Defined Small Molecules Produced by Himalayan Medicinal Plants Display Immunomodulatory Properties
Abstract
1. Introduction
2. Results
2.1. Medicinal Plants, Isolated Phytochemicals and Selected Compounds for In Vitro Assay
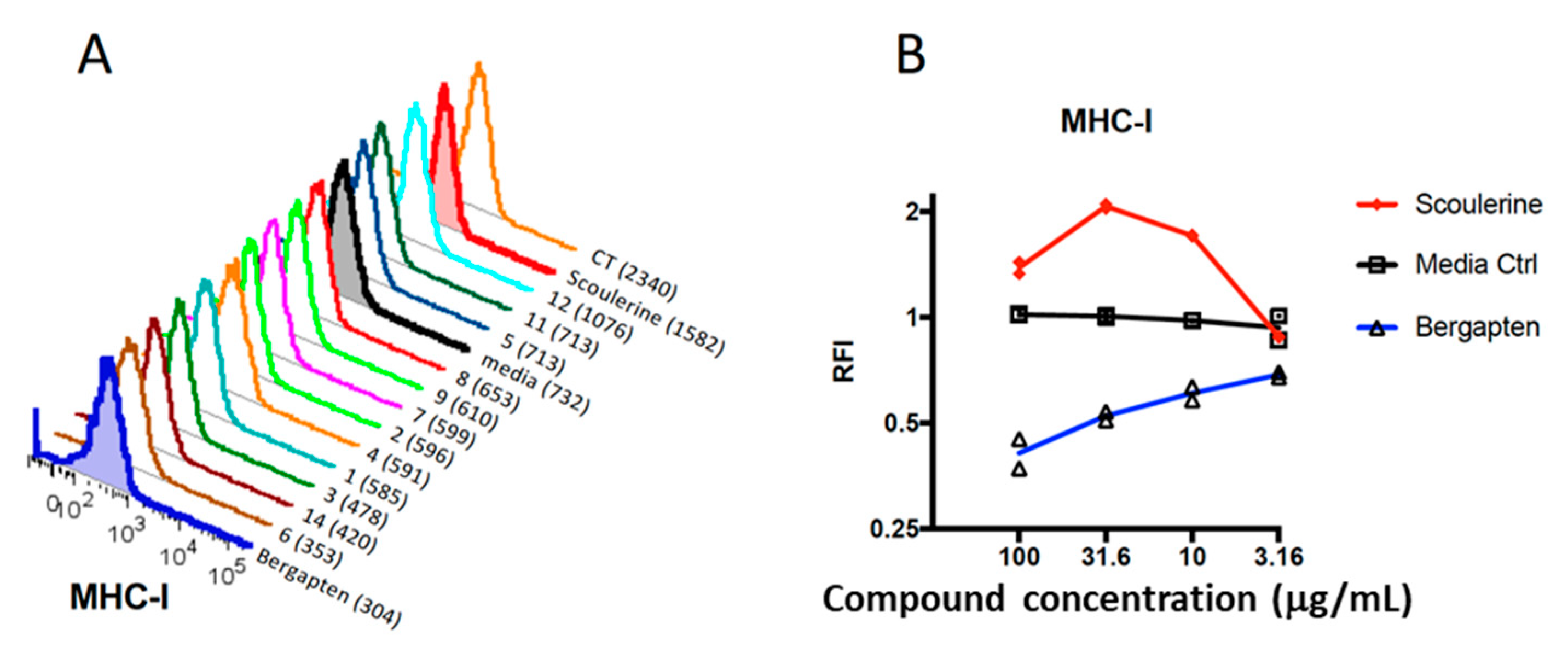
2.2. Plant Compounds Showed Immunomodulatory Activities in Dendritic Cell (DC)-Based Immunoassay
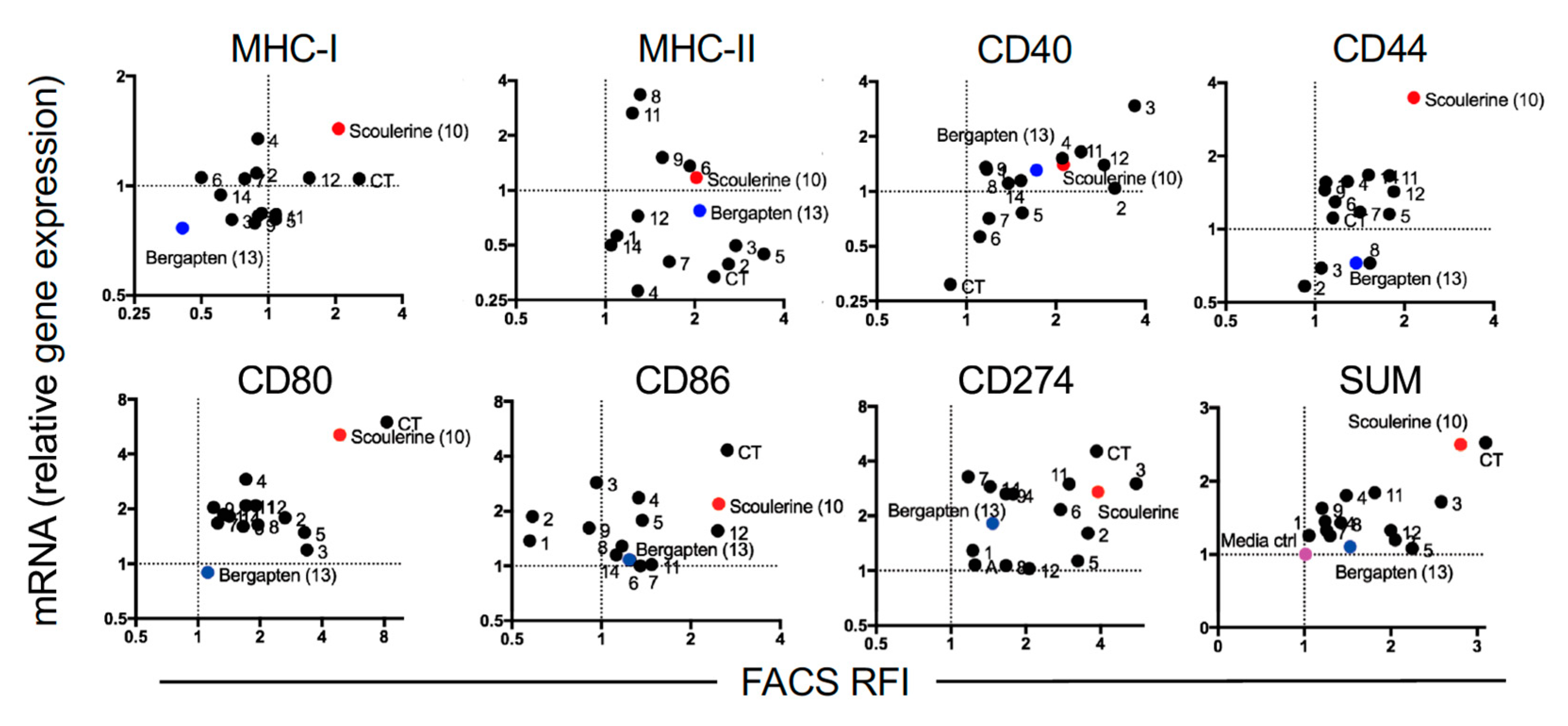
2.3. Modulation of Gene Expression by Plant Compounds
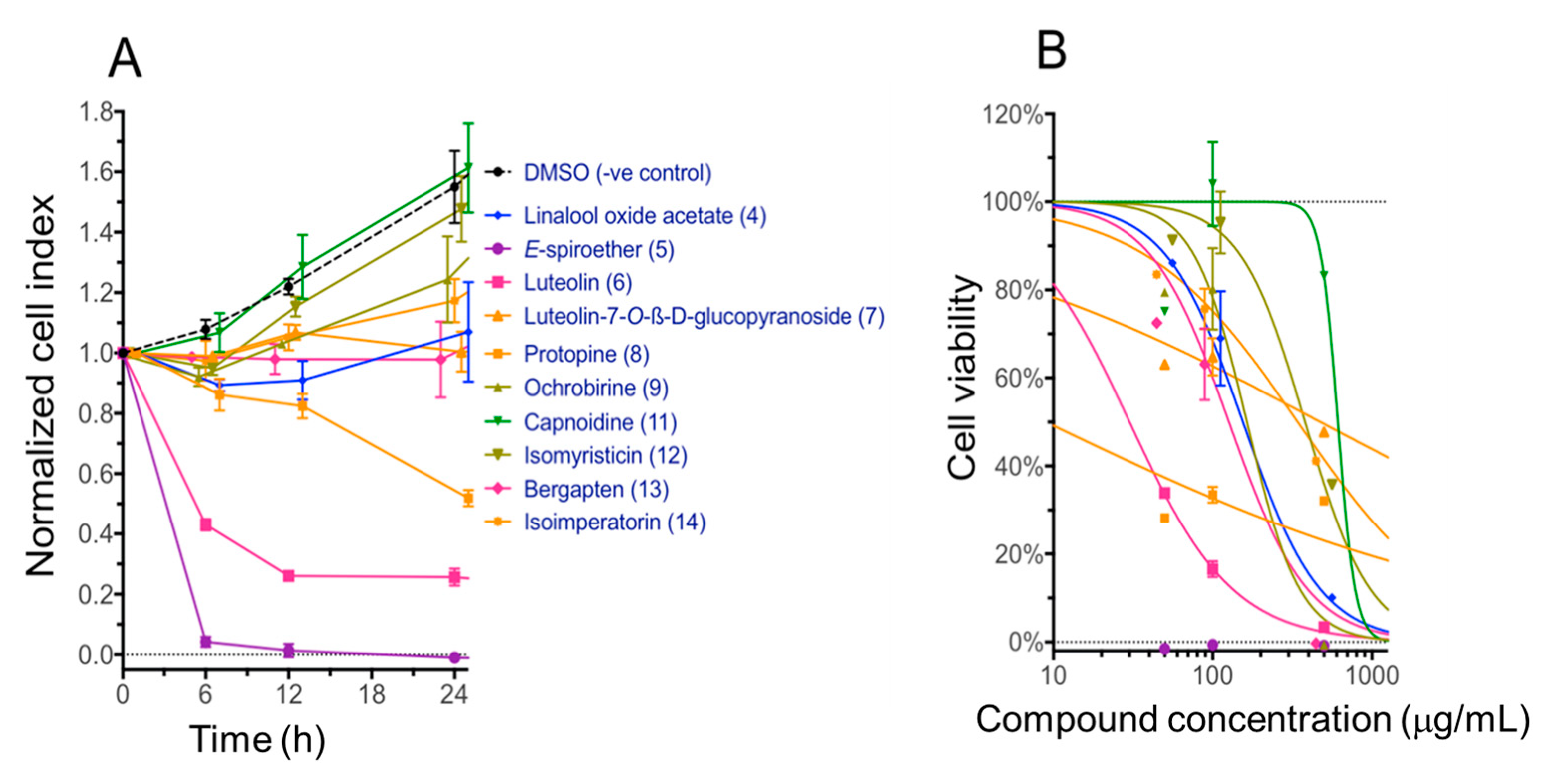
2.4. Cytotoxicity of Compounds with the Immortalized Non-Cancerous H69 Human Cholangiocyte Cell Line
3. Discussion
4. Materials and Methods
4.1. Collection and Extraction of Medicinal Plants
4.2. Isolation and Preparation of Compounds for In Vitro Screening Assays
4.3. DC Assay Method and Flow Cytometry
4.4. Gene Expression Analysis
4.5. Determining Cytotoxicity Using xCELLigence RTCA System
4.6. IC50 Calculations of Cytotoxicity
4.7. Research Ethics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P. Therapeutic applications of natural products in herbal medicines, biodiscovery programs, and biomedicine. J. Biol. Active Prod. Nat. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Landau, E. From a Tree, a “Miracle” Called Aspirin. CNN Health: Matters of the Heart. 2010. Available online: http://edition.cnn.com/2010/HEALTH/12/22/aspirin.history/index.html (accessed on 18 September 2018).
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Amirkia, V.; Heinrich, M. Alkaloids as drug leads—A predictive structural and biodiversity-based analysis. Phytochem. Lett. 2014, 10, xlviii–liii. [Google Scholar] [CrossRef]
- Wood, L. Botanical and Plant Derived Drugs—Analysis, Trends and Forecasts to 201. Research and Markets. 2017. Available online: https://www.businesswire.com/news/home/20171212005666/en/Botanical-Plant-Derived-Drugs-Analysis-Trends-Forecasts (accessed on 19 September 2018).
- Wen, C.C.; Chen, H.M.; Yang, N.S. Developing phytocompounds from medicinal plants as immunomodulators. Adv. Bot. Res. 2012, 62, 198–244. [Google Scholar]
- Gordon, J.R.; Ma, Y.; Churchman, L.; Gordon, S.A.; Dawicki, W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front. Immunol. 2014, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The dendritic cell lineage: Ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed]
- Pooley, J.L.; Heath, W.R.; Shortman, K. Cutting edge: Intravenous soluble antigen is presented to CD4 T cells by CD8 -dendritic cells, but cross-presented to CD8 T Cells by CD8 dendritic cells. J. Immunol. 2001, 166, 5327–5330. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, M.; Guilliams, M.; Vanheerswynghels, M.; Deswarte, K.; Branco-Madeira, F.; Toussaint, W.; Vanhoutte, L.; Neyt, K.; Killeen, N.; Malissen, B.; et al. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 2013, 38, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Granot, T.; Senda, T.; Carpenter, D.J.; Matsuoka, N.; Weiner, J.; Gordon, C.L.; Miron, M.; Kumar, B.; Griesemer, A.; Ho, S.H.; et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity 2017, 46, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Jongbloed, S.L.; Kassianos, A.J.; McDonald, K.J.; Clark, G.J.; Ju, X.; Angel, C.E.; Chen, C.J.J.; Dunbar, P.R.; Wadley, R.B.; Jeet, V.; et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010, 207, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2016, 17, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Oh-e, G.; Oshikawa, T.; Furuichi, S.; Tano, T.; Ahmed, S.U.; Akashi, S.; Miyake, K.; Takeuchi, O.; Akira, S.; et al. Toll-like receptor 4 mediates the antitumor host response induced by a 55-kilodalton protein isolated from Aeginetia indica L., a parasitic plant. Clin. Diagn. Lab. Immunol. 2004, 11, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Aldahlawi, A.M. Modulation of dendritic cell immune functions by plant components. J. Microsc. Ultrastruct. 2016, 4, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bradley, W.G.; Widen, R.H.; Weiser, A.M.; Powers, J.J.; Fountain, L.B.; Punjwani, P.; Lofgren, S.M.; Hadzic, T.; Klein, R.; Green, W.H.; et al. The novel differentiation of human blood mononuclear cells into CD1a-negative dendritic cells is stimulated in the absence of exogenous cytokines by an extract prepared from pinecones. Int. Immunopharmacol. 2003, 3, 209–223. [Google Scholar] [CrossRef]
- Benson, J.M.; Pokorny, A.J.; Rhule, A.; Wenner, C.A.; Kandhi, V.; Cech, N.B.; Shepherd, D.M. Echinacea pupurea extracts modulate murine dendritic cell fate and function. Food Chem. Toxicol. 2010, 48, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-Y.; Cho, H.; Ahn, S.-C.; Oh, Y.-H.; Lee, C.-M.; Park, Y.-M. Resveratrol inhibits phenotypic and functional maturation of murine bone marrow-derived dendritic cells. Int. Immunopharmacol. 2004, 4, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Murakami, T.; Oppenheim, J.J.; Howard, O.M.Z. Triptolide, a constituent of immunosuppressive Chinese herbal medicine, is a potent suppressor of dendritic-cell maturation and trafficking. Blood 2005, 106, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Kure, C.; Timmer, J.; Stough, C. The immunomodulatory effects of plant extracts and plant secondary metabolites on chronic neuroinflammation and cognitive aging: A mechanistic and empirical review. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Apte, S.H.; Stephenson, R.J.; Simerska, P.; Groves, P.L.; Aljohani, S.; Eskandari, S.; Toth, I.; Doolan, D.L. Systematic evaluation of self-adjuvanting lipopeptide nano-vaccine platforms for the induction of potent CD8(+) T-cell responses. Nanomed. Lond. 2016, 11, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Reznikoff, G.; Dranoff, G.; Rock, K.L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 1997, 158, 2723–2730. [Google Scholar] [PubMed]
