Perfluoropropionic Acid (CF3CF2C(O)OH): Three Conformations and Dimer Formation
Abstract
1. Introduction
2. Results and Discussion
2.1. Quatum Chemical Calculations
2.1.1. Monomer
2.1.2. Dimer
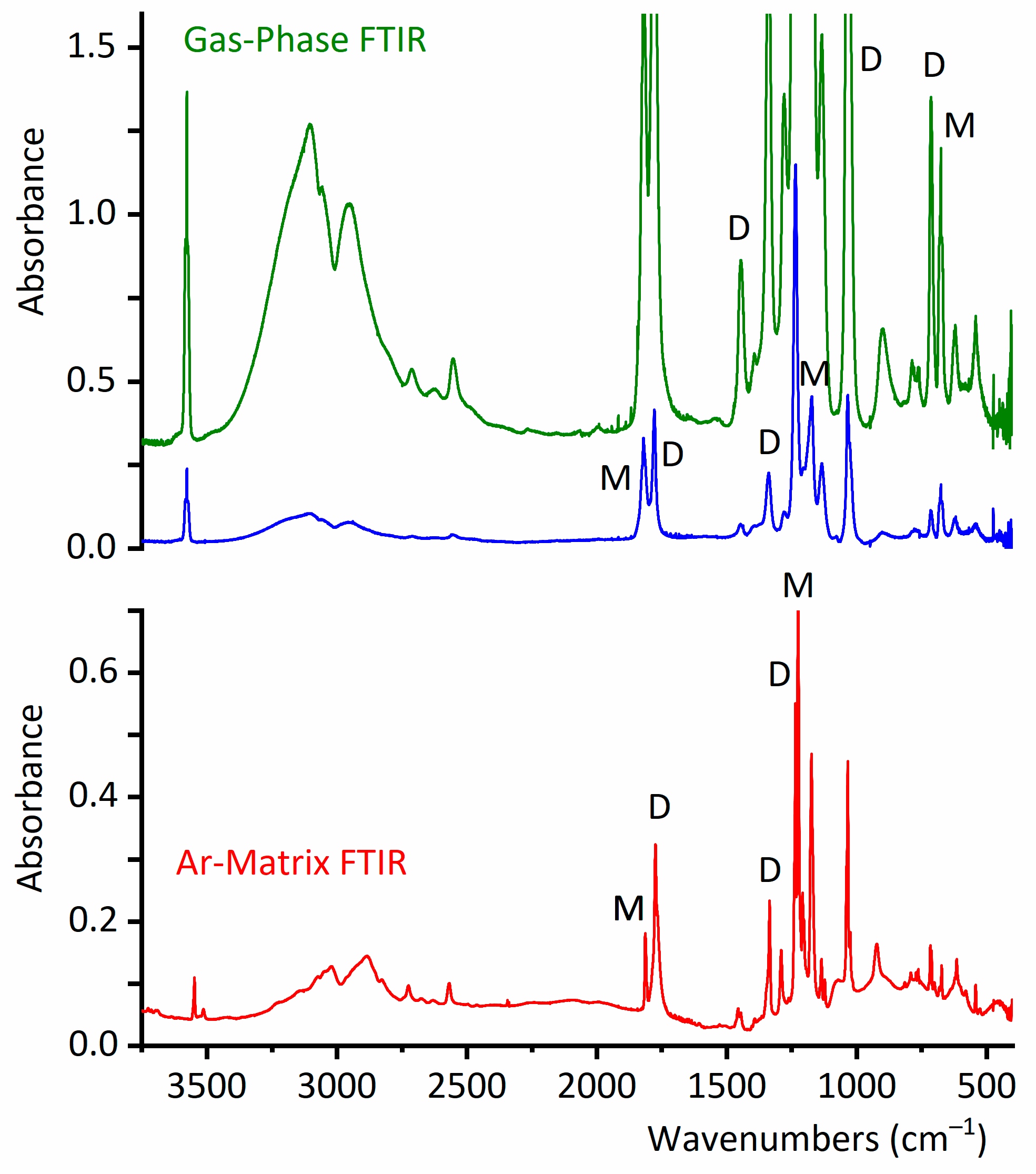
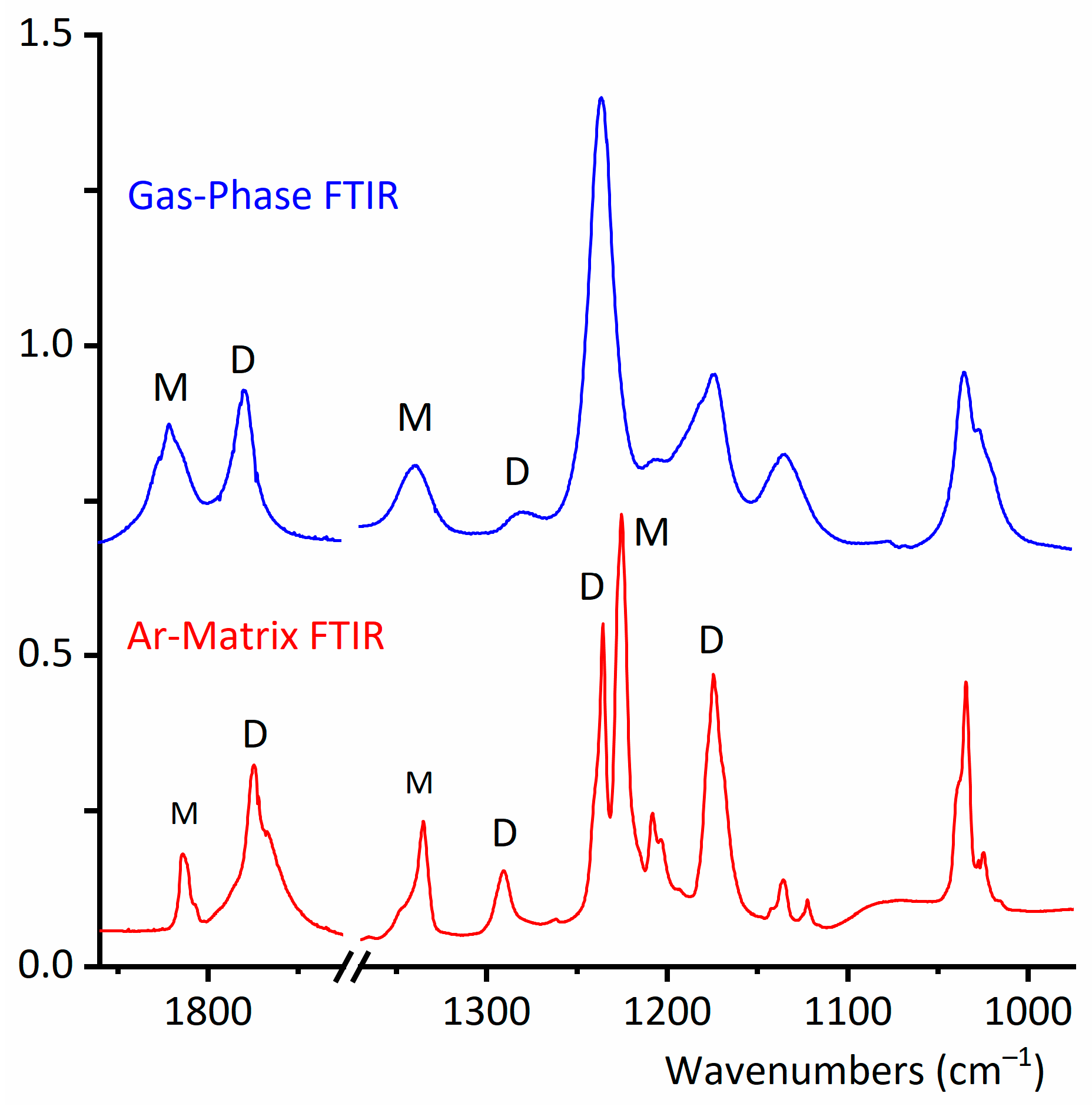
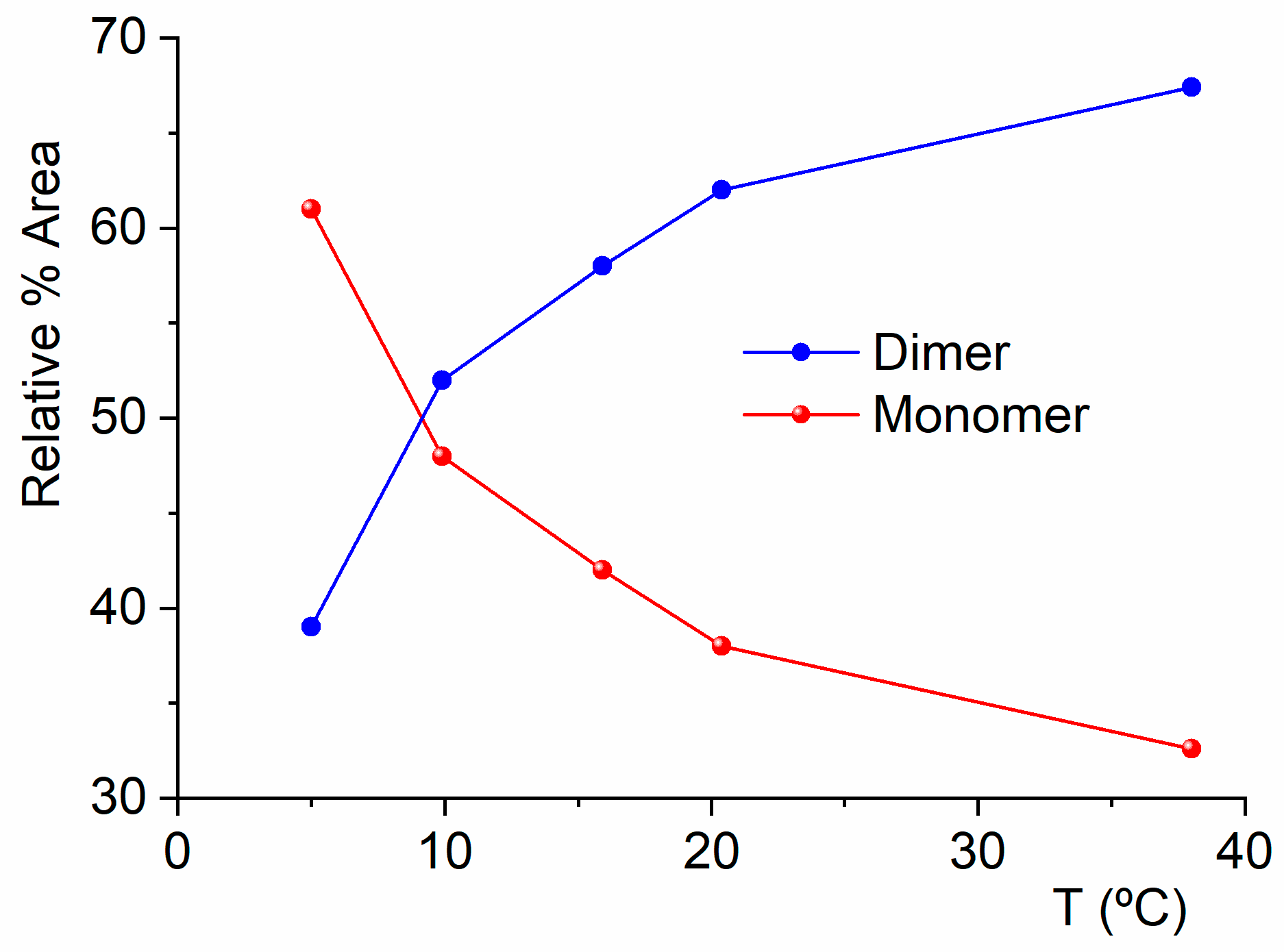
2.2. Experimental Results
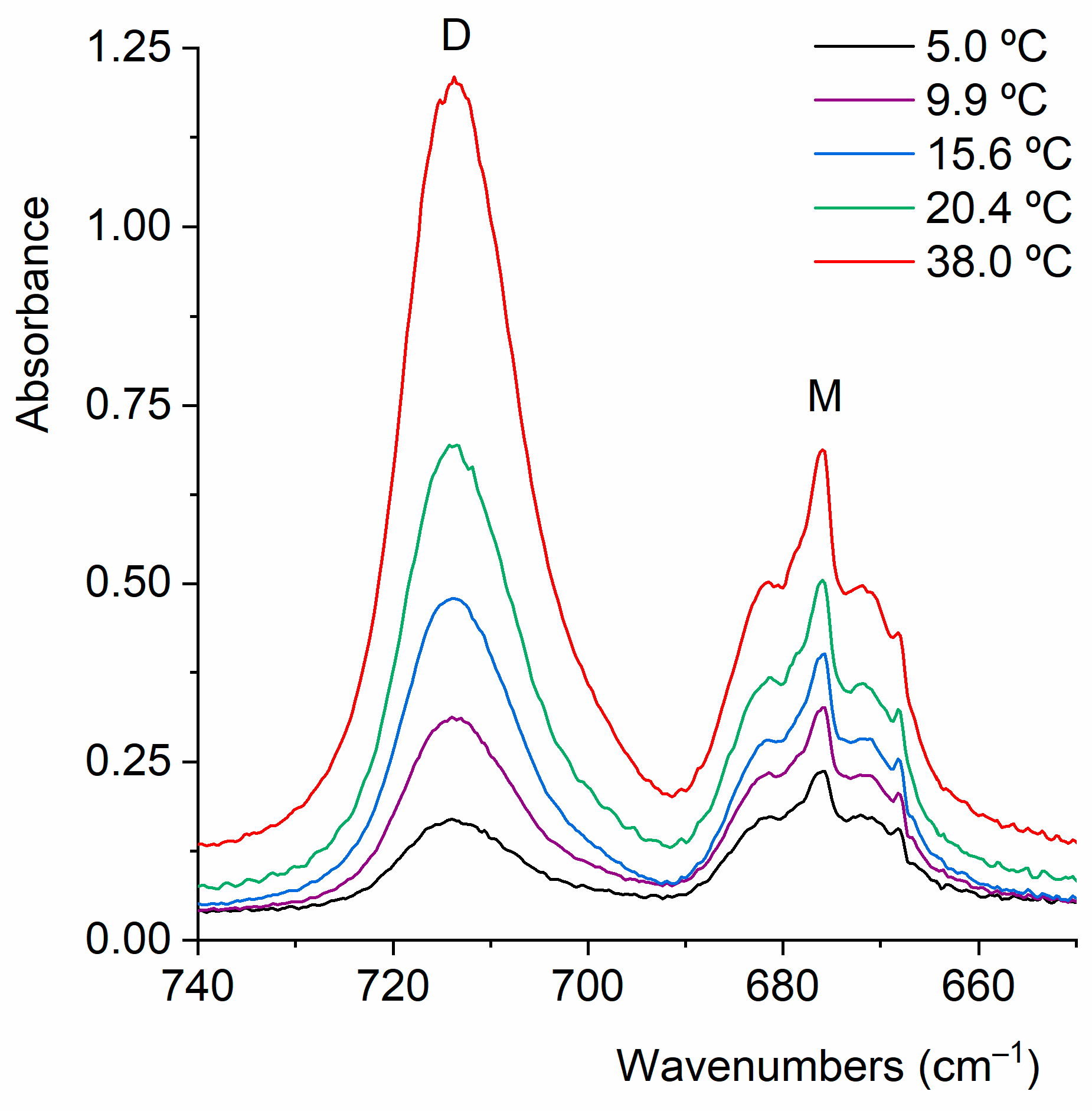
2.2.1. Gas-Phase FTIR Spectra
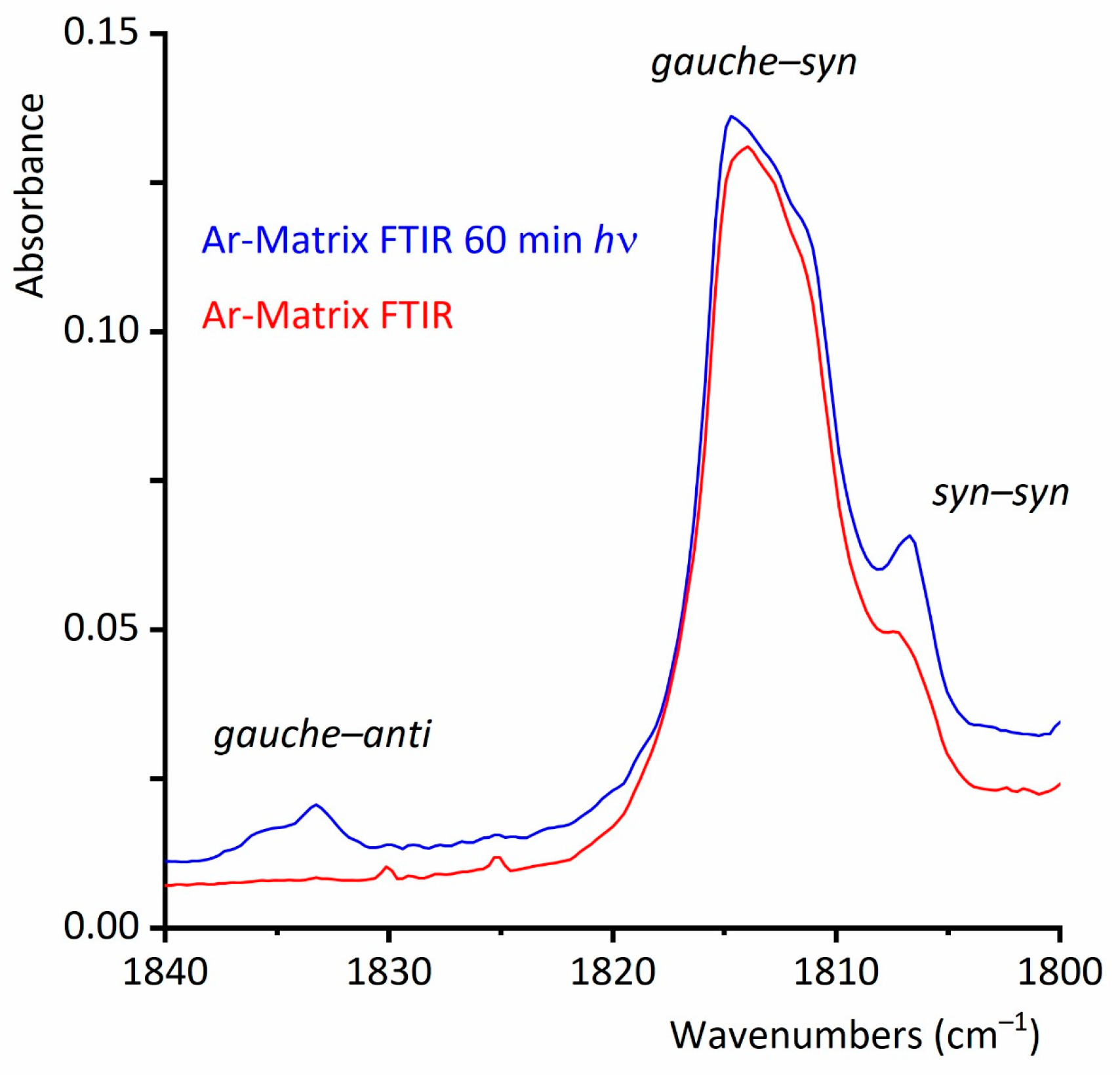
2.2.2. FTIR Spectrum of CF3CF2C(O)OH Isolated in Solid Argon
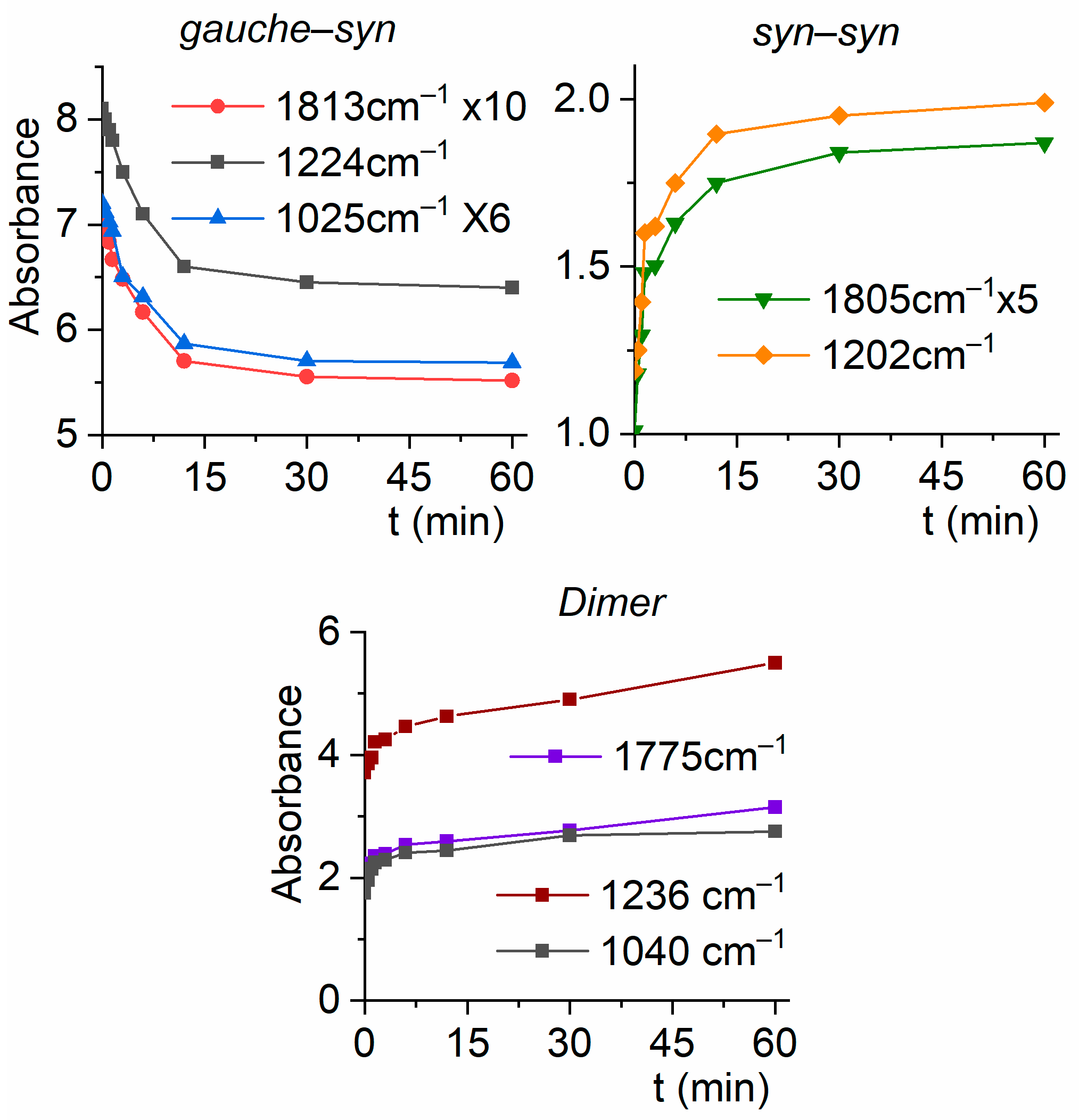
2.2.3. Matrix FTIR Spectra of CF3CF2C(O)OH After Broadband UV–Vis Irradiation
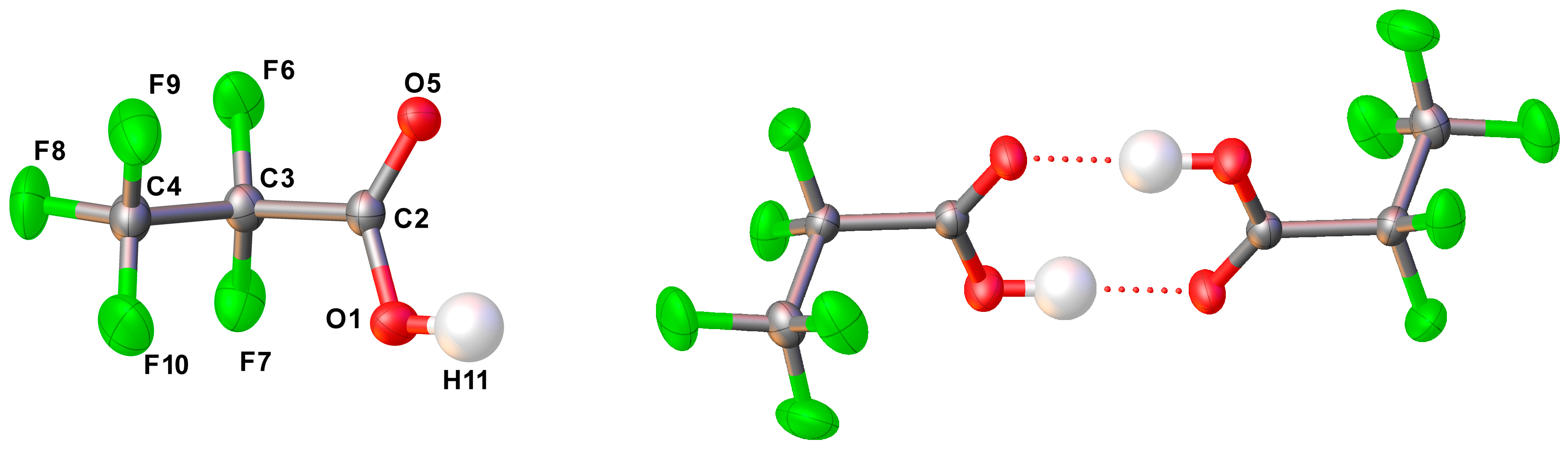
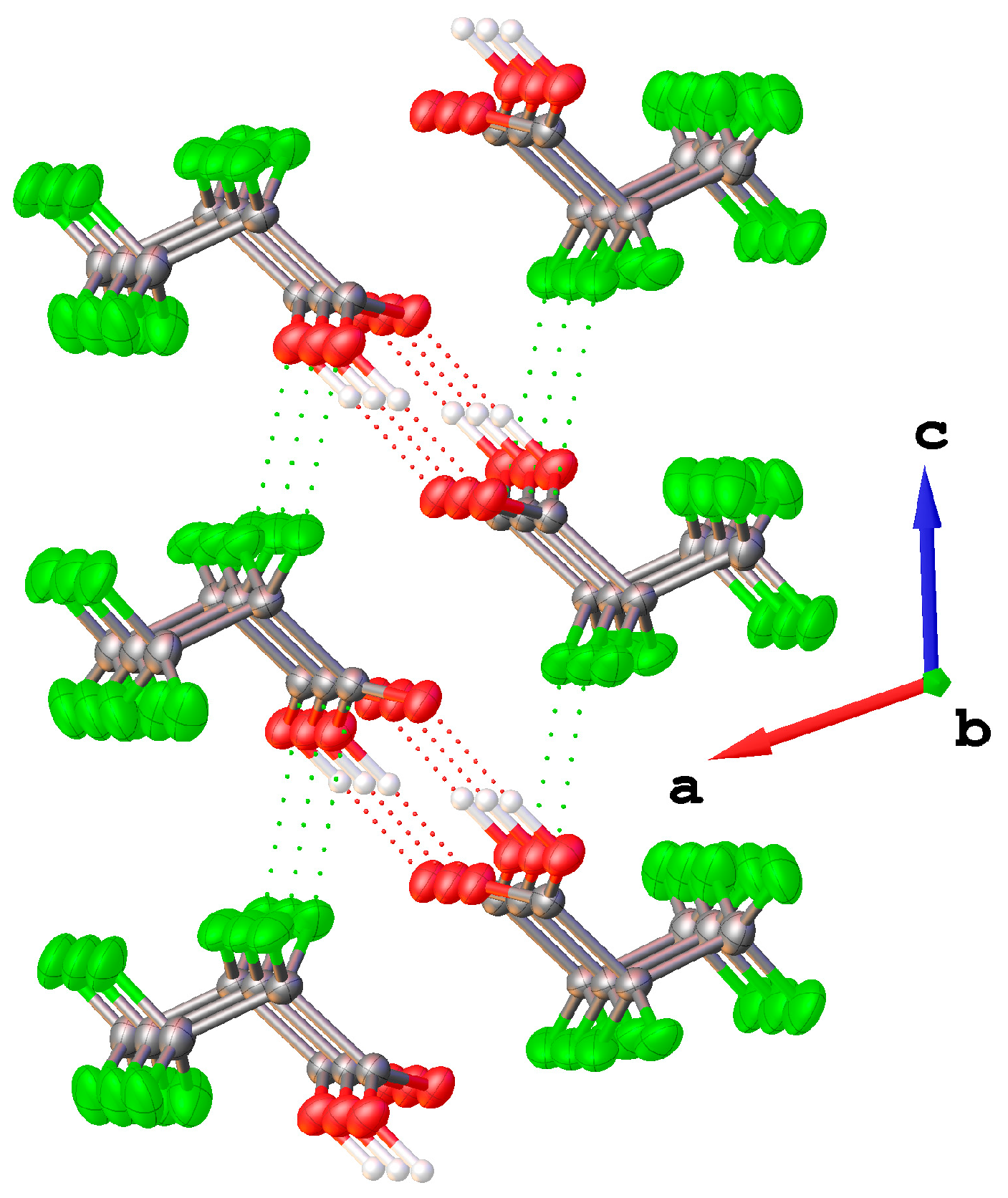
2.2.4. Solid State Structure
3. Materials and Methods
3.1. CF3CF2C(O)OH
3.2. Quantum Chemical Calculations
3.3. Infrared Spectroscopy
3.4. Matrix Isolation Experiments
3.5. X-Ray Diffraction Analysis
4. Conclusions
Dedication
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Susarla, N.; Ahmed, S. Estimating cost and energy demand in producing lithium hexafluorophosphate for Li-ion battery electrolyte. Ind. Eng. Chem. Res. 2019, 58, 3754–3766. [Google Scholar] [CrossRef]
- Moody, C.A.; Martin, J.W.; Kwan, W.C.; Muir, D.C.G.; Mabury, S.A. Monitoring perfluorinated surfactants in biota and surface water samples following an accidental release of fire-fighting foam into Etobicoke Creek. Environ. Sci. Technol. 2002, 36, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, H.; Li, F.; Zhou, Q. Occurrence and fate of perfluorinated acids in two wastewater treatment plants in Shanghai, China. Environ. Sci. Pollut. Res. 2015, 22, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Kannan, K.; Taniyasu, S.; Horii, Y.; Petrick, G.; Gamo, T. A global survey of perfluorinated acids in oceans. Mar. Pollut. Bull. 2005, 51, 658–668. [Google Scholar] [CrossRef]
