“Pepper”: Different Spices, One Name—Analysis of Sensory and Biological Aspects
Abstract
:1. Introduction
2. The Characteristics of the Six Pepper Spices
2.1. Strengths of the Six Pepper Spices
2.1.1. Chemosensory Characteristics
2.1.2. Multi-Faceted Bioactivity
2.1.3. Natural Additive for Food Preservation
2.2. Weaknesses of the Six Pepper Spices
2.2.1. Instability of Aromatic Compounds
2.2.2. Long Supply Chain
2.3. Opportunities of the Six Pepper Spices
2.4. Threats of the Six Pepper Spices
2.4.1. Impactful Chemosensory Characteristics
2.4.2. Adverse Reactions
2.4.3. Interaction with Drugs
3. Key Molecules and Properties of Selected “Peppers”
3.1. Black Pepper (Piper nigrum L.)
3.2. Cubeb Pepper (Piper cubeba L.f.)
3.3. Long Pepper (Piper longum L.)
3.4. Pink Pepper (Schinus terebinthifolius Raddi)
3.5. Allspice (Pimenta dioica L. Merrill)
3.6. Japanese Pepper (Zanthoxylum piperitum DC.)
4. Review Methodology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Cetó, X.; Sarma, M.; del Valle, M. Analysis of spices & herbs and its phenolic content by means of an electronic tongue. LWT 2024, 191, 115578. [Google Scholar] [CrossRef]
- USDA. “Spices and Herbs”, Forest Service—United States Department of Agriculture. Available online: https://www.fs.usda.gov/wildflowers/ethnobotany/food/spices.shtml#:~:text=Spices%20and%20herbs%20are%20defined,of%20as%20non%2Dwoody%20plants (accessed on 20 September 2024).
- Bano, H.; Jahan, N.; Makbul, S.A.A.; Kumar, B.; Husain, S.; Sayed, A. Effect of Piper cubeba L. fruit on ethylene glycol and ammonium chloride induced urolithiasis in male Sprague Dawley rats. Integr. Med. Res. 2018, 7, 358–365. [Google Scholar] [CrossRef]
- Drissi, B.; Mahdi, I.; Yassir, M.; Ben Bakrim, W.; Bouissane, L.; Sobeh, M. Cubeb (Piper cubeba L.f.): A comprehensive review of its botany, phytochemistry, traditional uses, and pharmacological properties. Front. Nutr. 2022, 9, 1048520. [Google Scholar] [CrossRef] [PubMed]
- Bishal, A.; Ali, K.A.; Ghosh, S.; Parua, P.; Bandyopadhyay, B.; Mondal, S.; Jana, M.; Datta, A.; Das, K.K.; Debnath, B. Natural dyes: Its Origin, Categories and Application on Textile Fabrics in brief. Nat. Dyes 2023, 12, 9780–9802. [Google Scholar]
- Chittaragi, D.; Menon, J.S. Bio-Colours From Spices. In Herbs and Spices—New Advances; Ivanišová, E., Ed.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Kačániová, M.; Čmiková, N.; Ban, Z.; Garzoli, S.; Elizondo-Luevano, J.H.; Ben Hsouna, A.; Ben Saad, R.; Bianchi, A.; Venturi, F.; Kluz, M.I.; et al. Enhancing the Shelf Life of Sous-Vide Red Deer Meat with Piper nigrum Essential Oil: A Study on Antimicrobial Efficacy against Listeria monocytogenes. Molecules 2024, 29, 4179. [Google Scholar] [CrossRef]
- Leja, K.B.; Czaczyk, K. The industrial potential of herbs and spices—A mini review. Acta Sci. Pol. Technol. Aliment. 2016, 15, 353–368. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Abdallah, E.M.; Alanazi, N.A.; Ed-Dra, A.; Jamal, A.; Idriss, H.; Alshammari, A.S.; Shommo, S.A.M. Spices as Sustainable Food Preservatives: A Comprehensive Review of Their Antimicrobial Potential. Pharmaceuticals 2023, 16, 1451. [Google Scholar] [CrossRef]
- Da Silva Dannenberg, G.; Funck, G.D.; Mattei, F.J.; da Silva, W.P.; Fiorentini, Â.M. Antimicrobial and antioxidant activity of essential oil from pink pepper tree (Schinus terebinthifolius Raddi) in vitro and in cheese experimentally contaminated with Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2016, 36, 120–127. [Google Scholar] [CrossRef]
- Parichanon, P.; Ascrizzi, R.; Tani, C.; Sanmartin, C.; Taglieri, I.; Macaluso, M.; Flamini, G.; Pieracci, Y.; Venturi, F.; Conti, B. The protective combined effect of chitosan and essential oil coatings on cheese and cured meat against the oviposition of Piophila casei. Food Biosci. 2023, 56, 103132. [Google Scholar] [CrossRef]
- Jarquin-Enriquez, L.; Ibarra-Torres, P.; Jimenez-Islas, H.; Flores-Martínez, N. Pimenta dioica: A review on its composition, phytochemistry, and applications in food technology. Int. Food Res. J. 2021, 28, 893–904. [Google Scholar] [CrossRef]
- Taglieri, I.; Tonacci, A.; Flamini, G.; Díaz-Guerrero, P.; Ascrizzi, R.; Bachi, L.; Procissi, G.; Billeci, L.; Venturi, F. Novel Perspectives for Sensory Analysis Applied to Piperaceae and Aromatic Herbs: A Pilot Study. Foods 2025, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Asensio, C.M.; Grosso, N.R.; Juliani, H.R. Quality characters, chemical composition and biological activities of oregano (Origanum spp.) Essential oils from Central and Southern Argentina. Ind. Crop. Prod. 2015, 63, 203–213. [Google Scholar] [CrossRef]
- Bonfanti, C.; Iannì, R.; Mazzaglia, A.; Lanza, C.M.; Napoli, E.M.; Ruberto, G. Emerging cultivation of oregano in Sicily: Sensory evaluation of plants and chemical composition of essential oils. Ind. Crop. Prod. 2012, 35, 160–165. [Google Scholar] [CrossRef]
- Douglas, M.; Heyes, J.; Smallfield, B. Herbs, Spices and Essential Oils: Post-Harvest Operations in Developing Countries. 2005. Available online: https://openknowledge.fao.org/handle/20.500.14283/AD420E (accessed on 20 September 2024).
- Mangalakumari, C.K.; Sreedharan, V.P.; Mathew, A.G. Studies on Blackening of Pepper (Piper nigrum, Linn) During Dehydration. J. Food Sci. 1983, 48, 604–606. [Google Scholar] [CrossRef]
- MAPA; FEN. Características Nutricionales de los Principales Alimentos de Nuestra Dieta. 13-Condimentos y Aperitivos: Pimienta. Ministerio de Agricultura, Pesca y Alimentación de España & Fundación Española de Nutrición. Available online: https://www.mapa.gob.es/es/ministerio/servicios/informacion/pimienta_tcm30-102753.pdf (accessed on 26 September 2024).
- Ahmad, H.; Khera, R.A.; Hanif, M.A.; Ayub, M.A. Cubeb. In Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–164. [Google Scholar] [CrossRef]
- Maiti, S.; Kumari, P. Cultivation of Long pepper.pdf. National Research Centre for Medicinal and Aromatic Plants. 2002. Available online: https://www.dmapr.org.in/Publications/bulletine/Cultivation%20of%20long%20pepper.pdf (accessed on 1 October 2024).
