Effect of Ultra-High Pressure on the Extraction of the Free, Esterified, and Bound Phenolics from Dendrobium fimbriatum Hook: Chemical Constituents and Antioxidant Ability
Abstract
1. Introduction
2. Results
2.1. UHPLC-OE-MS Analysis of Chemical Constituents in DFH Extracts
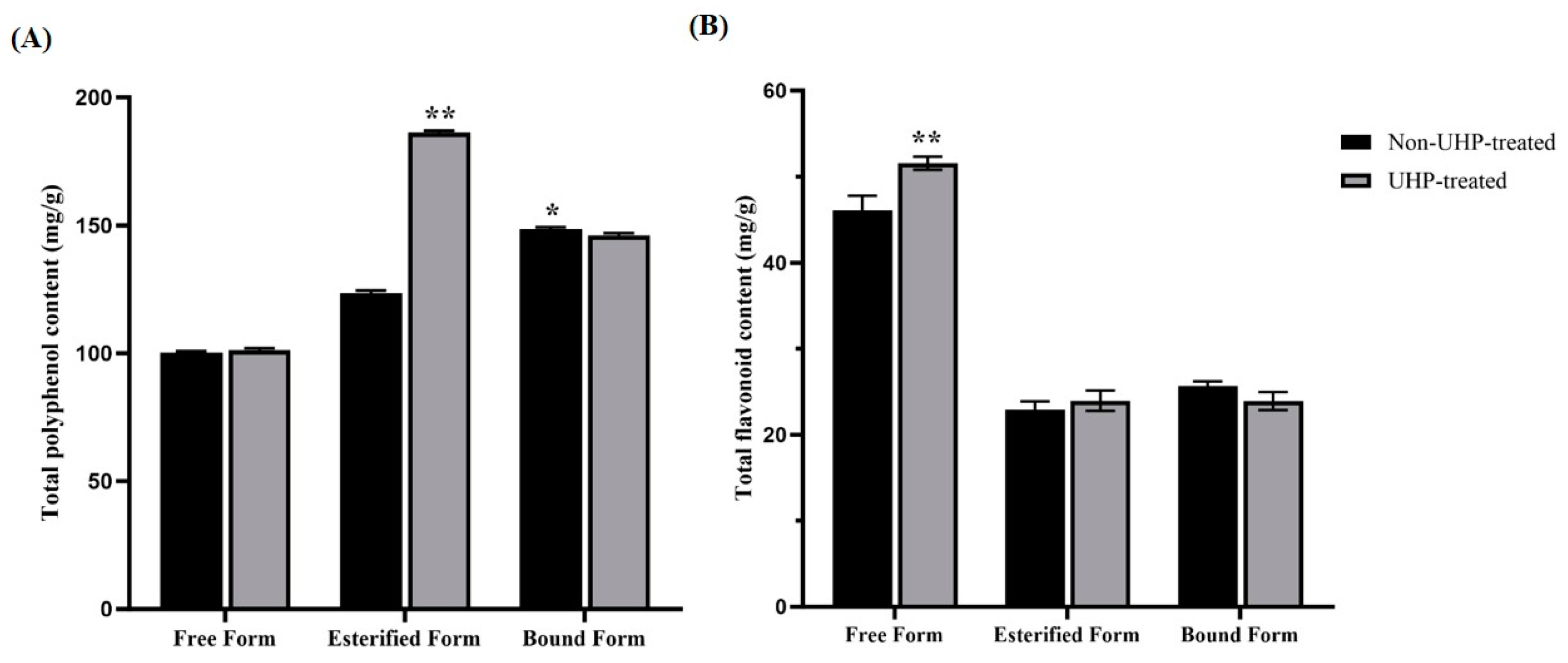
2.2. Determinations of Total Polyphenol Content (TPC) and Total Flavonoid Content (TFC) Levels in Different Forms of Phenolics in DFH
2.3. In Vitro Antioxidant Activity in Different DFH Extracts
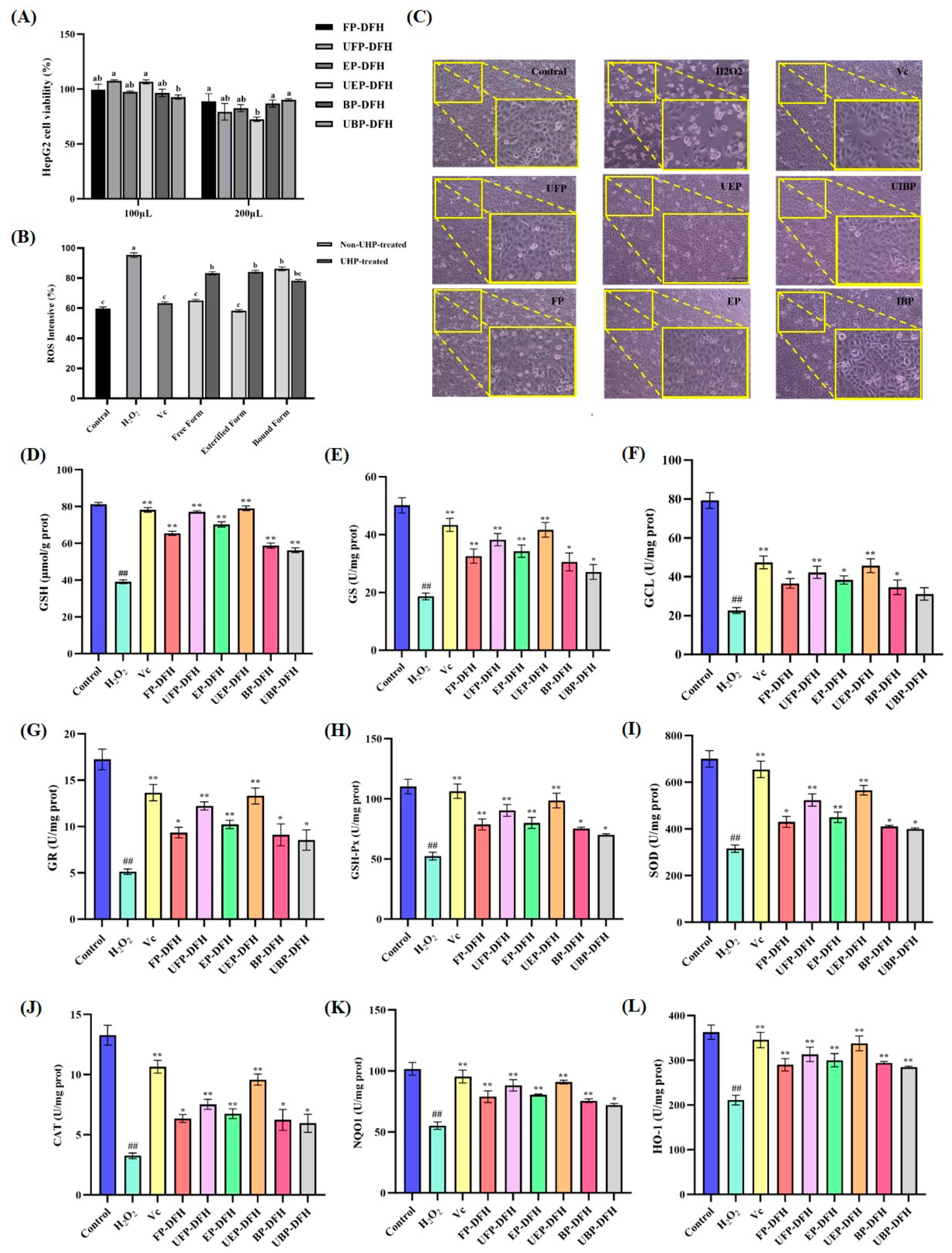
2.4. Cytoprotective Activity in Different DFH Extracts in H2O2-Treated HepG2 Cells
2.4.1. The Influence of Different DFH Extracts on H2O2-Induced HepG2 Cell Viability and Morphology
2.4.2. Effect of DFH Phenolic Fractions on ROS Production in H2O2-Induced HepG2 Cells
2.4.3. Effect of DFH Phenolic Fractions on Glutathione (GSH) Synthesis and Metabolism in H2O2-Induced HepG2 Cells
2.4.4. Effect of DFH Phenolic Fractions on Activities of Antioxidant Enzymes in H2O2-Induced HepG2 Cells
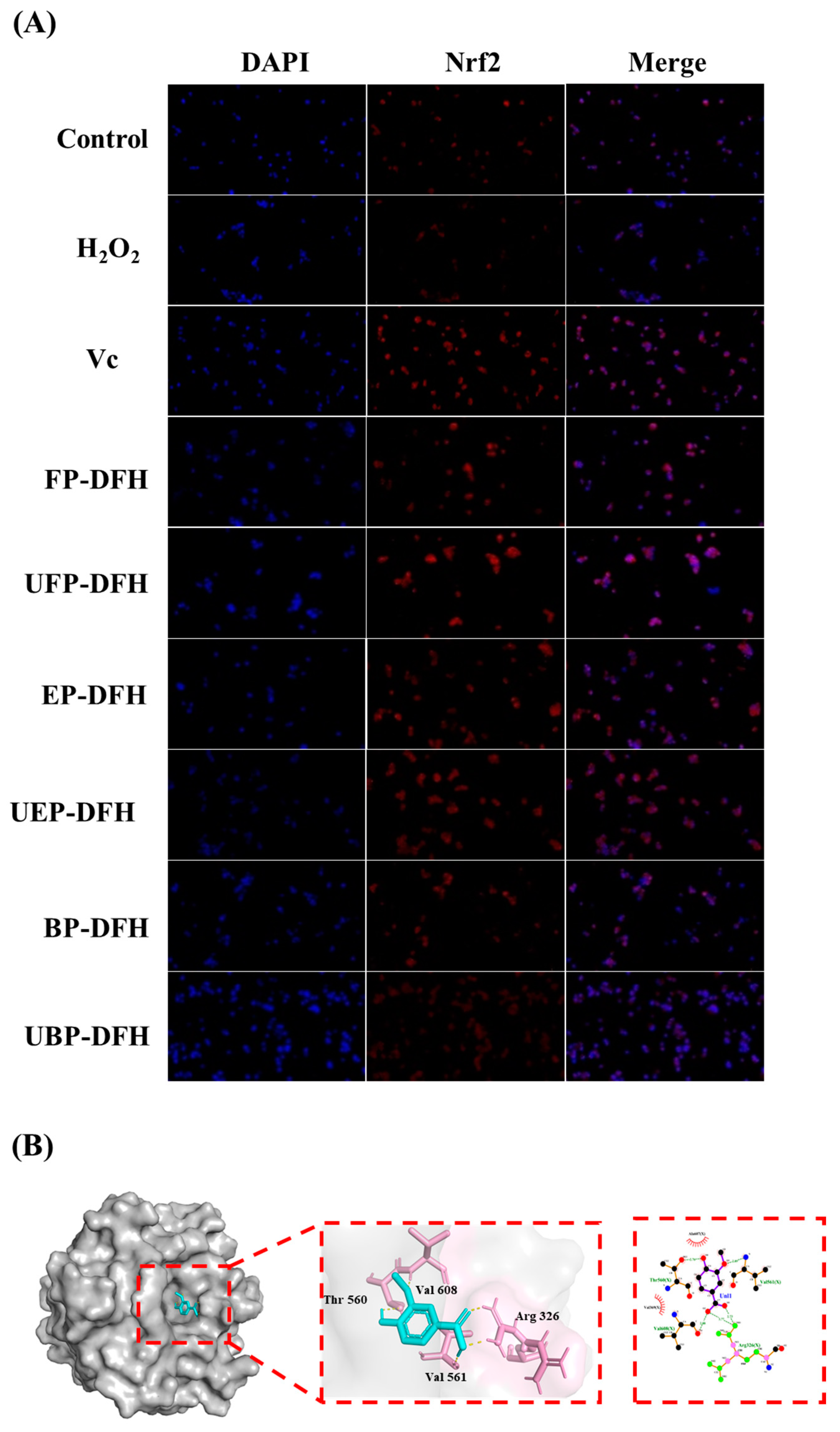
2.5. DAPI Staining Results and Molecular Docking Analysis
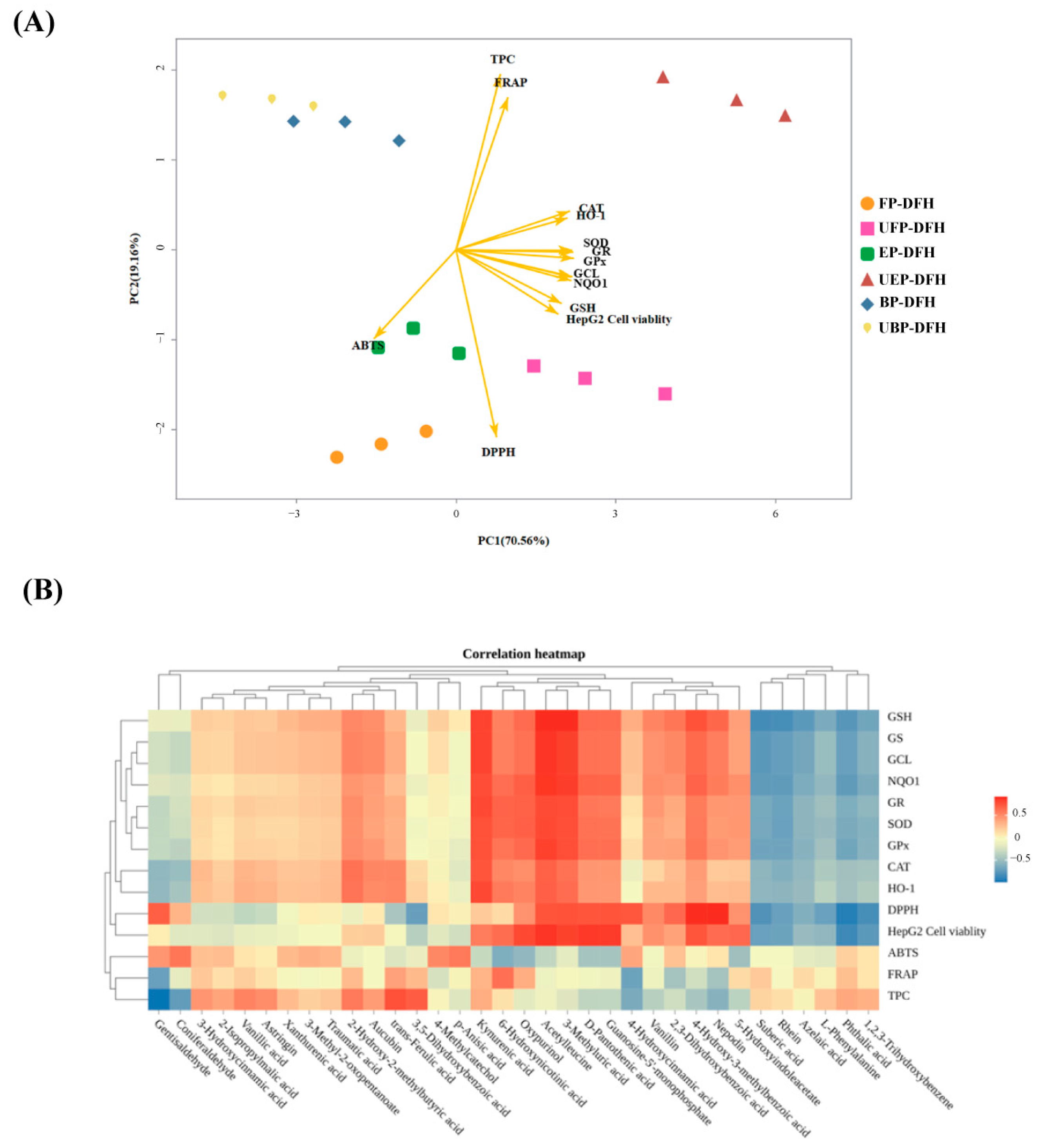
2.6. Principal Component and Relationship Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Collection and Preparation
4.3. Preparation of Free, Esterified, and Bound Phenolics
4.4. Chemical Constituent Analysis of DFH Extracts
4.5. Metabolic Diversity and Difference Analysis
4.6. Determination of Total Polyphenol (TPC) and Total Flavonoid (TFC) Content
4.6.1. Determination of TPC Level
4.6.2. Determination of TFC Level
4.7. Antioxidant Capacity of DFH Phenolics in Different Forms
4.7.1. DPPH Free Radical Scavenging Activity
4.7.2. ABTS Radical Scavenging Activity
4.7.3. Ferric-Reducing Antioxidant Capacity (FRAP) Assay
4.8. Cell Experiments
4.8.1. Estimation of DFH Extracts on HepG2 Cell Viability
4.8.2. Determination of Intracellular ROS Content
4.8.3. Determining Glutathione (GSH) Pathways in H2O2-Induced HepG2 Cells
4.8.4. Estimating Antioxidant Enzyme Activities
4.9. Immunofluorescence Analysis of Nrf2 Protein by DAPI Staining
4.10. Molecular Docking Analysis
