Analgesic-Like Activity of Essential Oil Constituents: An Update
Abstract
:1. Introduction
2. Results
2.1. p-Cymene
2.2. Carvacrol
2.3. Linalool
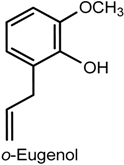
2.4. Eugenol
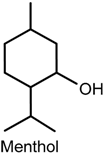
2.5. Menthol
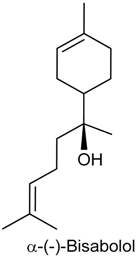
2.6. (−)-α-Bisabolol
2.7. Cinnamaldehyde
2.8. Citronellal
2.9. Citronellol
2.10. Citronellyl Acetate
2.11. α-Phellandrene
2.12. α-Terpineol
2.13. Vanillin
2.14. Borneol
2.15. Myrtenol
2.16. Pulegone
2.17. Citral
2.18. Thymol
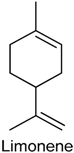
2.19. Limonene
2.20. Nerol
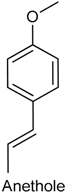
2.21. Anethole
2.22. Nerolidol
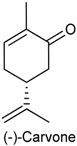
2.23. (−)-Carvone
2.24. Farnesol
2.25. β-Caryophyllene
2.26. α,β-Epoxy-Carvone
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franco, M.R.B. Aroma e Sabor de Alimentos: Temas Atuais; Varela Editora e Livraria Ltda: São Paulo, Brazil, 2003. [Google Scholar]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; Nóbrega, F.F.F.; Lima, M.R.V.; Almeida, R.N. Pharmacological Activity of (R)-(+)-pulegone, a chemical constituent of essential oils. Z. Naturforsch. 2011, 66, 353–359. [Google Scholar] [CrossRef]
- De Sousa, D.P. Analgesic-like activity of essential oils constituents. Molecules 2011, 16, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Sarmento-Neto, J.F.; Do Nascimento, L.G.; Felipe, C.F.; de Sousa, D.P. Analgesic Potential of Essential Oils. Molecules 2015, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.N.; Agra, M.F.; Maior, F.N.; de Sousa, D.P. Essential oils and their constituents: Anticonvulsant activity. Molecules 2011, 16, 2726–2742. [Google Scholar] [CrossRef] [PubMed]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. Sesquiterpenes from Essential Oils and Anti-Inflammatory Activity. Nat. Prod. Commun. 2015, 10, 1767–1774. [Google Scholar]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; Dos Reis Barreto de Oliveira, R.; de Sousa, D.P. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.A.; Andrade, L.N.; de Sousa, E.B.; de Sousa, D.P. Antitumor phenylpropanoids found in essential oils. Biomed. Res. Int. 2015, 2015, 392674. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P. Bioactive Essential Oils and Cancer; Springer International Publishing: New York, NY, USA, 2015. [Google Scholar]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; de Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 2014, 953451. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; de Almeida Soares Hocayen, P.; Andrade, L.N.; Andreatini, R.A. Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models. Molecules 2015, 20, 18620–18660. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.A.; Andrade, L.N.; de Sousa, E.B.; de Sousa, D.P. Anti-ulcer activity of essential oil constituents. Molecules 2014, 19, 5717–5747. [Google Scholar] [CrossRef] [PubMed]
- IASPPainTerminology. Available online: http://www.iasppain.org/AM/Template.cfm?Section=Pain_Definitions&Template=/CM/HTMLDisplay.cfm&ContentID=1728#Pain (accessed on 8 December 2010).
- Oliveira, F.A.; Costa, C.L.S.; Chaves, M.H.; Almeida, F.R.C.; Cavalcante, I.J.M.; Lima, A.F.; Lima-Júnior, R.C.P.; Silva, R.M.; Campos, A.R.; Santos, F.A.; et al. Attenuation of capsaicin-induced acute and visceral nociceptive pain by α- and β-amyrin, a triterpene mixture isolated from Protium heptaphyllum resin in mice. Life Sci. 2005, 77, 2942–2952. [Google Scholar] [CrossRef] [PubMed]
- Bispo, M.D.; Mourão, R.H.V.; Franzotti, E.M.; Bomfim, K.B.R.; Arrigoni-Blank, M.F.; Moreno, M.P.N.; Marchioro, M.; Antoniolli, A.R. Antinociceptive and antiedematogenic effects of the aqueous extract of Hyptis pectinata leaves in experimental animals. J. Etnopharmacol. 2001, 76, 81–86. [Google Scholar] [CrossRef]
- Quintans, J.S.S.; Menezes, P.P.; Santos, M.R.V.; Bonjardim, L.R.; Almeida, J.R.G.S.; Gelain, D.P.; Araújo, A.A.S.; Quintans-Júnior, L.J. Improvement of p-cymene antinociceptive and anti-inflammatory effects by inclusion in β-cyclodextrin. Phytomedicine 2013, 20, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.F.; Quintans-Júnior, L.J.; Cavalcanti, S.C.H.; Oliveira, M.G.B.; Guimarães, A.G.; Cunha, E.S.; Melo, M.S.; Santos, M.R.V.; Araújo, A.A.S.; Bonjardim, L.R. p-Cymene reduces orofacial nociceptive response in mice. Braz. J. Pharmacogn. 2011, 21, 1138–1143. [Google Scholar] [CrossRef]
- Bonjardim, L.R.; Cunha, E.S.; Guimaraes, A.G.; Santana, M.F.; Oliveira, M.G.B.; Serafini, M.R.; Araujo, A.A.S.; Antoniolli, A.R.; Cavalcanti, S.C.H.; Santos, M.R.V.; et al. Evaluation of the Anti-Infl ammatory and Antinociceptive Properties of p-Cymene in Mice. Z. Naturforsch. 2012, 67, 15–21. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Lorenzetti, B.B.; Poole, S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br. J. Pharmacol. 1993, 110, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.M.; Verri, W.A., Jr.; Silva, J.S.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Dray, A. Inflammatory mediators of pain. Br. J. Anaesth. 1995, 75, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.Q.; Poole, S.; Lorenzetti, B.B.; Ferreira, S.H. The pivotal role of tumour necrosis factor α in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992, 107, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.F.; Guimarães, A.G.; Chaves, D.O.; Silva, J.C.; Bonjardim, L.R.; Júnior, W.L.; Ferro, J.N.S.; Barreto, E.O.; Santos, F.E.; Soares, M.B.P.; et al. The anti-hyperalgesic and anti-inflammatory profiles of p-cymene: Evidence for the involvement of opioid system and cytokines. Pharm. Biol. 2015, 53, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Reale, M.; Fiore, S.; Cancelli, A.; Angeletti, P.U.; Dinarello, C.A. Recombinant interleukin 1 and tumor necrosis factor acting in synergy to release thromboxane, 6-KETO-PGF1 and PGE2 by human neutrophils. Scand. J. Rheumatol. Suppl. 1998, 75, 318–324. [Google Scholar]
- Kassama, T.; Miwa, Y.; Isozaki, T.; Odai, T.; Adachi, M.; Kunkel, S.L. Neutrophil-derived cytokines potential therapeutic targets in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 273–279. [Google Scholar] [CrossRef]
- Yang, E.J.; Yim, E.Y.; Song, G.; Kim, G.O.; Hyun, C.G. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW 264.7 macrophages by Jeju plant extracts. Interdiscip. Toxicol. 2009, 2, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Tao, Y.X.; Zhao, C.; Doré, S.; Liaw, W.J.; Raja, S.N.; Johns, R.A. Differential roles of neuronal and endothelial nitric oxide synthases during carrageenan-induced inflammatory hyperalgesia. Neuroscience 2004, 128, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experimental Models. Toxicology 2013, 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.S.; Santana, M.T.; Guimarães, A.G.; Siqueira, R.S.; de Sousa, D.P.; Santos, M.R.V.; Bonjardim, L.R.; Araujo, A.A.S.; Onofre, A.S.C.; Lima, J.T.; et al. Bioassay-guided evaluation of central nervous system effects of citronellal in rodents. Braz. J. Pharmacogn. 2011, 21, 697–703. [Google Scholar] [CrossRef]
- Dresch, M.T.K.; Rossato, S.B.; Kappel, V.D.; Biegelmeyer, R.; Hoff, M.L.; Mayorga, P.; Zuanazzi, J.A.; Henriques, A.T.; Moreira, J.C. Optimization and validation of an alternative method to evaluate total reactive antioxidant potential. Anal. Biochem. 2009, 385, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3120. [Google Scholar] [CrossRef] [PubMed]
- Koparal, A.T.; Zeytinoglu, M. Effects of carvacrol on a human nonsmall cell lung cancer (NSCLC) cell line, A549. Cytotecnology 2005, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother. Res. 2007, 21, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Guimarães, A.G.; Araújo, B.E.S.; Oliveira, G.F.; Santana, M.T.; Moreira, F.V.; Santos, M.R.V.; Cavalcanti, S.C.H.; de Lucca, J.W.; Botelho, M.A.; et al. Carvacrol, (−)-borneol and citral reduce convulsant activity in rodents. Afr. J. Biotechnol. 2010, 9, 6566–6572. [Google Scholar]
- Melo, M.G.D.; Santos, J.P.A.; Serafini, M.R.; Caregnato, F.F.; Pasquali, M.A.; Rabelo, T.K.; Da Rocha, R.F.; Araújo, A.A.; Da Silva, F.A.; Moreira, J.C.; et al. Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite. Toxicol. In Vitro 2011, 25, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.; Antoniolli, A.R.; Bonjardim, L.R.; Silva, F.A.; Santos, J.P.; Rocha, R.F.; Moreira, J.C.; et al. Bioassayguided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin. Pharmacol. Toxicol. 2010, 107, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.H.C.; Riosa, E.R.V.; Rocha, N.F.M.; Citóa, M.C.O.; Fernandes, M.L.; de Sousa, D.P.; Vasconcelos, S.M.M.; Sousa, F.C.F. Antinociceptive activity of carvacrol (5-isopropyl-2-methylphenol) in mice. J. Pharm. Pharmacol. 2012, 64, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a component of thyme oil, activates PPAR α and γ, and suppresses COX-2 expression. J. Lipid. Res. 2010, 51, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Xavier, M.A.; de Santana, M.T.; Camargo, E.A.; Santos, C.A.; Brito, F.A.; Barreto, E.O.; Cavalcanti, S.C.H.; Antoniolli, A.R.; Oliveira, R.C.M.; et al. Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Silva, F.V.; Xaviera, M.A.; Santos, M.R.V.; Rita, C.M.; Oliveira, R.C.M.; Oliveira, M.G.B.; Oliveira, A.P.; de Souza, C.C.; Quintans-Júnior, L.J. Orofacial Analgesic-Like Activity of Carvacrol in Rodents. Z. Naturforsch. 2012, 67, 481–485. [Google Scholar] [CrossRef]
