Evaluation of Polycyclic Aromatic Hydrocarbons (PAHs) in Pork Meat Cooked with Two Different Methods
Abstract
1. Introduction
2. Results and Discussion
2.1. Samples and Cooking Conditions
2.2. Weight Loss and Fat Content After Cooking
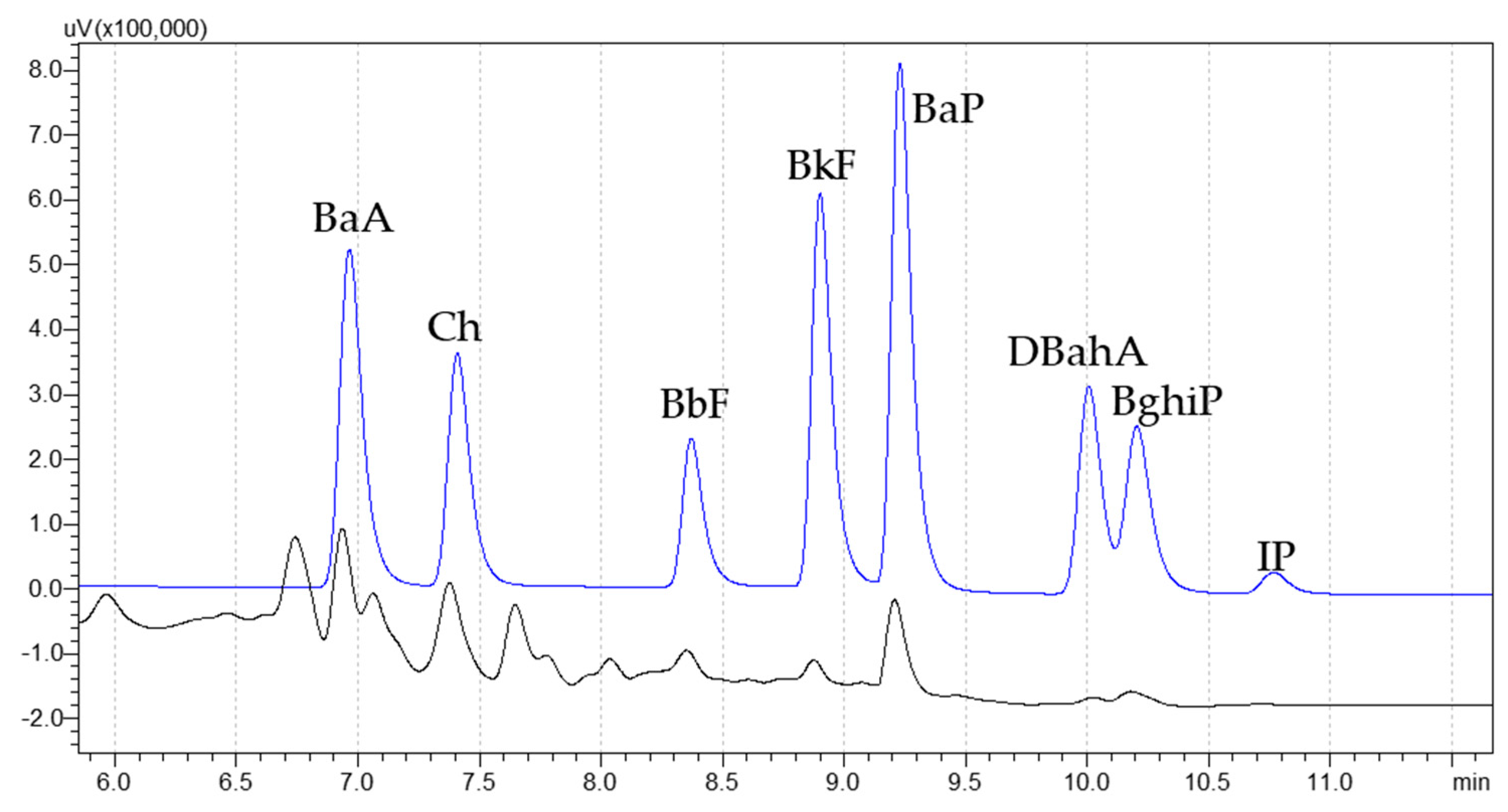
2.3. Extraction and Analytical Determination
2.4. Method Performances
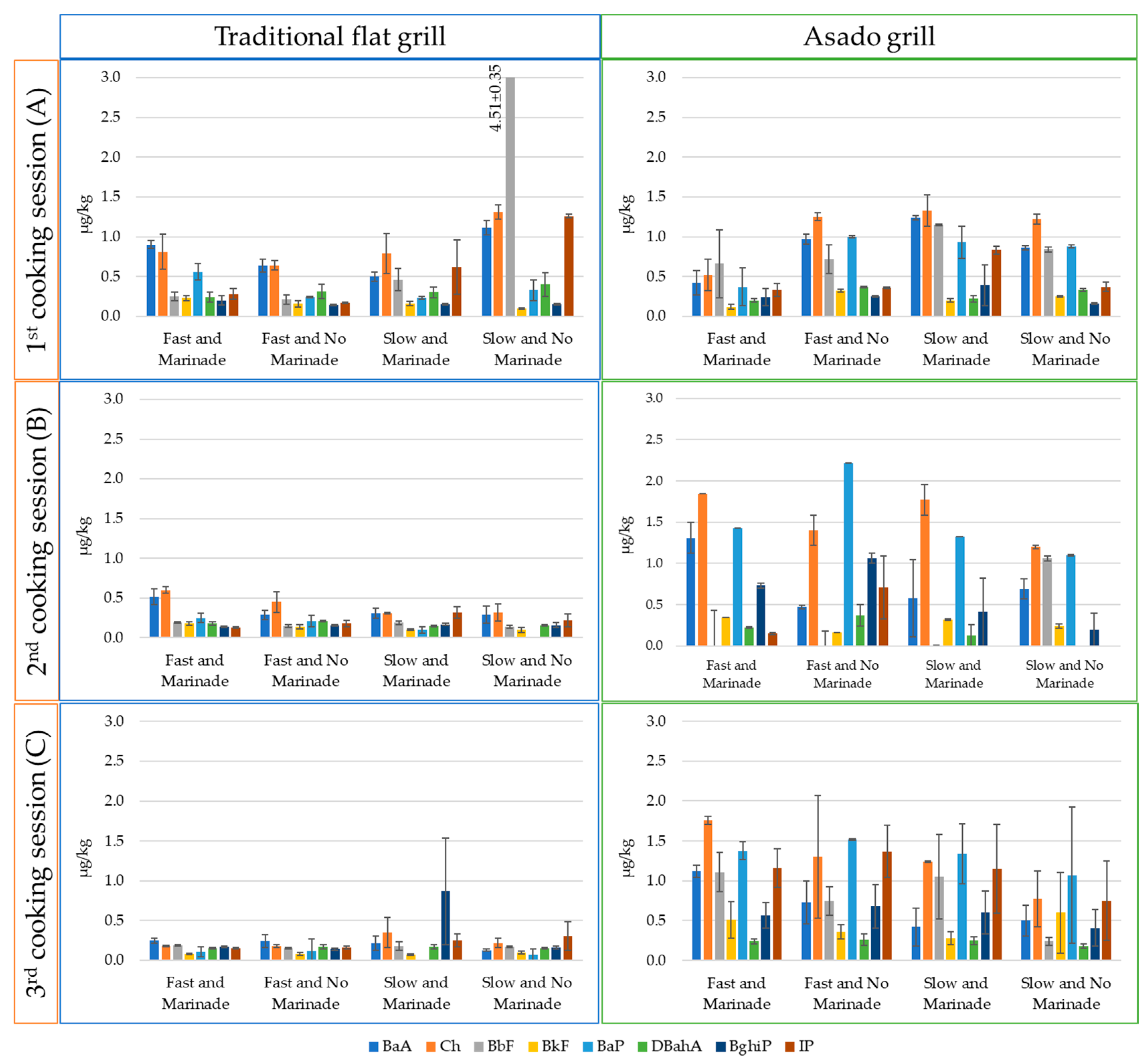
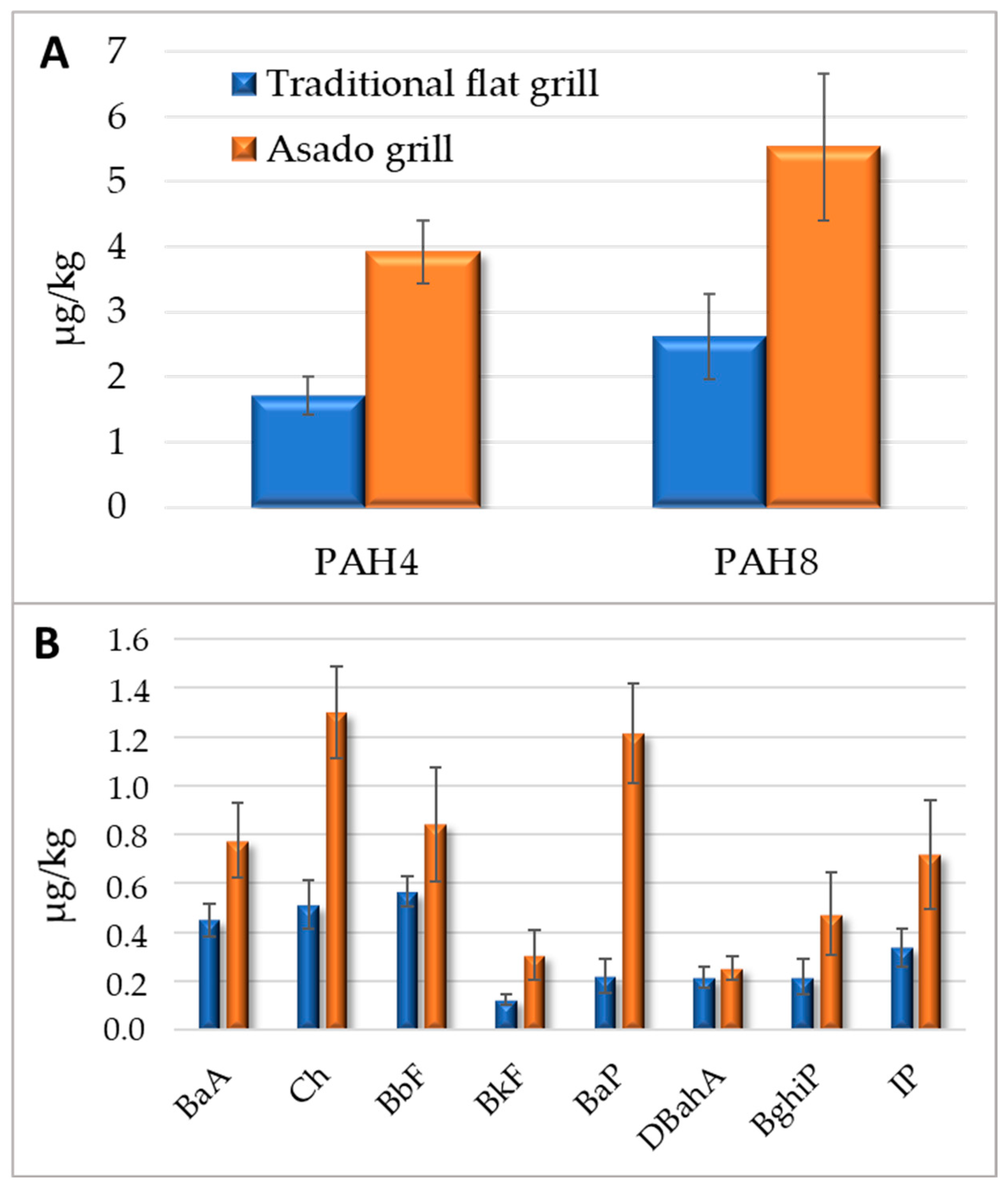
2.5. PAHs Related to the Different Cooking Conditions
3. Materials and Methods
3.1. Reagents and Standards
3.2. Meat Samples
3.3. Cooking Equipment
3.4. Cooking Conditions and Sampling Plan
3.5. Weight Loss and Fat Content Determination
3.6. PAH Extraction and Purification
3.7. UHPLC Analysis
3.8. Method Performance Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BaA | Benzo[a]anthracene |
| BaP | Benzo[a]pyrene |
| BbF | Benzo[b]fluoranthene |
| BghiP | Benzo[g,h,i]perylene |
| BkF | Benzo[k]fluoranthene |
| Ch | Chrysene |
| DBahA | Dibenzo[a,h]anthracene |
| EFSA | European Food Safety Authority |
| FLD | Fluorometric detector |
| IP | Indeno [1,2,3-cd]pyrene |
| MAE | Microwave-assisted extraction |
| MAS | Microwave-assisted saponification |