4.11. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hilal, B.; Khan, M.M.; Fariduddin, Q. Recent advancements in deciphering the therapeutic properties of plant secondary metabolites: Phenolics, terpenes, and alkaloids. Plant Physiol. Biochem. 2024, 211, 108674. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, Y.; Wang, Z.; Khan, A.; Zhou, W.; Zhao, T.; Cao, J.; Cheng, G.; Cai, S. Phenolic constituents, antioxidant and cytoprotective activities of crude extract and fractions from cultivated artichoke inflorescence. Ind. Crops Prod. 2020, 143, 111433. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Prakash, O.; Baskaran, R.; Kudachikar, V.B. Characterization, quantification of free, esterified and bound phenolics in Kainth (Pyrus pashia Buch.-Ham. Ex D.Don) fruit pulp by UPLC-ESI-HRMS/MS and evaluation of their antioxidant activity. Food Chem. 2019, 299, 125114. [Google Scholar] [CrossRef]
- Park, S.K.; Hyun, S.H.; In, G.; Park, C.K.; Kwak, Y.S.; Jang, Y.J.; Kim, B.; Kim, J.H.; Han, C.K. The antioxidant activities of Korean Red Ginseng (Panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. J. Ginseng Res. 2021, 45, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xiao, Y.; Yan, Z.; Duan, Y.; Hu, K.; Cai, M.; Zhang, H. Poria cocos polysaccharide and lotus seedpod polyphenols synergistically inhibit CRFK cell damage induced by magnesium ammonium phosphate via activating Nrf2/HO-1 signaling pathway. Food Biosci. 2025, 66, 106184. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Pei, X.; Liu, X.; Dai, C.; Li, C.; Li, L.; Zhang, J.; Xiao, X.; Tang, S. Molecular mechanism of olaquindox-induced hepatotoxicity and the hepatic protective role of curcumin. Food Chem. Toxicol. 2020, 145, 111727. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zheng, L.; Yu, P.; Jiang, Q.; Wu, Y.; Huang, C.; Yin, B. Characterization and application of lignin–carbohydrate complexes from lignocellulosic materials as antioxidants for scavenging in vitro and in vivo reactive oxygen species. ACS Sustain. Chem. Eng. 2020, 8, 256–266. [Google Scholar] [CrossRef]
- Venkateswara Rao, M.; Sunil, C.K.; Rawson, A.; Chidanand, D.V.; Venkatachlapathy, N. Modifying the plant proteins techno-functionalities by novel physical processing technologies: A review. Crit. Rev. Food Sci. Nutr. 2021, 63, 4070–4091. [Google Scholar] [CrossRef]
- Sun, M.; Wei, X.; Wang, H.; Xu, C.; Wei, B.; Zhang, J.; He, L.; Xu, Y.; Li, S. Structure restoration of thermally denatured collagen by ultrahigh pressure treatment. Food Bioprocess Technol. 2020, 13, 367–378. [Google Scholar] [CrossRef]
- Floros, J.D.; Newsome, R.; Fisher, W.; Barbosa-Cánovas, G.V.; Chen, H.; Dunne, C.P.; German, J.B.; Hall, R.L.; Heldman, D.R.; Karwe, M.V.; et al. Feeding the world today and tomorrow: The importance of food science and technology. Compr. Rev. Food Sci. Food Saf. 2010, 9, 572–599. [Google Scholar] [CrossRef]
