Insights into the Involvement of TRPA1 Channels in the Neuro-Inflammatory Machinery of Trigeminal Neuralgia
Abstract
1. Introduction
2. Results
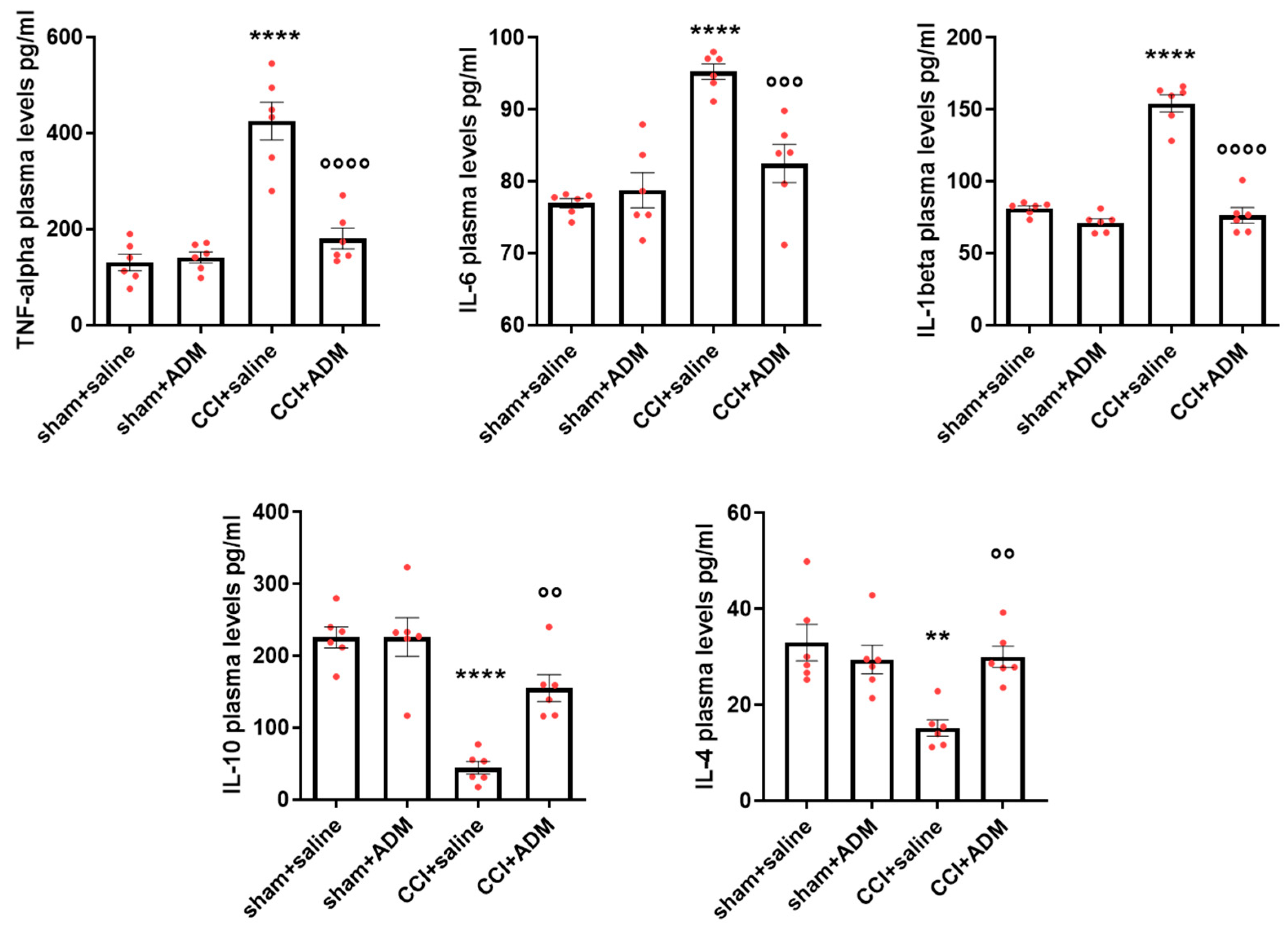
2.1. Cytokine Modulation
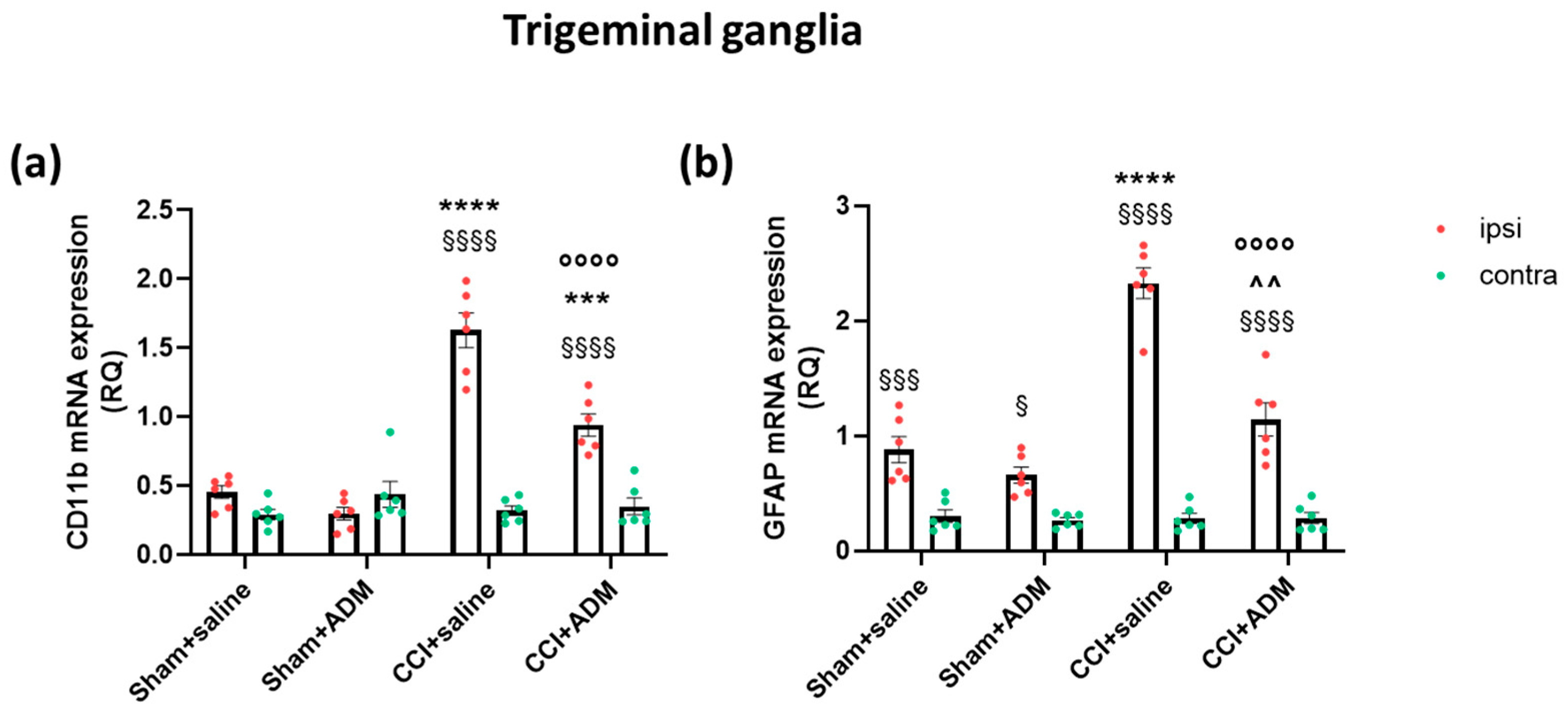
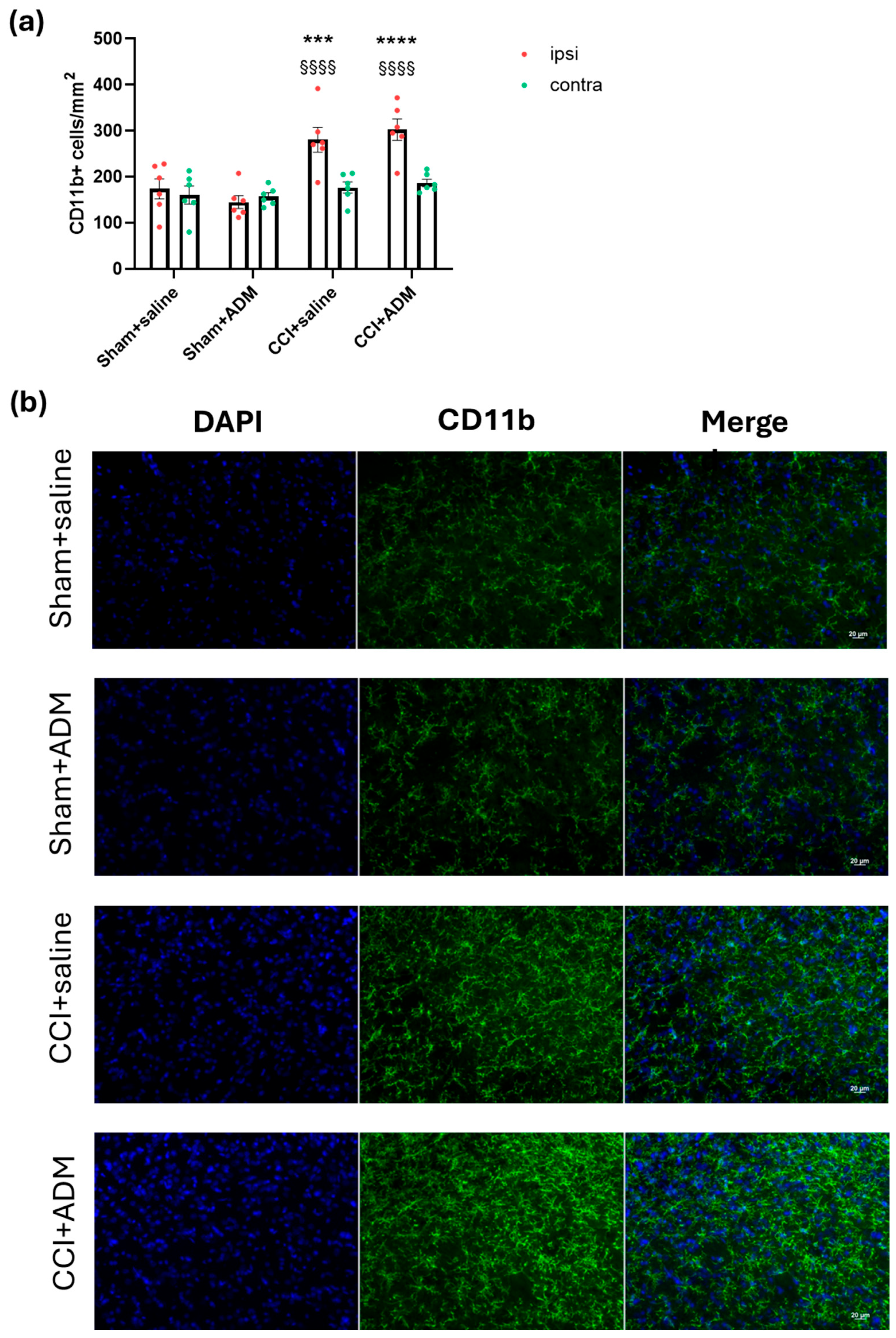
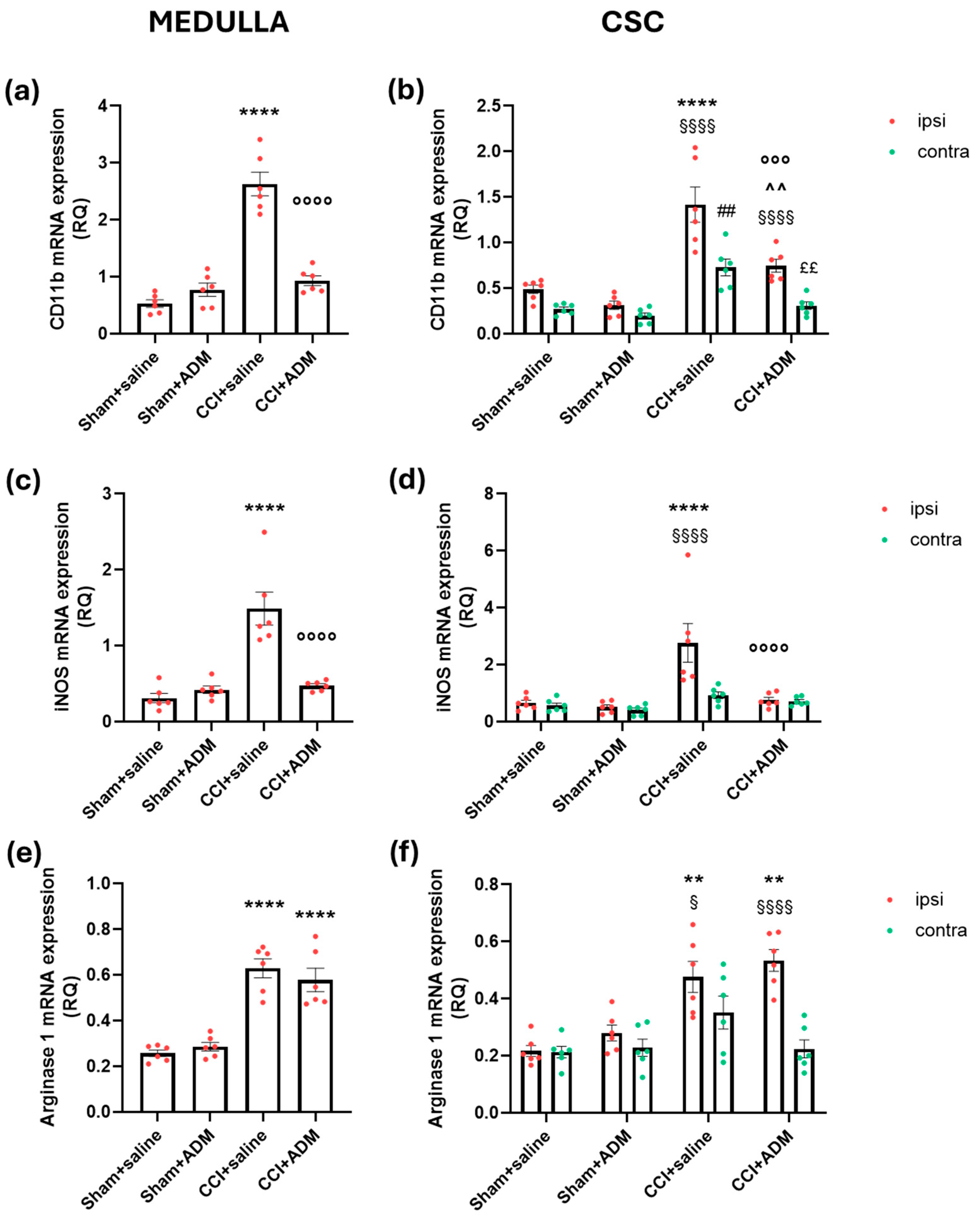
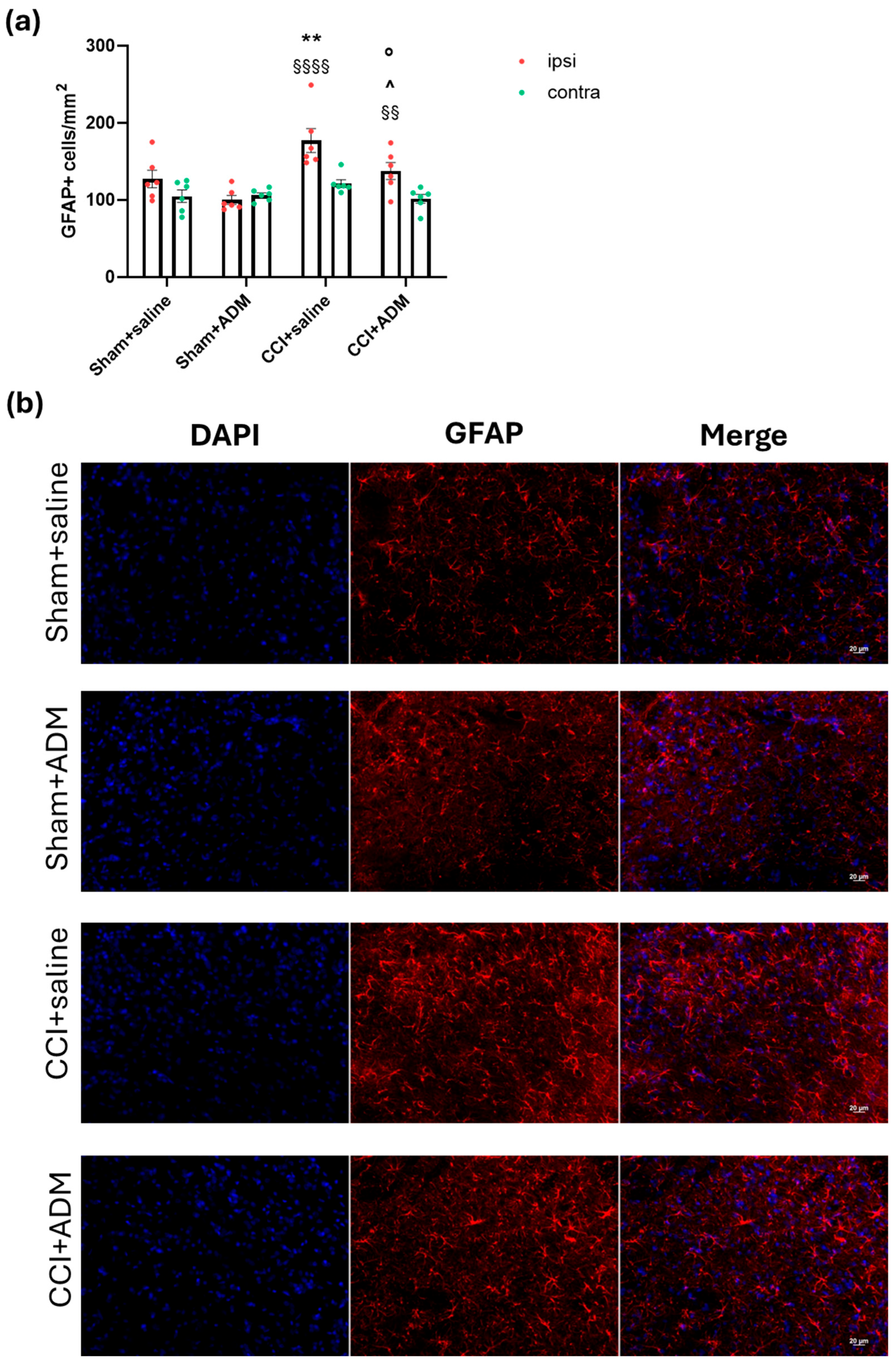
2.2. Glia Modulation
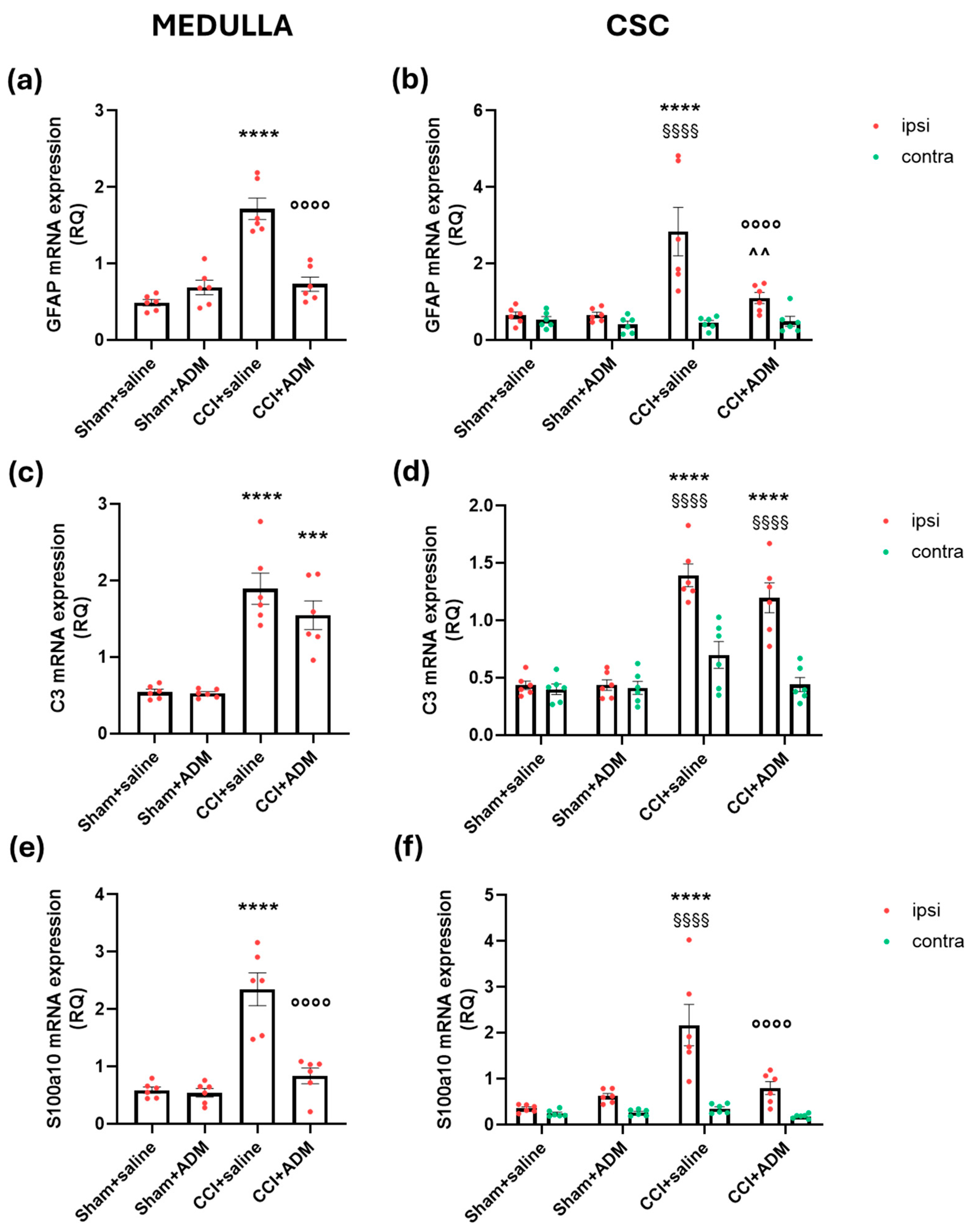
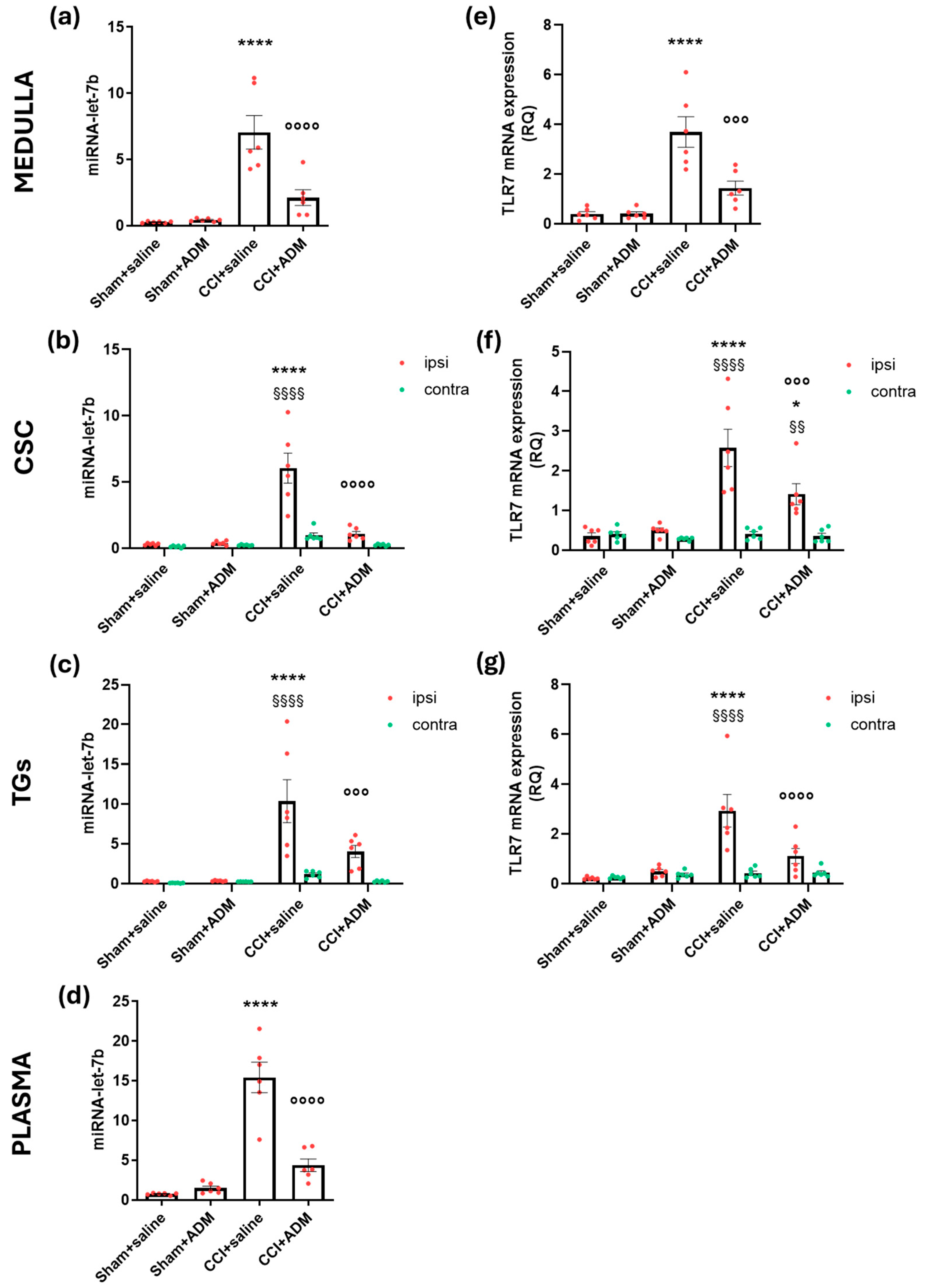
2.3. Changes in TLR-Related Pathways
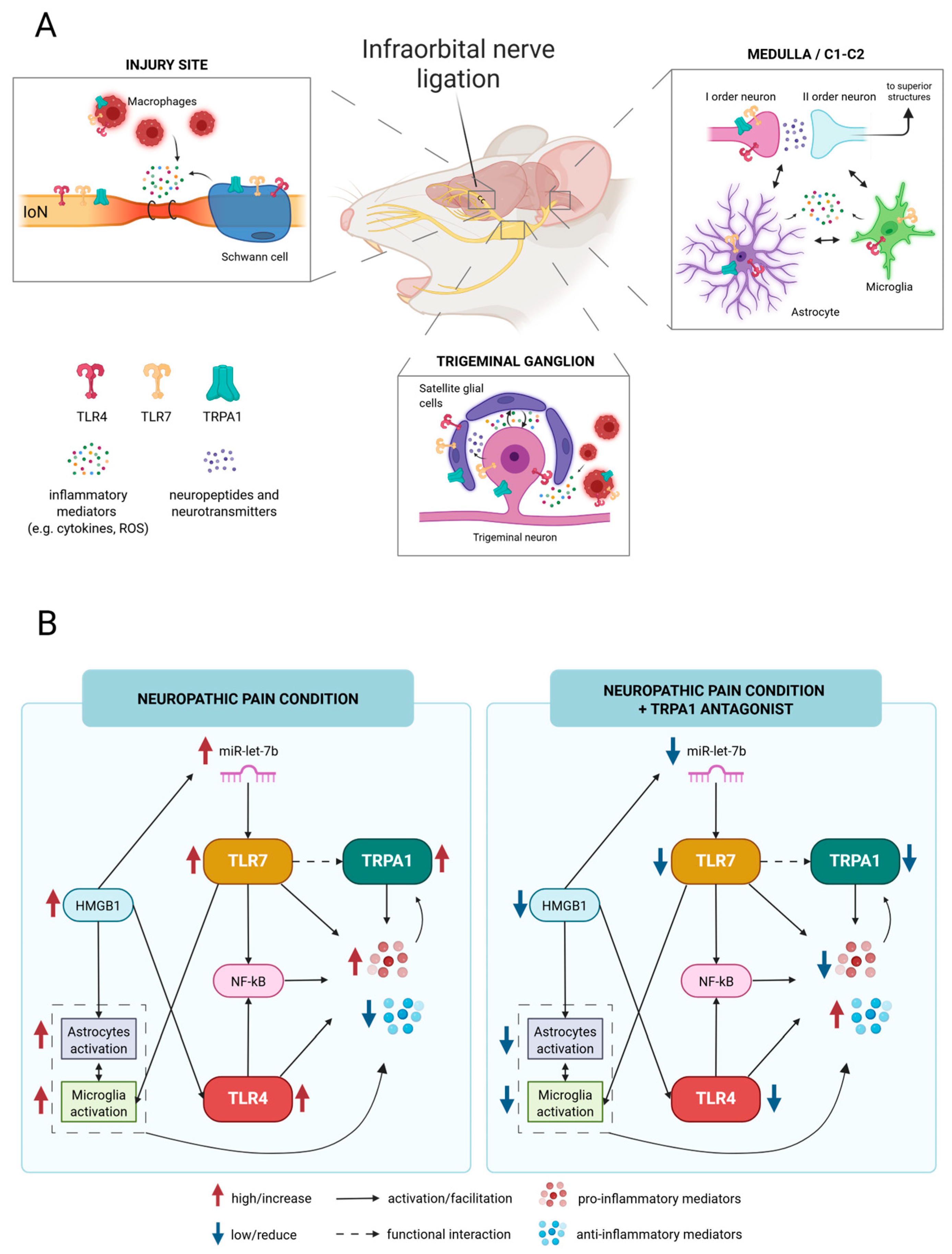
3. Discussion
3.1. TRPA1 as an Inflammatory Modulator Through the Crosstalk with the TLR-Related Pathways
3.2. Overview
3.3. Limitations of the Study
4. Materials and Methods
4.1. Experimental Plan
4.2. Immunofluorescence Staining
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Real-Time Polymerase Chain Reaction (rt-PCR)
4.5. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| C3 | complement 3 |
| CCI | chronic constriction injury |
| CD11b | cluster of differentiation molecule 11b |
| CSC | cervical spinal cord |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GFAP | glial fibrillary acidic protein |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| ELISA | enzyme-linked immunosorbent assay |
| HMGB1 | high-mobility group box 1 |
| NF-Kb | nuclear factor kappa B |
| rt-PCR | real-time polymerase chain reaction |
| S100a10 | S100 Calcium-Binding Protein A10 |
| TG | trigeminal ganglion |
| TLR | Toll-like receptors |
| TN | trigeminal neuralgia |
| TNC | trigeminal nucleus caudalis |
| TNF-alpha | tumor necrosis factor-alpha |
| TRPA1 | transient receptor potential ankyrin type-1 |
| U6 | RNU6-6P |
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd Edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Maarbjerg, S.; Di Stefano, G.; Bendtsen, L.; Cruccu, G. Trigeminal Neuralgia—Diagnosis and Treatment. Cephalalgia 2017, 37, 648–657. [Google Scholar] [CrossRef]
- Chen, Q.; Yi, D.I.; Perez, J.N.J.; Liu, M.; Chang, S.D.; Barad, M.J.; Lim, M.; Qian, X. The Molecular Basis and Pathophysiology of Trigeminal Neuralgia. Int. J. Mol. Sci. 2022, 23, 3604. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xie, M.E.; Jackson, C.M. Trigeminal Neuralgia: Current Approaches and Emerging Interventions. J. Pain Res. 2021, 14, 3437–3463. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chang, B.; Li, S. Relationship of Inflammation With Trigeminal Neuralgia. J. Craniofac. Surg. 2020, 31, e110–e113. [Google Scholar] [CrossRef]