- So, M.K.; Miyake, Y.; Yeung, W.Y.; Ho, Y.M.; Taniyasu, S.; Rostkowski, P.; Yamashita, N.; Zhou, B.S.; Shi, X.J.; Wang, J.X.; et al. Perfluorinated compounds in the Pearl River and Yangtze River of China. Chemosphere 2007, 68, 2085–2095. [Google Scholar] [CrossRef]
- Ellis, D.A.; Mabury, S.A.; Martin, J.W.; Muir, D.C.G. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 2001, 412, 321–324. [Google Scholar] [CrossRef]
- Tian, Y.; Yao, Y.; Chang, S.; Zhao, Z.; Zhao, Y.Y.; Yuan, X.J.; Wu, F.C.; Sun, H.W. Occurrence and phase distribution of neutral and ionizable per- and polyfluoroalkyl substances (PFASs) in the atmosphere and plant leaves around landfills: A case study in Tianjin, China. Environ. Sci. Technol. 2018, 52, 1301–1310. [Google Scholar] [CrossRef]
- Martin, J.W.; Mabury, S.A.; Solomon, K.R.; Muir, D.C.G. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss). Environ. Tox. Chem. 2003, 22, 189–195. [Google Scholar] [CrossRef]
- Martin, J.W.; Mabury, S.A.; Solomon, K.R.; Muir, D.C.G. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Tox. Chem. 2003, 22, 196–204. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Preliminary Risk Assessment of the Developmental Toxicity Associated with Exposure to Perfluorooctanoic Acid and Its Salts; Office of Pollution Prevention and Toxics, RiskAssessment Division: Washington, DC, USA, 2003.
- Berthiaume, J.; Wallace, K.B. Perfluorooctanoate, perflourooctanesulfonate, and N-ethyl perfluorooctanesulfonamido ethanol; peroxisome proliferation and mitochondrial biogenesis. Toxicol. Lett. 2002, 129, 23–32. [Google Scholar] [CrossRef]
