Tyr198 is the Essential Autophosphorylation Site for STK16 Localization and Kinase Activity
Abstract
:1. Introduction
2. Results
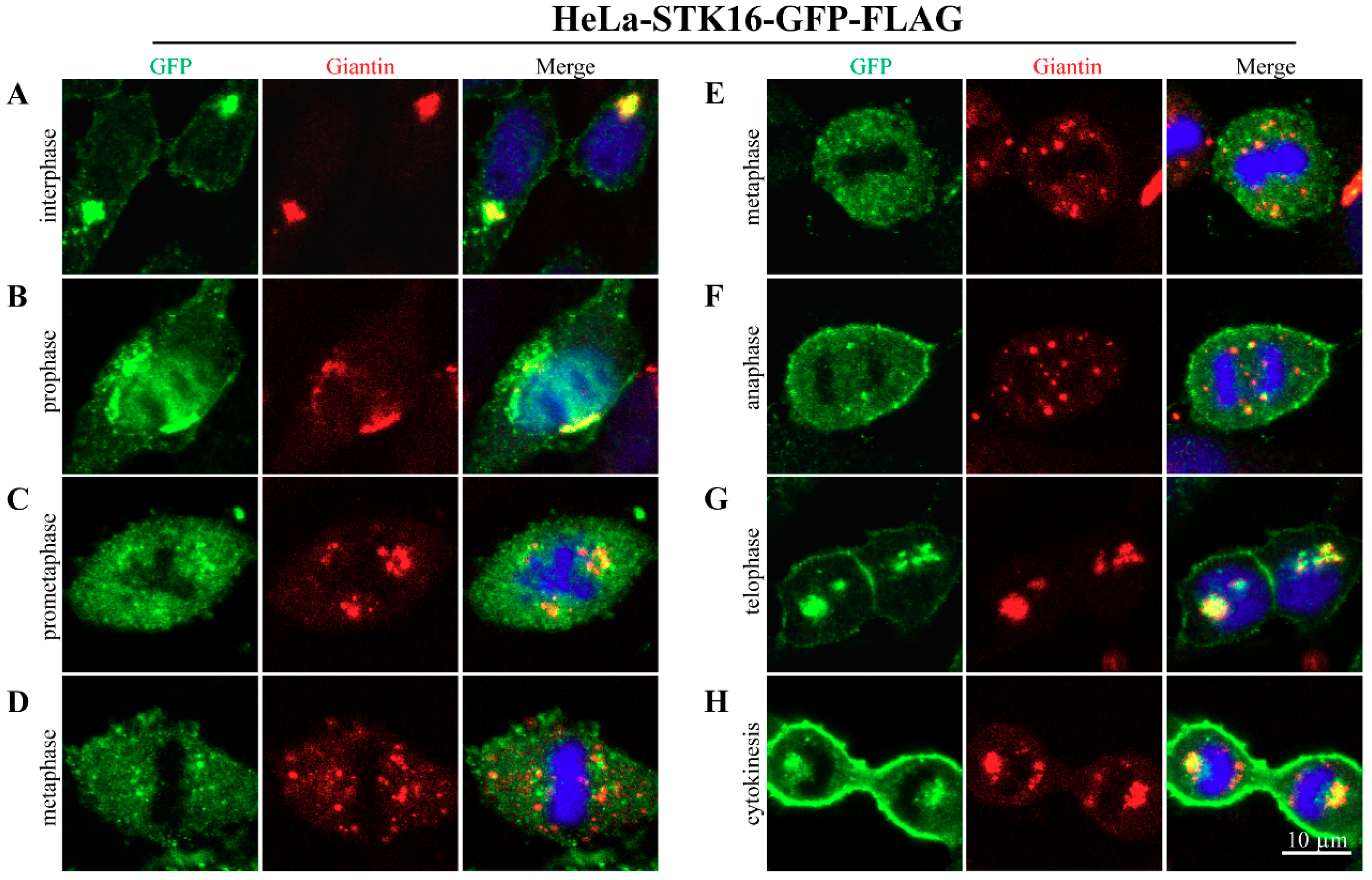
2.1. Subcellular Localization of STK16 in Mitosis
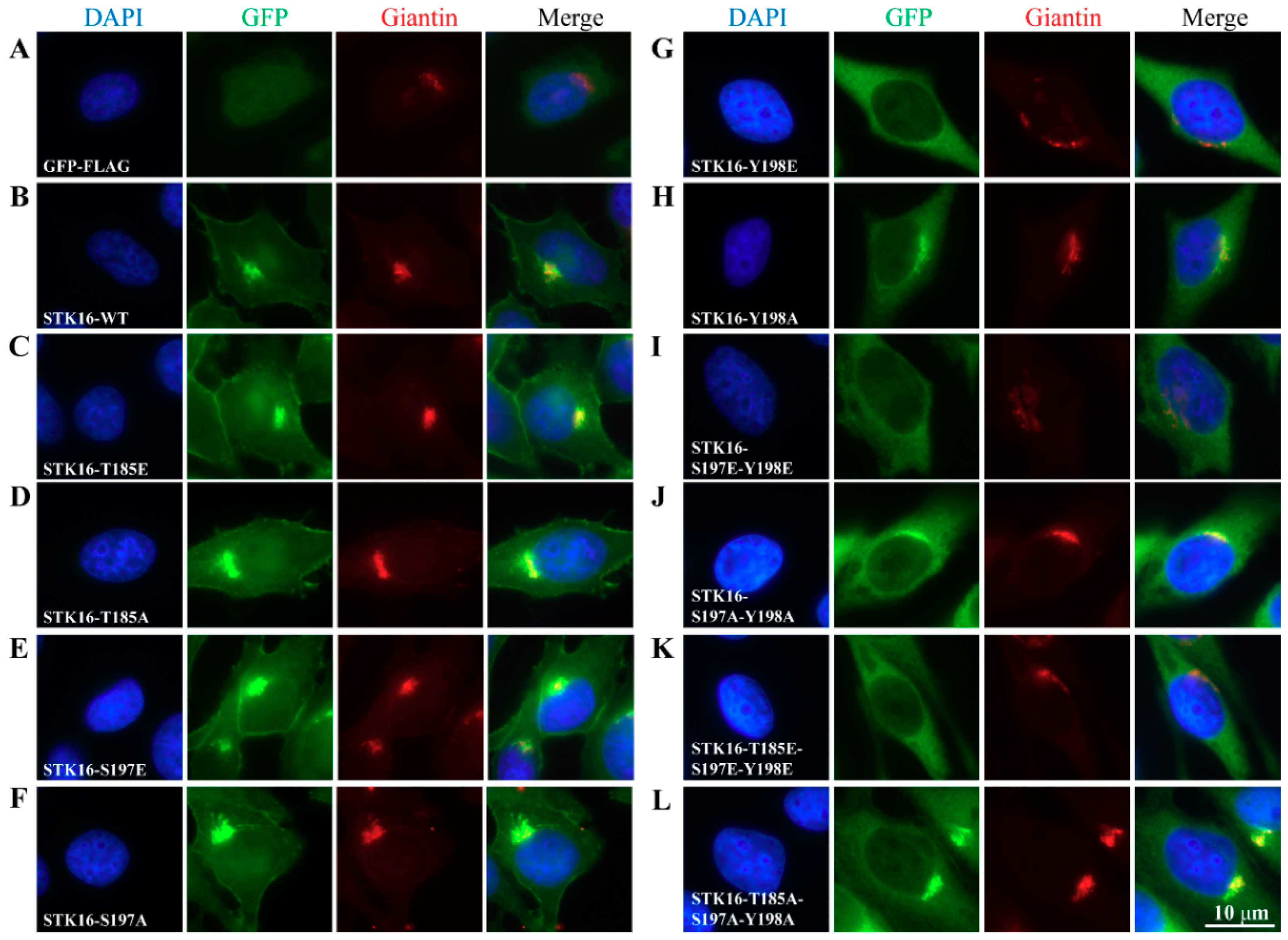
2.2. The Maintenance of the Golgi Apparatus Structure Depends on STK16-Tyr198 Phosphorylation
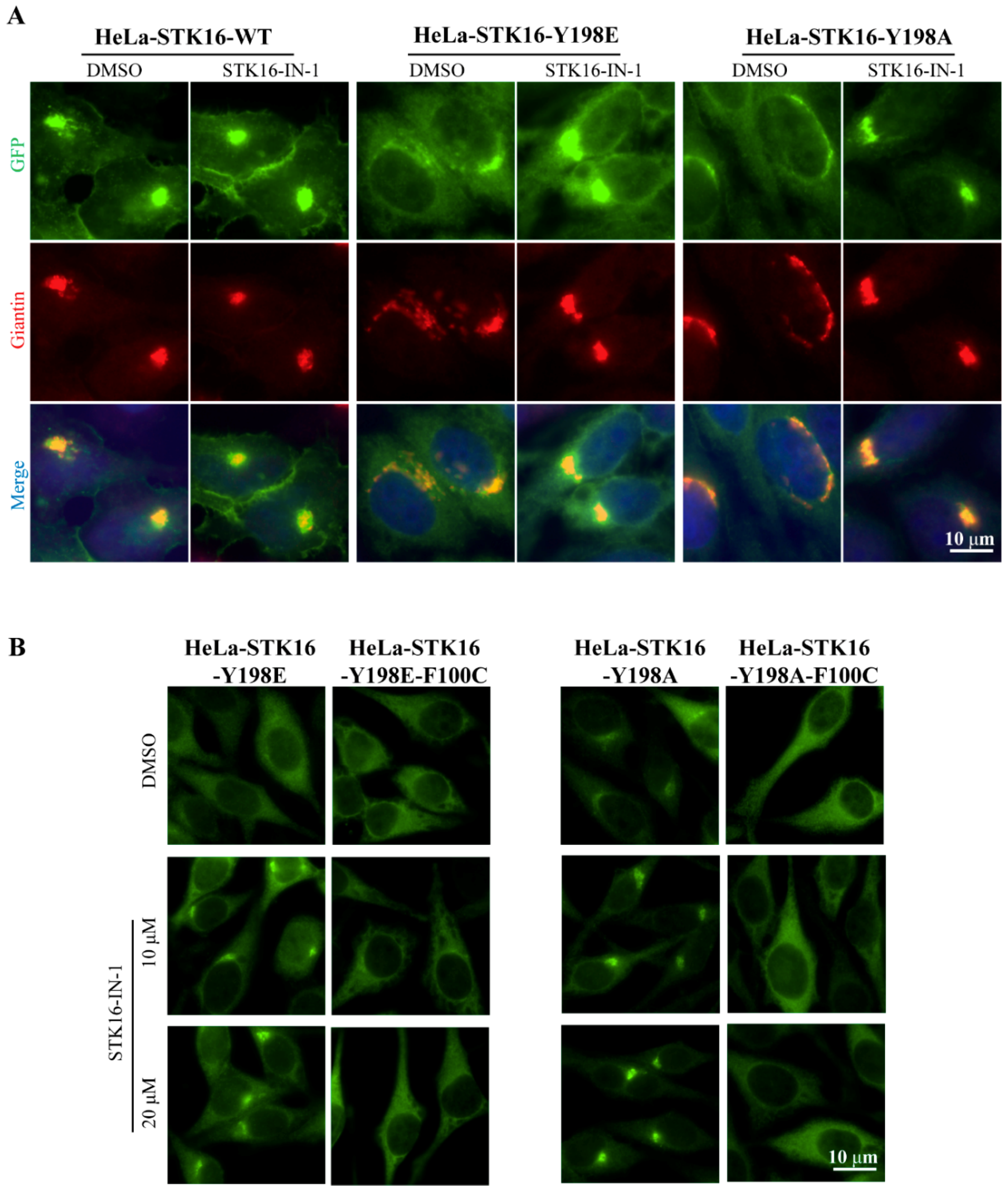
2.3. The Localization of STK16 to the Golgi Apparatus is Dependent on its Kinase Activity
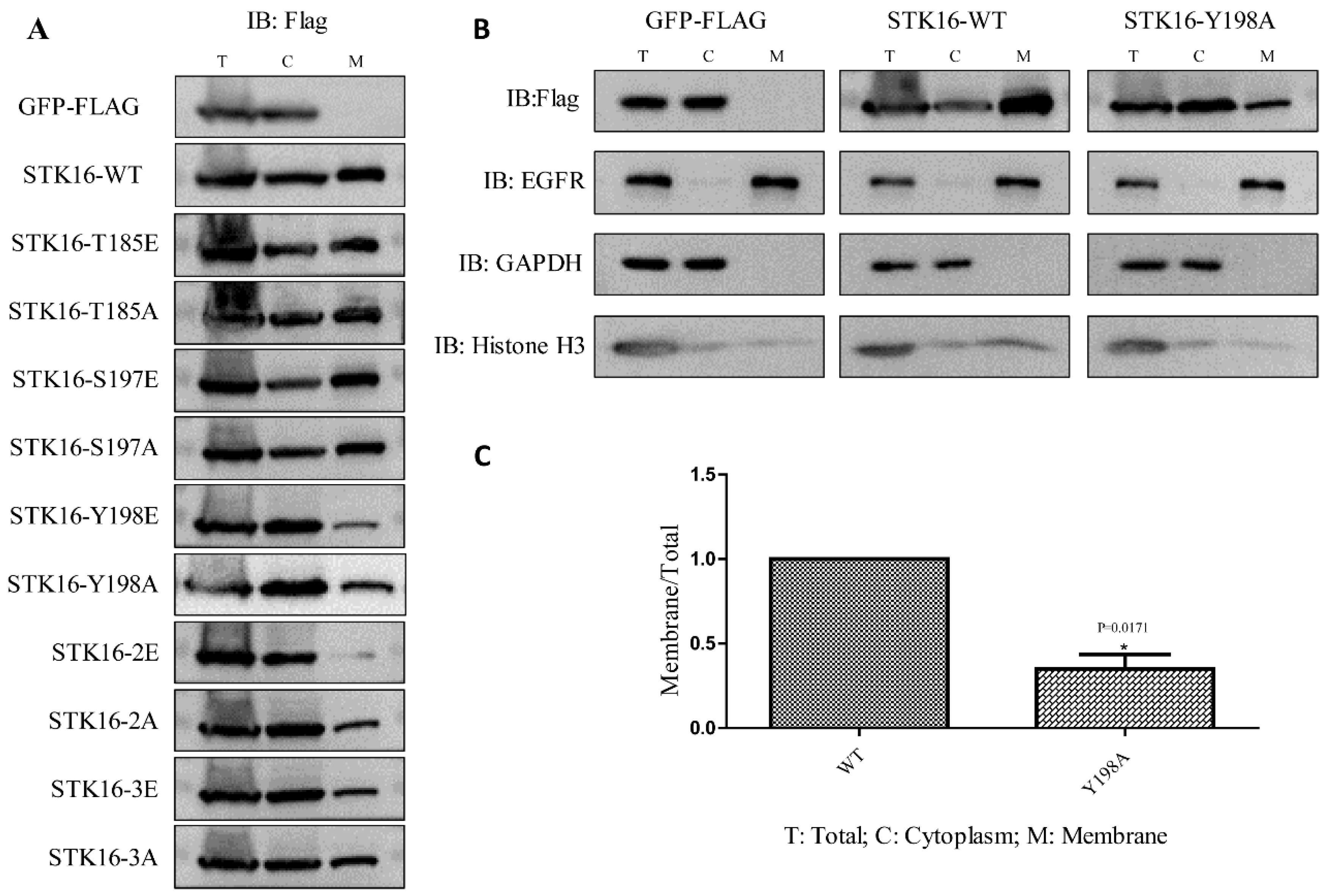
2.4. Phosphorylation at Tyr198 Affects the Localization of STK16 on the Cell Membrane
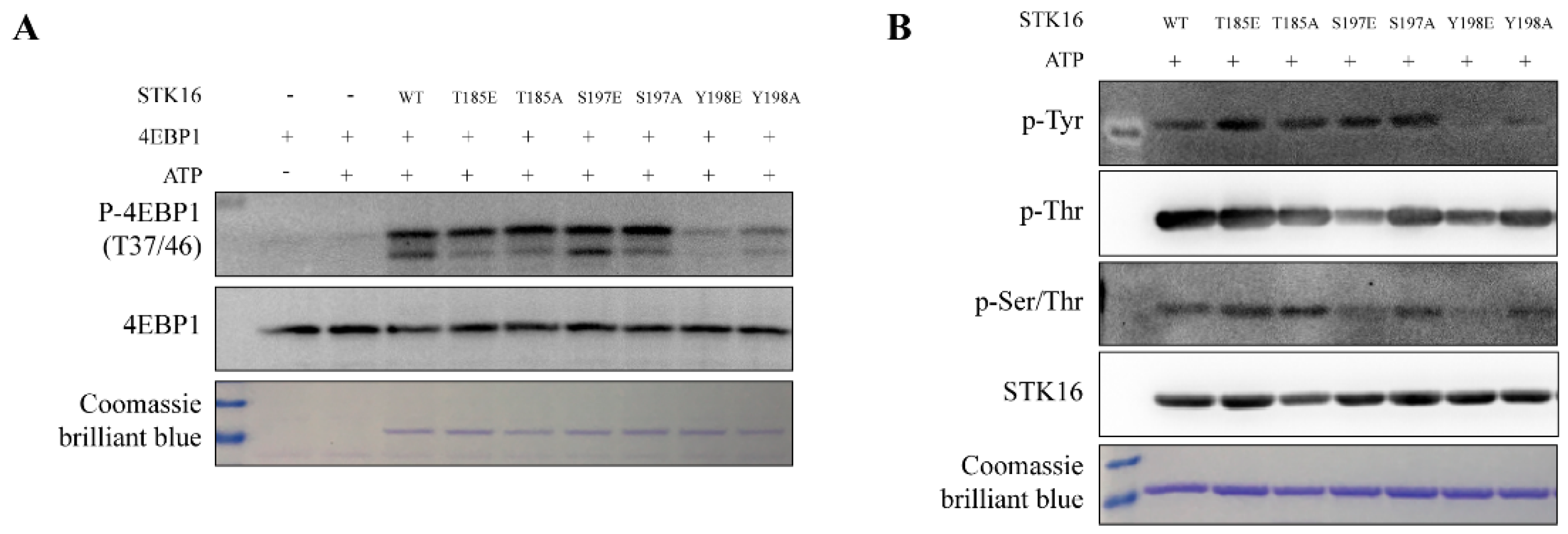
2.5. STK16’s Kinase Activity is Differentially Regulated by its Phosphorylation on Ser197/Tyr198
2.6. The Effect of STK16 on Cell Cycle is Also Dependent on Tyr198 Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Establishment of Stable Cell Lines
4.2. Reagents
4.3. Construction of Prokaryotic Expression Vector and Protein Purification
4.4. Immunofluorescence
4.5. Western Blots
4.6. In Vitro Phosphorylation Assay
4.7. Cell Synchronization
4.8. Cell Cycle Analysis by FACs
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ligos, J.M.; Gerwin, N.; Fernández, P.; Gutierrez-Ramos, J.C.; Bernad, A. Cloning, expression analysis, and functional characterization of PKL12, a member of a new subfamily of ser/thr kinases. Biochem. Biophys. Res. Commun. 1998, 249, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Stairs, D.B.; Perry Gardner, H.; Ha, S.I.