Electrochemical Degradation of Venlafaxine on Platinum Electrodes: Identification of Transformation Products by LC-MS/MS and In Silico Ecotoxicity Assessment
Abstract
1. Introduction
2. Results
2.1. Evaluation of Anode Material for the Electrochemical Degradation of VFX
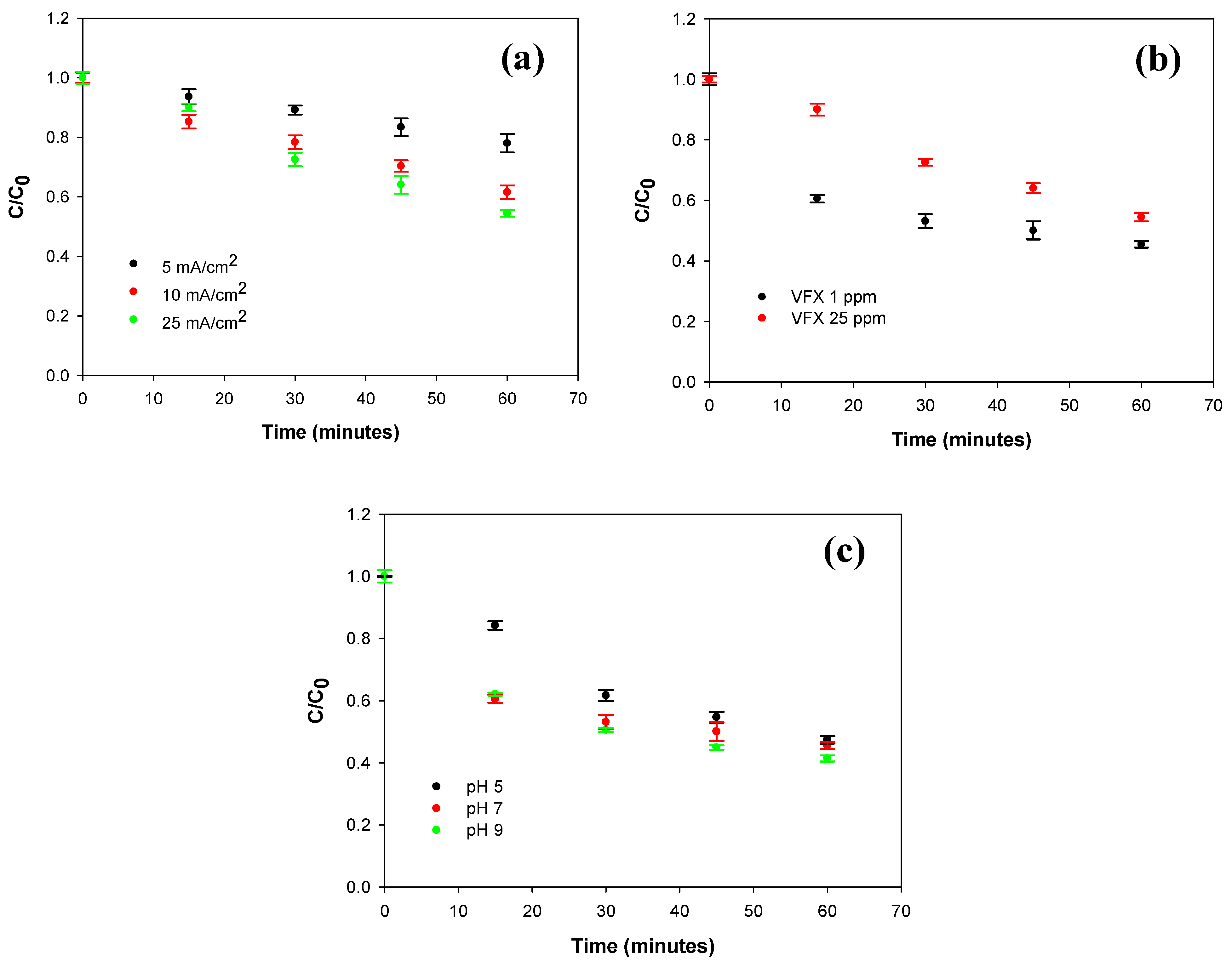
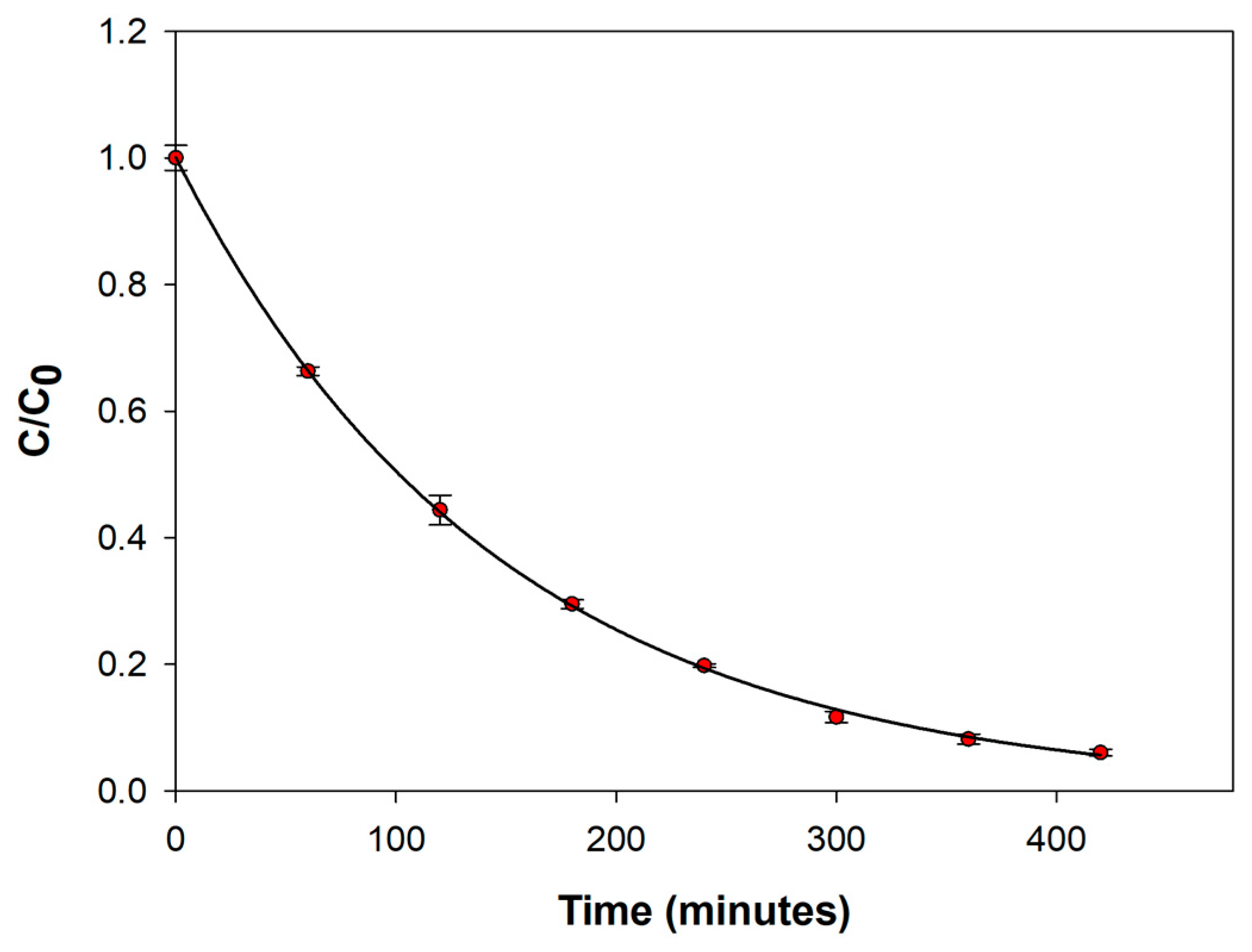
2.2. Optimization of the Degradation Conditions
2.3. Structural Elucidation of Venlafaxine and Degradation Products by LC-ESI-LIT-MSn and LC-ESI-Orbitrap-MS
2.3.1. LC-ESI-Orbitrap-MS Studies of Venlafaxine and Its Degradation Products
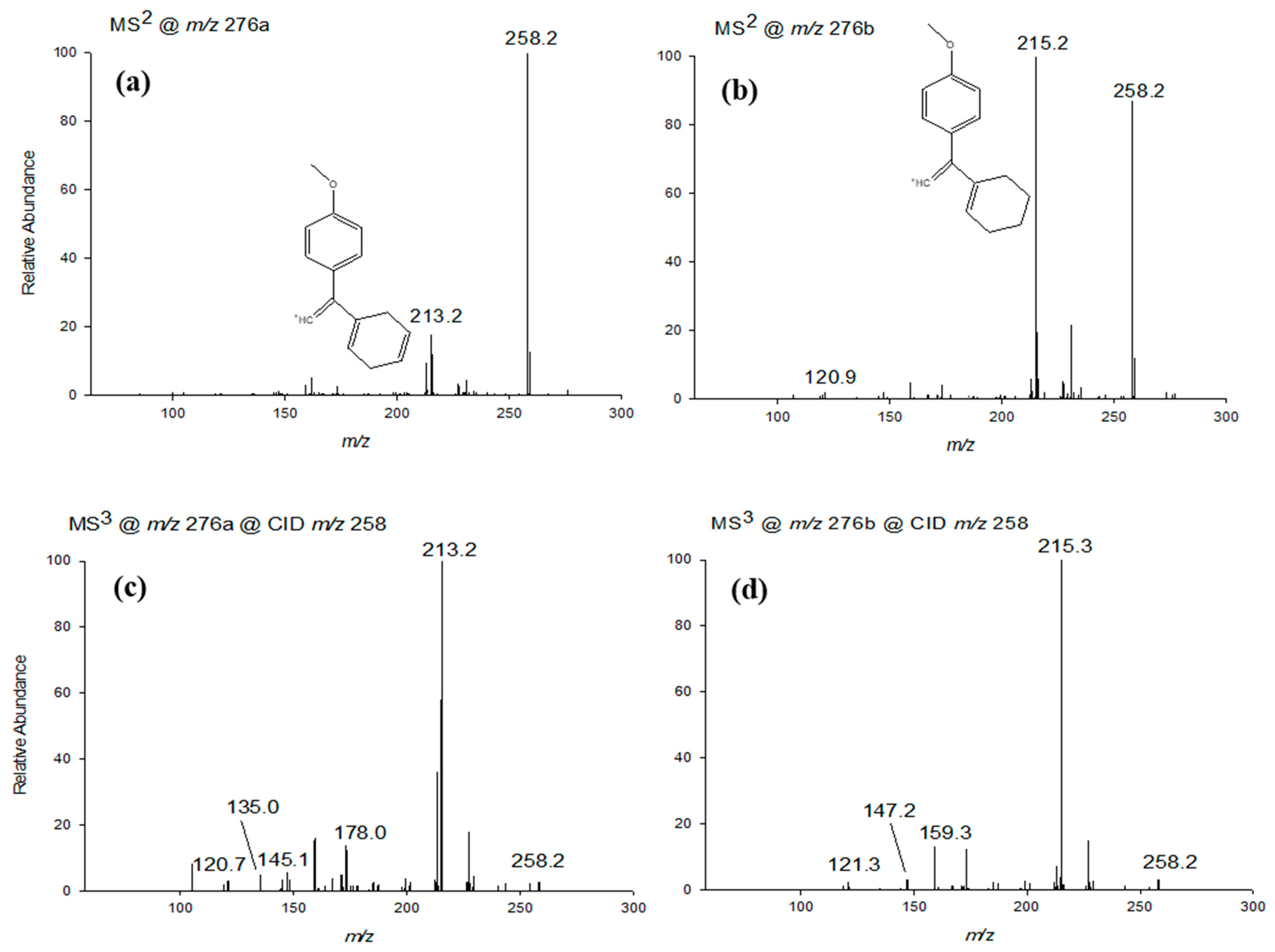
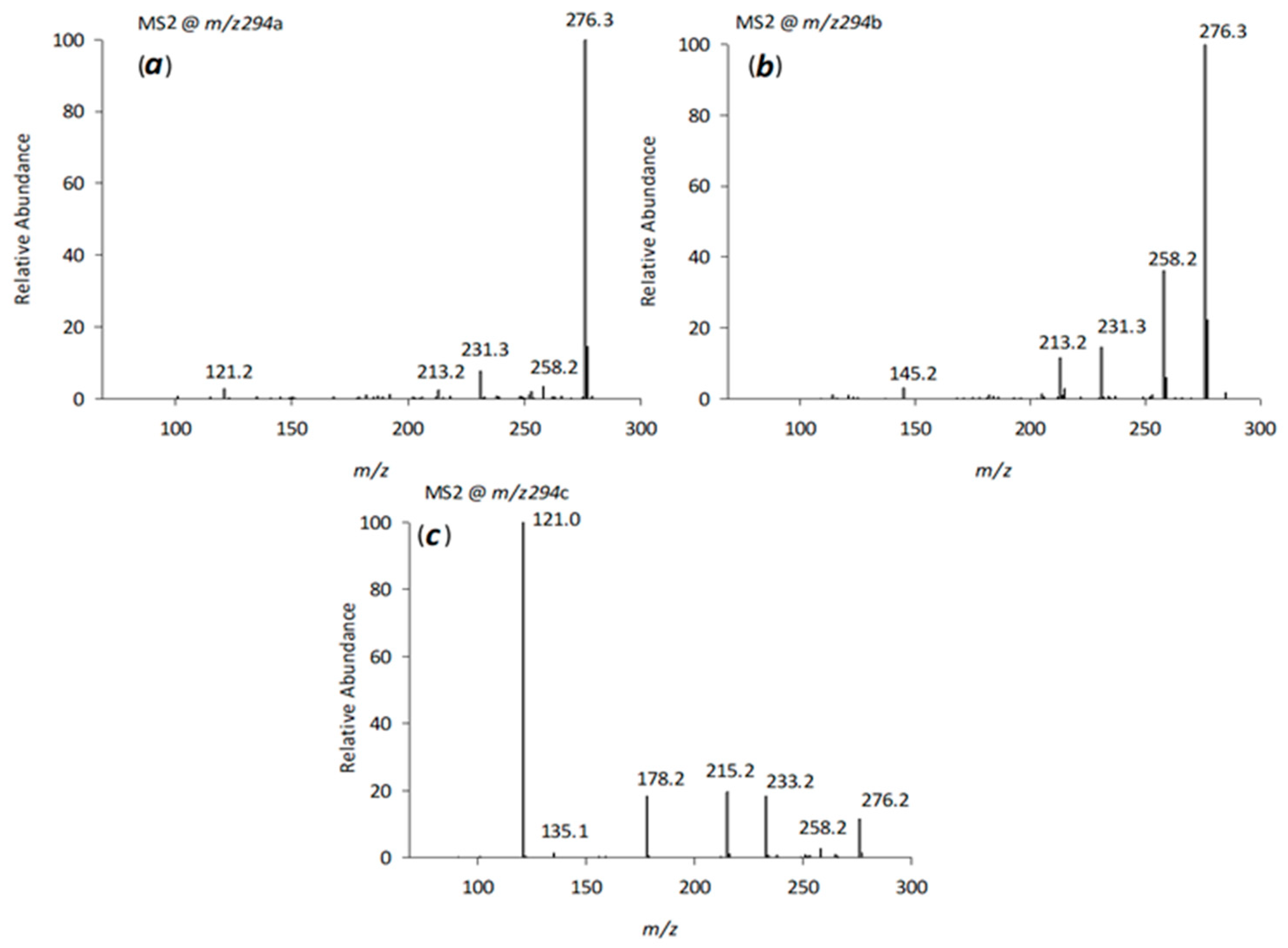
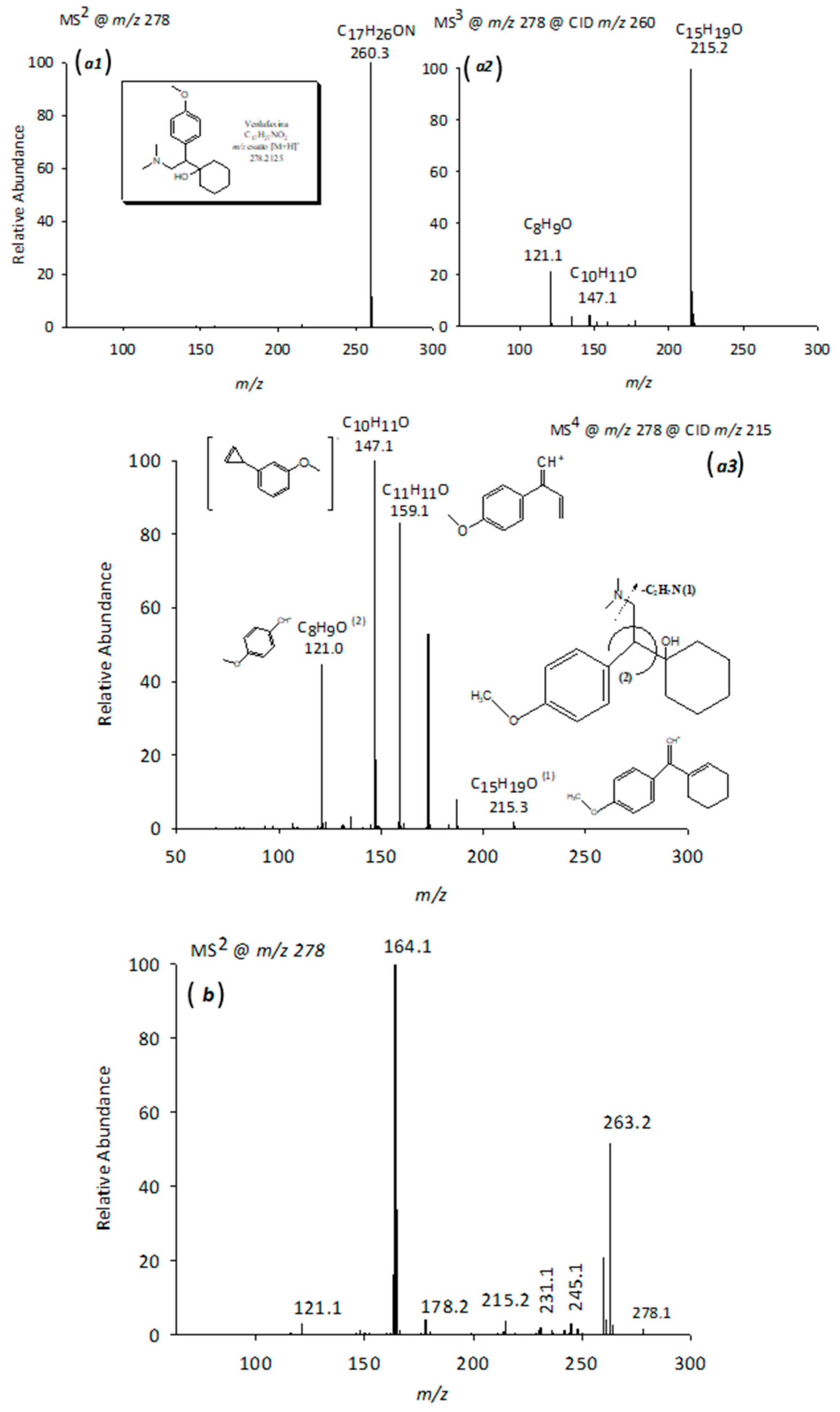
2.3.2. LC-ESI-CID-MSn Studies of VFX Degradation Products
2.4. Evaluation of the Ecotoxicity of Venlafaxine Degradation Products
2.5. Electrochemical Degradation of Venlafaxine: Advantages of Platinum Electrodes
3. Materials and Methods
3.1. Materials
3.2. Electrochemical Experiments
3.2.1. Cyclic Voltammetry and Differential Pulse Voltammetry
3.2.2. Galvanostatic Electrolysis
3.3. LC-UV Conditions
3.4. LC-MS/MS Analysis
3.5. In Silico Toxicity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Polanco-Rodriguez, A. Emerging Pollutants in Water and Wastewater: UNESCO-Sida Project Case-Studies. Case-Study from Latin America: Mexico–Maya Area. In Proceedings of the World Water Week, Swedent, Stockholm, Sweden, 28 August–2 September 2016. [Google Scholar]
- Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Sosa-Hernández, J.E.; Parra-Arroyo, L.; López-Pacheco, I.Y.; Barcelò, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Antidepressant drugs as emerging contaminants: Occurrence in urban and non-urban waters and analytical methods for their detection. Sci. Total Environ. 2021, 757, 143722. [Google Scholar] [CrossRef]
- De Souza, L.P.; Sanches-Neto, F.O.; Mitsuyoshi Yuki, G., Jr.; Ramos, B.; Lastre-Acosta, A.M.; Carvalho-Silva, V.H.; Silva Costa, A.C. Photochemical environmental persistence of venlafaxine in an urban water reservoir: A combined experimental and computational investigation. Process Saf. Environ. Prot. 2022, 166, 478–490. [Google Scholar] [CrossRef]
- Bisesi, J.H.J.; Sweet, L.E.; van den Hurk, P.; Klaine, S.J. Effects of an antidepressant mixture on the brain serotonin and predation behavior of hybrid striped bass. Environ. Toxicol. Chem. 2016, 35, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32022D1307&qid=1658824912292 (accessed on 15 March 2025).
- O’Flynn, D.; Lawler, J.; Yusuf, A.; Parle-McDermott, A.; Harold, D.; Cloughlin, T.M.; Holland, L.; Regan, F.; White, B. A review of pharmaceutical occurrence and pathways in the aquatic environment in the context of a changing climate and the COVID-19 pandemic. Anal. Methods 2021, 13, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Pandis, P.K.; Kalogirou, P.K.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, G.; Argirusis, A.A. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. Chem. Eng. 2022, 6, 8. [Google Scholar] [CrossRef]
