Abstract
Applicability of in vitro biotinylated ubiquitin for evaluation of endogenous ubiquitin conjugation and analysis of ubiquitin-associated protein-protein interactions has been investigated. Incubation of rat brain mitochondria with biotinylated ubiquitin followed by affinity chromatography on avidin-agarose, intensive washing, tryptic digestion of proteins bound to the affinity sorbent and their mass spectrometry analysis resulted in reliable identification of 50 proteins belonging to mitochondrial and extramitochondrial compartments. Since all these proteins were bound to avidin-agarose only after preincubation of the mitochondrial fraction with biotinylated ubiquitin, they could therefore be referred to as specifically bound proteins. A search for specific ubiquitination signature masses revealed several extramitochondrial and intramitochondrial ubiquitinated proteins representing about 20% of total number of proteins bound to avidin-agarose. The interactome analysis suggests that the identified non-ubiquitinated proteins obviously form tight complexes either with ubiquitinated proteins or with their partners and/or mitochondrial membrane components. Results of the present study demonstrate that the use of biotinylated ubiquitin may be considered as the method of choice for in vitro evaluation of endogenous ubiquitin-conjugating machinery in particular subcellular organelles and changes in ubiquitin/organelle associated interactomes. This may be useful for evaluation of changes in interactomes induced by protein ubiquitination under norm and various brain pathologies.
1. Introduction
Ubiquitin is a 76-residue protein, which is widely distributed in all eukaryotic cells [1–5]. The carboxyl group of Gly76 of ubiquitin forms an isopeptide bond with, most typically, the ɛ-amino group of a lysine residue within substrates [6]. ATP-dependent ubiquitin modification of various protein targets determines the role of this protein in numerous intracellular processes including regulation of gene expression, cell cycle and division, stress response, elimination of damaged proteins, DNA repair, import of proteins to mitochondria, assembly of ribosomes, apoptosis, etc. [1–5].
The ubiquitination process includes several stages, which involve several enzymes: ubiquitin activating enzyme, ubiquitin-conjugating enzyme, and ubiquitin ligase [7–8]. Taking into consideration the regulatory role of protein-protein interactions controlling the ubiquitination process [1–9], interactome, it is clear that ubiquitin and other components of the ubiquitn conjugating machinery as well as ubiquitinated proteins do potentially interact with many protein partners and therefore form a particular ubiquitin.
Levels of ubiquitinated proteins and ubiquitin binding partners are usually rather low for detection and therefore there is a clear need for the use of various strategies for their enrichment [6]. Isolation and identification of ubiquitinated proteins usually employ affinity chromatography purification, proteolytic digestion of eluted proteins, and analysis by mass spectrometry. Trypsinolysis of ubiquitinated proteins yields a unique peptide from the ubiquitination site containing a lysine residue with an isopeptide-linked glycine-glycine sequence [9]. This signature peptide can be identified by mass analysis due to its mass shift of 114.1 Da and the lack of proteolytic cleavage of modified lysine residues.
Proteomic analysis of ubiquitinated proteins is frequently based on the incorporation of tagged ubiquitin, which is used for subsequent affinity purification of ubiquitinated proteins [10]. However, the tag-based approach requires genetic manipulation with cells and/or multicellular organisms and therefore is basically inapplicable for analysis of clinically relevant samples [10]. This problem is frequently solved by employment of ubiquitin antibodies [11–12] adapted for affinity purification with some success [10]. However, detection of ubiquitinated proteins by means of antibody-based detection methods usually provides information about ubiquitination of a particular protein and leaves out of consideration possible alterations in the ubiquitin interactome.
An alternative method effective for protein purification is biotin tagging. The biotin-avidin binding (KD = 10−15 M) is the strongest known biochemical non-covalent interaction [13] and is resistant to much more stringent washes, minimizing nonspecific binding. Ubiquitin tagging by biotin usually involves genetic manipulations with microorganisms, cell cultures and whole macroorganisms [10]. Applicability of in vitro biotinylated ubiquitin for evaluation of endogenous ubiquitin conjugating activity and analysis of ubiquitin-associated protein-protein interactions in mammalian tissues and their subcellular fractions has not been investigated yet.
In this study we have performed proteomic profiling of proteins isolated using avidin-Agarose after incubation of rat brain mitochondrial fraction with biotinylated ubiquitin. Although it has been used in various studies on ubiquitination of soluble (non-mitochodnrial) proteins in different types of eukaryotic cells [10], this approach, however, has not been used either for analysis of ubiquitination of brain mitochondrial proteins or for analysis of the mitochondrial interactome. Previous studies have demonstrated that brain mitochondria do contain components of the protein ubiquitination machinery [14,15] and incorporation of exogenous ubiquitin into these organelles in vitro is accompanied by increased sensitivity of some enzymes to proteolysis [16,17].
