Impact of Fluorine in Manganese Citrate Synthesis on Structure and Value-Added Decomposition Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
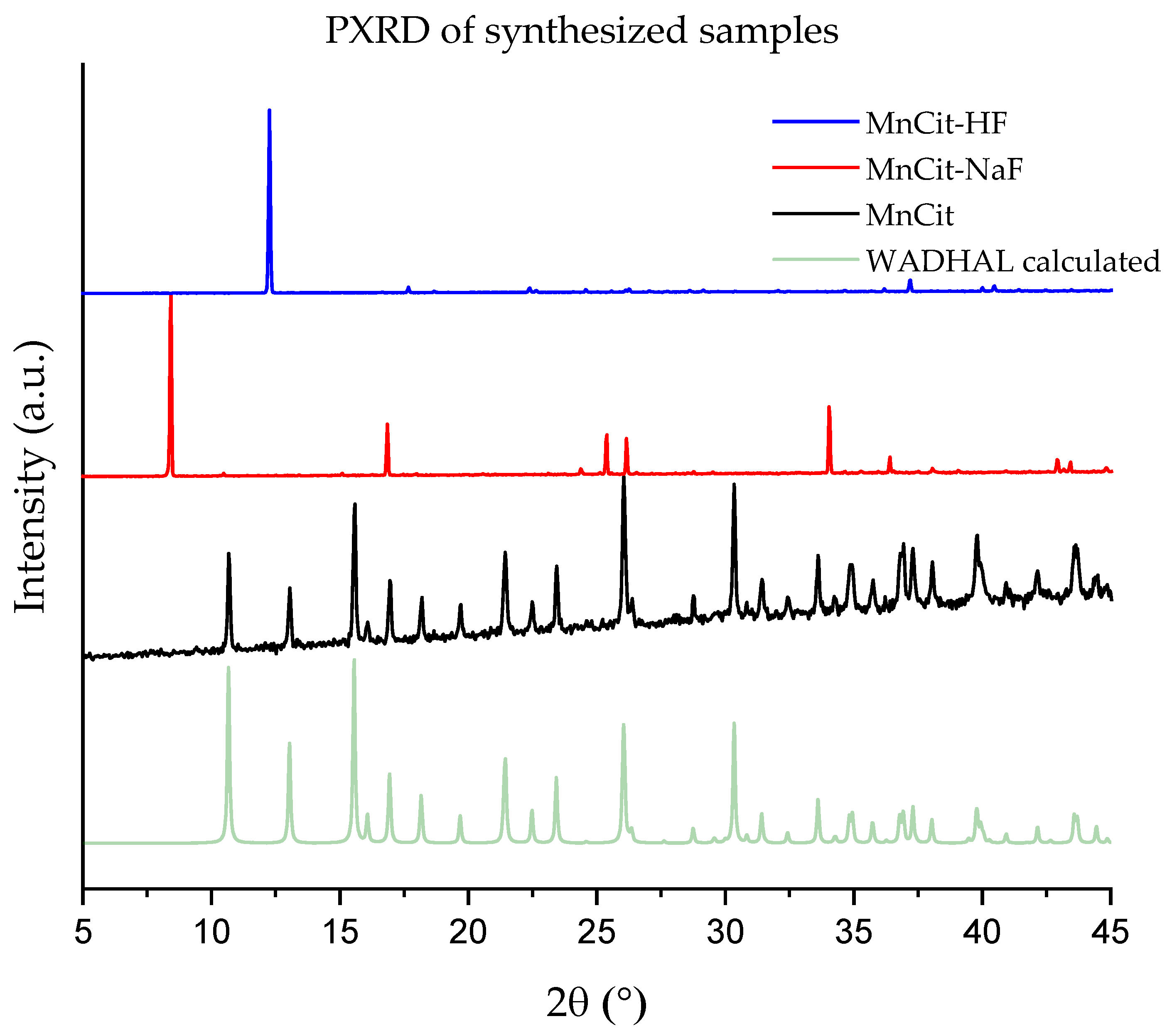
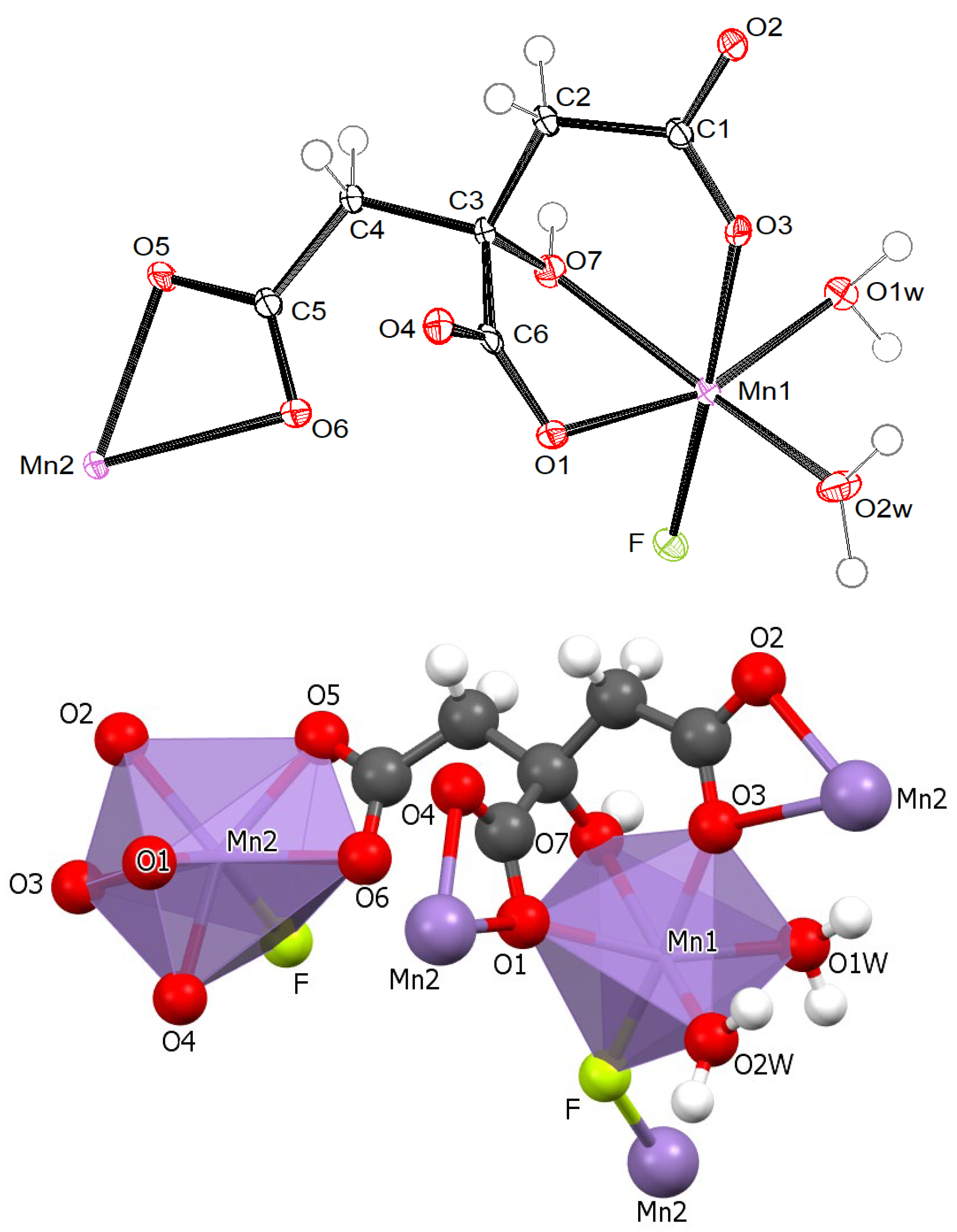
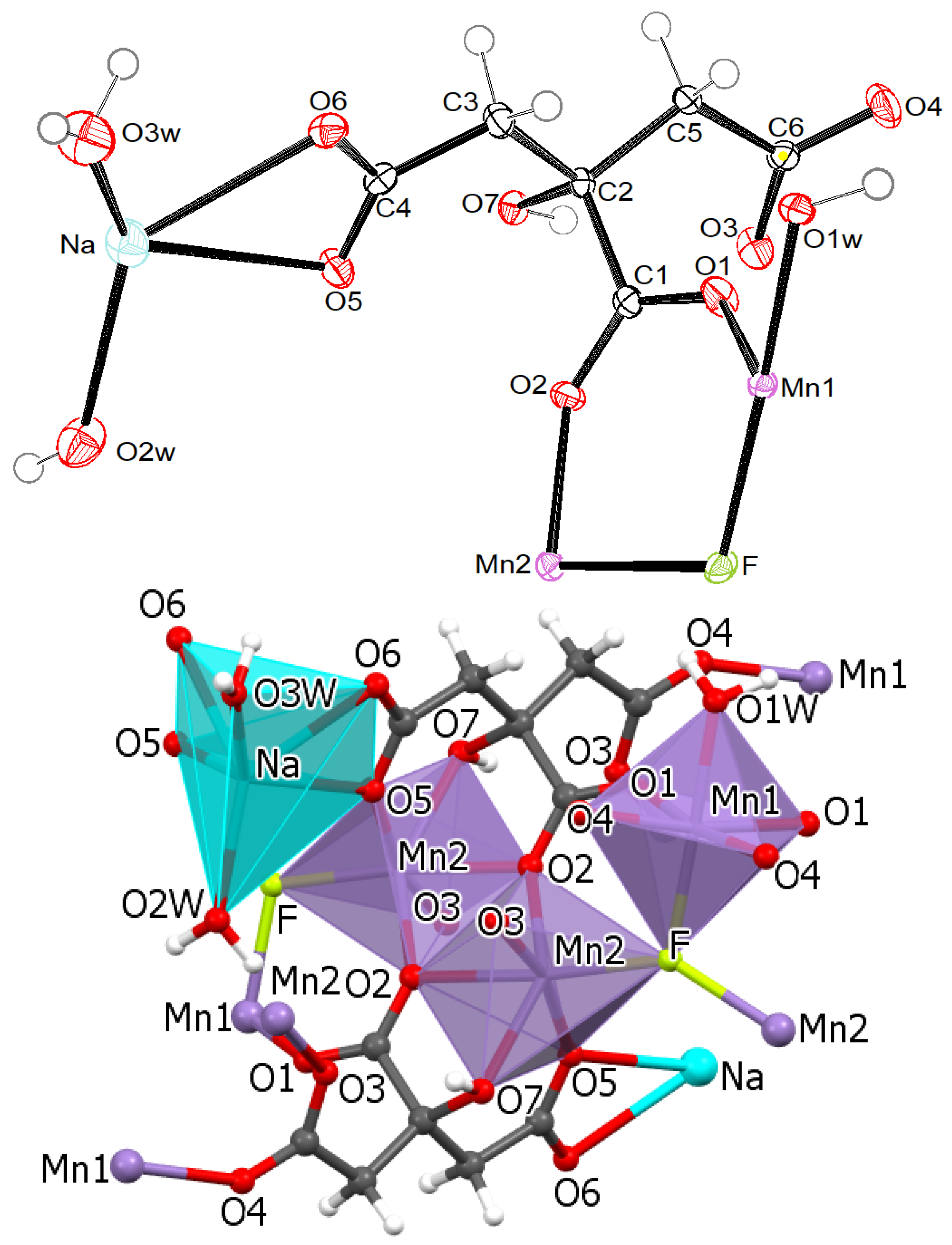
2.2. Crystal Structures
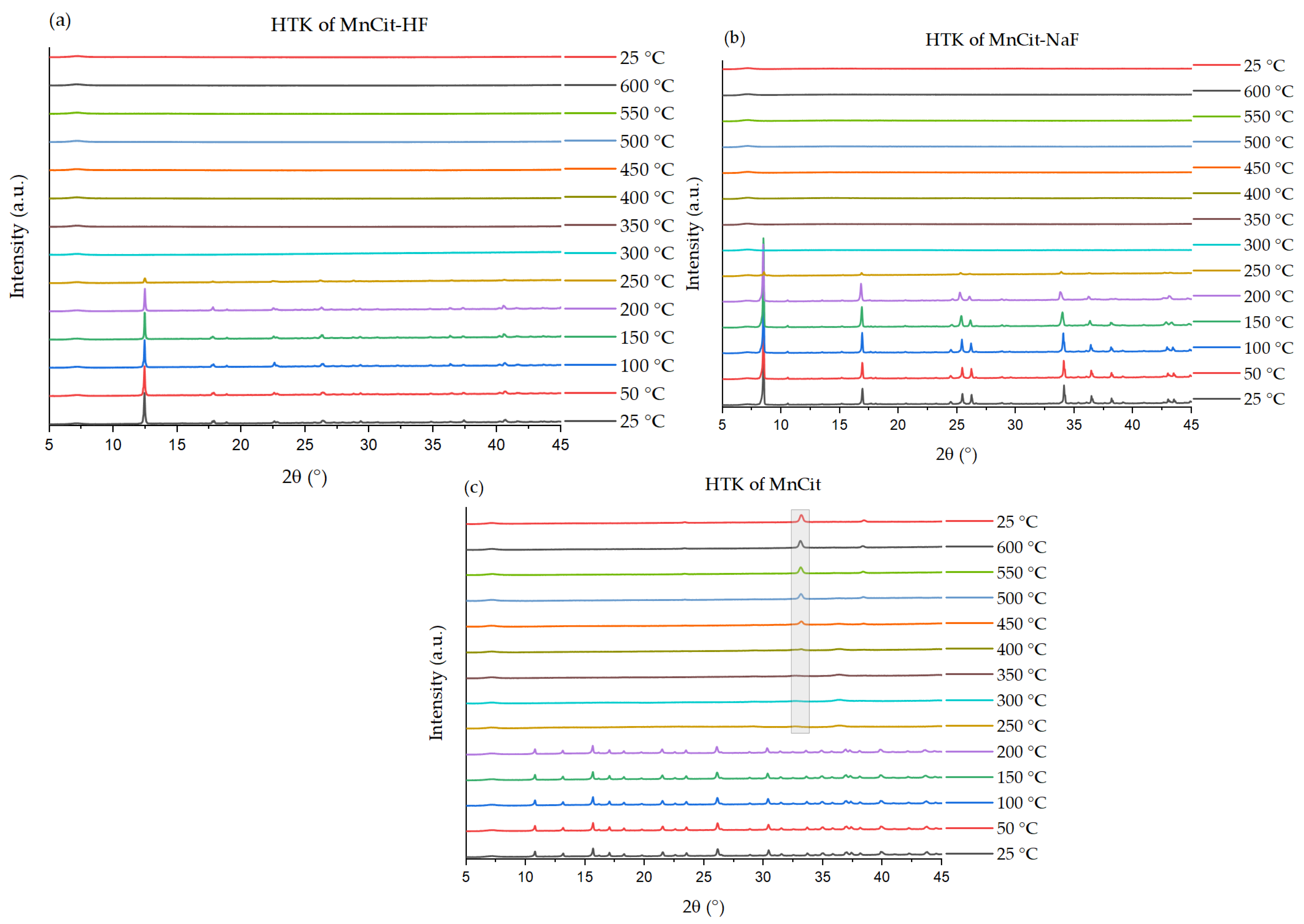
2.3. Analysis of Thermal Properties
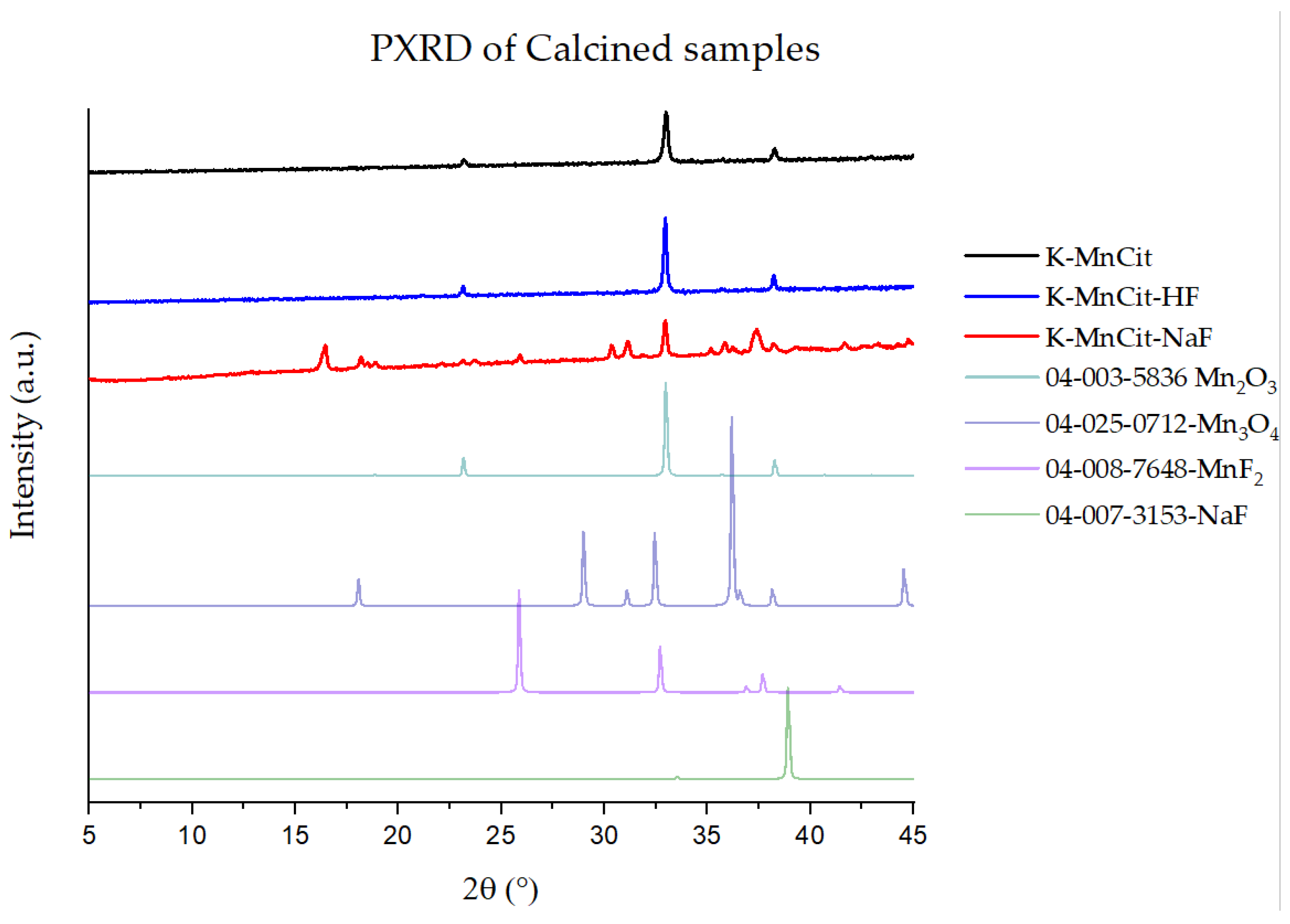
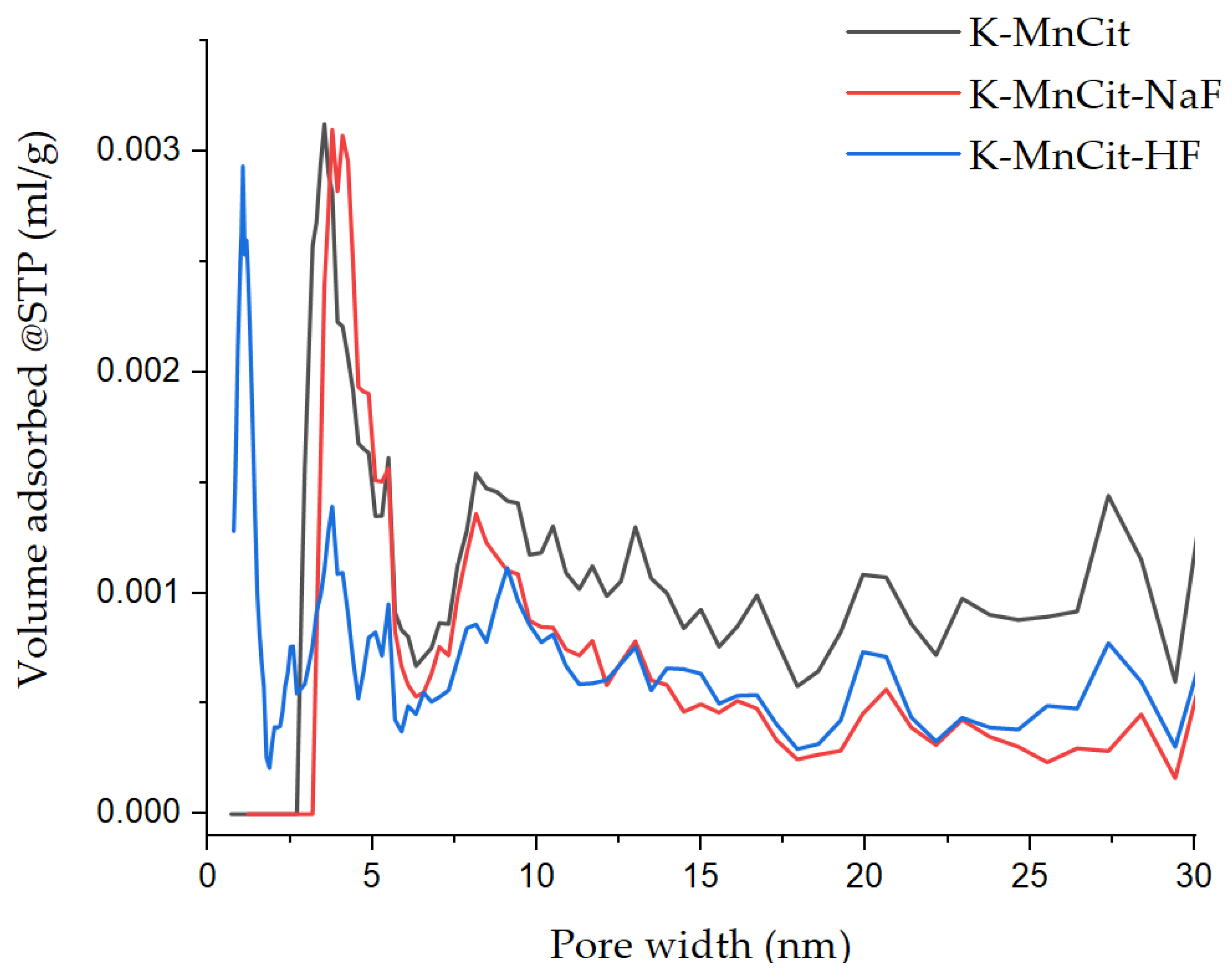
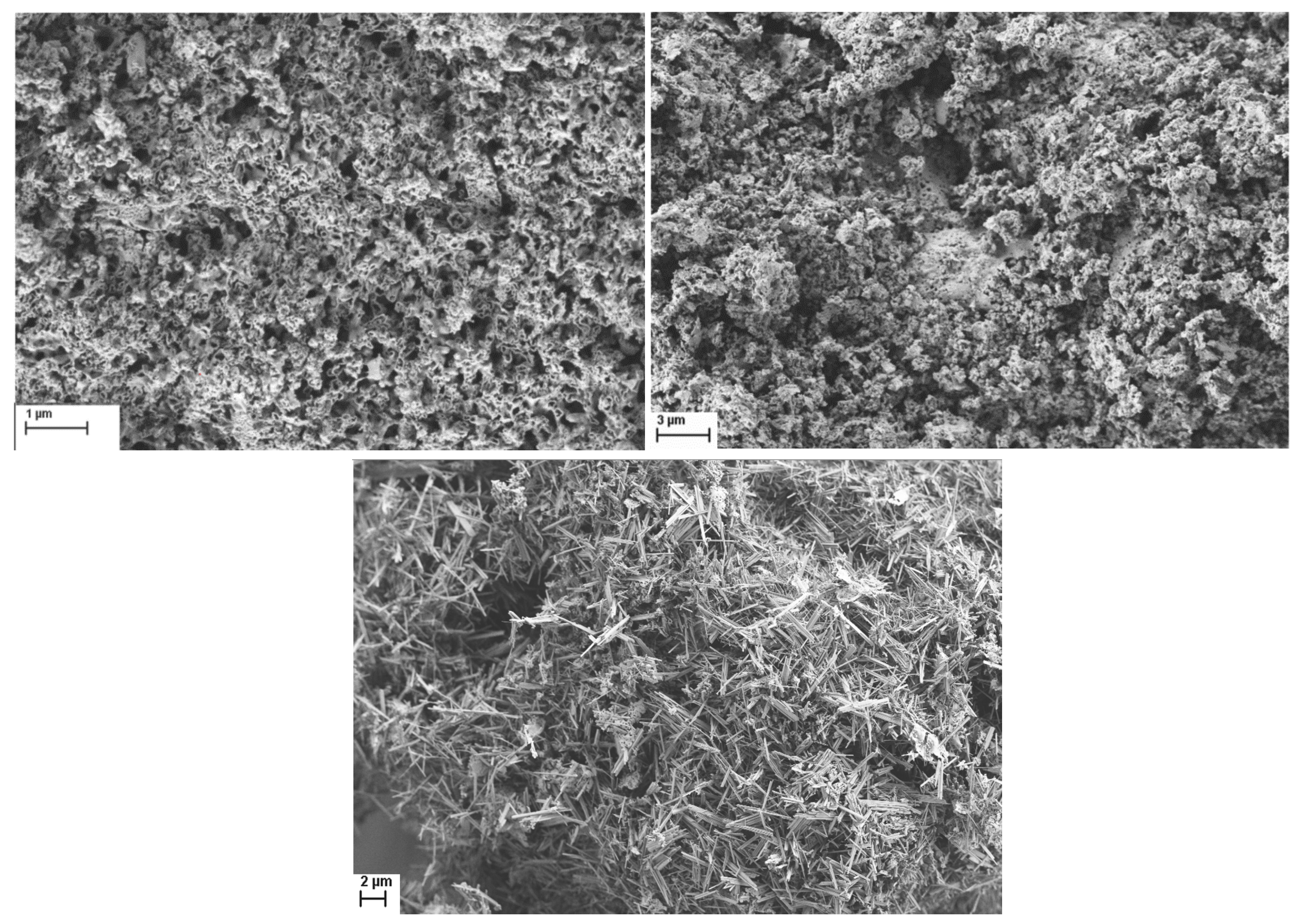
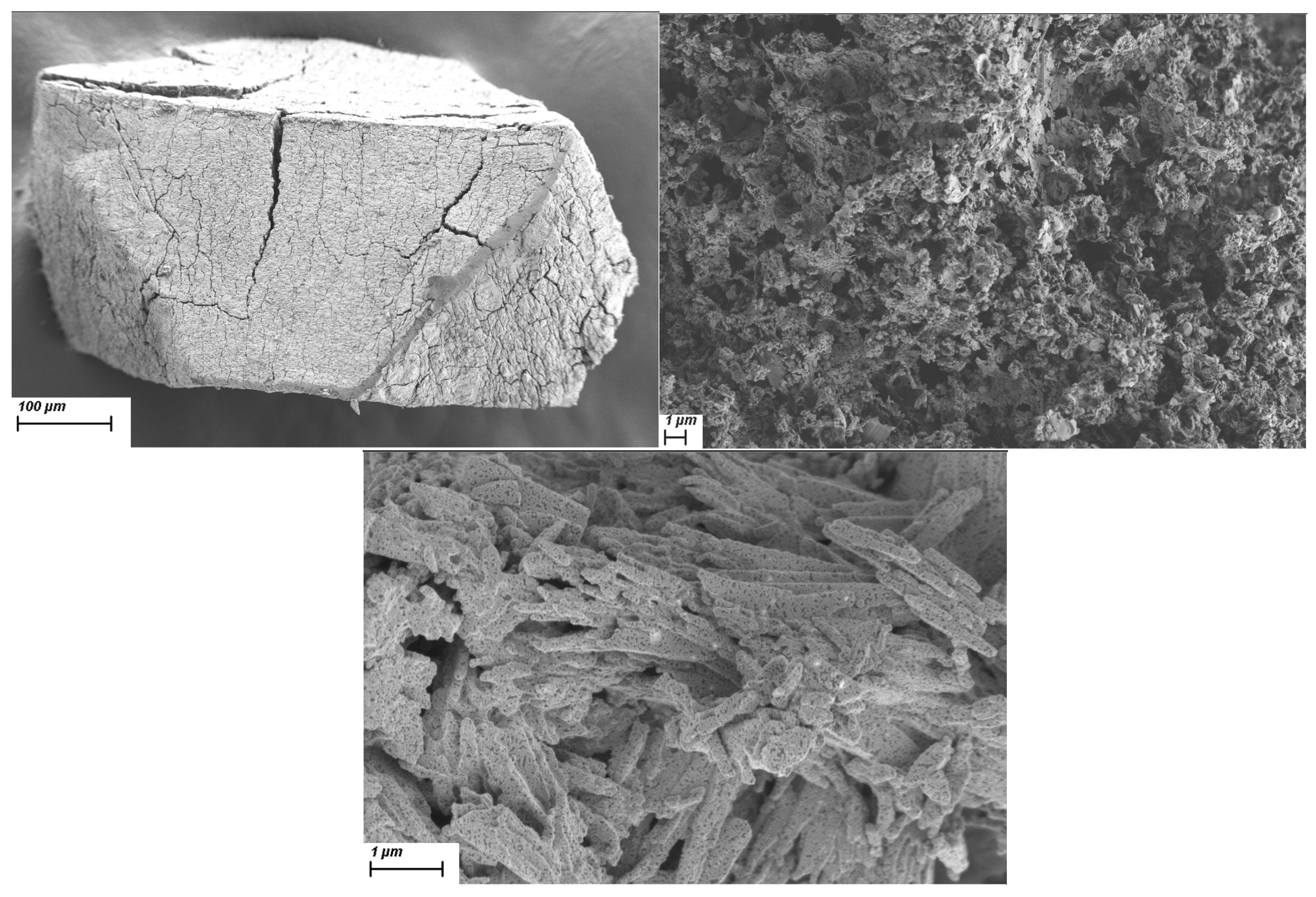
2.4. Mesoporous Oxide Formation
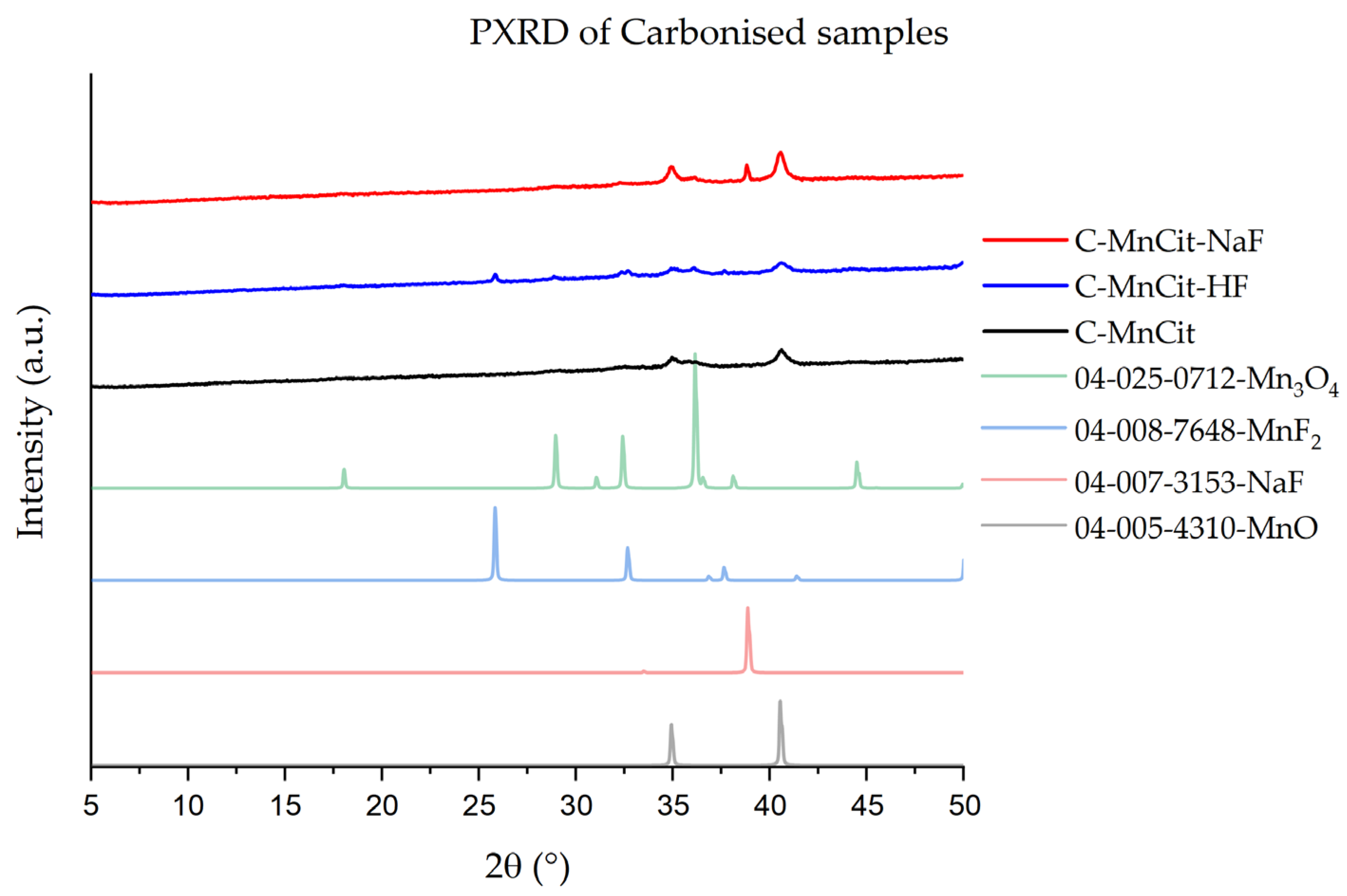
2.5. Carbonization of the Samples
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pudlo, M.; Demougeot, C.; Girard-Thernier, C. Arginase Inhibitors: A Rational Approach Over One Century. Med. Res. Rev. 2017, 37, 475–513. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Tušar, N.N.; Maučec, D.; Rangus, M.; Arčon, I.; Mazaj, M.; Cotman, M.; Pintar, A.; Kaučič, V. Manganese Functionalized Silicate Nanoparticles as a Fenton-Type Catalyst for Water Purification by Advanced Oxidation Processes (AOP). Adv. Funct. Mater. 2012, 22, 820–826. [Google Scholar] [CrossRef]
- Tušar, N.N.; Ristić, A.; Mali, G.; Mazaj, M.; Arčon, I.; Arčon, D.; Kaučič, V.; Logar, N.Z. MnOx Nanoparticles Supported on a New Mesostructured Silicate with Textural Porosity. Chem. A Eur. J. 2010, 16, 5783–5793. [Google Scholar] [CrossRef]
