Characterization of Catalase from Psychrotolerant Psychrobacter piscatorii T-3 Exhibiting High Catalase Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bacterial Identification
2.2. Purification of P. piscatorii T-3 Catalase
2.3. Molecular Mass and Spectroscopic Properties of the Catalase
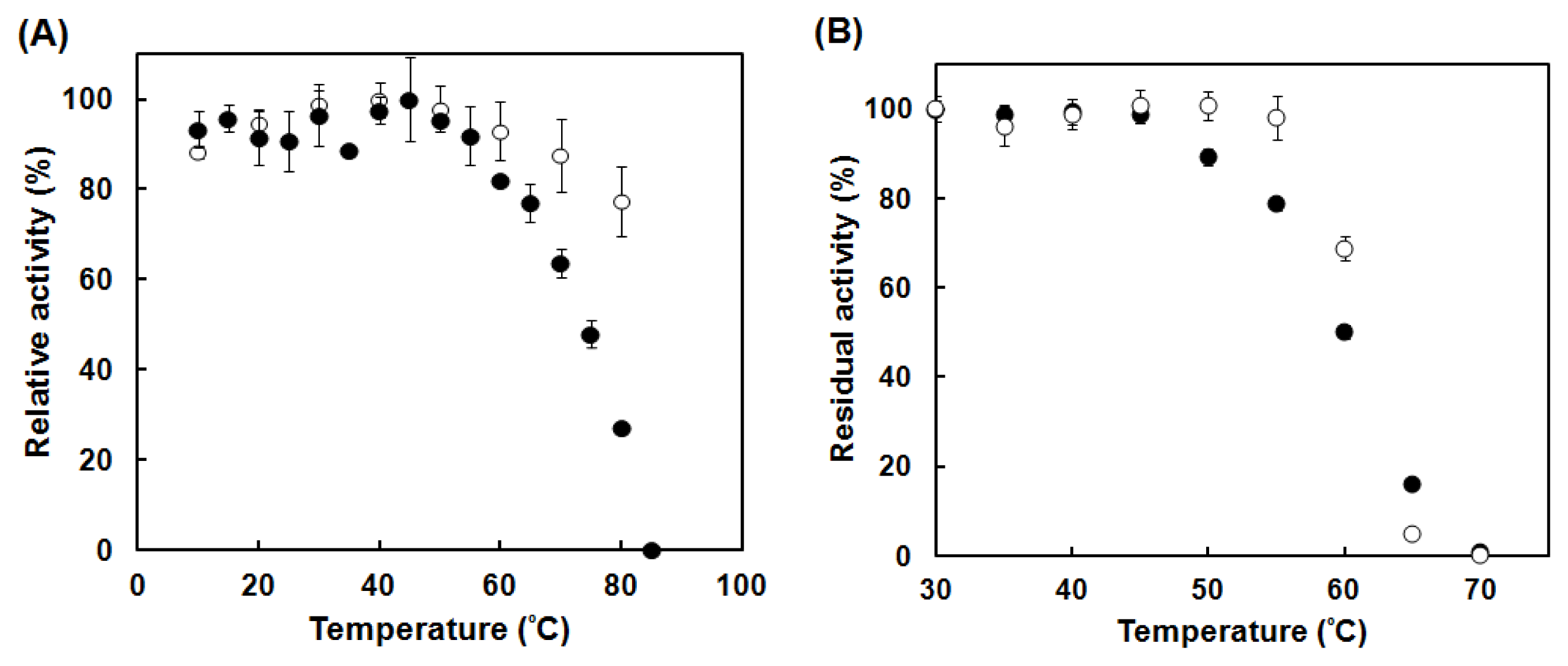
2.4. Enzymatic Characterization of the Catalase
2.5. DNA Gene Sequence of the Catalase
2.6. Discussion
3. Experimental Section
3.1. Chemicals and Enzyme
3.2. Bacterial Strain
3.3. Phenotypic Characterization of Strain T-3
3.4. 16 rRNA Sequencing
3.5. DNA Base Composition and DNA-DNA Hybridization
3.6. Enzyme Assay Condition
3.7. Purification of Catalase from Strain T-3(PktA)
3.8. Physical and Chemical Measurements
3.9. Protein Sequencing
3.10. Determination of Gene Sequence of PktA Catalase
4. Conclusions
Acknowledgments
References
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol 2000, 13, 135–160. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radical in Biology and Medicine, 3rd ed; Clarendon Press: Oxford, UK, 1999. [Google Scholar]
- Imlay, J.A.; Linn, S. DNA damage and oxygen radical toxicity. Science 1988, 240, 1302–1309. [Google Scholar]
- Rowe, L.A.; Degtyareva, N; Doetsch, P.W. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic. Biol. Med. 2008, 45, 1167–1177. [Google Scholar]
- Katsuwon, J.; Anderson, A.J. Characterization of catalase activities in root colonizing isolates of Pseudomonas putida. Can. J. Microbiol 1992, 38, 1026–1032. [Google Scholar]
- Rocha, E.R.; Selby, T.; Coleman, J.P.; Smith, C.J. Oxidative stress response in an anaerobe, Bacteroides fragilis: A role for catalase in protection against hydrogen peroxide. J. Bacteriol 1996, 178, 6895–6903. [Google Scholar]
- Visick, K.L.; Ruby, E.G. The periplasmic, group III catalase Vibrio fisheri is required for normal symbiotic competence and is induced both by oxidative stress and by approach to stationary phase. J. Bacteriol 1998, 180, 2087–2092. [Google Scholar]
- Loewen, P.C.; Klotz, M.G.; Hassett, D.J. Catalase—an “old” enzyme that continues to surprise us. ASM News 2000, 66, 76–82. [Google Scholar]
- Zamocky, M.; Furtmüller, P.G.; Obinger, C. Evolution of catalases from bacteria to humans. Antioxid. Redox Signal 2008, 10, 1527–1548. [Google Scholar]
- Klotz, M.G.; Klassen, G.R.; Loewen, P.C. Phylogenetic relationships among prokaryotic and eukaryotic catalases. Mol. Biol. Evol 1997, 14, 951–958. [Google Scholar]
