Integrative Histologic and Bioinformatics Analysis of BIRC5/Survivin Expression in Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
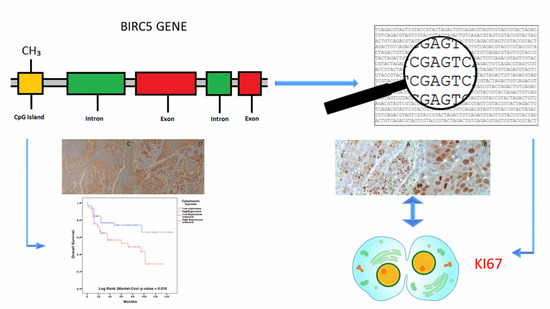
2.1. Bioinformatic Analyses
2.2. Tissue Micro Array (TMA) Immunohistochemistry (IHC) Analysis
3. Discussion
4. Materials and Methods
4.1. Patients Database
4.2. Comparison of BIRC5 Gene Expression between Tumor vs. Non-Tumor Samples
4.3. Analysis of BIRC5 Mutations, Methylation and Associated Network in OSCC from The Cancer Genome Atlas (TCGA) Database
4.4. Analysis of Survivin Expression in a Tissue Micro Array of OSCC, Leukoplakia and Healthy Mucosa Samples
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Tuyns, A.J.; Esteve, J.; Raymond, L.; Berrino, F.; Benhamou, E.; Blanchet, F.; Boffetta, P.; Crosignani, P.; del Moral, A.; Lehmann, W.; et al. Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France). Int. J. Cancer 1988, 41, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Massano, J.; Regateiro, F.S.; Januario, G.; Ferreira, A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, P.J. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J. Oral Pathol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hirata, R.M.; Jaques, D.A.; Chambers, R.G.; Tuttle, J.R.; Mahoney, W.D. Carcinoma of the oral cavity. An analysis of 478 cases. Ann. Surg. 1975, 182, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.J.; Helfrick, J.F.; Gard, D. Primary oral squamous cell carcinoma: A review of 92 cases. J. Oral Maxillofac. Surg. 1996, 54, 949–954. [Google Scholar] [CrossRef]
- Mashberg, A.; Merletti, F.; Boffetta, P.; Gandolfo, S.; Ozzello, F.; Fracchia, F.; Terracini, B. Appearance, site of occurrence, and physical and clinical characteristics of oral carcinoma in Torino, Italy. Cancer 1989, 63, 2522–2527. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, A.; Schulten, E.A.; Kostense, P.J.; Snow, G.B.; van der Waal, I. Tobacco and alcohol related to the anatomical site of oral squamous cell carcinoma. J. Oral Pathol. Med. 1993, 22, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Brandizzi, D.; Gandolfo, M.; Velazco, M.L.; Cabrini, R.L.; Lanfranchi, H.E. Clinical features and evolution of oral cancer: A study of 274 cases in Buenos Aires, Argentina. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, E544–E548. [Google Scholar] [PubMed]
- Hindle, I.; Downer, M.C.; Moles, D.R.; Speight, P.M. Is alcohol responsible for more intra-oral cancer? Oral Oncol. 2000, 36, 328–333. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Living with oral cancer: Epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010, 46, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; McLaughlin, J.K.; Winn, D.M.; Austin, D.F.; Greenberg, R.S.; Preston-Martin, S.; Bernstein, L.; Schoenberg, J.B.; Stemhagen, A.; Fraumeni, J.F., Jr. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988, 48, 3282–3287. [Google Scholar] [PubMed]
- Herrero, R.; Castellsague, X.; Pawlita, M.; Lissowska, J.; Kee, F.; Balaram, P.; Rajkumar, T.; Sridhar, H.; Rose, B.; Pintos, J.; et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J. Natl. Cancer Inst. 2003, 95, 1772–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, S.N.; Brown, J.S.; Woolgar, J.A.; Lowe, D.; Magennis, P.; Shaw, R.J.; Sutton, D.; Errington, D.; Vaughan, D. Survival following primary surgery for oral cancer. Oral Oncol. 2009, 45, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.; Warnakulasuriya, S.; Varela-Centelles, P.I.; Lopez-Jornet, P.; Suarez-Cunqueiro, M.; Diz-Dios, P.; Seoane, J. Is early diagnosis of oral cancer a feasible objective? Who is to blame for diagnostic delay? Oral Dis. 2010, 16, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.K.; Goel, A.; Mittal, R.D. Survivin: A molecular biomarker in cancer. Indian J. Med. Res. 2015, 141, 389–397. [Google Scholar] [PubMed]
