Abstract
We are describing a brief stereospecific synthesis of a model compound related to Saudin, with a lactone ketal backbone present in the natural product starting from a tricyclic epoxiketal.
Introduction
Saudin is a diterpene belonging to the labdane prefuranoid family; that was isolated from the toxic plant Cluytia richardiana (L), Euforbiaceae family, growing in Arabia Saudi, in 1985 [1]. The importance of this compound resides in its interesting potential biological properties as hypoglucemic agent.
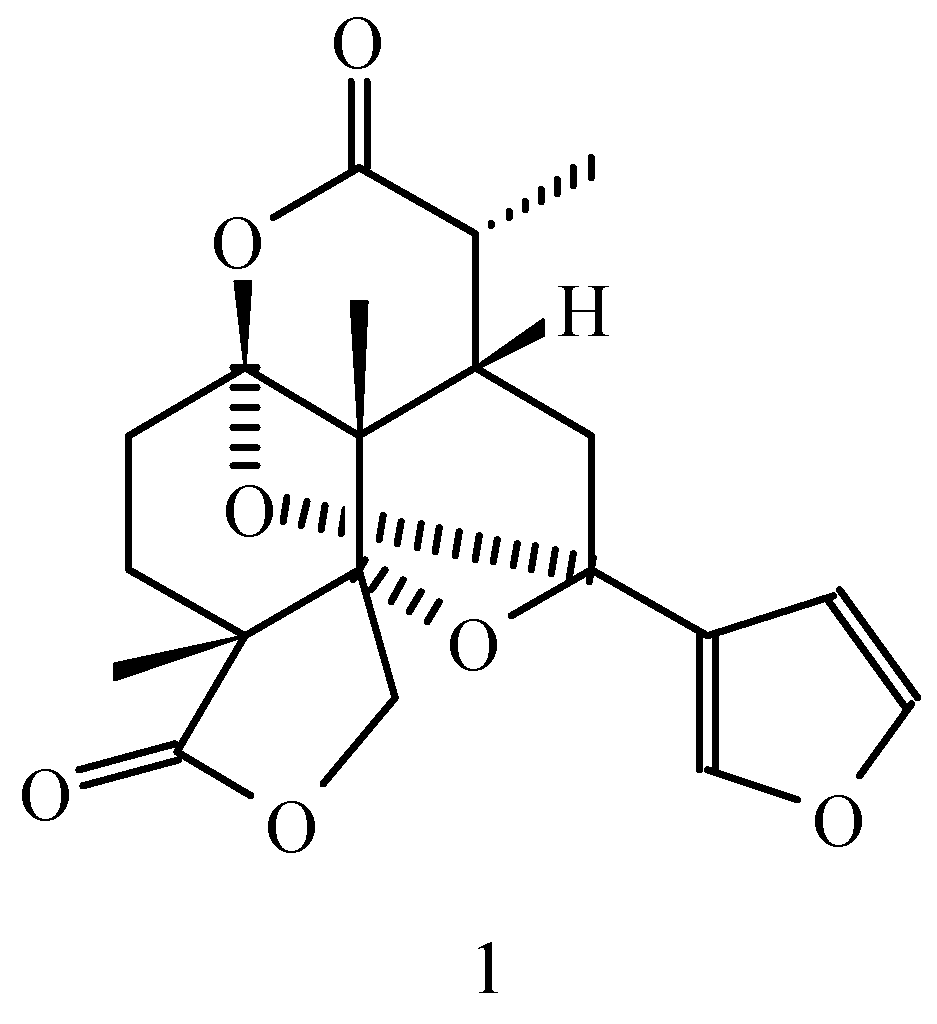
Continuing our efforts to the synthesis of intermediates related to Saudin, in this opportunity, we will present the synthesis of 2 which have the lactone-ketal structure found into the natural product with a 7 members ring instead of a 6 members as in Saudin.