- Gonzaga, I.M.D.; Almeida, C.V.S.; Mascaro, L.H. A Critical Review of Photo-Based Advanced Oxidation Processes to Pharmaceutical Degradation. Catalysts 2023, 13, 221. [Google Scholar] [CrossRef]
- Lambropoulou, D.; Evgenidou, E.; Saliverou, V.; Kosma, C.; Konstantinou, I. Degradation of venlafaxine using TiO2/UV process: Kinetic studies, RSM optimization, identification of transformation products and toxicity evaluation. J. Hazard. Mater. 2017, 323, 513–526. [Google Scholar] [CrossRef]
- Konstas, P.S.; Kosma, C.; Konstantinou, I.; Albanis, T. Photocatalytic treatment of pharmaceuticals in real hospital wastewaters for effluent quality amelioration. Water 2019, 11, 2165. [Google Scholar] [CrossRef]
- Hellauer, K.; Mergel, D.; Ruhl, A.S.; Filter, J.; Hübner, U.; Jekel, M.; Drewes, J.E. Advancing sequential managed aquifer recharge technology (SMART) using different intermediate oxidation processes. Water 2019, 9, 221. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, P.-S.; Jiang, Y.-X.; Sun, L.; Sun, X.-H. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef]
- Mordačíková, E.; Vojs, M.; Grabicová, K.; Marton, M.; Michniak, P.; Reháček, V.; Bořik, A.; Grabic, R.; Bruncko, J.; Mackul’ak, T.; et al. Influence of boron doped diamond electrodes properties on the elimination of selected pharmaceuticals from wastewater. J. Electroanal. Chem. 2020, 862, 114007. [Google Scholar] [CrossRef]
- Voigt, M.; Dluziak, J.M.; Wellen, N.; Langerbein, V.; Jaeger, M. Comparison of photoinduced and electrochemically induced degradation of venlafaxine. Environ. Sci. Pollut. Res. 2024, 31, 13442–13454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chang, B.; Sun, X.; Luo, H.; Wang, W.; Li, C. Chloride-mediated electrochemical degradation of the venlafaxine antidepressant. Environ. Technol. Innov. 2022, 25, 102189. [Google Scholar] [CrossRef]