- de Carvalho, R.O.; Machado, M.B.; Lopes, R.S.; Scherer, V.S.; Cruz, W.A. Agroindustry for drying pink pepper (Schinus terebinthifolius). Agric. Eng. Int. CIGR J. 2015, 2015, 177. [Google Scholar]
- Mitani, T.; Yawata, Y.; Yamamoto, N.; Okuno, Y.; Sakamoto, H.; Nishide, M.; Kayano, S.-I. Stabilization of Hydroxy-α-Sanshool by Antioxidants Present in the Genus Zanthoxylum. Foods 2023, 12, 3444. [Google Scholar] [CrossRef]
- Nixon, J.; Sinnakandu, P.; Chohan, M. Total phenolic content of microwaved, pan-heated and stewed, herbs and spices. Int. J. Gastron. Food Sci. 2024, 37, 100952. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Ju, R.; Chen, K.; Bhandari, B.; Wang, H. Advances in efficient extraction of essential oils from spices and its application in food industry: A critical review. Crit. Rev. Food Sci. Nutr. 2023, 63, 11482–11503. [Google Scholar] [CrossRef]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M.; Khan, M.A. Biological role of Piper nigrum L. (Black pepper): A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1945–S1953. [Google Scholar] [CrossRef]
- Biswas, P.; Ghorai, M.; Mishra, T.; Gopalakrishnan, A.V.; Roy, D.; Mane, A.B.; Mundhra, A.; Das, N.; Mohture, V.M.; Patil, M.T.; et al. Piper longum L.: A comprehensive review on traditional uses, phytochemistry, pharmacology, and health-promoting activities. Phytotherapy Res. 2022, 36, 4425–4476. [Google Scholar] [CrossRef]
- Vieira, J.d.S.; de Oliveira, V.S.; Carneiro, M.J.; da Silva, T.L.; Augusta, I.M.; de Carvalho, M.G.; Sawaya, A.C.H.F.; Saldanha, T. Phenolic composition and insights into the use of pink pepper (Schinus terebentifolius Raddi) fruit against lipid oxidation in food systems. Food Biosci. 2023, 53, 102556. [Google Scholar] [CrossRef]
- Yamasaki, K.; Fukutome, N.; Hayakawa, F.; Ibaragi, N.; Nagano, Y. Classification of Japanese Pepper (Zanthoxylum piperitum DC.) from Different Growing Regions Based on Analysis of Volatile Compounds and Sensory Evaluation. Molecules 2022, 27, 4946. [Google Scholar] [CrossRef] [PubMed]
- Graidist, P.; Martla, M.; Sukpondma, Y. Cytotoxic Activity of Piper cubeba Extract in Breast Cancer Cell Lines. Nutrients 2015, 7, 2707–2718. [Google Scholar] [CrossRef]
- Lim, T.K. Piper cubeba. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; pp. 311–321. [Google Scholar] [CrossRef]
- Kumar, S.; Kamboj, J.; Suman; Sharma, S. Overview for Various Aspects of the Health Benefits of Piper Longum Linn. Fruit. J. Acupunct. Meridian Stud. 2011, 4, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lakhera, S.; Devlal, K.; Ghosh, A.; Rana, M. In silico investigation of phytoconstituents of medicinal herb ‘Piper Longum’ against SARS-CoV-2 by molecular docking and molecular dynamics analysis. Results Chem. 2021, 3, 100199. [Google Scholar] [CrossRef]
- Li, D.; Wang, R.; Cheng, X.; Yang, J.; Yang, Y.; Qu, H.; Li, S.; Lin, S.; Wei, D.; Bai, Y.; et al. Chemical constituents from the fruits of Piper longum L. and their vascular relaxation effect on rat mesenteric arteries. Nat. Prod. Res. 2022, 36, 674–679. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Hamed, M.; Sila, A.; Nahdi, S.; Alwasel, S.; Harrath, A.H.; Tlili, N. Multidirectional insights on polysaccharides from Schinus terebinthifolius and Schinus molle fruits: Physicochemical and functional profiles, in vitro antioxidant, anti-genotoxicity, antidiabetic, and antihemolytic capacities, and in vivo anti-inflammatory and anti-nociceptive properties. Int. J. Biol. Macromol. 2020, 165, 2576–2587. [Google Scholar] [CrossRef]
- de Oliveira, V.S.; Augusta, I.M.; Braz, M.V.d.C.; Riger, C.J.; Prudêncio, E.R.; Sawaya, A.C.H.F.; Sampaio, G.R.; Torres, E.A.F.d.S.; Saldanha, T. Aroeira fruit (Schinus terebinthifolius Raddi) as a natural antioxidant: Chemical constituents, bioactive compounds and in vitro and in vivo antioxidant capacity. Food Chem. 2020, 315, 126274. [Google Scholar] [CrossRef]
- Monzote, L.; Machín, L.; González, A.; Scull, R.; Gutiérrez, Y.I.; Satyal, P.; Gille, L.; Setzer, W.N. Eugenol-Rich Essential Oil from Pimenta dioica: In Vitro and In Vivo Potentialities against Leishmania amazonensis. Pharmaceuticals 2023, 17, 64. [Google Scholar] [CrossRef]
- Murali, V.; Devi, V.M.; Parvathy, P.; Murugan, M. Phytochemical screening, FTIR spectral analysis, antioxidant and antibacterial activity of leaf extract of Pimenta dioica Linn. Mater. Today Proc. 2021, 45, 2166–2170. [Google Scholar] [CrossRef]
- Rao, P.S.; Navinchandra, S.; Jayaveera, K. An important spice, Pimenta dioica (Linn.) Merill: A Review. Int. Curr. Pharm. J. 2012, 1, 221–225. [Google Scholar] [CrossRef]
- Suárez, A.; Ulate, G.; Ciccio, J. Cardiovascular effects of ethanolic and aqueous extracts of Pimenta dioica in Sprague-Dawley rats. J. Ethnopharmacol. 1997, 55, 107–111. [Google Scholar] [CrossRef]
- Ji, Y.; Li, S.; Ho, C.-T. Chemical composition, sensory properties and application of Sichuan pepper (Zanthoxylum genus). Food Sci. Hum. Wellness 2019, 8, 115–125. [Google Scholar] [CrossRef]
- Luo, J.; Ke, J.; Hou, X.; Li, S.; Luo, Q.; Wu, H.; Shen, G.; Zhang, Z. Composition, structure and flavor mechanism of numbing substances in Chinese prickly ash in the genus Zanthoxylum: A review. Food Chem. 2022, 373, 131454. [Google Scholar] [CrossRef]
- Duan, Z.; Xie, H.; Yu, S.; Wang, S.; Yang, H. Piperine Derived from Piper nigrum L. Inhibits LPS-Induced Inflammatory through the MAPK and NF-κB Signalling Pathways in RAW264.7 Cells. Foods 2022, 11, 2990. [Google Scholar] [CrossRef]
- Elfahmi, V.; Ruslan, K.; Batterman, S.; Bos, R.; Kayser, O.; Woerdenbag, H.J.; Quax, W.J. Lignan profile of Piper cubeba, an Indonesian medicinal plant. Biochem. Syst. Ecol. 2007, 35, 397–402. [Google Scholar] [CrossRef]
- Yadav, V.; Krishnan, A.; Vohora, D. A systematic review on Piper longum L.: Bridging traditional knowledge and pharmacological evidence for future translational research. J. Ethnopharmacol. 2020, 247, 112255. [Google Scholar] [CrossRef]
- de Carvalho, M.C.R.D.; Barca, F.N.T.V.; Agnez-Lima, L.F.; de Medeiros, S.R.B. Evaluation of mutagenic activity in an extract of pepper tree stem bark (Schinus terebinthifolius Raddi). Environ. Mol. Mutagen. 2003, 42, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.G.S.; Alves, É.P.; Maia, C.M.d.A.; Brito, A.C.M.; Silva, D.R.; Freires, I.A.; Cavalcanti, Y.W.; Rehder, V.L.G.; Ruiz, A.L.T.G.; Duarte, M.C.T.; et al. Biological properties of Schinus terebinthifolia Raddi essential oil. Braz. J. Pharm. Sci. 2022, 58, e20417. [Google Scholar] [CrossRef]
- Carneiro, T.S.; Dutra, M.d.C.P.; Lima, D.A.; Araújo, A.J.d.B.; Constant, P.B.L.; Lima, M.d.S. Phenolic compounds in peel, seed and cold pressed pink pepper (Schinus terebinthifolia R.) oil and bioaccessibility of peel using a digestion model with intestinal barrier simulation. Food Biosci. 2022, 49, 101930. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Henry, F.C.; Valle, F.; Oliveira, D.B.; Santos Junior, A.C.; Resende, E.D.; Maia Junior, J.A.; Martins, M.L.L. Effect of the fruit aqueous extract of Brazilian pepper tree (Schinus terebinthifolius, Raddi) on selected quality parameters of frozen fresh pork sausage. J. Agric. Food Res. 2020, 2, 100055. [Google Scholar] [CrossRef]
- Barreira, C.F.T.; de Oliveira, V.S.; Chávez, D.W.H.; Gamallo, O.D.; Castro, R.N.; Júnior, P.C.D.; Sawaya, A.C.H.F.; Ferreira, M.d.S.; Sampaio, G.R.; Torres, E.A.F.d.S.; et al. The impacts of pink pepper (Schinus terebinthifolius Raddi) on fatty acids and cholesterol oxides formation in canned sardines during thermal processing. Food Chem. 2023, 403, 134347. [Google Scholar] [CrossRef]
- Fagundes, M.B.; Ballus, C.A.; Soares, V.P.; Ferreira, D.d.F.; Leães, Y.S.V.; Robalo, S.S.; Vendruscolo, R.G.; Campagnol, P.C.B.; Barin, J.S.; Cichoski, A.J.; et al. Characterization of olive oil flavored with Brazilian pink pepper (Schinus terebinthifolius Raddi) in different maceration processes. Food Res. Int. 2020, 137, 109593. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Arafah, M.; Gómez-Caravaca, A.M.; Contreras, M.d.M.; Nahdi, S.; Taamalli, A.; Allagui, M.S.; Alwasel, S.; Segura-Carretero, A.; et al. Schinus terebinthifolius fruits intake ameliorates metabolic disorders, inflammation, oxidative stress, and related vascular dysfunction, in atherogenic diet-induced obese rats. Insight of their chemical characterization using HPLC-ESI-QTOF-MS/MS. J. Ethnopharmacol. 2021, 269, 113701. [Google Scholar] [CrossRef]