- Ostertag, C.; Friedman, T.N.; Keough, M.B.; Kerr, B.J.; Sankar, T. Heightened Presence of Inflammatory Mediators in the Cerebrospinal Fluid of Patients with Trigeminal Neuralgia. Pain Rep. 2023, 8, e1117. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Zheng, W.; Wang, D.; Sun, C.; Dong, J.; Yu, W.; Du, Q. OTULIN’s Influence on Neuroinflammation and Pain Modulation in Trigeminal Neuralgia. CNS Neurosci. Ther. 2024, 30, e70006. [Google Scholar] [CrossRef]
- Shinoda, M.; Imamura, Y.; Hayashi, Y.; Noma, N.; Okada-Ogawa, A.; Hitomi, S.; Iwata, K. Orofacial Neuropathic Pain-Basic Research and Their Clinical Relevancies. Front. Mol. Neurosci. 2021, 14, 691396. [Google Scholar] [CrossRef]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef]
- Gualdani, R.; Ceruti, S.; Magni, G.; Merli, D.; Di Cesare Mannelli, L.; Francesconi, O.; Richichi, B.; la Marca, G.; Ghelardini, C.; Moncelli, M.R.; et al. Lipoic-Based TRPA1/TRPV1 Antagonist to Treat Orofacial Pain. ACS Chem. Neurosci. 2015, 6, 380–385. [Google Scholar] [CrossRef]
- Damasceno, M.B.M.V.; de Melo Júnior, J.d.M.A.; Santos, S.A.A.R.; Melo, L.T.M.; Leite, L.H.I.; Vieira-Neto, A.E.; Moreira, R.d.A.; Monteiro-Moreira, A.C.d.O.; Campos, A.R. Frutalin Reduces Acute and Neuropathic Nociceptive Behaviours in Rodent Models of Orofacial Pain. Chem. Biol. Interact. 2016, 256, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Demartini, C.; Greco, R.; Magni, G.; Zanaboni, A.M.; Riboldi, B.; Francavilla, M.; Nativi, C.; Ceruti, S.; Tassorelli, C. Modulation of Glia Activation by TRPA1 Antagonism in Preclinical Models of Migraine. Int. J. Mol. Sci. 2022, 23, 14085. [Google Scholar] [CrossRef]
- Marone, I.M.; De Logu, F.; Nassini, R.; De Carvalho Goncalves, M.; Benemei, S.; Ferreira, J.; Jain, P.; Li Puma, S.; Bunnett, N.W.; Geppetti, P.; et al. TRPA1/NOX in the Soma of Trigeminal Ganglion Neurons Mediates Migraine-Related Pain of Glyceryl Trinitrate in Mice. Brain 2018, 141, 2312–2328. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, G.; Benemei, S.; Materazzi, S.; De Logu, F.; De Siena, G.; Fusi, C.; Fortes Rossato, M.; Coppi, E.; Marone, I.M.; Ferreira, J.; et al. TRPA1 Mediates Trigeminal Neuropathic Pain in Mice Downstream of Monocytes/Macrophages and Oxidative Stress. Brain 2016, 139, 1361–1377. [Google Scholar] [CrossRef]
