Therapeutic Potential of Plants and Plant Derived Phytochemicals against Acetaminophen-Induced Liver Injury
Abstract
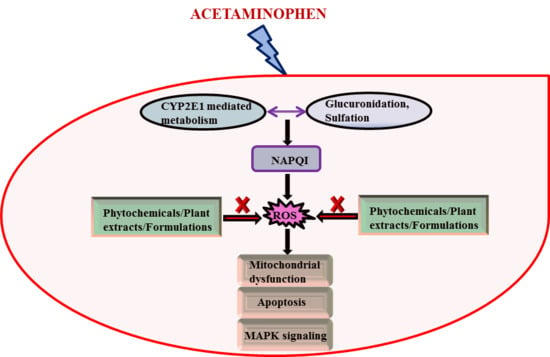
1. Introduction
1.1. Acanthoic Acid
1.2. Ajoene
1.3. Alpha Hederin
1.4. Amyrin
1.5. Andrographolide
1.6. Anthocyanins
1.7. Apigenin
1.8. Arjunolic Acid
1.9. Berberine
1.10. Bixin
1.11. Boswellic Acid
1.12. Brusatol
1.13. Caffeic Acid
1.14. Calamusins
1.15. Carnosic Acid
1.16. Chlorogenic Acid
1.17. Chrysin
1.18. Corynoline, Acetylcorynoline and Protopine
1.19. Curcumin
1.20. Diallyl Sulfide
1.21. Dioscin
1.22. Diosmin
1.23. (−)-Epigallocatechin-3-gallate
1.24. Esculetin
1.25. Ferulic Acid
1.26. Fulvotomentosides
1.27. Galangin
1.28. Gallic Acid
1.29. Genistein
1.30. Geranylgeranylacetone
1.31. Gingerol
1.32. Ginkgolide
1.33. Glycyrrhetinic Acid
1.34. Glycyrrhizin
1.35. Gomisin A
1.36. Guajavadimer A
1.37. Hesperidin
1.38. Homopterocarpin
1.39. Hyperoside
1.40. Isoquercitrin
1.41. Isorhamnetin
1.42. Kaempferol Derivatives
1.43. Lophirones
1.44. Lupeol
1.45. Luteolin
1.46. Magnolol
1.47. Meso-Zeaxanthin
1.48. Methoxypsoralen
1.49. Methyl Sulfonylmethane
1.50. Morin
1.51. Naphthoflavone
1.52. Naringenin
1.53. Oleanolic Acid
1.54. Paenol
1.55. Panaxatriol
1.56. Procyanidins
1.57. Pterostilbene
1.58. Punicalagin and Punicalin
1.59. Quercetin
1.60. Resveratrol
1.61. Rhein
1.62. Rutin
1.63. Saikosaponin D
1.64. Salidroside
1.65. Salvianolic Acids
1.66. Saponarin
1.67. Sauchinone
1.68. Schisandrol Derivatives
1.69. Sesamol
1.70. Silybin
1.71. Sweroside
1.72. Syringic Acid
1.73. Tannic Acid
1.74. Thymoquinone
1.75. Withferin A
1.76. Miscellaneous
2. Discussions
Funding
Conflicts of Interest
References
- Thomas, S.H. Paracetamol (acetaminophen) poisoning. Pharmacol. Ther. 1993, 60, 91–120. [Google Scholar] [CrossRef]
- Nelson, S.D. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990, 10, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Bessems, J.G.; Vermeulen, N.P. Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit. Rev. Toxicol. 2001, 31, 55–138. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.D.; Khairallah, E.A. Selective protein arylation and acetaminophen-induced hepatotoxicity. Drug Metab. Rev. 1997, 29, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; McGill, M.R.; Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: Lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012, 44, 88–106. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Jaeschke, H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Dara, L.; Win, S.; Than, T.A.; Yuan, L.; Abbasi, S.Q.; Liu, Z.X.; Kaplowitz, N. Regulation of drug-induced liver injury by signal transduction pathways: Critical role of mitochondria. Trends Pharmacol. Sci. 2013, 34, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, N.; Shinohara, M.; Saberi, B.; Gaarde, W.A.; Han, D.; Kaplowitz, N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008, 283, 13565–13577. [Google Scholar] [CrossRef] [PubMed]
- Saito, C.; Lemasters, J.J.; Jaeschke, H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010, 246, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kon, K.; Kim, J.S.; Jaeschke, H.; Lemasters, J.J. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 2004, 40, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Cover, C.; Mansouri, A.; Knight, T.R.; Bajt, M.L.; Lemasters, J.J.; Pessayre, D.; Jaeschke, H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005, 315, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Bajt, M.L.; Cover, C.; Lemasters, J.J.; Jaeschke, H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006, 94, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Gujral, J.S.; Knight, T.R.; Farhood, A.; Bajt, M.L.; Jaeschke, H. Mode of cell death after acetaminophen overdose in mice: Apoptosis or oncotic necrosis? Toxicol. Sci. 2002, 67, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat. Res. 2005, 569, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Jollow, D.J.; Mitchell, J.R.; Potter, W.Z.; Davis, D.C.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 1973, 187, 195–202. [Google Scholar] [PubMed]
- Raucy, J.L.; Lasker, J.M.; Lieber, C.S.; Black, M. Acetaminophen activation by human liver cytochromes P450IIE1 and P450IA2. Arch. Biochem. Biophys. 1989, 271, 270–283. [Google Scholar] [CrossRef]
- Laine, J.E.; Auriola, S.; Pasanen, M.; Juvonen, R.O. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica 2009, 39, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Wang, E.J.; Patten, C.; Lee, M.J.; Xiao, F.; Reuhl, K.R.; Yang, C.S. Protective effect of diallyl sulfone against acetaminophen-induced hepatotoxicity in mice. J. Biochem. Toxicol. 1996, 11, 11–20. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Manna, P.; Sil, P.C. Taurine protects acetaminophen-induced oxidative damage in mice kidney through APAP urinary excretion and CYP2E1 inactivation. Toxicology 2010, 269, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Polson, J.; Lee, W.M. AASLD position paper: The management of acute liver failure. Hepatology 2005, 41, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Smilkstein, M.J.; Knapp, G.L.; Kulig, K.W.; Rumack, B.H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N. Engl. J. Med. 1988, 319, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Casas-Grajales, S.; Muriel, P. Antioxidants in liver health. World J. Gastrointest. Pharmacol. Ther. 2015, 6, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Santillan, E.; Madrigal-Bujaidar, E.; Alvarez-Gonzalez, I.; Sumaya-Martinez, M.T.; Gutierrez-Salinas, J.; Bautista, M.; Morales-Gonzalez, A.; Garcia-Luna y Gonzalez-Rubio, M.; Aguilar-Faisal, J.L.; Morales-Gonzalez, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.S.; Prescott, L.F. Liver damage and impaired glucose tolerance after paracetamol overdosage. Br. Med. J. 1966, 2, 506–507. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Davis, D.C.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973, 187, 185–194. [Google Scholar] [PubMed]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Jiang, Y.Z.; Jin, X.J.; Lian, L.H.; Piao, J.Y.; Wan, Y.; Jin, H.R.; Joon Lee, J.; Nan, J.X. Acanthoic acid, a diterpene in Acanthopanax koreanum, protects acetaminophen-induced hepatic toxicity in mice. Phytomedicine 2010, 17, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.X.; Jin, X.J.; Lian, L.H.; Cai, X.F.; Jiang, Y.Z.; Jin, H.R.; Lee, J.J. A diterpenoid acanthoic acid from Acanthopanax koreanum protects against D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Biol. Pharm. Bull. 2008, 31, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Kaschula, C.H.; Hunter, R.; Stellenboom, N.; Caira, M.R.; Winks, S.; Ogunleye, T.; Richards, P.; Cotton, J.; Zilbeyaz, K.; Wang, Y.; et al. Structure-activity studies on the anti-proliferation activity of ajoene analogues in WHCO1 oesophageal cancer cells. Eur. J. Med. Chem. 2012, 50, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Yamada, N.; Nishikawa, T.; Fukuda, H.; Fujino, T. Protective effect of ajoene on acetaminophen-induced hepatic injury in mice. Biosci. Biotechnol. Biochem. 2001, 65, 2555–2557. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Mao, Q.; Klaassen, C.D. The effects of 10 triterpenoid compounds on experimental liver injury in mice. Toxicol. Sci. 1994, 22, 34–40. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Chaves, M.H.; Almeida, F.R.; Lima, R.C., Jr.; Silva, R.M.; Maia, J.L.; Brito, G.A.; Santos, F.A.; Rao, V.S. Protective effect of alpha- and beta-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J. Ethnopharmacol. 2005, 98, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Handa, S.S.; Sharma, A. Hepatoprotective activity of andrographolide against galactosamine & paracetamol intoxication in rats. Indian J. Med. Res. 1990, 92, 284–292. [Google Scholar] [PubMed]
- Visen, P.K.; Shukla, B.; Patnaik, G.K.; Dhawan, B.N. Andrographolide protects rat hepatocytes against paracetamol-induced damage. J. Ethnopharmacol. 1993, 40, 131–136. [Google Scholar] [CrossRef]
- Roy, P.; Das, S.; Auddy, R.G.; Saha, A.; Mukherjee, A. Engineered andrographolide nanoparticles mitigate paracetamol hepatotoxicity in mice. Pharm. Res. 2013, 30, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Mousa, H.M.; El-Mougy, S. The effect of a water extract and anthocyanins of hibiscus sabdariffa L on paracetamol-induced hepatoxicity in rats. Phytother. Res. 2003, 17, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choi, C.Y.; Lee, K.J.; Hwang, Y.P.; Chung, Y.C.; Jeong, H.G. Hepatoprotective effects of an anthocyanin fraction from purple-fleshed sweet potato against acetaminophen-induced liver damage in mice. J. Med. Food 2009, 12, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.; Wang, Z.; Gao, H.; Su, L.; Xie, J.; Chen, X.; Liang, H.; Wang, C.; Han, Y. Oral hepatoprotective ability evaluation of purple sweet potato anthocyanins on acute and chronic chemical liver injuries. Cell. Biochem. Biophys. 2014, 69, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, X.Y.; Xue, J.; Gu, Z.L.; Xie, M.L. Protective effect of apigenin on mouse acute liver injury induced by acetaminophen is associated with increment of hepatic glutathione reductase activity. Food Funct. 2013, 4, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Sinha, M.; Pal, P.; Sil, P.C. Arjunolic acid, a triterpenoid saponin, ameliorates arsenic-induced cyto-toxicity in hepatocytes. Chem. Biol. Interact. 2007, 170, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Das, J.; Manna, P.; Sil, P.C. Arjunolic acid, a triterpenoid saponin, prevents acetaminophen (APAP)-induced liver and hepatocyte injury via the inhibition of APAP bioactivation and JNK-mediated mitochondrial protection. Free Radic. Biol. Med. 2010, 48, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Janbaz, K.H.; Gilani, A.H. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia 2000, 71, 25–33. [Google Scholar] [CrossRef]