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Chodosh, L.A. Cloning and characterization of Krct, a member of a novel subfamily of serine/threonine kinases. Hum. Mol. Genet. 1998, 7, 2157–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurioka, K.; Nakagawa, K.; Denda, K.; Miyazawa, K.; Kitamura, N. Molecular cloning and characterization of a novel protein serine/threonine kinase highly expressed in mouse embryo. Biochim. Et Biophys. Acta 1998, 1443, 275–284. [Google Scholar] [CrossRef]
- Berson, A.E.; Young, C.; Morrison, S.L.; Fujii, G.H.; Sheung, J.; Wu, B.; Bolen, J.B.; Burkhardt, A.L. Identification and characterization of a myristylated and palmitylated serine/threonine protein kinase. Biochem. Biophys. Res. Commun. 1999, 259, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Takeuchi, M.; Deguchi, M.; Tsuji, T.; Gahara, Y.; Nagata, K. A novel transcriptional factor with Ser/Thr kinase activity involved in the transforming growth factor (TGF)-beta signalling pathway. Biochem. J. 2000, 350, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Ligos, J.M.; de Lera, T.L.; Hinderlich, S.; Guinea, B.; Sanchez, L.; Roca, R.; Valencia, A.; Bernad, A. Functional interaction between the Ser/Thr kinase PKL12 and N-acetylglucosamine kinase, a prominent enzyme implicated in the salvage pathway for GlcNAc recycling. J. Biol. Chem. 2002, 277, 6333–6343. [Google Scholar] [CrossRef]
