Research Progress of Photothermal Superhydrophobic Surfaces for Anti-Icing/Deicing
Abstract
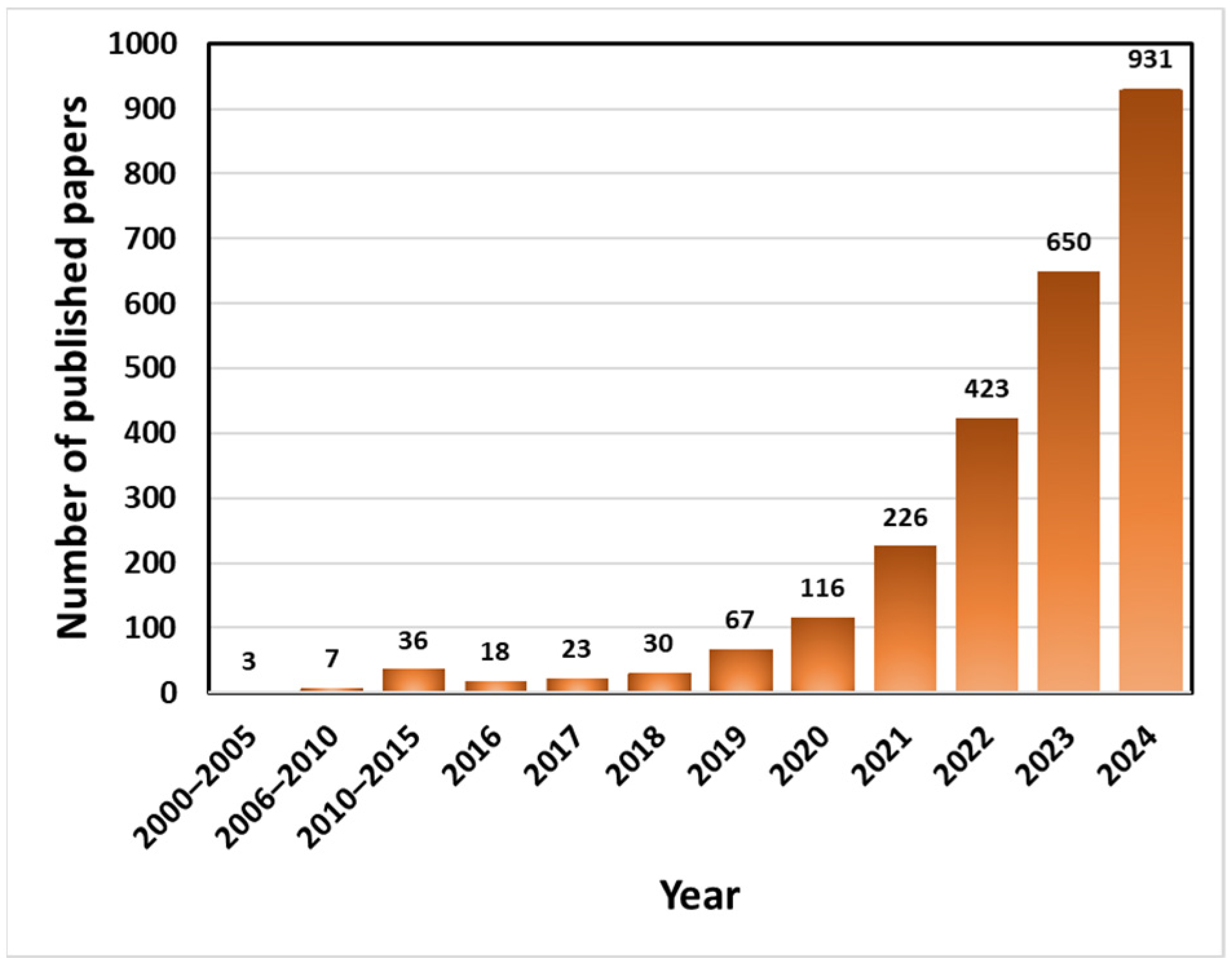
1. Introduction
2. Mechanism of Ice Formation and Photothermal Superhydrophobic Anti-Icing/Deicing
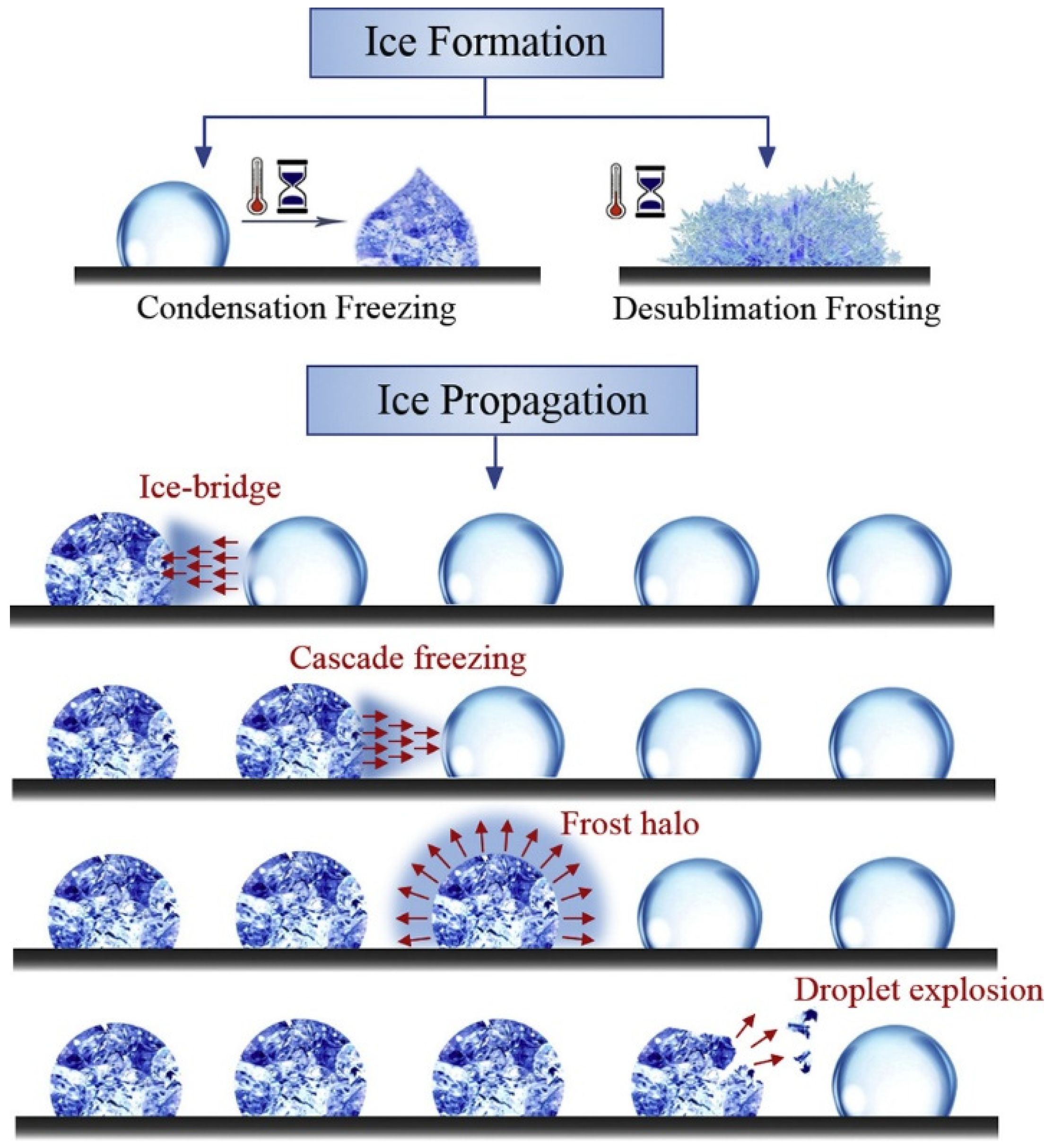
2.1. Ice Formation: Nucleation and Structural Diversity
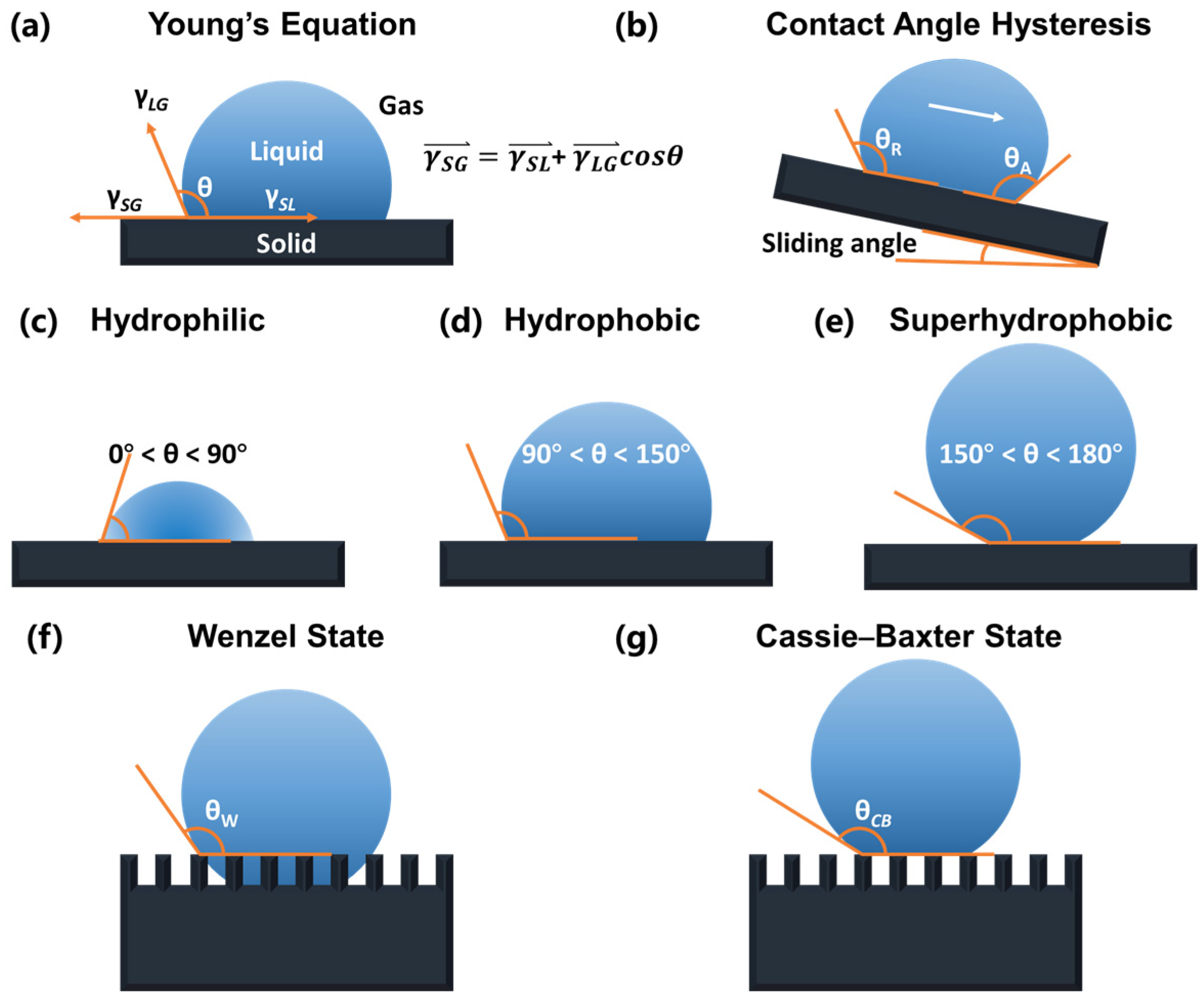
2.2. Anti-Icing/Deicing Mechanism of Superhydrophobic Materials
2.3. Anti-Icing/Deicing Mechanism of Photothermal Conversion Materials

2.4. Anti-Icing/Deicing Mechanism of Photothermal Superhydrophobic Materials
3. Advances in Photothermal Superhydrophobic Surfaces for Anti-Icing/Deicing
3.1. Metal-Based and Metallic-Compound-Based Photothermal Superhydrophobic Materials
3.1.1. Metal-Based
Copper
Silver
Gold
Other Metals
3.1.2. Metallic-Compound-Based
3.2. Carbon-Based Photothermal Superhydrophobic Materials
3.2.1. Carbon Nanotube
3.2.2. Graphene
3.2.3. Other Materials
3.3. Polymer-Based Photothermal Superhydrophobic Materials
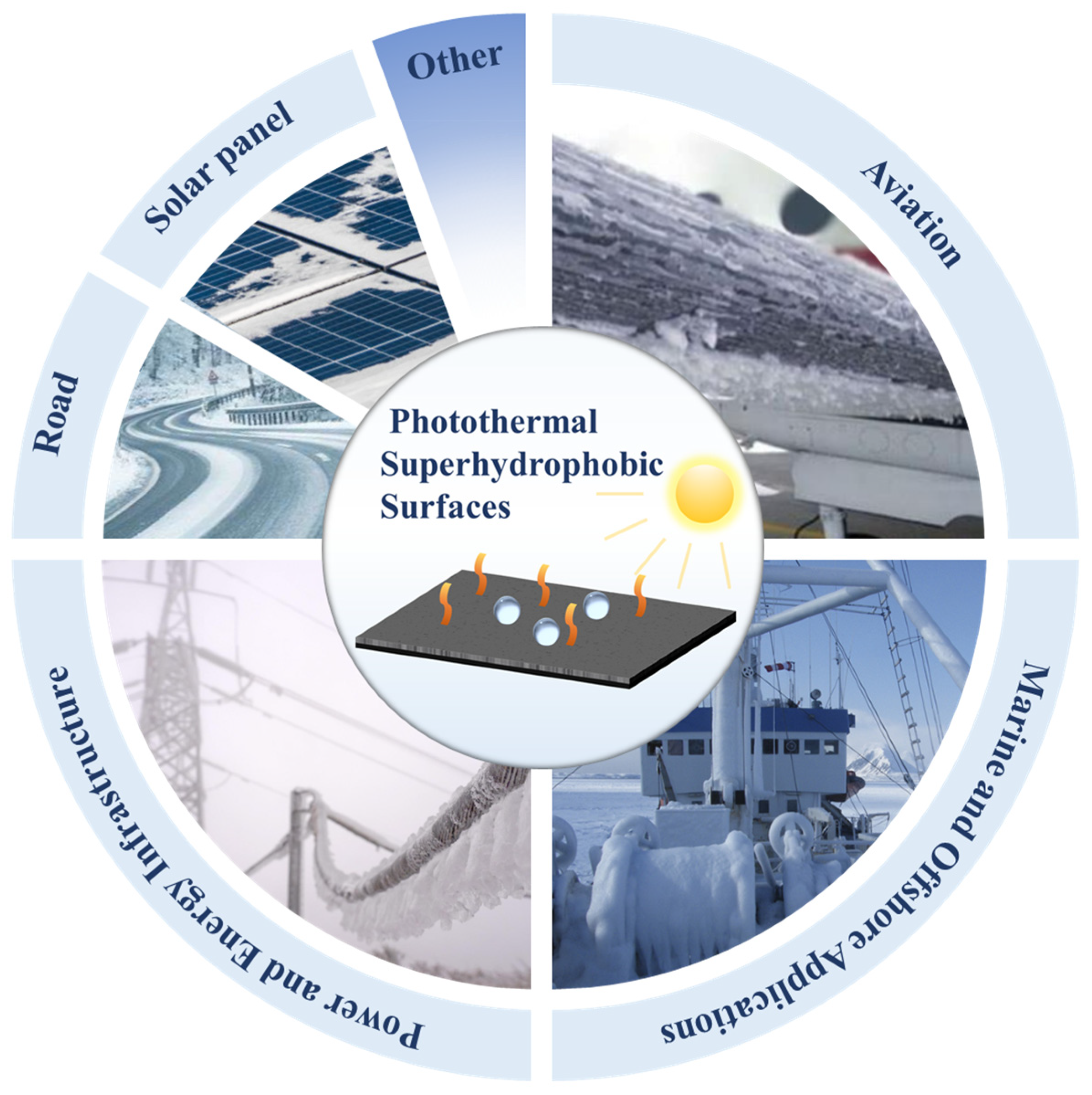
4. Application Scenarios of Photothermal Superhydrophobic Surfaces for Anti-Icing/Deicing
4.1. Aviation
4.2. Power and Energy Infrastructure
4.3. Marine and Offshore Applications
4.4. Other Application Scenarios
5. Challenges of Photothermal Superhydrophobic Surfaces for Anti-Icing/Deicing Applications
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.-H.; Wu, Z.; Su, Y.; Xu, Z. Aircraft flight characteristics in icing conditions. Prog. Aerosp. Sci. 2015, 74, 62–80. [Google Scholar] [CrossRef]
- Mortimer, A.R. A review of the icing problem for aerogenerators. Wind Eng. 1980, 4, 183–191. [Google Scholar]
- Rekuviene, R.; Saeidiharzand, S.; Mažeika, L.; Samaitis, V.; Jankauskas, A.; Sadaghiani, A.K.; Gharib, G.; Muganlı, Z.; Koşar, A. A review on passive and active anti-icing and de-icing technologies. Appl. Therm. Eng. 2024, 236, 123474. [Google Scholar] [CrossRef]
- Hudecz, A. Icing Problems of Wind Turbine Blades in Cold Climates. Ph. D. Thesis, Technical University of Denmark, Kongens Lyngby, Denmark, 2014. [Google Scholar]
- Fortin, G.; Perron, J. Wind turbine icing and de-icing. In Proceedings of the 47th AIAA Aerospace Sciences Meeting Including the New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 5–8 January 2009. [Google Scholar]
- Barjouei, A.S.; Gudmestad, O.T.; Barabady, J. Road transportation challenges in the Arctic. WIT Trans. Eng. Sci. 2020, 129, 229–240. [Google Scholar]
- Lotfi, A.; Virk, M.S. Railway operations in icing conditions: A review of issues and mitigation methods. Public Transp. 2023, 15, 747–765. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Xu, Y.-M.; Huang, Q. Progress on ultrasonic guided waves de-icing techniques in improving aviation energy efficiency. Renew. Sustain. Energy Rev. 2017, 79, 638–645. [Google Scholar] [CrossRef]
- Muthumani, A.; Fay, L.; Akin, M.; Wang, S.; Gong, J.; Shi, X. Correlating lab and field tests for evaluation of deicing and anti-icing chemicals: A review of potential approaches. Cold Reg. Sci. Technol. 2014, 97, 21–32. [Google Scholar] [CrossRef]
- Shiverskii, A.V.; Owais, M.; Mahato, B.; Abaimov, S.G. Electrical heaters for anti/de-icing of polymer structures. Polymers 2023, 15, 1573. [Google Scholar] [CrossRef]
- Gao, S.R.; Jiao, L.L.; Yi, M.C. Mechanism and research progress of anti/de-icing using hydrophobic/superhydrophobic surfaces. Acta Aerodyn. Sin. 2021, 39, 151–160. [Google Scholar]
- Huang, W.; Huang, J.; Guo, Z.; Liu, W. Icephobic/anti-icing properties of superhydrophobic surfaces. Adv. Colloid Interface Sci. 2022, 304, 102658. [Google Scholar] [CrossRef]
- Cong, Q.; Qin, X.; Chen, T.; Jin, J.; Liu, C.; Wang, M. Research progress of superhydrophobic materials in the field of anti-/de-icing and their preparation: A review. Materials 2023, 16, 5151. [Google Scholar] [CrossRef]
- Sethi, S.K.; Manik, G. Recent progress in super hydrophobic/hydrophilic self-cleaning surfaces for various industrial applications: A review. Polym. Plast. Technol. Eng. 2018, 57, 1932–1952. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Superhydrophobic surfaces and emerging applications: Non-adhesion, energy, green engineering. Curr. Opin. Colloid Interface Sci. 2009, 14, 270–280. [Google Scholar] [CrossRef]