- Demartini, C.; Greco, R.; Zanaboni, A.M.; Francesconi, O.; Nativi, C.; Tassorelli, C.; Deseure, K. Antagonism of Transient Receptor Potential Ankyrin Type-1 Channels as a Potential Target for the Treatment of Trigeminal Neuropathic Pain: Study in an Animal Model. Int. J. Mol. Sci. 2018, 19, 3320. [Google Scholar] [CrossRef]
- Shin, S.M.; Itson-Zoske, B.; Cai, Y.; Qiu, C.; Pan, B.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Satellite Glial Cells in Sensory Ganglia Express Functional Transient Receptor Potential Ankyrin 1 That Is Sensitized in Neuropathic and Inflammatory Pain. Mol. Pain 2020, 16, 1744806920925425. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Hegron, A.; Landini, L.; Jensen, D.D.; Latorre, R.; Ding, J.; Marini, M.; Souza Monteiro de Araujo, D.; Ramírez-Garcia, P.; et al. Schwann Cell Endosome CGRP Signals Elicit Periorbital Mechanical Allodynia in Mice. Nat. Commun. 2022, 13, 646. [Google Scholar] [CrossRef]
- Shigetomi, E.; Jackson-Weaver, O.; Huckstepp, R.T.; O’Dell, T.J.; Khakh, B.S. TRPA1 Channels Are Regulators of Astrocyte Basal Calcium Levels and Long-Term Potentiation via Constitutive D-Serine Release. J. Neurosci. 2013, 33, 10143–10153. [Google Scholar] [CrossRef] [PubMed]
- Billeter, A.T.; Galbraith, N.; Walker, S.; Lawson, C.; Gardner, S.A.; Sarojini, H.; Galandiuk, S.; Polk, H.C. TRPA1 Mediates the Effects of Hypothermia on the Monocyte Inflammatory Response. Surgery 2015, 158, 646–654. [Google Scholar] [CrossRef]
- Yao, K.; Dou, B.; Zhang, Y.; Chen, Z.; Li, Y.; Fan, Z.; Ma, Y.; Du, S.; Wang, J.; Xu, Z.; et al. Inflammation-the Role of TRPA1 Channel. Front. Physiol. 2023, 14, 1093925. [Google Scholar] [CrossRef]
- Landini, L.; Souza Monteiro de Araujo, D.; Titiz, M.; Geppetti, P.; Nassini, R.; De Logu, F. TRPA1 Role in Inflammatory Disorders: What Is Known So Far? Int. J. Mol. Sci. 2022, 23, 4529. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Planz, O. The Role of Toll-like Receptors (TLRs) and Their Related Signaling Pathways in Viral Infection and Inflammation. Int. J. Mol. Sci. 2023, 24, 6701. [Google Scholar] [CrossRef]
- Michot, B.; Casey, S.M.; Lee, C.S.; Erdogan, O.; Basu, H.; Chiu, I.; Gibbs, J.L. Lipopolysaccharide-Induced TRPA1 Upregulation in Trigeminal Neurons Is Dependent on TLR4 and Vesicular Exocytosis. J. Neurosci. 2023, 43, 6731–6744. [Google Scholar] [CrossRef]
- Park, C.-K.; Xu, Z.-Z.; Berta, T.; Han, Q.; Chen, G.; Liu, X.-J.; Ji, R.-R. Extracellular MicroRNAs Activate Nociceptor Neurons to Elicit Pain via TLR7 and TRPA1. Neuron 2014, 82, 47–54. [Google Scholar] [CrossRef]
