Interleukin-Mediated Pendrin Transcriptional Regulation in Airway and Esophageal Epithelia
Abstract
:1. Introduction
2. The Interleukins
3. Pendrin in the Airways
3.1. Molecular Mechanisms for Increased Pendrin Expression
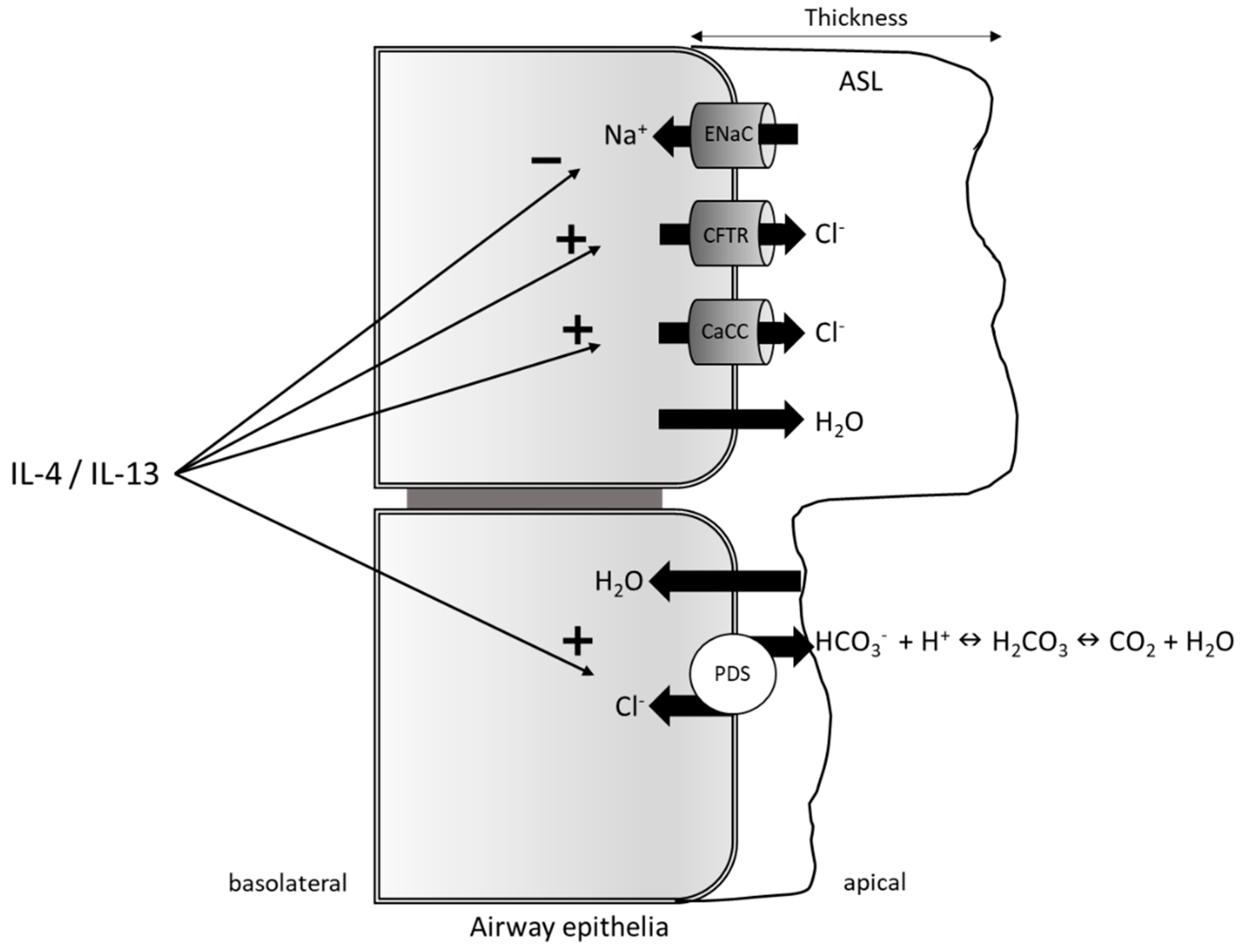
3.2. Pendrin as a Regulator of the Airway Surface Liquid
3.3. Pendrin and Mucus Production
3.4. Pendrin-Mediated Thiocyanate Secretion
4. Pendrin in the Esophagus
5. The Therapeutic Potential of Pendrin in Airway and Esophageal Inflammatory Diseases
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AHR | Airway hyperresponsiveness |
| ANO1 | Anoctamin 1 |
| AQP | Aquaporin |
| ASL | Airway surface liquid |
| BAL | Bronchoalveolar lavage |
| CA2 | Carbonic anhydrase |
| CACCs | Calcium-activated chloride channels |
| C/EBP | CCAAT/enhancer-binding protein |
| CBP | CREB-binding protein |
| CCL | Chemokine (C-C motif) ligand |
| CD4+ | Cluster of differentiation 4+ |
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| COPD | Chronic obstructive pulmonary disease |
| CREB | cAMP response element-binding |
| CXCL | Chemokine (C-X-C motif) ligand |
| DIS | Dilated intercellular spaces |
| DUOX | Dual oxidase |
| EE | Eosinophilic esophagitis |
| EnaC | Epithelial sodium channel |
| GAS | Gamma activated sequence |
| G-CSF | Granulocyte colony-stimulating factor |
| HEK | Human embryonic kidney |
| HNE | Human nasal epithelial |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| JAK | Janus kinase |
| KO | Knockout |
| MAPK | Mitogen-activated protein kinase |
| NCBE | Na+-driven Cl−/HCO3− exchanger |
| NcoA-1 | Steroid nuclear receptor co-activator 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NHE3 | Na+/H+ exchanger |
| NIS | Na+/I+ transporter |
| NKCC1 | Na+-K+-Cl− co-transporter |
| NKT | Natural killer T cells |
| ORF | Open reading frame |
| OSCN− | Hypothiocyanite |
| PI3K | Phosphoinositide-3-kinase |
| SCN− | Thiocyanate |
| SH2 | Src homology 2 |
| STAS | Sulfate transporter anti-sigma factor antagonist domain |
| STAT | Signal transducer and activator of transcription |
| TGF | Transforming growth factor |
| Th2 | T helper cell type 2 |
| TMD | Transmembrane domain |
| TNF | Tumor necrosis factor |
References
- Mount, D.B.; Romero, M.F. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004, 447, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Everett, L.A.; Glaser, B.; Beck, J.C.; Idol, J.R.; Buchs, A.; Heyman, M.; Adawi, F.; Hazani, E.; Nassir, E.; Baxevanis, A.D.; et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat. Genet. 1997, 17, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royaux, I.E.; Suzuki, K.; Mori, A.; Katoh, R.; Everett, L.A.; Kohn, L.D.; Green, E.D. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 2000, 141, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Porra, V.; Bernier-Valentin, F.; Trouttet-Masson, S.; Berger-Dutrieux, N.; Peix, J.L.; Perrin, A.; Selmi-Ruby, S.; Rousset, B. Characterization and semiquantitative analyses of pendrin expressed in normal and tumoral human thyroid tissues. J. Clin. Endocrinol. Metab. 2002, 87, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, D.; Sturlese, M.; Nies, F.; Kluge, M.; Bellanda, M.; Battistutta, R.; Oliver, D. Molecular architecture and the structural basis for anion interaction in prestin and SLC26 transporters. Nat. Commun. 2014, 5, 3622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geertsma, E.R.; Chang, Y.N.; Shaik, F.R.; Neldner, Y.; Pardon, E.; Steyaert, J.; Dutzler, R. Structure of a prokaryotic fumarate transporter reveals the architecture of the SLC26 family. Nat. Struct. Mol. Biol. 2015, 22, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Everett, L.A.; Morsli, H.; Wu, D.K.; Green, E.D. Expression pattern of the mouse ortholog of the Pendred’s syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc. Natl. Acad. Sci. USA 1999, 96, 9727–9732. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.M.; Hassell, K.A.; Royaux, I.E.; Green, E.D.; Chang, J.Y.; Shipley, G.L.; Verlander, J.W. Localization of pendrin in mouse kidney. Am. J. Physiol. Ren. Physiol. 2003, 284, F229–F241. [Google Scholar] [CrossRef]
- Dou, H.; Xu, J.; Wang, Z.; Smith, A.N.; Soleimani, M.; Karet, F.E.; Greinwald, J.H.; Choo, D. Co-expression of pendrin, vacuolar H+-ATPase α4-subunit and carbonic anhydrase II in epithelial cells of the murine endolymphatic sac. J. Histochem. Cytochem. 2004, 52, 1377–1384. [Google Scholar] [CrossRef]
- Nakaya, K.; Harbidge, D.G.; Wangemann, P.; Schultz, B.D.; Green, E.D.; Wall, S.M.; Marcus, D.C. Lack of pendrin HCO3− transport elevates vestibular endolymphatic Ca2+ by inhibition of acid-sensitive TRPV5 and TRPV6 channels. Am. J. Physiol. Ren. Physiol. 2007, 292, F1314–F1321. [Google Scholar] [CrossRef]
- Wangemann, P.; Nakaya, K.; Wu, T.; Maganti, R.J.; Itza, E.M.; Sanneman, J.D.; Harbidge, D.G.; Billings, S.; Marcus, D.C. Loss of cochlear HCO3− secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am. J. Physiol. Ren. Physiol. 2007, 292, F1345–F1353. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Hisatome, I.; Taniguchi, S.; Sasaki, N.; Yamamoto, Y.; Miake, J.; Fukui, H.; Shimizu, H.; Okamura, T.; Okura, T.; et al. Mechanism of iodide/chloride exchange by pendrin. Endocrinology 2004, 145, 4301–4308. [Google Scholar] [CrossRef] [PubMed]
- Kopp, P. Pendred’s syndrome and genetic defects in thyroid hormone synthesis. Rev. Endocr. Metab. Disord. 2000, 1, 109–221. [Google Scholar] [CrossRef]
- Royaux, I.E.; Wall, S.M.; Karniski, L.P.; Everett, L.A.; Suzuki, K.; Knepper, M.A.; Green, E.D. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc. Natl. Acad. Sci. USA 2001, 98, 4221–4226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlander, J.W.; Hassell, K.A.; Royaux, I.E.; Glapion, D.M.; Wang, M.E.; Everett, L.A.; Green, E.D.; Wall, S.M. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: Role of pendrin in mineralocorticoid-induced hypertension. Hypertension 2003, 42, 356–362. [Google Scholar] [CrossRef]