Synthesis Design
According to the following retrosynthetic analysis:
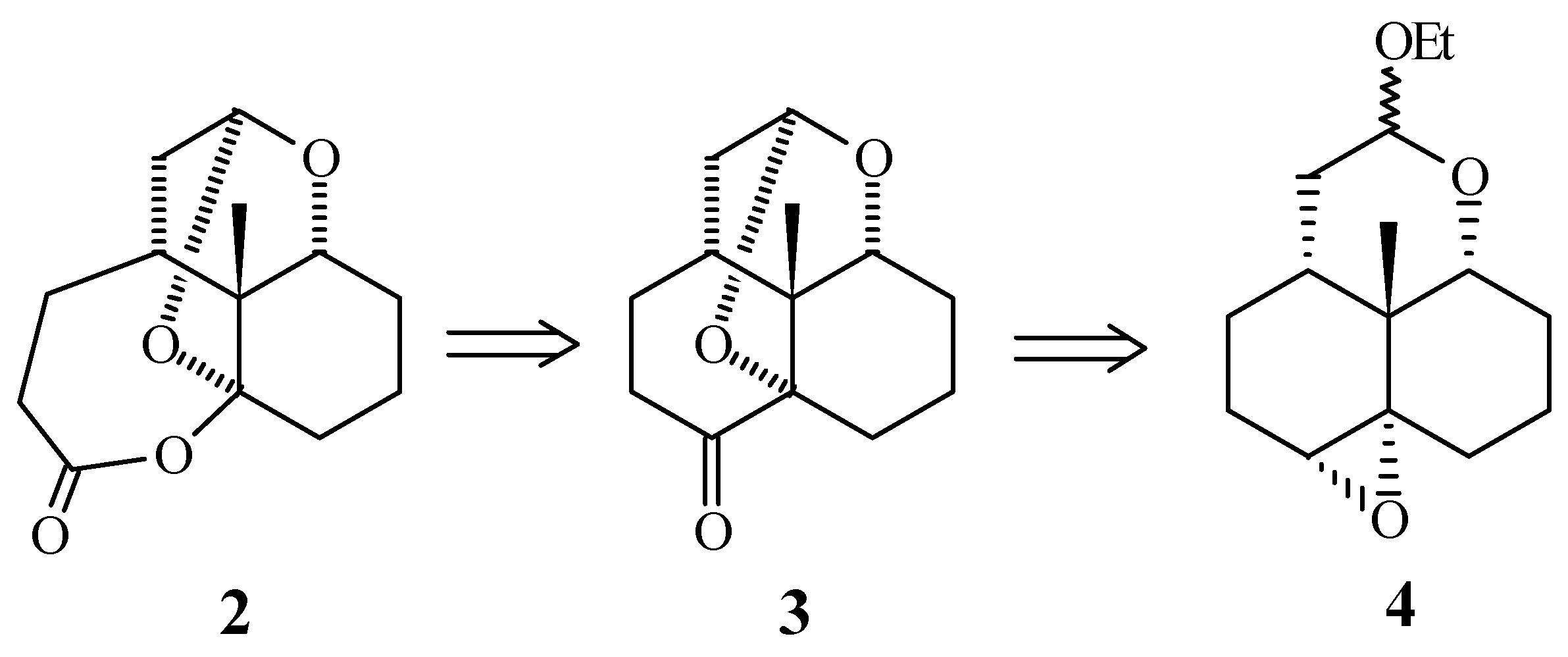
Compound 2 would be prepared from the intermediate 3 using a Baeyer-Villiger type reaction. In turn, compound 3 would be synthesize from the tetracyclic epoxiketal 4, by means of an epoxide cleavage followed by oxidation and cyclic ketal formation.
Experimental
The epoxyketal intermediate 4 was synthesized from the α- tetralone by a five steps sequence previously developed in our research group [2] that includes: a Birch-alkylation reaction, the stereospecific reduction of the carbonyl group, a regio and stereospecific epoxidation followed by a bromo ketal formation, and finally a radical cyclization [3]. After different alternatives we found that by treatment of 4 with Jones´s reagent, in acetone, compound 3 was obtained in good yield.
After oxidation of this compound under Baeyer Villiger conditions with solid hydrogen carbonate, the product 2 was obtained regioselectively. The lactone-ketal 2 was characterized using the spectroscopic methods and the comparison of the 13C NMR spectrum signals are in agreement with those reported for the natural product.
Acknowledgements:
We thank to Universidad Nacional de Rosario, CONICET and Agencia Nacional de Promoción Científica y Tecnología.
References and Notes
- Mossa, J. S.; Cassady, J. M.; Antoun, M. D.; Byrn, S. R.; McKenzie, A. T.; Kozlowski, J. F.; Main, P. J. Org. Chem. 1985, 50, 916–917.
- Labadie, G. R.; Cravero, R. M.; González-Sierra, M. Synth. Comm. 1996, 26, 4671–4684.
- Labadie, G. R. Tesis de Doctorado, UNR, 1999.