- Chen, R.; Meng, F.; Zhang, S.; Liu, Z. Effects of ultrahigh pressure extraction conditions on yields and antioxidant activity of ginsenoside from ginseng. Sep. Purif. Technol. 2009, 66, 340–346. [Google Scholar] [CrossRef]
- Chen, T.; He, S.; Zhang, J.; Wang, H.; Jia, Y.; Liu, Y.; Xie, M.; Cheng, G. Effects of ultra-high-pressure treatment on chemical composition and biological activities of free, esterified and bound phenolics from Phyllanthus emblica L. Fruits. Molecules 2024, 29, 3181. [Google Scholar] [CrossRef]
- Zhen, L.; He, S.; Xue, Q.; Liu, Y.; Cao, J.; Zhao, T.; Cheng, G.; Wang, Y. Influence of ultra-high-pressure pretreatment method on chemical constituents, antioxidant and cytoprotective activities of free, esterified, and bound phenolics from Anneslea fragrans Wall. Leaves. Plant Foods Hum. Nutr. 2023, 78, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, M.; Zhao, Z.; Khan, A.; Zhao, T.; Liu, Y.; Wang, Z.; Cheng, G. The influences of extraction methods on the chemical constituents of Lyonia ovalifolia (Wall.) Drude and intracellular protective effects based on metabolomics analysis. Food Chem. 2024, 456, 140031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X.; Zhu, X.; Hua, Y. Chemical constituents, bioactivities, and pharmacological mechanisms of Dendrobium officinale: A review of the past decade. J. Agric. Food Chem. 2023, 71, 14870–14889. [Google Scholar] [CrossRef]
- Xu, Z.; Li, L.; Xu, Y.; Wang, S.; Zhang, X.; Tang, T.; Yu, J.; Zhao, H.; Wu, S.; Zhang, C.; et al. Pesticide multi-residues in Dendrobium officinale Kimura et Migo: Method validation, residue levels and dietary exposure risk assessment. Food Chem. 2021, 343, 128490. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, F.; Liu, Y.; Khan, A.; Wang, Z.; Cheng, G. A comparative analysis of chemical constituents and antioxidant effects of Dendrobium fimbriatum Hook fractions with different polarities. Int. J. Mol. Sci. 2023, 24, 12646. [Google Scholar] [CrossRef]
- Ramos, S.; Alía, M.; Bravo, L.; Goya, L. Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (HepG2). J. Agric. Food Chem. 2005, 53, 1271–1280. [Google Scholar] [CrossRef]