- Luo, Q.T.; Fujita, T.; Jiang, C.Y.; Kumamoto, E. Carvacrol presynaptically enhances spontaneous excitatory transmission and produces outward current in adult rat spinal substantia gelatinosa neurons. Brain Res. 2014, 1592, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Fürst, S. Transmitters involved in antinociception in the spinal cord. Brain Res. Bull. 1999, 48, 129–141. [Google Scholar] [CrossRef]
- Roberts, K.; Shenoy, R.; Anand, P. A novel human volunteer pain model using contact heat evoked potentials (CHEP) following topical skin application of transient receptor potential agonists capsaicin, menthol and cinnamaldehyde. J. Clin. Neurosc. 2011, 18, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Joca, H.C.; Cruz-Mendes, Y.; Oliveira-Abreu, K.; Maia-Joca, R.P.M.; Barbosa, R.; Lemos, T.L.; Beirão, P.S.L.; Leal-Cardoso, J.H. Carvacrol Decreases Neuronal Excitability by Inhibition of Voltage-Gated Sodium Channels. J. Nat. Prod. 2012, 75, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, S.; Kuwahata, H.; Yagi, T.; Kishikawa, Y.; Komatsu, T.; Sakurada, T.; Nakamura, H. Intraplantar injection of linalool reduces paclitaxel-induced acute pain in mice. Biol. Res. 2012, 33, 175–181. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D.L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; de Montis, M.G.; Sechi, S.; Sircana, G.; D’Aquila, P.S.; Pippia, P. Effects of (−)-linalool in the acute hyperalgesia induced by carrageenan, l-glutamate and prostaglandin E2. Eur. J. Pharmacol. 2004, 497, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; Marzocco, S.; Popolo, A.; Pinto, A. (−)-Linalool inhibits in vitro NO formation: Probable involvement in the antinociceptive activity of this monoterpene compound. Life Sci. 2006, 78, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Viljoen, A.M. Linalool—A review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1183–1192. [Google Scholar]
- Venâncio, A.M.; Marchioro, M.; Estavam, C.S.; Melo, M.S.; Santana, M.T.; Onofre, A.S.C.; Guimarães, A.G.; Oliveira, M.G.B.; Alves, P.B.; Pimentel, H.C.; et al. Ocimum basilicum leaf essential oil and (−)-linalool reduce orofacial nociception in rodents: A behavioral and electrophysiological approach. Braz. J. Pharmacog. 2011, 21, 1043–1051. [Google Scholar] [CrossRef]
- Holanda-Pinto, S.A.; Pinto, L.M.S.; Guedes, M.A.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V.S. Antinoceptive effect of triterpenoid α,β-amyrin in rats on orofacial pain induced by formalin and capsaicin. Phytomedicine 2008, 15, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Bliss, T.V.; Skrede, K.K. Unit analysis of hippocampal polulation spikes. Exp. Brain. Res. 1971, 13, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Kuwahata, H.; Komatsu, T.; Katsuyama, S.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S.; Sakurada, T.; Kazuo Takahama, K. Peripherally injected linalool and bergamot essential oil attenuate mechanical allodynia via inhibiting spinal ERK phosphorylation. Pharmacol. Biochem. Behav. 2013, 103, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Galan, A.; Lopez-Garcia, J.A.; Cervero, F.; Laird, J.M. Activation of spinal extracelular signaling-regulated kinase-1 and -2 by intraplantar carrageenan in rodents. Neurosci. Lett. 2002, 322, 37–40. [Google Scholar] [CrossRef]
- Ji, R.R.; Befort, K.; Brenner, G.J.; Woolf, C.J. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J. Neurosci. 2002, 22, 478–485. [Google Scholar] [PubMed]
- Ji, R.R.; Baba, H.; Brenner, G.J.; Woolf, C.J. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat. Neurosci. 1999, 2, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Iwata, K.; Fukuoka, T.; Kondo, E.; Tokunaga, A.; Yamanaka, H.; Tachibana, T.; Liu, Y.; Noguchi, K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J. Neurosci. 2002, 22, 7737–7745. [Google Scholar] [PubMed]
- Komatsu, T.; Mizoguchi, H.; Sasaki, M.; Sakurada, C.; Tsuzuki, M.; Sakurada, S.; Sakurada, T. Inhibition of ERK phosphorylation by substance P N-terminal fragment decreases capsaicininduced nociceptive response. Neuropharmacology 2011, 61, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, S.; Otowa, A.; Kamio, S.; Sato, K.; Yagi, T.; Kishikawa, Y.; Komatsu, T.; Bagetta, G.; Sakurada, T.; Nakamura, H. Effect of plantar subcutaneous administration of bergamot essential oil and linalool on formalin-induced nociceptive behavior in mice. Biomed. Res. 2015, 36, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Harrasi, A.; Ali, L.; Hussain, J.; Rehman, N.U.; Mehjabeen; Ahmed, M.; Al-Rawahi, A. Analgesic effects of crude extracts and fractions of Omani frankincense obtained from traditional medicinal plant Boswellia sacra on animal models. Asian Pac. J. Trop. Med. 2014, 7S1, S485–S490. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, Y.J.; Li, Y.S.; Zhang, W.K.; Tang, H.B. α-Pinene, linalool, and1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, S.; Yamaguchi, R.; Ishikawa, S.; Sakurai, T.; Kajiya, K.; Kanmura, Y.; Kuwaki, T.; Kashiwadani, H. Odour-induced analgesia mediated by hypothalamic orexin neurons in mice. Sci. Rep. 2016, 6, 37129. [Google Scholar] [CrossRef] [PubMed]
- Chiou, L.C.; Lee, H.J.; Ho, Y.C.; Chen, S.P.; Liao, Y.Y.; Ma, C.H.; Fan, P.C.; Fuh, J.L.; Wang, S.J. Orexins/hypocretins: Pain regulation and cellular actions. Curr. Pharm. 2010, 16, 3089–3100. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Barreto, R.S.; Menezes, P.P.; Almeida, J.R.; Viana, A.F.; Oliveira, R.C.; Oliveira, A.P.; Gelain, D.P.; de Lucca Júnior, W.; Araújo, A.A. β-Cyclodextrin-complexed (−)-linalool produces antinociceptive effect superior to that of (−)-linalool in experimental pain protocols. Basic Clin. Pharmacol. Toxicol. 2013, 113, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Nöldner, M.; Germer, S.; Koch, E. Pharmacokinetics of linalool and linalyl acetate, the two main constituents of silexan, an essential oil from Lavandula angustifolia flowers, in rats. Planta Med. 2011, 77, 44. [Google Scholar] [CrossRef]
- Nascimento, S.S.; Camargo, E.A.; de Santana, J.M.; Araújo, A.A.S.; Menezes, P.P.; Lucca-Júnior, W.; Albuquerque-Júnior, R.L.C.; Bonjardim, L.R.; Quintans-Júnior, L.J. Linalool and linalool complexed in β-cyclodextrin produce anti-hyperalgesic activity and increase Fos protein expression in animal model for fibromyalgia. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Szabadics, J.; Erdelyi, L. Pre- and post-synaptic effects of eugenol and related compounds on Helix pomatia L. neurons. Acta Biol. Hung. 2000, 51, 265–273. [Google Scholar] [PubMed]
- Bó, W.D.; Luiz, A.P.; Martins, D.F.; Mazzardo-Martins, L.; Santos, A.R.S. Eugenol reduces acute pain in mice by modulating the glutamatergic and tumornecrosis factor α (TNF-α) pathways. Fundament. Clin. Pharmacol. 2013, 27, 517–525. [Google Scholar]
- Ohkubo, T.; Shibata, M. The selective capsaicin antagonista capsazepine abolishes the antinociceptive action of eugenol and guaiacol. J. Dent. Res. 1997, 76, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sim, Y.B.; Lee, J.K.; Kim, S.M.; Kang, Y.J.; Jung, J.S.; Suh, H.W. The Analgesic Effects and Mechanisms of Orally Administered Eugenol. Arch. Pharm. Res. 2011, 34, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Won, M.H.; Lee, J.C.; Kim, Y.H.; Song, D.K.; Suh, H.W.; Oh, Y.S.; Kim, J.H.; Shin, T.K.; Lee, Y.J.; Wie, M.B. Postischemic hypothermia induced by eugenol protects hippocampal neurons from global ischemia in gerbils. Neurosci. Lett. 1998, 254, 101–104. [Google Scholar] [CrossRef]
- Dallmeier, K.; Carlini, E.A. Anesthetic, hypothermic, myorelaxant and anticonvulsant effects of synthetic eugenol derivatives and natural analogues. Pharmacology 1981, 22, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lipton, J.M. Eugenol: Antipyretic activity in rabbits. Neuropharmacology 1987, 26, 1775–1778. [Google Scholar] [CrossRef]