- Upham, B.L.; Deocampo, N.D.; Wurl, B.; Trosko, J.E. Inhibition of gap junctional intercellular communication by perfluorinated fatty acids is dependent on the chain length of the fluorinated tail. Int. J. Cancer 1998, 78, 491–495. [Google Scholar] [CrossRef]
- Biegel, L.B.; Hurtt, M.E.; Frame, S.R.; Connor, J.O.; Cook, J.C. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol. Sci. 2001, 60, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.A.; Young, C.J.; Hurley, M.D.; Wallington, T.J.; Mabury, S.A. Atmospheric degradation of perfluoro-2-methyl-3-pentanone: Photolysis, hydrolysis and hydration. Environ. Sci. Technol. 2011, 45, 8030–8036. [Google Scholar] [CrossRef]
- Scott, B.F.; Spencer, C.; Mabury, S.A.; Muir, D.C.G. Environ. Poly and perfluorinated carboxylates in North American precipitation. Sci. Technol. 2006, 40, 7167–7174. [Google Scholar] [CrossRef]
- Young, C.J.; Hurley, M.l.D.; Wallington, T.J.; Mabury, S.A. Atmospheric chemistry of CF3CF2H and CF3CF2CF2CF2H: Kinetics and products of gas-phase reactions with Cl atoms and OH radicals, infrared spectra, and formation of perfluorocarboxylic acids. Chem. Phys. Lett. 2009, 473, 251–256. [Google Scholar] [CrossRef]
- Grubbs, G.S., II; Obenchain, D.A.; Frank, D.S.; Novick, S.E.; Cooke, S.A.; Serrato, A., III; Lin, W. A study of the monohydrate and dihydrate complexes of perfluoropropionic acid using Chirped-Pulse Fourier Transform Microwave (CP-FTMW) spectroscopy. J. Phys. Chem. A 2015, 119, 10475–10480. [Google Scholar] [CrossRef]
- Crowder, G.A. Infrared and Raman spectra of pentafluoropropionic acid. J. Fluorine Chem. 1972, 1, 385–389. [Google Scholar] [CrossRef]