- Lopez-Coral, A.; Striz, A.C.; Tuma, P.L. A Serine/Threonine Kinase 16-Based Phospho-Proteomics Screen Identifies WD Repeat Protein-1 As A Regulator Of Constitutive Secretion. Sci. Rep. 2018, 8, 13049. [Google Scholar] [CrossRef]
- In, J.G.; Striz, A.C.; Bernad, A.; Tuma, P.L. Serine/threonine kinase 16 and MAL2 regulate constitutive secretion of soluble cargo in hepatic cells. Biochem. J. 2014, 463, 201–213. [Google Scholar] [CrossRef] [Green Version]
- Eswaran, J.; Bernad, A.; Ligos, J.M.; Guinea, B.; Debreczeni, J.E.; Sobott, F.; Parker, S.A.; Najmanovich, R.; Turk, B.E.; Knapp, S. Structure of the human protein kinase MPSK1 reveals an atypical activation loop architecture. Structure 2008, 16, 115–124. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Li, B.; Wang, J.; Wang, W.; Liu, J.; Liu, Q.; Zhang, X. STK16 regulates actin dynamics to control Golgi organization and cell cycle. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Guinea, B.; Ligos, J.M.; de Lera, T.L.; Martín-Caballero, J.; Flores, J.; de la Pena, M.G.; García-Castro, J.; Bernad, A. Nucleocytoplasmic shuttling of STK16 (PKL12), a Golgi-resident serine/threonine kinase involved in VEGF expression regulation. Exp. Cell Res. 2006, 312, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, J.; Yang, X.; Li, B.; Wu, H.; Qi, S.; Chen, C.; Liu, X.; Yu, K.; Wang, W.; et al. Discovery of a Highly Selective STK16 Kinase Inhibitor. ACS Chem. Biol. 2016, 11, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto, G.J., Jr. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew. Chem. 2005, 44, 7342–7372. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I.; Derventzi, A.; Clark, B.F. Protein synthesis, posttranslational modifications, and aging. Ann. N. Y. Acad. Sci. 1992, 663, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Karve, T.M.; Cheema, A.K. Small changes huge impact: The role of protein posttranslational modifications in cellular homeostasis and disease. J. Amino Acids 2011, 2011, 207691. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Virshup, D.M. Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell Biol. 2007, 8, 139–148. [Google Scholar] [CrossRef]
- Huse, M.; Kuriyan, J. The conformational plasticity of protein kinases. Cell 2002, 109, 275–282. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Guderian, G.; Westendorf, J.; Uldschmid, A.; Nigg, E.A. Plk4 trans-autophosphorylation regulates centriole number by controlling betaTrCP-mediated degradation. J. Cell Sci. 2010, 123, 2163–2169. [Google Scholar] [CrossRef]
- Saul, V.V.; de la Vega, L.; Milanovic, M.; Kruger, M.; Braun, T.; Fritz-Wolf, K.; Becker, K.; Schmitz, M.L. HIPK2 kinase activity depends on cis-autophosphorylation of its activation loop. J. Mol. Cell Biol. 2013, 5, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Stites, E.C.; Yu, H.; Germino, E.A.; Meharena, H.S.; Stork, P.J.S.; Kornev, A.P.; Taylor, S.S.; Shaw, A.S. Allosteric activation of functionally asymmetric RAF kinase dimers. Cell 2013, 154, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Ashenberg, O.; Keating, A.E.; Laub, M.T. Helix bundle loops determine whether histidine kinases autophosphorylate in cis or in trans. J. Mol. Biol. 2013, 425, 1198–1209. [Google Scholar] [CrossRef]