2. Results
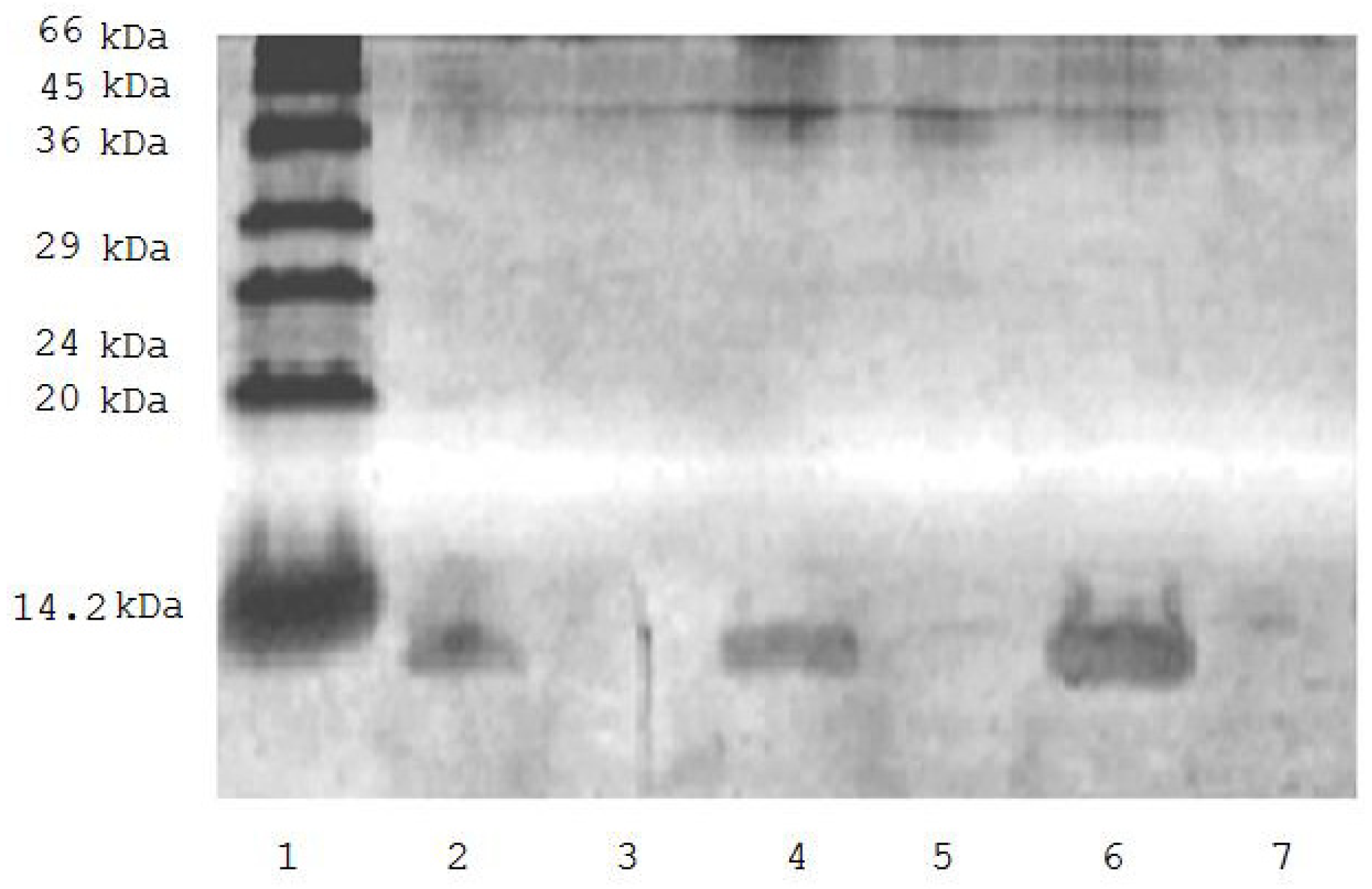
Figure 1 shows that incubation of biotinylated ubiquitin (0.9 mg/mL) with avidin-agarose suspension (1:1) resulted in complete elimination of the protein from the incubation medium (Figure 1) thus suggesting its effective binding to the sorbent. This allowed us to use the biotinylated ubiquitin for evaluation of the effect of ubiquitin on mitochondrial proteomic profiling.
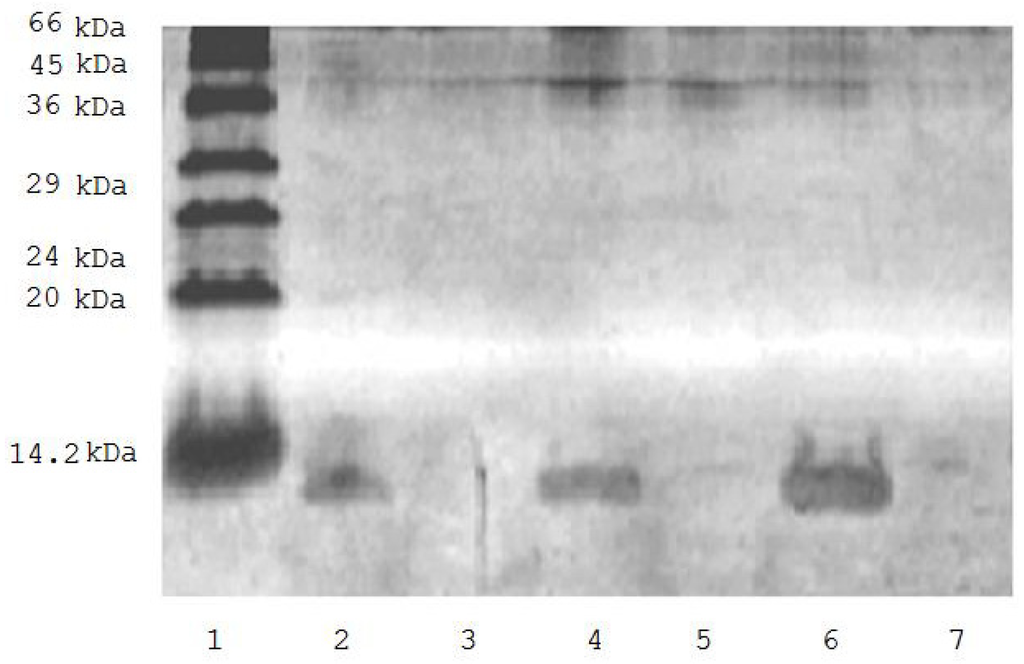
Figure 1.
SDS-PAGE of solution of biotinylated ubiquitin before (tracks 2, 4, 6) and after incubation (tracks 3, 5, 7) with avidin-agarose. 1. low molecular weight markers; 2. 0.375 μg of biotinylated ubiquitin; 4. 0.75 μg biotinylated ubiquitin; 6. 0.150 μg of biotinylated ubiquitin; 3, 5, and 7 are the same as in 2, 4, and 6 but after incubation with avidin-agarose.
Incubation of rat brain mitochondrial fraction with biotinylated ubiquitin followed by loading of cleared Triton X-100 lysates onto the avidin-agarose, intensive washing of the affinity sorbent and subsequent proteomic analysis of eluted proteins resulted in identification of 50 individual proteins (Table 1).