- Chen, O.; Jiang, C.; Berta, T.; Powell Gray, B.; Furutani, K.; Sullenger, B.A.; Ji, R.-R. MicroRNA Let-7b Enhances Spinal Cord Nociceptive Synaptic Transmission and Induces Acute and Persistent Pain through Neuronal and Microglial Signaling. Pain 2024, 165, 1824–1839. [Google Scholar] [CrossRef] [PubMed]
- Szabo-Pardi, T.A.; Barron, L.R.; Lenert, M.E.; Burton, M.D. Sensory Neuron TLR4 Mediates the Development of Nerve-Injury Induced Mechanical Hypersensitivity in Female Mice. Brain Behav. Immun. 2021, 97, 42–60. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Han, G.; Wu, S.; Du, S.; Zhang, Y.; Liu, W.; Jiang, B.; Zhang, L.; Xia, S.; Jia, S.; et al. Toll-like Receptor 7 Contributes to Neuropathic Pain by Activating NF-ΚB in Primary Sensory Neurons. Brain Behav. Immun. 2020, 87, 840–851. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Zhu, C.; Sun, S.; Wu, S.; Wang, L.; Wang, Y.; Ge, Z. Toll-like Receptors and Their Role in Neuropathic Pain and Migraine. Mol. Brain 2022, 15, 73. [Google Scholar] [CrossRef]
- Kochi, T.; Nakamura, Y.; Ma, S.; Hisaoka-Nakashima, K.; Wang, D.; Liu, K.; Wake, H.; Nishibori, M.; Irifune, M.; Morioka, N. Pretreatment with High Mobility Group Box-1 Monoclonal Antibody Prevents the Onset of Trigeminal Neuropathy in Mice with a Distal Infraorbital Nerve Chronic Constriction Injury. Molecules 2021, 26, 2035. [Google Scholar] [CrossRef]
- Di Stefano, G.; Yuan, J.-H.; Cruccu, G.; Waxman, S.G.; Dib-Hajj, S.D.; Truini, A. Familial Trigeminal Neuralgia—A Systematic Clinical Study with a Genomic Screen of the Neuronal Electrogenisome. Cephalalgia 2020, 40, 767–777. [Google Scholar] [CrossRef]
- Wu, C.; Xie, N.; Lian, Y.; Xu, H.; Chen, C.; Zheng, Y.; Chen, Y.; Zhang, H. Central Antinociceptive Activity of Peripherally Applied Botulinum Toxin Type A in Lab Rat Model of Trigeminal Neuralgia. Springerplus 2016, 5, 431. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Gonçalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann Cell TRPA1 Mediates Neuroinflammation That Sustains Macrophage-Dependent Neuropathic Pain in Mice. Nat. Commun. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed]
- Giacco, V.; Flower, G.; Artamonova, M.; Hunter, J.; Padilla Requerey, A.; Hamilton, N.B. Transient Receptor Potential Ankyrin-1 (TRPA1) Agonists Suppress Myelination and Induce Demyelination in Organotypic Cortical Slices. Glia 2023, 71, 1402–1413. [Google Scholar] [CrossRef]