- Klotz, M.G.; Loewen, P.C. The molecular evolution of catalase hydroperoxidase: Evidence for multiple lateral transfer of gene between prokaryota and from bacteria into eukaryote. Mol. Biol. Evol 2003, 20, 1098–1112. [Google Scholar]
- Yumoto, I.; Yamazaki, K.; Kawasaki, K.; Ichise, N.; Morita, N.; Hoshino, T.; Okuyama, H. Isolation of Vibrio sp. S-1 exhibiting extraordinarily high catalase activity. J. Ferment. Bioeng 1998, 85, 113–116. [Google Scholar]
- Yumoto, I.; Iwata, H.; Sawabe, T.; Ueno, K.; Ichise, N.; Matsuyama, H.; Okuyama, H.; Kawasaki, K. Characterization of a facultatively psychrophilic bacterium, Vibrio rumoiensis sp. nov., that exhibits high catalase activity. Appl. Environ. Microbiol 1999, 65, 67–72. [Google Scholar]
- Ichise, N.; Morita, N.; Hoshino, T.; Kawasaki, K.; Yumoto, I.; Okuyama, H. A mechanism of resistance to hydrogen peroxide in Vibrio rumoiensis S-1. Appl. Environ. Microbiol 1999, 65, 73–79. [Google Scholar]
- Ichise, N.; Hirota, K.; Ichihashi, D.; Nodasaka, Y.; Morita, N.; Okuyama, H.; Yumoto, I. H2O2 tolerance of Vibrio rumoiensis S-1T is attributable to the cellular catalase activity. J. Biosci. Bioeng 2008, 106, 39–45. [Google Scholar]
- Yumoto, I.; Ichihashi, D.; Iwata, H.; Istokovics, A.; Ichise, N.; Matsuyama, H.; Okuyama, H.; Kawasaki, K. Purification and characterization of a catalase from the facultative psychrophilic bacterium Vibrio rumoiensis S-1T exhibiting high catalase activity. J. Bacteriol 2000, 182, 1903–1909. [Google Scholar]
- Ichise, N.; Morita, N.; Kawasaki, K.; Yumoto, I.; Okuyama, H. Gene cloning and expression of the catalase from the hydrogen peroxide-resistant bacterium Vibrio rumoiensis S-1 and its subcellular localization. J. Biosci. Bioeng 2000, 90, 530–534. [Google Scholar]
- Yumoto, I.; Hishinuma-Narisawa, M.; Hirota, K.; Shingyo, T.; Takebe, F.; Nodasaka, Y.; Matsuyama, H.; Hara, I. Exiguobacterium oxidotolerans sp. nov., a novel alkaliphile exhibiting high catalase activity. Int. J. Syst. Evol. Microbiol 2004, 54, 2013–2017. [Google Scholar]
- Takebe, F.; Hara, I.; Matsuyama, H.; Yumoto, I. Effect of H2O2 under low- and high-aeration-level conditions on growth and catalase activity in Exiguobacterium oxidotolerans T-2-2T. J. Biosci. Bioeng 2007, 104, 464–469. [Google Scholar]
- Hara, I.; Ichise, N.; Kojima, K.; Kondo, H.; Ohgiya, S.; Matsuyama, H.; Yumoto, I. Relationship between the size of the bottleneck 15 Å away from iron in the main channel and reactivity of catalase corresponding to the molecular size of substrates. Biochemistry 2007, 46, 11–22. [Google Scholar]