- Siepi, F.; Gatti, V.; Camerini, S.; Crescenzi, M.; Soddu, S. HIPK2 catalytic activity and subcellular localization are regulated by activation-loop Y354 autophosphorylation. Biochim. Et Biophys. Acta 2013, 1833, 1443–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, A.; Frame, S.; Cohen, P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem. J. 2004, 377, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Sorrell, F.J.; Szklarz, M.; Azeez, K.R.A.; Elkins, J.M.; Knapp, S. Family-wide structural analysis of human numb-associated protein kinases. Structure 2016, 24, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Ayala, I.; Colanzi, A. Mitotic inheritance of the Golgi complex and its role in cell division. Biol. Cell 2017, 109, 364–374. [Google Scholar] [CrossRef]
- Colanzi, A.; Sütterlin, C. Signaling at the Golgi during mitosis. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 118, pp. 383–400. [Google Scholar]
- Corda, D.; Barretta, M.L.; Cervigni, R.I.; Colanzi, A. Golgi complex fragmentation in G2/M transition: An organelle-based cell-cycle checkpoint. Iubmb Life 2012, 64, 661–670. [Google Scholar] [CrossRef]
- Sohda, M.; Misumi, Y.; Yoshimura, S.; Nakamura, N.; Fusano, T.; Sakisaka, S.; Ogata, S.; Fujimoto, J.; Kiyokawa, N.; Ikehara, Y. Depletion of vesicle-tethering factor p115 causes mini-stacked Golgi fragments with delayed protein transport. Biochem. Biophys. Res. Commun. 2005, 338, 1268–1274. [Google Scholar] [CrossRef]
- Nolen, B.; Taylor, S.; Ghosh, G. Regulation of protein kinases: Controlling activity through activation segment conformation. Mol. Cell 2004, 15, 661–675. [Google Scholar] [CrossRef]
- Kannan, N.; Neuwald, A.F. Did protein kinase regulatory mechanisms evolve through elaboration of a simple structural component? J. Mol. Biol. 2005, 351, 956–972. [Google Scholar] [CrossRef]
- Soundararajan, M.; Roos, A.K.; Savitsky, P.; Filippakopoulos, P.; Kettenbach, A.N.; Olsen, J.V.; Gerber, S.A.; Eswaran, J.; Knapp, S.; Elkins, J.M. Structures of Down syndrome kinases, DYRKs, reveal mechanisms of kinase activation and substrate recognition. Structure 2013, 21, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Krishnankutty, A.; Kimura, T.; Saito, T.; Aoyagi, K.; Asada, A.; Takahashi, S.I.; Ando, K.; Ohara-Imaizumi, M.; Ishiguro, K.; Hisanaga, S.I. In vivo regulation of glycogen synthase kinase 3beta activity in neurons and brains. Sci. Rep. 2017, 7, 8602. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-t.; Wang, S.; Rothenberg, M.; Jan, L.Y.; Jan, Y.N. Numb-associated kinase interacts with the phosphotyrosine binding domain of Numb and antagonizes the function of Numb in vivo. Mol. Cell. Biol. 1998, 18, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, M.S.; Jan, L.Y.; Jan, Y.N. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter. Cell 1994, 76, 477–491. [Google Scholar] [CrossRef]