- Gutiérrez-Martínez, E.; Planès, R.; Anselmi, G.; Reynolds, M.; Menezes, S.; Adiko, A.C.; Saveanu, L.; Guermonprez, P. Cross-presentation of cell-associated antigens by MHC Class I in dendritic cell subsets. Front. Immunol. 2015, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Imai, T.; Kajiwara, C.; Mizukami, S.; Nakai, A.; Nakayama, T.; Udono, H. Essential role of endogenous heat shock protein 90 of dendritic cells in antigen cross-presentation. J. Immunol. 2010, 185, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, G.; Yan, H.; Nacif-Pimenta, R.; Matchimakul, P.; Bridger, J.; Mann, V.H.; Smout, M.J.; Brindley, P.J.; Knight, M. Cytometric analysis, genetic manipulation and antibiotic selection of the snail embryonic cell line Bge from Biomphalaria glabrata, the intermediate host of Schistosoma mansoni. Int. J. Parasitol. 2015, 45, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Taweechotipatr, M.; Tonsomboon, A.; Rattanajak, R.; Kamchonwongpaisan, S. Evaluation of an ethnopharmacologically selected Bhutanese medicinal plants for their major classes of phytochemicals and biological activities. J. Ethnopharmacol. 2011, 137, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Wangdi, T.; Qusar, N.; Nettles, J.; Craig, S.R.; Schrempf, M.; Wangchuk, P. Geopharmaceuticals of Himalayan Sowa Rigpa medicine: Ethnopharmacological uses, mineral diversity, chemical identification and current utilization in Bhutan. J. Ethnopharmacol. 2018, 223, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Sastraruji, T.; Taweechotipatr, M.; Rattanajak, R.; Tonsomboon, A.; Kamchonwongpaisan, S. Phytochemical and biological activity studies of the Bhutanese medicinal plant Corydalis crispa. Nat. Prod. Commun. 2012, 7, 575–580. [Google Scholar] [PubMed]
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Willis, A.C.; Kamchonwongpaisan, S. Antimalarial alkaloids from a Bhutanese traditional medicinal plant Corydalis dubia. J. Ethnopharmacol. 2012, 143, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Keller, P.A.; Stephen, G.P.; Korth, J.; Samten; Taweechotipatr, M.; Rattanajak, R.; Kamchonwongpaisan, S. Antimicrobial, antimalarial and cytoxicity activities of constituents of a Bhutanese variety of Ajania nubigena. Nat. Prod. Commun. 2013, 8, 733–736. [Google Scholar]