- Lee, K.-I.; Lee, H.-T.; Lin, H.-C.; Tsay, H.-J.; Tsai, F.-C.; Shyue, S.-K.; Lee, T.-S. Role of Transient Receptor Potential Ankyrin 1 Channels in Alzheimer’s Disease. J. Neuroinflammation 2016, 13, 92. [Google Scholar] [CrossRef]
- Bosson, A.; Paumier, A.; Boisseau, S.; Jacquier-Sarlin, M.; Buisson, A.; Albrieux, M. TRPA1 Channels Promote Astrocytic Ca2+ Hyperactivity and Synaptic Dysfunction Mediated by Oligomeric Forms of Amyloid-β Peptide. Mol. Neurodegener. 2017, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Naert, R.; López-Requena, A.; Talavera, K. TRPA1 Expression and Pathophysiology in Immune Cells. Int. J. Mol. Sci. 2021, 22, 11460. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Hamel, K.A.; Morvan, M.G.; Yu, S.; Leff, J.; Guan, Z.; Braz, J.M.; Basbaum, A.I. Dorsal Root Ganglion Macrophages Contribute to Both the Initiation and Persistence of Neuropathic Pain. Nat. Commun. 2020, 11, 264. [Google Scholar] [CrossRef]
- Iwata, K.; Katagiri, A.; Shinoda, M. Neuron-Glia Interaction Is a Key Mechanism Underlying Persistent Orofacial Pain. J. Oral Sci. 2017, 59, 173–175. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, M.; Cui, S.; Liang, W.; Jia, Z.; Guo, F.; Ou, W.; Wu, Y.; Zhang, S. The Core of Maintaining Neuropathic Pain: Crosstalk between Glial Cells and Neurons (Neural Cell Crosstalk at Spinal Cord). Brain Behav. 2023, 13, e2868. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An Update on Reactive Astrocytes in Chronic Pain. J. Neuroinflamm. 2019, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zheng, Y.; Yu, J.; Ying, X.; Gu, X.; Tan, Q.; Tu, W.; Lou, X.; Yang, G.; Li, M.; et al. Electroacupuncture Alleviates Neuropathic Pain Caused by SNL by Promoting M2 Microglia Polarization through PD-L1. Int. Immunopharmacol. 2023, 123, 110764. [Google Scholar] [CrossRef]
- Lee, S.M.; Cho, Y.S.; Kim, T.H.; Jin, M.U.; Ahn, D.K.; Noguchi, K.; Bae, Y.C. An Ultrastructural Evidence for the Expression of Transient Receptor Potential Ankyrin 1 (TRPA1) in Astrocytes in the Rat Trigeminal Caudal Nucleus. J. Chem. Neuroanat. 2012, 45, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Meotti, F.C.; Figueiredo, C.P.; Manjavachi, M.; Calixto, J.B. The Transient Receptor Potential Ankyrin-1 Mediates Mechanical Hyperalgesia Induced by the Activation of B1 Receptor in Mice. Biochem. Pharmacol. 2017, 125, 75–83. [Google Scholar] [CrossRef]