- Vivoli, E.; Cappon, A.; Milani, S.; Piombanti, B.; Provenzano, A.; Novo, E.; Masi, A.; Navari, N.; Narducci, R.; Mannaioni, G.; et al. NLRP3 inflammasome as a target of berberine in experimental murine liver injury: Interference with P2X7 signalling. Clin. Sci. 2016, 130, 1793–1806. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.P.; Manjunath, K.; Bhagawati, S.T.; Thippeswamy, B.S. Bixin loaded solid lipid nanoparticles for enhanced hepatoprotection—Preparation, characterisation and in vivo evaluation. Int. J. Pharm. 2014, 473, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Hu, L.H.; Yin, M.C. Alleviative effects from boswellic acid on acetaminophen-induced hepatic injury. Biomedicine 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Olayanju, A.; Copple, I.M.; Bryan, H.K.; Edge, G.T.; Sison, R.L.; Wong, M.W.; Lai, Z.Q.; Lin, Z.X.; Dunn, K.; Sanderson, C.M.; et al. Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity-implications for therapeutic targeting of Nrf2. Free Radic. Biol. Med. 2015, 78, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine 2004, 11, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Zheng, Z.; Shi, L.; Sheng, Y.; Wei, H.; Wang, Z.; Ji, L. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic. Biol. Med. 2016, 91, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Shi, L.; Sheng, Y.; Zheng, Z.; Wei, H.; Wang, Z.; Ji, L. Caffeic acid attenuated acetaminophen-induced hepatotoxicity by inhibiting ERK1/2-mediated early growth response-1 transcriptional activation. Chem. Biol. Interact. 2016, 260, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.Y.; Liang, D.; Luo, H.; Liu, Y.F.; Ni, G.; Zhang, Q.J.; Li, L.; Si, Y.K.; Sun, H.; Chen, R.Y.; et al. Bioactive sesquiterpenoids from the rhizomes of Acorus calamus. J. Nat. Prod. 2012, 75, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shen, Z.; Yu, H.; Lu, G.; Yu, Y.; Liu, X.; Zheng, P. Carnosic acid protects against acetaminophen-induced hepatotoxicity by potentiating Nrf2-mediated antioxidant capacity in mice. Korean J. Physiol. Pharmacol. 2016, 20, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Dickmann, L.J.; VandenBrink, B.M.; Lin, Y.S. In vitro hepatotoxicity and cytochrome P450 induction and inhibition characteristics of carnosic acid, a dietary supplement with antiadipogenic properties. Drug Metab. Dispos. 2012, 40, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sheng, Y.; Lu, B.; Ji, L. The therapeutic detoxification of chlorogenic acid against acetaminophen-induced liver injury by ameliorating hepatic inflammation. Chem. Biol. Interact. 2015, 238, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Sheng, Y.C.; Jiang, P.; Wei, H.; Ji, L.L. Chlorogenic acid prevents acetaminophen-induced liver injury: The involvement of CYP450 metabolic enzymes and some antioxidant signals. J. Zhejiang Univ. Sci. B 2015, 16, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Eaton, E.A.; Walle, U.K.; Lewis, A.J.; Hudson, T.; Wilson, A.A.; Walle, T. Flavonoids, potent inhibitors of the human P-form phenolsulfotransferase. Potential role in drug metabolism and chemoprevention. Drug Metab. Dispos. 1996, 24, 232–237. [Google Scholar] [PubMed]
- Morimitsu, Y.; Sugihara, N.; Furuno, K. Inhibitory effect of flavonoids on sulfo- and glucurono-conjugation of acetaminophen in rat cultured hepatocytes and liver subcellular preparations. Biol. Pharm. Bull. 2004, 27, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Pingili, R.B.; Pawar, A.K.; Challa, S.R. Systemic exposure of Paracetamol (acetaminophen) was enhanced by quercetin and chrysin co-administration in Wistar rats and in vitro model: Risk of liver toxicity. Drug Dev. Ind. Pharm. 2015, 41, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.L.; Liu, G.T. Protective action of corynoline, acetylcorynoline and protopine against experimental liver injury in mice. Yao Xue Xue Bao 1997, 32, 331–336. [Google Scholar] [PubMed]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. An assessment of the potential of protopine to inhibit microsomal drug metabolising enzymes and prevent chemical-induced hepatotoxicity in rodents. Pharmacol. Res. 1998, 38, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Donatus, I.A.; Sardjoko; Vermeulen, N.P. Cytotoxic and cytoprotective activities of curcumin. Effects on paracetamol-induced cytotoxicity, lipid peroxidation and glutathione depletion in rat hepatocytes. Biochem. Pharmacol. 1990, 39, 1869–1875. [Google Scholar] [CrossRef]
- Kheradpezhouh, E.; Panjehshahin, M.R.; Miri, R.; Javidnia, K.; Noorafshan, A.; Monabati, A.; Dehpour, A.R. Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N.-acetyl cysteine. Eur. J. Pharmacol. 2010, 628, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.I.; Omar, S.A.; El-Guendi, M.I.; Abdelmegid, L.A. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem. Toxicol. 2010, 48, 3246–3261. [Google Scholar] [CrossRef] [PubMed]
- Bulku, E.; Stohs, S.J.; Cicero, L.; Brooks, T.; Halley, H.; Ray, S.D. Curcumin exposure modulates multiple pro-apoptotic and anti-apoptotic signaling pathways to antagonize acetaminophen-induced toxicity. Curr. Neurovasc. Res. 2012, 9, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Somanawat, K.; Thong-Ngam, D.; Klaikeaw, N. Curcumin attenuated paracetamol overdose induced hepatitis. World J. Gastroenterol. 2013, 19, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, J.B.; Wang, C.; Xu, Z.; Nie, H.; Qin, X.Y.; Chen, X.M.; Gong, Q. Curcumin protects against acetaminophen-induced apoptosis in hepatic injury. World J. Gastroenterol. 2013, 19, 7440–7446. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Abdo Nassan, M.; Ismail, T.A. Immunohistochemical and molecular study on the protective effect of curcumin against hepatic toxicity induced by paracetamol in Wistar rats. BMC Complement. Altern. Med. 2014, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Yoo, J.S.; Lin, M.; Wang, E.J.; Yang, C.S. Protective effects of diallyl sulfide on acetaminophen-induced toxicities. Food Chem. Toxicol. 1996, 34, 963–969. [Google Scholar] [CrossRef]
- Wang, E.J.; Li, Y.; Lin, M.; Chen, L.; Stein, A.P.; Reuhl, K.R.; Yang, C.S. Protective effects of garlic and related organosulfur compounds on acetaminophen-induced hepatotoxicity in mice. Toxicol. Appl. Pharmacol. 1996, 136, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Chhabra, S.K.; Hong, J.Y.; Smith, T.J. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J. Nutr. 2001, 131, 1041S–1045S. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cong, X.; Zheng, L.; Xu, L.; Yin, L.; Peng, J. Dioscin, a natural steroid saponin, shows remarkable protective effect against acetaminophen-induced liver damage in vitro and in vivo. Toxicol. Lett. 2012, 214, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lahouel, M.; Boulkour, S.; Segueni, N.; Fillastre, J.P. The flavonoids effect against vinblastine, cyclophosphamide and paracetamol toxicity by inhibition of lipid-peroxydation and increasing liver glutathione concentration. Pathol. Biol. 2004, 52, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.T.; Yang, Y.C.; Chang, C.H.; Yang, H.T.; Yin, M.C. Protective effects of (-)-epigallocatechin-3-gallate against acetaminophen-induced liver injury in rats. Biomedicine 2015, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Janbaz, K.H.; Shah, B.H. Esculetin prevents liver damage induced by paracetamol and CCL4. Pharmacol. Res. 1998, 37, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, R.X. Sodium ferulate alleviated paracetamol-induced liver toxicity in mice. Zhongguo Yao Li Xue Bao 1994, 15, 81–83. [Google Scholar] [PubMed]
- Yuan, J.; Ge, K.; Mu, J.; Rong, J.; Zhang, L.; Wang, B.; Wan, J.; Xia, G. Ferulic acid attenuated acetaminophen-induced hepatotoxicity though down-regulating the cytochrome P 2E1 and inhibiting toll-like receptor 4 signaling-mediated inflammation in mice. Am. J. Transl. Res. 2016, 8, 4205–4214. [Google Scholar] [PubMed]
- Liu, Y.P.; Liu, J.; Jia, X.S.; Mao, Q.; Madhu, C.; Klaassen, C.D. Protective effects of fulvotomentosides on acetaminophen-induced hepatotoxicity. Zhongguo Yao Li Xue Bao 1992, 13, 209–212. [Google Scholar] [PubMed]
- Liu, J.; Liu, Y.; Klaassen, C.D. The effect of Chinese hepatoprotective medicines on experimental liver injury in mice. J. Ethnopharmacol. 1994, 42, 183–191. [Google Scholar] [PubMed]