- Zhong, X.; Xie, S.-Z.; Guo, Z.-G. The challenge of superhydrophobicity: Environmentally facilitated Cassie–Wenzel transitions and structural design. Adv. Sci. 2024, 11, 2305961. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, Z.; Jiang, G.; Luo, X.; Chen, C.; Hu, X.; Zhang, H.; Zhong, M. Spontaneous dewetting transitions of droplets during icing & melting cycle. Nat. Commun. 2022, 13, 378. [Google Scholar]
- Xie, Z.-T.; Wang, H.; Geng, Y.; Li, M.; Deng, Q.; Tian, Y.; Chen, R.; Zhu, X.; Liao, Q. Carbon—Based photothermal superhydrophobic materials with hierarchical structure enhances the anti-icing and photothermal deicing properties. ACS Appl. Mater. Interfaces 2021, 13, 48308–48321. [Google Scholar] [CrossRef] [PubMed]
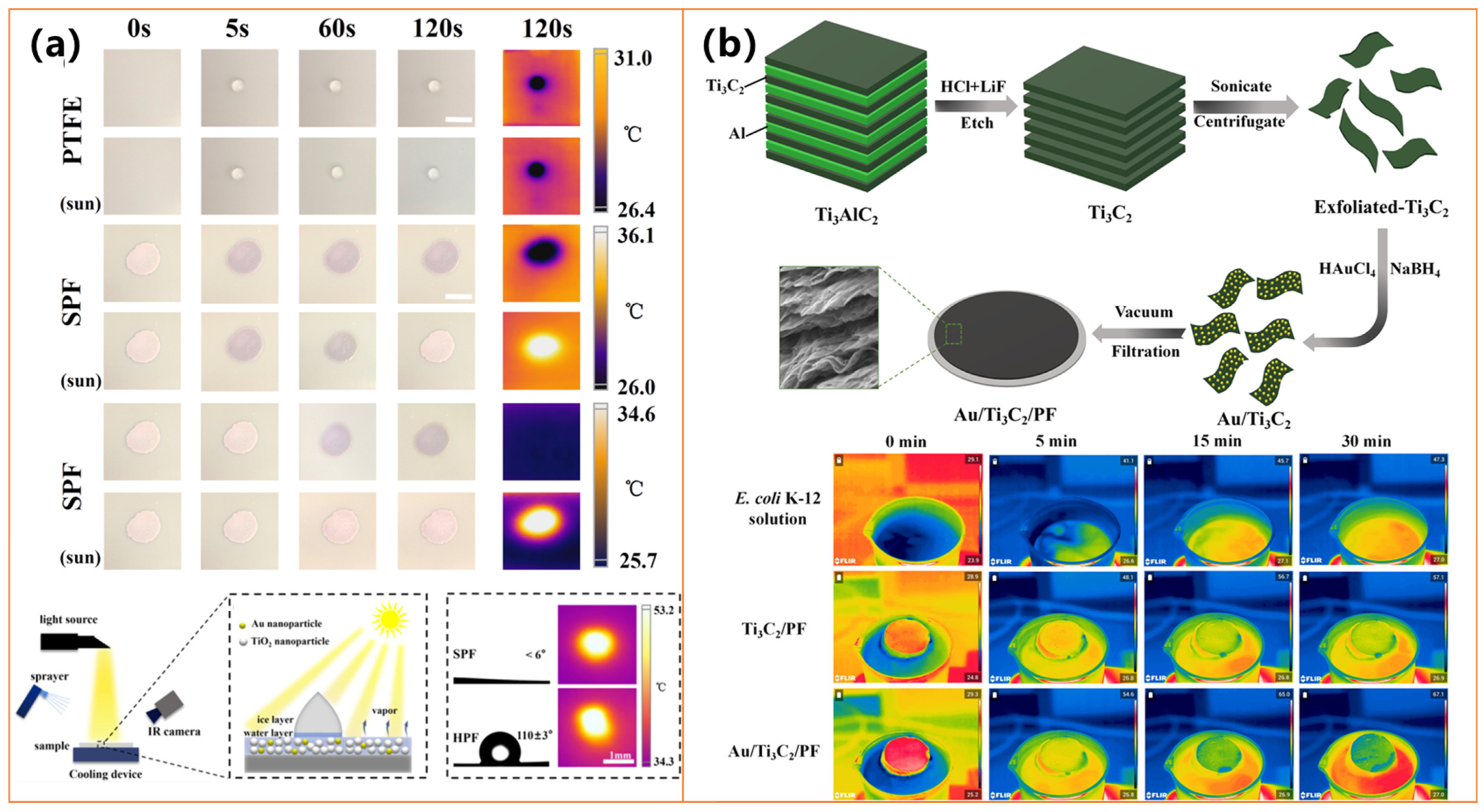
- Qu, W.; Zhao, H.; Zhang, Q.; Xia, D.; Tang, Z.; Chen, Q.; He, C.; Shu, D. Multifunctional Au/Ti3C2 photothermal membrane with antibacterial ability for stable and efficient solar water purification under the full spectrum. ACS Sustain. Chem. Eng. 2021, 9, 11372–11387. [Google Scholar] [CrossRef]
- Xiao, X.; Wei, X.; Wei, J.; Wang, J. Multifunctional Fe3O4-based photothermal superhydrophobic composite coating for efficient anti-icing/deicing. Sol. Energy Mater. Sol. Cells 2023, 256, 112313. [Google Scholar] [CrossRef]
- Wang, B.-Q.; Jing, Z.; Zhao, M.; Yu, P.; Ashalley, E.; Li, P.; Ma, C.; Tong, X.; Caputo, R.; Govorov, A.O.; et al. Ultraflexible photothermal superhydrophobic coating with multifunctional applications based on plasmonic TiN nanoparticles. Adv. Opt. Mater. 2022, 10, 2200168. [Google Scholar] [CrossRef]
- Xiang, T.-F.; Chen, X.; Lv, Z.; Tong, W.; Cao, J.; Shen, Y.; Liao, B.; Xie, Y.; Zhang, S. Stable photothermal solid slippery surface with enhanced anti-icing and de-icing properties. Appl. Surf. Sci. 2023, 624, 157178. [Google Scholar] [CrossRef]
- Cheng, S.-J.; Latthe, S.S.; Nakata, K.; Xing, R.; Liu, S.; Fujishima, A. Recent advancements in design, development and demands of photothermal superhydrophobic materials. Mater. Today Chem. 2024, 35, 101868. [Google Scholar] [CrossRef]
- Chen, H.-L.; Wu, S.-L.; Wang, H.-L.; Wu, Q.-Y.; Yang, H.-C. Photothermal devices for sustainable uses beyond desalination. Adv. Energy Sustain. Res. 2021, 2, 2000056. [Google Scholar] [CrossRef]
- Li, M.; Ma, R.; Yang, C.; Wang, L.; Lv, S.; Zhao, X.; Pan, M.; Zhu, J.; Xu, H. Photothermal and Hydrophobic Surfaces with Nano-Micro Structure: Fabrication and Their Anti-Icing Properties. Nanomaterials 2025, 15, 378. [Google Scholar] [CrossRef] [PubMed]
- DeMott, P.J.; Prenni, A.J.; Liu, X.; Kreidenweis, S.M.; Petters, M.D.; Twohy, C.H.; Richardson, M.S.; Eidhammer, T.; Rogers, D.C. Predicting global atmospheric ice nuclei distributions and their impacts on climate. Proc. Natl. Acad. Sci. USA 2010, 107, 11217–11222. [Google Scholar] [CrossRef] [PubMed]
- Storelvmo, T.; Tan, I. The Wegener-Bergeron-Findeisen process–Its discovery and vital importance for weather and climate. Meteorol. Z. 2015, 24, 455–461. [Google Scholar] [CrossRef]
- Korolev, A. Limitations of the Wegener–Bergeron–Findeisen mechanism in the evolution of mixed-phase clouds. J. Atmos. Sci. 2007, 64, 3372–3375. [Google Scholar] [CrossRef]
- Ono, A. The shape and riming properties of ice crystals in natural clouds. J. Atmos. Sci. 1969, 26, 138–147. [Google Scholar] [CrossRef]
- Schlamp, R.J.; Pruppacher, H.R.; Hamielec, A.E. A numerical investigation of the efficiency with which simple columnar ice crystals collide with supercooled water drops. J. Atmos. Sci. 1975, 32, 2330–2337. [Google Scholar] [CrossRef]
- Yancheshme, A.A.; Momen, G.; Jafari Aminabadi, R. Mechanisms of ice formation and propagation on superhydrophobic surfaces: A review. Adv. Colloid Interface Sci. 2020, 279, 102155. [Google Scholar] [CrossRef]
- Li, S.-B. Investigation of Aircraft Icing Process Based on Numerical Modeling and Machine Learning Methods. In Proceedings of the Argonne Leadership Computing Facility, Virtual, 29 July 2021; Available online: https://www.alcf.anl.gov/events/investigation-aircraft-icing-process-based-numerical-modeling-and-machine-learning-methods (accessed on 29 July 2021).
