18 | 18.1 | 29.1 | 9.6 | 85.7 |
| 19 | 30 | 35 | 6 | 73.8 |
| 20 | 30 | 35 | 6 | 73.8 |
| Source | DF | SS | F Value | p Value |
|---|---|---|---|---|
| Model | 9 | 11,969.90 | 10.99 | 0.0004 |
| A | 1 | 83.58 | 0.6906 | 0.4253 |
| B | 1 | 3657.29 | 30.22 | 0.0003 |
| C | 1 | 410.76 | 3.39 | 0.0952 |
| AB | 1 | 1133.83 | 9.37 | 0.0120 |
| AC | 1 | 25.49 | 0.2106 | 0.6561 |
| BC | 1 | 181.45 | 1.50 | 0.2488 |
| A2 | 1 | 16.33 | 0.1350 | 0.7210 |
| B2 | 1 | 5.97 | 0.0494 | 0.8286 |
| C2 | 1 | 6248.17 | 51.63 | <0.0001 |
| Residual | 10 | 1210.21 | ||
| Lack of fit | 5 | 1210.21 | ||
| = 0.9082 | ||||
| Adj = 0.8255 |
| Compound | HOMO (eV) | LUMO (eV) | ||
|---|---|---|---|---|
| ERY | −5.28 | −0.71 | 4.57 | - |
| Fe3O4 | −5.80 | −3.54 | 2.26 | - |
| Fe3O4-ERY | −5.24 | −2.51 | 2.73 | 4.03 |
| Fe6O8 | −6.41 | −4.10 | 2.31 | - |
| Fe6O8-ERY | −5.32 | −3.33 | 1.98 | 2.22 |
| Fe9O12 | −5.46 | −4.06 | 1.40 | - |
| Fe9O12-ERY | −4.79 | −3.23 | 1.55 | 3.00 |
| Fe12O16 | −5.53 | −3.94 | 1.59 | - |
| Fe12O16-ERY | −4.50 | −2.79 | 1.71 | 3.62 |
| Compound | HOMO (eV) | LUMO (eV) | ||
|---|---|---|---|---|
| ERY | −5.28 | −0.71 | 4.57 | - |
| CB | −6.32 | −1.57 | 4.76 | - |
| CB-ERY | −5.49 | −1.50 | 3.99 | 1.60 |
| CB-Fe9O12 | −4.67 | −2.92 | 1.75 | - |
| CB-Fe9O12-ERY | −4.32 | −2.75 | 1.56 | 2.38 |
| T (°C) | |||
|---|---|---|---|
| Time (h) | 400 | 500 | 600 |
| 2 | 27.3% | 44.7% | 20.7% |
| 4 | ~0 | 10.0% | 40.7% |
| 8 | ~0 | ~0 | 12.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, Y.-C.; Wu, Y.-Q.; Ye, R.-H.; Hung, H.-M.; Hong, G.-B. Magnetically Modified Bentonite for Optimized Erythromycin Removal via RSM and DFT Analysis. Molecules 2025, 30, 1792. https://doi.org/10.3390/molecules30081792
Hung Y-C, Wu Y-Q, Ye R-H, Hung H-M, Hong G-B. Magnetically Modified Bentonite for Optimized Erythromycin Removal via RSM and DFT Analysis. Molecules. 2025; 30(8):1792. https://doi.org/10.3390/molecules30081792
Chicago/Turabian StyleHung, Ying-Chieh, Yu-Qi Wu, Ru-Hau Ye, Hsiu-Min Hung, and Gui-Bing Hong. 2025. "Magnetically Modified Bentonite for Optimized Erythromycin Removal via RSM and DFT Analysis" Molecules 30, no. 8: 1792. https://doi.org/10.3390/molecules30081792
APA StyleHung, Y.-C., Wu, Y.-Q., Ye, R.-H., Hung, H.-M., & Hong, G.-B. (2025). Magnetically Modified Bentonite for Optimized Erythromycin Removal via RSM and DFT Analysis. Molecules, 30(8), 1792. https://doi.org/10.3390/molecules30081792