- Dalenogare, D.P.; Souza Monteiro de Araújo, D.; Landini, L.; Titiz, M.; De Siena, G.; De Logu, F.; Geppetti, P.; Nassini, R.; Trevisan, G. Neuropathic-like Nociception and Spinal Cord Neuroinflammation Are Dependent on the TRPA1 Channel in Multiple Sclerosis Models in Mice. Cells 2023, 12, 1511. [Google Scholar] [CrossRef]
- Liu, T.; Gao, Y.-J.; Ji, R.-R. Emerging Role of Toll-like Receptors in the Control of Pain and Itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Pan, R.; Chen, M.; Wang, X.; Kong, E.; Yu, W.; Sun, Y.; Wu, F. Lentiviral-mediated Inducible Silencing of TLR4 Attenuates Neuropathic Pain in a Rat Model of Chronic Constriction Injury. Mol. Med. Rep. 2018, 18, 5545–5551. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Jiang, J.; Song, J.; Zhu, W.; Zhang, F.; Shao, M.; Xu, H.; Ma, X.; Lyu, F. TLR4 Promotes Microglial Pyroptosis via LncRNA-F630028O10Rik by Activating PI3K/AKT Pathway after Spinal Cord Injury. Cell Death Dis. 2020, 11, 693. [Google Scholar] [CrossRef]
- He, L.; Cao, J.; Jiang, B.-C.; Yang, J.-J.; Tao, Y.-X.; Ai, Y. C/EBPβ Participates in Nerve Trauma-Induced TLR7 Upregulation in Primary Sensory Neurons. Mol. Neurobiol. 2022, 59, 2629–2641. [Google Scholar] [CrossRef]
- Orlova, I.A.; Alexander, G.M.; Qureshi, R.A.; Sacan, A.; Graziano, A.; Barrett, J.E.; Schwartzman, R.J.; Ajit, S.K. MicroRNA Modulation in Complex Regional Pain Syndrome. J. Transl. Med. 2011, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- von Schack, D.; Agostino, M.J.; Murray, B.S.; Li, Y.; Reddy, P.S.; Chen, J.; Choe, S.E.; Strassle, B.W.; Li, C.; Bates, B.; et al. Dynamic Changes in the MicroRNA Expression Profile Reveal Multiple Regulatory Mechanisms in the Spinal Nerve Ligation Model of Neuropathic Pain. PLoS ONE 2011, 6, e17670. [Google Scholar] [CrossRef]
- Coleman, L.G.; Zou, J.; Crews, F.T. Microglial-Derived MiRNA Let-7 and HMGB1 Contribute to Ethanol-Induced Neurotoxicity via TLR7. J. Neuroinflamm. 2017, 14, 22. [Google Scholar] [CrossRef]
- Al-Ofi, E.A.; Al-Ghamdi, B.S. High-Mobility Group Box 1, an Endogenous Ligand of Toll-like Receptors 2 and 4, Induces Astroglial Inflammation via Nuclear Factor Kappa B Pathway. Folia Morphol. 2019, 78, 10–16. [Google Scholar] [CrossRef]
- Sekiguchi, F.; Domoto, R.; Nakashima, K.; Yamasoba, D.; Yamanishi, H.; Tsubota, M.; Wake, H.; Nishibori, M.; Kawabata, A. Paclitaxel-Induced HMGB1 Release from Macrophages and Its Implication for Peripheral Neuropathy in Mice: Evidence for a Neuroimmune Crosstalk. Neuropharmacology 2018, 141, 201–213. [Google Scholar] [CrossRef]