Table 1.
Proteomic identification of rat brain ubiquitin binding proteins: M, EM, PM designate mitochondrial, extramitochondrial and plasma membrane localization, respectively; “?” precise localization remains unknown at the moment. Here and in subsequent Tables each protein was identified at least in three independent experiments.
The same incubation of the rat brain mitochondrial fraction without biotinylated ubiquitin (control) followed by the same affinity chromatography fractionation resulted in identification of some highly abundant (and mostly) cytoskeletal proteins (Table 2).

Table 2.
Proteomic identification of proteins from control rat brain mitochondria incubated without biotinylated ubiquitin.
All these proteins (Table 1) preferentially associated with both outer and inner mitochondrial compartments bound to avidin-agarose only after preincubation of brain mitochondria with biotinylated ubiqutin. They could be referred to the following functional groups of proteins/enzymes: (1) Proteins (enzymes) involved in carbohydrate metabolism and energy generation; (2) Proteins involved in cytoskeleton formation and exocytosis; (3) Antioxidant/protective proteins; (4) Proteins/enzymes involved in signal transduction and regulation of enzyme activity; (5) Proteins of fatty acid metabolism; (6) Transporters; (7) Ubiquitin and related proteins.
Search for specific ubiquitination signature masses (+114.04 Da) revealed several ubiquitinated proteins of both extramitochondrial and intramitochondrial compartments (Table 3). Some of them had more than one ubiquitination site. Thus results of these experiments suggest that in vitro ubiquitination by means of exogenous biotinylated ubiquitin involves both external and intrinsic mitochondrial proteins.

Table 3.
Proteomic identification of rat brain proteins containing ubiquitin signatures.
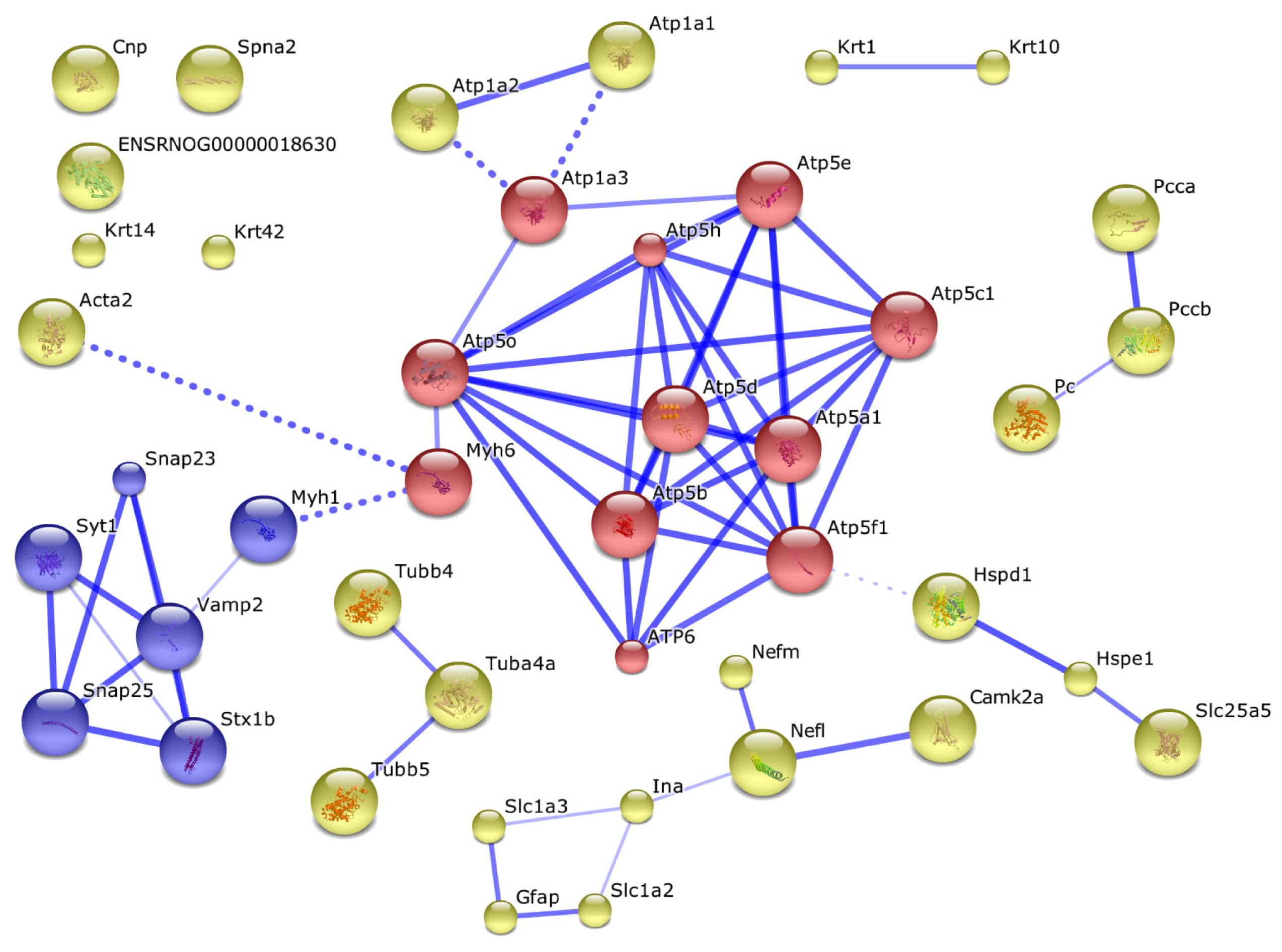
Other proteins obviously formed complexes with the ubiquitination proteins or they could be involved into formation of mitochondrial interactome (Figure 2).