- Hansman, R.J., Jr.; Turnock, S.R. Investigation of surface water behavior during glaze ice accretion. J. Aircr. 1989, 26, 140–147. [Google Scholar] [CrossRef]
- Work, A.H., Jr. The Measurement of the Adhesion of Glaze Ice. Available online: https://ir.library.louisville.edu/etd/2987/ (accessed on 22 March 2025).
- The Washington Times. Heavy Snow Causes Jet to Pull Stationary Wheelie at Airport. 17 January 2019. Available online: https://www.washingtontimes.com/news/2019/jan/17/heavy-snow-causes-jet-to-pull-stationary-wheelie-a/ (accessed on 17 January 2019).
- Kako, T.; Nakajima, A.; Irie, H.; Kato, Z.; Uematsu, K.; Watanabe, T.; Hashimoto, K. Adhesion and sliding of wet snow on a super-hydrophobic surface with hydrophilic channels. J. Mater. Sci. 2004, 39, 547–555. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farhadi, S.; Nose, K.; Du, X.W. Superhydrophobic surfaces: Are they really ice-repellent? Langmuir 2011, 27, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Transportation Safety Board of Canada. Aviation Investigation Report A14W0181: Severe Icing Encounter and Forced Landing. 20 November 2014. Available online: https://www.tsb.gc.ca/eng/rapports-reports/aviation/2014/a14w0181/a14w0181.html (accessed on 22 March 2025).
- Ferrari, M.; Cirisano, F. Superhydrophobic coating solutions for deicing control in aircraft. Appl. Sci. 2023, 13, 11684. [Google Scholar] [CrossRef]
- Bragg, M.B. Rime Ice Accretion and Its Effect on Airfoil Performance. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 1981. [Google Scholar]
- NASA Glenn Research Center. Ground Icing: Cues—Anticipating Contamination; NASA: Washington, DC, USA, 2016. Available online: https://aircrafticing.grc.nasa.gov/2_2_2_1.html (accessed on 22 March 2025).
- Ahlmann, H.W. On the formation of hoarfrost and its relation to glacial growth. J. Geol. 1929, 37, 275–280. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Chen, Y.; Yan, Z.; Liu, X.; Su, Y.; Xiao, L.; Li, R.; Li, T.; Wu, J.; et al. Robust anti-icing double-layer superamphiphobic composite coatings for heat exchangers. Prog. Org. Coat. 2024, 197, 108814. [Google Scholar] [CrossRef]
- Cole, J.; Sand, W. Statistical study of aircraft icing accidents. In Proceedings of the 29th Aerospace Sciences Meeting, Reno, NV, USA, 7–10 January 1991. [Google Scholar]
- Li, T.-Y.; Li, J.-J. Analysis of icing accident in South China power grids in 2008 and it’s countermeasures. In Proceedings of the CIRED 2009—20th International Conference and Exhibition on Electricity Distribution—Part 1, Prague, Czech Republic, 8–11 June 2009. [Google Scholar]
- Solangi, A.R. Icing effects on power lines and anti-icing and de-icing methods. Master’s Thesis, UiT The Arctic University of Norway, Tromsø, Norway, 2018. [Google Scholar]
- Jiang, X.-L.; Jiang, X.; Zhao, J.; Luo, B.; Zhang, J.; Huang, C. Survey and analysis of ice accidents of early 2008 in southern China. In Proceedings of the IWAIS XIII, Andermatt, Switzerland, 1–5 June 2009. [Google Scholar]
- Sun, H.-Y.; Lin, G.; Jin, H.; Bu, X.; Cai, C.; Jia, Q.; Ma, K.; Wen, D. Experimental investigation of surface wettability induced anti-icing characteristics in an ice wind tunnel. Renew. Energy 2021, 179, 1179–1190. [Google Scholar] [CrossRef]
- Gao, Y.-L.; Qu, L.; He, B.; Dai, K.; Fang, Z.; Zhu, R. Study on effectiveness of anti-icing and deicing performance of super-hydrophobic asphalt concrete. Constr. Build. Mater. 2018, 191, 270–280. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Fujishima, A. Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf. Molecules 2014, 19, 4256–4283. [Google Scholar] [CrossRef]
- Samaha, M.A.; Vahedi Tafreshi, H.; Gad-el-Hak, M. Superhydrophobic surfaces: From the lotus leaf to the submarine. Comptes Rendus Mécanique 2012, 340, 18–34. [Google Scholar] [CrossRef]
- Bouillant, A.; Mouterde, T.; Bourrianne, P.; Lagarde, A.; Clanet, C.; Quéré, D. Leidenfrost wheels. Nat. Phys. 2018, 14, 1188–1192. [Google Scholar] [CrossRef]
- Zhong, L.-S.; Guo, Z.-G. Effect of surface topography and wettability on the Leidenfrost effect. Nanoscale 2017, 9, 6219–6236. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Desai, S. The nanoscale Leidenfrost effect. Nanoscale 2019, 11, 12139–12151. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.-J.; Liu, J.; Koynov, K.; Straub, B.; Hinduja, C.; Roismann, I.; Berger, R.; Li, X.; Vollmer, D.; Steffen, W.; et al. Contact angle hysteresis. Curr. Opin. Colloid Interface Sci. 2022, 59, 101574. [Google Scholar] [CrossRef]
- Seveno, D.; Blake, T.D.; De Coninck, J. Young’s equation at the nanoscale. Phys. Rev. Lett. 2013, 111, 096101. [Google Scholar] [CrossRef] [PubMed]
- Rogers, F.J.; Young, D.A. New, thermodynamically consistent, integral equation for simple fluids. Phys. Rev. A 1984, 30, 999. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Gong, X. Special oleophobic and hydrophilic surfaces: Approaches, mechanisms, and applications. J. Mater. Chem. A 2017, 5, 3759–3773. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Mognetti, B.M.; Kusumaatmaja, H.; Yeomans, J.M. Drop dynamics on hydrophobic and superhydrophobic surfaces. Faraday Discuss. 2010, 146, 153–165. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Kumar, S.V.; Bobji, M.; Chakradhar, R.; Basu, B.J. Effect of wettability and surface roughness on ice-adhesion strength of hydrophilic, hydrophobic and superhydrophobic surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Eral, H.B.; ’t Mannetje, D.J.C.M.; Oh, J.M. Contact angle hysteresis: A review of fundamentals and applications. Colloid Polym. Sci. 2013, 291, 247–260. [Google Scholar] [CrossRef]
- Joanny, J.F.; De Gennes, P.-G. A model for contact angle hysteresis. J. Chem. Phys. 1984, 81, 552–562. [Google Scholar] [CrossRef]
- Miwa, M.; Nakajima, A.; Fujishima, A.; Hashimoto, K.; Watanabe, T. Effects of the surface roughness on sliding angles of water droplets on superhydrophobic surfaces. Langmuir 2000, 16, 5754–5760. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Cansoy, C.E. Range of applicability of the Wenzel and Cassie—Baxter equations for superhydrophobic surfaces. Langmuir 2009, 25, 14135–14145. [Google Scholar] [CrossRef]
- Azadi Tabar, M.; Barzegar, F.; Ghazanfari, M.H.; Mohammadi, M. On the applicability range of Cassie—Baxter and Wenzel equation: A numerical study. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 399. [Google Scholar] [CrossRef]
- Wolansky, G.; Marmur, A. Apparent contact angles on rough surfaces: The Wenzel equation revisited. Colloids Surf. A Physicochem. Eng. Asp. 1999, 156, 381–388. [Google Scholar] [CrossRef]
- Nosonovsky, M. On the range of applicability of the Wenzel and Cassie equations. Langmuir 2007, 23, 9919–9920. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Nypelö, T.; Österberg, M.; Laine, J.; Alava, M. Modifying the wettability of surfaces by nanoparticles: Experiments and modeling using the Wenzel law. Langmuir 2010, 26, 14563–14566. [Google Scholar] [CrossRef]