- Shi, J.Z.; Liu, G.T. Protective effect of the fulvotomentosides on paracetamol-induced hepatotoxicity in mice. Yao Xue Xue Bao 1995, 30, 311–314. [Google Scholar] [PubMed]
- Tsai, M.S.; Chien, C.C.; Lin, T.H.; Liu, C.C.; Liu, R.H.; Su, H.L.; Chiu, Y.T.; Wang, S.H. Galangin Prevents Acute Hepatorenal Toxicity in Novel Propacetamol-Induced Acetaminophen-Overdosed Mice. J. Med. Food 2015, 18, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.K.; Sabina, E.P.; Ramya, S.R.; Preety, P.; Patel, S.; Mandal, N.; Mishra, P.P.; Samuel, J. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Pharmacol. 2010, 62, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.J.; Rong, Y.; Li, P.F.; Dong, W.L.; Zhang, D.Y.; Zhang, L.; Cui, M.J. Genistein protection against acetaminophen-induced liver injury via its potential impact on the activation of UDP-glucuronosyltransferase and antioxidant enzymes. Food Chem. Toxicol. 2013, 55, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Krausz, K.; Chen, J.; Ge, X.; Li, J.; Gelboin, H.L.; Gonzalez, F.J. Identification of CYP1A2 as the main isoform for the phase I hydroxylated metabolism of genistein and a prodrug converting enzyme of methylated isoflavones. Drug Metab. Dispos. 2003, 31, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.N.; Nation, R.L.; Milne, R.W.; Reynolds, G.D.; Evans, A.M. The effects of phytoestrogenic isoflavones on the formation and disposition of paracetamol sulfate in the isolated perfused rat liver. J. Pharm. Pharmacol. 2003, 55, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wei, W.; Luo, J.; Jin, Y.; Dai, Z. Genistein promotes the metabolic transformation of acetaminophen to glucuronic acid in human L-O2, HepG2 and Hep3b cells via the Nrf2/Keap1 pathway. Food Funct. 2016, 7, 4683–4692. [Google Scholar]
- Nishida, T.; Matsura, T.; Nakada, J.; Togawa, A.; Kai, M.; Sumioka, I.; Minami, Y.; Inagaki, Y.; Ishibe, Y.; Ito, H.; et al. Geranylgeranylacetone protects against acetaminophen-induced hepatotoxicity by inducing heat shock protein 70. Toxicology 2006, 219, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sabina, E.P.; Pragasam, S.J.; Kumar, S.; Rasool, M. 6-gingerol, an active ingredient of ginger, protects acetaminophen-induced hepatotoxicity in mice. Zhong Xi Yi Jie He Xue Bao 2011, 9, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, G.; Chen, J.; Chang, T.K. Ginkgolide A contributes to the potentiation of acetaminophen toxicity by Ginkgo biloba extract in primary cultures of rat hepatocytes. Toxicol. Appl. Pharmacol. 2006, 217, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.; Kim, S.H. Effect of Glycyrrhiza glabra roots and glycyrrhizin on the glucuronidation in rats. Planta Med. 1997, 63, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Shieh, D.E.; Yen, M.H. Hepatoprotective effect of the fractions of Ban-zhi-lian on experimental liver injuries in rats. J. Ethnopharmacol. 1997, 56, 193–200. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, T.; Li, P.; Mao, Q. The protection of glycyrrhetinic acid (GA) towards acetaminophen (APAP)-induced toxicity partially through fatty acids metabolic pathway. Afr. Health Sci. 2015, 15, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Y.; Luo, M.; Li, X.D.; He, P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem. Biol. Interact. 2009, 181, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Murawaki, Y.; Kawasaki, H. Preventive effect of gomisin A, a lignan component of shizandra fruits, on acetaminophen-induced hepatotoxicity in rats. Biochem. Pharmacol. 1993, 46, 1081–1085. [Google Scholar] [CrossRef]
- Shiota, G.; Yamada, S.; Kawasaki, H. Rapid induction of hepatocyte growth factor mRNA after administration of gomisin A, a lignan component of shizandra fruits. Res. Commun. Mol. Pathol. Pharmacol. 1996, 94, 141–146. [Google Scholar] [PubMed]
- Li, C.J.; Ma, J.; Sun, H.; Zhang, D.; Zhang, D.M. Guajavadimer A, a Dimeric Caryophyllene-Derived Meroterpenoid with a New Carbon Skeleton from the Leaves of Psidium guajava. Org. Lett. 2016, 18, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.T.; Arjumand, W.; Nafees, S.; Seth, A.; Ali, N.; Rashid, S.; Sultana, S. Hesperidin alleviates acetaminophen induced toxicity in Wistar rats by abrogation of oxidative stress, apoptosis and inflammation. Toxicol. Lett. 2012, 208, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Akinmoladun, A.C.; Olaleye, M.T.; Komolafe, K.; Adetuyi, A.O.; Akindahunsi, A.A. Effect of homopterocarpin, an isoflavonoid from Pterocarpus erinaceus, on indices of liver injury and oxidative stress in acetaminophen-provoked hepatotoxicity. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Jiang, Z.; Wang, J.; Zhang, X.; Melzig, M.F. Protective effect of hyperoside against acetaminophen (APAP) induced liver injury through enhancement of APAP clearance. Chem. Biol. Interact. 2016, 246, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wang, M.; Chen, C.; Zhang, X.; Melzig, M.F. Hepatoprotective effect of isoquercitrin against acetaminophen-induced liver injury. Life Sci. 2016, 152, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Xie, W.; Jiang, Z.; Wang, M.; Wang, J.; Zhao, H.; Zhang, X. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, attenuates acetaminophen (APAP)-induced liver injury through activation of Nrf-2. Xenobiotica 2016, 46, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Kupeli, E.; Orhan, D.D.; Yesilada, E. Effect of Cistus laurifolius L. leaf extracts and flavonoids on acetaminophen-induced hepatotoxicity in mice. J. Ethnopharmacol. 2006, 103, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; Soliman, G.M.; Abou El-Kassem, L.T.; Farrag, A.R.; Mahmoud, K.; Leon, F. A new flavonoid C-glycoside from Solanum elaeagnifolium with hepatoprotective and curative activities against paracetamol-induced liver injury in mice. Z. Naturforsch. C 2013, 68, 19–28. [Google Scholar] [PubMed]
- Ajiboye, T.O. Lophirones B and C Attenuate Acetaminophen-Induced Liver Damage in Mice: Studies on Hepatic, Oxidative Stress and Inflammatory Biomarkers. J. Biochem. Mol. Toxicol. 2016, 30, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kakkar, P. Lupeol protects against acetaminophen-induced oxidative stress and cell death in rat primary hepatocytes. Food Chem. Toxicol. 2012, 50, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.; Zhang, J.; Song, S.; Miao, R.; Liu, S.; Pang, Q.; Wu, Q.; Liu, C. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int. Immunopharmacol. 2015, 27, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Kassem, L.T.; Mohammed, R.S.; El Souda, S.S.; El-Anssary, A.A.; Hawas, U.W.; Mohmoud, K.; Farrag, A.R. Digalacturonide flavones from Egyptian Lantana camara flowers with in vitro antioxidant and in vivo hepatoprotective activities. Z. Naturforsch. C 2012, 67, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Tien, Y.H.; Chen, B.H.; Wang Hsu, G.S.; Lin, W.T.; Huang, J.H.; Lu, Y.F. Hepatoprotective and anti-oxidant activities of Glossogyne tenuifolia against acetaminophen-induced hepatotoxicity in mice. Am. J. Chin. Med. 2014, 42, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Lin, F.Y.; Liu, P.L.; Huang, Y.T.; Chiu, J.H.; Chang, Y.C.; Man, K.M.; Hong, C.Y.; Ho, Y.Y.; Lai, M.T. Antioxidative and hepatoprotective effects of magnolol on acetaminophen-induced liver damage in rats. Arch. Pharm. Res. 2009, 32, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Firdous, A.P.; Sindhu, E.R.; Kuttan, R. Hepato-protective potential of carotenoid meso-zeaxanthin against paracetamol, CCl4 and ethanol induced toxicity. Indian J. Exp. Biol. 2011, 49, 44–49. [Google Scholar] [PubMed]
- Liu, W.X.; Jia, F.L.; He, Y.Y.; Zhang, B.X. Protective effects of 5-methoxypsoralen against acetaminophen-induced hepatotoxicity in mice. World J. Gastroenterol. 2012, 18, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Bohlooli, S.; Mohammadi, S.; Amirshahrokhi, K.; Mirzanejad-Asl, H.; Yosefi, M.; Mohammadi-Nei, A.; Chinifroush, M.M. Effect of Methylsulfonylmethane Pretreatment on Aceta-minophen Induced Hepatotoxicity in Rats. Iran. J. Basic Med. Sci. 2013, 16, 896–900. [Google Scholar] [PubMed]
- Rizvi, F.; Mathur, A.; Kakkar, P. Morin mitigates acetaminophen-induced liver injury by potentiating Nrf2 regulated survival mechanism through molecular intervention in PHLPP2-Akt-Gsk3beta axis. Apoptosis 2015, 20, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Tuntaterdtum, S.; Chaudhary, I.P.; Cibull, M.; Robertson, L.W.; Blouin, R.A. Acetaminophen hepatotoxicity: Influence of phenobarbital and beta-naphthoflavone treatment in obese and lean Zucker rats. Toxicol. Appl. Pharmacol. 1993, 123, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, B.; Xing, G.; Wang, F.; Hu, Z. Protective effect of naringenin against acetaminophen-induced acute liver injury in metallothionein (MT)-null mice. Food Funct. 2013, 4, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.S.; McLean, A.E. Effects of phenolic antioxidants and flavonoids on DNA synthesis in rat liver, spleen, and testis in vitro. Toxicology 1999, 139, 243–253. [Google Scholar] [CrossRef]