- Lionnet, L.; Beaudry, F.; Vachon, P. Intrathecal eugenol administration alleviates neuropathic pain in male Sprague- Dawley rats. Phytother. Res. 2010, 24, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Park, C.K.; Kim, K.; Jung, S.J.; Kim, M.J.; Ahn, D.K.; Hong, S.D.; Kim, J.S.; Oh, S.B. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain 2009, 144, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Paula-Freire, L.I.G.; Molska, G.R.; Andersen, M.L.; Carlini, E.L.A. Ocimum gratissimum Essential Oil and Its Isolated Compounds (Eugenol and Myrcene) Reduce Neuropathic Pain in Mice. Planta Med. 2016, 82, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Ferland, C.E.; Beaudry, F.; Vachon, P. Antinociceptive Effects of Eugenol Evaluated in a Monoiodoacetate-induced Osteoarthritis Rat Model. Phytother. Res. 2012, 26, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.B.; Li, Y.S.; Miyano, K.; Nakata, Y. Phosphorylation of TRPV1 by neurokinin-1 receptor agonist exaggerates the capsaicin-mediated substance P release from cultured rat dorsal root ganglion neurons. Neuropharmacology 2008, 55, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Salo, P.T.; Theriault, E. Number, distribution and neuropeptide content of rat knee joint afferents. J. Anat. 1997, 190, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.H.; Carstens, M.I.; Carstens, E. Eugenol and carvacrol induce temporally desensitizing patterns of oral irritation and enhance innocuous warmth and noxious heat sensation on the tongue. Pain 2013, 154, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Tian, M.; Yu, J.; Shang, M.Y.; Cai, S.Q. Studies on morphology and aristolochic acid analogue constituents of Asarum campaniflorum and a comparison with two official species of Asari Radix et Rhizoma. J. Nat. Med. 2010, 64, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.C.; Criddle, D.N.; Coelho-de-Souza, A.N.; Monte, F.J.; Jaffar, M.; Leal-Cardoso, J.H. Relaxant and antispasmodic actions of methyleugenol on guinea-pig isolated ileum. Planta Med. 2000, 66, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Tabakoff, B.; Levinson, S.R.; Heinbockel, T. Inhibition of Nav1.7 channels by methyl eugenol as a mechanism underlying its antinociceptive and anesthetic actions. Acta Pharmacol. Sin. 2015, 36, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Fônseca, D.V.; Salgado, P.R.R.; Aragão Neto, H.C.; Golzio, A.M.F.O.; Marcelo, R.D.; Caldas Filho, M.R.; Melo, C.G.; Leite, F.C.; Piuvezam, M.R.; Pordeus, L.C.M.; et al. Ortho-eugenol exhibits anti-nociceptive and anti-inflammatory activities. Int. Immunopharmacol. 2016, 38, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.E. TRPM8 is the Principal Mediator of Menthol-induced Analgesia of Acute and Inflammatory. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on l-menthol. Food Chem. Toxicol. 2008, 46, S218–S223. [Google Scholar] [CrossRef] [PubMed]
- Green, B.G.; McAuliffe, B.L. Menthol desensitization of capsaicin irritation. Evidence of a short-term anti-nociceptive effect. Physiol. Behav. 2000, 68, 631–639. [Google Scholar] [CrossRef]
- Borhani Haghighi, A.; Motazedian, S.; Rezaii, R.; Mohammadi, F.; Salarian, L.; Pourmokhtari, M.; Khodaei, S.; Vossoughi, M.; Miri, R. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: A randomised, double-blind, placebo-controlled, crossed-over study. Int. J. Clin. Pract. 2010, 64, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Kiuchi, T.; Furuta, K. Efficacy and safety profile of a topical methyl salicylate and menthol patch in adult patients with mild to moderate muscle strain: A randomized, double-blind, parallel-group, placebo-controlled, multicenter study. Clin. Ther. 2010, 32, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.H.; Sawyer, C.M.; Carstens, M.I.; Tsagareli, M.G.; Tsiklauri, N.; Carstens, E. Topical application of l-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav. Brain. Res. 2010, 212, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Tian, Y.; Gao, R.; Li, H.; Zhao, X.; Barrett, J.E.; Hu, H. Central Mechanisms of Menthol-Induced Analgesia. J. Pharmacol. Exp. Ther. 2012, 343, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.; Attal, N.; Willer, J.C.; Bouhassira, D. Psychophysical study of the effects of topical application of menthol in healthy volunteers. Pain 2006, 122, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Wasner, G.; Naleschinski, D.; Binder, A.; Schattschneider, J.; McLachlan, E.M.; Baron, R. The effect of menthol on cold allodynia in patients with neuropathic pain. Pain Med. 2008, 9, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Gentry, C.; Stoakley, N.; Andersson, D.A.; Bevan, S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol. Pain 2010, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, W.M.; Bifolck-Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Hwang, S.W.; Miyamoto, T.; Dubin, A.E.; Patapoutian, A.; Story, G.M. More than cool: Promiscuous relationships of menthol and other sensory compounds. Mol. Cell. Neurosci. 2006, 32, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Karashima, Y.; Talavera, K.; Everaerts, W.; Janssens, A.; Kwan, K.Y.; Vennekens, R.; Nilius, B.; Voets, T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, M.; Uchida, K.; Suzuki, Y.; Matsui, H.; Shimada, T.; Fujita, F.; Tominaga, M. Reciprocal effects of capsaicin and menthol on thermosensation through regulated activities of TRPV1 and TRPM8. J. Physiol. Sci. 2016, 66, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Hosokawa, H.; Okazawa, M.; Kandachi, M.; Sawada, Y.; Yamanaka, K.; Matsumura, K.; Kobayashi, S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain. Res. Mol. Brain. Res. 2015, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Vichnewski, W.; Takahashi, A.M.; Nasi, A.M.T.; Gonçalves, D.C.R.G.; Dias, D.A.; Lopes, J.N.C.; Goedken, V.L.; Gutierrez, A.B.; Herz, W. Sesquiterpene lactones and other constituents from Eremanthus seidelli, E. goyazensis and Vanillosmopsis erythropappa. Phytochemistry 1989, 29, 1441–1451. [Google Scholar] [CrossRef]
- De Lira, P.N.; Andrade, E.H.; Sousa, P.J.; Silva, N.N. Essential oil composition of three Peperomia species from the Amazon, Brazil. Nat. Prod. Commun. 2009, 4, 427–430. [Google Scholar] [PubMed]
- Reynolds, J.E.F. Martindale, the Extra Pharmacopoeia; The Pharmaceutical: London, UK, 1996. [Google Scholar]
- Rocha, N.F.M.; Rios, E.R.V.; Carvalho, A.M.R.; Cerqueira, G.S.; Lopes, A.A.; Leal, L.K.A.M.; Dias, M.L.; de Sousa, D.P.; Sousa, F.C.F. Anti-nociceptive and anti-inflammatory activities of (−)-α-bisabolol in rodents. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 384, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.F.; Marçalo, A.; Martin, J.; Fernández, I.D.; Maldonado, H.; Vaisberg, A.J.; Hammond, G.B. (+) epi-α-bisabolol [correction of bisbolol] is the wound-healing principle of Peperomia galioides: Investigation of the in vivo wound-healing activity of related terpenoids. J. Nat. Prod. 2001, 64, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, S.B.; Leal, L.K.; Nogueira, N.A.; Campos, A.R. Bisabolol-induced gastroprotection against acute gastric lesions: Role of prostaglandins, nitric oxide, and KATP+ channels. J. Med. Food. 2009, 12, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.; Martini, M.V.; de Oliveira, C.M.; Cunha, S.; de Carvalho, J.E.; Ruiz, A.L.; da Silva, C.C. Antitumor activity of (−)-α-bisabolol-based thiosemicarbazones against human tumor cell lines. Eur. J. Med. Chem. 2010, 45, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Dal Sasso, M.; Fonti, E.; Culici, M. Antioxidant activity of bisabolol: Inhibitory effects on chemiluminescence of human neutrophil bursts and cell-free systems. Pharmacology 2009, 83, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Morales-Yuste, M.; Morillas-Márquez, F.; Martín-Sánchez, J.; Valero-López, A.; Navarro-Moll, M.C. Activity of (−) α-bisabolol against Leishmania infantum promastigotes. Phytomedicine 2010, 17, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.M.; Gonçalves, J.C.; Cruz, J.S.; Araújo, D.A. Evaluation of the sesquiterpene (−)-α-bisabolol as a novel peripheral nervous blocker. Neurosci. Lett. 2010, 472, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.O.; Leite, L.H.I.; Sampaio, R.S.; Araruna, M.K.A.; Menezea, I.R.A.; Costa, J.G.M.; Campos, A.R. (−)-α-Bisabolol attenuates visceral nociception and inflammation in mice. Fitoterapia 2011, 82, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.O.; Fernandes, C.N.; Menezes, I.R.A.; Costa, J.G.M.; Campos, A.R. Attenuation of visceral nociception by α-bisabolol in mice: Investigation of mechanisms. Org. Med. Chem. Lett. 2012, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Nurulain, S.; Prytkova, T.; Sultan, A.M.; Ievglevskyi, O.; Lorke, D.; Yang, K.H.S.; Petroianu, G.; Howarth, F.C.; Kabbani, N.; Oz, M. Inhibitory actions of bisabolol on α7-nicotinicacetylcholine receptors. Neuroscience 2015, 306, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed]
- Minae, B.; Sardari, M.; Sharifi, H.; Abadi, M.S.R.; Sadeghpour, O. Stachys lavandulifolia Vahl. and its relation with marmazad activities in traditional manuscripts. Iran. Red Crescent Med. J. 2015, 17, 19932. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.S.S.; Quintans, J.S.S.; Amarante, R.K.L.; Nascimento, T.S.; Amarante, R.S.; Barreto, A.S.; Pereira, E.W.M.; Duarte, M.C.; Coutinho, H.D.M.; Menezes, I.R.A.; et al. Evidence for the involvement of TNF-α and IL-1β in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl. (Lamiaceae) essential oil and (−)-α-bisabolol, its main compound, in mice. J. Ethnopharmacol. 2016, 191, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complem. Alternat. Med. 2014, 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Khan, A.A.; Musaddiq, I.A.M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of eugenol and cinnamaldehyde against the human gastric pathogen Helicobacter Pylori. Ann. Clin. Microbil. Antimicrob. 2005, 4, 1–7. [Google Scholar]