- Yu, B.; Han, Q.; Li, C.; Zhu, Y.; Jin, X.; Dai, Z. Influencing factors of venlafaxine degradation at boron-doped diamond anode. Arab. J. Chem. 2022, 15, 103463. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Guo, Z.; Zhang, W.; Li, H.; Huang, W. Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants. Sep. Purif. Technol. 2018, 212, 802–821. [Google Scholar] [CrossRef]
- Brosler, P.; Girão, A.; Silva, R.; Tedim, J.; Oliveira, F. In-house vs. commercial boron-doped diamond electrodes for electrochemical degradation of water pollutants: A critical review. Front. Mater. 2023, 10, 1020649. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Ryl, J. Structural and electrochemical heterogeneities of boron-doped diamond surfaces. Curr. Opin. Electrochem. 2022, 31, 100876. [Google Scholar] [CrossRef]
- Chaplin, B.; Wylie, I.; Zeng, H.; Carlisle, J.; Farrell, J. Characterization of the performance and failure mechanisms of boron-doped ultrananocrystalline diamond electrodes. J. Appl. Electrochem. 2011, 41, 1329–1340. [Google Scholar] [CrossRef]
- Lu, X.-R.; Ding, M.-H.; Zhang, C.; Tang, W.-Z. Comparative study on stability of boron doped diamond coated titanium and niobium electrodes. Diam. Relat. Mater. 2019, 93, 26–33. [Google Scholar] [CrossRef]