- Uemura, T.; Shepherd, S.; Ackerman, L.; Jan, L.Y.; Jan, Y.N. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell 1989, 58, 349–360. [Google Scholar] [CrossRef]
- Fukushima, K.; Wang, M.; Naito, Y.; Uchihashi, T.; Kato, Y.; Mukai, S.; Yabuta, N.; Nojima, H. GAK is phosphorylated by c-Src and translocated from the centrosome to chromatin at the end of telophase. Cell Cycle 2017, 16, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Nagamori, I.; Yabuta, N.; Nojima, H. GAK, a regulator of clathrin-mediated membrane traffic, also controls centrosome integrity and chromosome congression. J. Cell Sci. 2009, 122, 3145–3152. [Google Scholar] [CrossRef] [Green Version]
- Tanenbaum, M.E.; Vallenius, T.; Geers, E.F.; Greene, L.; Makela, T.P.; Medema, R.H. Cyclin G-associated kinase promotes microtubule outgrowth from chromosomes during spindle assembly. Chromosoma 2010, 119, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wang, Y. Golgi structure formation, function, and post-translational modifications in mammalian cells. F1000Res 2017, 6, 2050. [Google Scholar] [CrossRef]
- Valente, C.; Colanzi, A. Mechanisms and regulation of the mitotic inheritance of the Golgi complex. Front. Cell Dev. Biol. 2015, 3, 79. [Google Scholar] [CrossRef]