| PAH | Polycyclic aromatic hydrocarbons |
| PAH4 | Sum of chrysene, benzo[a]anthracene, benzo[a]pyrene, and benzo[b]fluoranthene |
| PAH8 | Sum of PAH4 and benzo[k]fluoranthene, dibenzo[a,h]anthracene, benzo[g,h,i]perylene, and indeno [1,2,3-cd]pyrene |
| SPE | Solid-phase extraction |
| UHPLC | Ultra-high performance liquid chromatography |
References
- Ježek, F.; Kameník, J.; Macharáčková, B.; Bogdanovičová, K.; Bednář, J. Cooking of Meat: Effect on Texture, Cooking Loss and Microbiological Quality—A Review. Acta Vet. Brno 2019, 88, 487–496. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A. Thermal Processing Food-Related Toxicants: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3579–3596. [Google Scholar] [CrossRef] [PubMed]
- Cartus, A.; Schrenk, D. Current Methods in Risk Assessment of Genotoxic Chemicals. Food Chem. Toxicol. 2017, 106, 574–582. [Google Scholar] [CrossRef]
- Park, K.C.; Pyo, H.S.; Kim, W.S.; Yoon, K.S. Effects of Cooking Methods and Tea Marinades on the Formation of Benzo[a]Pyrene in Grilled Pork Belly (Samgyeopsal). Meat Sci. 2017, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Yettella, R.R.; Kim, J.S.; Kwon, K.; Kim, M.C.; Min, D.B. Effects of Grilling and Roasting on the Levels of Polycyclic Aromatic Hydrocarbons in Beef and Pork. Food Chem. 2011, 129, 1420–1426. [Google Scholar] [CrossRef]