- Cansoy, C.E.; Erbil, H.Y.; Akar, O.; Akin, T. Effect of pattern size and geometry on the use of Cassie–Baxter equation for superhydrophobic surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2011, 386, 116–124. [Google Scholar] [CrossRef]
- Banerjee, S. Simple derivation of Young, Wenzel and Cassie—Baxter equations and its interpretations. arXiv 2008, arXiv:0808.1460. [Google Scholar]
- Polster, D.; Graaf, H.; Baumgärtel, T.; von Borczyskowski, C.; Benedikt, U.; Auer, A.A. Beyond Cassie’s Law: A Theoretical and Experimental Study of Mixed Alkyl Monolayers. Langmuir 2010, 26, 8301–8308. [Google Scholar] [CrossRef]
- McHale, G.; Ledesma-Aguilar, R.; Neto, C. Cassie’s Law Reformulated: Composite Surfaces from Superspreading to Superhydrophobic. Langmuir 2023, 39, 11028–11035. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Sarshar, M.A.; Song, D.; Swarctz, C.; Lee, J.; Choi, C.-H. Anti-icing or deicing: Icephobicities of superhydrophobic surfaces with hierarchical structures. Langmuir 2018, 34, 13821–13827. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-B.; Chen, H.; Wang, G.; Liu, A. Recent progress in preparation and anti-icing applications of superhydrophobic coatings. Coatings 2018, 8, 208. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, K.; Tao, C.; Zhao, Y.; Li, X.; Zhu, K.; Yuan, X. Strategies for anti-icing: Low surface energy or liquid-infused? RSC Adv. 2016, 6, 70251–70260. [Google Scholar] [CrossRef]
- Nanev, C.N.; Hodzhaoglu, F.V.; Dimitrov, I.L. Kinetics of insulin crystal nucleation, energy barrier, and nucleus size. Cryst. Growth Des. 2011, 11, 196–202. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Dong, L.; Shu, X.; Yang, Y.; Feng, P.; Ran, Q. Recent advancements in photothermal anti-icing/deicing materials. Chem. Eng. J. 2023, 469, 143924. [Google Scholar] [CrossRef]
- Liu, J.-J.; He, C.Y.; Liu, B.H.; Wang, Z.Q.; Zhao, S.J.; Lu, Z.W.; Zhang, Y.Z.; Tang, Z.Q.; Gao, X.H.; Aday, X. A robust photo-thermal and electr -thermal superhydrphobic surface for all-weather anti-icing/deicing. Chem. Eng. J. 2024, 489, 151338. [Google Scholar] [CrossRef]
- Cheng, S.-M.; Guo, P.; Wang, X.; Che, P.; Han, X.; Jin, R.; Heng, L.; Jiang, L. Photothermal slippery surface showing rapid self-repairing and exceptional anti-icing/deicing property. Chem. Eng. J. 2022, 431, 133411. [Google Scholar] [CrossRef]
- Xie, Z.-T.; Wang, H.; Li, M.; Tian, Y.; Deng, Q.; Chen, R.; Zhu, X.; Liao, Q. Photothermal trap with multi-scale micro-nano hierarchical structure enhances light absorption and promote photothermal anti-icing/deicing. Chem. Eng. J. 2022, 435, 135025. [Google Scholar] [CrossRef]
- Ding, X.-G.; Liow, C.H.; Zhang, M.; Huang, R.; Li, C.; Shen, H.; Liu, M.; Zou, Y.; Gao, N.; Zhang, Z.; et al. Surface plasmon resonance enhanced light absorption and photothermal therapy in the second near-infrared window. J. Am. Chem. Soc. 2014, 136, 15684–15693. [Google Scholar] [CrossRef]
- Loeb, S.; Li, C.-H.; Kim, J.-H. Solar photothermal disinfection using broadband-light absorbing gold nanoparticles and carbon black. Environ. Sci. Technol. 2018, 52, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Michinobu, T. Conjugated photothermal materials and structure design for solar steam generation. Beilstein J. Nanotechnol. 2023, 14, 454–466. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, J.; Kundrat, D.M.; Bonmarin, M.; Krupczak, J., Jr.; Thomas, S.V.; Lyu, M.; Shi, D. Photonically—Activated molecular excitations for thermal energy conversion in porphyrinic compounds. J. Phys. Chem. C 2019, 124, 1575–1584. [Google Scholar] [CrossRef]
- Ren, Y.-T.; Yan, Y.-Y.; Qi, H. Photothermal conversion and transfer in photothermal therapy: From macroscale to nanoscale. Adv. Colloid Interface Sci. 2022, 308, 102753. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Chen, M.; He, Y.; Zhu, J.; Kim, D.R. Enhancement of photo-thermal conversion using gold nanofluids with different particle sizes. Energy Convers. Manag. 2016, 112, 21–30. [Google Scholar] [CrossRef]
- Xie, Z.-T.; Tian, Y.; Shao, Y.; Wang, H.; Chen, R.; Zhu, X.; Liao, Q. Recent progress in anti-icing and deicing applications of the photothermal conversion materials. Prog. Org. Coat. 2023, 184, 107834. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Chen, H.; Zhu, Y.; Liu, X.; Wang, Z.; Chen, J. A robust superhydrophobic anti-icing/de-icing composite coating with electrothermal and auxiliary photothermal performances. Compos. Sci. Technol. 2022, 227, 109578. [Google Scholar] [CrossRef]
- Wei, X.-P.; Wei, J.; Feng, Y.; Wang, J. Photothermal hydrophobic coating with light-trapping and thermal isolated effects for efficient photothermal anti-icing/de-icing. Prog. Org. Coat. 2023, 179, 107550. [Google Scholar] [CrossRef]
- Zheng, W.-W.; Teng, L.; Lai, Y.; Zhu, T.; Li, S.; Wu, X.; Cai, W.; Chen, Z.; Huang, J. Magnetic responsive and flexible composite superhydrophobic photothermal film for passive anti-icing/active deicing. Chem. Eng. J. 2022, 427, 130922. [Google Scholar] [CrossRef]
- Murakami, D.; Jinnai, H.; Takahara, A. Wetting transition from the Cassie–Baxter state to the Wenzel state on textured polymer surfaces. Langmuir 2014, 30, 2061–2067. [Google Scholar] [CrossRef]
- Giacomello, A.; Meloni, S.; Chinappi, M.; Casciola, C.M. Cassie–Baxter and Wenzel states on a nanostructured surface: Phase diagram, metastabilities, and transition mechanism by atomistic free energy calculations. Langmuir 2012, 28, 10764–10772. [Google Scholar] [CrossRef]
- Li, X.-Y.; Lin, X.; Wu, Y.; Zhang, Z.; Hu, T.; Jiang, L.; Xiao, T.; Tan, X. Superhydrophobic surface with good mechanical robustness and stable Cassie—Baxter state throughout freezing and thawing processes. Surf. Interfaces 2025, 56, 105619. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, L.; Zhang, S.; Huang, H.-X. Superhydrophobic SiC/CNTs coatings with photothermal deicing and passive anti-icing properties. ACS Appl. Mater. Interfaces 2018, 10, 36505–36511. [Google Scholar] [CrossRef]
- Chu, F.-Q.; Hu, Z.; Feng, Y.; Lai, N.C.; Wu, X.; Wang, R. Advanced anti-icing strategies and technologies by macrostructured photothermal storage superhydrophobic surfaces. Adv. Mater. 2024, 36, 2402897. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wei, Y.; Feng, Y.; Chu, F. Anti-icing and deicing characteristics of photothermal superhydrophobic surfaces based on metal nanoparticles and carbon nanotube materials. Energy 2024, 286, 129656. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Liu, P.; Luo, R.; Chen, B.; Li, J.; Yang, F.; Zhou, H.; Zeng, J.; Xing, L.; Guo, J. Design and fabrication of superhydrophobic photothermal coating on copper mesh and its applications on anti-corrosion, anti-icing and oil-water separation. Prog. Org. Coat. 2024, 188, 108243. [Google Scholar] [CrossRef]