- Altieri, D.C. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 2003, 22, 8581–8589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sah, N.K.; Khan, Z.; Khan, G.J.; Bisen, P.S. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006, 244, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Adida, C.; Altieri, D.C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997, 3, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, S.P.; McNeish, I.A. Survivin: A protein with dual roles in mitosis and apoptosis. Int. Rev. Cytol. 2005, 247, 35–88. [Google Scholar] [CrossRef]
- Lo Muzio, L.; Pannone, G.; Staibano, S.; Mignogna, M.D.; Rubini, C.; Mariggio, M.A.; Procaccini, M.; Ferrari, F.; de Rosa, G.; Altieri, D.C. Survivin expression in oral squamous cell carcinoma. Br. J. Cancer 2003, 89, 2244–2248. [Google Scholar] [CrossRef] [PubMed]
- Luscombe, N.M.; Greenbaum, D.; Gerstein, M. What is bioinformatics? A proposed definition and overview of the field. Methods Inf. Med. 2001, 40, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Eisen, M.B.; Brown, P.O. DNA arrays for analysis of gene expression. Methods Enzymol. 1999, 303, 179–205. [Google Scholar] [PubMed]
- Cheung, V.G.; Morley, M.; Aguilar, F.; Massimi, A.; Kucherlapati, R.; Childs, G. Making and reading microarrays. Nat. Genet. 1999, 21, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Duggan, D.J.; Bittner, M.; Chen, Y.; Meltzer, P.; Trent, J.M. Expression profiling using cDNA microarrays. Nat. Genet. 1999, 21, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, R.J.; Fodor, S.P.; Gingeras, T.R.; Lockhart, D.J. High density synthetic oligonucleotide arrays. Nat. Genet. 1999, 21, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Velculescu, V.E.; Zhang, L.; Vogelstein, B.; Kinzler, K.W. Serial analysis of gene expression. Science 1995, 270, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, M. Integrative database analysis in structural genomics. Nat. Struct. Mol. Biol. 2000, 7, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; Khwaja, S.S.; Baker, C.M.; Gay, H.A.; Thorstad, W.L.; Daly, M.D.; Lewis, J.S., Jr.; Wang, X. Prognostic microRNA signatures derived from The Cancer Genome Atlas for head and neck squamous cell carcinomas. Cancer Med. 2016, 5, 1619–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Jensen, M.A.; Zenklusen, J.C. A Practical Guide to The Cancer Genome Atlas (TCGA). Methods Mol. Biol. 2016, 1418, 111–141. [Google Scholar] [PubMed]
- Creighton, C.J. Making Use of Cancer Genomic Databases. Curr. Protoc. Mol. Biol. 2018, 121. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.; Xu, H.; Shan, X.; Liu, B.; Wang, K.; Cai, Z. Clinicopathological and prognostic significance of survivin expression in patients with oral squamous cell carcinoma: Evidence from a meta-analysis. PLoS ONE 2015, 10, e0116517. [Google Scholar] [CrossRef] [PubMed]
- Sgaramella, N.; Coates, P.J.; Strindlund, K.; Loljung, L.; Colella, G.; Laurell, G.; Rossiello, R.; Muzio, L.L.; Loizou, C.; Tartaro, G.; et al. Expression of p16 in squamous cell carcinoma of the mobile tongue is independent of HPV infection despite presence of the HPV-receptor syndecan-1. Br. J. Cancer 2015, 113, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polz-Gruszka, D.; Morshed, K.; Stec, A.; Polz-Dacewicz, M. Prevalence of Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in oral and oropharyngeal squamous cell carcinoma in south-eastern Poland. Infect. Agent Cancer 2015, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Melchers, L.J.; Mastik, M.F.; Samaniego Cameron, B.; van Dijk, B.A.; de Bock, G.H.; van der Laan, B.F.; van der Vegt, B.; Speel, E.J.; Roodenburg, J.L.; Witjes, M.J.; et al. Detection of HPV-associated oropharyngeal tumours in a 16-year cohort: More than meets the eye. Br. J. Cancer 2015, 112, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Belobrov, S.; Cornall, A.M.; Young, R.J.; Koo, K.; Angel, C.; Wiesenfeld, D.; Rischin, D.; Garland, S.M.; McCullough, M. The role of human papillomavirus in p16-positive oral cancers. J. Oral