- Su, M.; Yu, T.; Zhang, H.; Wu, Y.; Wang, X.; Li, G. The antiapoptosis effect of glycyrrhizate on HepG2 cells induced by hydrogen peroxide. Oxid. Med. Cell. Longev. 2016, 2016, 6849758. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, J.; Chen, Y.; Liu, Y.; Huang, S.; Zhong, X.; Cheng, Y.; Wang, Z. Nrf2 mediates the protective effects of homocysteine by increasing the levels of GSH content in HepG2 cells. Mol. Med. Rep. 2017, 16, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.; Nemat Alla, M.M. Kinetics of inhibition of isoproturon to glutathione-associated enzymes in wheat. Physiol. Mol. Biol. Plants 2020, 26, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zeng, Y.; Lee, T.D.; Yang, Y.; Ou, X.; Chen, L.; Haque, M.; Rippe, R.; Lu, S.C. Role of AP-1 in the coordinate induction of rat glutamate-cysteine ligase and glutathione synthetase bytert-butylhydroquinone. J. Biol. Chem. 2002, 277, 35232–35239. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Zou, B.; Xiao, G.; Xu, Y.; Wu, J.; Yu, Y.; Fu, M. Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chem. 2018, 255, 23–30. [Google Scholar] [CrossRef]
- Yu, X.; Mansouri, A.; Liu, Z.; Gao, R.; Li, K.; Chen, C.; Huang, Y.; Chen, Z.; Chen, S.; Lu, Y.; et al. NRF2 activation induced by PML-RARα promotes microRNA 125b-1 expression and confers resistance to chemotherapy in acute promyelocytic leukemia. Clin. Transl. Med. 2021, 11, e418. [Google Scholar] [CrossRef]
- Ren, L.; Fan, J.; Yang, Y.; Liu, X.; Wang, B.; Bian, X.; Wang, D.; Xu, Y.; Liu, B.; Zhu, P.; et al. Identification, in silico selection, and mechanism study of novel antioxidant peptides derived from the rice bran protein hydrolysates. Food Chem. 2023, 408, 135230. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of Kynurenic Acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.-D.; Xue, Q.-W.; Zhao, T.-R.; Khan, A.; Wang, Y.-F.; Liu, Y.-P.; Cao, J.-X.; Cheng, G.-G. The Effect of ultra-high pretreatment on free, esterified and insoluble-bound phenolics from mango leaves and their antioxidant and cytoprotective activities. Food Chem. 2022, 368, 130864. [Google Scholar] [CrossRef]
- Navarro-Baez, J.E.; Martínez, L.M.; Welti-Chanes, J.; Buitimea-Cantúa, G.V.; Escobedo-Avellaneda, Z. High hydrostatic pressure to increase the biosynthesis and extraction of phenolic compounds in food: A review. Molecules 2022, 27, 1502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Xue, Q.; Zhen, L.; Wang, Y.; Cao, J.; Liu, Y.; Khan, A.; Zhao, T.; Cheng, G. Effect of ultra-high pressure pretreatment on the phenolic profiles, antioxidative activity and cytoprotective capacity of different phenolic fractions from Que Zui Tea. Food Chem. 2023, 409, 135271. [Google Scholar] [CrossRef]
- Huchzermeyer, B.; Menghani, E.; Khardia, P.; Shilu, A. Metabolic pathway of natural antioxidants, antioxidant enzymes and ROS providence. Antioxidants 2022, 11, 761. [Google Scholar] [CrossRef]