- Tušar, N.N.; Mali, G.; Arčon, I.; Kaučič, V.; Ghanbari-Siahkali, A.; Dwyer, J. Framework cobalt and manganese in MeAPO-31 (Me=Co, Mn) molecular sieves. Microporous Mesoporous Mater. 2002, 55, 203–216. [Google Scholar] [CrossRef]
- Tušar, N.N.; Logar, N.Z.; Arčon, I.; Thibault-Starzyk, F.; Ristić, A.; Rajić, N.; Kaučič, V. Manganese-Containing Silica-Based Microporous Molecular Sieve MnS-1: Synthesis and Characterization. Chem. Mater. 2003, 15, 4745–4750. [Google Scholar] [CrossRef]
- Šuligoj, A.; Trendafilova, I.; Maver, K.; Pintar, A.; Ristić, A.; Dražić, G.; Abdelraheem, W.H.M.; Jagličić, Z.; Arčon, I.; Logar, N.Z.; et al. Multicomponent Cu-Mn-Fe silica supported catalysts to stimulate photo-Fenton-like water treatment under sunlight. J. Environ. Chem. Eng. 2023, 11, 110369. [Google Scholar] [CrossRef]
- Zhou, H.; Su, W.; Xing, Y.; Wang, J.; Zhang, W.; Jia, H.; Yue, T. Progress of catalytic oxidation of VOCs by manganese-based catalysts. Fuel 2024, 366, 131305. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Mosa, I.M.; Poyraz, A.S.; Biswas, S.; El-Sawy, A.M.; Song, W.; Luo, Z.; Chen, S.-Y.; Rusling, J.F.; He, J.; et al. Robust Mesoporous Manganese Oxide Catalysts for Water Oxidation. ACS Catal. 2015, 5, 1693–1699. [Google Scholar] [CrossRef]
- Pilgrim, B.S.; Champness, N.R. Metal-Organic Frameworks and Metal-Organic Cages—A Perspective. Chempluschem 2020, 85, 1842–1856. [Google Scholar] [CrossRef]