- Sharma, U.K.; Sharma, A.K.; Pandey, A.K. Medicinal attributes of major phenylpropanoids present in cinnamon. BMC Complement Altern. Med. 2016, 31, 156. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.S.; Lee, J.K.; Choi, Y.J.; Saitoh, S.I.; Miyake, K.; Hwanq, D.H.; Lee, J.Y. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem. Pharmacol. 2008, 75, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, H.D.R.; Mohammad, D.Q.; Farzane, S.M.D.; Mohammadreza, N.Y.; Seyyed, M.B. Comparative effect of Cinnamon essential oil, diclofenac and morphine on acute and chronic pain in mice. Int. J. Med. Lab. 2016, 3, 92–103. [Google Scholar]
- Churihar, R.; Solanki, P.; Vyas, S.; Hemant Tanwani, H.; Shubham Atal, S. Analgesic activity of cinnamaldehyde per se and it’s interaction with diclofenac sodium and pentazocine in swiss albino mice. Int. J. Phamacog. 2016, 3, 97–102. [Google Scholar]
- Trongtokit, Y.; Rongsriyam, Y.; Komalamisra, N.; Apiwathnasorn, C. Comparative Repellency of 38 Essential Oils against Mosquito Bites. Phytother. Res. 2005, 19, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Aakanksha, W.; Shivesh, J.; Vinod, K.N.; Dev, M.P. Chemical analysis and therapeutic uses of citronella oil from Cymbopogon winterianus: A short review. Int. J. Adv. Res. 2013, 1, 504–521. [Google Scholar]
- Lu, Y.; Khoo, T.J.; Wiart, C. Antioxidant activity determination of citronellal and crude extracts of Cymbopogon citratus by 3 different methods. Pharmacol. Pharm. 2014, 5, 395–400. [Google Scholar] [CrossRef]
- Leite, B.L.S.; Bonfim, R.R.; Antoniolli, A.R.; Tomazzi, S.M.; Araújo, A.A.S.; Blank, A.F.; Estevam, C.S.; Cambui, E.V.F.; Bonjardim, L.R.; Albuquerque Júnior, R.L.C.; et al. Assessment of antinociceptive, anti-inflammatory and antioxidant properties of Cymbopogon winterianus leaf essential oil. Pharm. Biol. 2010, 48, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Rocha, R.F.; Caregnato, F.F.; Moreira, J.C.F.; Silva, F.A.; Araújo, A.A.S.; Santos, J.P.A.; Melo, M.S.; Sousa, D.P.; Bonjardim, L.R.; et al. Antinociceptive action and redox properties of citronellal, an essential oil present in Lemongrass. J. Med. Food 2011, 14, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.T.; Oliveira, M.B.; Santana, M.F.; de Sousa, D.P.; Santana, D.G.; Camargo, E.A.; Oliveira, A.P.; Almeida, J.R.G.S.; Quintans-Júnior, L.J. Citronellal, a monoterpene present in Java citronella oil, attenuates mechanical nociception response in mice. Pharm. Biol. 2013, 51, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Tavares, E.S.; Julião, L.S.; Lopes, D.; Bizzo, H.R.; Lage, C.L.S.; Leitão, S.G. Análise do óleo essencial de folhas de quimiotipos de Lippia alba (Mill.) N.E. Br. (Verbenaceae) cultivados em condições semelhantes. Braz. J. Pharmacog. 2005, 15, 1–5. [Google Scholar] [CrossRef]
- Chanthai, S.; Prachakoll, S.; Ruangviriyachai, C.; Luthria, D.L. Influence of extraction methodologies on the analysis of five major volatile aromatic compounds of citronella grass (Cymbopogon nardus) and lemongrass (Cymbopogon citratus) grown in Thailand. J. AOAC Int. 2012, 95, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.R.R.; Bhattacharya, A.K.; Mallavarapu, G.R.; Ramesh, S. Yellowing and crinkling disease and its impact in the yield and composition of the essential oil of citronella (Cymbopogon winterianus Jowitt.). Flavour Frag. J. 2004, 19, 344–350. [Google Scholar]
- Katsukawa, M.; Nakata, R.; Koeji, S.; Hori, K.; Takahashi, S.; Inoue, H. Citronellol and geraniol, components of rose oil, activate peroxisome proliferator-activated receptor α and γ and suppress cyclooxygenase-2 expression. Biosci. Biotechnol. Biochem. 2011, 75, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.S.; Zhang, H.; Setzer, W.N. Composition of geranium (Pelargonium graveolens) essential oil from Tajikistan. Am. J. Essent. Oil. Nat. Prod. 2014, 2, 13–16. [Google Scholar]
- Viana, G.S.; Vale, T.G.; Pinho, R.S.; Matos, F.J. Antinociceptive effect of the essential oil from Cymbopogon citratus in mice. J. Ethnopharmacol. 2000, 70, 323–327. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Kameli, A.; Ferhat, M.A.; Saidi, F.; Mekarnia, M. Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan J. Med. 2013, 7, 22520. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.G.; Guimarães, A.G.; Quintans, J.S.S.; Santos, M.R.V.; de Sousa, D.P.; Passos, D.B., Jr.; Lucca Junior, W.; Brito, F.A.; Barreto, E.O.; Oliveira, A.P.; et al. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. J. Nat. Med. 2012, 66, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.G.; Santos, P.L.; Prado, D.S.; Santana, M.T.; Araújo, A.A.; Bonjardim, L.R.; Santos, M.R.; de Lucca Júnior, W.; Oliveira, A.P.; Quintans-Júnior, L.J. Citronellol reduces orofacial nociceptive behaviour in mice—Evidence of involvement of retrosplenial cortex and periaqueductal grey areas. Basic Clin. Pharmacol. Toxicol. 2013, 112, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.G.; Santos, P.L.; Quintans, J.S.S.; de Lucca Júnior, W.; Araújo, A.A.S.; Saravanan, S.; Menezes, I.R.A.; Coutinho, H.D.M.; Quintans-Júnior, L.J. Citronellol, a natural acyclic monoterpene, attenuates mechanical hyperalgesia response in mice: Evidence of the spinal cord lamina I inhibition. Chem. Biol. Interact. 2015, 239, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.R.V.; Rocha, N.F.M.; Carvalho, A.M.R.; Vasconcelos, L.F.; Dias, M.L.; Sousa, D.P.; Sousa, F.C.F.; Fonteles, M.M.F. TRP and ASIC channels mediate the antinociceptive effect of citronellyl acetate. Chem. Biol. Interact. 2013, 203, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Loumouamou, A.N.; Silou, T.; Mapola, G.; Chalchat, J.C.; Figuérédo, G. Yield and composition of essential oils from Eucalyptus citriodora x Eucalyptus torelliana, a hybrid species growing in Congo-Brazzaville. J. Essent. Oil Res. 2009, 21, 295–299. [Google Scholar] [CrossRef]
- Paik, S.Y.; Koh, K.H.; Beak, S.M.; Paek, S.H.; Kim, J.A. The essential oils from Zanthoxylum schinifolium pericarp induce apoptosis of HepG2 human hepatoma cells through increased production of reactive oxygen species. Biol. Pharm. Bull. 2005, 28, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.; Matos, F.J. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, H.D.A.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; Silva, F.V.; Cunha, F.V.M.; Wanderley, C.W.S.; Pinheiro, G.; Cândido, A.G.F.; Wong, D.V.T.; et al. α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Abbasi, N. Hypolipidemic activity of Anethum graveolens in rats. Phytother. Res. 2008, 22, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Essien, E.E.; Ogunwande, I.A.; Setzer, W.N.; Ekundayo, O. Chemical composition, antimicrobial, and cytotoxicity studies on S. erianthum and S. macranthum essential oils. Pharm. Biol. 2012, 50, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.F.; Camara, C.A.; Moraes, M.M.; Ramos, C.S. Essential oil composition and acaricidal activity of Schinus terebinthifolius from Atlantic Forest of Pernambuco, Brazil against Tetranychusurticae. Nat. Prod. Commun. 2012, 7, 129–132. [Google Scholar]
- Singh, G.; Singh, O.P.; Maurya, S. Chemical and biocidal investigation on essential oil of some Indian Curcuma species. Prog. Cryst. Growth Charact. Mater. 2002, 45, 75–81. [Google Scholar] [CrossRef]
- Asbaghian, S.; Shafaghat, A.; Zarea, K.; Kasimov, F.; Salimi, F. Comparison of volatile constituents, and antioxidant and antibacterial activities of the essential oils of Thymus caucasicus, T. kotschyanus and T. vulgaris. Nat. Prod. Commun. 2011, 6, 137–140. [Google Scholar]
- Arjouni, M.Y.; Bahri, F.; Romane, A.; El Fels, M.A. Chemical composition and antimicrobial activity of essential oil of Cupressus atlantica. Nat. Prod. Commun. 2011, 6, 1519–1522. [Google Scholar] [PubMed]
- Vitalini, S. Traditional uses of medicinal plants in Valvestino (Italy). J. Ethnopharmacol. 2009, 121, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Erazo, S.; Delporte, C.; Negrete, R.; Garcıa, R.; Zaldıvar, M.; Iturra, G.; Caballero, E.; Lopez, J.L.; Backhouse, N. Constituents and biological activities of Schinus polygamus. J. Ethnopharmacol. 2006, 107, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, A.; Takaki, I.; Bersani-Amado, L.E.; Dantas, J.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Antiinflammatory and antinociceptive activities of Zingiber officinale roscoe essential oil in experimental animal models. Indian J. Pharmacol. 2006, 38, 58–59. [Google Scholar]
- Lima, D.F.; Brandão, M.S.; Moura, J.B.; Leitão, J.M.; Carvalho, F.A.; Miúra, L.M.; Leite, J.R.; Sousa, D.P.; Almeida, F.R. Antinociceptive activity of the monoterpene α-phellandrene in rodents: Possible mechanisms of action. J. Pharm. Pharmacol. 2012, 64, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Siani, A.C.; Nakamura, M.J.; Das Neves, G.P.; Monteiro, S.S.; Ramos, M.F.S. Leaf essential oil from three exotic Myrtaceae species growing in the Botanical Garden of Rio de Janeiro, Brazil. Am. J. Plant Sci. 2016, 7, 834–840. [Google Scholar] [CrossRef]
