Abstract
Full and unambiguous asssignment of all 1H- and 13C-NMR resonances of the free bases as well as the hydrochloride salts of the antiarrhythmic agent propafenone and a thiophene analogue in different solutions (DMSO-d6, CDCl3) is reported.
Introduction
Propafenone (1) is a class Ic antiarrhythmic drug with β-adrenoreceptor blocking and calcium antagonistic activity [1,2]. Moreover, 1 and related compounds have been identified to be highly effective modulators of multidrug resistance [3,4,5].
Although some NMR data of propafenone-like molecules have been reported [5,6,7,8] in most cases full assignments are missing and to the best of our knowledge no 13C-NMR data for the parent compound 1 have been published. Thus, the present communication deals with the completely assigned 1H- and 13C-NMR spectra of propafenone and its thiophene analogue 2 [9], obtained by combined application of one and two-dimensional standard NMR techniques.
Results and Discussion
Complete and unambiguous assignments of all proton and carbons resonances were achieved on the basis of chemical shift considerations, coupling information (APT [10] and 'gated decoupled' 13C-NMR spectra), and NOE-difference [11], COSY45 [12], HMQC [13], and 1D-TOCSY [14] spectra as well as on long-range INEPT experiments with selective excitation [15]. The numbering of atoms used in the discussion and in Table 1 and Table 2 is given in the formulas of Scheme 1.
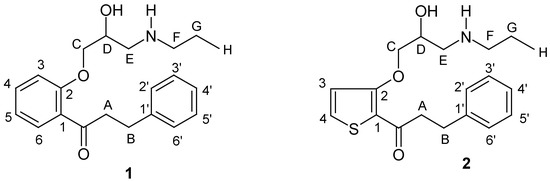
Scheme 1.
Propafenone (1) and its Thiophene Analogue (2)
Scheme 1.
Propafenone (1) and its Thiophene Analogue (2)
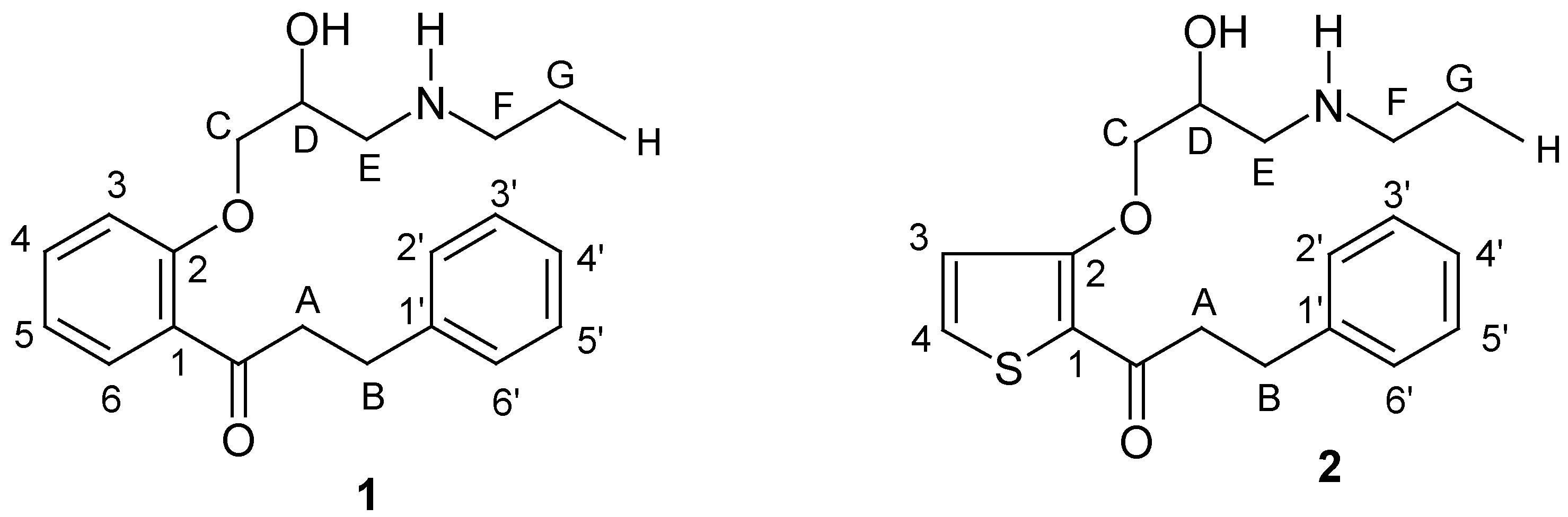
1H-NMR Spectra
Whereas the aromatic region of the 1H-NMR spectra of the investigated compounds is easy to interpret (AX-system for thiophene protons of 2, four different signals of H-3, H-4, H-5, and H-6 of propafenone (1) - with the long-range coupling 5J(H-3,H-6) not resolved) the aliphatic part of the spectra is much more complex. Although - at first sight - the signal of protons HB in some cases seem to have a pseudo-triplet structure, the nuclei attached to carbons A and B give rise to a spin-system consisting of four non-equivalent