New Insight Regarding the Relationship Between Enantioselective Toxicity Difference and Enantiomeric Toxicity Interaction from Chiral Ionic Liquids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single Enantiomer Toxicity
2.2. Enantiomer Mixture CRC and CTC
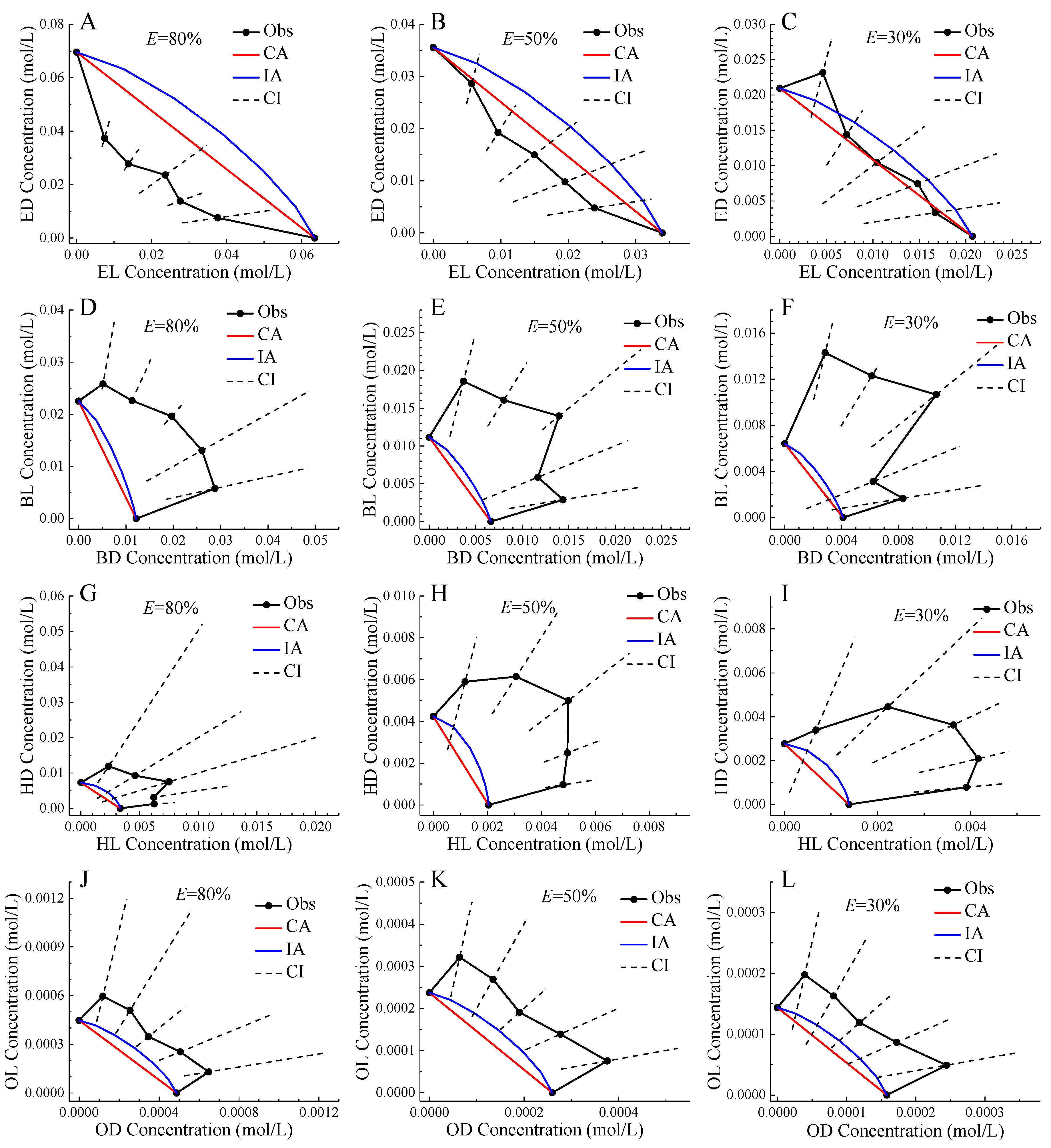
2.3. Enantiomer Mixture Toxicity Assessment Based on Isobole
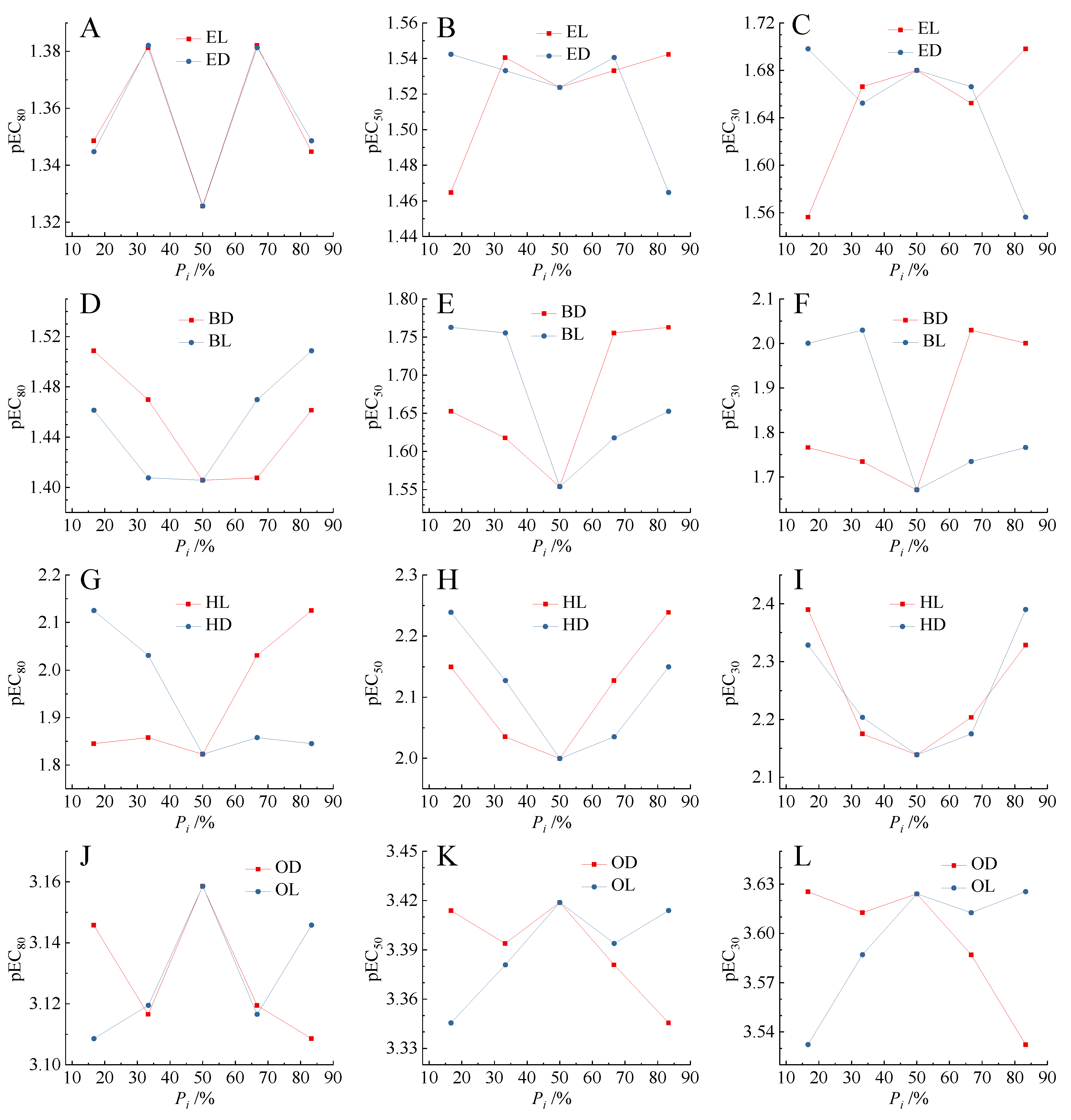
2.4. Relationship between Mixture Toxicity and Enantiomer Concentration Proportions
2.5. Implications
3. Materials and Methods
3.1. Chemicals
3.2. Photobacterium Toxicity Test
3.3. Experimental Design and Toxicity Evaluation of Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, W.; Gan, J.; Schlenk, D.; Jury, W.A. Enantioselectivity in environmental safety of current chiral insecticides. Proc. Natl. Acad. Sci. USA 2005, 102, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.; He, H.; Pham-Huy, C. Chiral drugs: an overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Lee, S.T.; Green, B.T.; Welch, K.D.; Pfister, J.A.; Panter, K.E. Stereoselective potencies and relative toxicities of coniine enantiomers. Chem. Res. Toxicol. 2008, 21, 2061–2064. [Google Scholar] [CrossRef] [PubMed]
- Masurel, D.; Houghton, P.J.; Young, C.L.; Wainer, I.W. Efficacy, toxicity, pharmacokinetics, and in vitro metabolism of the enantiomers of ifosfamide in mice. Cancer Res. 1990, 50, 252–255. [Google Scholar] [PubMed]
- Zhu, Y.; Liu, W.; Qi, S.; Wang, H.; Wang, Y.; Deng, G.; Zhang, Y.; Li, S.; Ma, C.; Wang, Y.; et al. Stereoselective glucuronidation metabolism, pharmacokinetics, anti-amnesic pharmacodynamics, and toxic properties of vasicine enantiomers in vitro and in vivo. Eur. J. Pharm. Sci. 2018, 123, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, C.J. Asymmetric (ADMA) and symmetric (SDMA) dimethylarginines in chronic kidney disease: a clinical approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef] [Green Version]
- Neale, P.A.; Branch, A.; Khan, S.J.; Leusch, F.D.L. Evaluating the enantiospecific differences of non-steroidal anti-inflammatory drugs (NSAIDs) using an ecotoxicity bioassay test battery. Sci. Total Environ. 2019, 694, 133659. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, X.; Dong, F.; Duan, H.; Shao, X.; Chen, X.; Yang, T.; Wang, G.; Zheng, Y. Ecological toxicity reduction of dinotefuran to honeybee: New perspective from an enantiomeric level. Environ. Int. 2019, 130, 104854. [Google Scholar] [CrossRef]
- Pan, X.; Cheng, Y.; Dong, F.; Liu, N.; Xu, J.; Liu, X.; Wu, X.; Zheng, Y. Stereoselective bioactivity, acute toxicity and dissipation in typical paddy soils of the chiral fungicide propiconazole. J. Hazard. Mater. 2018, 359, 194–202. [Google Scholar] [CrossRef]