- Purcaro, G.; Barp, L.; Moret, S. Determination of Hydrocarbon Contamination in Foods. A Review. Anal. Methods 2016, 8, 5755–5772. [Google Scholar] [CrossRef]
- European food Safety Authority (EFSA). Polycyclic Aromatic Hydrocarbons in Food—Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 724, 1–114. [Google Scholar] [CrossRef]
- Marques, C.; Fiolet, T.; Frenoy, P.; Severi, G.; Mancini, F.R. Association between Polycyclic Aromatic Hydrocarbons (PAH) Dietary Exposure and Mortality Risk in the E3N Cohort. Sci. Total Environ. 2022, 840, 156626. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, S.Y.; Moon, J.S.; Kim, S.H.; Kang, D.H.; Yoon, H.J. Effects of Grilling Procedures on Levels of Polycyclic Aromatic Hydrocarbons in Grilled Meats. Food Chem. 2016, 199, 632–638. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Ionas, A.C. Formation and Mitigation of PAHs in Barbecued Meat—A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3553–3568. [Google Scholar] [CrossRef]
- Chaemsai, S.; Kunanopparat, T.; Srichumpuang, J.; Nopharatana, M.; Tangduangdee, C.; Siriwattanayotin, S. Reduction of the Polycyclic Aromatic Hydrocarbon (PAH) Content of Charcoal Smoke during Grilling by Charcoal Preparation Using High Carbonisation and a Preheating Step. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 385–390. [Google Scholar] [CrossRef]
- Kozliak, E.; Sulkes, M.; Smoliakova, I.P.; Alhroub, I.; Nespor, B.; Yao, B.; Kubátová, A. Pathways toward PAH Formation during Fatty Acid and Triglyceride Pyrolysis. J. Phys. Chem. A 2020, 124, 7559–7574. [Google Scholar] [CrossRef]
- Hitzel, A.; Pöhlmann, M.; Schwägele, F.; Speer, K.; Jira, W. Polycyclic Aromatic Hydrocarbons (PAH) and Phenolic Substances in Meat Products Smoked with Different Types of Wood and Smoking Spices. Food Chem. 2013, 139, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Djinovic-Stojanovic, J.; Popovic, A.; Spiric, A.; Jira, W. Emission of Polycyclic Aromatic Hydrocarbons from Beech Wood Combustion. Energy Sources Part A Recovery Util. Environ. Eff. 2013, 35, 328–336. [Google Scholar] [CrossRef]
- Available online: https://www.epicentro.iss.it/trichinella/ (accessed on 12 March 2025).