protons (ABMN), with the accurate coupling constants and chemical shifts not directly extractable from the higher order multiplets. Thus, in Table 1 only the centers of the signals due to protons A and B are given. The protons of the O-CH2-CH(OH)-CH2-N substructure formally establish an ABMXY spin-system, the chiral carbon center D causing more or less non-equivalence of the adjacent diastereotopic protons HC and HC', as well as of HE and HE' (see Figure 1 for 1•HCl in DMSO-d6). In DMSO-d6 solutions, the signal due to HD of 1•HCl is additionally split by a vicinal coupling to the acidic OH proton. However, in many cases the corresponding chemical shifts and coupling constants of this substructure can be determined with sufficient accuracy. In principle, methylene protons HF/HF' and HG/HG' of the propylamino moiety are also non-equivalent, the signal of HF/HF' showing more deviation from a first order pattern than that of HG/HG' (Figure 1). Expectedly, hydrochloride salt formation in general leads to larger chemical shifts for the proton signals of the aminoalcohol moiety compared with those of the corresponding free bases.
13C-NMR Spectra
The 13C chemical shifts and some selected 13C,1H spin coupling constants are collected in Table 2. The data show a high degree of consistency, in nearly all cases the chemical shifts for carbons of the aminoalcohol chain (carbons C-H) are somewhat reduced when switching from the free bases to the hydrochloride salts (the opposite trend as observed for the corresponding 1H chemical shifts). It should be mentioned that a good estimation of the 13C chemical shifts in 1 could be performed using the CSEARCH-program [16], the difference between the predicted (also given in Table 2) and observed values is less than 3.8 ppm for all carbon atoms.
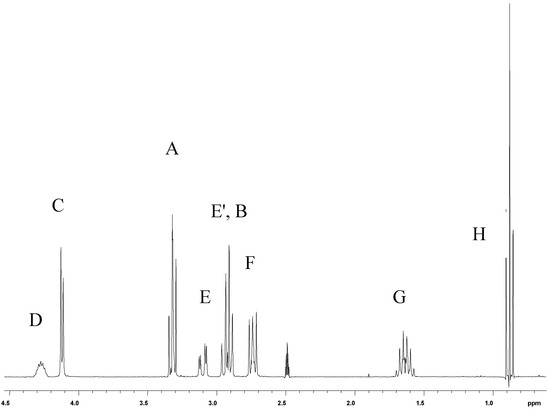
Figure 1.
Aliphatic Part of the 1H-NMR Spectrum of 1•HCl (in DMSO-d6 Solution)
Figure 1.
Aliphatic Part of the 1H-NMR Spectrum of 1•HCl (in DMSO-d6 Solution)
Conclusions
We have presented the complete 1H- and 13C-NMR chemical shifts of propafenone (1) and its thiophene analogue 2 as well as some selected spin-spin coupling constants.