- Deng, A.; Yin, Y.; Liu, Y.; Xu, Y.; He, H.; Yang, S.; Qin, Q.; Sun, D.; Li, S. Unlocking the potential of MOF-Derived Carbon-Based nanomaterials for water purification through advanced oxidation processes: A comprehensive review on the impact of process parameter modulation. Sep. Purif. Technol. 2023, 318, 123998. [Google Scholar] [CrossRef]
- Cui, S.; Tang, Y.; Cui, W.; Li, G.; Xiao, X.; Tao, K.; Han, L. Self-sacrificing MOF-74 to amorphous CoMoS4 hollow tube with nanosheet surface for high stability supercapacitors. J. Alloys Compd. 2024, 1003, 175709. [Google Scholar] [CrossRef]
- Ding, B.; Wang, J.; Chang, Z.; Xu, G.; Hao, X.; Shen, L.; Dou, H.; Zhang, X. Self-Sacrificial Template-Directed Synthesis of Metal–Organic Framework-Derived Porous Carbon for Energy-Storage Devices. ChemElectroChem 2016, 3, 668–674. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Hou, F.; Yang, Y.; Dong, H.; Liu, N.; Wang, Y.; Cui, L. Synthesis of highly efficient Mn2O3 catalysts for CO oxidation derived from Mn-MIL-100. Appl. Surf. Sci. 2017, 411, 27–33. [Google Scholar] [CrossRef]
- Cheng, H.; Li, J.; Meng, T.; Shu, D. Advances in Mn-Based MOFs and Their Derivatives for High-Performance Supercapacitor. Small 2024, 20, 2308804. [Google Scholar] [CrossRef]
- Yin, C.; Pan, C.-L.; Pan, Y.; Hu, J. Hollow Mn–Co–O@C Yolk–Shell Microspheres with Carbon Shells as Cathodes Derived from a Double-Metal MOF for Aqueous Zinc-Ion Batteries. ACS Sustain. Chem. Eng. 2023, 11, 12397–12405. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Wang, T.; Fu, N.; Yang, Z. Manganese-based MOF interconnected carbon nanotubes as a high-performance cathode for rechargeable aqueous zinc-ion batteries. J. Energy Storage 2024, 76, 109873. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, K.; Liu, S.; Cao, X.; Wu, K.; Cheong, W.-C.; Chen, Z.; Wang, Y.; Li, Y.; Liu, Y.; et al. Core–Shell ZIF-8@ZIF-67-Derived CoP Nanoparticle-Embedded N-Doped Carbon Nanotube Hollow Polyhedron for Efficient Overall Water Splitting. J. Am. Chem. Soc. 2018, 140, 24. [Google Scholar] [CrossRef]
- Li, R.; Crystengcomm; Nagamuthu, S.; Ryu, K.-S. MOF-derived microstructural interconnected network porous Mn2O3/C as negative electrode material for asymmetric supercapacitor device. CrystEngComm 2019, 21, 1442–1451. [Google Scholar] [CrossRef]
- Singh, A.; Singh, B.; Verma, S. Manganese-Based Metal-Organic Frameworks and Their Derivatives for Electrochemical Water Splitting: Recent Advances and Future Outlook. Chem. Asian J. 2025, 20, e202401522. [Google Scholar] [CrossRef]
- Lai, L.S.; Yeong, Y.F.; Ani, N.C.; Lau, K.K.; Shariff, A.M. Effect of Synthesis Parameters on the Formation of Zeolitic Imidazolate Framework 8 (ZIF-8) Nanoparticles for CO2 Adsorption. Part. Sci. Technol. 2014, 32, 520–528. [Google Scholar] [CrossRef]