- Di, S.; Cang, T.; Qi, P.; Wang, X.; Xu, M.; Wang, Z.; Xu, H.; Wang, Q.; Wang, X. A systemic study of enantioselectivity of isocarbophos in rice cultivation: Enantioselective bioactivity, toxicity, and environmental fate. J. Hazard. Mater. 2019, 375, 305–311. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, L.; Liu, K.; Guo, F.; Gao, L.; Liu, W. Activity, toxicity, molecular docking, and environmental effects of three imidazolinone herbicides enantiomers. Sci. Total Environ. 2018, 622–623, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, C.; Chen, Z.; Ali, B.A.; Wen, Y. Dichlorprop induced structural changes of LHCⅡ chiral macroaggregates associated with enantioselective toxicity to Scnedesmus obliquus. Aquat. Toxicol. 2019, 206, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, S.; He, Y.; Xu, Y.; Shi, D.; Yang, F.; Yu, W.; Zhu, W.; He, L. Identifying metabolic perturbations and toxic effects of rac-metalaxyl and metalaxyl-M in mice using integrative NMR and UPLC-MS/MS based metabolomics. Int. J. Mol. Sci. 2019, 20, 5457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, D.; Caballero, J. Is it reliable to use common molecular docking methods for comparing the binding affinities of enantiomer pairs for their protein target? Int. J. Mol. Sci. 2016, 17, 525. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Liu, W.; Li, L.; Gan, J. Single and joint acute toxicity of isocarbophos enantiomers to Daphnia magna. J. Agric. Food Chem. 2008, 56, 4273–4277. [Google Scholar] [CrossRef]
- Raffa, R.B.; Friderichs, E.; Reimann, W.; Shank, R.P.; Codd, E.E.; Vaught, J.L.; Jacoby, H.I.; Selve, N. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J. Pharmacol. Exp. Ther. 1993, 267, 331–340. [Google Scholar]
- Hinfray, N.; Tebby, C.; Piccini, B.; Bourgine, G.; Aït-Aïssa, S.; Porcher, J.M.; Pakdel, F.; Brion, F. Mixture concentration-response modeling reveals antagonistic effects of estradiol and genistein in combination on brain aromatase gene (cyp19a1b) in zebrafish. Int. J. Mol. Sci. 2018, 19, 1047. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.L.; Tao, S.S.; Zhou, M.; Han, B.J.; Yuan, H.Q. Integrative assessment of mixture toxicity of three ionic liquids on acetylcholinesterase using a progressive approach from 1D point, 2D curve, to 3D surface. Int. J. Mol. Sci. 2019, 20, 5330. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.P.T.; Cho, C.W.; Yun, Y.S. Environmental fate and toxicity of ionic liquids: A review. Water Res. 2010, 44, 352–372. [Google Scholar] [CrossRef]