- Carbone, M.E.; Ciriello, R.; Guerrieri, A.; Salvi, A.M. XPS investigation on the chemical structure of a very thin, insulating, film synthesized on platinum by electropolymerization of o-aminophenol (oAP) in aqueous solution at neutral pH. Surf. Interface Anal. 2014, 46, 1081–1085. [Google Scholar] [CrossRef]
- Diaf, H.; Pereira, A.; Mélinon, P.; Blanchard, N.; Bourquard, F. Revisiting thin film of glassy carbon. Phys. Rev. Mater. 2020, 4, 066002. [Google Scholar] [CrossRef]
- Heard, D.M.; Lennox, J.J. Electrode Materials in Modern Organic Electrochemistry. Angew. Chem. Int. Ed. 2020, 59, 18866–18884. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.B.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Application, 2nd ed.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Xie, J.; Zhang, C.; Waite, T.D. Hydroxyl radicals in anodic oxidation systems: Generation, identification and quantification. Water Res. 2022, 217, 118425. [Google Scholar] [CrossRef]
- Chen, L.; Lei, C.; Li, Z.; Yang, B.; Zhang, X.; Lei, L. Electrochemical activation of sulfate by BDD anode in basic medium for efficient removal of organic pollutants. Chemosphere 2018, 210, 516–523. [Google Scholar] [CrossRef]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2017, 313, 128–133. [Google Scholar] [CrossRef]
- Rabaaoui, N.; Allagui, M.S. Anodic oxidation of salicylic acid on BDD electrode: Variable effects and mechanisms of degradation. J. Hazard. Mater. 2012, 243, 187–192. [Google Scholar] [CrossRef]
- Santoke, H.; Song, W.; Cooper, W.J.; Peake, B.M. Advanced oxidation treatment and photochemical fate of selected antidepressant pharmaceuticals in solutions of Suwannee River humic acid. J. Hazard. Mater. 2012, 217–218, 382–390. [Google Scholar] [CrossRef]