- Wu, W.-Z.; Bao, L.; Chen, X.; Gong, X.; Wu, M. Facile Fabrication of Photothermal Superhydrophobic Copper Foam Using the Ultrafast Electroplating Approach. ACS Appl. Mater. Interfaces 2025, 17, 12973–12983. [Google Scholar] [CrossRef]
- He, X.-T.; Liu, X.; Lu, J.; Liu, H.; Wu, Z.; Xu, H.; Tao, W.; Li, Z. Facile fabrication of fluorine-free photo-thermal super-hydrophobic coating with hierarchical structure for efficient anti-icing and de-icing applications. Prog. Org. Coat. 2024, 194, 108543. [Google Scholar] [CrossRef]
- Dong, Y.-X.; Lin, Y.; Du, C.; Zhou, C.; Yang, S. Manipulating hydropathicity/hydrophobicity properties to achieve anti-corrosion copper-based membrane toward high-efficient solar water purification. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128755. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Xu, Q.; Xu, N.; Deng, W.; Chu, H. Preparation of photothermal superhydrophobic iron foam coating and its application in passive anti-icing/active de-icing and anti-corrosion. Int. J. Therm. Sci. 2025, 211, 109745. [Google Scholar] [CrossRef]
- Pakdel, E.; Sharp, J.; Kashi, S.; Bai, W.; Gashti, M.P.; Wang, X. Antibacterial superhydrophobic cotton fabric with photothermal, self-cleaning, and ultraviolet protection functionalities. ACS Appl. Mater. Interfaces 2023, 15, 34031–34043. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, S.; Chen, W.; Yang, X.; Dong, S.; Xing, T.; Zhao, Y.; Chen, G. Robust multifunctional superhydrophobic, photocatalytic and conductive fabrics with electro-/photo-thermal self-healing ability. J. Colloid Interface Sci. 2022, 614, 1–11. [Google Scholar] [CrossRef]
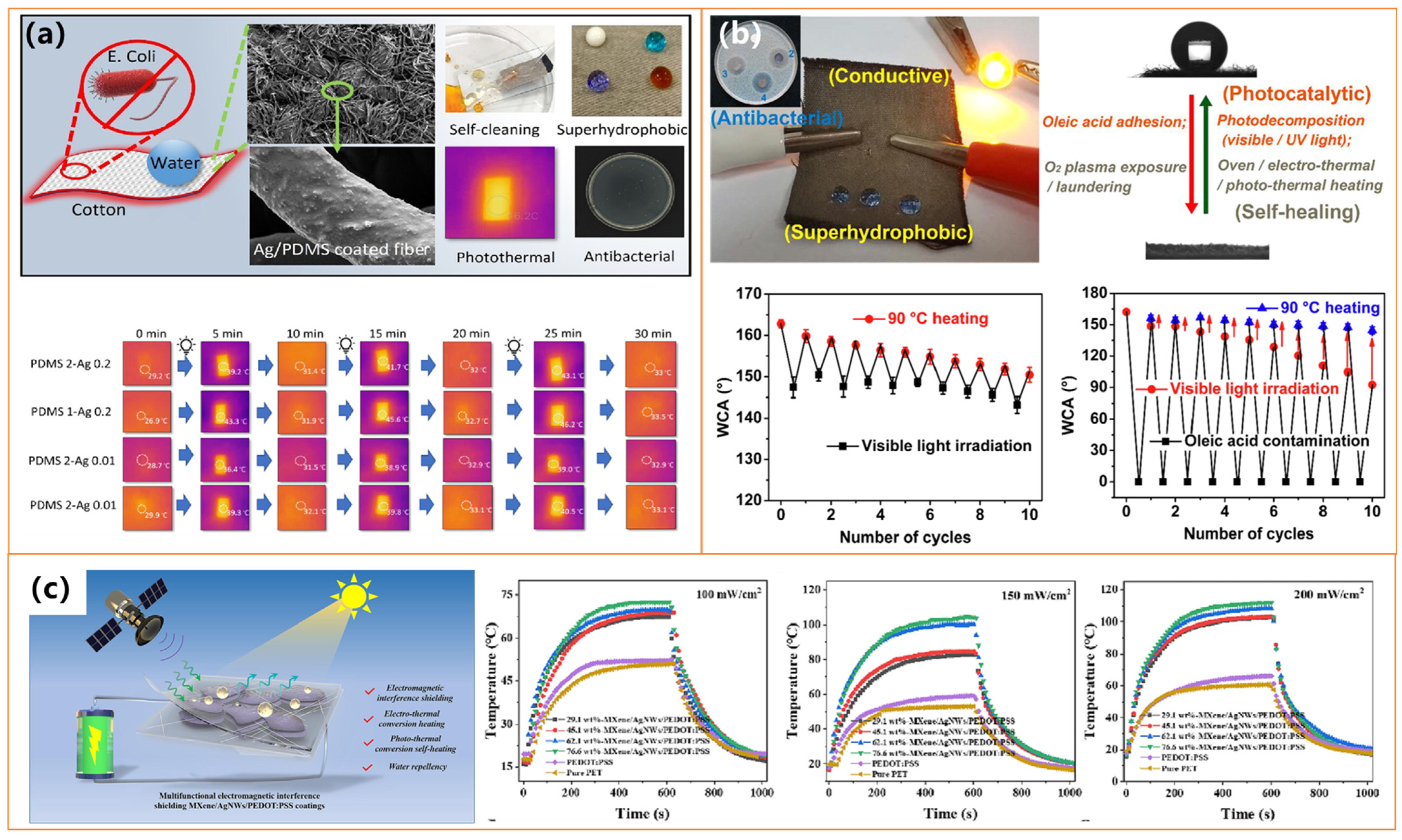
- Chang, G.-J.; Zeng, L.; Xie, L.; Xue, B.; Zheng, Q. Ultrathin multifunctional electromagnetic interference shielding MXene/AgNWs/PEDOT:PSS coatings with superior electro/photo-thermal transition ability and water repellency. Chem. Eng. J. 2023, 470, 144033. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Li, P.; Yu, P.; Leydecker, T.; Bayer, I.S.; Losic, D.; Neogi, A.; Wang, Z. Efficient photothermal deicing employing superhydrophobic plasmonic MXene composites. Adv. Compos. Hybrid Mater. 2022, 5, 3035–3044. [Google Scholar] [CrossRef]
- Tao, F.; Zheng, J.; Wang, L.; Yuan, Y.; Wan, F.; Xu, W.; Huang, Z.; Wang, S.; Huang, Y. Plasmonic photothermal film for defogging and anti-icing/deicing on PTFE. J. Alloys Compd. 2021, 866, 158827. [Google Scholar] [CrossRef]
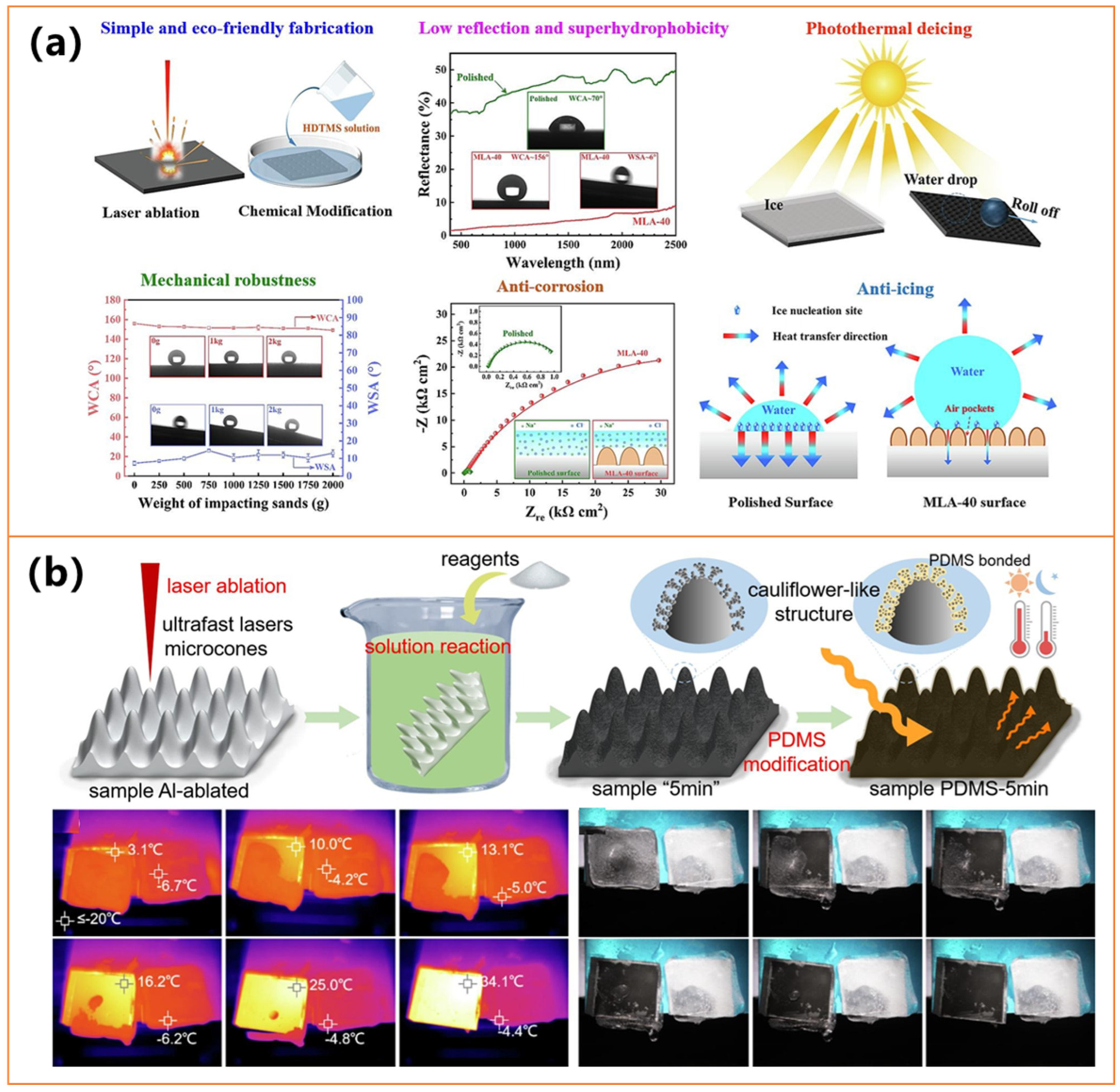
- Li, X.-Y.; Su, H.; Li, H.; Tan, X.; Lin, X.; Wu, Y.; Xiong, X.; Li, Z.; Jiang, L.; Xiao, T.; et al. Photothermal superhydrophobic surface with good corrosion resistance, anti-/de-icing property and mechanical robustness fabricated via multiple-pulse laser ablation. Appl. Surf. Sci. 2024, 646, 158944. [Google Scholar] [CrossRef]
- Li, N.-B.; Zhang, Y.; Zhi, H.; Tang, J.; Shao, Y.; Yang, L.; Sun, T.; Liu, H.; Xue, G. Micro/nano-cactus structured aluminium with superhydrophobicity and plasmon-enhanced photothermal trap for icephobicity. Chem. Eng. J. 2022, 429, 132183. [Google Scholar] [CrossRef]
- Chen, C.-H.; Tian, Z.; Luo, X.; Jiang, G.; Hu, X.; Wang, L.; Peng, R.; Zhang, H.; Zhong, M. Cauliflower-like micro-nano structured superhydrophobic surfaces for durable anti-icing and photothermal de-icing. Chem. Eng. J. 2022, 450, 137936. [Google Scholar] [CrossRef]
- Wei, J.; Wei, X.; Hou, M.; Wang, J. Fluorine-free photothermal superhydrophobic copper oxide micro -/nanostructured coatings for anti-icing/de-icing applications. ACS Appl. Nano Mater. 2023, 6, 9928–9938. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, C.; Zhong, L.; Zhu, L.; Chen, H.; Hou, Y.; Zheng, Y. Robust photothermal superhydrophobic coatings with dual-size micro/nano structure enhance anti-/de-icing and chemical resistance properties. Chem. Eng. J. 2022, 446, 137461. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Fan, X.; Li, X.; Zhang, Z.; Zhang, Y.; Chen, Z.; Ge, S.; Wang, Y.; Zhu, M. Micro-structure design of black ceramic composite surface towards photothermal superhydrophobic anti-icing. Chem. Eng. J. 2024, 498, 155101. [Google Scholar] [CrossRef]
- Hou, M.-T.; Jiang, Z.; Sun, W.; Chen, Z.; Chu, F.; Lai, N.C. Efficient photothermal anti-/deicing enabled by 3D Cu2-xS encapsulated phase change materials mixed superhydrophobic coatings. Adv. Mater. 2024, 36, 2310312. [Google Scholar] [CrossRef]
- Zhou, Z.-T.; Tan, S.; Sun, W.; Guan, X.; Xu, T.; Ji, G. A cost-effective photothermal superhydrophobic coating with micro-and nano-graded structures for efficient solar energy harvesting. J. Mater. Sci. Technol. 2024, 198, 158–165. [Google Scholar] [CrossRef]
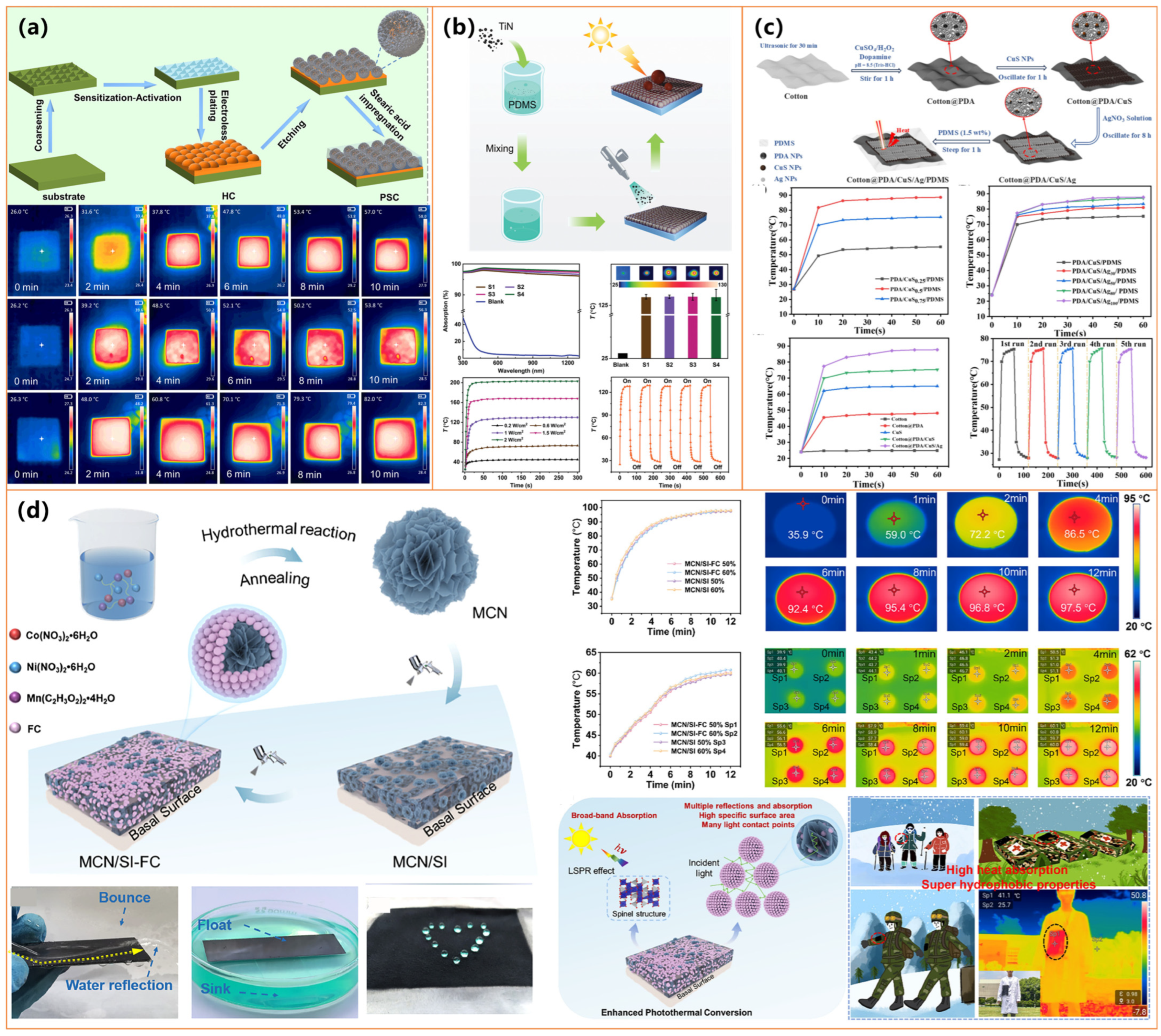
- Zheng, G.-L.; Jiang, Z.; Cui, Y.; Zhou, M.; Yu, Y.; Wang, P.; Wang, Q. Photothermal, superhydrophobic, conductive, and anti-UV cotton fabric loaded with polydimethylsiloxane-encapsulated copper sulfide nanoflowers. Int. J. Biol. Macromol. 2024, 265, 130650. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-C.; Huo, L.; Wang, L.; Huang, X.; Li, J.; Guo, Z.; Gao, Q.; Hu, M.; Xue, H.; Gao, J. Superhydrophobic and multi-responsive fabric composite with excellent electro-photo-thermal effect and electromagnetic interference shielding performance. Chem. Eng. J. 2020, 391, 123537. [Google Scholar] [CrossRef]
- Wang, Z.-T.; Cheng, H.; Chen, R.; Wang, M.-X.; Jiang, N.; Lu, Z.; Yang, H. Omnipotent antibacterial cotton fabrics with superhydrophobic and photothermal properties. Int. J. Biol. Macromol. 2025, 290, 138901. [Google Scholar] [CrossRef]
- Wu, L.-S.; Liu, P.; Hua, X.; Guo, Z.; Liu, W. Photothermal superhydrophobic membrane based on breath figure: Anti-icing and deicing. Chem. Eng. J. 2024, 480, 147553. [Google Scholar] [CrossRef]
- Li, X.-Y.; Li, H.; Su, H.; Tan, X.; Lin, X.; Wu, Y.; Jiang, L.; Xiao, T.; Tan, X. Substrate-Independent superhydrophobic coating capable of photothermal-induced repairability for multiple damages fabricated via simple blade coating. Langmuir 2024, 40, 9449–9461. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, D.; Zhang, D.; Ma, L.; Wang, J.; Huang, Y.; Chen, M.; Qian, H.; Li, X. A durable and photothermal superhydrophobic coating with entwinned CNTs-SiO2 hybrids for anti-icing applications. Chem. Eng. J. 2021, 423, 130238. [Google Scholar] [CrossRef]
- Peng, C.; Yang, D.; You, Z.; Ruan, D.; Guan, P.; Ye, Z.; Ning, Y.; Zhao, N.; Yang, F. A Multilayer Photothermal Superhydrophobic Anti-Icing Coating Applied to Asphalt Pavement with Remarkable Wear Resistance. ACS Appl. Mater. Interfaces 2024, 16, 35588–35603. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.-L.; Liu, K.; Ma, C.; Sun, P.; Liang, L. Constructing a hierarchical coating with photothermal superhydrophobic property by spraying and modification on polyurethane foam for anti-icing and deicing. Appl. Therm. Eng. 2024, 236, 121907. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Fan, X.; Zhu, W.; Li, X.; Cai, M.; Huang, Y.; He, C.; Zhang, L.; Lin, B.; Zhu, M. Probing photothermal superhydrophobic behaviors of graphene&SiO2 hybrid coating for anti-freeze application. Ceramics Int. 2023, 49, 33020–33028. [Google Scholar]