- Purcaro, G.; Moret, S.; Bučar-Miklavčič, M.; Conte, L.S. Ultra-High Performance Liquid Chromatographic Method for the Determination of Polycyclic Aromatic Hydrocarbons in a Passive Environmental Sampler. J. Sep. Sci. 2012, 35, 922–928. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission Regulation (EU) No. 836/2011 of 19 August 2011 Amending Regulation (EC) No 333/2007 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Lead, Cadmium, Mercury, Inorganic Tin, 3-MCPD and Benzo(a)Pyrene in Foodstuffs. Off. J. Eur. Union 2011, L215, 9–16. [Google Scholar]
- European Commission (EC). Commission Regulation (EU) No 835/2011 of 19 August 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Polycyclic Aromatic Hydrocarbons in Foodstuffs. Off. J. Eur. Union 2011, L215, 4–8. [Google Scholar]
- Sahin, S.; Ulusoy, H.I.; Alemdar, S.; Erdogan, S.; Agaoglu, S. The Presence of Polycyclic Aromatic Hydrocarbons (PAHs) in Grilled Beef, Chicken and Fish by Considering Dietary Exposure and Risk Assessment. Food Sci. Anim. Resour. 2020, 40, 675–688. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Y.; Feng, Y.; Song, W.; Cao, F.; Zhang, Y.; Li, Q.; Yang, X.; Chen, J. Different Formation Mechanisms of PAH during Wood and Coal Combustion under Different Temperatures. Atmos. Environ. 2020, 222, 117084. [Google Scholar] [CrossRef]
- Kao, T.H.; Chen, S.; Huang, C.W.; Chen, C.J.; Chen, B.H. Occurrence and Exposure to Polycyclic Aromatic Hydrocarbons in Kindling-Free-Charcoal Grilled Meat Products in Taiwan. Food Chem. Toxicol. 2014, 71, 149–158. [Google Scholar] [CrossRef]
- Chen, B.H.; Chen, Y.C. Formation of Polycyclic Aromatic Hydrocarbons in the Smoke from Heated Model Lipids and Food Lipids. J. Agric. Food Chem. 2001, 49, 5238–5243. [Google Scholar] [CrossRef] [PubMed]
- Anjum, Z.; Shehzad, F.; Rahat, A.; Shah, H.U.; Khan, S. Effect of Marination and Grilling Techniques in Lowering the Level of Polyaromatic Hydrocarbons and Heavy Metal in Barbecued Meat. Sarhad J. Agric. 2019, 35, 639–646. [Google Scholar] [CrossRef]
- Oz, F.; Yuzer, M.O. The Effects of Cooking on Wire and Stone Barbecue at Different Cooking Levels on the Formation of Heterocyclic Aromatic Amines and Polycyclic Aromatic Hydrocarbons in Beef Steak. Food Chem. 2016, 203, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kazerouni, N.; Sinha, R.; Hsu, C.-H.; Greenberg, A.; Rothman, N. Analysis of 200 Food Items for Benzo[a]Pyrene and Estimation of Its Intake in an Epidemiologic Study. Food Chem. Toxicol. 2001, 39, 423–436. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Abas, F.; Sakar, Z.I. Determination of Polycyclic Aromatic Hydrocarbons in Grilled Meat. Food Control 2010, 21, 606–610. [Google Scholar] [CrossRef]
- Viegas, O.; Yebra-Pimentel, I.; Martínez-Carballo, E.; Simal-Gandara, J.; Ferreira, I.M.P.L.V.O. Effect of Beer Marinades on Formation of Polycyclic Aromatic Hydrocarbons in Charcoal-Grilled Pork. J. Agric. Food Chem. 2014, 62, 2638–2643. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Faridah, A.; Zaidul, I.S.M. Effects of Marinating on the Formation of Polycyclic Aromatic Hydrocarbons (Benzo[a]Pyrene, Benzo[b]Fluoranthene and Fluoranthene) in Grilled Beef Meat. Food Control 2012, 28, 420–425. [Google Scholar] [CrossRef]
- Onopiuk, A.; Kołodziejczak, K.; Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Szpicer, A.; Stelmasiak, A.; Poltorak, A. Influence of Plant Extract Addition to Marinades on Polycyclic Aromatic Hydrocarbon Formation in Grilled Pork Meat. Molecules 2022, 27, 175. [Google Scholar] [CrossRef]