- Wang, B.R.; Hu, Y.; Pan, Z.; Wang, J. MOF-derived manganese oxide/carbon nanocomposites with raised capacitance for stable asymmetric supercapacitor. RSC Adv. 2020, 10, 34403–34412. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, W.; Hao, S.; Wu, H. Bimetal Metal–Organic Framework-Derived Ni–Mn@Carbon/Reduced Graphene Oxide as a Cathode for an Asymmetric Supercapacitor with High Energy Density. Langmuir 2023, 39, 12510–12519. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Wang, Z.; Zhao, M.; Xiao, F.; Zhang, L.; Yang, L.; Wu, Z.; Bai, J.; He, P. MOF/bacterial cellulose derived octahedral MnO/carbon nanofiber network: A hybrid for peroxymonosulfate activation toward degradation of tetracycline. J. Alloys Compd. 2023, 968, 171896. [Google Scholar] [CrossRef]
- Ebrahimi-Koodehi, S.; Ghodsi, F.E.; Mazloom, J. Ni/Mn metal–organic framework decorated bacterial cellulose (Ni/Mn-MOF@BC) and nickel foam (Ni/Mn-MOF@NF) as a visible-light photocatalyst and supercapacitive electrode. Sci. Rep. 2023, 13, 19260. [Google Scholar] [CrossRef]
- Li, W.-Z.; Guo, F.-Y.; Li, J.; Zhang, X.-S.; Liu, Y.; Luan, J. Fabrication of bimetallic MOF-74 derived materials for high-efficiency adsorption of iodine. Dalt. Trans. 2024, 53, 13370–13383. [Google Scholar] [CrossRef]
- Ni, C.; Chen, R.; Hao, C.; Lu, Y.; Wu, J.; Shen, Y.; Wang, X. High-performance asymmetric supercapacitor constructed by MOF-derived Mn-Ni-Co sulfide hollow cages and Typha pollen-derived carbon. J. Alloys Compd. 2023, 960, 170807. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, L.; Liu, X.; Ai, L. Bioinspired Cobalt-Citrate Metal-Organic Framework as an Efficient Electrocatalyst for Water Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 7193–7201. [Google Scholar] [CrossRef]