- Quentin, F.; Chambrey, R.; Trinh-Trang-Tan, M.M.; Fysekidis, M.; Cambillau, M.; Paillard, M.; Aronson, P.S.; Eladari, D. The Cl−/ HCO3− exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am. J. Physiol. Ren. Physiol. 2004, 287, F1179–F1188. [Google Scholar] [CrossRef] [PubMed]
- Vallet, M.; Picard, N.; Loffing-Cueni, D.; Fysekidis, M.; Bloch-Faure, M.; Deschênes, G.; Breton, S.; Meneton, P.; Loffing, J.; Aronson, P.S.; et al. Pendrin regulation in mouse kidney primarily is chloride-dependent. J. Am. Soc. Nephrol. 2006, 17, 2153–2163. [Google Scholar] [CrossRef]
- Di Valentin, E.; Crahay, C.; Garbacki, N.; Hennuy, B.; Guéders, M.; Noël, A.; Foidart, J.M.; Grooten, J.; Colige, A.; Piette, J.; et al. New asthma biomarkers: Lessons from murine models of acute and chronic asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L185–L197. [Google Scholar] [CrossRef]
- Kuperman, D.A.; Lewis, C.C.; Woodruff, P.G.; Rodriguez, M.W.; Yang, Y.H.; Dolganov, G.M.; Fahy, J.V.; Erle, D.J. Dissecting asthma using focused transgenic modeling and functional genomics. J. Allergy Clin. Immunol. 2005, 116, 305–311. [Google Scholar] [CrossRef]
- Nakagami, Y.; Favoreto, S.; Zhen, G.; Park, S.W.; Nguyenvu, L.T.; Kuperman, D.A.; Dolganov, G.M.; Huang, X.; Boushey, H.A.; Avila, P.C.; et al. The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J. Immunol. 2008, 181, 2203–2210. [Google Scholar] [CrossRef]
- Nakao, I.; Kanaji, S.; Ohta, S.; Matsushita, H.; Arima, K.; Yuyama, N.; Yamaya, M.; Nakayama, K.; Kubo, H.; Watanabe, M.; et al. Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J. Immunol. 2008, 180, 6262–6269. [Google Scholar] [CrossRef] [PubMed]
- Nofziger, C.; Vezzoli, V.; Dossena, S.; Schönherr, T.; Studnicka, J.; Nofziger, J.; Vanoni, S.; Stephan, S.; Silva, M.E.; Meyer, G.; et al. STAT6 links IL-4/IL-13 stimulation with pendrin expression in asthma and chronic obstructive pulmonary disease. Clin. Pharmacol. Ther. 2011, 90, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, N.; Caci, E.; Sondo, E.; Caputo, A.; Rhoden, K.; Pfeffer, U.; di Candia, M.; Bandettini, R.; Ravazzolo, R.; Zegarra-Moran, O.; et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: Role of pendrin and anion channels. J. Immunol. 2007, 178, 5144–5153. [Google Scholar] [CrossRef] [PubMed]