- El Gizawy, H.A.; Boshra, S.A.; Mostafa, A.; Mahmoud, S.H.; Ismail, M.I.; Alsfouk, A.A.; Taher, A.T.; Al-Karmalawy, A.A. Pimenta dioica (L.) Merr. Bioactive Constituents Exert Anti-SARS-CoV-2 and Anti-Inflammatory Activities: Molecular Docking and Dynamics, In Vitro, and In Vivo Studies. Molecules 2021, 26, 5844. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hara, S.; Kawai, Y.; Nakatani, N. Antioxidative phenylpropanoids from berries of Pimenta dioica. Phytochemistry 1999, 52, 1307–1312. [Google Scholar] [CrossRef]
- Nitta, Y.; Kikuzaki, H.; Ueno, H. Inhibitory activity of Pimenta dioica extracts and constituents on recombinant human histidine decarboxylase. Food Chem. 2009, 113, 445–449. [Google Scholar] [CrossRef]
- Silva, A.V.; Yerena, L.R.; Necha, L.L.B. Chemical profile and antifungal activity of plant extracts on Colletotrichum spp. isolated from fruits of Pimenta dioica (L.) Merr. Pestic. Biochem. Physiol. 2021, 179, 104949. [Google Scholar] [CrossRef]
- Ivane, N.M.A.; Haruna, S.A.; Zekrumah, M.; Elysé, F.K.R.; Hassan, M.O.; Hashim, S.B.; Tahir, H.E.; Zhang, D. Composition, mechanisms of tingling paresthesia, and health benefits of Sichuan pepper: A review of recent progress. Trends Food Sci. Technol. 2022, 126, 1–12. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, H.J.; Park, J.-C.; Hong, J.; Yang, W.M. Zanthoxylum piperitum reversed alveolar bone loss of periodontitis via regulation of bone remodeling-related factors. J. Ethnopharmacol. 2017, 195, 137–142. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, H.; Ha, I.J.; Yang, W.M. Zanthoxylum piperitum alleviates the bone loss in osteoporosis via inhibition of RANKL-induced c-fos/NFATc1/NF-κB pathway. Phytomedicine 2021, 80, 153397. [Google Scholar] [CrossRef] [PubMed]
- Gusson-Zanetoni, J.P.; da Silva, J.S.G.M.; Picão, T.B.; Cardin, L.T.; Prates, J.; de Sousa, S.O.; Henrique, T.; Oliani, S.M.; Tajara, E.H.; e Silva, M.L.A.; et al. Effect of Piper cubeba total extract and isolated lignans on head and neck cancer cell lines and normal fibroblasts. J. Pharmacol. Sci. 2021, 148, 93–102. [Google Scholar] [CrossRef]
- Hashimoto, K.; Satoh, K.; Kase, Y.; Ishige, A.; Kubo, M.; Sasaki, H.; Nishikawa, S.; Kurosawa, S.; Yakabi, K.; Nakamura, T. Modulatory Effect of Aliphatic Acid Amides from Zanthoxylum piperitum on Isolated Gastrointestinal Tract. Planta Medica 2001, 67, 179–181. [Google Scholar] [CrossRef]
- Hatano, T.; Inada, K.; Ogawa, T.-O.; Ito, H.; Yoshida, T. Aliphatic acid amides of the fruits of Zanthoxylum piperitum. Phytochemistry 2004, 65, 2599–2604. [Google Scholar] [CrossRef]
- Grinevicius, V.M.; Andrade, K.S.; Ourique, F.; Micke, G.A.; Ferreira, S.R.; Pedrosa, R.C. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. cultivar Bragantina through cell cycle arrest and apoptosis induction. J. Supercrit. Fluids 2017, 128, 94–101. [Google Scholar] [CrossRef]
- Fan, D.; Zhou, C.; Chen, C.; Li, X.; Ma, J.; Hu, Y.; Li, G.; Ruan, J.; Wu, A.; Li, L.; et al. Lignans from the genus Piper L. and their pharmacological activities: An updated review. Fitoterapia 2023, 165, 105403. [Google Scholar] [CrossRef]
- Niwa, A.M.; Marcarini, J.C.; Sartori, D.; Maistro, E.L.; Mantovani, M.S. Effects of (−)-cubebin (Piper cubeba) on cytotoxicity, mutagenicity and expression of p38 MAP kinase and GSTa2 in a hepatoma cell line. J. Food Compos. Anal. 2013, 30, 1–5. [Google Scholar] [CrossRef]
- Rajalekshmi, D.S.; Kabeer, F.A.; Madhusoodhanan, A.R.; Bahulayan, A.K.; Prathapan, R.; Prakasan, N.; Varughese, S.; Nair, M.S. Anticancer activity studies of cubebin isolated from Piper cubeba and its synthetic derivatives. Bioorganic Med. Chem. Lett. 2016, 26, 1767–1771. [Google Scholar] [CrossRef]
- Lima, T.C.; Lucarini, R.; Volpe, A.C.; de Andrade, C.Q.; Souza, A.M.; Pauletti, P.M.; Januário, A.H.; Símaro, G.V.; Bastos, J.K.; Cunha, W.R.; et al. In vivo and in silico anti-inflammatory mechanism of action of the semisynthetic (−)-cubebin derivatives (−)-hinokinin and (−)-O-benzylcubebin. Bioorganic Med. Chem. Lett. 2017, 27, 176–179. [Google Scholar] [CrossRef]
- Phong, N.V.; Anh, D.T.N.; Chae, H.Y.; Yang, S.Y.; Kwon, M.J.; Min, B.S.; Kim, J.A. Anti-inflammatory activity and cytotoxicity against ovarian cancer cell lines by amide alkaloids and piperic esters isolated from Piper longum fruits: In vitro assessments and molecular docking simulation. Bioorganic Chem. 2022, 128, 106072. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agrofor-Estree Database: A Tree Reference And Selection Guide; World Agroforestry Centre: Nairobi, Kenya, 2009; Available online: https://www.worldagroforestry.org/output/agroforestree-database (accessed on 15 November 2024).
- Chaudhari, A.K.; Singh, V.K.; Dwivedy, A.K.; Das, S.; Upadhyay, N.; Singh, A.; Dkhar, M.S.; Kayang, H.; Prakash, B.; Dubey, N.K. Chemically characterised Pimenta dioica (L.) Merr. essential oil as a novel plant based antimicrobial against fungal and aflatoxin B1 contamination of stored maize and its possible mode of action. Nat. Prod. Res. 2020, 34, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crop. Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Nair Prabhakaran, K.P. Agronomy and Economy of Black Pepper and Cardamom: The “King” and “Queen” of Spices, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Liang, J.; Sun, J.; Chen, P.; Frazier, J.; Benefield, V.; Zhang, M. Chemical analysis and classification of black pepper (Piper nigrum L.) based on their country of origin using mass spectrometric methods and chemometrics. Food Res. Int. 2021, 140, 109877. [Google Scholar] [CrossRef]
- Silva, B.G.; Cefali, L.C.; Rosa, P.d.T.V.e.; Franco, J.G.; Mazzola, P.G.; Fileti, A.M.F.; Foglio, M.A. Phytocosmetic Containing Pink Pepper Extracts Obtained by Sustainable Extraction. Chem. Biodivers. 2022, 19, e202200273. [Google Scholar] [CrossRef]
- Dawid, C.; Henze, A.; Frank, O.; Glabasnia, A.; Rupp, M.; Büning, K.; Orlikowski, D.; Bader, M.; Hofmann, T. Structural and Sensory Characterization of Key Pungent and Tingling Compounds from Black Pepper (Piper nigrum L.). J. Agric. Food Chem. 2012, 60, 2884–2895. [Google Scholar] [CrossRef]
- Moss, R.; Fisher, C.; Gorman, M.; Knowles, S.; LeBlanc, J.; Ritchie, C.; Schindell, K.; Ettinger, L.; McSweeney, M.B. Effect of Piperine on Saltiness Perception. Foods 2023, 12, 296. [Google Scholar] [CrossRef]
- Zaveri, M.; Khandhar, A.; Patel, S.; Patel, A. Chemistry and pharmacology of Piper longum L. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 67–76. [Google Scholar]
- Rajopadhye, A.; Upadhye, A.; Mujumdar, A. HPTLC method for analysis of piperine in fruits of Piper species. J. Planar Chromatogr. Mod. TLC 2011, 24, 57–59. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Pandian, A.; Warkentin, T.D. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: A review. Clin. Phytoscience 2021, 7, 52. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential Microwave-Ultrasound-Assisted Extraction for Isolation of Piperine from Black Pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Milenković, A.N.; Stanojević, L.P. Black pepper: Chemical composition and biological activities. Adv. Technol. 2021, 10, 40–50. [Google Scholar] [CrossRef]
- Spence, C. The king of spices: On pepper’s pungent pleasure. Int. J. Gastron. Food Sci. 2024, 35, 100900. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; Kumar, N.V.A.; Salehi, B.; Cho, W.C.; Sharifi-Rad, J. Piperine-A Major Principle of Black Pepper: A Review of Its Bioactivity and Studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Yang, G.; Chambers, E.; Wang, H. Flavor lexicon development (in English and Chinese) and descriptive analysis of Sichuan pepper. J. Sens. Stud. 2021, 36, e12636. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, X.; Battino, M.; Wei, X.; Shi, J.; Zhao, L.; Liu, S.; Xiao, J.; Shi, B.; Zou, X. A comparative overview on chili pepper (Capsicum genus) and sichuan pepper (Zanthoxylum genus): From pungent spices to pharma-foods. Trends Food Sci. Technol. 2021, 117, 148–162. [Google Scholar] [CrossRef]
- Sugai, E.; Morimitsu, Y.; Kubota, K. Quantitative Analysis of Sanshool Compounds in Japanese Pepper (Xanthoxylum piperitum DC.) and Their Pungent Characteristics. Biosci. Biotechnol. Biochem. 2005, 69, 1958–1962. [Google Scholar] [CrossRef]