Experimental
The NMR spectra were obtained using a Varian UnityPlus spectrometer (300 MHz for 1H, 75 MHz for 13C) from DMSO-d6 and CDCl3 solutions (concentrations approximately 0.1 M, 1•HCl in CDCl3 had a much lower concentration due to solubility problems) at 28 °C. The center of the solvent signal was used as internal standard which was related to TMS with δ 7.26 ppm (1H, CDCl3), δ 2.49 ppm (1H, DMSO-d6), δ 77.0 ppm (13C, CDCl3), and δ 39.5 ppm (13C, DMSO-d6). The digital resolution was 0.2 Hz/data point for the 1H-NMR spectra and 0.5 Hz/data point for the 13C-NMR spectra. Propafenone hydrochloride was obtained from Sigma-Aldrich Chemical Company (USA), its thiophene analogue 2•HCl was prepared according to the literature [9]. The corresponding free bases were obtained by treatment of aqueous solutions of the hydrochlorides with an excess of potassium carbonate and subsequent extraction with dichloromethane. The base 2 gave a satisfactory elemental analysis (calcd. for C19H25NO3S: C 65.68; H 7.25; N 4.03. Found: C 65.52; H 7.21; N 3.93) and showed a melting point of 60 °C (the re-solidified product had a mp of 71 °C).

Table 1.
1H- Chemical Shifts and Selected 1H,1H-Coupling Constants.
| Compound | 1 | 1•HCl | 2 | 2•HCl | ||||
|---|---|---|---|---|---|---|---|---|
| Solvent | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 |
| 'aromatic' H-atoms' | ||||||||
| 3 | 7.14 | 6.96 | 7.16 | 6.99 | 7.12 | 6.85 | 7.15 | 6.87 |
| 4 | 7.49 | 7.42 | 7.50 | 7.50 | 7.86 | 7.48 | 7.86 | 7.44 |
| 5 | 6.99 | 7.00 | 7.01 | 7.05 | --- | --- | --- | --- |
| 6 | 7.52 | 7.65 | 7.53 | 7.74 | --- | --- | --- | --- |
| 2',6' | 7.22 | 7.24 | 7.21-7.28 | 7.21 | 7.24 | 7.25 | 7.23-7.30 | 7.24 |
| 3',5' | 7.23 | 7.27 | 7.21-7.28 | 7.30 | 7.25 | 7.28 | 7.23-7.30 | 7.28 |
| 4' | 7.15 | 7.17 | 7.15 | 7.20 | 7.16 | 7.18 | 7.15 | 7.15 |
| ‘aliphatic' H-atoms | ||||||||
| A | 3.33 | 3.33 | 3.33 | 3.32 | 3.21 | 3.23 | 3.22 | 3.13 |
| B | 2.90 | 3.03 | 2.91 | 3.01 | 2.90 | 3.02 | 2.92 | 2.99 |
| C, C' | 4.08, 4.01 | 4.06 | 4.14 | 4.20 | 4.19, 4.13 | 4.14, 4.13 | 4.26 | 4.27, 4.22 |
| D | 3.90 | 4.00 | 4.32 | 4.54 | 3.86 | 3.96 | 4.26 | 4.60 |
| E, E' | 2.64, 2.58 | 2.79, 2.69 | 3.11, 2.94 | 3.43, 3.14 | 2.61, 2.56 | 2.78, 2.69 | 3.11, 2.93 | 3.27, 3.13 |
| F | 2.41 | 2.53 | 2.74 | 2.94 | 2.39 | 2.51 | 2.74 | 2.88 |
| G | 1.37 | 1.49 | 1.66 | 1.97 | 1.36 | 1.46 | 1.65 | 1.88 |
| H | 0.83 | 0.91 | 0.87 | 1.01 | 0.83 | 0.91 | 0.88 | 0.96 |
| NH and OH | * | 2.70 (2H) | 9.15 (2H), 5.96 | 9.40, 8.84, 1.65 | 4.99 (1 H) | 2.15 (2H) | 9.13 (2H), 5.90 | 9.28, 8.70 |
* Not unequivocally identified
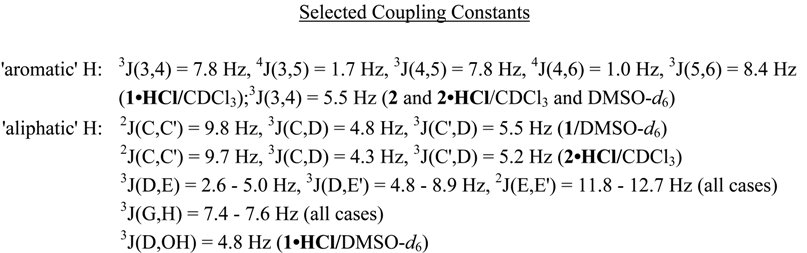

Table 2.