- Wangchuk, P.; Pyne, S.G.; Keller, P.A.; Taweechotipatr, M.; Kamchonwongpaisan, S. Phenylpropanoids and furanocoumarins as antibacterial and antimalarial constituents of the Bhutanese medicinal plant Pleurospermum amabile. Nat. Prod. Commun. 2014, 9, 957–960. [Google Scholar] [PubMed]
- Wangchuk, P.; Navarro, S.; Shepherd, C.; Keller, P.A.; Pyne, S.G.; Loukas, A. Diterpenoid alkaloids of Aconitum laciniatum and mitigation of inflammation by 14-O-acetylneoline in a murine model of ulcerative colitis. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Taweechotipatr, M. Inhibition of TNF-α production in LPS-activated THP-1 monocytic cells by the crude extracts of seven Bhutanese medicinal plants. J. Ethnopharmacol. 2013, 148, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Apte, S.H.; Redmond, A.M.; Groves, P.L.; Schussek, S.; Pattinson, D.J.; Doolan, D.L. Subcutaneous cholera toxin exposure induces potent CD103+ dermal dendritic cell activation and migration. Eur. J. Immunol. 2013, 43, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Habartova, K.; Havelek, R.; Seifrtova, M.; Kralovec, K.; Cahlikova, L.; Chlebek, J.; Cermakova, E.; Mazankova, N.; Marikova, J.; Kunes, J.; et al. Scoulerine affects microtubule structure, inhibits proliferation, arrests cell cycle and thus culminates in the apoptotic death of cancer cells. Sci. Rep. 2018, 8, 4829. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Loukas, A. Techniques and Technologies for the Biodiscovery of Novel Small Molecule Drug Lead Compounds From Natural Products. In Natural Products and Drug Discovery; Mandal, S.C., Mandal, V., Konishi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 16; pp. 435–465. [Google Scholar] [CrossRef]
- Spelman, K.; Burns, J.; Nichols, D.; Winters, N.; Ottersberg, S.; Tenborg, M. Modulation of cytokine expression by traditional medicines: A review of herbal immunomodulators. Altern. Med. Rev. 2006, 11, 128–150. [Google Scholar] [PubMed]
- Wilkinson, J.A.; Wahlqvist, M.L.; Clark, J. New Food and Pharmaceutical Products from Agriculture; Rural Industries Research and Development Corporation: Wagga Wagga, Australia, 2002; pp. 1–30. [Google Scholar]
- Prance, G.T.; Chadwick, D.J.; Marsh, J. Ethnobotany and the search for new drug discovery. In Ethnobotany and the Search for New Drugs; Chadwick, D.J., Marsh, J., Eds.; John Wiley and Sons: England, UK, 1994; p. 185. [Google Scholar]
- Mizumoto, N.; Gao, J.; Matsushima, H.; Ogawa, Y.; Tanaka, H.; Takashima, A. Discovery of novel immunostimulants by dendritic-cell–based functional screening. Blood 2005, 106, 3082–3089. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Flower, D.R. Systematic identification of small molecule adjuvants. Expert Opin. Drug Discov. 2012, 7, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Kensil, C.R.; Kammer, R. QS-21: A water-soluble triterpene glycoside adjuvant. Expert Opin. Investig. Drugs 1998, 7, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Evans, T.G.; McElrath, M.J.; Matthews, T.; Montefiori, D.; Weinhold, K.; Wolff, M.; Keefer, M.C.; Kallas, E.G.; Corey, L.; Gorse, G.J.; et al. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immmunization in humans. Vaccine 2001, 19, 2080–2091. [Google Scholar] [CrossRef]
