As part of a research programme targeting novel indole-like molecules as potential cannabinoid agonists [1,2,3,4] we synthesised methyl 3-(2-(N-morpholino)ethyl-5-methoxybenzofuran-2-carboxylate.
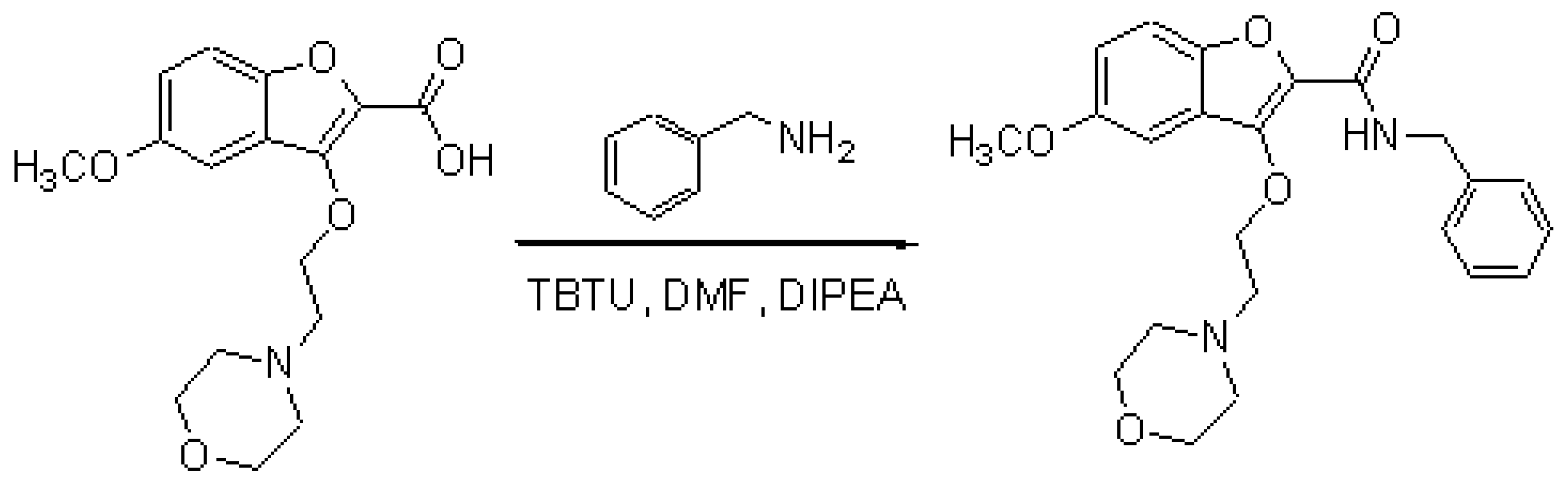
5-Methoxy-3-(2-ethyl morpholine) benzofuran-2-carboxylic acid (50.0 mg, 0.15 mmol) and benzylamine (18.2 mg, 0.17 mmol) and TBTU (55.0 mg, 0.17 mmol) were dissolved in anhydrous DMF (3.0 mL) and to this stirring solution was added DIPEA (33.0 mL, 0.19 mmol). The solution was stirred at room temperature under an atmosphere of nitrogen for 3.0 hours. The DMF was evaporated under reduced pressure and water was added and the aqueous layer was extracted with ethyl acetate. The organic layers were dried over magnesium sulphate, filtered and evaporated under reduced pressure to afford the desired benzofuran-2-carboxamide as an orange gum. This was further purified by column chromatography eluting with (chloroform/methanol) (99/1) to afford (21.39 mg, 33.5 %) of the desired N-benzyl-3-(2-morpholine)ethoxy-5-methoxy benzofuran-2-carboxamide as yellow crystals
M.p. 118-120 °C.
MS : 411 (M+1).+.
IR: 3100, 3000, 1660, 1600, 1500, 1450, 1400, 1300, 1290, 1105, 1100, 1030, 1050, 870, 750.
1H NMR (300 MHz, DMSO-d6): 2.35 (m, 3H, CH2 +CH), 2.65 (m, 2H, CH2), 3.40 (m, 2H, 2 x CH2), 3.81 (d, J = 4.2 Hz, 2H, NHCH2), 4.03 (3H, s, OCH3), 4.53 (m, 3H, CH2 + CH), 7.06 (dd, J = 2.2, J = 6.4 Hz, 1H, H-6), 7.24 (d, J = 2.67 Hz, 1H, H-4), 7.49 (d, J = 9.0 Hz, 1H, H-7), 8.56 (m, 1H, NH).
HPLC retention time = 8.87 minutes, Gradient conditions 30 B/70 A to 90 B/10 A over 20 minutes a (B = 90 % CH3CN, 10 % H2O)/ (A = 0.1 % TFA in H2O) using Zorbax 4.6 mm x 250 mm.
Supplementary materials
Supplementary File 1Supplementary File 2References
- Ward, S.J.; Mastriani, D.; Casiano, F.; Arnold, R. J. Pharmacol. Exp. 1990, 255, 1230–1239.
- Bell, M.R.; D'Ambra, T.E.; Kumar, V.; Eissenstat, M.A.; Herrmann, J.L.; Wetzel, J.R.; Rosi, D.; Philion, R.E.; Daum, S.J.; Hlasta, D.J.; Kullnig, R.K.; Ackerman, J.H.; Haubrich, D.R.; Luttinger, D.A.; Baizman, E.R.; Miller, M.S.; Ward, S.J. J. Med. Chem. 1991, 34, 1099–1110. [PubMed]
- Ward, S.J.; Miller, M.S.; Luttinger, D.A.; Eissenstat, M.A.; Bell, M.R. Neurosci. Abstr. 1988, 14, 324.
- D'Ambra, T.E.; Estep, K.G.; Bell, M.R.; Eissenstat, M.A.; Josef, K.A.; Ward, S.J.; Haycock, D.A.; Baizman, E.R.; Casiano, F.M.; Belgin, N.C.; Chippari, S.M.; Grego, J.D.; Kullnig, R.K.; Daley, G.T. J. Med. Chem. 1992, 35, 124–135. [PubMed]
- Sample availability: available from the authors and MDPI.
© 2001 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/