Pathol. Med. 2018, 47, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lodi, G.; Franchini, R.; Bez, C.; Sardella, A.; Moneghini, L.; Pellegrini, C.; Bosari, S.; Manfredi, M.; Vescovi, P.; Carrassi, A. Detection of survivin mRNA in healthy oral mucosa, oral leucoplakia and oral cancer. Oral Dis. 2010, 16, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Lim, K.Y.; Kim, J.W.; Nam, I.W.; Lee, J.H.; Myoung, H. Stage and mRNA expression of survivin in lymph node as prognostic indicators in patients with oral squamous cell carcinoma. Cancer Lett. 2005, 224, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.; Uzawa, K.; Shibahara, T.; Yokoe, H.; Noma, H.; Tanzawa, H. Expression of an inhibitor of apoptosis, survivin, in oral carcinogenesis. J. Dent. Res. 2003, 82, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shi, L.; Yang, X.; Ye, D.; Wang, T.; Dong, C.; Guo, W.; Liao, Y.; Song, H.; Xu, D.; et al. Nuclear survivin promoted by acetylation is associated with the aggressive phenotype of oral squamous cell carcinoma. Cell Cycle 2017, 16, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, A.; Mascitti, M.; Rubini, C.; Bambini, F.; Giannatempo, G.; lo Russo, L.; Sartini, D.; Emanuelli, M.; Procaccini, M.; Lo Muzio, L. Nuclear Survivin as a Prognostic Factor in Squamous-Cell Carcinoma of the Oral Cavity. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hung, H.C.; Kuo, R.C.; Chiang, C.P.; Kuo, M.Y. Survivin expression predicts poorer prognosis in patients with areca quid chewing-related oral squamous cell carcinoma in Taiwan. Oral Oncol. 2005, 41, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Farnebo, L.; Tiefenbock, K.; Ansell, A.; Thunell, L.K.; Garvin, S.; Roberg, K. Strong expression of survivin is associated with positive response to radiotherapy and improved overall survival in head and neck squamous cell carcinoma patients. Int. J. Cancer 2013, 133, 1994–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Muzio, L.; Farina, A.; Rubini, C.; Pezzetti, F.; Stabellini, G.; Laino, G.; Santarelli, A.; Pannone, G.; Bufo, P.; de Lillo, A.; et al. Survivin as prognostic factor in squamous cell carcinoma of the oral cavity. Cancer Lett. 2005, 225, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Engels, K.; Knauer, S.K.; Metzler, D.; Simf, C.; Struschka, O.; Bier, C.; Mann, W.; Kovacs, A.F.; Stauber, R.H. Dynamic intracellular survivin in oral squamous cell carcinoma: Underlying molecular mechanism and potential as an early prognostic marker. J. Pathol. 2007, 211, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Freier, K.; Pungs, S.; Sticht, C.; Flechtenmacher, C.; Lichter, P.; Joos, S.; Hofele, C. High survivin expression is associated with favorable outcome in advanced primary oral squamous cell carcinoma after radiation therapy. Int. J. Cancer 2007, 120, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Jane, C.; Nerurkar, A.V.; Shirsat, N.V.; Deshpande, R.B.; Amrapurkar, A.D.; Karjodkar, F.R. Increased survivin expression in high-grade oral squamous cell carcinoma: A study in Indian tobacco chewers. J. Oral Pathol. Med. 2006, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, J.; Wang, L.; Tian, Z.; Zhang, P.; Xu, Q.; Zhang, C.; Wei, F.; Chen, W. Prognostic significance of p21, p27 and survivin protein expression in patients with oral squamous cell carcinoma. Oncol. Lett. 2013, 6, 381–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhosale, P.G.; Cristea, S.; Ambatipudi, S.; Desai, R.S.; Kumar, R.; Patil, A.; Kane, S.; Borges, A.M.; Schaffer, A.A.; Beerenwinkel, N.; et al. Chromosomal Alterations and Gene Expression Changes Associated with the Progression of Leukoplakia to Advanced Gingivobuccal Cancer. Transl. Oncol. 