- Wolffenbuttel, A.N.; Zamboni, A.; Santos, M.K.; Borille, B.T.; Augustin, O.A.; Mariotti, K.C.; Leal, M.B.; Limberger, P.R. Chemical components of Citrus essential oils from Brazil. Nat. Prod. J. 2015, 5, 14–27. [Google Scholar] [CrossRef]
- Bibak, H.; Ali, A. Essential oil composition of stems, leaves and flowers of Nepeta dschuparensis Bornm from Kerman, Iran. J. Essent. Oil Bear. Plants 2017, 20, 597–600. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.J.; Oliveira, M.G.; Santana, M.F.; Santana, M.T.; Guimarães, A.G.; Siqueira, J.S.; de Sousa, D.P.; Almeida, R.N. α-Terpineol reduces nociceptive behavior in mice. Pharm Biol. 2011, 49, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, P.K.; Mishra, P.S. A review on the vanillin derivatives showing various biological activities. Int. J. Pharm.Tech. Res. 2012, 4, 266–279. [Google Scholar]
- Shoeb, A.; Chowta, M.; Pallempati, G.; Rai, A.; Singh, A. Evaluation of antidepressant activity of vanillin in mice. Indian J. Pharmacol. 2013, 45, 141–144. [Google Scholar] [PubMed]
- Kinga, A.A.; Shaughnessy, D.T.; Murea, K.; Leszczynska, J.; Ward, W.O.; Umbach, D.M. Anti mutagenicity of cinnamaldehyde and vanillin in human cells: Global gene expression and possible role of DNA damage and repair. Mutat. Res. 2007, 616, 609. [Google Scholar]
- Makni, M.; Chtourou, Y.; Fetoui, H.; Garoui el, M.; Boudawara, T.; Zeghal, N. Evaluation of the antioxidant, anti-inflammatory and hepato protective properties of vanillin in carbon tetrachloride-treated rats. Eur. J. Pharmacol. 2011, 668, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Soares, A.K.; de Sousa, D.P. Overview of the Role of Vanillin on Redox Status and Cancer Development. Oxid. Med. Cell. Longev. 2016, 2016, 9734816. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Sim, Y.; Choi, S.; Seo, Y.; Kwon, M.; Lee, J.; Suh, W.H. Antinociceptive profiles and mechanisms of orally administered vanillin in the mice. Arch. Pharm. Res. 2009, 32, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Rathnakar, U.P.; Srikanth, D.; Menezes, V.H.; Shenoy, K.; Ashok, A.; Sahana, D.; Nishchal, B.S.; Shivaprakash, G.; Udupa, A.L. Evaluation of antinociceptive activity of Vanillin mediated through opioid receptors. Drug Invent. Today 2012, 4, 674–676. [Google Scholar]
- Srikanth, D.; Vishma, H.M.; Nischal, S.; Rathnakar, U.P.; Shiv, P.G.; Sahana, D.A.; Ashok, S.K.; Udupa, A.L. Evaluation of anti-inflammatory property of vanillin in carrageenan induced paw edema model in rats. Int. J. Bioassays 2013, 2, 269–271. [Google Scholar]
- Sharan, A. Anti-inflammatory and antinociceptive activity of vanillin. Chem. Sci. J. 2016, 7, 3. [Google Scholar]
- Granger, R.E.; Campbell, E.L.; Johnston, G.A. (+)- And (−)-borneol: Efficacious positive modulators of GABA action at human recombinant α1β2γ2L GABAA receptors. Biochem. Pharmacol. 2005, 69, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, J.C.; Oliveira, N.N.; Arcanjo, D.D.; Quintans-Júnior, L.J.; Cavalcanti, S.C.; Santos, M.R.; Oliveira, R.C.; Oliveira, A.P. Investigation of mechanisms involved in (−)-borneol-induced vasorelaxant response on rat thoracic aorta. Basic Clin. Pharmacol. Toxicol. 2012, 110, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A. Camphor in the Edo era–camphor and borneol for medicines. Yakushigaku Zasshi 2000, 35, 49–54. [Google Scholar] [PubMed]
- Zhong, W.; Cui, Y.; Yu, Q.; Xie, X.; Liu, Y.; Wei, M.; Ci, X.; Peng, L. Modulation of LPS-stimulated pulmonary inflammation by borneol in murine acute lung injury model. Inflammation 2014, 37, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, L.; Lan, X.; Zhang, T.T.; Sun, J.H.; Du, G.H. Protection by borneol on cortical neurons against oxygen-glucose deprivation/reperfusion: Involvement of anti-oxidation and anti-inflammation through nuclear transcription factor γ B signaling pathway. Neurosci. 2011, 176, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.G.S.; Souza, G.R.; Silva, J.; Saraiva, S.R.G.L.; Oliveira Júnior, R.G.; Quintans, J.S.S.; Barreto, R.S.S.; Bonjardim, L.R.; Cavalcanti, S.C.H.; Quintans Júnior, L.J. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013, 2013, 808460. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shen, Y.Y.; Li, J.; Lin, Y.H.; Luo, C.X.; Zhu, D.Y. (+)-Borneol alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. Eur. J. Pharmacol. 2015, 757, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Padalia, R.C.; Yadav, A.; Chauhan, A. Essential Oil Composition of Aralia cachemirica from Uttarakhand, India. Rec. Nat. Prod. 2010, 4, 163–166. [Google Scholar]
- Mockute, D.; Judzentiene, A. The myrtenol chemotype of essential oil of Tanacetum vulgare L. var. vulgare (tansy) growing wild in the Vilnius region. Chemija 2003, 14, 103–107. [Google Scholar]
- Bell, S.G.; Chen, X.; Sowden, R.J.; Xu, F.; Williams, J.N.; Wong, L.L.; Rao, Z. Molecular recognition in (+)-α-pinene oxidation by cytochrome P450cam. J. Am. Chem. Soc. 2003, 125, 705–714. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; Nóbrega, F.F.F.; Claudino, F.S.; Almeida, R.N.; Leite, J.R.; Mattei, R. Pharmacological effects of the monoterpene α,β-epoxy-carvone in mice. Rev. Bras. Farmacogn. 2007, 17, 170–175. [Google Scholar] [CrossRef]
- Santos, M.R.V.; Moreira, F.V.; Fraga, B.P.; de Sousa, D.P.; Bonjardim, L.R.; Quintans-Junior, L.J. Cardiovascular effects of monoterpenes: A review. Rev. Bras. Pharmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef]
- Ngan, L.T.M.; Moon, J.K.; Kim, J.H.; Shibamoto, T.; Ahn, Y.J. Growth-inhibiting effects of Paeonia lactiflora root steam distillate constituents and structurally related compounds on human intestinal bacteria. World J. Microbiol. Biotechnol. 2012, 28, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.; Salvadori, M.S.; Sousa, F.B.M.; Santos, M.S.; Carvalho, N.S.; Sousa, D.P.; Gomes, B.S.; Oliveira, F.A.; Barbosa, A.L.R.; Freitas, R.M.; et al. Evaluation of the anti-inflammatory and antinociceptive effects of myrtenol, a plant derived monoterpene alcohol, in mice. Flavour Fragr. J. 2014, 29, 184–192. [Google Scholar] [CrossRef]
- Solis-Quispe, L.; Tomaylla-Cruz, C.; Callo-Choquelvica, Y.; Solís-Quispe, A.; Rodeiro, I.; Hernández, I.; Fernández, M.D.; Pino, J.A. Chemical composition, antioxidant and antiproliferative activities of essential oil from Schinus areira L. and Minthostachys spicata (Benth.) Epl. grown in Cuzco, Peru. J. Essent. Oil Res. 2015, 28, 234–240. [Google Scholar] [CrossRef]
- Mikaili, P.; Mojaverrostami, S.; Moloudizargari, M.; Aghajanshakeri, S. Pharmacological and therapeutic effects of Mentha longifolia L. and its main constituent, menthol. Anc. Sci. Life 2013, 33, 131–138. [Google Scholar] [PubMed]
- Lorenzo, D.; Paz, D.; Dellacassa, E.; Davies, P.; Vila, R.; Salvador, C. Essential oils of Mentha pulegium and Mentha rotundifolia from Uruguay. Braz. Arch. Biol. Technol. 2002, 45, 519–524. [Google Scholar] [CrossRef]
- Devi, R.C.; Sim, S.M.; Ismail, R. Spasmolytic effect of citral and extracts of Cymbopogon citratus on isolated rabbit ileum. J. Smooth Muscle Res. 2011, 47, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Olorunnisola, S.K.; Asiyanbi, H.T.; Hammed, A.M.; Simsek, S. Biological properties of lemongrass: An overview. Int. Food Res. J. 2014, 21, 455–462. [Google Scholar]
- Nishijima, C.M.; Ganev, E.G.; Mazzardo-Martins, L.; Martins, D.F.; Rocha, L.R.M.; Santos, A.R.S.; Hiruma-Lima, C.A. Citral: A monoterpene with prophylactic and therapeutic anti-nociceptive effects in experimental models of acute and chronic pain. Eur. J. Pharmacol. 2014, 736, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.; Lima, F.C.; Lahlou, S.; Magalhães, P.J.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam. Clin. Pharmacol. 2010, 24, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Angeles-López, G.; Pérez-Vásquez, A.; Hernández-Luis, F.; Déciga-Campos, M.; Bye, R.; Linares, E.; Mata, R. Antinociceptive effect of extracts and compounds from Hofmeisteria schaffneri. J. Ethnopharmacol. 2010, 131, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; Carvalho, M.D.B.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response, Evid. Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaji, I.; Karaki, S.; Kuwahara, A. Effects of luminal thymol on epithelial transport in human and rat colon. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1132–G1143. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Wang, C.; Fujita, T.; Jiang, C.Y.; Kumamoto, E. Action of thymol on spontaneous excitatory transmission in adult rat spinal substantia gelatinosa neurons. Neurosci. Lett. 2015, 606, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, E.; Laudicina, V.A.; Germanà, M.A. Current and potential use of Citrus essential oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Jezler, C.N.; Oliveira, A.R.M.F.; Batista, R.S.; Oliveira, R.A.; Silva, D.C.; Costa, L.C.B. Lippia alba morphotypes cidreira and melissa exhibit signifcant differences in leaf characteristics and essential oil profile. Braz. J. Pharmacog. 2013, 23, 217–223. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia dracunculus L. (Tarragon): A critical review of its traditional use, chemical composition, pharmacology, and safety. J. Agr. Food Chem. 2011, 59, 11367–11384. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Lee, N.H.; Hyun, C.G. Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. J. Oleo Sci. 2010, 59, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kaimoto, T.; Hatakeyama, Y.; Takahashi, K.; Imagawa, T.; Tominaga, M.; Ohta, T. Involvement of transient receptor potential A1 channel in algesic and analgesic actions of the organic compound limonene. Eur. J. Pain 2016, 20, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Reyes, R.; Aguirre-Hernández, E.; García-Argáez, A.; Soto-Hernández, M.; Linares, E.; Bye, R.; Heinze, G.; Martínez-Vázquez, M. Comparative chemical composition of Agastache mexicana subsp. mexicana and A. mexicana subsp. xolocotziana. Biochem. Syst. Ecol. 2004, 32, 685–694. [Google Scholar] [CrossRef]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef] [PubMed]