- Chapman, P.B.; Morrissey, D.M.; Panageas, K.S.; Hamilton, W.B.; Zhan, C.; Destro, A.N.; Williams, L.; Israel, R.J.; Livingston, P.O. Induction of Antibodies against GM2 Ganglioside by Immunizing Melanoma Patients Using GM2-Keyhole Limpet Hemocyanin + QS21 Vaccine: A Dose-Response Study. Clin. Cancer Res. 2000, 6, 874. [Google Scholar] [PubMed]
- Wangchuk, P.; Bremner, J.B.; Samten; Rattanajak, R.; Kamchonwongpaisan, S. Antiplasmodial agents from the Bhutanese medicinal plant Corydalis calliantha. Phytother. Res. 2010, 24, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Chlebek, J.; De Simone, A.; Hošťálková, A.; Opletal, L.; Pérez, C.; Pérez, D.I.; Havlíková, L.; Cahlíková, L.; Andrisano, V. Application of BACE1 immobilized enzyme reactor for the characterization of multifunctional alkaloids from Corydalis cava (Fumariaceae) as Alzheimer’s disease targets. Fitoterapia 2016, 109, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Sastraruji, T.; Taweechotipatr, M.; Keller, P.A.; Pyne, S.G. Anti-inflammatory, anti-bacterial and anti-acetylcholinesterase activities of two isoquinoline alkaloids-scoulerine and cheilanthifoline. Nat. Prod. Commun. 2016, 11, 1801–1804. [Google Scholar]
- Ko, F.N.; Yu, S.M.; Su, M.J.; Wu, Y.C.; Teng, C.M. Pharmacological activity of (-)-discretamine, a novel vascular alpha-adrenoceptor and 5-hydroxytryptamine receptor antagonist, isolated from Fissistigma glaucescens. Br. J. Pharmacol. 1993, 110, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, G.; Zhang, Q.; Wu, H.; Wu, Y. Fingerprint analysis of the fruits of Cnidium monnieri extract by high-performance liquid chromatography–diode array detection–electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 43, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, J.; Jiang, Y.; Zhou, T.; Fan, G.; Wu, Y. Separation and determination of coumarins in Fructus cnidii extracts by pressurized capillary electrochromatography using a packed column with a monolithic outlet frit. J. Pharm. Biomed. Anal. 2009, 50, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Bauri, A.K.; Foro, S.; Nhu Do, Q.N. Crystal structure of bergapten: A photomutagenic and photobiologically active furanocoumarin. Acta Crystallogr. E Crystallogr Commun. 2016, 72, 1194–1196. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Montesi, F.; Parnigotto, P.P. Antiproliferative activity and phototoxicity of some methyl derivatives of 5-methoxypsoralen and 5-methoxyangelicin. Pharmacol. Toxicol. 1998, 82, 193–198. [Google Scholar] [CrossRef] [PubMed]