13C-NMR Chemical Shifts and Selected 13C,1H Coupling Constants.
| Compound | 1 | 1•HCl | 2 | 2•HCl | |||||
|---|---|---|---|---|---|---|---|---|---|
| Solvent | DMSO-d6 | CDCl3 | * | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 | DMSO-d6 | CDCl3 |
| sp2-hybridized C-atoms | |||||||||
| 1 | 128.0 | 128.4 | 125.5 | 128.0 | 128.0 | 121.5 | 122.9 | 121.7 | 121.4 |
| 2 | 157.5 | 157.6 | 159.1 | 157.1 | 157.1 | 159.8 | 159.2 | 159.0 | 159.3 |
| 3 | 113.1 | 113.0 | 115.9 | 113.2 | 113.2 | 118.1 | 116.9 | 118.0 | 117.1 |
| 4 | 133.4 | 133.3 | 132.5 | 133.5 | 134.5 | 133.7 | 132.5 | 133.4 | 132.2 |
| 5 | 120.4 | 121.0 | 121.4 | 120.7 | 121.3 | --- | --- | --- | --- |
| 6 | 129.5 | 130.2 | 130.7 | 129.5 | 130.9 | --- | --- | --- | --- |
| 1' | 141.3 | 141.5 | 140.7 | 141.3 | 141.0 | 141.3 | 141.6 | 141.2 | 141.2 |
| 2',6' | 128.2 | 128.3 | 128.7 | 128.3 | 128.4 | 128.2 | 128.3 | 128.1 | 128.4 |
| 3',5' | 128.1 | 128.3 | 128.4 | 128.2 | 128.6 | 128.1 | 128.3 | 128.0 | 128.5 |
| 4' | 125.6 | 125.9 | 126.3 | 125.7 | 126.2 | 125.7 | 125.9 | 125.5 | 126.1 |
| C=O | 201.1 | 201.5 | 201.8 | 201.0 | 201.7 | 191.3 | 192.1 | 191.1 | 192.0 |
| sp3-hybridized C-atoms | |||||||||
| A | 44.5 | 45.1 | 41.3 | 44.4 | 43.4 | 42.1 | 43.1 | 41.9 | 43.0 |
| B | 29.7 | 30.2 | 30.6 | 29.7 | 30.2 | 29.5 | 30.1 | 29.3 | 30.1 |
| C | 71.2 | 71.4 | 72.0 | 70.4 | 71.5 | 74.4 | 74.0 | 73.4 | 74.2 |
| D | 67.9 | 67.8 | 69.3 | 64.8 | 64.8 | 68.1 | 67.7 | 64.8 | 65.0 |
| E | 52.3 | 51.8 | 52.4 | 49.8 | 51.6 | 52.1 | 51.5 | 48.7 | 51.5 |
| F | 51.2 | 51.6 | 52.3 | 48.8 | 50.7 | 51. 3 | 51.5 | 48.7 | 50.6 |
| G | 22.5 | 23.1 | 23.1 | 18.7 | 19.4 | 22.7 | 23.2 | 18.5 | 19.3 |
| H | 11.6 | 11.6 | 11.9 | 10.9 | 11.2 | 11.7 | 11.6 | 10.7 | 11.1 |
* 13C-NMR chemical shifts estimated by the CSEARCH-program [16] using neuronal network technology
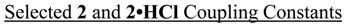
1J(C4,H4) = 171.7 (2/DMSO-d6), 169.7 (2/CDCl3), 171.7 (2•HCl/DMSO-d6), 170.7 (2•HCl/CDCl3)
2J(C4,H5) = 4.5 Hz (all cases); 2J(C5,H4) = 4.5 Hz (all cases)
1J(C5,H5) = 188.8 (2/DMSO-d6), 186.3 (2/CDCl3), 188.9 (2•HCl/DMSO-d6), 187.1 (2•HCl/CDCl3)
References and Notes
- Petrik, W.; Sachse, R. (Helopharm) Verfahren zur Herstellung neuer, therapeutisch wertvoller Derivate des 2'-Hydroxy-3-phenyl-propiophenons und deren Salze. DE 2001431, Jan. 6, 1970. Chem. Abstr. 1971, 75, 151538f. [Google Scholar]
- Bryson, H. M.; Palmer, K. J.; Langtry, H. D.; Fitton, A. Propafenone. A Reappraisal of its Pharmacology, Pharmacokinetics and Therapeutic Use in Cardiac Arrhythmias. Drugs 1993, 45, 85–130. [Google Scholar]
- Chiba, P.; Ecker, G.; Tell, B.; Moser, A.; Schmid, D.; Drach, J. Modulation of PGP-Mediated Multidrug-Resistance by Propafenone Analogs. Proc. Am. Assoc. Cancer Res. 1994, 35, 357. [Google Scholar]
- Ecker, G.; Fleischhacker, W.; Chiba, P. Pharmakologisch wirksame o-Acylaryloxypropanolamine mit tertiärem und quartärem Stickstoff. Austrian Patent Appl. 15A 963/93-1, 17 May 1993. [Google Scholar]