- González-Ramírez, A.E.; González-Trujano, M.E.; Pellicer, F.; López-Muñoz, F.J. Anti-nociceptive and anti-inflammatory activities of the Agastache mexicana extracts by using several experimental models in rodents. J. Ethnopharmacol. 2012, 142, 700–705. [Google Scholar] [PubMed]
- González-Ramírez, A.E.; González-Trujano, M.E.; Orozco-Suárez, S.A.; Alvarado-Vásquez, N.; López-Muñoz, F.J. Nerol alleviates pathologic markers in the oxazolone-induced colitis model. Eur. J. Pharmacol. 2016, 776, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Friedman, J.; Dudai, N.; Larkov, O.; Cohen, Y.; Bar, E.; Ravid, U.; Putievsky, E.; Lewinsohn, E. Biosynthesis of estragole and t-anethole in bitter fennel (Foeniculum vulgare Mill. var. vulgare) chemotypes. Changes in SAM: Phenylpropene O-methyltransferase activities during development. Plant. Sci. 2002, 163, 1047–1053. [Google Scholar] [CrossRef]
- Lucca, P.S.R.; Nóbrega, L.H.P.; Alves, L.F.A.; Cruz-Silva, C.T.A.; Pacheco, F.P. The insecticidal potential of Foeniculum vulgare Mill., Pimpinella anisum L. and Caryophillus aromaticus L. to control aphid on kale plants. Rev. Bras. Plantas Med. 2015, 17, 585–591. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Costache, I.I.; Miron, A. Anethole and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 247–267. [Google Scholar] [PubMed]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-Anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.J. Isolation and identification of anethole from Pimpinella anisum L. fruit oil: An antimicrobial study. J. Pharma. Res. 2009, 2, 915–919. [Google Scholar]
- Freire, R.S.; Morais, S.M.; Catunda, F.E.A., Jr.; Pinheiro, D.C.S.N. Synthesis and antioxidant, anti-inflammatory and gastroprotector activities of anethole and related compounds. Bioorganic Med. Chem. 2005, 13, 4353–4358. [Google Scholar] [CrossRef] [PubMed]
- Domiciano, T.P.; Dalalio, M.M.D.O.; Silva, E.L.; Ritter, A.M.; Estevão-Silva, C.F.; Ramos, F.S.; Caparroz-Assef, S.M.; Cuman, R.K.; Bersani-Amado, C.A. Inhibitory effect of anethole in nonimmune acute inflammation. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, C.; Galeotti, N.; Mazzanti, G. Local anaesthetic activity of monoterpenes and phenylpropanes of essential oils. Planta Med. 2001, 67, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.M.V.; Ames, F.Q.; Otani, F.; de Oliveira, R.M.W.; Cuman, R.K.N.; Bersani-Amado, C.A. Effects of anethole in nociception experimental models. Evid. Based Complement. Altern. Med. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.M.V.; Domiciano, T.P.; Verri, W.A., Jr.; Zarpelon, A.C.; Da Silva, L.G.; Barbosa, C.P.; Natali, M.R.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Antihypernociceptive activity of anethole in experimental inflammatory pain. Inflammopharmacology 2013, 21, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A sesquiterpene alcohol with Multi-faceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Koudou, J.; Abena, A.A.; Ngaissona, P.; Bessiére, J.M. Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia 2005, 76, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Klopell, F.C.; Lemos, M.; Sousa, J.P.B.; Comunello, E.; Maistro, E.L.; Bastos, J.K.; Andrade, S.F. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Z. Naturforsch. 2007, 62, 537–542. [Google Scholar] [CrossRef]
- Fonsêca, D.V.; Salgado, P.R.; de Carvalho, F.L.; Salvadori, M.G.; Penha, A.R.; Leite, F.C.; Borges, C.J.; Piuvezam, M.R.; Pordeus, L.C.; Sousa, D.P.; et al. Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABAergic system and proinflammatory cytokines. Fundam. Clin. Pharmacol. 2016, 30, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Terzi, V.A. Carvone (Mentha spicata L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2015; pp. 309–316. [Google Scholar]
- Nogoceke, F.P.; Barcaro, I.M.; de Sousa, D.P.; Andreatini, R. Antimanic-like effects of (R)-(−)-carvone and (S)-(+)-carvone in mice. Neurosci. Lett. 2016, 21, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Salim, E.R.A.; Abu-Goukh, A.B.A.; Khalid, H.E.S.; El Hassan, G.M. Carvone content and chemical compositon in spearmint (Mentha spicata var. viridis L.) as affected by herb storage under ambient temperature. J. Food Nutr. Popul. Health 2016, 1, 1–7. [Google Scholar]
- Gonçalves, J.C.; Oliveira, F.S.; Benedito, R.B.; de Sousa, D.P.; de Almeida, R.N.; de Araújo, D.A. Antinociceptive activity of (−)-carvone: Evidence of association with decreased peripheral nerve excitability. Biol. Pharm. Bull. 2008, 31, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.R.; Silveira, A.L.; de Souza, H.D.N.; Nery, A.A.; Prado, V.F.; Prado, M.A.M.; Ulrich, H.; Araújo, D.A.M. The monoterpene (−)-carvone: A novel agonist of TRPV1 Channels. Cytom. Part A 2013, 83, 212219. [Google Scholar] [CrossRef] [PubMed]
- Melo, N.I.; Carvalho, C.E.; Fracarolli, L.; Cunha, W.R.; Veneziani, R.C.S.; Martins, C.H.G.; Crotti, A.E.M. Antimicrobial activity of the essential oil of Tetradenia riparia (Hochst.) Codd. (Lamiaceae) against cariogenic bacteria. Braz. J. Microbiol. 2015, 46, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Grajales-Conesa, J.; Ramírez, V.M.; Cruz-López, L.; Guillén, D.S. Effect of Citrus floral extracts on the foraging behavior of the stingless bee Scaptotrigona pectoralis (Dalla Torre). Rev. Bras. Entomol. 2012, 56, 76–80. [Google Scholar] [CrossRef]
- Qamar, W.; Sultana, S. Farnesol ameliorates massive inflammation, oxidative stress and lung injury induced by intratracheal instillation of cigarette smoke extract in rats: An initial step in lung chemoprevention. Chem. Biol. Interact. 2008, 176, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Júnior, W.M.; Benedito, R.B.; Pereira, W.B.; Torres, P.A.; Ramos, C.A.F.; Costa, J.P.; Tomé, A.R.; de Sousa, D.P.; de Freitas, R.M.; Diniz, M.F.F.M.; et al. Farnesol: Antinociceptive effect and histopathological analysis of the striatum and hippocampus of mice. Fundam. Clin. Pharmacol. 2013, 27, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Jagan, M.R.L.; Sakariah, K.K. Volatile constituents from Cinnamomum zeylanicum fruit stalks and their antioxidant activities. J. Agric. Food Chem. 2003, 51, 4344–4348. [Google Scholar] [CrossRef] [PubMed]
- Mockute, D.; Bernotiene, G.; Judzentiene, A. Chemical composition of essential oils of Origanum vulgare L. growing in Lithuania. Biologija 2004, 4, 44–49. [Google Scholar]
- Menon, A.N.; Padmakumari, K.P.; Jayalekshmy, A. Essential oil composition of four major cultivars of black pepper (Piper nigrum L.)-IV. J. Essent. Oil Res. 2011, 14, 84–86. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. β-Caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.; Malingre, T.; Battermann, S.; Boss, R. Mono- and sesquiterpene hydrocarbons of essential oil of Cannabis sativa. Phytochemistry 1975, 14, 814–815. [Google Scholar] [CrossRef]
- Vijayalaxmi, A.; Vasudha, B.; Nazia, B.; Kowmudi, V.; Naveen, K.Y.; Yogesh, R. Anti-arthritc and anti-inflammatory actvity of β caryophyllene against freund’s complete adjuvant induced arthrits in wistar rats. J. Bone Rep. Recommend. 2015, 1, 1–10. [Google Scholar]
- Martin, S.; Padilla, E.; Ocete, M.A.; Galvez, J.; Jiménez, J.; Zarzuelo, A. Anti-inflammatory activity of the essential oil of Bupleurum fruticescens. Planta Med. 1993, 59, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, S.; Mizoguchi, H.; Kuwahata, H.; Komatsu, T.; Nagaoka, K.; Nakamura, H.; Bagetta, G.; Sakurada, T.; Sakurada, S. Involvement of peripheral cannabinoid and opioid receptors in β-caryophyllene-induced antinociception. Eur. J. Pain 2013, 17, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, B.P.J.; Menezes, C.T.; Silva, J.P.; de Sousa, D.P.; Batista, J.S. Antiulcer effect of epoxy-carvone. Braz. J. Pharmacog. 2012, 22, 144–149. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Shafi, P.M.; Abraham, G.T. Analysis of the essential oil of the roots of the medicinal plant Kaempferia galanga L. (Zingiberaceae) from South India. Acta Pharm. Turc. 2001, 43, 107–110. [Google Scholar]