- March, K.L.; Patton, B.L.; Wilensky, R.L.; Hathaway, D.R. 8-Methoxypsoralen and longwave ultraviolet irradiation are a novel antiproliferative combination for vascular smooth muscle. Circulation 1993, 87, 184–191. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Aquila, S.; Morelli, C.; Guido, C.; Santoro, M.; Perrotta, I.; Mauro, L.; Giordano, F.; Nigro, A.; Andò, S.; Panno, M.L. Bergapten drives autophagy through the up-regulation of PTEN expression in breast cancer cells. Mol. Cancer 2015, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Danheiser, R.L.; Trova, M.P. Synthesis of linear furocoumarins via a photochemical aromatic annulation strategy. An efficient total synthesis of bergapten. Synlett. 1995, 1995, 573–574. [Google Scholar] [CrossRef]
- Oda, K.; Nishizono, N.; Tamai, Y.; Yamaguchi, Y.; Yoshimura, T.; Wada, K.; Machida, M. An efficient synthesis of bergapten. Heterocycles 2005, 65, 1985–1988. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS version 12: Software for guided crystal structure analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPEFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Grubman, S.A.; Perrone, R.D.; Lee, D.W.; Murray, S.L.; Rogers, L.C.; Wolkoff, L.I.; Mulberg, A.E.; Cherington, V.; Jefferson, D.M. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am. J. Physiol. Gastrointest. Liver Physiol. 1994, 266, G1060–G1070. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.M.; Jefferson, D.M. Culturing hepatocytes and other differentiated cells. Hepatology 1984, 4, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Dastpeyman, M.; Bansal, P.S.; Wilson, D.; Sotillo, J.; Brindley, P.; Loukas, A.; Smout, M.J.; Daly, N.L. Structural variants of a liver fluke derived granulin peptide potently stimulate wound healing. J. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Giacomin, P.R.; Pearson, M.S.; Smout, M.J.; Loukas, A. Identification of lead chemotherapeutic agents from medicinal plants against blood flukes and whipworms. Sci. Rep. 2016, 6, 32101. [Google Scholar] [CrossRef] [PubMed]
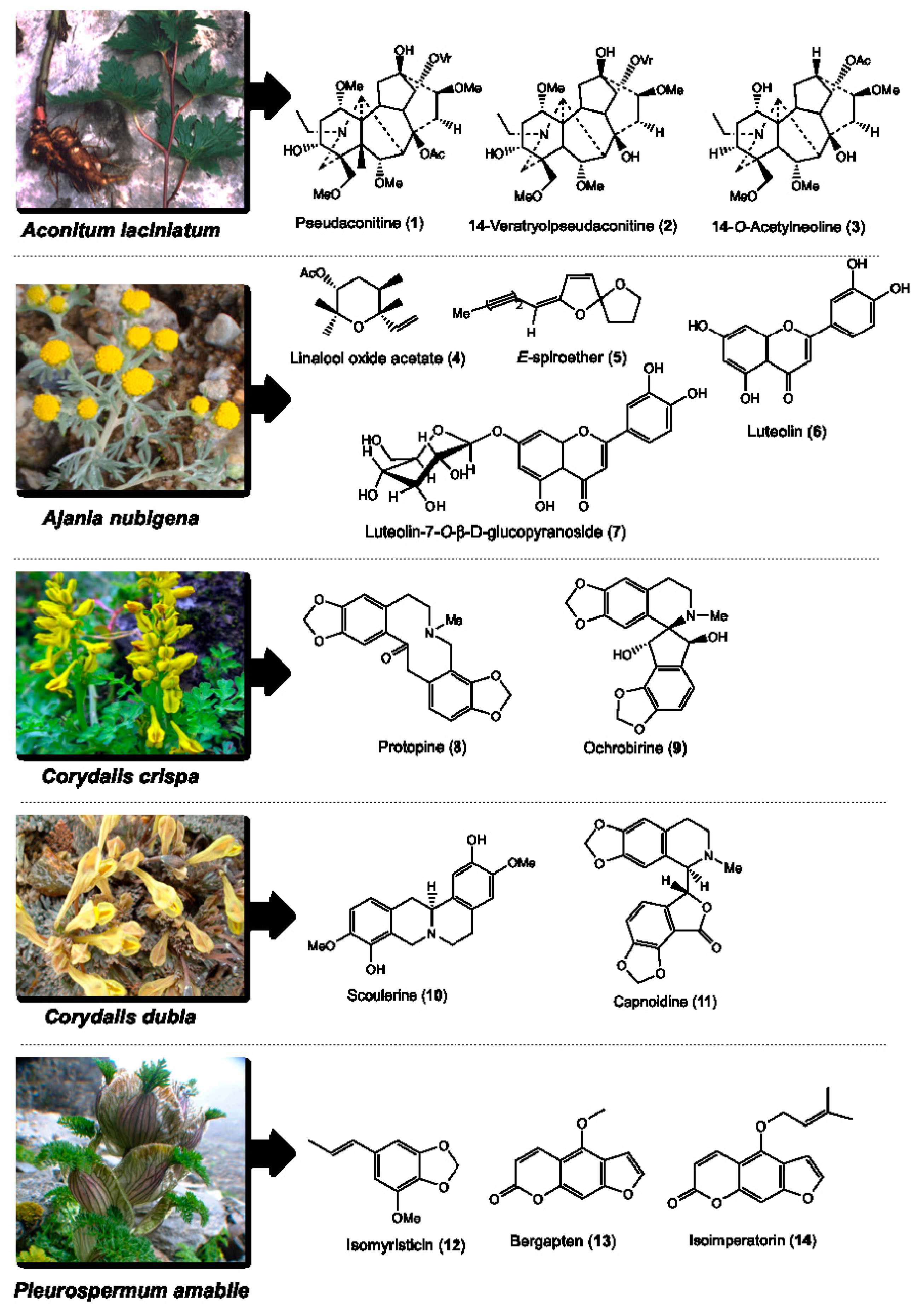
| Botanical Name | Voucher Specimen Number | Traditional Uses [36] | Parts Used | Total Compounds Isolated and the Weight Obtained [31,32,33,34,35] | Class of Phytochemical | Major Compounds Selected for DC and Cytotoxicity Assays |
|---|---|---|---|---|---|---|
| Aconitum laciniatum (Ranunculaceae) | 93 | Parasite infections, leprosy, bone diseases, mumps and gout | Tuber | Pseudaconitine (1.9 g), 14-veratroylpseudaconine (28.7 mg), 14-O-acetylneoline (46.9 mg), neoline (428.5 mg), senbusine A (13.8 mg) | Diterpenoid alkaloids | Pseudaconitine (1) 14-veratroylpseudaconine (2) 14-O-acetylneoline (3) |