- Zizzamia, A.R.; Tesoro, C.; Bianco, G.; Bufo, S.A.; Ciriello, R.; Brienza, M.; Scrano, L.; Lelario, F. Efficient photooxidation processes for the removal of sildenafil from aqueous environments: A comparative study. Case Stud. Chem. Environ. Eng. 2024, 9, 100708. [Google Scholar] [CrossRef]
- La Farré, M.; García, M.J.; Tirapu, L.; Ginebreda, A.; Barceló, D. Wastewater toxicity screening of non-ionic surfactants by Toxalert® and Microtox® bioluminescence inhibition assays . Anal. Chim. Acta 2001, 427, 181–189. [Google Scholar] [CrossRef]
- Dominguez, J.R.; González, T.; Correia, S.E.; Núñez, M.M. Emerging Contaminants Decontamination of WWTP Effluents by BDD Anodic Oxidation: A Way towards Its Regeneration. Water 2023, 15, 1668. [Google Scholar] [CrossRef]
- Cembalo, G.; Ciriello, R.; Tesoro, C.; Guerrieri, A.; Bianco, G.; Lelario, F.; Acquavia, M.A.; Di Capua, A. An Amperometric Biosensor Based on a Bilayer of Electrodeposited Graphene Oxide and Co-Crosslinked Tyrosinase for L-Dopa Detection in Untreated Human Plasma. Molecules 2023, 28, 5239. [Google Scholar] [CrossRef]
- Madden, J.C.; Enoch, S.J.; Hewitt, M.; Cronin, M.T.D. Pharmaceuticals in the environment: Good practice in predicting acute ecotoxicological effects. Toxicol. Lett. 2009, 185, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.epa.gov/tsca-screening-tools/ecological-structure-activity-relationships-ecosar-predictive-model(accessed in November 2024).