- Yumoto, I.; Hirota, K.; Kimoto, H.; Nodasaka, Y.; Matsuyama, H.; Yoshimune, K. Psychrobacter piscatorii sp. nov., a psychrotolerant bacterium exhibiting high catalase activity isolated from an oxidative environment. Int. J. Syst. Evol. Microbiol 2010, 60, 205–208. [Google Scholar]
- Kimoto, H.; Matsuyama, H.; Yumoto, I.; Yoshimune, K. Heme content of recombinant catalase from Psychrobacter sp. T-3 altered by host Escherichia coli growth conditions. Protein Expr. Purif 2008, 59, 357–359. [Google Scholar]
- Nakayama, M.; Nakajima-Kambe, T.; Katayama, H.; Higuchi, K.; Kawasaki, Y.; Fujii, R. High catalase production by Rhizobium radiobacter strain 2-1. J. Biosci. Bioeng 2008, 106, 554–558. [Google Scholar]
- Switala, J.; Loewen, P.C. Diversity of properties among catalases. Arch. Biochem. Biophys 2002, 401, 145–154. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acid Res 1994, 22, 4673–4680. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol 1987, 4, 406–425. [Google Scholar]
- Zámocký, M.; Koller, F. Understanding the structure and function of catalases: Clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol 1999, 72, 19–66. [Google Scholar]
- Bakermans, C.; Ayala-del-Río, H.L.; Ponder, M.A.; Vishnivetskaya, T.; Gilichinsky, D.; Thomashow, M.F.; Tiedje, J.M. Psychrobacter cryohalolentis sp. nov. and Psychrobacter arcticus sp. nov., isolated from Siberian permafrost. Int. J. Syst. Evol. Microbiol 2006, 56, 1285–1291. [Google Scholar]
- Loewntzen, M.S.; Moe, E.; Jouve, H.M.; Willassen, N.P. Cold adapted features of Vibrio salmonicida catalase: characterization and comparison to the mesophilic counterpart from Proteus mirabilis. Extremophiles 2006, 10, 427–440. [Google Scholar]
- Yamaguchi, H.; Sugiyama, K.; Hosoya, M.; Takahashi, S.; Nakayama, T. Gene cloning and biochemical characterization of a catalase from Gluconobacter oxydans. J. Biosci. Bioeng 2011, 111, 522–527. [Google Scholar]
- Wang, H.; Tokushige, Y.; Shinoyama, H.; Fujii, T.; Urakami, T. Purification and characterization of thermostable catalase from culture broth of Thermoascus aurantiacus. J. Ferment. Bioeng 1998, 85, 169–173. [Google Scholar]
- Rodrigues, D.F.; da C Jesus, E.; Ayala-Del-Río, H.L.; Pellizari, V.H.; Gilichinsky, D.; Sepulveda-Torres, L.; Tiedje, J.M. Biogeography of two cold-adapted genera: Psychrobacter and Exiguobacterium. ISME J 2009, 3, 658–665. [Google Scholar]
- Barrow, G.I.; Feltham, R.K.A. (Eds.) Cowan and Steel’s Manual for the Identification of Medical Bacteria, 3rd ed; Cambridge University Press: Cambridge, UK, 1993.