| Ajania nubigena (Asteraceae) | 73 | Allays abscess, swelling, tumor, fever, coughs, epistaxis and kidney infection | Aerial | Linalool oxide acetate (1.2 g), chamazulene (2.6 mg), (E)-spiroether (87.0 mg), (Z)-spiroether (6.7 mg), p-hydroxyacetophenone (11.3 mg), oxyanin B (18.6 mg), luteolin (618.0 mg), luteolin-7-O-β-d-glucopyranoside (41.3 mg) | Terpenes and flavonoids | Linalool oxide acetate (4) (E)-spiroether (5) luteolin (6) luteolin-7-O-β-d-glucopyranoside (7) |
| Corydalis crispa (Fumariaceae) | 78 | Allays blood, liver and bile disorders, and febrifuge | Whole | Protopine (1 g), 13-oxoprotopine (17.6 mg), 13-oxocryptopine (4.5 mg), stylopine (5 mg), coreximine (1 mg), rheagenine (1 mg), ochrobirine (60.6 mg), sibiricine (0.8 mg), bicuculline (8 mg) | Isoquinoline alkaloids | Protopine (8) ochrobirine (9) |
| Corydalis dubia (Fumariaceae) | 14 | Allays neuralgia, tuberculosis, and blood, liver, heart, lung, pancreas and kidney infections | Whole | Dubiamine (6.9 mg), scoulerine (9.4 mg), cheilanthifoline (15.1 mg), protopine (160 g), capnoidine (80.3 mg), bicuculline (18.3 mg), corydecumbine (12.3 mg), hydrastine (1.3 mg) | Isoquinoline alkaloids | Scoulerine (10) capnoidine (11) |
| Pleurospermum amabile (Umbelliferae) | 29 | Anti-dote, febrifuge, and dyspepsia | Aerial | (E)-isomyristicin (185.3 mg), (E)-isoapiol (30.7 mg), methyl eugenol (44.7 mg), (E)-isoelemicin (3.4 mg), psoralen (23.8 mg), bergapten (2.5 g), isoimperatorin (143.1 mg), isopimpinellin (93.8 mg), oxypeucedanin hydrate (109.7 mg), oxypeucedanin methanolate (295.2 mg) | Phenylpropanoids and furanocoumarins | (E)-isomyristicin (12) bergapten (13) isoimperatorin (14) |
| Comp. | Conc. (µg/mL) | % Live Cell (FACS) | FACS | PCR | SUM FACS + PCR | Ave. | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MHC-I | MHC-II | CD40 | CD44 | CD80 | CD86 | CD274 | SUM | MHC-I | MHC-II | CD40 | CD44 | CD80 | CD86 | CD274 | SUM | |||||
| 1 | 316.00 | 99.20 | 0.90 | 1.10 | 1.17 | 1.09 | 1.33 | 0.57 | 1.22 | 7.38 | 0.83 | 0.57 | 1.32 | 1.56 | 1.86 | 1.37 | 1.29 | 8.80 | 16.18 | 1.16 |
| 2 | 1000.00 | 94.20 | 0.88 | 2.60 | 3.15 | 0.92 | 2.64 | 0.59 | 3.56 | 14.34 | 1.08 | 0.39 | 1.04 | 0.58 | 1.79 | 1.87 | 1.61 | 8.37 | 22.71 | 1.62 |
| 3 | 1000.00 | 94.20 | 0.69 | 2.75 | 3.68 | 1.05 | 3.38 | 0.96 | 5.56 | 18.07 | 0.81 | 0.50 | 2.94 | 0.69 | 1.19 | 2.87 | 3.01 | 12.01 | 30.07 | 2.15 |
| 4 | 316.00 | 97.20 | 0.90 | 1.29 | 2.10 | 1.29 | 1.71 | 1.33 | 1.78 | 10.39 | 1.35 | 0.28 | 1.52 | 1.57 | 2.91 | 2.36 | 2.64 | 12.63 | 23.02 | 1.64 |
| 5 | 100.00 | 94.10 | 1.08 | 3.42 | 1.54 | 1.78 | 3.29 | 1.37 | 3.24 | 15.72 | 0.81 | 0.45 | 0.76 | 1.15 | 1.48 | 1.78 | 1.13 | 7.57 | 23.28 | 1.66 |