- Ben Khemis, I.; Mechi, N.; Ben Lamine, A. Investigation of mouse eugenol olfactory receptor activated by eugenol, vanillin and ethyl vanillin: Steric and energetic characterizations. Int. J. Biol. Macromol. 2020, 163, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Issaoui, M.; Delgado, A.M.; Caruso, G.; Micali, M.; Barbera, M.; Atrous, H.; Ouslati, A.; Chammem, N. Phenols, Flavors, and the Mediterranean Diet. J. AOAC Int. 2020, 103, 915–924. [Google Scholar] [CrossRef]
- Jungbauer, A.; Medjakovic, S. Anti-inflammatory properties of culinary herbs and spices that ameliorate the effects of metabolic syndrome. Maturitas 2012, 71, 227–239. [Google Scholar] [CrossRef]
- Srinivasan, K. Spices as influencers of body metabolism: An overview of three decades of research. Food Res. Int. 2005, 38, 77–86. [Google Scholar] [CrossRef]
- Srinivasan, K. Antioxidant Potential of Spices and Their Active Constituents. Crit. Rev. Food Sci. Nutr. 2014, 54, 352–372. [Google Scholar] [CrossRef]
- Khajuria, A.; Thusu, N.; Zutshi, U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: Influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine 2002, 9, 224–231. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.; Yrjönen, T.; Vuorela, H.; Holm, Y. Bioactive compounds from medicinal plants: Focus on Piper species. S. Afr. J. Bot. 2017, 112, 54–69. [Google Scholar] [CrossRef]
- Senay, T.L. Systematic Review on Spices and Herbs Used in Food Industry. Am. J. Ethnomedicine 2020, 7, 1–10. [Google Scholar]
- Alrashidi, A.A.; Noumi, E.; Snoussi, M.; De Feo, V. Chemical Composition, Antibacterial and Anti-Quorum Sensing Activities of Pimenta dioica L. Essential Oil and Its Major Compound (Eugenol) against Foodborne Pathogenic Bacteria. Plants 2022, 11, 540. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Shad, E.; Ziaee, E.; Yousefabad, S.H.A.; Golmakani, M.T.; Azizinia, M. Biodegradable Chitosan Coating Incorporated with Black Pepper Essential Oil for Shelf Life Extension of Common Carp (Cyprinus carpio) during Refrigerated Storage. J. Food Prot. 2016, 79, 986–993. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Djebbi, T.; Ascrizzi, R.; Bedini, S.; Farina, P.; Sanmartin, C.; Ben Jemâa, J.M.; Bozzini, M.F.; Flamini, G.; Conti, B. Physicochemical and repellent properties of chitosan films loaded with essential oils for producing an active packaging effective against the food pest Sitophilus oryzae. J. Stored Prod. Res. 2024, 106, 102297. [Google Scholar] [CrossRef]
- Bhatia, S.; Shah, Y.A.; Al-Harrasi, A.; Jawad, M.; Koca, E.; Aydemir, L.Y. Novel applications of black pepper essential oil as an antioxidant agent in sodium caseinate and chitosan based active edible films. Int. J. Biol. Macromol. 2024, 254, 128045. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.C.M.; Sediyama, M.A.N.; Machado, I.; Bitencourt, E.P.; Pinto, C.M.F.; Donzelles, S.M.L. Effect of drying temperature on yield and phytochemical quality of essential oil extracted from Schinus terebinthifolius. Rev. Bras. Agropecuária Sustentável 2021, 11, 71–77. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Krishnaraj, S.; Gunaseelan, R.; Arunmozhi, M.; Sumandiran, C. Supply chain perspective and logistics of spices in Indian retail industry. Mater. Today Proc. 2022, 48, 119–124. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Spices, Condiments, Extra Virgin Olive Oil and Aromas as Not Only Flavorings, but Precious Allies for Our Wellbeing. Antioxidants 2021, 10, 868. [Google Scholar] [CrossRef]
- Da Silva Acácio, R.; Pamphile-Adrian, A.J.; Florez-Rodriguez, P.P.; de Freitas, J.D.; Goulart, H.F.; Santana, A.E.G. Dataset of Schinus terebinthifolius essential oil microencapsulated by spray-drying. Data Brief 2023, 47, 108927. [Google Scholar] [CrossRef]
- Schöll, I.; Jensen-Jarolim, E. Allergenic Potency of Spices: Hot, Medium Hot, or Very Hot. Int. Arch. Allergy Immunol. 2004, 135, 247–261. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Durjava, M.F.; Kouba, M.; López-Alonso, M.; Puente, S.L.; Marcon, F.; et al. Safety and efficacy of feed additives prepared from Piper nigrum L.: Black pepper oil and black pepper oleoresin for use in all animal species and a supercritical extract for use in dogs and cats (FEFANA asbl). EFSA J. 2022, 20, e07599. [Google Scholar] [CrossRef]
- Ziegenhagen, R.; Heimberg, K.; Lampen, A.; Hirsch-Ernst, K.I. Safety Aspects of the Use of Isolated Piperine Ingested as a Bolus. Foods 2021, 10, 2121. [Google Scholar] [CrossRef]
- Abdul-Jalil, T.Z.; Nasser, Z.A. Piper cubeba: Phytochemical and pharmacological review of a routinely used spices. Int. J. Pharm. Res. 2020, 1, 761–768. [Google Scholar] [CrossRef]
- Carvalho, M.; Melo, A.; Aragão, C.; Raffin, F.; Moura, T. Schinus terebinthifolius Raddi: Chemical composition, biological properties and toxicity. Rev. Bras. Plantas Med. 2013, 15, 158–169. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; Kolar, B.; et al. Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA J. 2012, 10, 2663. [Google Scholar] [CrossRef]
- Ajazuddin; Alexander, A.; Qureshi, A.; Kumari, L.; Vaishnav, P.; Sharma, M.; Saraf, S.; Saraf, S. Role of herbal bioactives as a potential bioavailability enhancer for Active Pharmaceutical Ingredients. Fitoterapia 2014, 97, 1–14. [Google Scholar] [CrossRef]
- Db, M.; Sreedharan, S.; Mahadik, K. Role of Piperine as an Effective Bioenhancer in Drug Absorption. Pharm. Anal. Acta 2018, 9, 1000591. [Google Scholar] [CrossRef]
- Fan, R.; Qin, X.-W.; Hu, R.-S.; Hu, L.-S.; Wu, B.-D.; Hao, C.-Y. Studies on the chemical and flavour qualities of white pepper (Piper nigrum L.) derived from grafted and non-grafted plants. Eur. Food Res. Technol. 2020, 246, 2601–2610. [Google Scholar] [CrossRef]
- Li, Y.-X.; Zhang, C.; Pan, S.; Chen, L.; Liu, M.; Yang, K.; Zeng, X.; Tian, J. Analysis of chemical components and biological activities of essential oils from black and white pepper (Piper nigrum L.) in five provinces of southern China. LWT 2019, 117, 108644. [Google Scholar] [CrossRef]
- Orav, A.; Stulova, I.; Kailas, T.; Müürisepp, M. Effect of Storage on the Essential Oil Composition of Piper nigrum L. Fruits of Different Ripening States. J. Agric. Food Chem. 2004, 52, 2582–2586. [Google Scholar] [CrossRef]
- Dong, Y.; Yin, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. A distinct structural mechanism underlies TRPV1 activation by piperine. Biochem. Biophys. Res. Commun. 2019, 516, 365–372. [Google Scholar] [CrossRef]
- Slack, J.P. Molecular Pharmacology of Chemesthesis. In Chemosensory Transduction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 375–391. [Google Scholar] [CrossRef]
- Bryant, B.P.; Mezine, I. Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res. 1999, 842, 452–460. [Google Scholar] [CrossRef]
- Luebke, B. The Good Scents Company Information System. The Good Scents Company. Available online: www.thegoodscentscompany.com (accessed on 26 November 2024).
- Gutierrez, R.M.P.; Gonzalez, A.M.N.; Hoyo-Vadillo, C. Alkaloids from Piper: A Review of its Phytochemistry and Pharmacology. Mini-Reviews Med. Chem. 2013, 13, 163–193. [Google Scholar] [CrossRef]
- Rauscher, F.M.; Sanders, R.A.; Watkins, J.B. Effects of piperine on antioxidant pathways in tissues from normal and streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2000, 14, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef] [PubMed]
- Sabina, E.P.; Nasreen, A.; Vedi, M.; Rasool, M. Analgesic, Antipyretic and Ulcerogenic Effects of Piperine: An Active Ingredient of Pepper. J. Pharm. Sci. 2013, 5, 203. [Google Scholar]
- Friedman, M.; Levin, C.E.; Lee, S.-U.; Lee, J.-S.; Ohnisi-Kameyama, M.; Kozukue, N. Analysis by HPLC and LC/MS of Pungent Piperamides in Commercial Black, White, Green, and Red Whole and Ground Peppercorns. J. Agric. Food Chem. 2008, 56, 3028–3036. [Google Scholar] [CrossRef]
- Park, K.-R.; Leem, H.H.; Cho, M.; Kang, S.W.; Yun, H.-M. Effects of the amide alkaloid piperyline on apoptosis, autophagy, and differentiation of pre-osteoblasts. Phytomedicine 2020, 79, 153347. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Acree, T.; Arn, H. FlavorNet. Available online: http://www.flavornet.org (accessed on 28 November 2024).