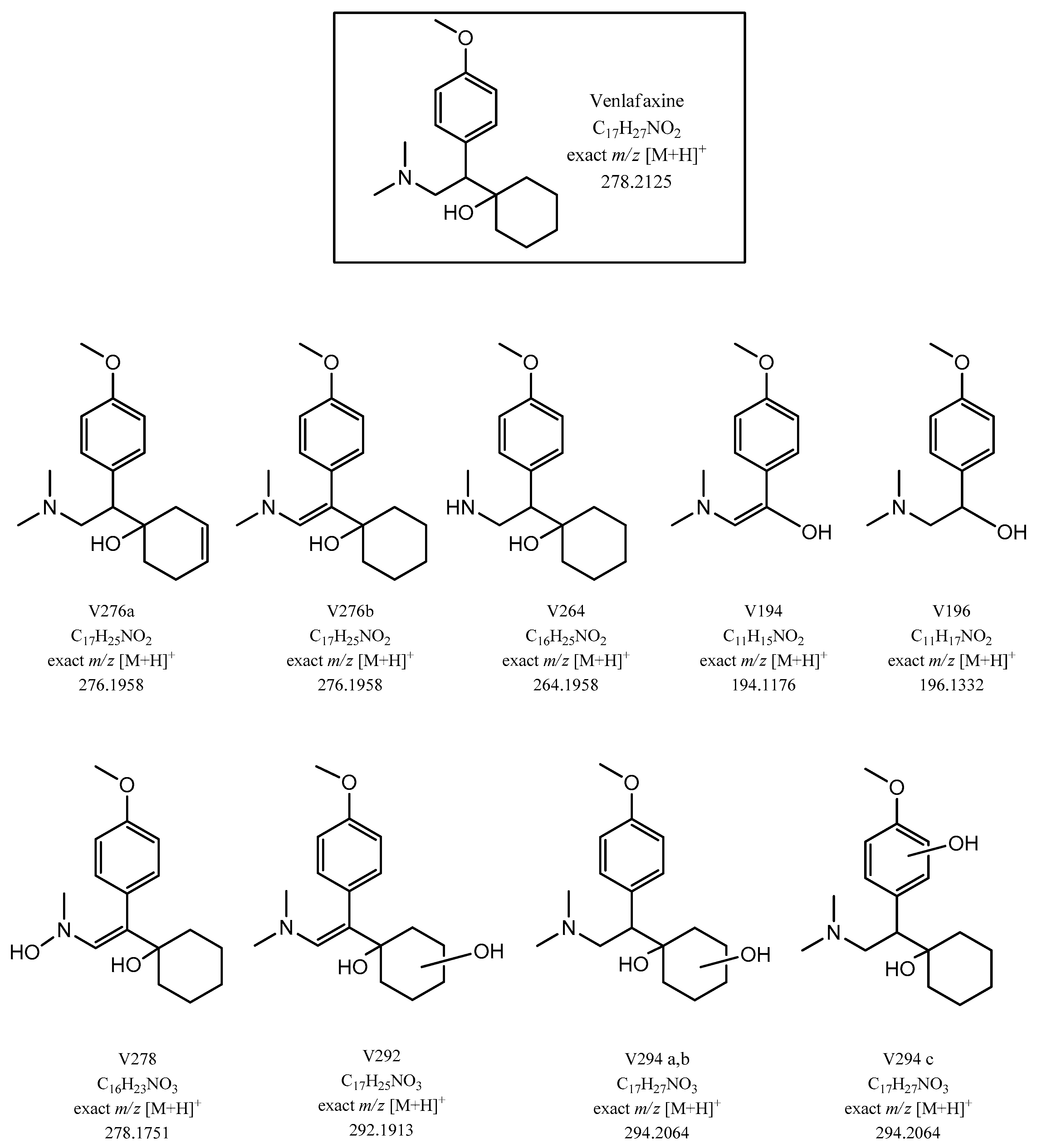

| N. | Compounds | Retention Time (min) | Molecular Formula | 1 [M+H]+ m/z Accurate | Error (ppm) | Main Fragments [M+H]+ m/z Nominal |
|---|---|---|---|---|---|---|
| 1 | VFX | 9.24 | C17H27O2N | 278.2111 | −1.42 | 260; 215;159; 147; 121; 58 |
| 2 | V276a | 8.80 | C17H25O2N | 276.1954 | −1.47 | 258; 246; 213; 178; 145; 135; 121 |
| 3 | V276b | 9.48 | C17H25O2N | 276.1956 | −0.89 | 258; 246; 215; 159; 147; 121 |
| 4 | V264 (N-desmethylVFX) | 8.65 | C16H25O2N | 264.1956 | −0.70 | 246; 215; 121 |
| 5 | V196 | 4.46 | C11H17O2N | 196.1333 | 0.28 | 178; 163; 147; 135 |
| 6 | V194 | 5.19 | C11H15O2N | 194.1176 | 0.28 | 149; 121; 58 |
| 7 | V292 | 8.28 | C17H25O3N | 292.1903 | −1.40 | 274; 256; 121 |
| 8 | V294a | 8.49 | C17H27O3N | 294.2061 | −1.09 | 276; 258; 231; 213; 121 |
| 9 | V294b | 8.83 | C17H27O3N | 294.2059 | −1.60 | 276; 258; 231; 213; 145 |
| 10 | V294c | 9.47 | C17H27O3N | 294.2060 | −1.30 | 276; 258; 233; 215; 178; 135; 121 |
| 11 | V278 | 8.22 | C16H23O3N | 278.1749 | −0.72 | 263; 245; 231; 178; 164; 121 |
| Electrode Material | Electrolyte Composition | pH | Current Density | Initial VFX Concentration | Degradation Efficiency | Identified Transformation Products | Toxicity Data | References |
|---|---|---|---|---|---|---|---|---|
| Pt | 0.1 M Na2SO4 | 9 | 25 mA/cm2 | 25 mg/L | 94% (7 h) | 10 by-products identified by LC-MS/MS and HRMS | in silico (ECOSAR) | This study |
| BDD | HCl | 3 | not reported | 20 mg/L | not detailed (deduced value of ~100% after around 3 h) | 4 by-products identified by LC-HRMS | in silico (QSAR) | [16] |
| BDD | 0.1 M Na2SO4 0.02 M NaCl | 6.5 | 100 mA/cm2 | 25 mg/L | 98% (5 min) | 16 by-products (electrolyte NaCl) + 6 by-products (electrolyte Na2SO4) identified by GC-MS | toxicity test on Chlorella Vulgaris | [17] |