| 6 | 10.00 | 95.70 | 0.50 | 1.92 | 1.11 | 1.17 | 1.66 | 1.25 | 1.47 | 9.08 | 1.05 | 1.36 | 0.57 | 1.30 | 1.60 | 1.07 | 1.82 | 8.78 | 17.86 | 1.28 |
| 7 | 100.00 | 96.50 | 0.78 | 1.64 | 1.19 | 1.42 | 1.24 | 1.35 | 1.17 | 8.80 | 1.05 | 0.41 | 0.71 | 1.18 | 1.67 | 1.00 | 3.28 | 9.29 | 18.09 | 1.29 |
| 8 | 100.00 | 93.60 | 0.94 | 1.31 | 1.38 | 1.53 | 1.95 | 1.17 | 1.67 | 9.95 | 0.84 | 3.35 | 1.11 | 0.73 | 1.64 | 1.29 | 1.07 | 10.01 | 19.96 | 1.43 |
| 9 | 100.00 | 97.90 | 0.87 | 1.55 | 1.16 | 1.08 | 1.19 | 0.91 | 1.67 | 8.43 | 0.79 | 1.52 | 1.36 | 1.45 | 2.03 | 1.61 | 2.64 | 11.41 | 19.84 | 1.42 |
| 10 | 31.60 | 83.80 | 2.07 | 2.03 | 2.12 | 2.15 | 4.89 | 2.48 | 3.91 | 19.64 | 1.43 | 1.17 | 1.40 | 3.47 | 5.10 | 2.19 | 2.71 | 17.48 | 37.12 | 2.65 |
| 11 | 316.00 | 96.80 | 1.08 | 1.23 | 2.43 | 1.78 | 1.71 | 1.47 | 2.99 | 12.70 | 0.83 | 2.66 | 1.65 | 1.66 | 2.09 | 1.02 | 2.99 | 12.89 | 25.59 | 1.83 |
| 12 | 100.00 | 92.00 | 1.53 | 1.29 | 2.90 | 1.84 | 1.91 | 2.46 | 2.07 | 14.00 | 1.05 | 0.72 | 1.40 | 1.43 | 2.09 | 1.56 | 1.03 | 9.28 | 23.27 | 1.66 |
| 13 | 100.00 | 86.90 | 0.41 | 2.08 | 1.72 | 1.38 | 1.12 | 1.24 | 2.76 | 10.70 | 0.77 | 0.78 | 1.30 | 0.73 | 0.90 | 1.08 | 2.17 | 7.72 | 18.41 | 1.32 |
| 14 | 31.60 | 95.40 | 0.61 | 1.05 | 1.52 | 1.51 | 1.42 | 1.12 | 1.44 | 8.68 | 0.94 | 0.50 | 1.15 | 1.67 | 1.83 | 1.15 | 2.90 | 10.13 | 18.81 | 1.34 |
| CT | 1.00 | 92.70 | 2.56 | 2.32 | 0.88 | 1.15 | 8.25 | 2.65 | 3.86 | 21.67 | 1.04 | 0.34 | 0.31 | 1.11 | 6.00 | 4.32 | 4.54 | 17.66 | 39.33 | 2.81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wangchuk, P.; Apte, S.H.; Smout, M.J.; Groves, P.L.; Loukas, A.; Doolan, D.L. Defined Small Molecules Produced by Himalayan Medicinal Plants Display Immunomodulatory Properties. Int. J. Mol. Sci. 2018, 19, 3490. https://doi.org/10.3390/ijms19113490
Wangchuk P, Apte SH, Smout MJ, Groves PL, Loukas A, Doolan DL. Defined Small Molecules Produced by Himalayan Medicinal Plants Display Immunomodulatory Properties. International Journal of Molecular Sciences. 2018; 19(11):3490. https://doi.org/10.3390/ijms19113490
Chicago/Turabian StyleWangchuk, Phurpa, Simon H. Apte, Michael J. Smout, Penny L. Groves, Alex Loukas, and Denise L. Doolan. 2018. "Defined Small Molecules Produced by Himalayan Medicinal Plants Display Immunomodulatory Properties" International Journal of Molecular Sciences 19, no. 11: 3490. https://doi.org/10.3390/ijms19113490
APA StyleWangchuk, P., Apte, S. H., Smout, M. J., Groves, P. L., Loukas, A., & Doolan, D. L. (2018). Defined Small Molecules Produced by Himalayan Medicinal Plants Display Immunomodulatory Properties. International Journal of Molecular Sciences, 19(11), 3490. https://doi.org/10.3390/ijms19113490