- Tseng, M.Y.; Su, Y.H.; Lai, Y.S.; Pan, F.; Kung, P.Y. Cobalt-Citrate Metal-Organic-Framework UTSA-16 on TiO2 Nanoparticles. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: London, UK, 2020; Volume 720, p. 012008. [Google Scholar]
- Deng, Y.F.; Zhou, Z.H. Manganese citrate complexes: Syntheses, crystal structures and thermal properties. J. Coord. Chem. 2009, 62, 778–788. [Google Scholar] [CrossRef]
- Matzapetakis, M.; Karligiano, N.; Bino, A.; Dakanali, M.; Raptopoulou, C.P.; Tangoulis, V.; Terzis, A.; Giapintzakis, J.; Salifoglou, A. Manganese citrate chemistry: Syntheses, spectroscopic studies, and structural characterizations of novel mononuclear, water-soluble manganese citrate complexes. Inorg. Chem. 2000, 39, 4044–4051. [Google Scholar] [CrossRef]
- Deng, Y.F.; Zhou, Z.H.; Wan, H.L.; Ng, S.W. Δ-aqua-S-citrato(2−)manganese(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, m310–m312. [Google Scholar] [CrossRef]
- Glusker, J.P.; Carrell, H.L. X-Ray crystal analysis of the substrates of aconitase. XI. Manganous citrate decahydrate. J. Mol. Struct. 1973, 15, 151–159. [Google Scholar] [CrossRef]