- Li, Y.; Wang, E.; Patten, C.J.; Chen, L.; Yang, C.S. Effects of flavonoids on cytochrome P450-dependent acetaminophen metabolism in rats and human liver microsomes. Drug Metab. Dispos. 1994, 22, 566–571. [Google Scholar] [PubMed]
- Liu, J.; Liu, Y.; Madhu, C.; Klaassen, C.D. Protective effects of oleanolic acid on acetaminophen-induced hepatotoxicity in mice. J. Pharmacol. Exp. Ther. 1993, 266, 1607–1613. [Google Scholar] [PubMed]
- Liu, J.; Liu, Y.; Parkinson, A.; Klaassen, C.D. Effect of oleanolic acid on hepatic toxicant-activating and detoxifying systems in mice. J. Pharmacol. Exp. Ther. 1995, 275, 768–774. [Google Scholar] [PubMed]
- Abdel-Zaher, A.O.; Abdel-Rahman, M.M.; Hafez, M.M.; Omran, F.M. Role of nitric oxide and reduced glutathione in the protective effects of aminoguanidine, gadolinium chloride and oleanolic acid against acetaminophen-induced hepatic and renal damage. Toxicology 2007, 234, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Q.; Lu, Y.F.; Pi, J. New insights into generalized hepatoprotective effects of oleanolic acid: Key roles of metallothionein and Nrf2 induction. Biochem. Pharmacol. 2008, 76, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Reisman, S.A.; Aleksunes, L.M.; Klaassen, C.D. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem. Pharmacol. 2009, 77, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Rounds, B.V.; Gribble, G.W.; Suh, N.; Wang, Y.; Sporn, M.B. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophages. Bioorg. Med. Chem. Lett. 1998, 8, 2711–2714. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Q.; Xu, Y.; Chen, Y.; Deng, Y.; Zhi, F.; Qian, K. Attenuating Oxidative Stress by Paeonol Protected against Acetaminophen-Induced Hepatotoxicity in Mice. PLoS ONE 2016, 11, e0154375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W.; Li, P.; Deng, M.C.; Yang, S.L.; Yang, L. The inhibitory effect of intestinal bacterial metabolite of ginsenosides on CYP3A activity. Biol. Pharm. Bull. 2004, 27, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Luo, F.; Tang, X.; Li, K.; Hu, X.; Bai, J. Panaxatriol saponin ameliorated liver injury by acetaminophen via restoring thioredoxin-1 and pro-caspase-12. Liver Int. 2014, 34, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Gum, S.I.; Cho, M.K. The amelioration of N-acetyl-p-benzoquinone imine toxicity by ginsenoside Rg3: The role of Nrf2-mediated detoxification and Mrp1/Mrp3 transports. Oxid. Med. Cell. Longev. 2013, 2013, 957947. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Bak, M.J.; Jun, M.; Kong, A.N.; Ho, C.T.; Jeong, W.S. Antioxidant defense and hepatoprotection by procyanidins from almond (Prunus amygdalus) skins. J. Agric. Food Chem. 2014, 62, 8668–8678. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed el, S.M.; Mansour, A.M.; Nady, M.E. Protective effects of pterostilbene against acetaminophen-induced hepatotoxicity in rats. J. Biochem. Mol. Toxicol 2015, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Hsu, Y.F.; Lin, T.C.; Hsu, H.Y. Antioxidant and hepatoprotective effects of punicalagin and punicalin on acetaminophen-induced liver damage in rats. Phytother. Res. 2001, 15, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Janbaz, K.H.; Shah, B.H. Quercetin exhibits hepatoprotective activity in rats. Biochem. Soc. Trans. 1997, 25, S619. [Google Scholar] [CrossRef] [PubMed]
- Bousova, I.; Skalova, L. Inhibition and induction of glutathione S-transferases by flavonoids: Possible pharmacological and toxicological consequences. Drug Metab. Rev. 2012, 44, 267–286. [Google Scholar] [CrossRef] [PubMed]
- El-Shafey, M.M.; Abd-Allah, G.M.; Mohamadin, A.M.; Harisa, G.I.; Mariee, A.D. Quercetin protects against acetaminophen-induced hepatorenal toxicity by reducing reactive oxygen and nitrogen species. Pathophysiology 2015, 22, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.; Badr-Eldin, S.M.; Tawfik, M.K.; Ahmed, T.A.; El-Say, K.M.; Badr, J.M. Design and optimization of self-nanoemulsifying delivery system to enhance quercetin hepatoprotective activity in paracetamol-induced hepatotoxicity. J. Pharm. Sci. 2014, 103, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Sheng, Y.C.; Zheng, Z.Y.; Shi, L.; Wang, Z.T. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic. Biol. Med. 2015, 85, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Wojnarova, L.; Kutinova Canova, N.; Farghali, H.; Kucera, T. Sirtuin 1 modulation in rat model of acetaminophen-induced hepatotoxicity. Physiol. Res. 2015, 64, S477. [Google Scholar] [PubMed]
- Wang, Y.; Jiang, Y.; Fan, X.; Tan, H.; Zeng, H.; Chen, P.; Huang, M.; Bi, H. Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathways. Toxicol. Lett. 2015, 236, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; McGill, M.R.; Xie, Y.; Bajt, M.L.; Jaeschke, H. Resveratrol prevents protein nitration and release of endonucleases from mitochondria during acetaminophen hepatotoxicity. Food Chem. Toxicol. 2015, 81, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, Y.; Sugiyama, S.; Horie, T. Th1/Th2 cytokine balance as a determinant of acetaminophen-induced liver injury. Chem. Biol. Interact. 2009, 179, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Toklu, H.Z.; Sehirli, A.O.; Velioglu-Ogunc, A.; Cetinel, S.; Gedik, N. Protective effects of resveratrol against acetaminophen-induced toxicity in mice. Hepatol. Res. 2006, 35, 62–68. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Du, K.; Weemhoff, J.L.; Jaeschke, H. Critical review of resveratrol in xenobiotic-induced hepatotoxicity. Food Chem. Toxicol. 2015, 86, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Darvesh, A.S.; Politis, T.; McGory, R. Resveratrol and liver disease: From bench to bedside and community. Liver Int. 2010, 30, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Zhou, G.D.; Yang, H.B.; Wang, J.B.; Shan, L.M.; Li, R.S.; Xiao, X.H. Rhein protects against acetaminophen-induced hepatic and renal toxicity. Food Chem. Toxicol. 2011, 49, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia 2002, 73, 557–563. [Google Scholar] [CrossRef]
- Liu, A.; Tanaka, N.; Sun, L.; Guo, B.; Kim, J.H.; Krausz, K.W.; Fang, Z.; Jiang, C.; Yang, J.; Gonzalez, F.J. Saikosaponin d protects against acetaminophen-induced hepatotoxicity by inhibiting NF-kappaB and STAT3 signaling. Chem. Biol. Interact. 2014, 223, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Piao, D.M.; Han, X.H.; Nan, J.X. Protective effects of salidroside against acetaminophen-induced toxicity in mice. Biol. Pharm. Bull. 2008, 31, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Ding, W.; Wang, Y.; Hu, Z.; Wang, Z. An LC-MS/MS method for the determination of salidroside and its metabolite p-tyrosol in rat liver tissues. Pharm. Biol. 2014, 52, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cheung, C.M.; Yang, J.M.; Or, P.M.; Lee, W.Y.; Yeung, J.H. Danshen (Salvia miltiorrhiza) water extract inhibits paracetamol-induced toxicity in primary rat hepatocytes via reducing CYP2E1 activity and oxidative stress. J. Pharm. Pharmacol. 2015, 67, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Zhai, X.; Wang, G.; Tian, X.; Gao, D.; Shi, L.; Wu, H.; Fan, Q.; Peng, J.; Liu, K.; et al. Salvianolic acid B protects against acetaminophen hepatotoxicity by inducing Nrf2 and phase II detoxification gene expression via activation of the PI3K and PKC signaling pathways. J. Pharmacol. Sci. 2015, 127, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, R.; Vitcheva, V.; Kondeva-Burdina, M.; Krasteva, I.; Manov, V.; Mitcheva, M. Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma Wend. on paracetamol-induced liver damage in rats. Biomed. Res. Int. 2013, 2013, 757126. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kim, Y.C.; Moon, A. Sauchinone, a lignan from Saururus chinensis, inhibits staurosporine-induced apoptosis in C6 rat glioma cells. Biol. Pharm. Bull. 2003, 26, 1428–1430. [Google Scholar] [CrossRef] [PubMed]
- Kay, H.Y.; Kim, Y.W.; Ryu, D.H.; Sung, S.H.; Hwang, S.J.; Kim, S.G. Nrf2-mediated liver protection by sauchinone, an antioxidant lignan, from acetaminophen toxicity through the PKCdelta-GSK3beta pathway. Br. J. Pharmacol. 2011, 163, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Fan, X.; Wang, Y.; Tan, H.; Chen, P.; Zeng, H.; Huang, M.; Bi, H. Hepato-protective effects of six schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chem. Biol. Interact. 2015, 231, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Fan, X.; Wang, Y.; Chen, P.; Zeng, H.; Tan, H.; Gonzalez, F.J.; Huang, M.; Bi, H. Schisandrol B protects against acetaminophen-induced hepatotoxicity by inhibition of CYP-mediated bioactivation and regulation of liver regeneration. Toxicol. Sci. 2015, 143, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.M.; Wang, Y.; Tan, H.S.; Yu, T.; Fan, X.M.; Chen, P.; Zeng, H.; Huang, M.; Bi, H.C. Schisandrol B protects against acetaminophen-induced acute hepatotoxicity in mice via activation of the NRF2/ARE signaling pathway. Acta pharmacol. Sin. 2016, 37, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.R.; Hsu, D.Z.; Liu, M.Y. The protective effect of sesamol against mitochondrial oxidative stress and hepatic injury in acetaminophen-overdosed rats. Shock 2009, 32, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.R.; Chien, S.P.; Hsu, D.Z.; Liu, M.Y. Anti-hepatotoxic effects of 3,4-methylenedioxyphenol and N-acetylcysteine in acutely acetaminophen-overdosed mice. Hum. Exp. Toxicol. 2011, 30, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.; Garrido, A.; Guerra, R.; Valenzuela, A. Acetaminophen hepatotoxicity in rats is attenuated by silybin dihemisuccinate. Prog. Clin. Biol. Res. 1988, 280, 375–378. [Google Scholar] [PubMed]