- Wu, C.-C.; Chang, C.-Y.; Tzeng, C.-Y.; Huang, J.-H.; Hung, C.-J.; Chen, W.-Y.; Liao, S.-L.; Kuan, Y.-H.; Chen, C.-J. Preventive Intrathecal Injection of Bupivacaine Alleviated Microglia Activation and Neuropathic Pain in a Rat Model of Chronic Constriction Injury. Int. J. Mol. Sci. 2022, 23, 7197. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q. Dexmedetomidine Relieved Neuropathic Pain and Inflammation Response Induced by CCI through HMGB1/TLR4/NF-ΚB Signal Pathway. Biol. Pharm. Bull. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Bai, L.; Li, S.; Li, L.; Dou, Z.; Huang, Y.; Li, Y.; Lv, Y. Dexmedetomidine Alleviates CCI-Induced Neuropathic Pain via Inhibiting HMGB1-Mediated Astrocyte Activation and the TLR4/NF-ΚB Signaling Pathway in Rats. Neurotox. Res. 2020, 38, 723–732. [Google Scholar] [CrossRef]
- Bautista, D.M.; Pellegrino, M.; Tsunozaki, M. TRPA1: A Gatekeeper for Inflammation. Annu. Rev. Physiol. 2013, 75, 181–200. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Steinhoff, M.; Dolly, J.O. TNFα Induces Co-Trafficking of TRPV1/TRPA1 in VAMP1-Containing Vesicles to the Plasmalemma via Munc18-1/Syntaxin1/SNAP-25 Mediated Fusion. Sci. Rep. 2016, 6, 21226. [Google Scholar] [CrossRef]
- Hatano, N.; Itoh, Y.; Suzuki, H.; Muraki, Y.; Hayashi, H.; Onozaki, K.; Wood, I.C.; Beech, D.J.; Muraki, K. Hypoxia-Inducible Factor-1α (HIF1α) Switches on Transient Receptor Potential Ankyrin Repeat 1 (TRPA1) Gene Expression via a Hypoxia Response Element-like Motif to Modulate Cytokine Release. J. Biol. Chem. 2012, 287, 31962–31972. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; de la Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO Cooperatively Regulate Vascular Tone by Activating a Neuroendocrine HNO–TRPA1–CGRP Signalling Pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef] [PubMed]
- Waite, P.M.E.; Ashwell, K.W.S. Trigeminal Sensory System. In The Human Nervous System; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1093–1124. [Google Scholar]