- Rillema, J.A.; Hill, M.A. Pendrin transporter carries out iodide uptake into MCF-7 human mammary cancer cells. Exp. Biol. Med. (Maywood) 2003, 228, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, L.; Mian, C.; Caillou, B.; Talbot, M.; Filetti, S.; Schlumberger, M.; Bidart, J.M. Na+/I− symporter and Pendred syndrome gene and protein expressions in human extra-thyroidal tissues. Eur. J. Endocrinol. 2001, 144, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Bidart, J.M.; Lacroix, L.; Evain-Brion, D.; Caillou, B.; Lazar, V.; Frydman, R.; Bellet, D.; Filetti, S.; Schlumberger, M. Expression of Na+/I− symporter and Pendred syndrome genes in trophoblast cells. J. Clin. Endocrinol. Metab. 2000, 85, 4367–4372. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Royaux, I.E.; Everett, L.A.; Mori-Aoki, A.; Suzuki, S.; Nakamura, K.; Sakai, T.; Katoh, R.; Toda, S.; Green, E.D.; et al. Expression of PDS/Pds, the Pendred syndrome gene, in endometrium. J. Clin. Endocrinol. Metab. 2002, 87, 938. [Google Scholar] [CrossRef] [PubMed]
- Alesutan, I.; Daryadel, A.; Mohebbi, N.; Pelzl, L.; Leibrock, C.; Voelkl, J.; Bourgeois, S.; Dossena, S.; Nofziger, C.; Paulmichl, M.; et al. Impact of bicarbonate, ammonium chloride, and acetazolamide on hepatic and renal SLC26A4 expression. Cell. Physiol. Biochem. 2011, 28, 553–558. [Google Scholar] [CrossRef]
- Zuo, L.; Fulkerson, P.C.; Finkelman, F.D.; Mingler, M.; Fischetti, C.A.; Blanchard, C.; Rothenberg, M.E. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R α2-inhibited pathway. J. Immunol. 2010, 185, 660–669. [Google Scholar] [CrossRef]
- Adams, K.M.; Abraham, V.; Spielman, D.; Kolls, J.K.; Rubenstein, R.C.; Conner, G.E.; Cohen, N.A.; Kreindler, J.L. IL-17A induces Pendrin expression and chloride-bicarbonate exchange in human bronchial epithelial cells. PLoS ONE 2014, 9, e103263. [Google Scholar] [CrossRef]
- Corren, J. Asthma phenotypes and endotypes: An evolving paradigm for classification. Discov. Med. 2013, 15, 243–249. [Google Scholar] [PubMed]
- Maddox, L.; Schwartz, D.A. The pathophysiology of asthma. Annu. Rev. Med. 2002, 53, 477–498. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Mediators of chronic obstructive pulmonary disease. Pharmacol. Rev. 2004, 56, 515–548. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Jones, C.E. Regulation of mucin expression in respiratory diseases. Biochem. Soc. Trans. 2009, 37, 877–881. [Google Scholar] [CrossRef]
- Lappalainen, U.; Whitsett, J.A.; Wert, S.E.; Tichelaar, J.W.; Bry, K. Interleukin-1β causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell Mol. Biol. 2005, 32, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.; Mingler, M.K.; Vicario, M.; Abonia, J.P.; Wu, Y.Y.; Lu, T.X.; Collins, M.H.; Putnam, P.E.; Wells, S.I.; Rothenberg, M.E. IL-13 involvement in eosinophilic esophagitis: Transcriptome analysis and reversibility with glucocorticoids. J. Allergy Clin. Immunol. 2007, 120, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Hogan, S.P.; Brandt, E.B.; Rothenberg, M.E. IL-5 promotes eosinophil trafficking to the esophagus. J. Immunol. 2002, 168, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.L.; Weinberg, A.D.; English, M.; Huston, G. IL-4 directs the development of Th2-like helper effectors. J. Immunol. 1990, 145, 3796–3806. [Google Scholar]