- Ge, H.L.; Liu, S.S.; Su, B.X.; Zhu, X.W. Two-stage prediction of the effects of imidazolium and pyridinium ionic liquid mixtures on luciferase. Molecules 2014, 19, 6877–6890. [Google Scholar] [CrossRef] [Green Version]
- Baudequin, C.; Baudoux, J.; Levillain, J.; Cahard, D.; Gaumont, A.C.; Plaquevent, J.C. Ionic liquids and chirality: opportunities and challenges. Tetrahedron Asymmetry 2003, 14, 3081–3093. [Google Scholar] [CrossRef]
- Sedghamiz, T.; Bahrami, M. Chiral ionic liquid interface as a chiral selector for recognition of propranolol enantiomers: A molecular dynamics simulations study. J. Mol. Liq. 2019, 292, 111441. [Google Scholar] [CrossRef]
- Yu, S.F.; Lindeman, S.; Tran, C.D. Chiral ionic liquids: synthesis, properties, and enantiomeric recognition. J. Org. Chem. 2008, 73, 2576–2591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.R.; Wang, S.H.; Yun, M.X.; Li, X.Y.; Wang, J.J.; Sun, Z.J. The toxic effects of ionic liquids on the activities of acetylcholinesterase and cellulase in earthworms. Chemosphere 2009, 77, 313–318. [Google Scholar] [CrossRef]
- Ranke, J.; Molter, K.; Stock, F.; Bottin-Weber, U.; Poczobutt, J.; Hoffmann, J.; Ondruschka, B.; Filser, J.; Jastorff, B. Biological effects of imidazolium ionic liquids with varying chain lengths in acute Vibrio fischeri and WST-1 cell viability assays. Ecotoxicol. Environ. Saf. 2004, 58, 396–404. [Google Scholar] [CrossRef]
- Cho, C.W.; Jeon, Y.C.; Pham, T.P.T.; Vijayaraghavan, K.; Yun, Y.S. The ecotoxicity of ionic liquids and traditional organic solvents on microalga Selenastrum capricornutum. Ecotoxicol. Environ. Saf. 2008, 71, 166–171. [Google Scholar] [CrossRef]
- Kumar, R.A.; Papaiconomou, N.; Lee, J.M.; Salminen, J.; Clark, D.S.; Prausnitz, J.M. In vitro cytotoxicities of ionic liquids: effect of cation rings, functional groups, and anions. Environ. Toxicol. 2009, 24, 388–395. [Google Scholar] [CrossRef]
- Matzke, M.; Stolte, S.; Arning, U.; Uebers, U.; Filser, J. Imidazolium based ionic liquids in soils: effects of the side chain length on wheat (Triticum aestivum) and cress (Lepidium sativum) as affected by different clays and organic matter. Green Chem. 2008, 10, 584–591. [Google Scholar] [CrossRef]
- Costello, D.M.; Brown, L.M.; Lamberti, G.A. Acute toxic effects of ionic liquids on zebra mussel (Dreissena polymorpha) survival and feeding. Green Chem. 2009, 11, 548–553. [Google Scholar] [CrossRef]