- Lu, Q.; Shu, Y.; Wang, L.; Li, G.; Zhang, S.; Gu, W.; Sun, Y.; Hua, W.; Huang, L.; Chen, F.; et al. The Protective Effect of Veronica ciliata Fisch. Extracts on Relieving Oxidative Stress-Induced Liver Injury via Activating AMPK/P62/Nrf2 Pathway. J. Ethnopharmacol. 2021, 270, 113775. [Google Scholar] [CrossRef] [PubMed]
- Mashitoa, F.M.; Shoko, T.; Slabbert, R.M.; Shai, J.L.; Sultanbawa, Y.; Sivakumar, D. A practical chemometric approach using UPLC–QTOF/MS tool to investigate three varieties of pumpkinspecies and in vitro bioactivities. Food Biosci. 2021, 43, 101229. [Google Scholar] [CrossRef]
- Lana, J.V.; Rios, A.; Takeyama, R.; Santos, N.; Pires, L.; Santos, G.S.; Rodrigues, I.J.; Jeyaraman, M.; Purita, J.; Lana, J.F. Nebulized glutathione as a key antioxidant for the treatment of oxidative stress in neurodegenerative conditions. Nutrients 2024, 16, 2476. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Vázquez-Velasco, M.; González-Torres, L.; Benedí, J.; Sánchez-Muniz, F.J.; Morales-González, J.A.; Jaramillo-Morales, O.A.; Valadez-Vega, C.; Bautista, M. Effect of extract and ellagic acid from Geranium schiedeanum on the antioxidant defense system in an induced-necrosis model. Antioxidants 2018, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Xiao, W.; Wu, D.; Bai, M.; Zhao, Q.; Li, Y. Esketamine alleviated cardiomyocyte ferroptosis induced by oxygen–glucose deprivation/reoxygenation (OGD/R) via cyclic GMP-AMP synthase interactor. Cytotechnology 2025, 77, 57. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, C.; Song, J.; Ji, X.; Wang, W. Cytidine-gold nanoclusters as peroxidase mimetic for colorimetric detection of glutathione (GSH), glutathione disulfide (GSSG) and glutathione reductase (GR). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119316. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Zheng, Y.; Zhao, J.; Yu, H.; Zhu, J.; Li, D. Neuroprotective effects and mechanisms of procyanidins in vitro and in vivo. Molecules 2021, 26, 2963. [Google Scholar] [CrossRef]
- Mason, R.P.; Sherratt, S.C.R.; Dawoud, H.; Malinski, T.; Bhatt, D. Eicosapentaenoic acid (EPA) increases heme oxygenase-1 expression in endothelial cells under conditions of inflammation unlike docosahexaenoic acid (DHA). J. Am. Coll. Cardiol. 2021, 77, 1829. [Google Scholar] [CrossRef]