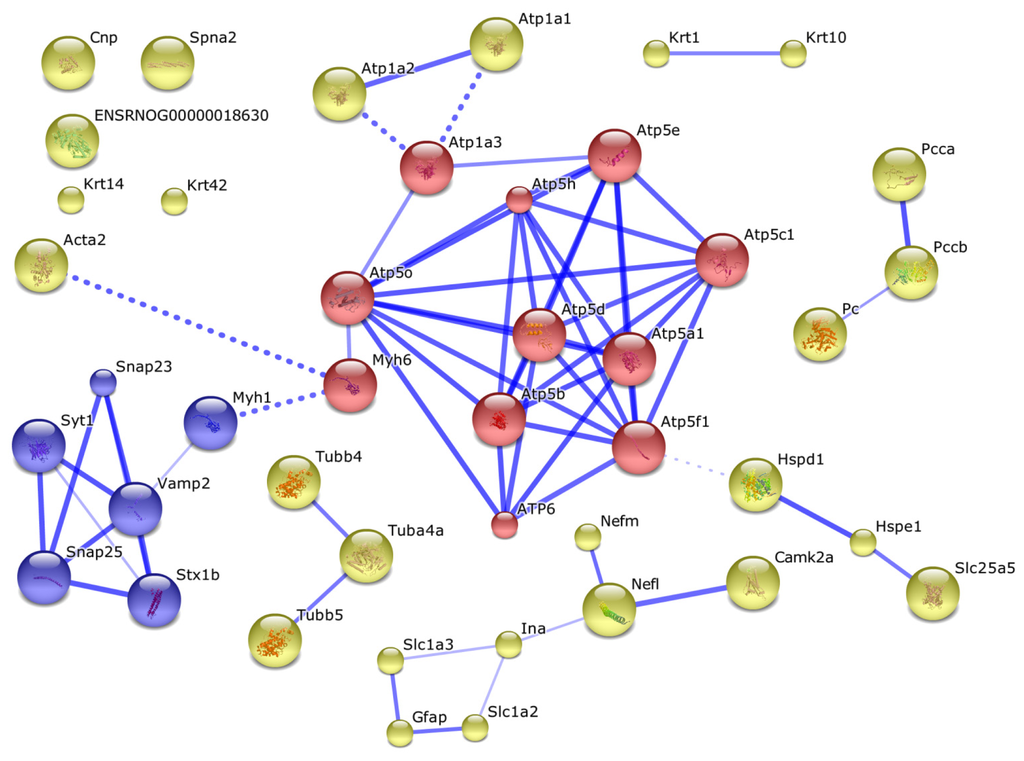
Figure 2.
The interactome of identified proteins, containing ubiquitin signatures. Solid blue lines designate direct interactions between proteins, and dashed blue lines demonstrate the most confident interactions between the cluster of intramitochondrial proteins and clusters of extramitochondrial proteins involved in cytoskeleton formation and carbohydrate metabolism and other proteins. Acronym names of the proteins shown in this figure are listed in Table 3. Intramitochondrial proteins are shown as red circles. Proteins indirectly interacting with mitochondrial components via more than one linker protein are shown as unconnected circles.
3. Discussion
Results of this study provide clear evidence that incubation of the rat brain mitochondrial fraction with biotinylated ubiquitin in vitro results in direct ubiquitination of some extramitochondrial and intramitochondrial proteins. In these experiments we have used the crude rat brain mitochondrial fraction, which has been originally used in pilot experiments performed to investigate the effect of exogenously added ubiquitin on proteolytic sensitivity of mitochondrial enzymes [16,17]. Since the employed system results in ubiquitination of both extramitochondrial (n = 10) and intramitochondrtial (n = 2) proteins it appears that under our experimental conditions components of the ubiquitin conjugation machinery associated with both extramitochondrial and mitochondrial compartments have used biotinylated ubiquitin. However, the proportion of directly ubiquitinated proteins represents not more than 20% of the total number of identified proteins specifically bound to avidin-agarose.
Previous proteomic studies of endogenous ubiquitination of human myocardial proteins demonstrated that more than 200 distinct proteins were bound to the affinity column (S5a-agarose) and only 27 proteins (i.e. less than 10%) were identified as directly ubiquitinated proteins [18]. Other proteins were obviously bound to the column in association with ubiquitinated proteins [18]. It should be noted that in accordance with data by Weekes et al. [18], we found alpha-chain of ATP synthase and myosin as direct targets for ubiquitination, while glyceraldehyde-3-phosphate dehydrogenase, creatine kinase M type which were found to be directly ubiquitinated in hearts of cardiac patients [18] lacked specific ubiquitin signature masses.
Using a transgenic mouse expressing octahistidine/Flag-tagged ubiquitin (HisF-Ub) in the heart Jeon et al. [19] identified 121 ubiquitinated proteins, including more than 40 mitochondrial proteins. Although about 10 proteins were also found in our study only two of them (alpha subunit of ATP synthase, sodium-potassium-transporting ATPase subunit alpha-3) contained specific ubiquitin signature masses.
Thus, it appears that in vitro incorporation of exogenous ubiquitin to rat brain mitochondria is accompanied by direct ubiquitination of some extramitochondrial and intramitochondrial proteins. However, it should be noted that among 12 identified proteins that contained specific ubiquitin signatures only pyruvate carboxylase and alpha subunit of ATP synthase are located in mitochondrial matrix or associated with the inner surface of the inner mitochondrial membrane, respectively. Although others have extramitochondrial localization, certain evidence exists that most of them (even plasma membrane proteins) may interact with mitochondria and thus participate in mitochondrial interactome formation (Figure 2). Our results suggest that ubiquitination of some proteins at both sides of the mitochondrial membranes significantly influences the mitochondrial interactome (evaluated in our experiments by the avidin-agarose affinity chromatography probing) (Figure 2). This is consistent with literature data on the dependence of mitochondrial functioning on functional competence of the ubiquitin-proteasomal machinery [20–22].