- Pretti, C.; Chiappe, C.; Baldetti, I.; Brunini, S.; Monni, G.; Intorre, L. Acute toxicity of ionic liquids for three freshwater organisms: Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Ecotoxicol. Environ. Saf. 2009, 72, 1170–1176. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Zhang, L.; Wen, Y.; Liu, W. Enantioselective toxicities of chiral ionic liquids 1-alkyl-3-methylimidazolium lactate to aquatic algae. Aquat. Toxicol. 2014, 154, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, J.; Zhang, X.; Xia, Y.; Li, Y.; Du, S. Enantioselective oxidative stress caused by chiral ionic liquids forms of 1-alkyl-3-methyl imidazolium tartrate on Scenedesmus obliquus. Sci. Total Environ. 2017, 595, 819–827. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Stock, F.; Hoffmann, J.; Ranke, J.; Störmann, R.; Ondruschka, B.; Jastorff, B. Effects of ionic liquids on the acetylcholinesterase – a structure–activity relationship consideration. Green Chem. 2004, 6, 286–290. [Google Scholar] [CrossRef]
- Lin, Z.; Yu, H.; Gao, S.; Cheng, J.; Wang, L. Development of the fragment constant method for estimating the partition coefficients of nonionic organic mixtures. Arch. Environ. Contam. Toxicol. 2001, 41, 255–260. [Google Scholar]
- Zhu, X.W.; Liu, S.S.; Ge, H.L.; Liu, Y. Comparison between the short-term and the long-term toxicity of six triazine herbicides on photobacteria Q67. Water Res. 2009, 43, 1731–1739. [Google Scholar] [CrossRef]
- Stolte, S.; Arning, J.; Bottin-Weber, U.; Matzke, M.; Stock, F.; Thiele, K.; Uerdingen, M.; Welz-Biermann, U.; Jastorff, B.; Ranke, J. Anion effects on the cytotoxicity of ionic liquids. Green Chem. 2006, 8, 621–629. [Google Scholar] [CrossRef]
- Schulz, P.S.; Muller, N.; Bosmann, A.; Wasserscheid, P. Effective chirality transfer in ionic liquids through ion-pairing effects. Angew. Chem. Int. Ed. 2007, 46, 1293–1295. [Google Scholar] [CrossRef]
- Zeng, L.X.; He, Y.J.; Dai, Z.F.; Wang, J.; Cao, Q.; Zhang, Y.L. Chiral induction, memory, and amplification in porphyrin homoaggregates based on electrostatic interactions. Chem. Phys. Chem. 2009, 10, 954–962. [Google Scholar] [CrossRef]
- Sun, Y.P.; Johnson, E.R. Analysis of joint action of insecticides against house flies. J. Econ. Entomol. 1960, 53, 887–892. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.S.; Yu, M.; Chen, F. Application of the combination index integrated with confidence intervals to study the toxicological interactions of antibiotics and pesticides in Vibrio qinghaiensis sp.-Q67. Environ. Toxicol. Pharmacol. 2015, 39, 447–456. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, W.; Hu, H.; Ma, X.; Li, Q.; Song, X.; Ren, X.; Ma, Y. Joint toxicity of methoxyfenozide and lufenuron on larvae of Spodoptera exigua Hübner (Lepidoptera: Noctuidae). J. Asia Pac. Entomol. 2019, 22, 795–801. [Google Scholar] [CrossRef]