- Nishida-Tamehiro, K.; Kimura, A.; Tsubata, T.; Takahashi, S.; Suzuki, H. Antioxidative enzyme NAD(P)H quinone oxidoreductase 1 (NQO1) modulates the differentiation of Th17 cells by regulating ROS levels. PLoS ONE 2022, 17, e0272090. [Google Scholar] [CrossRef] [PubMed]
- Tonolo, F.; Fiorese, F.; Moretto, L.; Folda, A.; Scalcon, V.; Grinzato, A.; Ferro, S.; Arrigoni, G.; Bindoli, A.; Feller, E.; et al. Identification of new peptides from fermented milk showing antioxidant properties: Mechanism of action. Antioxidants 2020, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, B.; Wang, X.; Yu, Q.; Fang, R. Epidermal growth factor, through alleviating oxidative stress, protect IPEC-J2 cells from lipopolysaccharides-induced apoptosis. Int. J. Mol. Sci. 2018, 19, 848. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X.; Zhang, H.; Song, Y.; Li, T.; Liu, X.; Liu, Y.; Guo, L.; Wang, F.; Yang, T.; et al. Epigallocatechin Gallate Upregulates NRF2 to Prevent Diabetic Nephropathy via Disabling KEAP1. Free Radic. Biol. Med. 2017, 108, 840–857. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Priya Reddy, Y.N.; Oelmüller, R. Lipid peroxidation and stress-induced signalling molecules in systemic resistance mediated by azelaic acid/AZELAIC ACID INDUCED1: Signal initiation and propagation. Physiol. Mol. Biol. Plants 2024, 30, 305–316. [Google Scholar] [CrossRef]
- Mentese, A.; Demir, S.; Kucuk, H.; Yulug, E.; Alemdar, N.T.; Demir, E.A.; Aliyazicioglu, Y. Vanillic acid abrogates cisplatin-induced ovotoxicity through activating Nrf2 pathway. Tissue Cell 2023, 84, 102161. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Y.; Jia, Y.; Pang, M.; Cheng, G.; Cai, S. Phenolic profiles, antioxidant activities and cytoprotective effects of different phenolic fractions from oil palm (Elaeis guineensis Jacq.) fruits treated by ultra-high pressure. Food Chem. 2019, 288, 68–77. [Google Scholar] [CrossRef]
- Javanmardi, J. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Lue, B.-M.; Nielsen, N.S.; Jacobsen, C.; Hellgren, L.; Guo, Z.; Xu, X. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human Low density lipoprotein assays. Food Chem. 2010, 123, 221–230. [Google Scholar] [CrossRef]
- Luo, A.; He, X.; Zhou, S.; Fan, Y.; He, T.; Chun, Z. In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 2009, 45, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Kaced, A.; Belkacemi, L.; Chemat, S.; Taibi, N.; Bensouici, C.; Boussebaa, W.; Menaa, S.; Abou Mustapha, M. Assessment of L-DOPA, bioactive molecules and antioxidant activities of the local Algerian legume Tadelaght (Vigna mungo L. Hepper) extract. Food Biosci. 2024, 61, 104902. [Google Scholar] [CrossRef]