- Voehringer, D.; Shinkai, K.; Locksley, R.M. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity 2004, 20, 267–277. [Google Scholar] [CrossRef]
- Zhong, W.; Su, W.; Zhang, Y.; Liu, Q.; Wu, J.; Di, C.; Zhang, Z.; Xia, Z. Basophils as a primary inducer of the T helper type 2 immunity in ovalbumin-induced allergic airway inflammation. Immunology 2014, 142, 202–215. [Google Scholar] [CrossRef] [Green Version]
- Gessner, A.; Mohrs, K.; Mohrs, M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J. Immunol. 2005, 174, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Pathogenesis of asthma. Clin. Exp. Allergy 2008, 38, 872–897. [Google Scholar] [CrossRef] [PubMed]
- Rael, E.L.; Lockey, R.F. Interleukin-13 signaling and its role in asthma. World Allergy Organ. J. 2011, 4, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Takeshita, T.; Ishii, N.; Nakamura, M.; Watanabe, S.; Arai, K.; Sugamura, K. Sharing of the interleukin-2 (IL-2) receptor γ chain between receptors for IL-2 and IL-4. Science 1993, 262, 1874–1877. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.J.; Noguchi, M.; Russell, S.M. Sharing of a common γ chain, γ c, by the IL-2, IL-4, and IL-7 receptors: Implications for X-linked severe combined immunodeficiency (XSCID). Adv. Exp. Med. Biol. 1994, 365, 225–232. [Google Scholar] [PubMed]
- Russell, S.M.; Keegan, A.D.; Harada, N.; Nakamura, Y.; Noguchi, M.; Leland, P.; Friedmann, M.C.; Miyajima, A.; Puri, R.K.; Paul, W.E. Interleukin-2 receptor γ chain: A functional component of the interleukin-4 receptor. Science 1993, 262, 1880–1883. [Google Scholar] [CrossRef] [PubMed]
- Zurawski, S.M.; Vega, F.; Huyghe, B.; Zurawski, G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 1993, 12, 2663–2670. [Google Scholar] [CrossRef]
- Andrews, A.L.; Holloway, J.W.; Puddicombe, S.M.; Holgate, S.T.; Davies, D.E. Kinetic analysis of the interleukin-13 receptor complex. J. Biol. Chem. 2002, 277, 46073–46078. [Google Scholar] [CrossRef]
- Takeda, K.; Tanaka, T.; Shi, W.; Matsumoto, M.; Minami, M.; Kashiwamura, S.; Nakanishi, K.; Yoshida, N.; Kishimoto, T.; Akira, S. Essential role of Stat6 in IL-4 signalling. Nature 1996, 380, 627–630. [Google Scholar] [CrossRef]
- Hou, J.; Schindler, U.; Henzel, W.J.; Ho, T.C.; Brasseur, M.; McKnight, S.L. An interleukin-4-induced transcription factor: IL-4 Stat. Science 1994, 265, 1701–1706. [Google Scholar] [CrossRef]
- Schindler, U.; Wu, P.; Rothe, M.; Brasseur, M.; McKnight, S.L. Components of a Stat recognition code: Evidence for two layers of molecular selectivity. Immunity 1995, 2, 689–697. [Google Scholar] [CrossRef]
