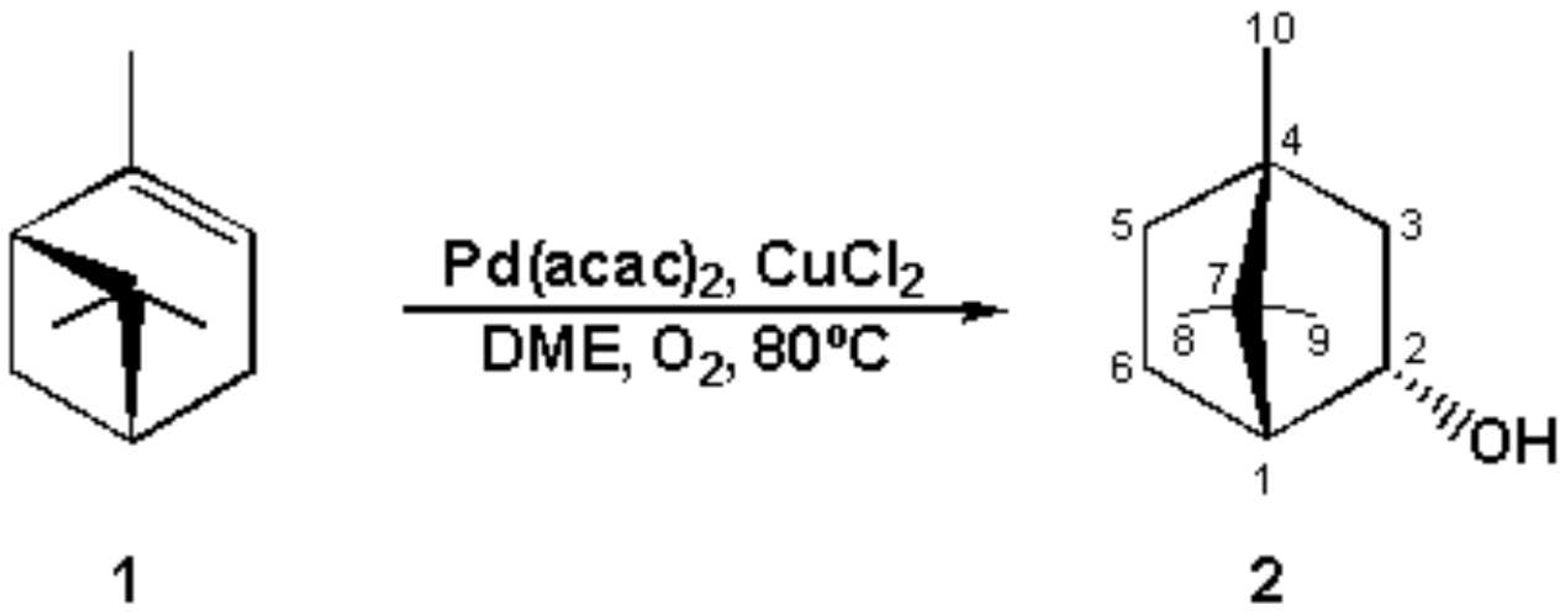
A mixture of Pd(acac)2 (41.26 mg, 0.12 mmol) and CuCl2 (168 mg, 1.25 mmol) [1] in 10 mL of 1,2-dimethoxyethan (DME) was stirred for 30 mn at 80°C under oxygen atmosphere, was added α -pinene (425 mg, 3.12 mmol). The mixture was stirred for another 18h at he same temperature. The evolution of reaction was controlled by GC. When the reaction was completed, 5mL of water and hexane/EtOAc (1:1, 20 mL) were added. The phases were separated and the aqueous one was extracted with hexane/EtOAc (1:1, 4x20mL), and the combined organic phases were washed with water, dried over MgSO4. Removal of the solvent under reduced pressure and purification of the residue by flash chromatography, using hexane as eluent, gave the compound 2 (45%) as a colorless oil [2].
1H NMR (300MHz, CDCl3) 0.87(6H; 8-Me and 9-Me) 0.92(s; 3H; 10-Me) 1.30(3H, m) 1.70(2H, m) 2,06(1H, m) 2.45(1H, m, 1-CH) 4.16(1H, ddd; J2ax, 3ax 10.8 J2ax, 1eq 4.2 J2ax, 3eq 2.4Hz, 2-CH).
13C NMR (75MHz, CDCl3) 13.27(CH3, C-10); 18.48 and 20.58 (CH3, C-8 and C-9) 28.03 and 28.12 (CH2, C-6 and C-5) 40.16 (CH2, C-3) 44.92 (CH, C-1) 47.86 (quat, C-7) 50.83(quat, C-4); 68.01(CH, C-2).
Supplementary materials
Supplementary File 1Supplementary File 2References
- EL Firdoussi, L.; Baqqa, A.; Allaoud, S.; Aitallal, B.; Karim, A.; Castanet, Y.; Mortreux, A. J. Mol. Cat. 1998, 135, 11.
- Bohlmann, F.; Zeisberg, R. Organic Magnetic Resonance 1975, 7, 426.
- Sample Availability: Available from the authors and from MDPI.
© 2001 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/