- Cedergreen, N.; Streibig, J.C. Can the choice of endpoint lead to contradictory results of mixture-toxicity experiments? Environ. Toxicol. Chem. 2005, 24, 1676–1683. [Google Scholar] [CrossRef]
- Backhaus, T.; Scholze, M.; Grimme, L.H. The single substance and mixture toxicity of quinolones to the bioluminescent bacterium Vibrio fischeri. Aquat. Toxicol. 2000, 49, 49–61. [Google Scholar] [CrossRef]
- Grote, M.; Brack, W.; Walter, H.A.; Altenburger, R. Light as a confounding factor for toxicity assessment of complex contaminated sediments. Environ. Toxicol. Chem. 2005, 24, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Faust, M.; Altenburger, R.; Backhaus, T.; Blanck, H.; Boedeker, W.; Gramatica, P.; Hamer, V.; Scholze, M.; Vighi, M.; Grimme, L.H. Predicting the joint algal toxicity of multi-component s-triazine mixtures at low-effect concentrations of individual toxicants. Aquat. Toxicol. 2001, 56, 13–32. [Google Scholar] [CrossRef]
- Altenburger, R.; Nendza, M.; Schuurmann, G. Mixture toxicity and its modeling by quantitative structure-activity relationships. Environ. Toxicol. Chem. 2003, 22, 1900–1915. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, L.; Diao, J.; Zhou, Z. Enantioselective toxic effects and degradation of myclobutanil enantiomers in Scenedesmus obliquus. Chirality 2013, 25, 858–864. [Google Scholar] [CrossRef]
- Ge, H.L.; Liu, S.S.; Su, B.X.; Xu, Z. Mathematical derivation of concentration addition, independent action and effect summation models. Appl. Mech. Mater. 2013, 361-363, 1054–1057. [Google Scholar] [CrossRef]
- Lin, Z.; Zhong, P.; Kong, D.; Yin, K.; Cai, Z. The ratios of individual chemicals in a mixture determine the degree of joint effect: the climax hypothesis. Arch. Environ. Contam. Toxicol. 2005, 49, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.Y.; Lin, Z.F.; Yin, D.Q. Quantitative structure activity relationships (QSAR) for binary mixtures at non-equitoxic ratios based on toxic ratios-effects curves. Dose Response 2012, 11, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short, G.F., III; Staunton, J.E.; Jin, X.; et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Tobert, J.A.; Cirillo, V.J.; Hitzenberger, G.; James, I.; Pryor, J.; Cook, T.; Buntinx, A.; Holmes, I.B.; Lutterbeck, P.M. Enhancement of uricosuric properties of indacrinone by manipulation of the enantiomer ratio. Clin. Pharmacol. Ther. 1981, 29, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Liu, S.S.; Song, X.Q.; Ge, H.L. Prediction for the mixture toxicity of six organophosphorus pesticides to the luminescent bacterium Q67. Ecotox. Environ. Safe. 2008, 71, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Scholze, M.; Boedeker, W.; Faust, M.; Backhaus, T.; Altenburger, R.; Grimme, L.H. A general best-fit method for concentration-response curves and the estimation of low-effect concentrations. Environ. Toxicol. Chem. 2001, 20, 448–457. [Google Scholar] [CrossRef]