- Kotanides, H.; Reich, N.C. Requirement of tyrosine phosphorylation for rapid activation of a DNA binding factor by IL-4. Science 1993, 262, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Ehret, G.B.; Reichenbach, P.; Schindler, U.; Horvath, C.M.; Fritz, S.; Nabholz, M.; Bucher, P. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J. Biol. Chem. 2001, 276, 6675–6688. [Google Scholar] [CrossRef] [PubMed]
- Seidel, H.M.; Milocco, L.H.; Lamb, P.; Darnell, J.E.; Stein, R.B.; Rosen, J. Spacing of palindromic half sites as a determinant of selective STAT (signal transducers and activators of transcription) DNA binding and transcriptional activity. Proc. Natl. Acad. Sci. USA 1995, 92, 3041–3045. [Google Scholar] [CrossRef] [PubMed]
- Sehra, S.; Bruns, H.A.; Ahyi, A.N.; Nguyen, E.T.; Schmidt, N.W.; Michels, E.G.; von Bülow, G.U.; Kaplan, M.H. IL-4 is a critical determinant in the generation of allergic inflammation initiated by a constitutively active Stat6. J. Immunol. 2008, 180, 3551–3559. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, D.; Schofield, B.; Wills-Karp, M.; Grusby, M.J. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J. Exp. Med. 1998, 187, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Tsuji, T.; Matsuzaki, J.; Jinushi, T.; Ashino, S.; Teramura, T.; Chamoto, K.; Tanaka, Y.; Asakura, Y.; Sakurai, T.; et al. STAT6-mediated signaling in Th2-dependent allergic asthma: Critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. Int. Immunol. 2004, 16, 1497–1505. [Google Scholar] [CrossRef]
- Shen, C.H.; Stavnezer, J. Interaction of stat6 and NF-κB: Direct association and synergistic activation of interleukin-4-induced transcription. Mol. Cell. Biol. 1998, 18, 3395–3404. [Google Scholar] [CrossRef]
- Gray, M.J.; Poljakovic, M.; Kepka-Lenhart, D.; Morris, S.M. Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPβ. Gene 2005, 353, 98–106. [Google Scholar] [CrossRef]
- McDonald, C.; Reich, N.C. Cooperation of the transcriptional coactivators CBP and p300 with Stat6. J. Interferon Cytokine Res. 1999, 19, 711–722. [Google Scholar] [CrossRef]
- Litterst, C.M.; Pfitzner, E. An LXXLL motif in the transactivation domain of STAT6 mediates recruitment of NCoA-1/SRC-1. J. Biol. Chem. 2002, 277, 36052–36060. [Google Scholar] [CrossRef]
- Yao, Z.; Painter, S.L.; Fanslow, W.C.; Ulrich, D.; Macduff, B.M.; Spriggs, M.K.; Armitage, R.J. Human IL-17: A novel cytokine derived from T cells. J. Immunol. 1995, 155, 5483–5486. [Google Scholar] [PubMed]
- Fossiez, F.; Djossou, O.; Chomarat, P.; Flores-Romo, L.; Ait-Yahia, S.; Maat, C.; Pin, J.J.; Garrone, P.; Garcia, E.; Saeland, S.; et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996, 183, 2593–2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awane, M.; Andres, P.G.; Li, D.J.; Reinecker, H.C. NF-κB-inducing kinase is a common mediator of IL-17-, TNF-α-, and IL-1 β-induced chemokine promoter activation in intestinal epithelial cells. J. Immunol. 1999, 162, 5337–5344. [Google Scholar] [PubMed]