In order to minimize nonspecific adsorption of proteins onto the affinity sorbent we have employed the washing system (see Experimental Section 3.6), which is frequently used in affinity purification of antibodies [23]. Reliable detection of 50 proteins (Table 1) suggests that proteins bound nonspecifically to the affinity sorbent have been removed during the sorbent washing, and non-ubiquitinated proteins obviously form tight complexes either with ubiquitinated proteins or with their partners and/or mitochondrial membrane components. Results of the interactome analysis seem to support our viewpoint (Figure 2).
It should be noted that good evidence exists in the literature that some plasma membrane proteins interact with mitochondrial interactome. For example, vesicle-associated membrane protein 2 (Vamp 2), which is highly expressed in secretory vesicles and located in synaptic vesicle membrane, interacts with myosin heavy chain 4 associated with myosin heavy chain 6 [24,25]. Myosin 6 directly interacts with ATP-synthase subunit O. Considering this branch of the interactome (Figure 2) one can see that it can be further extended from Vamp 2 to synaptosomal-associated proteins 23 and 25 (SNAP23 and SNAP25) [26,27]; functional activity of this complex is regulated by synaptotagmin-1 associated with syntaxin [28]. Synaptotagmin-1, a protein associated with the vesicular membrane, is considered as a Ca2+ sensor for neurotransmitter release [29] and a regulator of Vamp 2 functioning. Synaptotagmin-1 also forms complexes with SNAP 23 and SNAP 25 proteins and thus promotes Vamp 2-dependent O-glycosylation [30]. These complex-forming proteins interact with of mitochondrial ATP synthase subunits via myosins, containing nucleotide-binding domains [31,32]. The interaction between actin and the ATP-synthase complex also involves myosin 6 subunits. Some syntaxin proteins may form a functional complex with the sodium-dependent glutamate/aspartate transporter 2 (GLT-1), which is strictly required for regulation of glutamante uptake by cAMP in the central nervous system [33]. This cAMP-dependent regulation of glutamante uptake is indirectly associated with mitochondrial ATP-synthase subunits and adenine nucleotide translocase (ANT) [34,35]. The glial fibrillary acidic protein (GFAP) plays a key role during the development of the central nervous system [36,37]; it is considered as a marker that distinguishes astrocytes from other glial cells [38,39]. GFAP acts as a link between Vamp 2 and SNAP 23/SNAP 25 proteins and also between ANT and synaptotagmin [40]. Impairments of such interactions have been recently demonstrated using a murine model of Alzheimer’s disease [41].
Thus, even brief consideration of the interactome data indicates that the non-ubiquitinated proteins co-isolated on avidin-agarose together with the biotin-tagged ubiquitinated proteins represent co-isolated components of mitochondrial interactome sub-complexes rather than nonspecific contaminants. Existence of such interactome sub-complexes is reasonably documented in the literature [24–41].
Thus, using biotinylated ubiquitin and proteomic methodologies supplemented by bioinformatic analysis, it is possible not only to find direct ubiquitination targets in rat brain mitochondria but also to get valuable information on the mitochondrial interactome influenced by protein ubiquitination.
Although biotinylated ubiquitin has limited (if any) applicability for in vivo studies, the proposed algorithm employing identification of both ubiquitinated proteins and specific protein complexes co-isolated with ubiquitinated proteins would be useful for mapping of certain mitochondrial (sub) interactomes.
4. Experimental Section
4.1. Chemicals
Avidin agarose, biotin, ubiquitin, DTT, iodoacetamide, trypsin, Tris (hydroxymethyl) aminomethane, guanidine hydrochloride, ammonium hydrocarbonate, potassium phosphate, sodium phosphate, sodium chloride, magnesium chloride, potassium chloride, Triton X-100, sucrose, EDTA, ATP, creatine phosphokinase, creatine phosphate, bacitracin, aprotinin, ACN, formic acid, CBB R-250, CBB G-250 were purchased from Sigma-Aldrich (USA).
Acrylamide, N,N′-methylenebisacrylamide, Low molecular weight protein standards, SDS, ammonium persulfate, TEMED were from Bio-Rad (USA).
4.2. Animals and Preparation of Brain Mitochondrial Fraction
Male Wistar rats (250 g–300 g) obtained from the Stolbovaya nursery (Russian Academy of Medical Sciences) were used in the experiments that were performed at least one week after their arrival from the nursery. Animals received a standard laboratory chow and water ad libitum and their decapitation was performed between 11.00 and 13.00. The brains were immediately dissected and homogenized in the isolation mixture of the following composition: 0.32 M sucrose, 1 mM EDTA, 10 mM Tris-HCl buffer, pH 7.5, using Ultra-Turrax T 10 homogenizer at a low speed, to obtain 30% w/v homogenate. Rat brain mitochondria were isolated as described in [42]. In each experiment we used brains from 5 animals.
4.3. Biotinylated Ubiquitin
Biotinylated ubiquitin was prepaed following the protocol developed for antibody biotinylation [43].
4.4. SDS-PAGE
SDS-PAGE was performed according to Laemmli in 12% gel [44].
4.5. Sample Preparation
Mitochondria (protein concentration, measured by the method of Bradford [45] was 2 mg/mL) were incubated with biotinylated ubiquitin (30 min, 37 °C) in 1 mL of incubation mixture containing 50 mM Tris-HCl, pH 7.5, 2 mM MgCl2, 3 mM dithiotreitol, 4 mM ATP, ATP-regenerating system (2 ME/mL creatine phosphokinase and 10 mM creatine phosphate), biotinylated ubiquitin (10 μM). The control mitochondria were incubated without ubiquitin. The reaction was terminated by cooling and subsequent centrifuging the incubation mixture (30 min, 16,000g).