- Campos, R.; Garrido, A.; Guerra, R.; Valenzuela, A. Silybin dihemisuccinate protects against glutathione depletion and lipid peroxidation induced by acetaminophen on rat liver. Planta Med. 1989, 55, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Arancibia, C.; Campos, R.; Valenzuela, A. Acetaminophen does not induce oxidative stress in isolated rat hepatocytes: Its probable antioxidant effect is potentiated by the flavonoid silybin. Pharmacol. Toxicol. 1991, 69, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Malandrino, S.; Magistretti, M.J. Protective activity of silipide on liver damage in rodents. Jpn. J. Pharmacol. 1992, 60, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.D.; Chen, J.; Cao, J.; Wen, X.D.; Li, P. Determination of sweroside in rat plasma and bile for oral bioavailability and hepatobiliary excretion. Chem. Pharm. Bull. 2009, 57, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zeng, W.; He, C.; Bligh, S.W.; Liu, Q.; Yang, L.; Wang, Z. Characterization of metabolites of sweroside in rat urine using ultra-high-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry and NMR spectroscopy. J. Mass Spectrom. 2014, 49, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Raja, B. Protective effects of syringic acid against acetaminophen-induced hepatic damage in albino rats. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, Q.; Han, X.; Zhang, Y.; Zhang, X.; Chu, X.; Zhang, F.; Chu, L. Multi-targeted protection of acetaminophen-induced hepatotoxicity in mice by tannic acid. Int. Immunopharmacol. 2017, 47, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Nagi, M.N.; Almakki, H.A.; Sayed-Ahmed, M.M.; Al-Bekairi, A.M. Thymoquinone supplementation reverses acetaminophen-induced oxidative stress, nitric oxide production and energy decline in mice liver. Food Chem. Toxicol. 2010, 48, 2361–2365. [Google Scholar] [CrossRef] [PubMed]
- Jadeja, R.N.; Urrunaga, N.H.; Dash, S.; Khurana, S.; Saxena, N.K. Withaferin-A Reduces Acetaminophen-Induced Liver Injury in Mice. Biochem. Pharmacol. 2015, 97, 122–132. [Google Scholar] [CrossRef] [PubMed]
- He, Y.C.; Zou, Y.; Peng, C.; Liu, J.L.; He, C.J.; Guo, L.; Xie, X.F.; Xiong, L. Penthorin A and B, two unusual 2,4′-epoxy-8,5′-neolignans from Penthorum chinese. Fitoterapia 2015, 100, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Sakran, M.; Selim, Y.; Zidan, N. A new isoflavonoid from seeds of Lepidium sativum L. and its protective effect on hepatotoxicity induced by paracetamol in male rats. Molecules 2014, 19, 15440–15451. [Google Scholar] [CrossRef] [PubMed]
- Palabiyik, S.S.; Karakus, E.; Halici, Z.; Cadirci, E.; Bayir, Y.; Ayaz, G.; Cinar, I. The protective effects of carvacrol and thymol against paracetamol-induced toxicity on human hepatocellular carcinoma cell lines (HepG2). Hum. Exp. Toxicol. 2016, 35, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Koner, B.C.; Jayanthi, S.; Ramachandra Rao, K.; Rajesh, B.; Pradhan, S.C. Hepatoprotective activity of picroliv, curcumin and ellagic acid compared to silymarin on paracetamol induced liver toxicity in mice. Fundam. Clin. Pharmacol. 2009, 23, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Bi, J.; Gu, J.; Deng, Y.; Liu, C. Astaxanthin pretreatment attenuates acetaminophen-induced liver injury in mice. Int. Immunopharmacol. 2017, 45, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Day, Y.J.; Lee, H.C.; Liou, J.T.; Chou, A.H.; Liu, F.C. ERK Signaling Pathway Plays a Key Role in Baicalin Protection Against Acetaminophen-Induced Liver Injury. Am. J. Chin. Med. 2017, 45, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Day, Y.J.; Lee, H.C.; Liou, J.T.; Chou, A.H.; Liu, F.C. Baicalin Attenuates IL-17-Mediated Acetaminophen-Induced Liver Injury in a Mouse Model. PLoS ONE 2016, 11, e0166856. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Jiang, P.; Lu, B.; Sheng, Y.; Wang, X.; Wang, Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J. Nutr. Biochem. 2013, 24, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, S.; Cheng, H.; Lv, H.; Cheng, G.; Ci, X. Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3beta pathway. Free Radic. Biol. Med. 2016, 101, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Wang, H.; Zhao, M.; Yagai, T.; Chai, Y.; Krausz, K.W.; Xie, C.; Cheng, X.; Zhang, J.; Che, Y.; et al. Glycyrrhizin Protects against Acetaminophen-Induced Acute Liver Injury via Alleviating Tumor Necrosis Factor alpha-Mediated Apoptosis. Drug Metab. Dispos. 2016, 44, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Thangaraj, P.; Lima, B.D.; Chandran, R.; de Souza Araujo, A.A.; Narain, N.; Serafini, M.R.; Junior, L.J. Effects of luteolin and quercetin 3-beta-d-glucoside identified from Passiflora subpeltata leaves against acetaminophen induced hepatotoxicity in rats. Biomed. Pharmacother. 2016, 83, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, A.C.; da Silva, T.P.; de Araujo, G.R.; Araujo, C.M.; da Silva, R.C.; Lima, W.G.; Bezerra, F.S.; Costa, D.C. Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem. Biol. Interact. 2017, 263, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, A.C.; da Silva, R.C.; Rossoni, J.V.J.; Figueiredo, V.P.; Talvani, A.; Cangussu, S.D.; Bezerra, F.S.; Costa, D.C. Lycopene pretreatment improves hepatotoxicity induced by acetaminophen in C57BL/6 mice. Bioorg. Med. Chem. 2017, 25, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Ko, S.Y.; Jun, M.; Jeong, W.S. Quercitrin from Toona sinensis (Juss.) M.Roem. Attenuates Acetaminophen-Induced Acute Liver Toxicity in HepG2 Cells and Mice through Induction of Antioxidant Machinery and Inhibition of Inflammation. Nutrients 2016, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, B.A.; Ritter, A.M.; Ames, F.Q.; Goncalves, O.H.; Leimann, F.V.; Bracht, L.; Natali, M.R.; Cuman, R.K.; Bersani-Amado, C.A. Acetaminophen-induced hepatotoxicity: Preventive effect of trans anethole. Biomed. Pharmacother. 2017, 86, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Chartoumpekis, D.V.; Wakabayashi, N.; Skoko, J.J.; Yagishita, Y.; Singh, S.V.; Kensler, T.W. Withaferin A induces Nrf2-dependent protection against liver injury: Role of Keap1-independent mechanisms. Free Radic. Biol. Med. 2016, 101, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Ki, S.H.; Lee, J.R.; Lee, S.J.; Kim, C.W.; Kim, S.C.; Kim, S.G. Liquiritigenin, an aglycone of liquiritin in Glycyrrhizae radix, prevents acute liver injuries in rats induced by acetaminophen with or without buthionine sulfoximine. Chem. Biol. Interact. 2006, 161, 12538. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Kakkar, P. Lupeol prevents acetaminophen-induced in vivo hepatotoxicity by altering the Bax/Bcl-2 and oxidative stress-mediated mitochondrial signaling cascade. Life Sci. 2012, 90, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Reddy, A.G.; Kumar, B.K.; Madhuri, D.; Boobalan, G.; Reddy, M.A. Protective effect of rutin in comparison to silymarin against induced hepatotoxicity in rats. Vet. World 2017, 10, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, V.; Hanelt, S.; Nastevska, C.; El-Bahay, C.; Rohrdanz, E.; Kahl, R. Antioxidants protect primary rat hepatocyte cultures against acetaminophen-induced DNA strand breaks but not against acetaminophen-induced cytotoxicity. Toxicology 2003, 191, 179–187. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Bullock, P.; Klaassen, C.D. Suppression of liver cytochrome P450 by alpha-hederin: Relevance to hepatoprotection. Toxicol. Appl. Pharmacol. 1995, 134, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Jaeschke, H. Sterile inflammation in acute liver injury: Myth or mystery? Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Lahon, K.; Das, S. Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in Albino rats. Pharm. Res. 2011, 3, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Chandan, B.K.; Saxena, A.K.; Shukla, S.; Sharma, N.; Gupta, D.K.; Suri, K.A.; Suri, J.; Bhadauria, M.; Singh, B. Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J. Ethnopharmacol. 2007, 111, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, L.; Bhatt, J.; Patel, J. Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum DC in CCl4 induced hepatic damage in rats. J. Ethnopharmacol. 2010, 127, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, D.; Wang, H.; Di, L.; Zhou, X.; Xu, T.; Yang, X.; Liu, Y. The hepatoprotective and antifibrotic effects of Saururus chinensis against carbon tetrachloride induced hepatic fibrosis in rats. J. Ethnopharmacol. 