- Iacobellis, N.S.; Lo Cantore, P.; Capasso, F.; Senatore, F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J. Agric. Food Chem. 2005, 53, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Ohloff, G. Der stereochemische verlauf der alkalischen epoxydation von α,β-ungesättigten carbonylverbindungen der cyclischen monoterpenreihe. Tetrahedron 1963, 19, 1091–1099. [Google Scholar] [CrossRef]
- Arruda, T.A.; Antunes, R.M.P.; Catão, R.M.R.; Lima, E.O.; de Sousa, D.P.; Nunes, X.P.; Pereira, M.S.V.; Barbosa-Filho, J.M.; da Cunha, E.V.L. Preliminary study of the antimicrobial activity of Mentha x villosa Hudson essential oil, rotundifolone ant its analogues. Rev. Bras. Farmacogn. 2006, 16, 307–311. [Google Scholar] [CrossRef]
- Almeida, R.N.; de Sousa, D.P.; Nóbrega, F.F.F.; Claudino, F.S.; Araújo, D.A.M.; Leite, J.R.; Mattei, R. Anticonvulsant effect of a natural compound α,β-epoxy-carvone and its action on the nerve excitability. Neurosci. Lett. 2008, 443, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, M.L.; Oliveira, L.E.G.; Santos, C.C.M.P.; de Sousa, D.P.; de Almeida, R.N.; Araújo, D.A.M. Antinociceptive and anti-inflammatory effects of the monoterpene α,β-epoxy-carvone in mice. J. Nat. Med. 2013, 63, 743–749. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; de Sousa Oliveira, F.; de Almeida, R.N. Evaluation of the central activity of hydroxydihydrocarvone. Biol. Pharm. Bull. 2006, 29, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.S.; de Sousa, D.P.; de Almeida, R.N. Antinociceptive effect of hydroxydihydrocarvone. Biol. Pharm. Bull. 2008, 31, 588–591. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Chessa, M.L.; Moretti, M.D.; Serra, G.; Pippia, P. (−)-Linalool produces antinociception in two experimental models of pain. Eur. J. Pharmacol. 2003, 460, 37–41. [Google Scholar] [CrossRef]
- Peana, A.T.; de Montis, M.G.; Nieddu, E.; Spano, M.T.; D’Aquila, P.S.; Pippia, P. Profile of spinal and supra-spinal antinociception of (−)-linalool. Eur. J. Pharmacol. 2004, 485, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.T.; Rubattu, P.; Piga, G.G.; Fumagalli, S.; Boatto, G.; Pippia, P.; de Montis, M.G. Involvement of adenosine A1 and A2A receptors in (−)-linalool-induced antinociception. Life Sci. 2006, 78, 2471–2474. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.A.; Werner, M.F.; Oliveira, E.C.; Burgos, L.; Pereira, P.; Brum, L.F.; Story, G.M.; Santos, A.R. The antinociceptive effect of (−)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J. Pain 2010, 11, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Letizia, C.S.; Cocchiara, J.; Lalko, J.; Api, A.M. Fragrance material review on linalool. Food Chem. Toxicol. 2003, 41, 943–964. [Google Scholar] [CrossRef]
| Compound | Experimental Protocol | Antinociceptive Activity and/or Mechanism | Animal Tested and/or Cell Line | Reference |
|---|---|---|---|---|
 | Formalin, capsaicin and glutamate-induced orofacial nociception | Decreased rubbing behavior | Male Swiss mice | [19] |
| Acetic acid-induced writhing, formalin and hot plate tests CG-induced inflammation | Reduced writhes, and liking time Increased latency time of liking and jumping behavior Reduced leukocyte migration | Male Swiss mice | [20] | |
| Tail flick test CG, TNF-α, PGE2 and dopamine-induced hypernociception CG-induced pleurisy LPS-induced NO secretion Fos protein immunofluorescence | Increased latency time response Decreased mechanical hypernociception Reduced leukocyte and neutrophils migration and TNF-α level Reduced NO production Increased c-Fos immunoreaction | Male Swiss mice Macrophage Periaqueductal grey neurons | [25] | |
| Acetic acid-induced writhing and formalin tests | Reduced writhes, and liking time | Male Swiss mice | [31] | |
 | Acetic acid-induced writhing test Formalin test Hot plate test Peritoneal permeability induced by acetic acid | Reduced the number of abdominal contortions Inhibited nociception in both the first phase and second phase Caused a significant latency prolongation Inhibited the acetic acid-induced peritoneal capillary permeability | Male albino Swiss mice | [241] |
 | Acetic acid-induced writhing, formalin and hot plate tests | Reduced writhes, and paw-liking time Increased latency time response | Male Swiss mice | [40] |
| CG, TNF-α, PGE2 and dopamine-induced hypernociception CG-induced paw edema CG-induced pleurisy LPS-induced nitrite secretion | Decreased mechanical hypernociception Decreased edema volume Reduced leukocyte migration and TNF-α level Reduced nitrite production | Male Swiss mice Macrophage | [42] | |
| Formalin, capsaicin and glutamate-induced orofacial nociception | Reduced face-rubbing behavior | Male Swiss mice | [43] | |
| Whole-cell voltage-clamp recordings | Increased secretion of l-glutamate Membrane hyperpolarization | Rat spinal cord | [44] | |
 | Whole-cell voltage-clamp recordings Intracellular recordings | Inhibition of excitability Generation of action potentials | Male and female Wistar rat sciatic nerve and dorsal root ganglia | [47] |
 | von Frey test (electronic version) | Reduced mechanical allodynia | Male ddY-strain mice (sciatic nerve ligation) | [56] |
| Formalin test | Reduced licking and biting behavior | Male ddY-strain mice | [62] | |
| Xylene-induced ear edema and formalin-induced hind paw edema COX-2 expression and inflammatory infiltrates immunohistochemistry | Reduced edema volume Decreased COX-2 expression and inflammatory infiltrates | Male Kunming mice | [64] | |
| Formalin and hot plate tests c-Fos immunohistochemistry | Increased latency time of hindpaw withdrawl Increased c-Fos expression in hypothalamic orexin neurons | Wild type mice (C57BL/6) Orexin neuron-ablated mice Orexin peptide-deficient mice | [65] | |
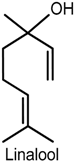 | Paclitaxel-induced acute pain (von Frey filaments) | Inhibiton of mechanical allodynia and hypernociception | Male ddY-strain mice | [48] |
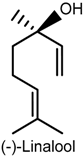 | Formalin, capsaicin and glutamate-induced orofacial nociception Field potential recordings | Reduced face-rubbing behavior Inhibition of field potentials | Male Swiss mice Hippocampal dentate gyrus | [53] |
| Acetic acid-induced writhing, formalin and hot plate tests CG-induced peritonitis | Reduced writhes, and paw-liking time Increased latency time response Inhibition of leukocyte migration and TNF-α level | Male Swiss mice | [67] | |
| Chronic noninflammatory muscle pain model c-Fos protein immunofluorescence | Inhibition of mechanical hypernociception Neuron activation | Male Swiss mice | [69] | |
 | Acetic acid-induced writhing and formalin tests Substance P and glutamate-induced nociceptive behavior | Reduced writhes, and paw-liking/biting behavior Reduced nociceptive response | Male ICR mice | [73] |
| Lingual irritation method | Oral irritation desensitization Increased innocuous warmth and noxious heat sensation | Human subjects | [83] | |
| Acetic acid-induced writhing test and glutamate-induced nociception Glutamate, AMPA, kainate, substance P and TNF-α-induced pain | Reduced writhes Reduced nociception Inhibition of biting behavior | Male Swiss mice | [71] | |
| Monoiodoacatate-induced osteoarthritis von Frey filaments method Spinal pain-related peptide analysis | Altered gait parameters Reduced mechanical allodynia Reduced expression of substance P and calcitonin gene-related peptide Increased dynorphin level | Sprague Dawley rats Spinal cord samples | [80] | |
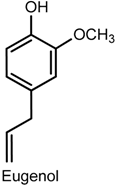 | Sciatic nerve constriction von Frey and hot plate tests Biochemical assay | Inhibition of mechanical hypernociception Increased latency time response Reduced IL-1β level | Male C57BL/6J mice Sciatic nerve | [79] |
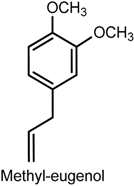 | Whole-cell patch-clamp recordings | Inhibition of peripheral nerve Nav1.7 currents | Chinese hamster ovary cells | [86] |
 | Acetic acid-induced writhing, glutamate and hot plate tests Acetic acid-induced peritoneal permeability CG-induced peritonitis | Reduced writhes and licking time Increased latency time response Inhibition of vascular permeability Reduced TNF-α and IL-1β levels | Male Swiss mice | [87] |
 | Complete Freund’s adjuvant-induced mechanical and thermal hypersensitivity Formalin-induced nociception Electrophysiological recording | Reduced mechanical and thermal allodynia Reduced paw-licking and lifting behavior Blockage of voltage-gated sodium and calcium channels Reduced neural excitability Blockage of spontaneous synaptic transmission | D1-male mice Mouse pup spinal cord dorsal horn neurons | [94] |
| Quantitative sensory testing | Provoked cold hypersensitivity | Human subjects | [46] | |
 | Acetic acid-induced writhing, hot plate, tail flick, and Complete Freund’s adjuvant tests Capsaicin-induced pain | Reduced writhes and licking, fliching and biting behavior | C57BL/6 wild-type mice Trp1-/- and Trpm8-/- mice | [88] |
| Calcium ion-imaging Whole-cell patch-clamp recordings Sensory irritation tests | Inhibition of TRPV1 currents Reduced skin irritation | Human embryonic kidney 293 cells Human subjects | [101] | |