- Xie, F.T.; Duan, L.M.; Chen, X.Y.; Cheng, P.; Xu, J.Q.; Ding, H.; Wang, T.G. Solvothermal synthesis and characterization of 2D to 3D metal-citrate coordination polymer linked by K+ ions: {K[Mn(C6H5O7)(H2O)]}n. Inorg. Chem. Commun. 2005, 8, 274–277. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Chen, F.; Ma, C.; Chen, C.; Liu, Q.; Liao, D.; Li, L. Homo- and hetero-metallic manganese citrate complexes: Syntheses, crystal structures and magnetic properties. Polyhedron 2005, 24, 1656–1668. [Google Scholar] [CrossRef]
- Qiu, H.; Pan, S.; Mutailipu, M. Fluorination strategy toward chemical and functional modification. Fundam. Res. 2025, 5, 640–653. [Google Scholar] [CrossRef]
- Majekodunmi, T.; Britton, D.; Montclare, J.K. Engineered Proteins and Materials Utilizing Residue-Specific Noncanonical Amino Acid Incorporation. Chem. Rev. 2024, 124, 9113–9135. [Google Scholar] [CrossRef]
- Zabukovec Logar, N.; Mali, G.; Rajic, N.; Jevtic, S.; Rangus, M.; Golobic, A.; Kaucic, V. Structure investigation of fluorinated aluminophosphate ULM-3 Al templated by 3-methylaminopropylamine. J. Solid State Chem. 2010, 183, 1055–1062. [Google Scholar] [CrossRef]
- He, S.; Zhou, X.; Li, Z.; Wang, J.; Ma, L.; Yang, S. Fluorine Doping Strengthens the Lithium-Storage Properties of the Mn-Based Metal–Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 26907–26914. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. IUCr SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP -3 for Windows—A version of ORTEP -III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D. Recent developments in the methods and applications of the bond valence model. Chem. Rev. 2009, 109, 6858–6919. [Google Scholar] [CrossRef] [PubMed]