| BDD | 0.1 M Na2SO4 0.02 M NaCl | 6.5 | 100 mA/cm2 | 25 mg/L | 98.5% (5 min) | not evaluated | not evaluated | [18] |
| BDD | waste water (specific electrical conductivity 1455 μS/cm) | 7.2 | 50 mA/cm2 | 1 mg/L | 99.9% (240 min) | 3 by-products (2 of which chlorinated) identified by LC-MS/MS and HRMS | not evaluated | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zizzamia, A.R.; Pasquariello, V.; Lelario, F.; Tesoro, C.; Ciriello, R. Electrochemical Degradation of Venlafaxine on Platinum Electrodes: Identification of Transformation Products by LC-MS/MS and In Silico Ecotoxicity Assessment. Molecules 2025, 30, 1881. https://doi.org/10.3390/molecules30091881
Zizzamia AR, Pasquariello V, Lelario F, Tesoro C, Ciriello R. Electrochemical Degradation of Venlafaxine on Platinum Electrodes: Identification of Transformation Products by LC-MS/MS and In Silico Ecotoxicity Assessment. Molecules. 2025; 30(9):1881. https://doi.org/10.3390/molecules30091881
Chicago/Turabian StyleZizzamia, Angelica R., Veronica Pasquariello, Filomena Lelario, Carmen Tesoro, and Rosanna Ciriello. 2025. "Electrochemical Degradation of Venlafaxine on Platinum Electrodes: Identification of Transformation Products by LC-MS/MS and In Silico Ecotoxicity Assessment" Molecules 30, no. 9: 1881. https://doi.org/10.3390/molecules30091881
APA StyleZizzamia, A. R., Pasquariello, V., Lelario, F., Tesoro, C., & Ciriello, R. (2025). Electrochemical Degradation of Venlafaxine on Platinum Electrodes: Identification of Transformation Products by LC-MS/MS and In Silico Ecotoxicity Assessment. Molecules, 30(9), 1881. https://doi.org/10.3390/molecules30091881