2009, 126, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Sun, J.; He, H.; Guo, H.; Zhang, S. Hepatoprotective effects of Ganoderma lucidum peptides against D-galactosamine-induced liver injury in mice. J. Ethnopharmacol. 2008, 117, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Jinno, K.; Kawagishi, H.; Arimoto, Y.; Suganuma, H.; Inakuma, T.; Sugiyama, K. Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d-galactosamine-induced liver injury. J. Agric. Food Chem. 2003, 51, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Kasimu, R.; Basnet, P.; Kadota, S.; Namba, T. Preventive effect of lithospermate B from Salvia miltiorhiza on experimental hepatitis induced by carbon tetrachloride or D-galactosamine/lipopolysaccharide. Planta Med. 1997, 63, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Madrigal-Santillan, E.; Morales-Gonzalez, A.; Esquivel-Soto, J.; Esquivel-Chirino, C.; Garcia-Luna, Y.G.-R.M.; Gayosso-de-Lucio, J.A.; Morales-Gonzalez, J.A. Hepatoprotective effect of silymarin. World J. Hepatol. 2014, 6, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Freitag, A.F.; Cardia, G.F.; da Rocha, B.A.; Aguiar, R.P.; Silva-Comar, F.M.; Spironello, R.A.; Grespan, R.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K. Hepatoprotective Effect of Silymarin (Silybum marianum) on Hepatotoxicity Induced by Acetaminophen in Spontaneously Hypertensive Rats. Evid. Based Complement. Alternat. Med. 2015, 2015, 538317. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Lin, L.C.; Hung, S.C.; Lin, C.H.; Chi, C.W.; Tsai, T.H. Hepatobiliary excretion of silibinin in normal and liver cirrhotic rats. Drug Metab. Dispos. 2008, 36, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.C.; Hernandez, M.; Richardson, W.H., 3rd; Betten, D.P.; Favata, M.; Riffenburgh, R.H.; Clark, R.F.; Tanen, D.A. Comparative treatment of alpha-amanitin poisoning with N-acetylcysteine, benzylpenicillin, cimetidine, thioctic acid, and silybin in a murine model. Ann. Emerg. Med. 2007, 50, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Papackova, Z.; Heczkova, M.; Dankova, H.; Sticova, E.; Lodererova, A.; Bartonova, L.; Poruba, M.; Cahova, M. Silymarin prevents acetaminophen-induced hepatotoxicity in mice. PLoS ONE 2018, 13, e0191353. [Google Scholar] [CrossRef] [PubMed]
- Lirussi, F.; Beccarello, A.; Zanette, G.; De Monte, A.; Donadon, V.; Velussi, M.; Crepaldi, G. Silybin-beta-cyclodextrin in the treatment of patients with diabetes mellitus and alcoholic liver disease. Efficacy study of a new preparation of an anti-oxidant agent. Diabetes Nutr. Metab. 2002, 15, 222–231. [Google Scholar] [PubMed]
- Huseini, H.F.; Larijani, B.; Heshmat, R.; Fakhrzadeh, H.; Radjabipour, B.; Toliat, T.; Raza, M. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Phytother. Res. 2006, 20, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, E.; Saffari, J.; Jafari-Dehkordi, E. Hepatoprotective action of Echinophora platyloba DC leaves against acute toxicity of acetaminophen in rats. J. Diet. Suppl. 2014, 11, 53–63. [Google Scholar] [CrossRef] [PubMed]
- de Avelar, C.R.; Pereira, E.M.; de Farias Costa, P.R.; de Jesus, R.P.; de Oliveira, L.P.M. Effect of silymarin on biochemical indicators in patients with liver disease: Systematic review with meta-analysis. World J. Gastroenterol. 2017, 23, 5004–5017. [Google Scholar] [CrossRef] [PubMed]
- Darvishi Khezri, H.; Salehifar, E.; Kosaryan, M.; Aliasgharian, A.; Jalali, H.; Hadian Amree, A. Potential Effects of Silymarin and Its Flavonolignan Components in Patients with beta-Thalassemia Major: A Comprehensive Review in 2015. Adv. Pharmacol. Sci. 2016, 2016, 3046373. [Google Scholar] [PubMed]
- Yang, Z.; Zhuang, L.; Lu, Y.; Xu, Q.; Chen, X. Effects and tolerance of silymarin (milk thistle) in chronic hepatitis C virus infection patients: A meta-analysis of randomized controlled trials. Biomed. Res. Int. 2014, 2014, 941085. [Google Scholar] [CrossRef] [PubMed]
- Huseini, H.F.; Alavian, S.M.; Heshmat, R.; Heydari, M.R.; Abolmaali, K. The efficacy of Liv-52 on liver cirrhotic patients: A randomized, double-blind, placebo-controlled first approach. Phytomedicine 2005, 12, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Chaphalkar, R.; Apte, K.G.; Talekar, Y.; Ojha, S.K.; Nandave, M. Antioxidants of Phyllanthus emblica L. Bark Extract Provide Hepatoprotection against Ethanol-Induced Hepatic Damage: A Comparison with Silymarin. Oxid. Med. Cell. Longev. 2017, 2017, 3876040. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Bandyopadhyay, S.; Ramasamy, A.; Mondal, S. Evaluation of hepatoprotective activity of aqueous extracts of leaves of Basella alba in albino rats. Nat. Prod. Res. 2015, 29, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Yahya, F.; Mamat, S.S.; Mahmood, N.D.; Mohtarrudin, N.; Taher, M.; Hamid, S.S.; Teh, L.K.; Salleh, M.Z. Hepatoprotective action of various partitions of methanol extract of Bauhinia purpurea leaves against paracetamol-induced liver toxicity: Involvement of the antioxidant mechanisms. BMC Complement. Altern. Med. 2016, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Qu, J.; Han, L.; Zhan, M.; Wu, L.X.; Zhang, Y.W.; Zhang, W.; Zhou, H.H. Inhibitory effects of phytochemicals on metabolic capabilities of CYP2D6(*)1 and CYP2D6(*)10 using cell-based models in vitro. Acta pharmacol. Sin. 2014, 35, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Vinken, M.; Jaeschke, H. Experimental models of hepatotoxicity related to acute liver failure. Toxicol. Appl. Pharmacol. 2016, 290, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Hewitt, N.J.; Albrecht, U.; Andersen, M.E.; Ansari, N.; Bhattacharya, S.; Bode, J.G.; Bolleyn, J.; Borner, C.; Bottger, J.; et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Xie, Y.; McGill, M.R.; Jaeschke, H. Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Yan, H.M.; Ramachandran, A.; Murray, G.J.; Rollins, D.E.; Jaeschke, H. HepaRG cells: A human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 2011, 53, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; McGill, M.R.; Dorko, K.; Kumer, S.C.; Schmitt, T.M.; Forster, J.; Jaeschke, H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2014, 279, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; McGill, M.R.; Williams, C.D.; Ramachandran, A. Current issues with acetaminophen hepatotoxicity—A clinically relevant model to test the efficacy of natural products. Life Sci. 2011, 88, 737–745. [Google Scholar] [CrossRef] [PubMed]

| Phytochemical | Dose of Phytochemical | Dose of APAP and Route | Efficacy and Major Mechanisms | CYP2E1 Inhibition | References |
|---|---|---|---|---|---|
| Acanthoic acid | 50, 100 mg/kg, p. o. 2h before APAP | 300 mg/kg, i. p. | LFT, antioxidants, anti-inflammatory, antiapoptotic and antinecrotic | No | [27] |
| Ajoene | 20,50,100 mg/kg, p. o., 2 & 24 h before APAP | 300 mg/kg, p. o. | LFT, GSH | No | [30] |
| Apigenin | 100, 200 mg/kg | 350 mg/kg, i. p. | LFT, antioxidants, H&E | No | [39] |
| Astaxanthin | 30, 60 mg/kg, p. o. × 14 days | 300 mg/kg, i. p. | LFT, antioxidants, pro-inflammatory cytokines, inhibition of JNK signal pathway and phosphorylation of ERK and P38 | No | [170] |
| Baicalin | 15, 30, 60 mg/kg, p. o. | 300 mg/kg, i. p. | LFT, cytokines, H&E, decrease hepatic phosphorylated extracellular signal-regulated kinase expression | No | [171,172] |
| Berberine | 1 or 5 mg/kg, i. p | 500 mg/kg, i. p. | LFT, mortality, NLRP3 inflammasome pathway | No | [43] |
| Boswellic acid | 0.05, 0.1% in diet × 4 weeks | 400 mg/kg, i. p. | LFT, antioxidants, cytokines and chemokines, toll-like receptor signaling and H&E | Yes | [45] |
| Carnosic acid | 100 mg/kg × 3 days | 400 mg/kg, i. p. | LFT, antioxidants, Nrf2/Keap pathway, H&E | No | [51] |
| Chlorogenic acid | 5, 10, 20 or 40 mg/kg × 7days | 300 mg/kg, i. g | LFT, antioxidants, antiapoptotic, ERK1/2, JNK, p38 kinases mediated MAPK pathway | No | [173] |
| Chlorogenic acid | 10, 20, 40 mg/kg at 1h after given AP | 400 mg/kg, and another 3h later | LFT, MPO, H&E, pro-inflamatory cytokines, chemokines, TLR3/4 and NFκB signaling | No | [53] |
| Corynoline, acetylcorynoline and protopine | 50, 100 mg/kg, 8 to 24 h before APAP | - | LFT, antioxidants | Yes | [58] |
| Esculentoside A | 2.5 mg/kg, i. p. twice in a day | 400, 900 mg/kg, i. p. | LFT, antioxidants, H&E, increases Nrf2 expression and phosphorylation of AMPK, Akt and GSK3β | No | [174] |
| Ferulic acid | 30, 100 mg/kg, p. o., t.d. × 3 days | 350 mg/kg, i. p. | LFT, antioxidants, H&E, MAPK and TLR4 pathway | Yes | [75] |
| Gallic acid | 100 mg/kg, i. p. 30 min after APAP | 900 mg/kg, i. p. | LFT, pro-inflammatory cytokines, antioxidants | No | [80] |
| 6-Gingerol | 30 mg/kg, 30 min after APAP | 900 mg/kg | LFT, antioxidants, comparable to the standard drug silymarin | No | [86] |
| Glycyrrhetinic acid | 500 mg/kg × 20 days before APAP | 400 mg/kg, i. p. | LFT, metabolism pathway of fatty acids, palmtioylcarnitine and oleoylcarnitine | No | [90] |
| Glycyrrhizin | Oral, i. p. and i. v. | 200-600 mg/kg, i. p. | LFT, antioxidants, pro-inflammatory cytokines, antiapptotic, H & E, only i. p., i. v. effective | Yes | [175] |