4.6. Avidin Agarose Chromatography
Avidin agarose chromatography was performed to enrich the samples before the identification of ubiquitinated proteins. The mitochondrial pellets obtained at the previous stage (about 2 mg of protein) were resuspended in 100 μL of calcium/magnesium-free PBS (CMF-PBS; 2.68 mM KCl, 1.5 mM K3PO4 (monobasic), 136.9 mM NaCl, 8.1 mM Na3PO4 (dibasic heptahydrate)), pH 7.4, containing 3% Triton X-100, and after 45 min incubation at 4 °C were dissolved in the same buffer (without Triton X-100) containing bacitracin and aprotinin. The final concentration of Triton X-100 was 1%, bacitracin, 0.01% and aprotinin, 0.03%. Three hundred microliters of washed avidin-agarose slurry was then added to each sample and after 1 h incubation (25 °C) the slurry was washed 10 times with CMF-PBS containing 0.5 M NaCl and 2 times with CMF-PBS.
4.7. Proteomic Identification of Ubiquitinated Proteins
Avidin agarose absorbed proteins were modified by reduction with 0.02 M DTT in 6 M guanidine hydrochloride, pH 6.8, (1 h, 37 °C), and subsequent carbamidomethylation of SH groups with 0.055 M iodoacetamide (1 h, 37 °C). After the same extraction procedure [46] the protein sediment was dissolved in 50 mM ammonium hydrocarbonate and sonicated in the sonication bath for 15 min at 4 °C. After trypsinolysis (1 mg trypsin/100 mg of sample protein, 37 °C, 1 h; then 2 mg trypsin/100 mg of sample protein, 37 °C, overnight) formic acid was added to the preparations up to 0.5%. The preparations were evaporated using a vacuum concentrator 5301 (Eppendorf, AG, Germany), then dissolved in 0.1% formic acid, and analyzed by LC-MS/MS. Reverse-phase nano-LCMS/MS was performed using an Agilent 1100 nano-flow HPLC-Chip cube system coupled to Agilent 6340 Ion Trap (Agilent Technologies, Palo Alto, CA, USA). Tryptic peptides were separated on the HPLC Chip (40 nL trap column, 75 mm × 43 mm analytical column, 5 mm C-18SB-ZX, Agilent Technologies) using a linear gradient of 5%–80% ACN in 0.1% formic acid over 60 min at a flow rate of 300 nL/min and detected by an ion trap in 300–1800 m/z range following the supplier’s recommendations. Mass spectra were acquired in positive-ion mode with automated data-dependent MS/MS on the five most intense ions from precursor MS scans. Protein identification was performed using Spectrum Mill MS Proteomics Workbench Rev A.03.03.078 (Agilent Technologies). Protein identifications were obtained by comparison of experimental data to the Swiss-Prot rat and mouse subset database. The following search parameters were used: trypsin was used as the cutting enzyme, mass tolerance for the monoisotopic peptide window was set to 1.6 Da, the MS/MS tolerance window was set to 0.5 Da, and two missed cleavage were allowed. Cysteine carbamidomethylation was chosen as a fixed modification and oxidized methionine was chosen as a variable modifications. The criteria of positive identification were set as follows: 70% minimum scored peak intensity, d-forward reverse score 42; at least two peptides identifications with a confident score 7 and summarized protein score 14 [47].
4.8. Identification of Ubiquitination Sites
Peptides with attached mono-ubiquitin and poly-ubiquitin moieties were searched against SwissProt Human proteins database. Branched and unbranched –GG signature peptide ions with the charge state of 2+, 3+ and 4+ generated by incomplete trypsin digestion were targeted for analysis by narrow (+/− 0.3 m/z) window extracted ion chromatography. Tandem MS/MS spectra of branched fragment ions shifted to the m/z value corresponding to the GG-moeity were isolated and analyzed for direct searching of ubiquitin-labeled peptides in samples. At least four branched fragment ions of the precursor with GG signature have to be founded for confident approval of ubiquitin-labeled peptides.
4.9. Prediction of a Mitochondrial Interactome/Protein Interaction Network
Prediction of interactions of proteins identified during the proteomic analysis was performed using an open access STRING version 9.0 software platform [48]. A list of identified proteins Uniprot/SwissProt IDs was used as input data and applied for interactive network processing. The confidence score at the level of 0.7 was chosen to view the best fitted proteins with approximate probability that a predicted link exists between selected proteins in the same metabolic map in the KEGG database. KMEANS clustering algorithm was selected for clustering the obtained proteins in the resultant network. The number of clusters was chosen as 5 and at least the confidence score of 0.9 was selected for each analyzing node in the resultant protein connectivity map.
5. Conclusions
Thus, results of the present study demonstrate that the use of biotinylated ubiquitin may be considered as the method of choice for in vitro evaluation of endogenous ubiquitin-conjugating machinery in particular subcellular organelles and changes in ubiquitin/organelle associated interactomes. We believe that our approach is a good supplement to traditionally used methods for detection of direct ubiquitination targets and evaluation of ubiquitin conjugated activity known in the literature [49–52].
This may be useful for evaluation of changes in mitochondrial interactomes induced by protein ubiquitination under normal and various brain pathologies, which still require better characterization.
Acknowledgments
This work was supported by grants from the Russian Foundation for Basic Research, grants no. 07-04-00803-a and 10-04-00530-b, and by the Russian State Contract no. 14.740.11.0761.