- Terayama, R.; Nagamatsu, N.; Ikeda, T.; Nakamura, T.; Rahman, O.I.F.; Sakoda, S.; Shiba, R.; Nishimori, T. Differential Expression of Fos Protein after Transection of the Rat Infraorbital Nerve in the Trigeminal Nucleus Caudalis. Brain Res. 1997, 768, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Panneton, W.M.; Pan, B.; Gan, Q. Somatotopy in the Medullary Dorsal Horn As a Basis for Orofacial Reflex Behavior. Front. Neurol. 2017, 8, 522. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Tassorelli, C. Antagonism of CGRP Receptor: Central and Peripheral Mechanisms and Mediators in an Animal Model of Chronic Migraine. Cells 2022, 11, 3092. [Google Scholar] [CrossRef]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | AACCTGCCAAGTATGATGAC | GGAGTTGCTGTTGAAGTCA |
| IL-10 | GCTCAGCACTGCTATGTTGC | CAGTAGATGCCGGGTGGTTC |
| IL-4 | CAACAAGGAACACCACGGAG | TTTCAGTGTTGTGAGCGTGG |
| GFAP | GATGTAGGAGTGGGTAGGGC | CCCTCTCCGCATCCATACTT |
| CD11b | GAAGCCTTGGCGTGTGATAG | GAGCAGTTTGTTCCCAAGGG |
| iNOS | TGGCCTCCCTCTGGAAAGA | GGTGGTCCATGATGGTCACAT |
| Arginase 1 | GACATCAACACTCCGCTGAC | TTGCCAATTCCCAGCTTGTC |
| C3 | TCAGGGTCCCAGCTACTAGT | TGTTCAGATGTCCAGTGGCT |
| S100a10 | TTCTATCACTAGTGGCGGGG | AAGGGTCCTGATCTGCTCAC |
| HMGB1 | GGAACGGTTTGCCTTGCTTA | ACTTGACAGAGGCAGGATCC |
| TLR4 | TATCGGTGGTCAGTGTGCTT | AGTCCTCATTCTGGCTCGAG |
| TLR7 | CTGTCCTTGAGTGGCCTACA | TCAGATACCCAGGCATGTCC |
| U6 | TGCGGGTGCTCGCTTCGGCAGC | CCAGTGCAGGGTCCGAGGT |
| miR-let-7b | CGGGGTGAGGTAGTAGGTTG | CAGGGAAGGCAGTAGGTTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demartini, C.; Greco, R.; Zanaboni, A.M.; Francavilla, M.; Facchetti, S.; Nativi, C.; Tassorelli, C. Insights into the Involvement of TRPA1 Channels in the Neuro-Inflammatory Machinery of Trigeminal Neuralgia. Molecules 2025, 30, 1884. https://doi.org/10.3390/molecules30091884
Demartini C, Greco R, Zanaboni AM, Francavilla M, Facchetti S, Nativi C, Tassorelli C. Insights into the Involvement of TRPA1 Channels in the Neuro-Inflammatory Machinery of Trigeminal Neuralgia. Molecules. 2025; 30(9):1884. https://doi.org/10.3390/molecules30091884
Chicago/Turabian StyleDemartini, Chiara, Rosaria Greco, Anna Maria Zanaboni, Miriam Francavilla, Sara Facchetti, Cristina Nativi, and Cristina Tassorelli. 2025. "Insights into the Involvement of TRPA1 Channels in the Neuro-Inflammatory Machinery of Trigeminal Neuralgia" Molecules 30, no. 9: 1884. https://doi.org/10.3390/molecules30091884
APA StyleDemartini, C., Greco, R., Zanaboni, A. M., Francavilla, M., Facchetti, S., Nativi, C., & Tassorelli, C. (2025). Insights into the Involvement of TRPA1 Channels in the Neuro-Inflammatory Machinery of Trigeminal Neuralgia. Molecules, 30(9), 1884. https://doi.org/10.3390/molecules30091884