- Kagarise, R.E. Relation between the electronegativities of adjacent substitutents and the stretching frequency of the carbonyl group. J. Am. Chem. Soc. 1955, 77, 1377–1379. [Google Scholar] [CrossRef]
- Statz, G.; Lippert, E. Far infrared spectroscopic studies on carboxylic acid solutions. Ber. Bunsen-Ges. 1967, 71, 673–679. [Google Scholar]
- Badawi, H.M.; Al-Khaldi, M.A.A.; Al-Sunaidi, Z.H.A.; Al-Abbad, S.S.A. Conformational properties and vibrational analyses of monomeric pentafluoropropionic acid CF3CF2COOH and pentafluoropropionamide CF3CF2CONH2. Can. J. Anal. Sci. Spectrosc. 2007, 52, 252–269. [Google Scholar]
- Grubbs, G.S., II; Serrato, A., III; Obenchain, D.A.; Cooke, S.A.; Novick, S.E.; Lin, W. The rotational spectrum of perfluoropropionic acid. J. Mol. Spectrosc. 2012, 275, 1–4. [Google Scholar] [CrossRef]
- Husted, D.R.; Ahlbrecht, A.H. The chemistry of the perfluoro acids and their derivatives. V. Perfluoropropionic acid. J. Am. Chem. Soc. 1953, 75, 1605–1608. [Google Scholar] [CrossRef]
- Collins, M.F.; Haywood, B.C. Vibration spectra of carboxylic acids by neutron spectroscopy. J. Chem. Phys. 1970, 52, 5740–5745. [Google Scholar] [CrossRef]
- Rontu, N.; Vaida, V. Vibrational spectroscopy of perfluorocarboxylic acids from the infrared to the visible regions. J. Phys. Chem. B 2008, 112, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Rontu, N.; Vaida, V. Vibrational spectroscopy of perfluoropropionic acid in the region between 1000 and 11000 cm−1. J. Mol. Spectros. 2006, 237, 19–26. [Google Scholar] [CrossRef]
- Cho, S.; Remucal, C.K.; Wei, H. Common and distinctive Raman spectral features for the identification and differentiation of per- and polyfluoroalkyl substances. ACS EST Water 2025, 5, 300–309. [Google Scholar] [CrossRef]