- Zhu, X.W.; Liu, S.S.; Ge, H.L.; Liu, Y. Comparison between two confidence intervals of dose-response relationships. China Environ. Sci. 2009, 29, 113–117. [Google Scholar]
- Dou, R.N.; Liu, S.S.; Mo, L.Y.; Liu, H.L.; Deng, F.C. A novel direct equipartition ray design (EquRay) procedure for toxicity interaction between ionic liquid and dichlorvos. Environ. Sci. Pollut. Res. 2011, 18, 734–742. [Google Scholar] [CrossRef]
- Faust, M.; Altenburger, R.; Backhaus, T.; Blanck, H.; Boedeker, W.; Gramatica, P.; Hamer, V.; Scholze, M.; Vighi, M.; Grimme, L.H. Joint algal toxicity of 16 dissimilarly acting chemicals is predictable by the concept of independent action. Aquat. Toxicol. 2003, 63, 43–63. [Google Scholar] [CrossRef]

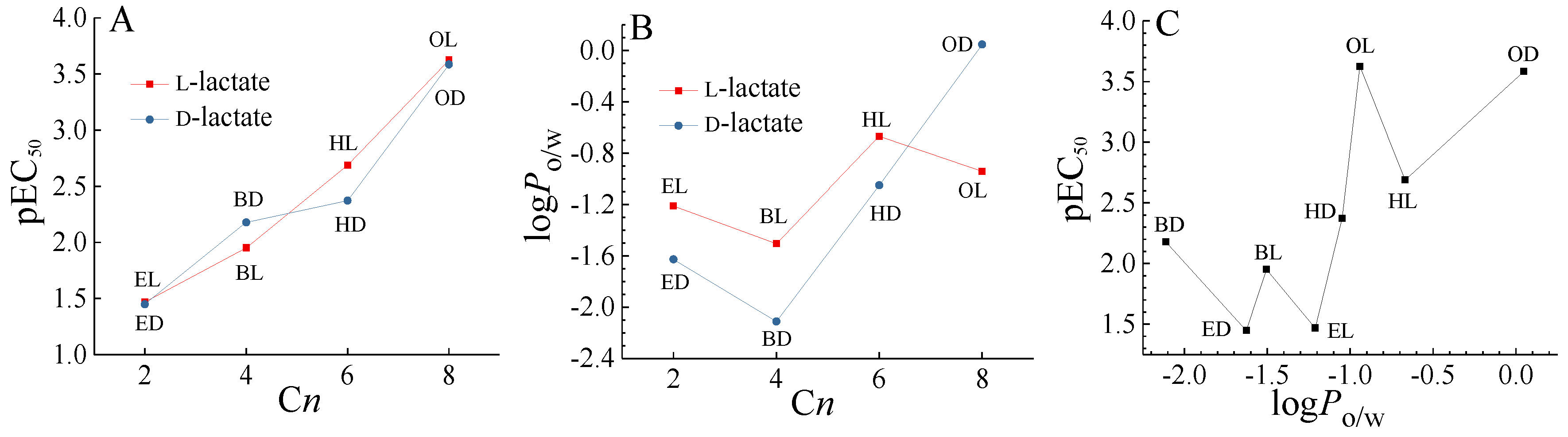
| Toxicants | Molar Ratio | C0 | a | b | R2 | RMSE | EC30 | EC50 | EC80 |
|---|---|---|---|---|---|---|---|---|---|
| EL | 1.05 × 10–1 | 4.178 | 3.093 | 0.913 | 0.075 | 2.07 × 10−2 | 3.39 × 10−2 | 6.35 × 10−2 | |
| ED | 1.01 × 10−1 | 3.821 | 2.890 | 0.910 | 0.072 | 2.09 × 10−2 | 3.56 × 10−2 | 6.96 × 10−2 | |
| BL | 9.51 × 10−2 | 5.020 | 2.759 | 0.978 | 0.054 | 6.41 × 10−3 | 1.12 × 10−2 | 2.25 × 10−2 | |
| BD | 9.00 × 10−2 | 6.596 | 3.196 | 0.958 | 0.088 | 4.11 × 10−3 | 6.63 × 10−3 | 1.22 × 10−2 | |
| HL | 7.92 × 10−2 | 10.19 | 3.925 | 0.988 | 0.053 | 1.38 × 10−3 | 2.04 × 10−3 | 3.35 × 10−3 | |
| HD | 7.83 × 10−2 | 8.155 | 3.592 | 0.980 | 0.065 | 2.77 × 10−3 | 4.24 × 10−3 | 7.28 × 10−3 | |
| OL | 7.91 × 10−3 | 10.70 | 3.053 | 0.983 | 0.057 | 1.44 × 10−4 | 2.37 × 10−4 | 4.48 × 10−4 | |
| OD | 6.79 × 10−3 | 10.68 | 3.081 | 0.979 | 0.063 | 1.58 × 10−4 | 2.60 × 10−4 | 4.88 × 10−4 | |
| E1 | 1:5 (EL:ED) | 1.02 × 10−1 | 10.26 | 7.255 | 0.938 | 0.074 | 2.78 × 10−2 | 3.43 × 10−2 | 4.48 × 10−2 |
| E2 | 2:4 (EL:ED) | 1.02 × 10−1 | 7.783 | 5.290 | 0.946 | 0.067 | 2.16 × 10−2 | 2.88 × 10−2 | 4.16 × 10−2 |
| E3 | 3:3 (EL:ED) | 1.03 × 10−1 | 6.114 | 4.253 | 0.850 | 0.113 | 2.09 × 10−2 | 2.99 × 10−2 | 4.72 × 10−2 |
| E4 | 4:2 (EL:ED) | 1.04 × 10−1 | 8.181 | 5.575 | 0.876 | 0.119 | 2.23 × 10−2 | 2.93 × 10−2 | 4.15 × 10−2 |
| E5 | 5:1 (EL:ED) | 1.04 × 10−1 | 6.210 | 4.264 | 0.882 | 0.096 | 2.00 × 10−2 | 2.87 × 10−2 | 4.52 × 10−2 |
| B1 | 1:5 (BD:BL) | 9.42 × 10−2 | 9.308 | 5.854 | 0.960 | 0.065 | 1.71 × 10−2 | 2.23 × 10−2 | 3.10 × 10−2 |
| B2 | 2:4 (BD:BL) | 9.33 × 10−2 | 8.839 | 5.690 | 0.972 | 0.053 | 1.84 × 10−2 | 2.41 × 10−2 | 3.39 × 10−2 |