- Al-Sayed, E.; Gad, H.A.; El-Kersh, D.M. Characterization of Four Piper Essential Oils (GC/MS and ATR-IR) Coupled to Chemometrics and Their anti-Helicobacter pylori Activity. ACS Omega 2021, 6, 25652–25663. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Lomarat, P.; Sripha, K.; Phanthong, P.; Kitphati, W.; Thirapanmethee, K.; Bunyapraphatsara, N. In vitro biological activities of black pepper essential oil and its major components relevant to the prevention of Alzheimer’s disease. Thai J. Pharm. Sci. 2015, 39, 94–101. [Google Scholar] [CrossRef]
- Mendes De Lacerda Leite, G.; Barbosa, M.d.O.; Lopes, M.J.P.; Delmondes, G.d.A.; Bezerra, D.S.; Araújo, I.M.; de Alencar, C.D.C.; Coutinho, H.D.M.; Peixoto, L.R.; Barbosa-Filho, J.M.; et al. Pharmacological and toxicological activities of α-humulene and its isomers: A systematic review. Trends Food Sci. Technol. 2021, 115, 255–274. [Google Scholar] [CrossRef]
- de Araújo-Filho, H.G.; dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytotherapy Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.S.O.; Babeanu, N.; Petruţa, C.; Radu, N. Limonene—A biomolecule with potential applications in regenerative medicine. Sci. Bull. Ser. F. Biotechnol. 2022, 26, 139–148. [Google Scholar]
- Soulimani, J.; Bouayed, R.; Joshi, R.K. Limonene: Natural monoterpene volatile compounds of potential therapeutic interest. Am. J. Essent. Oils Nat. Prod. 2019, 7, 1–10. [Google Scholar]
- Teoh, E.S. Secondary Metabolites of Plants. In Medicinal Orchids of Asia; Springer International Publishing: Cham, Switzerland, 2016; pp. 59–73. [Google Scholar] [CrossRef]
- Perazzo, F.; Rodrigues, I.; Maistro, E.; Souza, S.; Nanaykkara, N.; Bastos, J.; Carvalho, J.; de Souza, G. Anti-inflammatory and analgesic evaluation of hydroalcoholic extract and fractions from seeds of Piper cubeba L. (Piperaceae). Pharmacogn. J. 2013, 5, 13–16. [Google Scholar] [CrossRef]
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants 2020, 9, 1534. [Google Scholar] [CrossRef]
- Feng, T.; Wang, X.; Fan, C.; Wang, X.; Wang, X.; Cui, H.; Xia, S.; Huang, Q. The selective encapsulation and stabilization of cinnamaldehyde and eugenol in high internal phase Pickering emulsions: Regulating the interfacial properties. Food Chem. 2023, 401, 134139. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine science: Principles and applications. In Food Science and Technology International Series, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Srinivasan, K. Spices for Taste and Flavour: Nutraceuticals for Human Health. In Spices: The Elixir of Life; Original Publications: New Delhi, India, 2011; Chapter 2; pp. 43–62. [Google Scholar]
- Tan, K.H.; Nishida, R. Methyl Eugenol: Its Occurrence, Distribution, and Role in Nature, Especially in Relation to Insect Behavior and Pollination. J. Insect Sci. 2012, 12, 1–60. [Google Scholar] [CrossRef]
- Burdock, G.A.; Fenaroli, G. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2010. [Google Scholar]
- Centre International de Recherche sur le Cancer (Ed.) Some chemicals present in industrial and consumer products, food and drinking-water. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; No. 101; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Kuang, B.-C.; Wang, Z.-H.; Hou, S.-H.; Wang, M.-Q.; Zhang, J.-S.; Sun, K.-L.; Ni, H.-Q.; Gong, N.-Q. Methyl eugenol protects the kidney from oxidative damage in mice by blocking the Nrf2 nuclear export signal through activation of the AMPK/GSK3β axis. Acta Pharmacol. Sin. 2023, 44, 367–380. [Google Scholar] [CrossRef]
- Yin, L.; Sun, Z.; Ren, Q.; Su, X.; Zhang, D. Methyl eugenol induces potent anticancer effects in RB355 human retinoblastoma cells by inducing autophagy, cell cycle arrest and inhibition of PI3K/mTOR/Akt signalling pathway. J. BUON 2018, 23, 1174–1178. [Google Scholar] [PubMed]
- Macedo, A.; Martorano, L.; de Albuquerque, A.C.; Fiorot, R.; Carneiro, J.; Campos, V.; Vasconcelos, T.; Valverde, A.; Moreira, D.; dos Santo, F.M., Jr. Absolute Configuration of (−)-Cubebin, a Classical Lignan with Pharmacological Potential, Defined by Means of Chiroptical Spectroscopy. J. Braz. Chem. Soc. 2020, 31, 2030–2037. [Google Scholar] [CrossRef]
- Agrawal, N.; Goyal, D.; Goyal, A. A review on multi-therapeutic potential of (-)-cubebin: Experimental evidences. Nat. Prod. Res. 2023, 37, 4290–4301. [Google Scholar] [CrossRef]
- Bastos, J.K.; Carvalho, J.C.; de Souza, G.H.; Pedrazzi, A.H.; Sarti, S.J. Anti-inflammatory activity of cubebin, a lignan from the leaves of Zanthoxyllum naranjillo Griseb. J. Ethnopharmacol. 2001, 75, 279–282. [Google Scholar] [CrossRef]
- da Silva, R.; de Souza, G.H.; da Silva, A.A.; de Souza, V.A.; Pereira, A.C.; Royo, V.d.A.; e Silva, M.L.; Donate, P.M.; Araújo, A.L.d.M.; Carvalho, J.C.; et al. Synthesis and biological activity evaluation of lignan lactones derived from (−)-cubebin. Bioorganic Med. Chem. Lett. 2005, 15, 1033–1037. [Google Scholar] [CrossRef]
- Silva, M.L.A.; Coímbra, H.S.; Pereira, A.C.; Almeida, V.A.; Lima, T.C.; Costa, E.S.; Vinhólis, A.H.C.; Royo, V.A.; Silva, R.; Filho, A.A.S.; et al. Evaluation of Piper cubeba extract, (-)-cubebin and its semi-synthetic derivatives against oral pathogens. Phytother. Res. 2007, 21, 420–422. [Google Scholar] [CrossRef]
- Marcotullio, M.C.; Pelosi, A.; Curini, M. Hinokinin, an Emerging Bioactive Lignan. Molecules 2014, 19, 14862–14878. [Google Scholar] [CrossRef]