- Lee, E.; Featherstone, J.; Martens, J.; McMahon, T.B.; Hopkins, W.S. Fluorinated propionic acids unmasked: Puzzling fragmentation phenomena of the deprotonated species. J. Phys. Chem. Lett. 2024, 15, 3029–3036. [Google Scholar] [CrossRef]
- Berrueta Martínez, Y.; Bava, Y.B.; Cavasso Filho, R.L.; Erben, M.F.; Romano, R.M.; Della Védova, C.O. Valence and inner electronic excitation, ionization, and fragmentation of perfluoropropionic acid. J. Phys. Chem. A 2018, 122, 9842–9850. [Google Scholar] [CrossRef]
- Litvanová, K.; Klemetsrud, B.; Xiao, F.; Kubátová, A. Investigation of real-time gaseous thermal decomposition products of representative per- and polyfluoroalkyl substances (PFAS). J. Am. Soc. Mass Spectrom. 2025, 36, 108–118. [Google Scholar] [CrossRef]
- Wiens, J.P.; Miller, T.H.; Ard, S.G.; Viggiano, A.A.; Shuman, N.S. Elementary reactions leading to perfluoroalkyl substance degradation in an Ar+/e− Plasma. J. Phys. Chem. A 2022, 126, 9076–9086. [Google Scholar] [CrossRef]
- Wang, W.; Song, M.; Abusallout, I.; Hanigan, D. Thermal decomposition of two gaseous perfluorocarboxylic acids: Products and mechanisms. Environ. Sci. Technol. 2023, 57, 6179–6187. [Google Scholar] [CrossRef]
- Melin, T.R.L.; Harell, P.; Ali, B.; Loganathan, N.; Wilson, A.K. Thermochemistry of per- and polyfluoroalkyl substances. J. Comput. Chem. 2023, 44, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.S., Jr.; Mota, C.; Chaudhuri, P. Perfluoropropionic acid-driven nucleation of atmospheric molecules under ambient conditions. J. Phys. Chem. A 2022, 126, 8449–8458. [Google Scholar] [CrossRef] [PubMed]