| Hyperoside | 10, 50, 100 mg/kg, p. o. for 3 days before APAP | 300 mg/kg, i. p. | LFT, antioxidants, Nrf2/Keap pathway, Phase II enzymes | Yes | [97] |
| Isoquercitrin | 10, 20, or 50 mg/kg, p. o. for 3 days before APAP | 300 mg/kg, i. p. | LFT, Pro-inflammatory cytokines, antioxidants, NF-κB/MAPK pathway | Yes | [98] |
| Kaempferoll8-C-β-galactoside and C-glycoside | 25, 50, 75 mg/kg | 500 mg/kg | LFT, H&E, comparable to silymarin | No | [101] |
| Luteolin and quercetin 3-β-d-glucoside | 200, 400 mg/kg, p. o. for 14 days | 2 g/kg, p. o. × 14 days | LFT, antioxidants, H&E | No | [176] |
| Lycopene | 10, 100mg/kg, p. o. | 500 mg/kg, p. o. | LFT, antioxidants, MMP-2, H&E, morphometry | No | [177,178] |
| Naringenin | 200, 400, and 800 mg/kg, p. o. | 250 mg/kg, s. c. | LFT, antioxidants, H&E | No | [113] |
| Paeonol | 25, 50, 100 mg/kg, p. o., 6 and 24 h before APAP | 400 mg/kg, i. p. | LFT, antioxidants, chemokines and cytokines, JNK pathways | No | [122] |
| Fulvotomentosides, oleanolic acid, total saponins of Panax japonicus & Panax notoginseng, sweroside, oxymatrine, dimethyl dicarboxylate biphenyl, | - | - | LFT, H&E, Fulvomentosides found most potent, oleanic acid, total saponins of Panax japonicus and Panax notoginseng had moderate hepatoprotective effects, sweroside, oxymatrine and dimethyl dicarboxylate biphenyl had no effect on APAP toxicity | No | [77] |
| α-Hederin and sapindoside B | 20 mg/kg, s. c. twice | - | LFT, H&E, mortality | No | [78] |
| Procyanidins | 1 or 10 mg/kg, p. o. | 300 mg/kg, i. p. | LFT, enhanced Nrf2/ARE activity and phase II detoxifying/antioxidant enzymes | Yes | [126] |
| Rutin | 20 mg/kg, p. o. | 640 mg/kg, p. o. | LFT, antioxidants | No | [142] |
| Sodium ferulate | 100 mg/kg, p. o., q.d. × 10 days | 130 mg/kg, i. p. | LFT, antioxidants | No | [74] |
| Salidroside | 50, 100 mg/kg 2 h before APAP | 300 mg/kg, i. p. | LFT, pro-inflammatory cytokines, antioxidants, antiapoptotic, H&E, parallel with NAC | No | [144] |
| Salvianolic acid B | 25 and 50 mg/kg, i. g. × 3 days | 300 mg/kg, i. g. | LFT, antioxidants, Nrf2, HO-1 and Gclc activation of the PI3K and PKC pathways | Yes | [147] |
| Sauchinone | 6 h after APAP | 500 mg/kg, i. p. | LFT, antioxidants, H&E, Keap1/Nrf2 and GSK3β-PKCδ pathway | No | [150] |
| Schisandrol B | 200 mg/kg, p. o. for 3 days before APAP | 400 mg/kg, i. p. | LFT, H&E, antioxidants, Nrf2/ARE signaling pathway | No | [153] |
| Schisandrol B | 6.25, 25 and 100 mg/kg for 7 days before APAP | 400 mg/kg, i. p. | LFT, antioxidants, antiapoptotic (p53, p21, CCND1, PCNA, and BCL-2) | Yes | [152] |
| Schisandrin derivatives | 200 mg/kg/day, p. o. | 400 mg/kg, i. p. | LFT, antioxidants, H&E | Yes | [151] |
| Silipide | 400 mg/kg, p. o. | - | LFT, antioxidant activities | No | [159] |
| Quercitrin | 10, 50 mg/kg, p. o. × 7 days | 300 mg/kg, i. p. | LFT, antioxidants and Nrf2/ARE, anti-inflammatory, MAPK pathways including ERK, JNK, and p38 MAPK, comparable to silymarin | No | [179] |
| Tannic acid | 25, 50 mg/kg, p. o. × 3 days | 400 mg/kg, p. o. | LFT, antioxidants, pro-inflammatory cytokines, H&E, suppressed c-Fos, c-Jun, NF-κB (p65) and caspase-3, regulated Bax/Bcl-2, Nrf2 and HO-1 | No | [163] |
| Trans-anethole | 62.5, 125, 250 mg/kg, p. o. | 250 mg/kg, p. o. in mice | LFT, antioxidants, pro-inflammatory cytokines, morphometrics, H&E | No | [180] |
| Withaferin A | 7 mg/kg, p. o. in Nrf2 KO mice | 250 mg/kg, i. p. | LFT, Keap1-independent & Pten/PI3K/Akt-dependent | No | [181] |
| Phytochemical | Dose of Phytochemical | Route and Dose of APAP | Efficacy and Major Mechanisms | CYP2E1 Inhibition | References |
|---|---|---|---|---|---|
| Andrographolide | 200 mg/kg, i. p., 1, 4 & 7 h after APAP | 3 g/kg, p. o. | LFT, H&E, antioxidants | No | [33] |
| Berberine | 4 mg/kg; p. o. twice × 2 days or 4 mg/kg every 6 h | - | LFT, antioxidants | Yes | [42] |
| Chlorogenic acid | 40 mg/kg p. o. × 7 days | 300 mg/kg, intragastric | LFT, antioxidants LFT, antioxidants | Yes | [54] |
| Esculetin | 6 mg/kg | 640 mg/kg, p. o. | LFT, antioxidants | No | [73] |
| Gomisin A | 50 mg/kg | 750 mg/kg i. p. | LFT, antioxidants, antiapoptotic, H&E | No | [92] |
| Hesperidin | 100, 200 mg/kg × 14 days | 750 mg/kg, p. o. | LFT, antioxidants, antioapoptotic, H&E | No | [95] |
| Liquiritigenin & Schisandrin C derivative | p. o. or i. v., 2–4 days | LFT, H & E, liquiritigenin and combination showed protection while schisandrin C derivative failed | No | [182] | |
| Lupeol | 150 mg/kg, p. o. × 30 days | 1 g/kg | LFT, antioxidants, antiapoptotic, H&E | No | [183] |
| Magnolol | 0.01, 0.1, 1 µg/kg 0.5 h after APAP | 500 mg/kg, i. p. × 8 and 24 h | LFT, H&E, antioxidants | No | [107] |
| Pterostilbene | 50, 100 mg/kg, p. o. × 15 days before APAP | 800 mg/kg, i. p. | LFT, lipid profiles, pro-inflammatory cytokines, antioxidants, antiapoptotitic, antifibrotic, comparable to silymarin | No | [127] |
| Punicalagin and Punicalin | 1,5,12.5 or 25 mg/kg, i. p. | 500 mg/kg, i. p. | LFT, antioxidants, H&E | No | [128] |
| Rutin | 20 mg/kg, p. o. × 11 days | 500 mg/kg p. o. from day 1–3 in rats | LFT, H&E, TEM, antioxidants, comparable to silymarin | No | [184] |
| Saponarin | 80 mg/kg, p. o. × 7 days | 600 mg/kg, i. p. | LFT, antioxidants, H&E | Yes | [148] |
| Silybin | - | - | LFT, GSH and lipid peroxidation | No | [157] |
| Syringic acid | 25, 50 and 100 mg/kg p. o. | 750 mg/kg i. p. | LFT, H&E, comparable to silymarin | No | [162] |
| Phytochemicals | Dose of Phytochemical | Cells and Dose of APAP | Efficacy and Major Mechanisms | CYP2E1 Inhibition | References |
|---|---|---|---|---|---|
| Andrographolide | 0.75–12 mg/kg p. o. × 7 days | Rat hepatocytes | LFT, viability, more potent than silymarin | No | [34] |
| Lupeol | 10 μM | Rat hepatocytes, APAP (675 μM) | Maintaining redox and preventing mitochondria-mediated apoptosis | No | [103] |
| Paeonol | 20, 40, 80 μM | Mouse hepatocytes H2O2 or APAP | LDH, ROS and pro-inflammatory genes and reduced IKKα/β, IκBα and p65 phosphorylation | No | [122] |
| Silibin | 25 μM | Rat hepatocytes, APAP (25–30 mM) | Inhibited APAP toxicity, prevented DNA strand breaks formation | No | [185] |
| CalamusinsA-I | 10 μM | HepG2 cells | Weak hepatoprotective activities against APAP | No | [50] |
| α-Hederin | 10, 30 µM/kg, s. c. × 3 days | Rat liver microsomes | Dose-dependent suppression of liver cytochrome P450 enzymes | Yes | [186] |
| Saponarin | 60-0.006 μg/mL | Rat hepatocytes, APAP (100 μM) | Cell viability, LDH, GSH, MDA | Yes | [148] |
| Chlorogenic acid | 1, 10, 25, 50 and 100 μM/L | L-02 cells | LFT, cell viability | Yes | [54] |
| Procyanidins | 10, 25 and 50 μg/L | HepG2 cells | Enhanced phase II detoxifying and antioxidant enzymes and Nrf2/ARE activity | Yes | [126] |
| Thymol and carvacrol | 25, 50 and 100 µM | HepG2 cells | Antioxidants, pro-inflammatory cytokines, comparable to NAC | No | [168] |
| Plant Names | Plant Names | Plant Names | Plant Names | Plant Names |
|---|---|---|---|---|
| Abelmoschus moschatus | Boswellia ovalifoliolata | Eugenia jambolana | Mucuna capitataRoxb. | Sargassum tenerrimum |
| Abutilon indicum | Boswellia serrata | Fagonia olivieri | Mucuna pruriens | Sargassum variegatum |
| Acacia auriculiformis | Brassica juncea Linn. | Fermented ginseng | Muntingia calabura | Schisandra chinensis |
| Acacia indica | Bridelia micrantha | Fermented red ginseng | Musa paradisiaca | Schoenoplectus grossus |
| Acathopanax senticosus | Bryophylum pinnatum | Ficus exasperate | Musanga cecropioides | Scutia myrtina |
| Achillea wilhelmsii C. | Bupleurus spp. | Ficus hispida Linn | Mussaenda erythrophylla | Senecio scandens |
| Acronychia laurifolia | Caesalpinia bonduc Linn. | Ficus microcarpa Linn. | Myrica rubra Sieb. | Sesamum indicum |
| Adansonia digitata Linn. | Caesalpinia gilliesii | Ficus mollis | Nasturtium officinale | Sida acuta Burm. f. |
| Adhatoda vasica | Cajanus cajan | Ficus religisoa Linn. | Nauclea latifolia | Silene aprica |
| Aegle marmelos | Cajanus indicus | Flos lonicerae | Nigella sativa | Silybum marianum |
| Agaricus blazei | Calotropis procera | Foeniculum vulgare | Ocimum gratissimum | Smilax zeylanica Linn. |
| Ageratum conyzoides | Camelia sinesis | Fumaria indica | Opuntia robusta | Solanum alatum |
| Alcea rosea | Capparis sepiaria L. | Fumaria officinalis | Opuntia streptacantha | Solanum fastigiatum |
| Alchornea cordifolia | Caralluma umbellate | Fumaria parviflora | Ornithogalum saundersiae | Solanum indicum |
| Allium cepa | Cardiospermum halicacabum | Ganoderma amboinense | Oroxylum indicum | Solanum nigrum |
| Allium sativum | Carica papaya | Garcinia indica | Osbeckia octandra | Sophora flavescens |
| Alnus japonica | Carissa carandas Linn. | Garcinia kola | Oxalis corniculata | Sphaeranthus indicus |
| Aloe barbadensis | Carum copticum | Genista quadriflora | Oxalis strictalinn | Swertia chirata |
| Aloe vera | Cassia fistula | Gentiana manshurica | Paederia foetida | Swertia longifolia Boiss |
| Alpinia galanga | Cassia occidentalis L | Glossogyne tenuifolia | Paeonia anomala | Swertia punicea |
| Alstonia scholaris R. Br. | Ceiba pentandra Linn. | Glycosmis arborea | Pandanus odoratissimus | Swietenia mahagoni L. |