- Bobek, K.B.; Ezzat, N.S.; Jones, B.S.; Bian, Y.; Shaw, T.E.; Jurca, T.; Li, H.; Yuan, Y. Total Synthesis of Polysubstituted γ-Butyrolactone Lignans (−)-Hinokinin, (−)-Bicubebin B, and (−)-Isodeoxypodophyllotoxin via Oxime Carbonate Formation. Org. Lett. 2023, 25, 31–36. [Google Scholar] [CrossRef]
- Dash, M.; Singh, S.; Sahoo, S.; Dutt, M.; Kar, B. Metabolite profiling of Piper longum L. fruit volatiles by two-dimensional gas chromatography and time-of-flight mass spectrometry: Insights into the chemical complexity. Biotechnol. Appl. Biochem. 2024, 71, 670–680. [Google Scholar] [CrossRef]
- Zhao, Y.; Liao, P.; Chen, L.; Zhang, Y.; Wang, X.; Kang, Q.; Chen, X.; Sun, Y.; Jin, Y.; Yu, J.; et al. Characterization of the key aroma compounds in a novel Qingke baijiu of Tibet by GC-MS, GC×GC-MS and GC-O-MS. Food Chem. Adv. 2024, 4, 100589. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.P.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene?-humulene in experimental airways allergic inflammation. Br. J. Pharmacol. 2009, 158, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Luo, R.; Chen, X.-Q.; Ba, Y.-Y.; Zheng, L.; Guo, W.-W.; Wu, X. Identification and simultaneous quantification of five alkaloids in Piper longum L. by HPLC–ESI-MSn and UFLC–ESI-MS/MS and their application to Piper nigrum L. Food Chem. 2015, 177, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Belhoussaine, O.; El Kourchi, C.; Harhar, H.; El Moudden, H.; El Yadini, A.; Ullah, R.; Iqbal, Z.; Goh, K.W.; Goh, B.H.; Bouyahya, A.; et al. Phytochemical characterization and nutritional value of vegetable oils from ripe berries of Schinus terebinthifolia raddi and Schinus molle L., through extraction methods. Food Chem. X 2024, 23, 101580. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Lopes, N.A.; Nicoletti, V.R. Pink Pepper (Schinus terebinthifolius Raddi) from Extracts to application: Truths about a Fake Pepper. Food Rev. Int. 2023, 39, 5185–5214. [Google Scholar] [CrossRef]
- Barbosa, L.C.A.; Demuner, A.J.; Clemente, A.D.; de Paula, V.F.; Ismail, F.M.D. Seasonal variation in the composition of volatile oils from Schinus terebinthifolius raddi. Quimica Nova 2007, 30, 1959–1965. [Google Scholar] [CrossRef]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical Composition and Anticancer and Antioxidant Activities of Schinus molle L. and Schinus terebinthifolius Raddi Berries Essential Oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Tiyajamorn, T.; Bharathi, M.; Chaiyasut, C. A Narrative Review on the Bioactivity and Health Benefits of Alpha-Phellandrene. Sci. Pharm. 2022, 90, 57. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Lopes, N.A.; Menis-Henrique, M.E.C.; Janzantti, N.S.; Nicoletti, V.R. Modulation of volatile release and antimicrobial properties of pink pepper essential oil by microencapsulation in single- and double-layer structured matrices. Int. J. Food Microbiol. 2020, 335, 108890. [Google Scholar] [CrossRef]
- Kang, G.-Q.; Duan, W.-G.; Lin, G.-S.; Yu, Y.-P.; Wang, X.-Y.; Lu, S.-Z. Synthesis of Bioactive Compounds from 3-Carene (II): Synthesis, Antifungal Activity and 3D-QSAR Study of (Z)- and (E)-3-Caren-5-One Oxime Sulfonates. Molecules 2019, 24, 477. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Pandey, M.K.; Von Suskil, M.; Chitren, R.; Al-Odat, O.; Jonnalagadda, S.C.; Aggarwal, B.B. Cancer on fire: Role of inflammation in prevention and treatment. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Elsevier: Amsterdam, The Netherlands, 2022; pp. 605–626. [Google Scholar] [CrossRef]
- Saldanha, E.; Pai, R.J.; George, T.; D’souza, S.; Adnan, M.; Pais, M.; Naik, T.; D’souza, R.C.; D’cunha, R.; Baliga, M.S. Health Effects of Various Dietary Agents and Phytochemicals (Therapy of Acute Pancreatitis). In Therapeutic, Probiotic, and Unconventional Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 303–314. [Google Scholar] [CrossRef]
- Radice, M.; Durofil, A.; Buzzi, R.; Baldini, E.; Martínez, A.P.; Scalvenzi, L.; Manfredini, S. Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications. Life 2022, 12, 1602. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Kuttithodi, A.M.; Alfarhan, A.; Rajagopal, R.; Barcelo, D. Chemical Composition, Insecticidal and Mosquito Larvicidal Activities of Allspice (Pimenta dioica) Essential Oil. Molecules 2021, 26, 6698. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, P.; Vikram, K.; Singh, H. Fabrication and characterization of noble crystalline silver nanoparticles from Pimenta dioica leave extract and analysis of chemical constituents for larvicidal applications. Saudi J. Biol. Sci. 2022, 29, 1134–1146. [Google Scholar] [CrossRef]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, D.; Zhao, L.; Shi, B.; Xiao, J.; Liu, X.; Zekruman, M.; Hu, Y.; Ngouana, A.; Shi, J.; et al. Antagonistic interaction of phenols and alkaloids in Sichuan pepper (Zanthoxylum bungeanum) pericarp. Ind. Crop. Prod. 2020, 152, 112551. [Google Scholar] [CrossRef]
- Donald, G.R.; Fernandes, P.D.; Boylan, F. Antinociceptive Activity of Zanthoxylum piperitum DC. Essential Oil. Evidence-Based Complement. Altern. Med. 2016, 2016, 3840398. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Huang, J.; Himabindu, K.; Tewari, D.; Horbańczuk, J.O.; Xu, S.; Chen, Z.; Atanasov, A.G. Cardiovascular protective effect of black pepper (Piper nigrum L.) and its major bioactive constituent piperine. Trends Food Sci. Technol. 2021, 117, 34–45. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Mujumdar, A.S.; Deng, D. The control effect and mechanism of antioxidants on the flavor deterioration of Sichuan pepper essential oil during thermal processing. Food Biosci. 2024, 60, 104452. [Google Scholar] [CrossRef]