- Hercules, D.A.; Parrish, C.A.; Sayler, T.S.; Tice, K.T.; Williams, S.M.; Lowery, L.E.; Brady, M.E.; Coward, R.B.; Murphy, J.A.; Hey, T.A.; et al. Preparation of tetrafluoroethylene from the pyrolysis of pentafluoropropionate salts. J. Fluor. Chem. 2017, 196, 107–116. [Google Scholar] [CrossRef]
- Sablinskas, V.; Pucetaite, M.; Ceponkus, J.; Kimtys, L. Structure of propanoic acid dimers as studied by means of MIR and FIR spectroscopy. J. Mol. Struct. 2010, 976, 263–269. [Google Scholar]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Rev C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules (International Series of Monographs on Chemistry); Oxford University Press: New York, NJ, USA, 1989. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Foster, J.P.; Weinhold, F. Natural hybrid orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Weinhold, F. NBO 5.0; Theoretical Chemistry Institute, University of Wisconsin-Madison: Madison, WI, USA, 2004. [Google Scholar]
- Almond, M.J.; Downs, A.J. Spectroscopy of matrix isolated species. Adv. Spectrosc. 1989, 17, 1–505. [Google Scholar] [CrossRef]
- Dunkin, I.R. Matrix-Isolation Techniques: A Practical Approach; Oxford University Press: New York, NJ, USA, 1998. [Google Scholar]
- Perutz, R.N.; Turner, J.J. Pulsed matrix isolation. A comparative study. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1973, 69, 452–461. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. 2015, A71, 59–75. [Google Scholar]
- Kleemiss, F.; Dolomanov, O.V.; Bodensteiner, M.; Peyerimhoff, N.; Midgley, L.; Bourhis, L.J.; Genoni, A.; Malaspina, L.A.; Jayatilaka, D.; Spencer, J.L.; et al. Accurate crystal structures and chemical properties from NoSpherA2. Chem. Sci. 2021, 12, 1675–1692. [Google Scholar] [CrossRef] [PubMed]