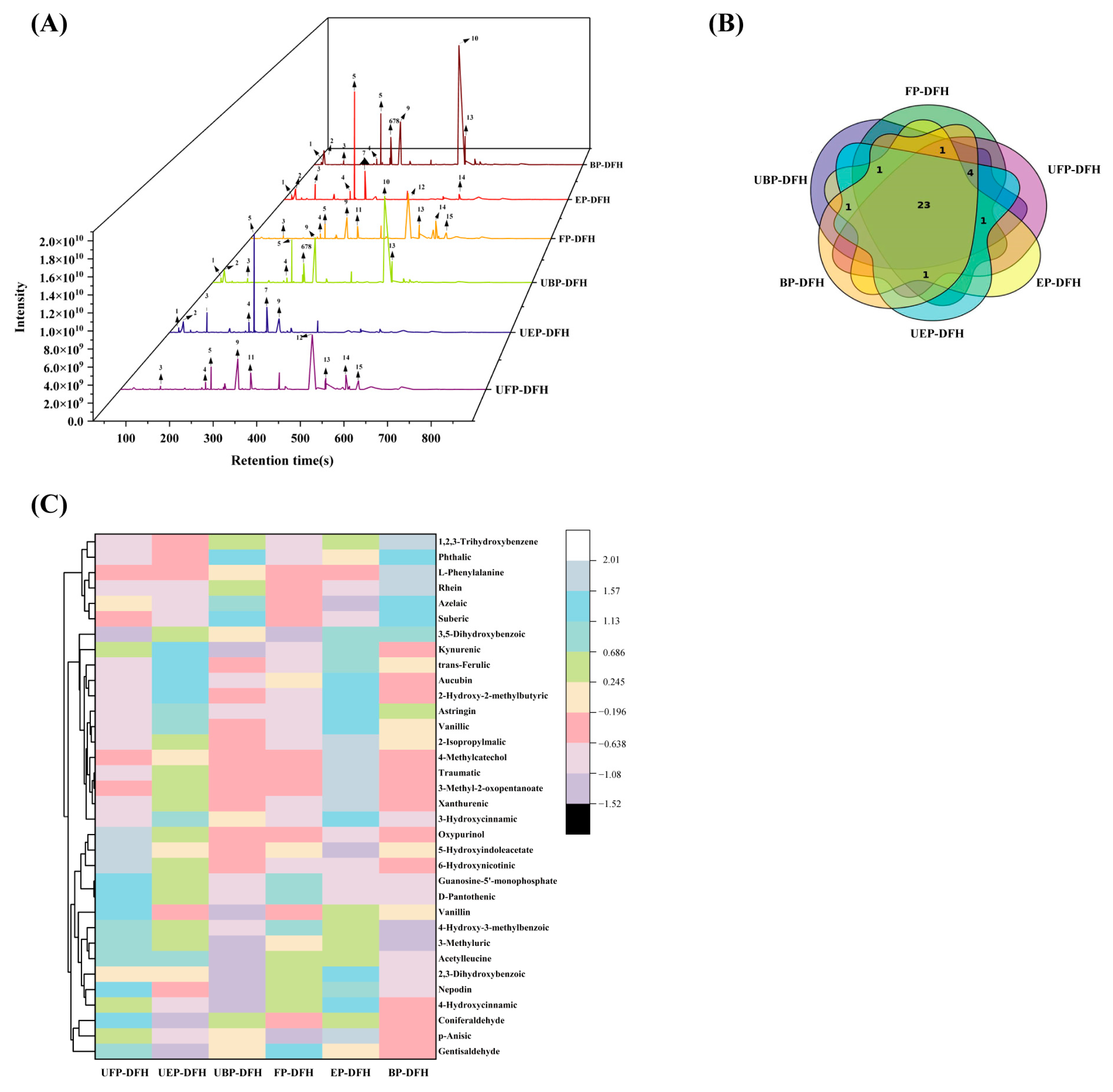

| No. | Compound | RT (min) | [M-H]- (m/z) | Molecular Formula | FP | UFP | EP | UEP | BP | UBP |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Coniferaldehyde | 0.50 | 177.0557 | C10H10O3 | 0.4995 | 0.5641 | 0.7070 | 0.5064 | 0.5503 | 0.6177 |
| 2 | Vanillin | 0.56 | 151.0404 | C8H8O3 | 1.5430 | 1.9668 | 2.3606 | 1.8610 | 1.9462 | 1.5387 |
| 3 | Gentisaldehyde | 0.66 | 137.024 | C7H6O3 | 0.4955 | 0.4559 | 0.5286 | 0.3846 | 0.4438 | 0.4551 |
| 4 | 1,2,3-Trihydroxybenzene | 0.83 | 125.0246 | C6H6O3 | 0.0883 | 0.1032 | 0.5325 | 0.2491 | 0.8958 | 0.5310 |
| 5 | Rhein | 1.03 | 283.0271 | C15H8O6 | 0.0189 | 0.0044 | 0.0030 | —— | 0.1026 | 0.0549 |
| 6 | Phthalic acid | 1.48 | 165.0195 | C8H6O4 | —— | —— | 0.5784 | 0.2747 | 0.9825 | 1.0042 |
| 7 | 4-Methylcatechol | 1.77 | 123.0451 | C7H8O2 | 0.1824 | 0.1571 | —— | 0.2920 | 0.2259 | 0.1935 |
| 8 | p-Anisic acid | 1.83 | 151.0404 | C8H8O3 | 0.5206 | 0.6708 | —— | 0.6720 | 0.6950 | 0.7531 |
| 9 | 2-Hydroxy-2-methylbutyric acid | 2.00 | 117.0557 | C5H10O3 | 26.2968 | 23.6317 | 172.1057 | 170.1849 | 43.5481 | 38.4994 |
| 10 | 2,3-Dihydroxybenzoic acid | 2.25 | 153.0194 | C7H6O4 | 1.7250 | 1.5524 | 2.8067 | 1.9010 | 1.4105 | 1.0499 |
| 11 | 6-Hydroxynicotinic acid | 2.80 | 138.0196 | C6H5NO3 | 0.0587 | 0.1927 | 0.0902 | 0.1664 | 0.1137 | 0.1133 |
| 12 | 2-Isopropylmalic acid | 2.97 | 175.0614 | C7H12O5 | 8.1956 | 10.0603 | 62.0692 | 35.6353 | 24.2040 | 21.0959 |
| 13 | 4-Hydroxy-3-methylbenzoic acid | 3.00 | 151.0402 | C8H8O3 | 2.5323 | 2.6305 | 2.8736 | 2.5382 | 0.7799 | 1.1939 |
| 14 | 3,5-Dihydroxybenzoic acid | 3.14 | 153.0192 | C7H6O4 | 3.7772 | 3.6628 | 11.0047 | 9.7879 | 10.5693 | 7.0524 |
| 15 | L-Phenylalanine | 3.26 | 164.0717 | C9H11NO2 | 0.0234 | 0.0415 | 0.0901 | 0.0738 | —— | 0.4390 |
| 16 | D-Pantothenic acid | 3.32 | 218.1035 | C9H17NO5 | 2.3129 | 2.8695 | 0.0286 | 2.1183 | 0.0402 | 0.0285 |
| 17 | Xanthurenic acid | 3.52 | 204.0301 | C10H7NO4 | 0.0095 | 0.0088 | —— | 1.0110 | 0.3960 | 0.1246 |
| 18 | 4-Hydroxycinnamic acid | 3.63 | 163.0404 | C9H8O3 | 0.9138 | 0.8933 | 1.3231 | 0.8071 | 0.8909 | 0.6438 |
| 19 | Aucubin | 3.70 | 405.1406 | C15H22O9 | 0.1438 | 0.0766 | 0.3219 | 0.2886 | 0.1333 | 0.0883 |
| 20 | Trans-Ferulic acid | 3.72 | 193.0506 | C10H10O4 | 0.4977 | 0.5037 | 2.2448 | 2.3360 | 1.3769 | 1.0619 |
| 21 | Kynurenic acid | 3.78 | 188.0357 | C10H7NO3 | 0.2485 | 1.9866 | 3.5634 | 3.5251 | 0.9928 | 0.0171 |