- Ribeiro-Filho, H.V.; De Souza Silva, C.M.; De Siqueira, R.J.; Lahlou, S.; Santos, A.A.D.; Magalhães, P.J.C. Biphasic cardiovascular and respiratory effects induced by β-citronellol. Eur. J. Pharmacol. 2016, 775, 96–105. [Google Scholar] [CrossRef]
- Iqbal, U.; Malik, A.; Sial, N.T.; Mehmood, M.H.; Nawaz, S.; Papadakis, M.; Fouad, D.; Ateyya, H.; Welson, N.N.; Alexiou, A.; et al. β-Citronellol: A potential anti-inflammatory and gastro-protective agent-mechanistic insights into its modulatory effects on COX-II, 5-LOX, eNOS, and ICAM-1 pathways through in vitro, in vivo, in silico, and network pharmacology studies. Inflammopharmacology 2024, 32, 3761–3784. [Google Scholar] [CrossRef] [PubMed]
- Billesbølle, C.B.; de March, C.A.; van der Velden, W.J.C.; Ma, N.; Tewari, J.; del Torrent, C.L.; Li, L.; Faust, B.; Vaidehi, N.; Matsunami, H.; et al. Structural basis of odorant recognition by a human odorant receptor. Nature 2023, 615, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Daly, M.; Minniti, N.; Daly, J. Sense of Taste (Effect on Behavior). In Encyclopedia of Human Behavior; Elsevier: Amsterdam, The Netherlands, 2012; pp. 373–378. [Google Scholar] [CrossRef]
- Haley, H.; McDonald, S.T. Spice and herb extracts with chemesthetic effects. In Chemesthesis, 1st ed.; McDonald, S.T., Bolliet, D.A., Hayes, J.E., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 32–47. [Google Scholar] [CrossRef]
- Benzaghta, M.A.; Elwalda, A.; Mousa, M.; Erkan, I.; Rahman, M. SWOT analysis applications: An integrative literature review. J. Glob. Bus. Insights 2021, 6, 55–73. [Google Scholar] [CrossRef]
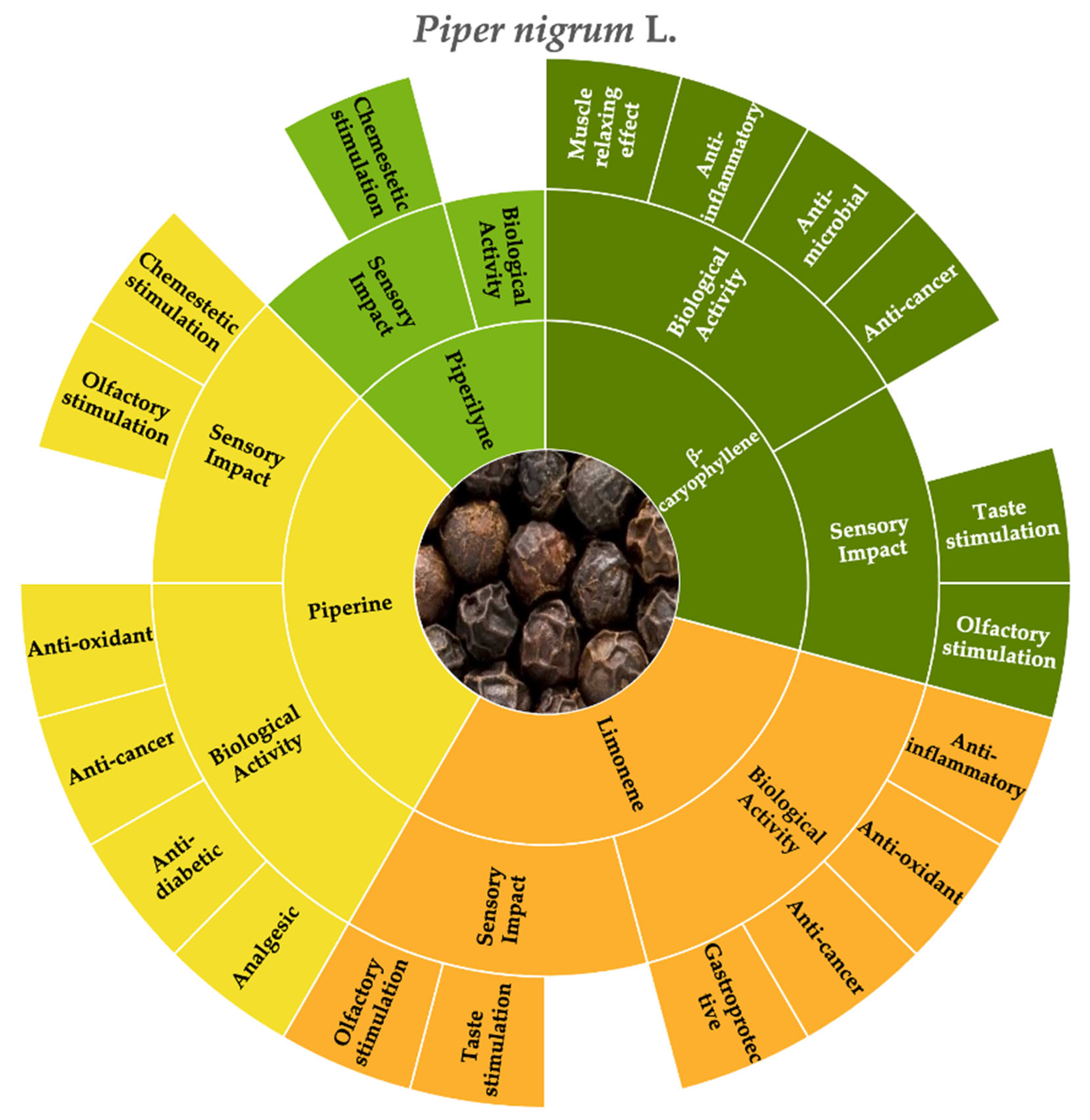
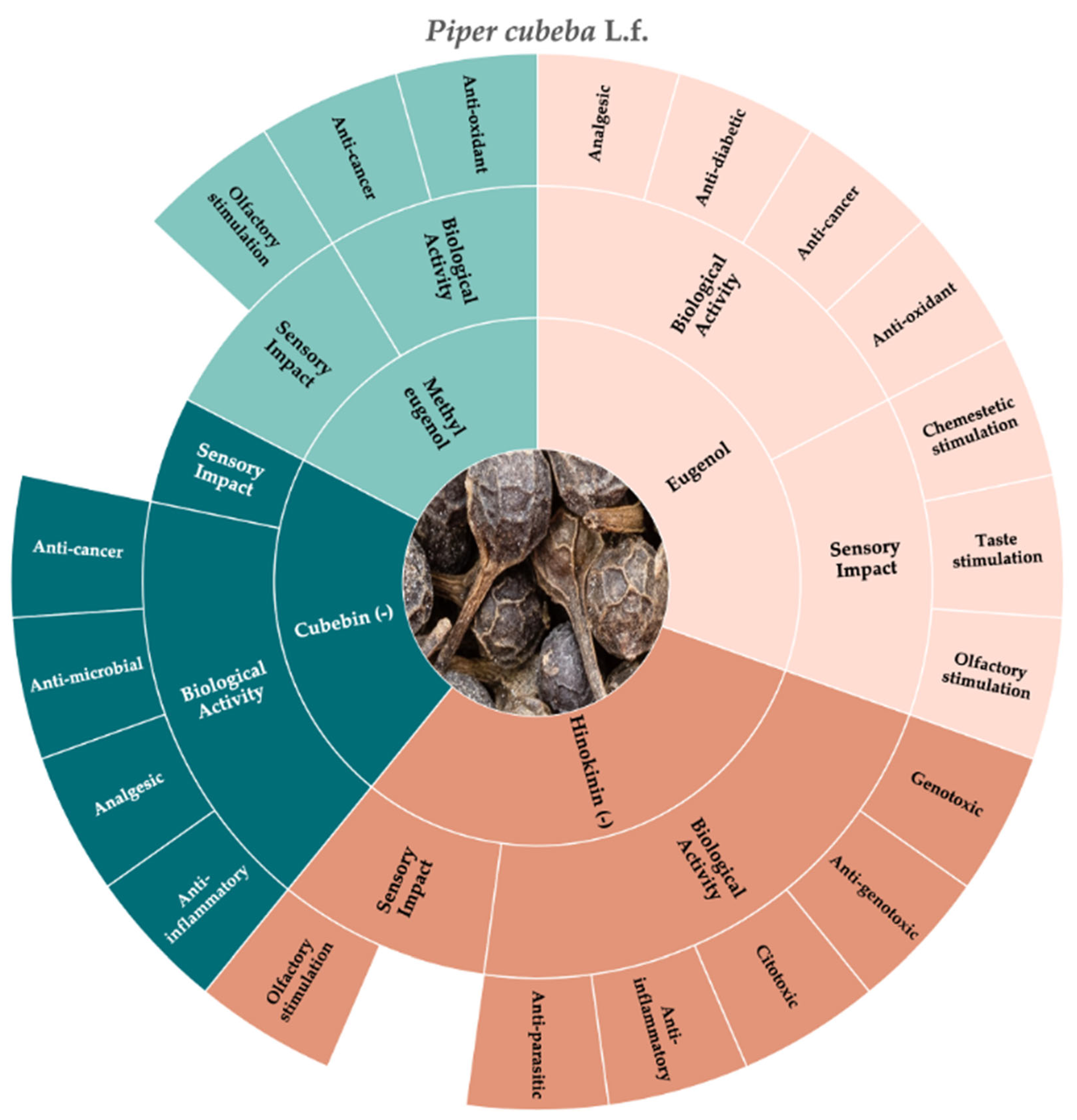

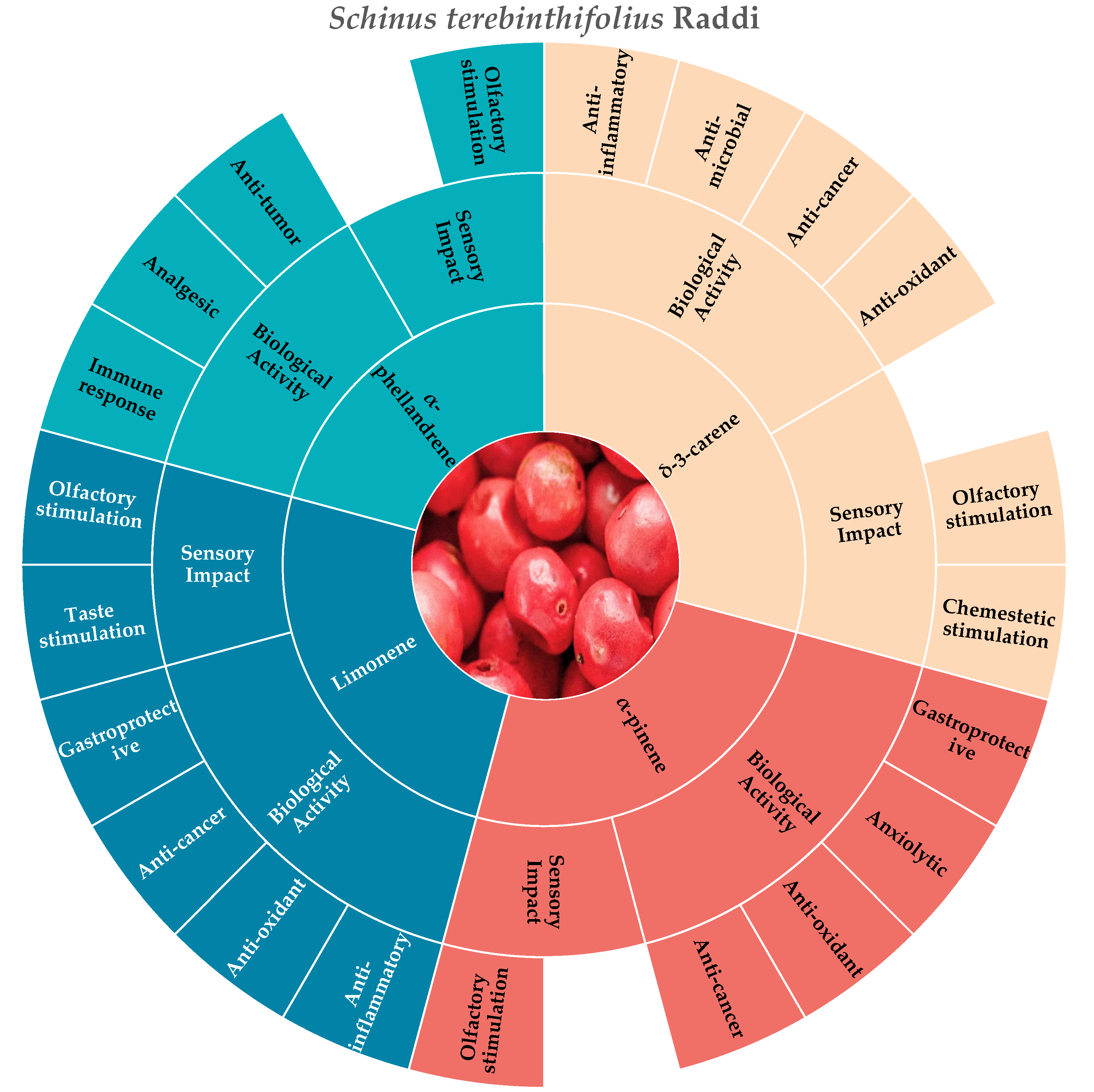
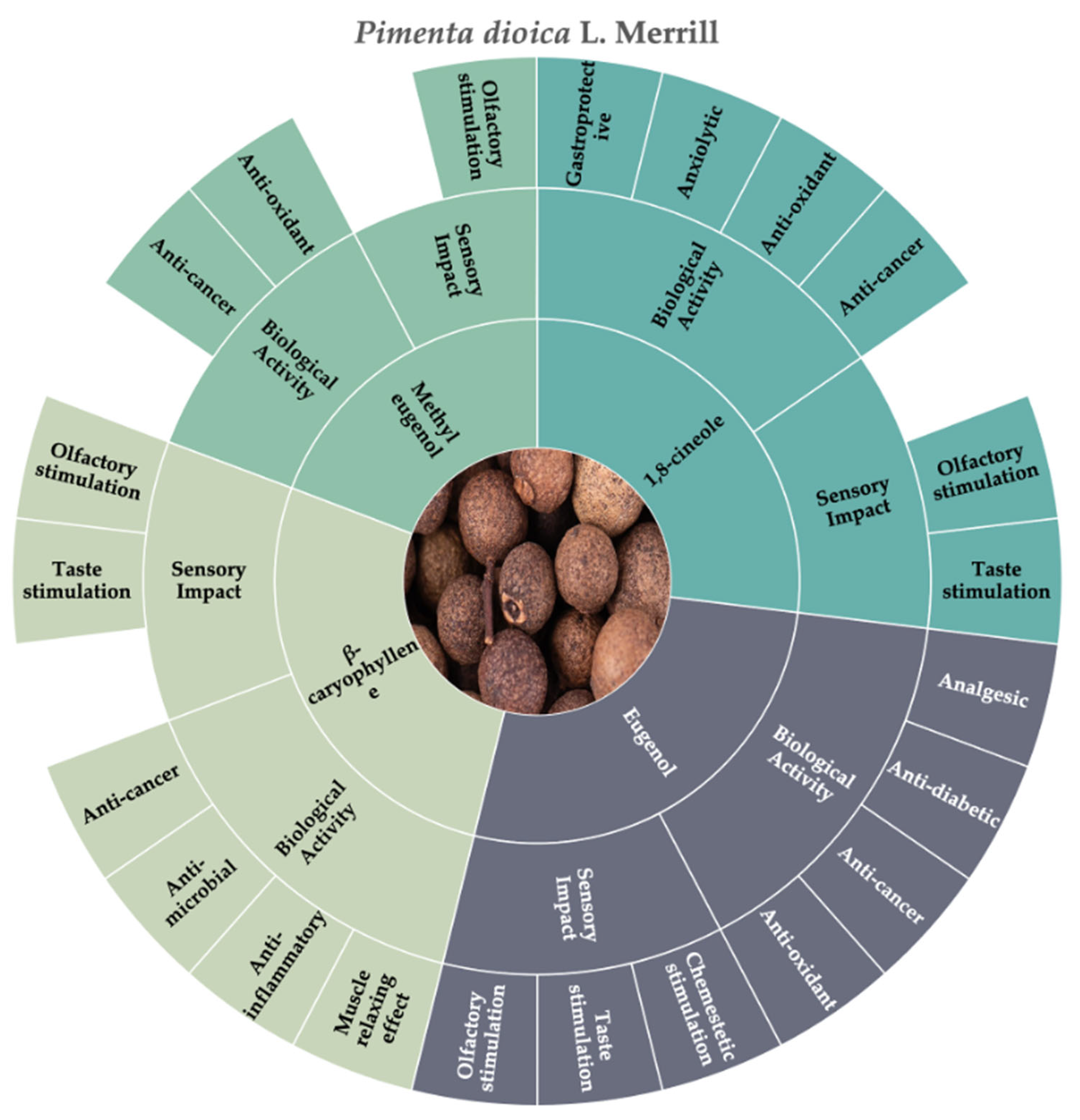

| Botanical Name | Family | Distribution Area | Common Name | Edible Organs | Method of Production |
|---|---|---|---|---|---|
| Piper nigrum L. | Piperaceae | Hainan, Yunnan, and Guangdong in China and Europe | (eng) black pepper, (fra) poivre noir, (esp) pimienta negra, (deu) schwarzer pfeffer, (ita) pepe nero | Fruit and bark | Berries are harvested at early ripening when they turn yellow. Then, berries are washed in hot water, and finally, they are sun-dried or dried by artificial methods [16,17,18]. |
| Piper cubeba L.f | Piperaceae | Sri Lanka, Sumatra, Malaysia, Southern Borneo, and Java | (eng) cubeb pepper, (fra) poivre cubèbe, (esp) pimienta cubeba, (deu) kubeben pfeffer, (ita) pepe cubebe | Fruit | The fruits are harvested by hand when ripe and separated from spikes. Then, the berries can be directly dried or immersed in water to remove the pericarp, and afterwards, they are dried for 3–4 days [19]. |
| Piper longum L. | Piperaceae | India, Malaysia, Indonesia, Singapore, Sri Lanka | (eng) long pepper, (fra) poivre long, (esp) pimienta larga, (deu) langer pfeffer, (ita) pepe lungo | Dried infructescence and leaves | The infructescence is harvested before ripening when the color is blackish green. Subsequently, the berries are dried in the sun for about 4–5 days [20]. |
| Schinus terebinthifolius Raddi | Anacardiacee | Central and North America, Europe, Asia, and Africa | (eng) pink pepper or false pepper, (fra) faux poivrier, (esp) pimienta de brasil o pimienta rosada, (deu) rosa pfeffer, (ita) pepe rosa | Fruit | The berries are harvested manually once they have reached maturity and then dried [21]. |
| Pimenta dioica (L.) Merrill | Myrtaceae | West Indies (Jamaica) and Central America (Cuba, Mexico, Brazil, Honduras, Guatemala, Belize) | (eng) Jamaica pepper or allspice, (fra) poivre de la jamaïque, (esp) pimienta de jamaica, (deu) jamaika pfeffer, (ita) pepe garofanato | Fruit and leaves | The berries harvested are left for up to 5 days in sacks to ferment. Then, they are dried from 5 to 10 days, depending on the weather, until the moisture content is about 12% [16]. |
| Zanthoxylum piperitum DC. | Rutaceae | Japan and Korea | (eng) Japanese pepper, Japanese prickly ash; (fra) poivre japonais, (esp) pimienta japonesa, (deu) japanischer Pfeffer, (ita) pepe giapponese | Fruit and leaves | Sansho fruits are harvested when ripe. After harvesting, the fruits are dried, and the seeds are removed, leaving dried pericarps [22]. |
| TARGET APPARATUS | SPICES | |||||
|---|---|---|---|---|---|---|
| Piper nigrum L. | Piper cubeba L.f | Piper longum L. | Schinus terebinthifolius Raddi | Pimenta dioica L. Merrill | Zanthoxylum piperitum DC. | |
| Metabolic effects | Anti-thyroid; treatment of obesity [25] | Cardiovascular; antidiabetic; hypocholesterolemic; treatment of renal disorders; anti-urolithiatic [3,4,19,29,30] | Antidiabetic treatment of insomnia and pyrexia; anti-hyperlipidemic; anti-obesity [4,26,31,32,33] | Antidiabetic; anti-hypertensive [27,34,35] | Antidiabetic; cardiovascular; hypotensive activity; increase in body weight [36,37,38,39] | Decreased lipids, appetite stimulant [40,41] |