References
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem 1998, 67, 425–479. [Google Scholar]
- Schwartz, A.L.; Ciechanover, A. Targeting proteins for destruction by the ubiquitin system: Implications for human pathobiology. Annu. Rev. Pharmacol. Toxicol 2009, 49, 73–96. [Google Scholar]
- Ciechanover, A. The ubiquitin-mediated proteolytic pathway: Mechanisms of action and cellular physiology. Biol. Chem. Hoppe-Seyler 1994, 375, 565–581. [Google Scholar]
- Schwartz, A.L.; Ciechanover, A. Ubiquitin-mediated protein modification and degradation. Am. J. Respir. Cell Mol. Biol 1992, 7, 463–468. [Google Scholar]
- Wilkinson, K.D. Ubiquitination and deubiquitination: Targeting of proteins for degradation by the proteasome. Semin. Cell Dev. Biol 2000, 11, 141–148. [Google Scholar]
- Peng, J. Evaluation of proteomic strategies for analyzing ubiquitinated proteins. BMB Rep 2008, 41, 177–183. [Google Scholar]
- Ciechanover, A.; Orian, A.; Schwartz, A.L. Ubiquitin-mediated proteolysis: Biological regulation via destruction. Bioessays 2000, 22, 442–451. [Google Scholar]
- Hershko, A. The ubiquitin system for protein degradation and some of its roles in the control of the cell-division cycle (Nobel lecture). Angew. Chem Int. Ed 2005, 44, 5932–5943. [Google Scholar]
- Peng, J.; Schwartz, D.; Elias, J.E.; Thoreen, C.C.; Cheng, D.; Marsischky, G.; Roelofs, J.; Finley, D.; Gygi, S.P. A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol 2003, 21, 921–926. [Google Scholar]
- Medvedev, A.E.; Kopylov, A.T.; Buneeva, O.A.; Zgoda, V.G.; Archakov, A.I. Affinity-based proteomic profiling: Problems and achievements. Proteomics 2012, 12, 621–637. [Google Scholar]
- Riederer, I.M.; Schiffrin, M.; Kövari, E.; Bouras, C.; Riederer, B.M. Ubiquitination and cysteine nitrosylation during aging and Alzheimer’s disease. Brain Res. Bull 2009, 80, 233–241. [Google Scholar]
- Shimada, Y.; Fukuda, T.; Aoki, K.; Yukawa, T. A protocol for immunoaffinity separation of the accumulated ubiquitin-protein conjugates solubilized with sodium dodecyl sulfate. Anal. Biochem 2008, 377, 77–82. [Google Scholar]
- Gitlin, G.; Bayer, E.A.; Wilchek, M. Studies on the biotin-binding site of avidin. Lysine residues involved in the active site. Biochem. J 1987, 242, 923–926. [Google Scholar]
- Magnani, M.; Serafini, G.; Antonelli, A.; Malatesta, M.; Gazzanelli, G. Evidence for a particulate location of ubiquitin conjugates and ubiquitin-conjugating enzymes in rabbit brain. J. Biol. Chem 1991, 266, 21018–21024. [Google Scholar]
- Kemeny, S.; Dery, D.; Loboda, Y.; Rovner, M.; Lev, T.; Zuri, D.; Finberg, J.M.P.; Larisch, S. Parkin Promotes Degradation of the Mitochondrial Pro-Apoptotic ARTS Protein. PLoS One 2012, 7, e38837. [Google Scholar]
- Buneeva, O.A.; Medvedeva, M.V.; Medvedev, A.E. Incorporation of ubiquitin into rat brain mitochondria is accompanied by increased proteolytic digestibility of MAO. Neurobiology 1999, 7, 257–261. [Google Scholar]
- Buneeva, O.A.; Medvedeva, M.V.; Medvedev, A.E. Ubiquitin and proteolytic degradation of monoamine oxidases. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem 2008, 2, 101–104. [Google Scholar]
- Weekes, J.; Morrison, K.; Mullen, A.; Wait, R.; Barton, P.; Dunn, M.J. Hyperubiquitination of proteins in dilated cardiomyopathy. Proteomics 2003, 3, 208–216. [Google Scholar]
- Jeon, H.B.; Choi, E.S.; Yoon, J.H.; Hwang, J.H.; Chang, J.W.; Lee, E.K.; Choi, H.W; Park, Z.Y.; Yoo, Y.J. A proteomics approach to identify the ubiquitinated proteins in mouse heart. Biochem. Biophys. Res. Comm. 2007, 357, 731–736. [Google Scholar]
- Sullivan, P.G.; Dragicevic, N.B.; Deng, J.H.; Bai, Y.; Dimayuga, E.; Ding, Q.; Chen, Q.; Bruce-Keller, A.J.; Keller, J.N. Proteasome inhibition alters neural mitochondrial homeostasis and mitochondria turnover. J. Biol. Chem 2004, 279, 20699–20707. [Google Scholar]
- Nakamura, N.; Hirose, S. Regulation of mitochondrial morphology by USP30, a deubiquitinating enzyme present in the mitochondrial outer membrane. Mol. Biol. Cell 2008, 19, 1903–1911. [Google Scholar]
- Cohen, M.M.; Leboucher, G.P.; Livnat-Levanon, G.; Glickman, M.H.; Weissman, A.M. Ubiquitin-proteasome dependent degradation of a mitofusin, a critical regulator of mitochondrial fusion. Mol. Biol. Cell 2008, 19, 2457–2464. [Google Scholar]
- Gagnon, P. Technology trends in antibody purification. J. Chrom. A 2012, 1221, 57–70. [Google Scholar]
- Takuma, T.; Arakawa, T.; Tajima, Y. Interaction of SNARE proteins in rat parotid acinar cells. Arch. Oral. Biol 2000, 45, 369–375. [Google Scholar]
- Jacobsson, G.; Meister, B. Molecular components of the exocytotic machinery in the rat pituitary gland. Endocrinology 1996, 137, 5344–5356. [Google Scholar]
- Nakahara, T.; Nakamura, K.; Tsutsumi, T.; Hashimoto, K.; Hondo, H.; Hisatomi, S.; Motomura, K.; Uchimura, H. Effect of chronic haloperidol treatment on synaptic protein mRNAs in the rat brain. Mol. Brain Res 1998, 61, 238–242. [Google Scholar]
- Cho, W.J.; Jeremic, A.; Jena, B.P. Direct interaction between SNAP-23 and l-type Ca2+ channel. J. Cell Mol. Med 2005, 9, 380–386. [Google Scholar]