- Champion, L.; Linder, M.I.; Kutay, U. Cellular reorganization during mitotic entry. Trends Cell Biol. 2017, 27, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Litvak, V.; Argov, R.; Dahan, N.; Ramachandran, S.; Amarilio, R.; Shainskaya, A.; Lev, S. Mitotic phosphorylation of the peripheral Golgi protein Nir2 by Cdk1 provides a docking mechanism for Plk1 and affects cytokinesis completion. Mol. Cell 2004, 14, 319–330. [Google Scholar] [CrossRef]
- Zhou, Y.; Atkins, J.B.; Rompani, S.B.; Bancescu, D.L.; Petersen, P.H.; Tang, H.; Zou, K.; Stewart, S.B.; Zhong, W. The mammalian Golgi regulates numb signaling in asymmetric cell division by releasing ACBD3 during mitosis. Cell 2007, 129, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Royle, S.J.; Bright, N.A.; Lagnado, L. Clathrin is required for the function of the mitotic spindle. Nature 2005, 434, 1152. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, J.; Ji, X.; Zhang, X. Tyr198 is the Essential Autophosphorylation Site for STK16 Localization and Kinase Activity. Int. J. Mol. Sci. 2019, 20, 4852. https://doi.org/10.3390/ijms20194852
Wang J, Liu J, Ji X, Zhang X. Tyr198 is the Essential Autophosphorylation Site for STK16 Localization and Kinase Activity. International Journal of Molecular Sciences. 2019; 20(19):4852. https://doi.org/10.3390/ijms20194852
Chicago/Turabian StyleWang, Junjun, Juanjuan Liu, Xinmiao Ji, and Xin Zhang. 2019. "Tyr198 is the Essential Autophosphorylation Site for STK16 Localization and Kinase Activity" International Journal of Molecular Sciences 20, no. 19: 4852. https://doi.org/10.3390/ijms20194852