2017, 10, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Sticht, C.; Freier, K.; Knopfle, K.; Flechtenmacher, C.; Pungs, S.; Hofele, C.; Hahn, M.; Joos, S.; Lichter, P. Activation of MAP kinase signaling through ERK5 but not ERK1 expression is associated with lymph node metastases in oral squamous cell carcinoma (OSCC). Neoplasia 2008, 10, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Cumbo, F.; Fiscon, G.; Ceri, S.; Masseroli, M.; Weitschek, E. TCGA2BED: Extracting, extending, integrating, and querying The Cancer Genome Atlas. BMC Bioinf. 2017, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Aquino, G.; Pannone, G.; Santoro, A.; Liguori, G.; Franco, R.; Serpico, R.; Florio, G.; de Rosa, A.; Mattoni, M.; Cozza, V.; et al. pEGFR-Tyr 845 expression as prognostic factors in oral squamous cell carcinoma: A tissue-microarray study with clinic-pathological correlations. Cancer Biol. Ther. 2012, 13, 967–977. [Google Scholar] [CrossRef] [PubMed]





| Variable | mRNA Expression | Methylation | Age | Stage | Grade |
|---|---|---|---|---|---|
| mRNA expression | ρ = 1 | ρ = −0.125 | ρ = 0.025 | ρ = 0.137 | ρ = 0.023 |
| p-value = 1 | p-value = 0.021 * | p-value = 0.644 | p-value = 0.015 * | p-value = 0.670 | |
| Methylation | ρ = 1 | ρ = 0.085 | ρ = 0.019 | ρ = −0.099 | |
| p-value = 1 | p-value = 0.119 | p-value = 0.735 | p-value = 0.070 | ||
| Age | ρ = 1 | ρ = −0.099 | ρ = 0.118 | ||
| p-value = 1 | p-value = 0.083 | p-value = 0.033 * | |||
| Stage | ρ = 1 | ρ = −0.094 | |||
| p-value = 1 | p-value = 0.104 | ||||
| Grade | ρ = 1 | ||||
| p-value = 1 |
| Variable | Overall Survival | Disease Free Survival | ||
|---|---|---|---|---|
| Hazard Ratio | p-Value | Hazard Ratio | p-Value | |
| mRNA expression | 1.182 | 0.008 * | 0.906 | 0.653 |
| Methylation rate | 2.350 | 0.577 | 0.004 | 0.068 |
| Grade | 0.779 | 0.209 | 0.799 | 0.657 |
| Stage | 0.965 | 0.637 | 1.806 | 0.021 * |
| Age | 0.996 | 0.474 | 1.007 | 0.694 |
| Gender | 0.997 | 0.988 | 0.574 | 0.259 |
| ClinicoPathological Parameter | Groups | Number |
|---|---|---|
| Age | ≥65 years old | 63/107 (58.9%) |
| <65 years old | 44/107 (41.1%) | |
| Gender | Male | 76/107 (71%) |
| Female | 31/107 (29%) | |
| Grade | G1 | 21/107 (19.6%) |
| G2/G3 | 86/107 (90.4%) | |
| Stage | St1/St2 | 39/107 (36.4%) |
| St3/St4 | 68/107 (63.6%) | |
| Subsite Involved | Tongue | 64/107 (59.8%) |
| Others sites | 43/107 (40.2%) |
| Clinicopathologic Factor | Overall Survival | |
|---|---|---|
| Hazard Ratio | p-Value | |
| Cytoplasmic Survivin | 2.040 | 0.045 * |
| Nuclear Survivin | 0.858 | 0.726 |
| Grade | 1.961 | 0.220 |
| Stage | 4.938 | 0.001 * |
| Age | 0.895 | 0.734 |
| Gender | 0.781 | 0.490 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troiano, G.; Guida, A.; Aquino, G.; Botti, G.; Losito, N.S.; Papagerakis, S.; Pedicillo, M.C.; Ionna, F.; Longo, F.; Cantile, M.; et al. Integrative Histologic and Bioinformatics Analysis of BIRC5/Survivin Expression in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2018, 19, 2664. https://doi.org/10.3390/ijms19092664
Troiano G, Guida A, Aquino G, Botti G, Losito NS, Papagerakis S, Pedicillo MC, Ionna F, Longo F, Cantile M, et al. Integrative Histologic and Bioinformatics Analysis of BIRC5/Survivin Expression in Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2018; 19(9):2664. https://doi.org/10.3390/ijms19092664
Chicago/Turabian StyleTroiano, Giuseppe, Agostino Guida, Gabriella Aquino, Gerardo Botti, Nunzia Simona Losito, Silvana Papagerakis, Maria Carmela Pedicillo, Franco Ionna, Francesco Longo, Monica Cantile, and et al. 2018. "Integrative Histologic and Bioinformatics Analysis of BIRC5/Survivin Expression in Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 19, no. 9: 2664. https://doi.org/10.3390/ijms19092664
APA StyleTroiano, G., Guida, A., Aquino, G., Botti, G., Losito, N. S., Papagerakis, S., Pedicillo, M. C., Ionna, F., Longo, F., Cantile, M., Pennella, A., Lo Russo, L., Di Gioia, G., Mariggiò, M. A., Lo Muzio, L., & Pannone, G. (2018). Integrative Histologic and Bioinformatics Analysis of BIRC5/Survivin Expression in Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences, 19(9), 2664. https://doi.org/10.3390/ijms19092664