| B3 | 3:3 (BD:BL) | 9.25 × 10−2 | 8.463 | 5.682 | 0.945 | 0.066 | 2.13 × 10−2 | 2.79 × 10−2 | 3.93 × 10−2 |
| B4 | 4:2 (BD:BL) | 9.16 × 10−2 | 3.885 | 2.422 | 0.855 | 0.111 | 9.34 × 10−3 | 1.76 × 10−2 | 3.91 × 10−2 |
| B5 | 5:1 (BD:BL) | 9.08 × 10−2 | 4.562 | 2.796 | 0.906 | 0.095 | 9.99 × 10−3 | 1.73 × 10−2 | 3.46 × 10−2 |
| H1 | 1:5 (HL:HD) | 7.84 × 10−2 | 5.580 | 2.766 | 0.869 | 0.130 | 4.07 × 10−3 | 7.08 × 10−3 | 1.43 × 10−2 |
| H2 | 2:4 (HL:HD) | 7.86 × 10−2 | 9.297 | 4.748 | 0.935 | 0.102 | 6.68 × 10−3 | 9.22 × 10−3 | 1.39 × 10−2 |
| H3 | 3:3 (HL:HD) | 7.87 × 10−2 | 9.164 | 4.766 | 0.927 | 0.102 | 7.26 × 10−3 | 1.00 × 10−2 | 1.50 × 10−2 |
| H4 | 4:2 (HL:HD) | 7.89 × 10−2 | 18.12 | 8.690 | 0.964 | 0.082 | 6.25 × 10−3 | 7.46 × 10−3 | 9.32 × 10−3 |
| H5 | 5:1 (HL:HD) | 7.90 × 10−2 | 16.22 | 7.409 | 0.977 | 0.070 | 4.69 × 10−3 | 5.77 × 10−3 | 7.50 × 10−3 |
| O1 | 1:5 (OD:OL) | 7.70 × 10−3 | 10.36 | 3.142 | 0.969 | 0.077 | 2.37 × 10−4 | 3.86 × 10−4 | 7.15 × 10−4 |
| O2 | 2:4 (OD:OL) | 7.50 × 10−3 | 9.944 | 3.038 | 0.960 | 0.085 | 2.44 × 10−4 | 4.04 × 10−4 | 7.65 × 10−4 |
| O3 | 3:3 (OD:OL) | 7.31 × 10−3 | 10.70 | 3.237 | 0.981 | 0.061 | 2.38 × 10−4 | 3.81 × 10−4 | 6.94 × 10−4 |
| O4 | 4:2 (OD:OL) | 7.13 × 10−3 | 10.53 | 3.223 | 0.971 | 0.073 | 2.59 × 10−4 | 4.16 × 10−4 | 7.59 × 10−4 |
| O5 | 5:1 (OD:OL) | 6.95 × 10−3 | 11.53 | 3.556 | 0.970 | 0.075 | 2.94 × 10−4 | 4.51 × 10−4 | 7.79 × 10−4 |
| E = 80% | E = 50% | E = 30% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mixtures | CTC | CTCUL | CTCLL | Interaction | CTC | CTCUL | CTCLL | Interaction | CTC | CTCUL | CTCLL | Interaction |
| E1 | 153 | 166 | 131 | synergism | 103 | 118 | 86 | additivity | 75 | 103 | 63 | additivity |
| E2 | 162 | 177 | 131 | synergism | 122 | 149 | 96 | additivity | 97 | 138 | 78 | additivity |
| E3 | 141 | 198 | 96 | additivity | 116 | 176 | 82 | additivity | 100 | 226 | 67 | additivity |
| E4 | 158 | 179 | 131 | synergism | 118 | 193 | 73 | additivity | 93 | 166 | 60 | additivity |
| E5 | 143 | 190 | 102 | synergism | 119 | 168 | 88 | additivity | 103 | 190 | 73 | additivity |
| B1 | 64 | 66 | 43 | antagonism | 45 | 74 | 35 | antagonism | 34 | 48 | 29 | antagonism |
| B2 | 52 | 52 | 38 | antagonism | 38 | 48 | 29 | antagonism | 29 | 45 | 28 | antagonism |
| B3 | 40 | 44 | 36 | antagonism | 30 | 34 | 18 | antagonism | 23 | 41 | 17 | antagonism |
| B4 | 37 | 66 | 20 | antagonism | 44 | 89 | 24 | antagonism | 50 | 201 | 25 | additivity |
| B5 | 38 | 59 | 23 | antagonism | 41 | 68 | 26 | antagonism | 44 | 109 | 26 | additivity |