- Li, D.-W.; Ma, L.; Zhang, B.; Chen, S. Facile fabrication of robust and photo-thermal super-hydrophobic coating with efficient ice removal and long-term corrosion protection. Chem. Eng. J. 2022, 450, 138429. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, C.; Xie, L.; Xue, B.; Jiang, Y. Superhydrophobic photothermal coating comprising SiO2-grafted Fe3O4 and Ag decorated reduced graphene for anti-icing and de-icing. Surf. Coat. Technol. 2024, 489, 131127. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Chen, Z.; Song, Z.; Meng, X.; Chen, X. Porous graphene-based photothermal superhydrophobic surface for robust anti-icing and efficient de-icing. Adv. Mater. Interfaces 2022, 9, 2201758. [Google Scholar] [CrossRef]
- Deng, Y.-T.; Du, F.; Chen, Z.; Lv, P.; Yin, Z.; He, M.; Xue, M.; Li, H. Functionalized Superhydrophobic Coatings with Electro-Photothermal Effect for All-Day Durable Anti-Icing. Adv. Mater. Interfaces 2024, 11, 2300869. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Xu, X.; Wu, M.; Zhao, Y.; Sun, F.; Xin, Q.; Zhou, Y.; Qin, M.; Zhou, Y.; Ding, C.; et al. Virus-Like Iron Oxide Minerals Inspired by Magnetotactic Bacteria: Towards an Outstanding Photothermal Superhydrophobic Platform on Universal Substrates. Adv. Funct. Mater. 2022, 32, 2201795. [Google Scholar] [CrossRef]
- Wu, S.-W.; Du, Y.; Alsaid, Y.; Wu, D.; Hua, M.; Yan, Y.; Yao, B.; Ma, Y.; Zhu, X.; He, X. Superhydrophobic photothermal icephobic surfaces based on candle soot. Proc. Natl. Acad. Sci. USA 2020, 117, 11240–11246. [Google Scholar] [CrossRef]
- Guo, C.-L.; Liu, K.; Zhang, T.; Sun, P.; Liang, L. Development of flexible photothermal superhydrophobic microarray by photolithography technology for anti-icing and deicing. Prog. Org. Coat. 2023, 182, 107675. [Google Scholar] [CrossRef]
- Xie, Z.-T.; Wang, H.; Deng, Q.; Tian, Y.; Shao, Y.; Chen, R.; Zhu, X.; Liao, Q. Heat transfer characteristics of carbon-based photothermal superhydrophobic materials with thermal insulation micropores during anti-icing/deicing. J. Phys. Chem. Lett. 2022, 13, 10237–10244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, C.; Jiang, B.; Lei, Y.; Li, Z.; Sun, K.; Zhang, Y. A robust photothermal superhydrophobic anti/de-icing composite coating fabricated from BP/SiO2 on a modified fluorocarbon coating base. Prog. Org. Coat. 2024, 196, 108725. [Google Scholar] [CrossRef]
- Xiong, Z.; Yu, H.-Y.; Gong, X. Designing photothermal superhydrophobic PET fabrics via in situ polymerization and 1,4-conjugation addition reaction. Langmuir 2022, 38, 8708–8718. [Google Scholar] [CrossRef]
- Xie, H.; Wei, J.; Duan, S.; Zhu, Q.; Yang, Y.; Chen, K.; Zhang, J.; Li, L.; Zhang, J. Non-fluorinated and durable photothermal superhydrophobic coatings based on attapulgite nanorods for efficient anti-icing and deicing. Chem. Eng. J. 2022, 428, 132585. [Google Scholar] [CrossRef]
- Jiang, B.-C.; Lei, Y.; Sun, K.; Chen, Q.; Zhang, F.; An, Y.; Zhang, Y.; Lin, Y.; Yuan, Y.; Liu, T.; et al. Superhydrophobic F-SiO2/tea polyphenol coating with high efficiency photothermal anti-icing and de-icing properties. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 132846. [Google Scholar] [CrossRef]
- Yu, B.; Sun, Z.; Liu, Y.; Wu, Y.; Zhou, F. Photo-thermal superhydrophobic sponge for highly efficient anti-icing and de-icing. Langmuir 2023, 39, 1686–1693. [Google Scholar] [CrossRef]
- Wu, W.-Z.; Zhang, Y.; Miao, S.; Wu, Y.; Gong, X. Photothermal superhydrophobic cotton fabric based on silver nanoparticles cross-linked by polydopamine and polyethylenimide. Langmuir 2023, 39, 15131–15141. [Google Scholar] [CrossRef]
- Ma, B.; Xiong, F.; Wang, H.; Wen, M.; Yang, J.; Qing, Y.; Chu, F.; Wu, Y. A gravity–inspired design for robust and photothermal superhydrophobic coating with dual–size lignin micro–nanospheres. J. Clean. Prod. 2024, 435, 140506. [Google Scholar] [CrossRef]
- Ding, S.-P.; Zhang, T.; Wu, M.; Wang, X. Photothermal dual-layer hydrophilic/hydrophobic composite nanofibrous membrane for efficient solar-driven membrane distillation. J. Membr. Sci. 2023, 680, 121740. [Google Scholar] [CrossRef]
- Peng, C.; Yang, D.; You, Z.; Ruan, D.; Guan, P.; Ye, Z.; Ning, Y.; Zhao, N.; Yang, F. A photothermal and superhydrophobic emulsified asphalt coating modified by CNTs and PTFE for anti-icing and de-icing applications. Constr. Build. Mater. 2024, 416, 135148. [Google Scholar] [CrossRef]
- Li, B.; Bai, J.; He, J.; Ding, C.; Dai, X.; Ci, W.; Zhu, T.; Liao, R.; Yuan, Y. A review on superhydrophobic surface with anti-icing properties in overhead transmission lines. Coatings 2023, 13, 301. [Google Scholar] [CrossRef]
- Gou, Y.-J.; Han, J.; Li, Y.; Qin, Y.; Li, Q.; Zhong, X. Research on anti-icing performance of graphene photothermal superhydrophobic surface for wind turbine blades. Energies 2023, 16, 408. [Google Scholar] [CrossRef]
- Nazari, A.; Eslami-Farsani, R. A review of photothermal and superhydrophobic polymer nanocomposites as anti-icing nanocoatings. In Polymer Composites; Wiley: New York, NY, USA, 2024. [Google Scholar]
- Qi, X.-Y.; Li, C.; Zou, X.; He, L.; Liu, Z.; Gao, Z. Super-hydrophobic graphene-based high elastic sponge with superior photothermal effect for efficient cleaning of oil contamination. Chem. Eng. J. 2023, 476, 146317. [Google Scholar] [CrossRef]
- Ji, H.; Guo, J.; Yang, K.; Jiang, J.; Xing, Z. Superhydrophobic/superoleophilic polyurethane/reduced graphene oxide/sponge for efficient oil-water separation and photothermal remediation. Process Saf. Environ. Prot. 2024, 191, 2653–2662. [Google Scholar] [CrossRef]
- Cui, Y.-C.; Zhang, L.; Xing, C.; Tan, Y. Anti-icing properties and application of superhydrophobic coatings on asphalt pavement. Constr. Build. Mater. 2024, 419, 135452. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Du, J.; Liu, G.; Ma, D.; Jia, F.; Klemeš, J.J.; Wang, J. A review of self-cleaning technology to reduce dust and ice accumulation in photovoltaic power generation using superhydrophobic coating. Renew. Energy 2022, 185, 1034–1061. [Google Scholar] [CrossRef]

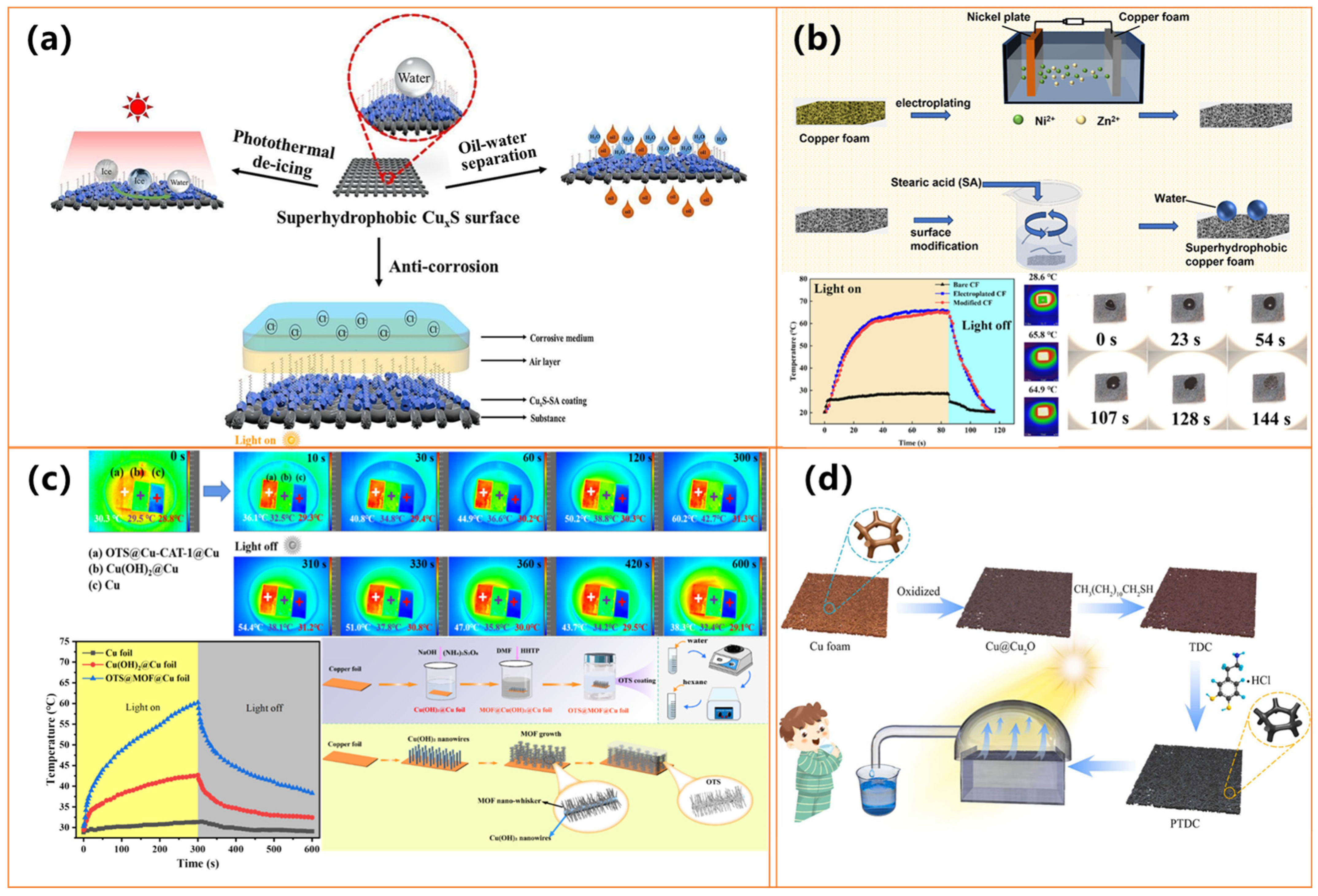
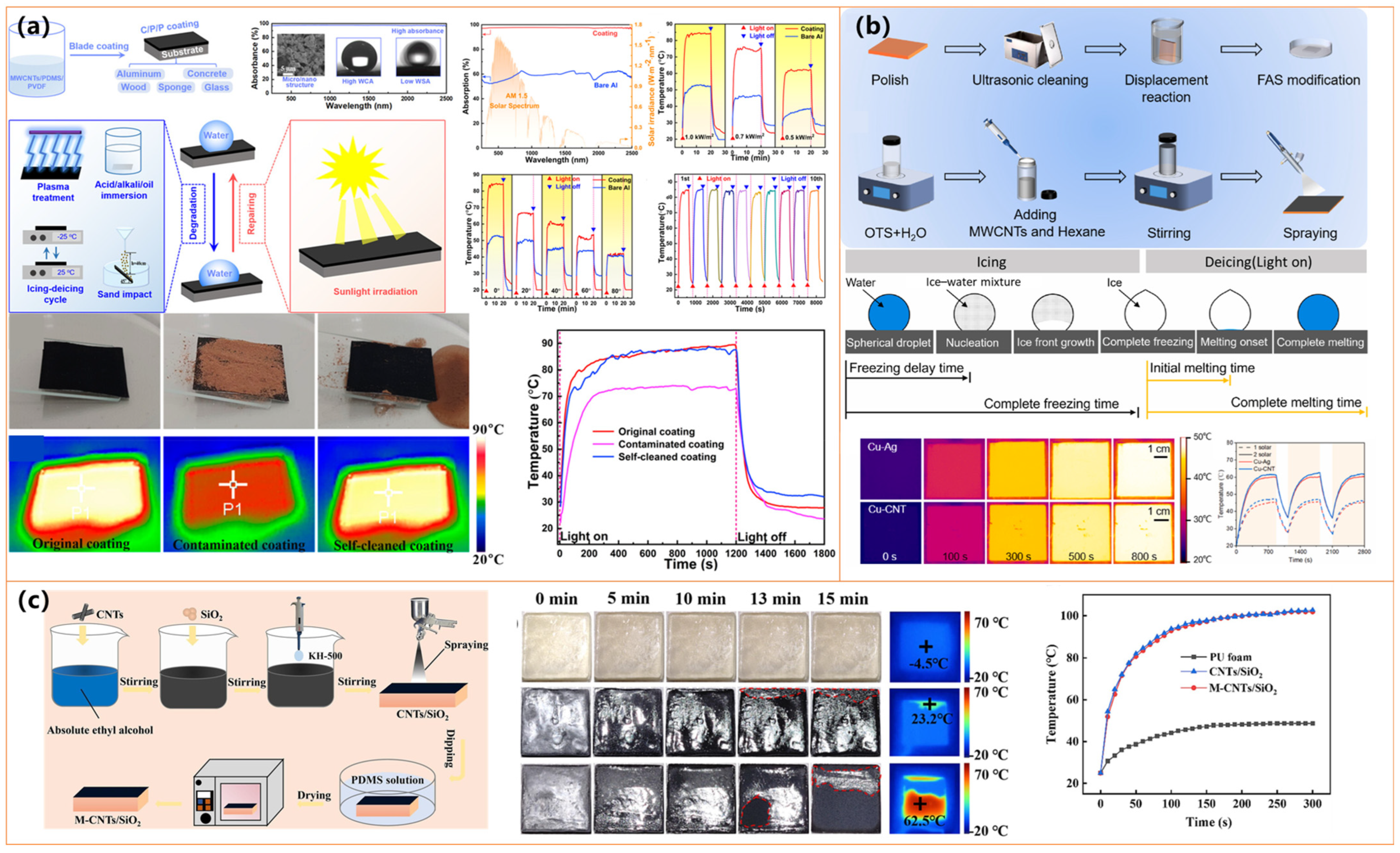
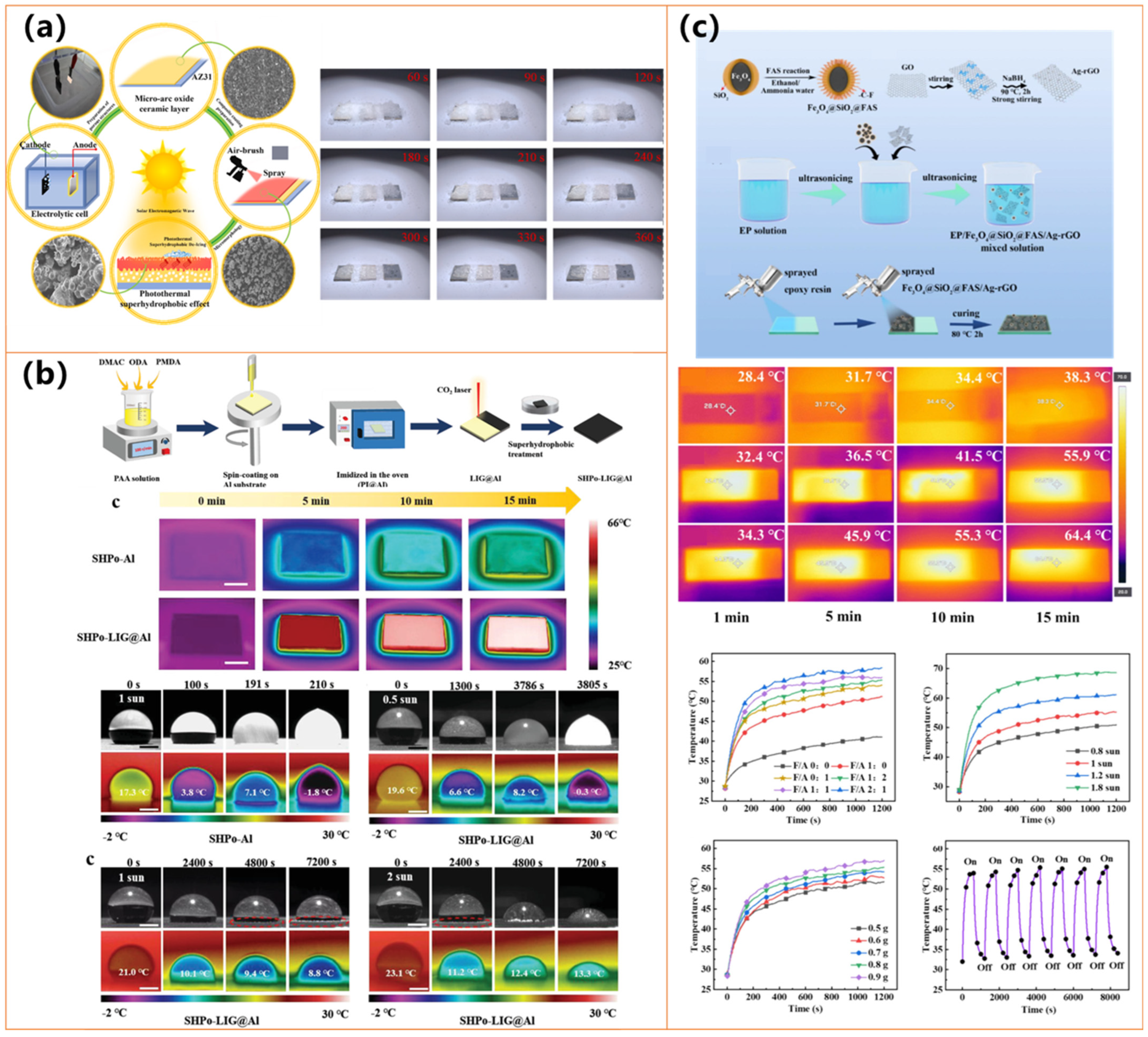
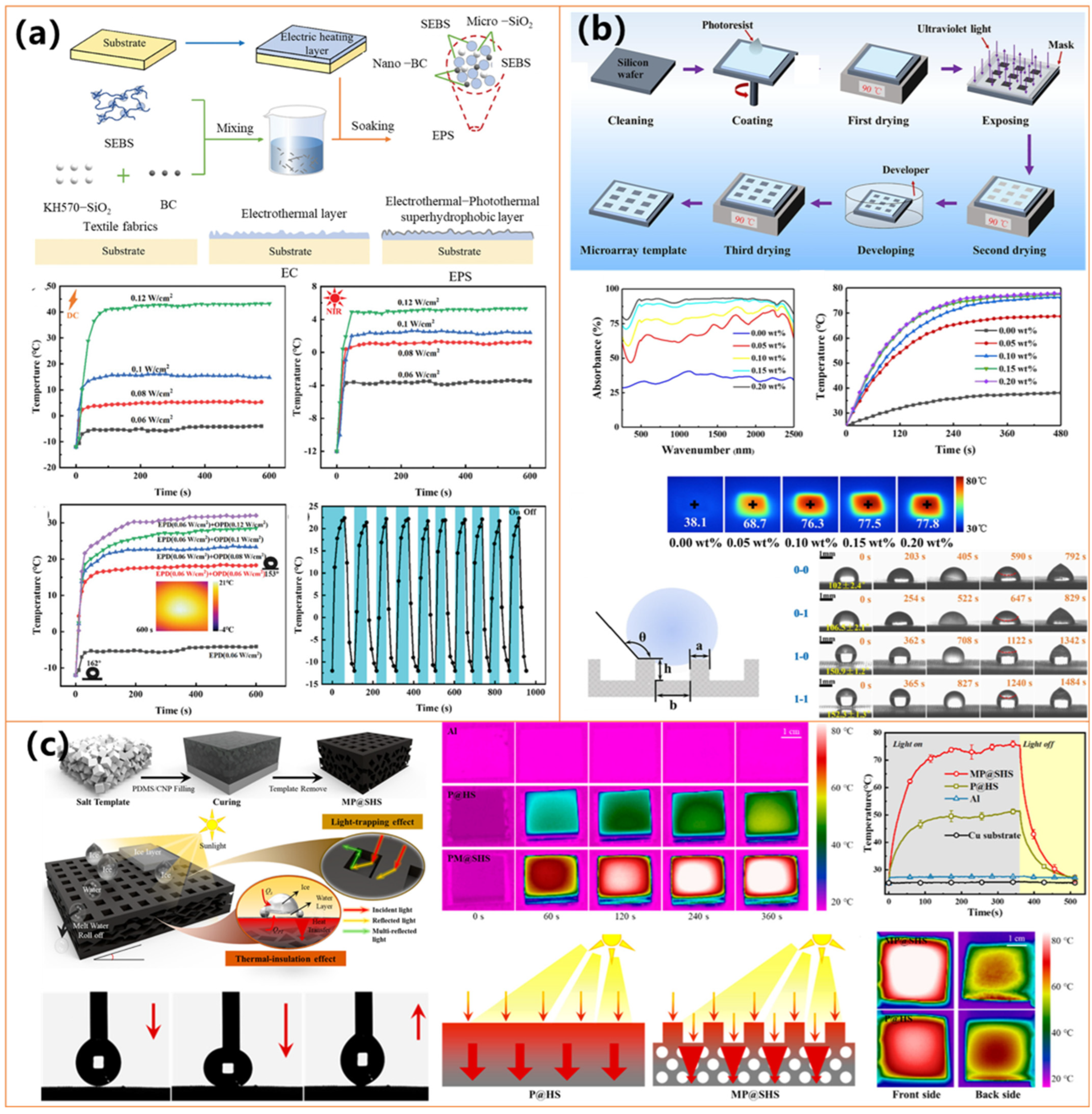
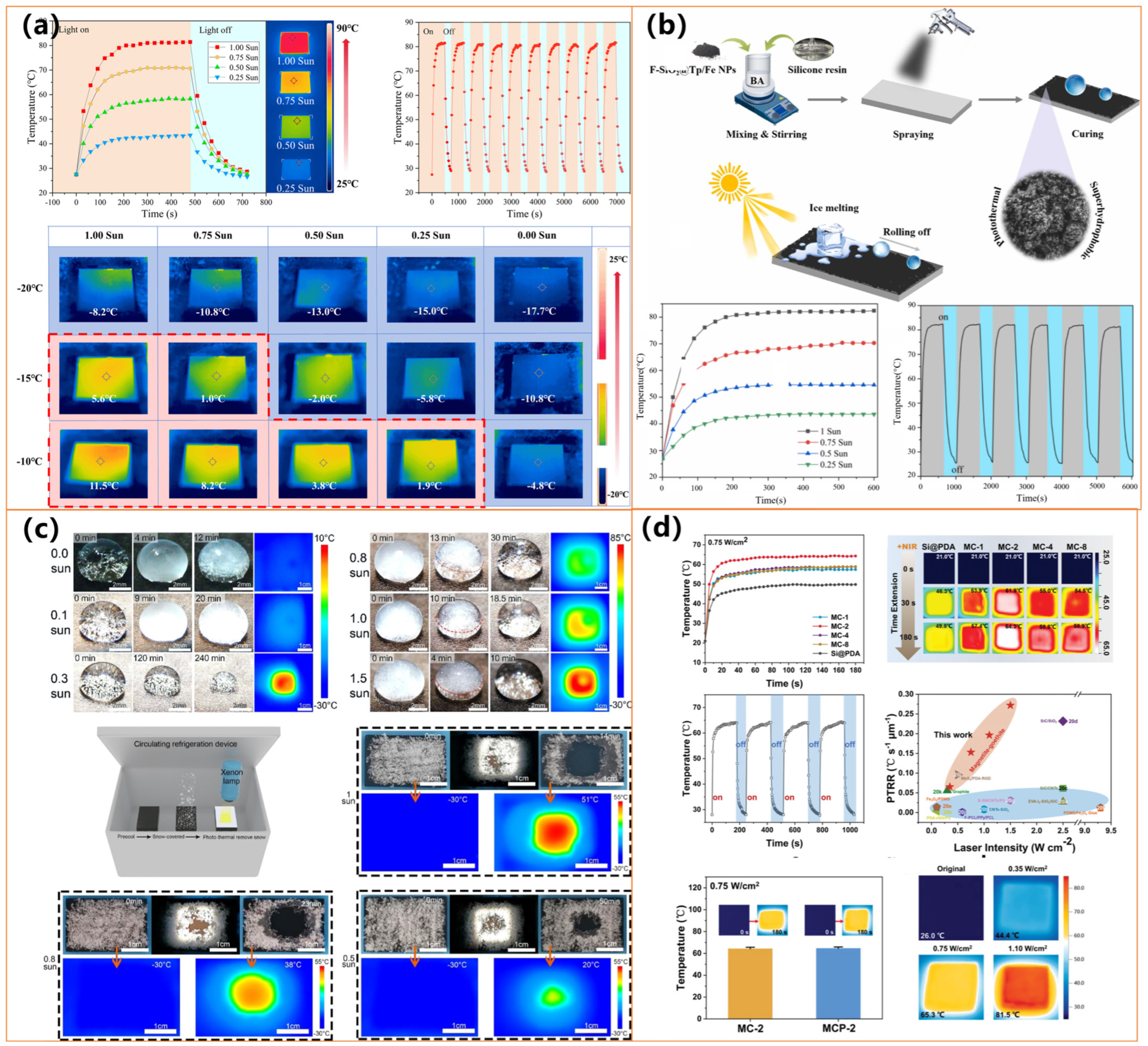
| Materials | Preparation Method | Water Contact Angle | Photothermal Performance | Anti-Icing/Deicing Ability | Durability& Other Features | Ref. |
|---|---|---|---|---|---|---|
| Copper mesh, CuxS (CuS/Cu2S), stearic acid | Hydrothermal growth of CuxS + SA | 169° | Temp ↑ to 48.1 °C (808 nm laser) | Freezing delay: time extended to 27 min; Fast deicing | UV, Acid/base, Bending, Abrasion, Ultrasonic; Long-term storage; Corrosion protection; Oil-water separation | [100] |