- Rose, M.; Holland, J.; Dowding, A.; Petch, S.R.G.; White, S.; Fernandes, A.; Mortimer, D. Investigation into the Formation of PAHs in Foods Prepared in the Home to Determine the Effects of Frying, Grilling, Barbecuing, Toasting and Roasting. Food Chem. Toxicol. 2015, 78, 1–9. [Google Scholar] [CrossRef]
- Akpambang, V.O.E.; Purcaro, G.; Lajide, L.; Amoo, I.A.; Conte, L.S.; Moret, S. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Commonly Consumed Nigerian Smoked/Grilled Fish and Meat. Food Addit. Contam.—Part A 2009, 26, 1096–1103. [Google Scholar] [CrossRef]
| Cooking Session | Grill Type | Cooking Mode | Heating Source | Climatic Conditions | |
|---|---|---|---|---|---|
| Temperature Range (°C) | Time Range (min) | ||||
| I | flat | slow | 246–367 | 135 | 6.3–10.4 °C; RH: 93%; cloudy day |
| fast | 464–544 | 100 | |||
| asado | slow | 249–350 | 178 | ||
| fast | 475–553 | 120 | |||
| II | flat | slow | 292–342 | 110 | 7.6–16.2 °C; RH: 36%; sunny day |
| fast | 423–523 | 80 | |||
| asado | slow | 282–352 | 140 | ||
| fast | 482–531 | 95 | |||
| III | flat | slow | 281–330 | 110 | 7.8–15.9 °C; RH: 38%; cloudy day |
| fast | 454–522 | 70 | |||
| asado | slow | 283–368 | 160 | ||
| fast | 447–533 | 110 | |||
| Traditional Flat Grill | Asado Grill | |||||||
|---|---|---|---|---|---|---|---|---|
| Cooking mode | Fast | Slow | Fast | Slow | ||||
| Marinade | yes | no | yes | no | yes | no | yes | no |
| Weight loss (%) | 29.2 | 37.2 | 34.8 | 34.4 | 31.6 | 35.8 | 29.0 | 32.2 |
| SD (%) | 6.7 | 7.6 | 5.7 | 4.1 | 4.8 | 6.7 | 5.1 | 6.0 |
| RSD (%) | 23.0 | 20.4 | 16.4 | 12.0 | 15.2 | 18.7 | 17.6 | 18.7 |
| Fat content (%) | 24.9 | 22.0 | 25.9 | 26.6 | 24.1 | 21.5 | 24.0 | 23.4 |
| SD (%) | 3.6 | 3.8 | 11.1 | 4.7 | 8.1 | 5.9 | 4.0 | 7.1 |
| RSD (%) | 14.4 | 17.3 | 43.0 | 17.6 | 33.4 | 27.3 | 16.6 | 30.4 |
| Grill Type | Asado Grill | Traditional Flat Grill | ||||||
|---|---|---|---|---|---|---|---|---|
| Heating source | flame from beech wood | charcoal from beech wood | ||||||
| Fat dripping | limited | yes | ||||||
| Cooking speed (to reach 72 °C at the core) | slow | fast | slow | fast | ||||
| Marinating | no | yes | no | yes | no | yes | no | yes |
| Cooking session | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Replicate number for each cooking session | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total pieces of meat cooked in the 3 sessions | 24 | 24 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conchione, C.; Socal, S.; Barp, L.; Moret, S. Evaluation of Polycyclic Aromatic Hydrocarbons (PAHs) in Pork Meat Cooked with Two Different Methods. Molecules 2025, 30, 1886. https://doi.org/10.3390/molecules30091886
Conchione C, Socal S, Barp L, Moret S. Evaluation of Polycyclic Aromatic Hydrocarbons (PAHs) in Pork Meat Cooked with Two Different Methods. Molecules. 2025; 30(9):1886. https://doi.org/10.3390/molecules30091886
Chicago/Turabian StyleConchione, Chiara, Silvia Socal, Laura Barp, and Sabrina Moret. 2025. "Evaluation of Polycyclic Aromatic Hydrocarbons (PAHs) in Pork Meat Cooked with Two Different Methods" Molecules 30, no. 9: 1886. https://doi.org/10.3390/molecules30091886
APA StyleConchione, C., Socal, S., Barp, L., & Moret, S. (2025). Evaluation of Polycyclic Aromatic Hydrocarbons (PAHs) in Pork Meat Cooked with Two Different Methods. Molecules, 30(9), 1886. https://doi.org/10.3390/molecules30091886