| Amaranthus caudatus | Centaurium erythraea | Glycosmis pentaphylla | Parinari curatellifolia | Syzygium aromaticum |
| Ambrosia maritima | Chelidonium majus | Gongronema latifolium | Pavonia zeylanica | Taraxacum officinale |
| Amorphophallus paeoniifolius | Cichorium endivia | Gossypium herbacium | Penthorum chinese | Taraxacum syriacum |
| Andrographis paniculata | Cichorium glandulosum | Gymnaster koraiensis | Pergularia daemia | Telfairia occidentalis |
| Anisochilus carnosus | Cinnamomum tamala | Gymnosporia montana | Phyllanthus acidus | Tephrosia purprea |
| Annona muricata | Cinnamomum zeylanicum | Gynostemma pentaphyllum | Phyllanthus amarus | Terminalia chebula |
| Anoectochilus formosanus | Cistus laurifolius Linn. | Gypsophila trichotoma | Phyllanthus emblica | Terminalia paniculata |
| Apium graveolens Linn. | Citrullus colocynthis | Haplophylum tuberculatum | Phyllanthus maderaspatensis | Tetracera loureiri |
| Apocynum venetum Linn. | Citrus hystrix | Harungana madagascariensis | Phyllanthus niruri Linn. | Teucrium poliumgeyrii |
| Aquilegia vulgaris | Citrus maxima | Hedyotis corymbosa | Phyllanthus polyphyllus | Teucrium stocksianum |
| Arctium lappa Linn | Citrus microcarpa | Hemodiscus indicus | Phyllanthus urinariae | Thymus vulgaris |
| Argania spinosa | Clausena dentata | Hibiscus hispidissimus | Piper methysticum | Tinospora cordifolia |
| Artemisia absinthium | Cleome chelidonii | Hibiscus sabdariffa L | Piper puberulum | Tournefortia sarmentosa |
| Artemisia capillaris | Clerodendron Inerme | Hippocratea africana | Pisonia aculeate | Trianthema portulacastrum |
| Artemisia maritima | Clitoria ternatea Linn. | Hippophae rhamnoides | Pittosporum neilgherrense | Tribulus terrestris Linn. |
| Artemisia pallens Walls | Cnidoscolus aconitifolius | Holostemma ada Kodien | Plantago major | Trichopus zeylanicus |
| Artemisia sacrorumLedeb. | Coldenia procumbens | Hordeum vulgare Linn. | Platycodon grandiflorum | Trichosanthes dioica |
| Artemisia scoparia | Conyza bonariensis | Hypericum perforatum | Pleurotus ostreatus | Trichosanthes lobata |
| Artichoke | Copaiba oil | Indigofera tinctoria Linn. | Pluchea arguta | Tridax procumbens Linn |
| Asparagus falcatus | Cornus officinalisSieb. | Iris spuria | Plumbago zeylanica | Trifolium alexandrinum |
| Asparagus racemosus | Corylus avellana | Ixeris chinensis | Polyalthia longifolia | Ulva reticulata |
| Asteracantha longifolia | Costus igneus | Khaya gradifoliola | Polygonum odoratum | Urtica dioica |
| Astragalus corniculatus | Crataegus songarica | Khaya senegalensis | Pongamia pinnata | Uvaria afzelli |
| Astragalus persicus | Croton zehntneri | Kigelia africana | Porphyra yezoensis | Vernonia amygdalina |
| Astragalus tournefortii | Cucurbita pepo | Kohautia grandiflora | Pouteria campechiana | Vigna angularis |
| Atropa acuminata | Cuscuta australis | Kombucha tea | Premna tomentosa | Vitellaria paradoxa |
| Auricularia polytricha | Cuscuta chinensis | Lawsonia inermis | Prosopis africana | Vitex doniana |
| Averrhoa bilimbi | Cyathea gigantea | Leea asiatica | Prosopis farcta | Wedelia calendulacea |
| Averrhoa carambola | Cynanchum atratum | Leonotis nepetifolia | Psidium guajava | Wedelia paludosa |
| Azadirachta indica | Cynara scolymus | Lepidium sativum Linn. | Pterocarpus osun Craib | Woodfordia fruticosa |
| Azolla microphylla | Cyperus scariosus | Lopatherum gracile | Pueraria lobata | Ximenia americana Linn. |
| Baccharis dracunculifolia | Cyperus segetum | Lophira lanceolata | Pyropia yezoensis | Xylopia aethiopica |
| Baccharis trimera | Dalbergia paniculata | Lycopersicum esculentum | Raphanus sativus | Zea mays Linn. |
| Balanites aegyptiaca | Desmodium adscendens | Lycopodium clavatum | Rhazya stricta | Zingiber officinale |
| Barleria prionitis Linn. | Dicranopteris linearis | Malva sylvestris Linn. | Rhodiola imbricata | Zingiber zerumbet |
| Basella alba | Dioscorea alata Linn. | Mangifera india | Rosa damascena | Zizyphus jujube |
| Bauhinia purpurea | Ecballium elaterium | Markhamia platycalyx | Rosa laevigataMichx | Zizyphus spina |
| Berberis aristata | Echinophora platyloba | Maytenus emerginata | Rosmarinus officinalis | |
| Beta vulgaris | Eclipta alba Hassk. | Melastoma malabathricum | Rubia cordifolia | |
| Bidens pilosa Linn. | Embelia ribes | Mesona palustris BL | Salacia oblonga | |
| Bixa orellana Linn. | Enantia chlorantha | Momordica charantia | Salvia miltiorrhiza | |
| Blumea mollis | Entada africana | Monochoria vaginalis | Santallum album | |
| Boehmeria nivea | Epaltes divaricate | Moringa oleifera Lam. | Sargassum binderi | |
| Boerhaavia diffusa | Eucalyptus maculata | Moutan cortex | Sargassum polycystum |
| S. No. | Polyherbal/Single HerbFormulation |
|---|---|
| 1 | 999 Ganmaoling® |
| 2 | A formulation of Andrographis paniculata, Tinospora cordifolia and Solanum nigrum |
| 3 | A polyherbal formulation containing eight herbs; Vasaguduchyadi Kwatha® |
| 4 | A polyherbal formulation containing a mixture of leaves of Gongronema latifolia, Ocimum gratissimum and Vernonia amygdalina |
| 5 | A polyherbal formulation containing aqueous extracts of Ocinnim larrilifolium, Crassocephaluin vitellitiurn, Guizotia scabra and Vernonia lasiopus |
| 6 | A polyherbal formulation containing extracts of Butea monosperma, Bauhinia variegata and Ocimum gratissimum |
| 7 | A polyherbal formulation containing Hydrocotyle asiatica, Tephrosia purpurea, Solanum nigrum, Citrullus colocynthis, Momordica charantia |
| 8 | A polyherbal formulation HP-4® is a combination of 80% alcoholic extract of leaves of Aloe vera, Bacopa monniera, Moringa oleifera and rhizome of Zingiber officinale |
| 9 | A polyherbal formulation, HD-03® |
| 10 | A polyherbal formulations containing five bioactive fractionated extracts of Butea monosperma, Bauhinia variegata and Ocimum gratissimum |
| 11 | A polyherbal formulation containing extracts of Andrographis paniculata Nees., Phyllanthus niruri Linn., Phyllanthus emblica Linn. |
| 12 | A polyherbal mixture of Tinospora cordifolia, Boerhavia diffusa, Phyllanthus amaraus, Euphorbia hirta, Wedelia chinensis |
| 13 | A polyherbal Siddha formulation, Karisalai Karpam® |
| 14 | A polyherbal Siddha medicine, Amukkara chooranam® |
| 15 | Ban-zhi-lian |
| 16 | Bazhen decoction |
| 17 | Biherbal formulations of Aerva lanata and Achyranthes aspera |
| 18 | Chai-Hu-Ching-Kan-Tang® |
| 19 | D-003® |
| 20 | DA-9601®, a quality-controlled extract of Artemisia asiatica |
| 21 | Fengxiang Yigankang® |
| 22 | Fourteen vitex honeys |
| 23 | Gn-3®, a stilbene polymer isolated from Gnetum parvifolium |
| 24 | Habb-e-Asgand®, polyherbal Unani formulation |
| 25 | Hepax®, a polyherbal formulation |
| 26 | Himoliv®, a polyherbal formulation |
| 27 | Huanglian-Jie-Du-Tang® |
| 28 | Hwang-hua-mih-tsay (Wedelia chinensis) |
| 29 | IH636 grape seed extract |
| 30 | Karisalai Karpam tablet® |
| 31 | Kava herbal dietary supplements |
| 32 | Liu weiwuling Tablets® |
| 33 | Livartho®, a polyherbal formulations consist of 10 active constituents of medicinal plants viz, Andrographis paniculata, Cichorium intybus, Tephrosia purpurea, Solanum nigrum, Phyllanthus amarus, Tinospora cordifolia, Eclipta alba, Berberis aristata, Piper longum and Emblica officinalis |
| 34 | Livina®, a polyherbal formulation |
| 35 | Majoon -e-Dabeed-ul-Ward |
| 36 | MAP, a Standardized Herbal Composition, Blend Comprising Myristica fragrans, Astragalus membranaceus and Poriacocos |
| 37 | Picroliv® |
| 38 | Polyherbal ayurvedic formulations, Liv 52®, Livergen®, Livokin®, Octogen®, Stimuliv®, Triphala® and Tefroliv®, Tritone® (Livosone) |
| 39 | Shekwasha® |
| 40 | Somanathitamrabhasma®, a tamra bhasma preparation containing shudhatamra, parada, gandhaka, haritala and manashila |
| 41 | ‘Teng-khia-u’ |
| 42 | Yang-Gan-Wan |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanya, S.B.; Venkataraman, B.; Meeran, M.F.N.; Goyal, S.N.; Patil, C.R.; Ojha, S. Therapeutic Potential of Plants and Plant Derived Phytochemicals against Acetaminophen-Induced Liver Injury. Int. J. Mol. Sci. 2018, 19, 3776. https://doi.org/10.3390/ijms19123776
Subramanya SB, Venkataraman B, Meeran MFN, Goyal SN, Patil CR, Ojha S. Therapeutic Potential of Plants and Plant Derived Phytochemicals against Acetaminophen-Induced Liver Injury. International Journal of Molecular Sciences. 2018; 19(12):3776. https://doi.org/10.3390/ijms19123776
Chicago/Turabian StyleSubramanya, Sandeep B., Balaji Venkataraman, Mohamed Fizur Nagoor Meeran, Sameer N. Goyal, Chandragouda R. Patil, and Shreesh Ojha. 2018. "Therapeutic Potential of Plants and Plant Derived Phytochemicals against Acetaminophen-Induced Liver Injury" International Journal of Molecular Sciences 19, no. 12: 3776. https://doi.org/10.3390/ijms19123776
APA StyleSubramanya, S. B., Venkataraman, B., Meeran, M. F. N., Goyal, S. N., Patil, C. R., & Ojha, S. (2018). Therapeutic Potential of Plants and Plant Derived Phytochemicals against Acetaminophen-Induced Liver Injury. International Journal of Molecular Sciences, 19(12), 3776. https://doi.org/10.3390/ijms19123776