- Hogg, P.J. Disulfide bonds as switches for protein function. Trends Biochem. Sci. 2003, 28, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Hilal, R.; El-Aaser, A.M. A comparative quantum chemical study of methyl acetate and S-methyl thioacetate Toward an understanding of the biochemical reactivity of esters of coenzyme A. Biophys. Chem. 1985, 2, 145–150. [Google Scholar] [CrossRef]
- Beck, I.T.; Mitchell, E.C.; Webb Hill, A.; Turney, J.M.; Rotavera, B.; Schaefer, H.F., III. Evaluating the importance of conformers for understanding the vacuum-ultraviolet spectra of oxiranes: Experiment and theory. J. Phys. Chem. A 2024, 128, 10906–10920. [Google Scholar] [CrossRef]
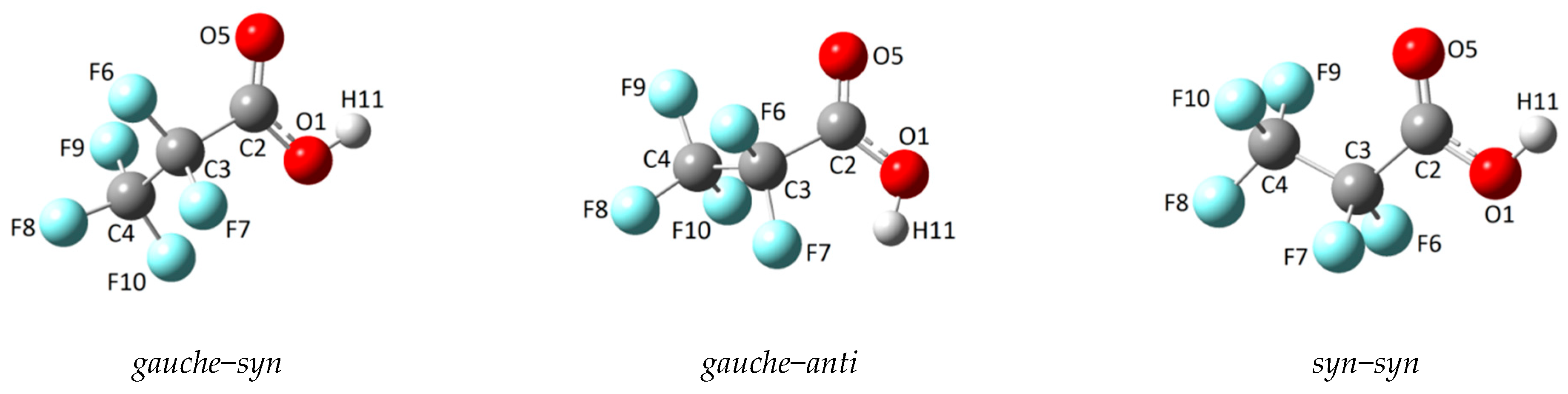

| Conformer | φ(C−C−C=O) | φ(O−C−O−H) | ΔE (kcal/mol) | ΔG (kcal/mol) | χ (%) |
|---|---|---|---|---|---|
| gauche–syn | 101.2 | −0.3 | 0.00 | 0.00 | 85.1 |
| syn–syn | −0.1 | 0.0 | 0.43 | 0.62 | 14.7 |
| gauche–anti | 82.3 | 176.6 | 3.37 | 3.64 | 0.2 |
| Parameter | X-Ray Diffraction | MP2/6-311+G(D) |
|---|---|---|
| r(F6−C3) | 1.338(2) | 1.344 |
| r(F7−C3) | 1.336(2) | 1.351 |
| r(F9−C4) | 1.319(2) | 1.336 |
| r(F8−C4) | 1.317(2) | 1.330 |
| r(F10−C4) | 1.304(2) | 1.332 |
| r(O5=C2) | 1.215(2) | 1.203 |
| r(O1−H11) | 0.97(3) | 0.971 |
| r(O1−C2) | 1.286(2) | 1.337 |
| r(C2−C3) | 1.545(2) | 1.542 |
| r(C4−C3) | 1.542(2) | 1.542 |
| α(H11−O1−C2) | 112.6(17) | 108.3 |
| α(O5−C2−O1) | 127.9(2) | 126.9 |
| α(O5−C2−C3) | 120.0(2) | 123.0 |
| α(O1−C2−C3) | 112.0(2) | 110.0 |
| α(F9−C4−F8) | 108.9(2) | 109.0 |
| α(F9−C4−F10) | 108.8(2) | 108.8 |
| α(F9−C4−C3) | 109.4(2) | 109.4 |
| α(F8−C4−F10) | 109.1(2) | 108.9 |
| α(F8−C4−C3) | 110.1(2) | 110.1 |
| α(F10−C4−C3) | 110.6(2) | 110.5 |
| α(F6−C3−C7) | 108.7(2) | 108.9 |
| α(F6−C3−C2) | 109.0(2) | 108.8 |
| α(F6−C3−C4) | 108.1(2) | 107.7 |