| Cu foil, Cu-CAT-1 MOF, OTS | In situ MOF growth on Cu foil + OTS modification | 155.8° | Rapid heating under xenon lamp; effective up to 480 s for deicing | Freezing delay: 870 s; Deicing: 480 s | Chemically stable in acid/base/salt solutions; | [102] |
| Cu@Cu2O foam, n-dodecanethiol, polydopamine | Oxidation + surface hydrophobic/hydrophilic functionalization | / | Photothermal conversion efficiency: 92%, Temp ↑ under 1 sun | / | Stable in continuous desalination, | [103] |
| Iron foam, CuO layer, PFDTES | Chemical etching + sintering + PFDTES modification | / | Temp ↑ to 55.8 °C (240 s under sunlight) | Freezing delay: 1220 s (from 221 s); Deicing in 251 s (0.2 W/cm2) | sandpaper abrasion (50 cycles); Water impact; Corrosion resistance | [104] |
| Cotton fabric, Ag NPs, PDMS | Dip-coating with Ag/PDMS in isopropanol, curing at 140 °C for 5 h | 171.3° | Surface temperature increases under NIR; deicing under −20 °C | Photothermal deicing; Freezing and melting delay | Maintains WCA and UPF after washing (AATCC-61); Abrasion (Martindale, sandpaper); UV shielding (UPF tested); Antibacterial | [105] |
| Cotton, Ag/CdS, PDA, PFDT, or NDM thiols | PDA-assisted Ag/CdS deposition + thiol self-assembly | / | Electro-/photothermal self-healing under IR, xenon lamp, Joule heating | / | Stable: 18 accelerated wash cycles (≈90 home cycles); Thermal/photo-healing; Corrosion resistance (electrochemically tested); Photocatalytic Methylene Blue degradation; Antibacterial; Electrically conductive | [106] |
| MXene (Ti3C2Tₓ), Ag nanowires, PEDOT: PSS | Drop-casting + hydrophobic spray with fluorosilane | / | Photo-/electrothermal conversion for self-heating in cold/damp | Effective under low-temperature/high-humidity conditions | Strong adhesion via interlocking; Stable under humidity and cold; EMI shielding (31.5 dB @ 10 µm) | [107] |
| MXene (Ti3C2Tx), Au NPs, SiO2@FAS, WPU | Two-step spray coating of MXene@Au-WPU + fSiO2 | 153° | photothermal deicing efficiency of 73.1% | Anti-icing time: 1053 s (−20 °C, RH 68%) | Stable high humidity, and multiple cycles; Corrosion resistance (pH 1–13) | [108] |
| Au/TiO2 NPs on PTFE, PDMS | Sol–gel synthesis + spin coating | / | Surface temp ↑ >25 °C (3-sun illumination) | Deicing under light (effective) | Stable during cyclic fogging and deicing; Low-cost, simple, low-noble-metal loading | [109] |
| Carbon steel, HDTMS | Laser ablation + HDTMS chemical modification | 156° | Temp ↑ from ~13 °C to ~65 °C (1 sun, 8 min) | Freezing delay 2547 s; Thawing (60 s, 0.5 sun) | WCA > 150° after 2000 g sand impact or 400 tape peels; No fluorine; Black surface | [110] |
| Fe particles, PDMS, candle soot (CS) | Spin coating + magnetic particle deposition + candle soot deposition | 154.5° | 1 mm of ice melted (237 s, 1 sun) | Freezing: 4.7× longer; Deicing in 237 s | Stable after 320 abrasion cycles; Acid/base/NaCl, liquid nitrogen; Water impact; Self-healing; Magnetically attachable; Fluorine-free | [93] |
| Aluminium alloy, Zn/ZnSb dendrites, PDMS | Femtosecond laser + chemical treatment + PDMS coating | 161.5° | ΔT = 48.5 °C (300 s, 1 sun) | Ice melted in <2 min | Durable after sand abrasion; Water impact; Tape peel; 3-month outdoor test; Absorptivity 97.3%, minimal surface change | [111] |
| Aluminium, FDTS (fluorinated silane) | Laser surface direct writing + thermal evaporation of FDTS | 161.2° | Solar absorption > 94.5%, ΔT > 60 °C, rapid heating | Strong anti-icing; Defrosting at −30 °C | Stable (−80–200 °C); Mechanical/thermal/cold tests passed; Self-cleaning; Light trapping; Plasmon-enhanced heat conversion | [112] |
| Materials | Preparation Method | Water Contact Angle | Photothermal Performance | Anti-Icing/Deicing Ability | Durability& Other Features | Ref. |
|---|---|---|---|---|---|---|
| GO@Fe3O4, PDMS, PF lubricant, CuO nano-grass on Cu | Etching Cu → CuO + lubricant infusion (PDMS/PF) with GO@Fe3O4 | / | ΔT = 71.7 °C (infrared, 120 s) | Icing-delay: 269 s, −10 °C; Deicing: photothermal melting; Extremely low ice adhesion strength | Stable over 12 months; Self-healing; Anti-aging; Self-healing | [22] |
| Electroless-plated Cu, etched CuO | Plating + surface etching to form micro/nano CuO | / | ΔT = 82 °C (10 min, simulated light) | Deicing time: 1/3 of the bare substrate | Friction-/water-impact-stable; Alkali-resistant; Strong adhesion resistance; Self-cleaning; Antifouling; Fluorine-free | [113] |
| Fe3O4 (dual-size NPs), PDMS, Epoxy | Spray coating of Fe3O4@SiO2@FAS + PDMS + epoxy layer | / | ΔT = 14.4 °C/min; Efficiency = 62.38%; | Deicing in 790 s under sunlight, rate: ~1.96 kg/m2·h | pH 1–14, 20-day corrosion, 90 tape peels, 30 sand impacts; | [114] |
| Black ceramic (Mg1−xCuxO), PDMS NPs | Plasma etching + black ceramic coating + PDMS vapor deposition | 156° | Up to 69.4 °C (200 mW/cm2) | Photothermal active + superhydrophobic passive melting strategy | Validated long-term thermal and mechanical performance; MD simulation; Cassie–Wenzel transition optimization | [115] |
| 3D Cu2−xS@Cu2O MPCM + OTS-based matrix | MPCM + hydrolysis + polycondensation with silane | / | Efficiency: 96.1%; Full-spectrum absorption | Efficient at low T, high humidity | 200-cycle phase change with minimal thermal change; Phase change thermal storage + LSPR Cu2−xS optimization | [116] |
| MCN (MnxCoNi1−xOy), PVDF, silicone resin | Solvothermal synthesis + two-step spray with silicone/PVDF | 155.79° | Temp ↑ to 97.5 °C (1 kW/m2) | / | Excellent mechanical durability; Adheres to multiple substrates; Low-cost; Hierarchical micro/nanostructure | [117] |
| Cotton, CuS nanoflowers, PDMS | TA polymerization + CuS deposition + PDMS encapsulation | 153.0 ± 0.4° | NIR- /unlight-responsive; Resistance drops (250 mW/cm2) | / | Maintains hydrophobicity after 40 sandpaper abrasion cycles; UV resistance; Oil–water separation | [118] |
| PP fabric, AgNPs, Fe3O4, PDMS | O2 plasma + AgNPs deposition + spray Fe3O4,/PDMS | / | Electro-photothermal effect; Joule-/light-responsive | / | Stable after bending, abrasion, and ultrasonic washing; EMI SE ≈ 56.1 dB (X-band); multi-responsive, | [119] |
| Cotton, PDA, CuS, AgNPs, PDMS | PDA/CuS/Ag deposition + PDMS encapsulation | / | Photothermal antibacterial (NIR and dark conditions); dual-light responsive | / | Stable after abrasion, bending, and ultrasonic washing; Omnipotent antibacterial; Self-cleaning, | [120] |
| Materials | Preparation Method | Water Contact Angle | Photothermal Performance | Anti-Icing/Deicing Ability | Durability& Other Features | Ref. |
|---|---|---|---|---|---|---|
| PMMA, FAS-modified MWCNTs | Breath figure method + spray-coating MWCNTs | 157° | Surface T ↑ to 80.9 °C (120 s, 1 sun) | Freezing delay: 1175 s; Deicing: 160 s; Defrosting: 521 s | Stable after water/sand impact; Simple fabrication; Porous micro-nanostructures | [24] |