- Abonyo, B.O.; Gou, D.; Wang, P.; Narasaraju, T.; Wang, Z.; Liu, L. Syntaxin 2 and SNAP-23 are required for regulated surfactant secretion. Biochemistry 2004, 43, 3499–3506. [Google Scholar]
- Kanno, E.; Fukuda, M.J. Increased plasma membrane localization of O-glycosylation-deficient mutant of synaptotagmin I in PC12 cells. J. Neurosci Res 2008, 86, 1036–1043. [Google Scholar]
- Fukuda, M.J. Vesicle-associated membrane protein-2/synaptobrevin binding to synaptotagmin I promotes O-glycosylation of synaptotagmin I. Biol. Chem 2002, 277, 30351–30358. [Google Scholar]
- Thomas, S.G.; Takahashi, M.; Neill, J.D.; Clarke, I.J. Components of the neuronal exocytotic machinery in the anterior pituitary of the ovariectomised ewe and the effects of oestrogen in gonadotropes as studied with confocal microscopy. Neuroendocrinology 1998, 67, 244–259. [Google Scholar]
- Risinger, C.; Bennett, M.K. Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms. J. Neurochem 1999, 72, 614–624. [Google Scholar]
- Martínez-López, I.; García, C.; Barber, T.; Viña, J.R.; Miralles, V.J. The l-glutamate transporters GLAST (EAAT1) and GLT-1 (EAAT2): Expression and regulation in rat lactating mammary gland. Mol. Membr. Biol 1998, 15, 237–242. [Google Scholar]
- Schlag, B.D.; Vondrasek, J.R.; Munir, M.; Kalandadze, A.; Zelenaia, O.A.; Rothstein, J.D.; Robinson, M.B. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol. Pharmacol 1998, 53, 355–369. [Google Scholar]
- Swanson, R.A.; Liu, J.; Miller, J.W.; Rothstein, J.D.; Farrell, K.; Stein, B.A.; Longuemare, M.C. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J. Neurosci 1997, 17, 932–940. [Google Scholar]
- Boyer, S.; Maunoury, R.; Gomès, D.; de Néchaud, B.; Hill, A.M.; Dupouey, P. Expression of glial fibrillary acidic protein and vimentin in mouse lens epithelial cells during development in vivo and during proliferation and differentiation in vitro: Comparison with the developmental appearance of GFAP in the mouse central nervous system. J. Neurosci. Res. 1990, 27, 55–64. [Google Scholar]
- Verderber, L.; Johnson, W.; Mucke, L.; Sarthy, V. Differential regulation of a glial fibrillary acidic protein-LacZ transgene in retinal astrocytes and Müller cells. Invest. Ophthalmol. Vis. Sci 1995, 36, 1137–1143. [Google Scholar]
- Yeh, C.Y.; Vadhwana, B.; Verkhratsky, A.; Rodríguez, J.J. Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer’s disease. ASN Neuro 2011, 3, 271–279. [Google Scholar]
- Salmaso, N.; Cossette, M.P.; Woodside, B. Pregnancy and maternal behavior induce changes in glia, glutamate and its metabolism within the cingulate cortex. PLoS One 2011, 6, e23529. [Google Scholar]
- Ardais, A.P.; Viola, G.G.; Costa, M.S.; Nunes, F.; Behr, G.A.; Klamt, F.; Moreira, J.C.; Souza, D.O.; Rocha, J.B.; Porciúncula, L.O. Acute treatment with diphenyl diselenide inhibits glutamate uptake into rat hippocampal slices and modifies glutamate transporters, SNAP-25, and GFAP immunocontent. Toxicol. Sci 2010, 113, 434–443. [Google Scholar]
- Cassano, T.; Serviddio, G.; Gaetani, S.; Romano, A.; Dipasquale, P.; Cianci, S.; Bellanti, F.; Laconca, L.; Romano, A.D.; Padalino, I; et al. Glutamatergic alterations and mitochondrial impairment in a murine model of Alzheimer disease. Neurobiol. Aging 2012, 33, 1121.e1–1121.e12. [Google Scholar]
- Medvedev, A.E.; Gorkin, V.Z. Endogenous stimulation of lipid peroxidation in brain increases proteolytic inactivation of mitochondrial monoamine oxidases. Int. J. Dev. Neurosci 1994, 12, 151–155. [Google Scholar]
- Kantor, A. Biotinylation of Antibodies protocol. Available online: http://www.drmr.com/abcon/Biotin.html accessed on 7 July 2004.
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Spector, T. Refinement of the Coomassie Blue method of protein quantitation. Anal. Biochem 1978, 86, 142–146. [Google Scholar]
- Walker, J.M. The protein Protocols Handbook; Humana Press Inc: Totowa, NY, USA, 2002; p. 1176. [Google Scholar]
- Kapp, E.A.; Schultz, F.; Conolly, F.M.; Chakel, J.A.; Meza, J.E.; Miller, C.A.; Fenyo, D.; Eng, J.K.; Adkins, J.N.; Omenn, G.S.; et al. An evaluation, comparison, and accurate benchmarking of several publicly available MS/MS search algorithms: sensitivity and specificity analysis. Proteomics 2005, 5, 3475–3490. [Google Scholar]
- Jensen, L.J.; Kuhn, M.; Stark, M.; Chaffron, S.; Creevey, C.; Muller, J.; Doerks, T.; Julien, P.; Roth, A.; Simonovic, M.; et al. STRING 8—A global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res 2009, 37, D412–D416. [Google Scholar]
- Merbl, Y.; Kirschner, M.W. Large-scale detection of ubiquitination substrates using cell extracts and protein microarrays. Proc. Natl. Acad. Sci. USA 2009, 106, 2543–2548. [Google Scholar]
- Hjerpe, R.; Rodríguez, M.S. Efficient approaches for characterizing ubiquitinated proteins. Biochem. Soc. Trans 2008, 36, 823–827. [Google Scholar]
- Correia, M.A.; Sadeghi, S.; Mundo-Paredes, E. Cytochrome P450 ubiquitination: branding for the proteolytic slaughter? Annu. Rev. Pharmacol. Toxicol 2005, 45, 439–464. [Google Scholar]
- Merbl, Y.; Kirschner, M.W. Large-scale detection of ubiquitination substrates using cell extracts and protein microarrays. Proc. Natl. Acad. Sci. USA 2009, 106, 2543–2548. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).