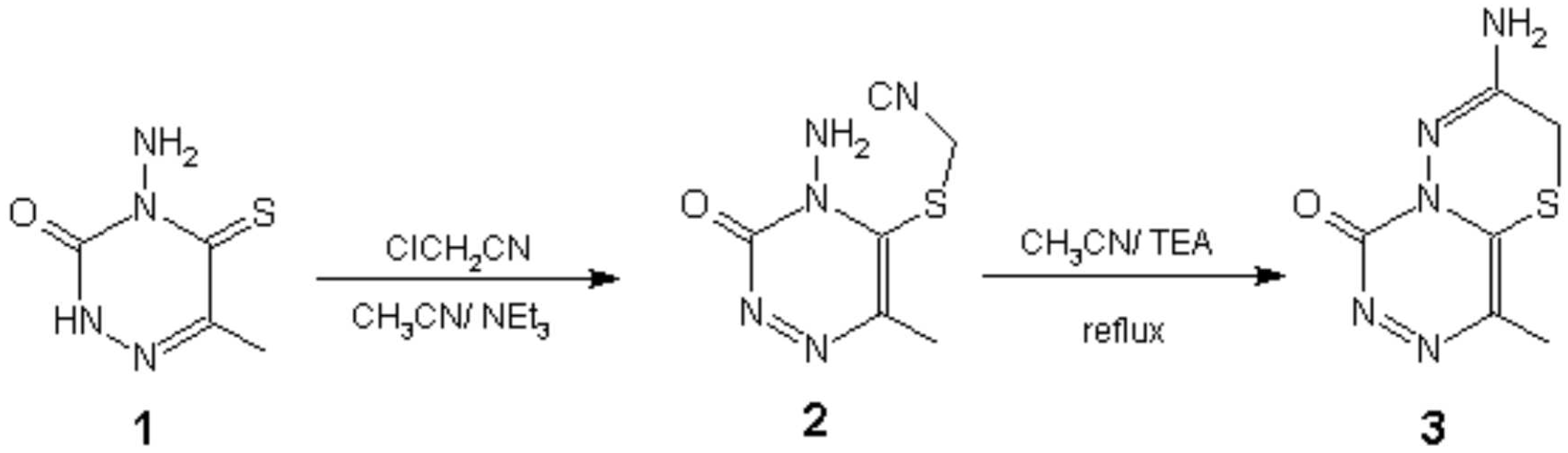
Condensation of 4-amino-6-methyl-5-thio-1,2,4-triazin-3-one 1 with chloro acetonitrile in the presence of triethey amine afforded the corresponding 5-thiocyanomethyl derivative 2. The latter was refluxed in a mixture of acetonitrile and triethylamine to give the title compound 3. Compound 1 (0.316 g, 2 mmol) was dissolved in a solution of acetonitrile (8 mL) and triethylamine (0.6 mL). To this solution chloro acetonitrile (0.2 mL, excess) was added. The reaction mixture was refluxed for 12 hrs at room temperature. The solvent was evaporated and the residue was washed with hot EtOH to afford 2. The latter (0.2 g, 1 mmol) was refluxed in a mixture of acetonitrile (5 mL) and triethylamine (0.3 mL) for 4 hrs. The solvent was evaporated and the residue was crystallized from CHCl3 to afford 3.
Selected Data for 2. Yield: 72%, mp.: 188-9° C, 1HNMR (CDCl3)δ, 2.3(s, 3H, Me), 4.1(s, 2H, CH2), 5.9(s, 2H, NH2, exchangeable with D2O). IR, (KBr disc): 3400, 3300, 2400, 1690, 1210, 1100 cm-1, M.S., m/z, M+, 196(3), 195(44), 194(12), 193(100), 177(47), 56(72).
Selected data for 3. Yield: 75%, mp.: 159-60° C, 1HNMR δ (CDCl3), 2.47(s, 3H, Me), 4.88(s, 2H, CH2), 6.55(s, 2H, NH2, exchangeable with D2O). IR, (KBr disc): 3450, 1690, 1400, 1110 cm-1, M.S., m/z, M+, 196(3), 195(11), 194(18), 73(100),42(23).
Supplementary materials
Supplementary File 1Supplementary File 2Reference
- Heravi, M.M.; Rajabzadeh, Gh.; Rahimizadeh, M.; Bakavoli, M.; Ghassemzadeh, M. Work on P, S, Si and related elements. Submitted in 2000.
- Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/