| α(F7−C3−C2) | 110.5(2) | 110.6 |
| α(F7−C3−C4) | 107.9(2) | 108.0 |
| α(C2−C3−C4) | 112.6(2) | 112.8 |
| τ(H11−O1−C2−O5) | −0.5(18) | −0.3 |
| τ(H11−O1−C2−C3) | −178.2(17) | 178.8 |
| τ(O5−C2−C3−F6) | 21.2(2) | −18.2 |
| τ(O5−C2−C3−F7) | 140.6(2) | −137.8 |
| τ(O5−C2−C3−C4) | −98.7(2) | 101.2 |
| τ(O1−C2−C3−F6) | −160.9(2) | 162.6 |
| τ(O1−C2−C3−F7) | −41.5(2) | 43.1 |
| τ(O1−C2−C3−C4) | 79.2(2) | −78.0 |
| τ(F9−C4−C3−F6) | −65.5(2) | 65.1 |
| τ(F9−C4−C3−F7) | 177.2(2) | −177.4 |
| τ(F9−C4−C3−C2) | 54.9(2) | −54.9 |
| τ(F8−C4−C3−F6) | 54.1(2) | −54.7 |
| τ(F8−C4−C3−F7) | −63.3(2) | 62.8 |
| τ(F8−C4−C3−C2) | 174.5(2) | −174.7 |
| τ(F10−C4−C3−F6) | 174.7(2) | −175.0 |
| τ(F10−C4−C3−F7) | 57.3(2) | −57.6 |
| τ(F10−C4−C3−C2) | −64.9(2) | 65.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Védova, C.O.; Romano, R.M.; Stammler, H.-G.; Mitzel, N.W. Perfluoropropionic Acid (CF3CF2C(O)OH): Three Conformations and Dimer Formation. Molecules 2025, 30, 1887. https://doi.org/10.3390/molecules30091887
Della Védova CO, Romano RM, Stammler H-G, Mitzel NW. Perfluoropropionic Acid (CF3CF2C(O)OH): Three Conformations and Dimer Formation. Molecules. 2025; 30(9):1887. https://doi.org/10.3390/molecules30091887
Chicago/Turabian StyleDella Védova, Carlos O., Rosana M. Romano, Hans-Georg Stammler, and Norbert W. Mitzel. 2025. "Perfluoropropionic Acid (CF3CF2C(O)OH): Three Conformations and Dimer Formation" Molecules 30, no. 9: 1887. https://doi.org/10.3390/molecules30091887
APA StyleDella Védova, C. O., Romano, R. M., Stammler, H.-G., & Mitzel, N. W. (2025). Perfluoropropionic Acid (CF3CF2C(O)OH): Three Conformations and Dimer Formation. Molecules, 30(9), 1887. https://doi.org/10.3390/molecules30091887