| H1 | 43 | 69 | 10 | antagonism | 51 | 113 | 37 | additivity | 58 | 352 | 26 | additivity |
| H2 | 38 | 124 | 13 | additivity | 34 | 48 | 23 | antagonism | 31 | 61 | 16 | antagonism |
| H3 | 31 | 126 | 11 | additivity | 28 | 39 | 19 | antagonism | 25 | 49 | 20 | antagonism |
| H4 | 44 | 46 | 21 | antagonism | 33 | 40 | 27 | antagonism | 27 | 38 | 23 | antagonism |
| H5 | 49 | 50 | 39 | antagonism | 39 | 45 | 31 | antagonism | 32 | 45 | 27 | antagonism |
| O1 | 64 | 86 | 32 | antagonism | 62 | 88 | 44 | antagonism | 62 | 112 | 41 | additivity |
| O2 | 60 | 83 | 27 | antagonism | 61 | 90 | 40 | antagonism | 61 | 119 | 38 | additivity |
| O3 | 67 | 81 | 44 | antagonism | 65 | 83 | 51 | antagonism | 63 | 97 | 45 | antagonism |
| O4 | 62 | 78 | 33 | antagonism | 61 | 82 | 43 | antagonism | 59 | 100 | 40 | additivity |
| O5 | 62 | 76 | 33 | antagonism | 57 | 76 | 41 | antagonism | 53 | 90 | 38 | antagonism |
| Chemicals | Abbreviation | Chemical Formula | Molecular Structure | Purity | Molecular Weight |
|---|---|---|---|---|---|
| [EMIM]D-Lac | ED | C9H16N2O3 | 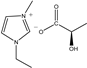 | 98% | 200.23 |
| [EMIM]L-Lac | EL | C9H16N2O3 | 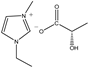 | 98% | 200.23 |
| [BMIM]D-Lac | BD | C11H20N2O3 | 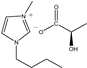 | 98% | 228.29 |
| [BMIM]L-Lac | BL | C11H20N2O3 |  | 98% | 228.29 |
| [HMIM]D-Lac | HD | C13H24N2O3 | 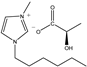 | 98% | 256.34 |
| [HMIM]L-Lac | HL | C13H24N2O3 | 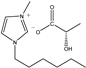 | 98% | 256.34 |
| [OMIM]D-Lac | OD | C15H28N2O3 |  | 98% | 284.39 |
| [OMIM]L-Lac | OL | C15H28N2O3 |  | 98% | 284.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, H.; Zhou, M.; Lv, D.; Wang, M.; Dong, C.; Wan, Y.; Zhang, Z.; Wang, S. New Insight Regarding the Relationship Between Enantioselective Toxicity Difference and Enantiomeric Toxicity Interaction from Chiral Ionic Liquids. Int. J. Mol. Sci. 2019, 20, 6163. https://doi.org/10.3390/ijms20246163
Ge H, Zhou M, Lv D, Wang M, Dong C, Wan Y, Zhang Z, Wang S. New Insight Regarding the Relationship Between Enantioselective Toxicity Difference and Enantiomeric Toxicity Interaction from Chiral Ionic Liquids. International Journal of Molecular Sciences. 2019; 20(24):6163. https://doi.org/10.3390/ijms20246163
Chicago/Turabian StyleGe, Huilin, Min Zhou, Daizhu Lv, Mingyue Wang, Cunzhu Dong, Yao Wan, Zhenshan Zhang, and Suru Wang. 2019. "New Insight Regarding the Relationship Between Enantioselective Toxicity Difference and Enantiomeric Toxicity Interaction from Chiral Ionic Liquids" International Journal of Molecular Sciences 20, no. 24: 6163. https://doi.org/10.3390/ijms20246163