- Chiba, P.; Burghofer, S.; Richter, E.; Tell, B.; Moser, A.; Ecker, G. Synthesis, Pharmacologic Activity, and Structure-Activity Relationships of a Series of Propafenone-Related Modulators of Multidrug Resistance. J. Med. Chem. 1995, 38, 2789–2793. [Google Scholar] [CrossRef] [PubMed]
- Ecker, G.; Chiba, P.; Hitzler, M.; Schmid, D.; Visser, K.; Cordes, H. P.; Csöllei, J.; Seydel, J. K.; Schaper, K.-J. Structure-Activity Relationship Studies on Benzofuran Analogs of Propafenone-Type Modulators of Tumor Cell Multidrug Resistance. J. Med. Chem. 1996, 39, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Studenik, C.; Lemmens-Gruber, R.; Heistracher, P.; Ecker, G.; Maxl, A.; Fleischhacker, W. Electromechanical Effects of Newly Synthetized Propafenone Derivatives on Isolated Guinea-Pig Heart Muscle Preparations. Arzneim.-Forsch./Drug Res. 1996, 46(I), 134–138. [Google Scholar]
- Sánz-García, T.; González-Gaitano, G.; Iza, N.; Gálvez-García, A.; Tardajos, G. NMR Study of the Inclusion Complex between β-Cyclodextrin and Propafenone. In Spectroscopy of Biological Molecules: New Directions, 8th European Conference of Biological Molecules September 1999; Kluwer Academic Publishers: Dodrecht, 1999; pp. 333–334. [Google Scholar]
- Binder, D.; Noe, C. R.; Holzer, W. Ein Thiophenanalogon des Propafenons. Arch. Pharm. (Weinheim) 1990, 323, 919–921. [Google Scholar] [CrossRef]
- Patt, S. L.; Shoolery, J. N. Attached Proton Test for Carbon-13 NMR. J. Magn. Reson. 1982, 46, 535–539. [Google Scholar]
- Neuhaus, D.; Williamson, M. P. The Nuclear Overhauser Effect in Structural and Conformational Analysis; VCH Publishers: New York - Weinheim - Cambridge, 1989; pp. 211–252. [Google Scholar]
- Bax, A.; Freeman, R. Investigation of Complex Networks of Spin-Spin Coupling by Two-Dimensional NMR. J. Magn. Reson. 1981, 44, 542–561. [Google Scholar]
- Bax, A.; Subramanian, S. Sensitivity-Enhanced Two-Dimensional Heteronuclear Shift Correlation NMR Spectroscopy. J. Magn. Reson. 1986, 67, 565–569. [Google Scholar]
- Davis, D. G.; Bax, A. Simplification of 1H NMR Spectra by Selective Excitation of Experimental Subspectra. J. Am. Chem. Soc. 1985, 107, 7197–7198. [Google Scholar] [CrossRef]
- Bax, A. Structure Determination and Spectral Assignment by Pulsed Polarization Transfer via Long-Range 1H-13C Couplings. J. Magn. Reson. 1984, 57, 314–318. [Google Scholar]
- Kalchhauser, H.; Robien, W. CSEARCH: A Computer Program for Identification of Organic Compounds and Fully Automated Assignment of Cabon-13 Nuclear Magnetic Resonance Spectra. J. Chem. Inform. Comput. Sci. 1985, 25, pp. 103–108, For more information and the performance of estimations see http://mailbox.univie.ac.at/~robienw8/csearch_server_info.html. [CrossRef]
- Sample Availability: Compound 2•HCl is available from MDPI.
© 2001 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