| Protective effects on tissue and organs | Antioxidant; hepatoprotective; anti-apoptotic [25,42] | Treatment of enteritis; antioxidant; hepatoprotective; renoprotective; prophylactic agent; anti-ulcer [3,4,19,30,43] | Antioxidant; hepatoprotective; renoprotective; anti-arthritis [26,31,32,33,44] | Antioxidant; cicatricial agent; hepatoprotective; treatment of urinary infections, rheumatism, and wounds; protective against doxorubicin-induced cardiotoxicity [27,35,45,46,47,48,49,50,51] | Antioxidant; as treatment for rheumatism [12,36,37,38,52,53,54,55] | Antioxidant; anti-osteoclastic; anti-osteoarthritic [40,56,57,58] |
| Effect on respiratory system | Respiratory disorder [25] | Anti-asthmatic [4,19,29,30,43,59] | As treatment for respiratory disorder (bronchitis, asthma) [26,31,32,44] | |||
| Effect on gastrointestinal system | Anti-diarrheal; anti-colon toxin; gastric ailments [25] | Treatment against dysentery, diarrhea [4,19,29,30,43,59] | As treatment for dysentery and stomach disease [26,31] | As treatment for diarrhea; cramp; indigestion; flatulence; nausea; carminative [36,37,38,39] | Digestant; induces ileum and distal colon contraction; gastrointestinal protection; relaxes gastric body; as treatment for digestive disorders [56,60,61] | |
| Effect on nervous system | Anti-depressant; neuroprotective; antispasmodic; analgesic [4,25,62] | Vasorelaxant; modulator of human monoamine; GABA transporter; anti-depressant; anti-nociceptive; analgesic neuroprotector [4,12,19,30,63,64,65] | Neuroprotective; anti-stress; anti-Parkinson and Alzheimer; nootropic; anti-convulsant; analgesic [26] | Analgesic [27,34] | As treatment for neuralgia; sedative; spasmolytic; analgesic; anesthetic; anti-depression; anti-stress [12,37,38,39,54] | |
| Effect on immunologic system | Anti-pyretic; immunomodulatory; anti-inflammatory; intermittent fever [25,42,62] | Antiproliferative; anti-inflammatory; immunomodulatory; anti-ulcer [4,19,29,30,59,64,65,66] | Immunomodulatory; anti-inflammatory [26,32,44,67] | Anti-inflammatory; anti-allergic; antiproliferative [27,34,35,47,50,51] | Anti-inflammatory; antiseptic [37] | Anti-inflammatory [40,56,58,61] |
| Enhanced activities | Pancreatic amylase, chymotrypsin activation, protease, lipase [25] | Wound healing [4,19] | Circulatory stimulant, vasorelaxant [26,33] | As stimulant in digestive disorders (purgatives) [37,38] | Enhanced transdermal drug penetration [40] | |
| Response to biotic factors | Insecticidal; larvicidal; pesticidal; antibacterial; antifungal [4,25,42,62] | Anti-protozoal; trypanocidal; antiviral; as treatment against syphilis gonorrhea; leishmanicidal; molluscicidal; insecticidal; acaricidal; anti-amebic; antibacterial; antifungal; antiparasitic [4,19,29,30,59,63,64,65] | Antibacterial; mosquito larvicidal; acaricidal; anti-amoebic; antifungal; antimicrobial; anthelmintic [26,31,33,44,67] | Antiviral; antimicrobial; antifungal; insecticidal; antibacterial; protective against multi-drug-resistant strains [27,35,47,48,50,68] | Antimicrobial; acaricidal; antifungal; nematicidal; inhibits Leishmania amazonesis; antibacterial; anti-SARS-Cov 2; antiparasitic [12,36,37,38,52,55,69,70] | Antimicrobial; vermicide; antiviral; as herbicide barrier [40,60] |
| Effect on genomic expression | Anti-mutagenic; inhibits transcription; inhibits cytochrome; anti-metastatic; antitumor | Anti-mutagenic; cytotoxicity; anticancer; antitumor; genotoxic [4,19,29,30,59,63,64,65] | Anticancer and tumor [4,26,31,32,33,44] | Antitumor [50] | Anticancer [37,38] | Control proliferation of osteosarcoma [58] |
| Adverse effects | Anti-spermatogenic; interactions with medical products; may affect pregnant or breastfeeding women; EO could be considered as irritant | Genotoxic at 1.5 g/kg; may irritate the gastrointestinal tract and kidneys; cytotoxic at 280 mM of (-) cubebin; irritant [19,30,64] | Emmenagogue; anti-conceptive; infertility action; ACE inhibitor; increases the weight of lungs and spleen [26,31,33] | May cause allergies, mutagenic properties [45] | Some EOs could be cytotoxic, irritant, corrosive, and phytotoxic [12] | |
| Other effects | Ciprofloxacin potentiator; enhances the bioavailability of some nutrients like vitamins, β-carotene, and selenium [25] | Anti-platelet-activating factor (PAF); CYP3A4 inhibitor; cytochrome P450 inhibitor; melanogenesis activity [4,30,63] | Aphrodisiac; anti-angiogenic; anti-platelet; anti-ulcer; photoprotective [26,33,67] | Cytoprotective [64]; aphrodisiac; digestive stimulant agent; rubefacient agent [38] | Prevention of toothache; use as skin-lifting agent [40,56] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Guerrero, P.; Panzani, S.; Sanmartin, C.; Muntoni, C.; Taglieri, I.; Venturi, F. “Pepper”: Different Spices, One Name—Analysis of Sensory and Biological Aspects. Molecules 2025, 30, 1891. https://doi.org/10.3390/molecules30091891
Díaz-Guerrero P, Panzani S, Sanmartin C, Muntoni C, Taglieri I, Venturi F. “Pepper”: Different Spices, One Name—Analysis of Sensory and Biological Aspects. Molecules. 2025; 30(9):1891. https://doi.org/10.3390/molecules30091891
Chicago/Turabian StyleDíaz-Guerrero, Pierina, Sofia Panzani, Chiara Sanmartin, Chiara Muntoni, Isabella Taglieri, and Francesca Venturi. 2025. "“Pepper”: Different Spices, One Name—Analysis of Sensory and Biological Aspects" Molecules 30, no. 9: 1891. https://doi.org/10.3390/molecules30091891
APA StyleDíaz-Guerrero, P., Panzani, S., Sanmartin, C., Muntoni, C., Taglieri, I., & Venturi, F. (2025). “Pepper”: Different Spices, One Name—Analysis of Sensory and Biological Aspects. Molecules, 30(9), 1891. https://doi.org/10.3390/molecules30091891