- Hugh, R.; Leifson, E. (Eds.) The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J. Bacteriol 1953, 66, 24–26.
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetic analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol 2011, 28, 2731–2739. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol 1980, 16, 111–120. [Google Scholar]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol 1981, 17, 368–376. [Google Scholar]
- Marmur, J. A procedure for the isolation of deoxyribonucleic acid from micro-organismss. J. Mol. Biol 1961, 3, 208–218. [Google Scholar]
- Tamaoka, J.; Komagata, K. Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol. Lett 1984, 25, 125–128. [Google Scholar]
- Esaki, T.; Hashimoto, Y.; Yabuuchi, E. Fluorometric deoxyribonucleic acid–deoxyribonucleic acid hybridization in micro-dilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int. J. Syst. Bacteriol 1989, 39, 224–229. [Google Scholar]
- Hildebraunt, A.G.; Roots, I. Reduced nicotinamide adenine phosphate (NADH)-dependent formation and breakdown of hydrogen peroxide during mixed function oxidation reactions in liver microsomes. Arch. Biochem. Biophys 1975, 171, 385–397. [Google Scholar]
- RØrth, M.; Jensen, P.K. Determination of catalase activity by means of the Clark oxygen electrode. Biochem. Biophys. Acta 1967, 139, 171–173. [Google Scholar]
- Laemmli, U.K.; Favre, M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol 1973, 80, 575–599. [Google Scholar]
- Edman, P.; Henschen, A. Sequence Determination. In Protein sequence determination, 2nd ed; Needlman, S.B., Ed.; Springer-Verlag: Berlin, Germany, 1975; pp. 232–279. [Google Scholar]



| Step | Total protein (mg) | Total activity (U × 103) | Specific activity (U·mg−1) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Crude extract | 1130 | 22,300 | 19,700 | 1.0 | 100 |
| DEAE-Toyopearl | 120 | 17,000 | 142,000 | 7.2 | 76 |
| Phenyl Sepharose | 22.2 | 4940 | 222,000 | 11.0 | 22 |
| Source | Vmax a | Km (mM) | Vmax/Km |
|---|---|---|---|
| Psychrobacter piscatorii T-3 | 235,000 | 75 | 3133 |
| Micrococcus luteus | 284,000 | 147 | 1931 |
| Bacteroides fragilis | 241,000 | 128 | 1883 |
| Helicobacter pylori | 250,000 | 108 | 2315 |
| Serratia marcescens | 228,000 | 180 | 1267 |
| Xanthomonas campestris | 244,000 | 64 | 3812 |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kimoto, H.; Yoshimune, K.; Matsuyma, H.; Yumoto, I. Characterization of Catalase from Psychrotolerant Psychrobacter piscatorii T-3 Exhibiting High Catalase Activity. Int. J. Mol. Sci. 2012, 13, 1733-1746. https://doi.org/10.3390/ijms13021733
Kimoto H, Yoshimune K, Matsuyma H, Yumoto I. Characterization of Catalase from Psychrotolerant Psychrobacter piscatorii T-3 Exhibiting High Catalase Activity. International Journal of Molecular Sciences. 2012; 13(2):1733-1746. https://doi.org/10.3390/ijms13021733
Chicago/Turabian StyleKimoto, Hideyuki, Kazuaki Yoshimune, Hidetoshi Matsuyma, and Isao Yumoto. 2012. "Characterization of Catalase from Psychrotolerant Psychrobacter piscatorii T-3 Exhibiting High Catalase Activity" International Journal of Molecular Sciences 13, no. 2: 1733-1746. https://doi.org/10.3390/ijms13021733
APA StyleKimoto, H., Yoshimune, K., Matsuyma, H., & Yumoto, I. (2012). Characterization of Catalase from Psychrotolerant Psychrobacter piscatorii T-3 Exhibiting High Catalase Activity. International Journal of Molecular Sciences, 13(2), 1733-1746. https://doi.org/10.3390/ijms13021733