| Ag NPs on Cu (Cu–Ag), MWCNTs on Cu (Cu–CNT) | Cu–Ag: displacement deposition; Cu–CNT: OTS/MWCNTs spray | Cu–Ag: 162.1°; Cu–CNT: 158.3° | T ↑ Cu–Ag: 16.58 °C; Cu–CNT: 17.58 °C (100 s, 2 sun) | Freezing delay: 1336 s (Cu–Ag), 2926 s (Cu–CNT); Melt: ~473 s/421 s | Stable under cold/wet cycling; High repeatability; Scalable; Low-tox reagents | [99] |
| MWCNTs@PDA/ODA, PVDF, PDMS | Blade-coating on diverse substrates | >150°, | ΔT ≈ 65 °C (1 sun) | Superhydrophobicity restored after icing–thawing by sunlight | Resistant to plasma, oil, acid/base, sand, icing; Repairable by photothermal effect | [122] |
| CNTs-SiO2 hybrids, Epoxy | One-step spraying | 159.3° | Rapid heating (Laser-induced) | Freezing delayed; Deicing: in seconds | Excellent chemical/ mechanical durability (tape-peel, friction); Hierarchical micro/nanostructure | [123] |
| AFP-modified emulsified asphalt, CNTs, SiC | Multilayer spray + curing | 161° | Photothermal efficiency 50.94% | Freezing time doubled; Deicing under 2/4 kW/m2 NIR | Stable after 500-wheel rolls and 100 abrasion cycles; AFP core remains anti-icing after surface wear | [124] |
| CNTs, SiO2, PDMS, PU foam | Spraying + PDMS modification | 157° | ΔT = 101.9 °C (1 kW/m2, 300 s) | Fast deicing | Stable after 10 freeze–thaw/20 friction cycles; Hierarchical structure | [125] |
| Graphene, SiO2, PDMS, Epoxy | Micro-arc oxidation on Mg alloy + spray coating | 162.2° | Stable at 49.56 °C (2 sun) | Long-lasting deicing | Chemical/mechanical durability; 200 cm under 300g force | [126] |
| SiO2, GO, TiC, MSR | One-step spraying of MSR + modified additives | 161.9° | / | Active deicing (808 nm NIR) | Stable after 60m of abrasion, kneading, corrosion, and plasma etch; Self-healing; Fluorine-free | [127] |
| Fe3O4@SiO2@FAS, Ag-rGO, Epoxy | Two-step spraying on epoxy base layer | 153.8° | ΔT = 58.8 °C (1 sun) | Freezing delay: 1498 s; Deicing: 120 s, 40 mW/cm2 | Stable under tape peel, friction, water, acid/base; Scalable | [128] |
| Laser-induced graphene (LIG) on PI/Al | Laser engraving + silanation on PI@Al | / | T ↑ to 65 °C (5 min, 1 sun); Absorption ≈98% | Retarded frost; Melting under 1–2 sun | Thermal, chemical, and corrosion stability; Scalable | [129] |
| EPS nanocomposite (Biochar, MWCNTs, SiO2, SEBS) | Blending + coating onto textiles | >150° | Maintaining T > 0 °C (−20 °C, PT/ET synergy) | Icing delayed 4×; Deicing time reduced 5× compared to PT only; All-weather anti-icing | UV, corrosion, and abrasion resistant; All-day effectiveness | [130] |
| Virus-like Fe3O4/Goethite nanostructures | Liquid-confined magnetite mineralization with PDA | / | Exceptional PT effect, comparable to carbon materials | Defrosting/deicing | Not quantified but implied via stability and reusability; Magnetic field assisted; Biocompatible; Scalable | [131] |
| Candle soot, SiO2 shell, PDMS brushes | Soot deposition + silica + PDMS grafting | / | ΔT = 53 °C (1 sun) | Prevents icing at −50 °C; Melts frost/ice: 300 s | Self-healing after plasma; Long-term stability; Eco-friendly; Scalable; Self-cleaning | [132] |
| CB-PDMS microarray | Photolithography + CB infusion | 151.1 ± 0.9° | Freezing: delay 87%; ΔT not specified | Deicing time reduced by ~43.1% vs. control | Flexible, stable under bending, and friction; Photolithographic precision; Cost-effective | [133] |
| MP@SHS (PDMS + CNPs with micropores) | Salt-template casting | 153.5 ± 0.5° | T ↑ to 75 °C (360 s, 1 sun); Absorbance = 98.3% | Freezing delay: ~648 s; Melting: ~129 s | Resistant to chemical/mechanical damage; Flexible | [134] |
| Materials | Preparation Method | Water Contact Angle | Photothermal Performance | Anti-Icing/Deicing Ability | Durability& Other Features | Ref. |
|---|---|---|---|---|---|---|
| PPy-coated basalt (BP), SiO2 NPs, fluorocarbon resin | Two-step spray with inverse infusion process | 155° ± 2° | Ice removal in 309 s (frost)/760 s (ice cube) under 1 sun | Icing delay: 656 s; Ice adhesion < 20 kPa after cycles | Stable after 300 sandpaper + 100 tape cycles | [135] |
| PET fabric, PPy, pentaerythritol tetraacrylate, octadecyl acrylate, APTES | In situ oxidative polymerization + 1,4-conjugation addition | 155.8° | T ↑ to 91 °C (simulated sunlight, 20 mA) | Not directly measured; Fabric suitable for photothermal deicing | Stable after UV, acid/base, organics, 25 abrasion cycles, 8 wash cycles; Self-cleaning; Fluorine-free | [136] |
| PPy/ATP@hexadecylPOS, silicone resin | Spray-coating PPy/ATP+silicone resin on Al | 162.7° | ΔT = 70 °C (3 min, 1 sun) | Fast deicing; Long-term anti-icing (4 weeks) | Mechanical, chemical, and thermal stability; Outdoor-tested; Fluorine-free; Eco-friendly; Hierarchical porous structure | [137] |
| F-SiO2@Tp/Fe, silicone resin | One-step spray-coating | 159° | ΔT = 81 °C (1 sun) | Icing delay ×3, stable de/defrosting cycles | Stable under abrasion, peeling, and corrosion | [138] |
| PU sponge, Fe3O4 NPs, PDA, fluorosilane | Deposition of Fe3O4 + PDA + fluorination | ≈150° | Surface T reached ~51 °C (1 sun) | No icing under 0.3 sun at −30 °C; Melts ice in 10 min under 1 sun | Self-cleaning; Self-healing; Stable in harsh conditions; Porous structure | [139] |
| Cotton fabric, PDA, PEI, GA@AgNPs, PDMS | Dipping and sequential coating | 159.6° | Surface T reached 70.4 °C (simulated sunlight, 20 A) | / | Resistant to washing, abrasion, chemicals, and high temperatures; Environmentally friendly; Low-cost | [140] |
| Dual-size LMNSs, epoxy resin, F13–TMS | Gravity-assisted sedimentation + immersion coating | 166.1° ± 0.8°, | T ↑ from ~13 °C to 112 °C in 60 s (laser) | Not specifically measured but implied to be effective | Abrasion distance > 320 cm, 210 tape cycles; Chemical/thermal Resistance; Renewable | [141] |
| PAN (AgNP-loaded), PVDF-PDMS | Electrospinning + immersion + reduction | / | Energy efficiency 75.2%; T sufficient for membrane distillation | Not tested for icing, tested for desalination | Stable over 10 h of distillation with low conductivity | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Yin, T.; Ma, J.; Zhou, Y.; Li, K.; Bao, J. Research Progress of Photothermal Superhydrophobic Surfaces for Anti-Icing/Deicing. Molecules 2025, 30, 1865. https://doi.org/10.3390/molecules30091865
Gao H, Yin T, Ma J, Zhou Y, Li K, Bao J. Research Progress of Photothermal Superhydrophobic Surfaces for Anti-Icing/Deicing. Molecules. 2025; 30(9):1865. https://doi.org/10.3390/molecules30091865
Chicago/Turabian StyleGao, Hui, Tianjun Yin, Jieyin Ma, Yuqin Zhou, Ke Li, and Jiayi Bao. 2025. "Research Progress of Photothermal Superhydrophobic Surfaces for Anti-Icing/Deicing" Molecules 30, no. 9: 1865. https://doi.org/10.3390/molecules30091865
APA StyleGao, H., Yin, T., Ma, J., Zhou, Y., Li, K., & Bao, J. (2025). Research Progress of Photothermal Superhydrophobic Surfaces for Anti-Icing/Deicing. Molecules, 30(9), 1865. https://doi.org/10.3390/molecules30091865