- (IUCr) Bond Valence Parameters. Available online: https://www.iucr.org/resources/data/datasets/bond-valence-parameters (accessed on 12 April 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škrjanc, A.; Trstenjak, E.; Opresnik, M.; Gabrijelčič, M.; Golobič, A.; Zabukovec Logar, N. Impact of Fluorine in Manganese Citrate Synthesis on Structure and Value-Added Decomposition Products. Molecules 2025, 30, 1794. https://doi.org/10.3390/molecules30081794
Škrjanc A, Trstenjak E, Opresnik M, Gabrijelčič M, Golobič A, Zabukovec Logar N. Impact of Fluorine in Manganese Citrate Synthesis on Structure and Value-Added Decomposition Products. Molecules. 2025; 30(8):1794. https://doi.org/10.3390/molecules30081794
Chicago/Turabian StyleŠkrjanc, Aljaž, Emanuela Trstenjak, Mojca Opresnik, Matej Gabrijelčič, Amalija Golobič, and Nataša Zabukovec Logar. 2025. "Impact of Fluorine in Manganese Citrate Synthesis on Structure and Value-Added Decomposition Products" Molecules 30, no. 8: 1794. https://doi.org/10.3390/molecules30081794
APA StyleŠkrjanc, A., Trstenjak, E., Opresnik, M., Gabrijelčič, M., Golobič, A., & Zabukovec Logar, N. (2025). Impact of Fluorine in Manganese Citrate Synthesis on Structure and Value-Added Decomposition Products. Molecules, 30(8), 1794. https://doi.org/10.3390/molecules30081794