 | Acetic acid-induced writhing and formalin tests CG and dextran-induced paw edema CG-induced peritonitis and Neutrophil myeloperoxidade (MPO) assay | Reduced writhes and paw-licking time Inhibition of edema formation Inhibition of MPO activity Reduced neutrophil migration and TNF-α migration | Male Swiss mice Rat | [107] |
| Cyclophosphamide and mustard oil-induced visceral nociception Croton oil, arachidonic acid and phenol-induced ear edema | Inhibition of nociceptive behavior Inhibition of ear edema formation | Male Swiss mice | [114] | |
| Acetic acid, formalin, capsaicin and mustard oil-induced visceral nocicpetion | Reduced writhes Inhibition of nociceptive behavior | Male Swiss mice | [115] | |
| Electrophysiological recordings Whole-cell patch-clamp recordings | Inhibition of α7-nicotinic acetylcholine receptors Inhibition of choline-induced currents | Frog oocytes (Xenopus laevis) Male Sprague Dawley rat hippocampus | [116] | |
 | Formalin test Capsaicin and glutamate-induced orofacial nociception CG-induced pleurisy | Reduced face-rubbing behavior Inhibition of nociceptive response Reduced TNF-α level | Male Swiss mice | [119] |
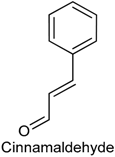 | Quantitative sensory testing | Provoked heat hypersensitivity | Human subjects | [46] |
| Acetic acid induced writhing Eddy’s hot plate methods | Reduced nociception Exhibited hyperalgesic behavior | Swiss albino mice | [125] | |
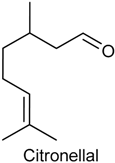 | Formalin test, capsaicin test and glutamate-induced nociception | Reduced the pain-related behaviors and decreased face-rubbing behavior | Male Swiss mice | [131] |
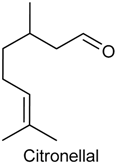 | Mechanical nociception induced by CG, TNF-α, PGE2 and DA | Reduced mechanical nociception induced by TNF-α, PGE2, DA and carrageenan | Male Swiss mice | [130] |
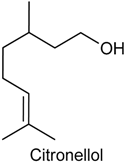 | Acetic acid-induced abdominal constrictions, formalin-induced nociception, hot plate test and carrageenan-induced pleurisy | Reduced nociception, inhibited neutrophil infiltration and decreased levels of TNF-α in the exudates | Male Swiss mice | [139] |
| Formaline and capsaicin tests, and glutamate-induced nociception | Decreased orofacial nociceptive behavior | Male Swiss mice | [140] | |
| Mechanical hyperalgesia induced by CG, TNF-α, PGE2 and DA | Attenuated mechanical hyperalgesia induced by CG, TNF-α, PGE2 and DA | Male Swiss mice | [141] | |
 | Acetic acid-induced writhing test and paw licking induced by formalin, glutamate, capsaicin, menthol, cinnamaldehyde, acidified saline, PMA and 8-Br-cAMP | Reduced the acute pain induced by acetic acid, formalin, glutamate, capsaicin, menthol, PMA and 8-Br-cAMP | Male Swiss mice | [142] |
 | Acetic acid-induced abdominal writhing, formalin, capsaicin and glutamate tests Mechanical hypernociception and carrageenan-induced inflammatory hypernociception | Decreased the nociceptive response Reduced the hypernociception index | Male Swiss mice | [156] |
 | Acetic acid writhing reflex, hot plate test, and formalin-, capsaicin- and glutamate-induced nociception | Showed central analgesic properties and reduced nociceptive response induced by acetic acid, formalin, glutamate and capsaicin | Male Swiss mice | [160] |
 | Eddy’s hot plate method | Increased the latency period, suggesting a potential central analgesic activity | Adult Wistar rats | [166] |
| Carrageenan induced paw edema | Decreased the paw volume | Adult Wistar rats | [167] | |
| Tail flick method and carrageenan induced rat paw edema | Showed antinociceptive effect and decreased the paw volume | Adult Wistar rats | [168] | |
 | Acetic acid-induced abdominal writhings, formalin-induced nociception, hot plate test, grip strength test and carrageenan-induced peritonitis | Reduced the antinociceptive behavior, increased the latency time and decreased the leukocyte migration to the peritoneal cavity | Male Swiss mice | [175] |
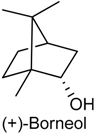 | Segmental spinal nerve ligation-induced neuropathic pain and complete Freund’s adjuvant-induced inflammatory pain | Attenuated mechanical hyperalgesia through activation spinal GABAergic transmission in the spinal cord | Male adult ICR mice | [176] |
 | Acetic acid-induced writhing Paw licking induced by formalin, glutamate and capsaicin Hot-plate test Paw edema induced by compound 48/80, serotonin, histamine or PGE2 Carrageenan-induced peritonitis | Inhibited acetic acid-induced nociception Decreased time of licking the paw Did not change the latency reaction time Reduced the paw edema and Reduced the cytokine levels | Male Swiss mice | [183] |
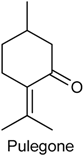 | Formalin test and hot plate test | Inhibited both phases of the formalin test and augmented the latency reaction time in hot plate | Male Swiss mice | [3] |
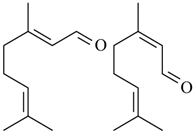 (geranial) (neral) Citral (= geranial + neral) | Acetic acid-induced writhing and formalin induced nociception | Inhibited the abdominal constrictions induced by acetic acid and reduced nociceptive behavior | Male Swiss mice and male Wistar rats | [131] |
| Formalin-induced nociception, Mechanical hyperalgesia Pain behaviour induced by glutamate, capsaicin, and phorbol 12-myristate 13-acetate | Inhibited formalin-induced licking Reduced mechanical hyperalgesia Inhibited the nociceptive response | Male Swiss mice | [189] | |
 | Whole-cell patch-clamp recording | Increased the frequency of spontaneous excitatory postsynaptic current | Adult Wistar rat | [194] |
 | Heterologous expression in HEK293 cells H2O2-induced nociception | Stimulated primary sensory neurons Reduced nociceptive behaviors via H2O2-induced TRPA1 activation | Adult Swiss mice | [199] |
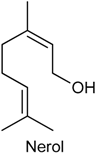 | Oxazolone-induced colitis model Nociceptive behavior assay | Alleviated pathological alterations induced by oxazolone Reduced the levels of proinflammatory cytokines (IL-13 and TNF-α) | Male BALB/c mice | [203] |
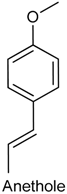 | Paw edema induced by carrageenan and Complete Freund’s adjuvant Mechanical hypernociception induced by prostaglandin, carrageenan and Complete Freund’s adjuvant | Reduced the paw edema and level of some cytokines (TNF-α, IL-1β and IL-17) Decreased hypernociceptive response induced by carrageenan but was not able to alter the PGE2-induced mechanical hypernociception | Male Swiss mice | [212] |
 | Acetic acid-induced writhing Formalin test Complete Freund adjuvant-induced pain (CFA) Hot-plate test Glutamate test | Reduced the total number of writhing Decreased paw licking times during the second phase of the formalin test Decreased peripheral nociception induced by CFA Did not alter the latency time in the hot plate test Reduced paw edema in the glutamate test | Male Swiss mice | [213] |
 | Dorsal root ganglia (DRG) neurons and TRPV1-expressing HEK293 cells | Did not provoke any membrane damage and promoted an elevation of the cytosolic calcium levels in DRG neurons | Old Wistar rat | [222] |
 | Acetic acid-induced writhing test ormalin test Hot plate test Carrageenan-induced paw edema Carrageenan-induced peritonitis | Decreased the total number of writhing Reduced the licking time in both phases Did not increase latency in the hot-plate test Decreased carrageenan-induced paw edema Inhibited the production or action of some proinflammatory cytokines | Male Swiss mice | [217] |
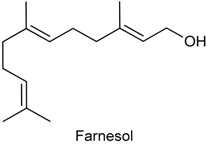 | Acetic acid-induced writhing test Formalin-induced nociception | Reduced the number of abdominal contortions Inhibited both phases of the pain stimulus | Male albino Swiss mice | [226] |
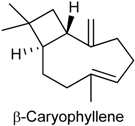 | Capsaicin test | Attenuated the capsaicin-induced nociceptive response and this effect was mediated by peripheral CB2 receptor activation | Male Swiss mice | [234] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cássia da Silveira e Sá, R.; Lima, T.C.; Da Nóbrega, F.R.; De Brito, A.E.M.; De Sousa, D.P. Analgesic-Like Activity of Essential Oil Constituents: An Update. Int. J. Mol. Sci. 2017, 18, 2392. https://doi.org/10.3390/ijms18122392
De Cássia da Silveira e Sá R, Lima TC, Da Nóbrega FR, De Brito AEM, De Sousa DP. Analgesic-Like Activity of Essential Oil Constituents: An Update. International Journal of Molecular Sciences. 2017; 18(12):2392. https://doi.org/10.3390/ijms18122392
Chicago/Turabian StyleDe Cássia da Silveira e Sá, Rita, Tamires Cardoso Lima, Flávio Rogério Da Nóbrega, Anna Emmanuela Medeiros De Brito, and Damião Pergentino De Sousa. 2017. "Analgesic-Like Activity of Essential Oil Constituents: An Update" International Journal of Molecular Sciences 18, no. 12: 2392. https://doi.org/10.3390/ijms18122392
APA StyleDe Cássia da Silveira e Sá, R., Lima, T. C., Da Nóbrega, F. R., De Brito, A. E. M., & De Sousa, D. P. (2017). Analgesic-Like Activity of Essential Oil Constituents: An Update. International Journal of Molecular Sciences, 18(12), 2392. https://doi.org/10.3390/ijms18122392