| 22 | Acetylleucine | 3.87 | 172.0979 | C8H15NO3 | 7.2885 | 8.1930 | 8.7119 | 10.6443 | 2.2280 | 0.2493 |
| 23 | Vanillic acid | 4.01 | 167.0348 | C8H8O4 | 120.8814 | 147.6204 | 1197.8593 | 834.2383 | 533.5137 | 370.9397 |
| 24 | Astringin | 4.05 | 405.1187 | C20H22O9 | 0.3206 | 0.3241 | 1.4720 | 1.0375 | 0.8699 | 0.4659 |
| 25 | Suberic acid | 4.50 | 173.0822 | C8H14O4 | 15.2357 | 16.6105 | 6.7092 | 13.5709 | 71.7242 | 69.5280 |
| 26 | Azelaic acid | 5.07 | 187.0977 | C9H16O4 | 175.1019 | 198.6162 | 30.8073 | 117.7309 | 448.1798 | 390.5198 |
| 27 | 3-Hydroxycinnamic acid | 5.11 | 163.0404 | C9H8O3 | 3.5035 | 1.4209 | 32.2576 | 21.8638 | 5.3865 | 13.8460 |
| 28 | Oxypurinol | 1.69 | 151.0264 | C5H4N4O2 | 0.6179 | —— | 0.3730 | 1.2113 | 0.4851 | 0.5641 |
| 29 | Traumatic acid | 4.47 | 227.1289 | C12H20O4 | 0.0546 | 0.0139 | —— | 1.8766 | 0.2041 | 0.1923 |
| 30 | Nepodin | 1.27 | 215.0736 | C13H12O3 | 0.2625 | 0.3039 | 0.3475 | 0.2099 | 0.1406 | 0.0642 |
| 31 | 3-Methyluric acid | 4.49 | 227.0467 | C6H6N4O3 | 0.3854 | 0.5446 | 0.6132 | 0.5731 | 0.1803 | 0.1786 |
| 32 | Guanosine-5′-monophosphate | 6.84 | 362.0525 | C10H14N5O8P | 1.6239 | 1.8488 | 0.0309 | 1.4545 | 0.0396 | —— |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Hu, J.; Cui, H.; Zheng, F.; Liu, Y.; Wang, Z.; Cheng, G. Effect of Ultra-High Pressure on the Extraction of the Free, Esterified, and Bound Phenolics from Dendrobium fimbriatum Hook: Chemical Constituents and Antioxidant Ability. Molecules 2025, 30, 1836. https://doi.org/10.3390/molecules30081836
Su Q, Hu J, Cui H, Zheng F, Liu Y, Wang Z, Cheng G. Effect of Ultra-High Pressure on the Extraction of the Free, Esterified, and Bound Phenolics from Dendrobium fimbriatum Hook: Chemical Constituents and Antioxidant Ability. Molecules. 2025; 30(8):1836. https://doi.org/10.3390/molecules30081836
Chicago/Turabian StyleSu, Qinge, Junbo Hu, Huimin Cui, Fangyuan Zheng, Yaping Liu, Zhengxuan Wang, and Guiguang Cheng. 2025. "Effect of Ultra-High Pressure on the Extraction of the Free, Esterified, and Bound Phenolics from Dendrobium fimbriatum Hook: Chemical Constituents and Antioxidant Ability" Molecules 30, no. 8: 1836. https://doi.org/10.3390/molecules30081836
APA StyleSu, Q., Hu, J., Cui, H., Zheng, F., Liu, Y., Wang, Z., & Cheng, G. (2025). Effect of Ultra-High Pressure on the Extraction of the Free, Esterified, and Bound Phenolics from Dendrobium fimbriatum Hook: Chemical Constituents and Antioxidant Ability. Molecules, 30(8), 1836. https://doi.org/10.3390/molecules30081836