- Chen, Y.; Thai, P.; Zhao, Y.H.; Ho, Y.S.; DeSouza, M.M.; Wu, R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 2003, 278, 17036–17043. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, M.J.; Wong, G.C.; Liu, X.K.; Yamamoto, H.; Kasayama, S.; Kirkwood, K.L.; Gaffen, S.L. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 2004, 279, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Qian, Y. IL-17/IL-17 receptor system in autoimmune disease: Mechanisms and therapeutic potential. Clin. Sci. (Lond.) 2012, 122, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Kao, C.Y.; Wachi, S.; Thai, P.; Ryu, J.; Wu, R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-κB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007, 179, 6504–6513. [Google Scholar] [CrossRef]
- Saleh, A.; Shan, L.; Halayko, A.J.; Kung, S.; Gounni, A.S. Critical role for STAT3 in IL-17A-mediated CCL11 expression in human airway smooth muscle cells. J. Immunol. 2009, 182, 3357–3365. [Google Scholar] [CrossRef]
- Hartupee, J.; Liu, C.; Novotny, M.; Sun, D.; Li, X.; Hamilton, T.A. IL-17 signaling for mRNA stabilization does not require TNF receptor-associated factor 6. J. Immunol. 2009, 182, 1660–1666. [Google Scholar] [CrossRef]
- Sun, D.; Novotny, M.; Bulek, K.; Liu, C.; Li, X.; Hamilton, T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nat. Immunol. 2011, 12, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louten, J.; Boniface, K.; de Waal Malefyt, R. Development and function of TH17 cells in health and disease. J. Allergy Clin. Immunol. 2009, 123, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Molet, S.; Hamid, Q.; Davoine, F.; Nutku, E.; Taha, R.; Pagé, N.; Olivenstein, R.; Elias, J.; Chakir, J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001, 108, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003, 170, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Starnes, T.; Robertson, M.J.; Sledge, G.; Kelich, S.; Nakshatri, H.; Broxmeyer, H.E.; Hromas, R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J. Immunol. 2001, 167, 4137–4140. [Google Scholar] [CrossRef] [PubMed]
- Vykhovanets, E.V.; Maclennan, G.T.; Vykhovanets, O.V.; Gupta, S. IL-17 Expression by macrophages is associated with proliferative inflammatory atrophy lesions in prostate cancer patients. Int. J. Clin. Exp. Pathol. 2011, 4, 552–565. [Google Scholar] [PubMed]
- Pavlov, O.; Selutin, A.; Pavlova, O.; Selkov, S. Macrophages are a source of IL-17 in the human placenta. Am. J. Reprod. Immunol. 2018, 80, e13016. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.L.; Keller, A.C.; Paget, C.; Fujio, M.; Trottein, F.; Savage, P.B.; Wong, C.H.; Schneider, E.; Dy, M.; Leite-de-Moraes, M.C. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007, 204, 995–1001. [Google Scholar] [CrossRef]
- Singh, S.P.; Zhang, H.H.; Foley, J.F.; Hedrick, M.N.; Farber, J.M. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J. Immunol. 2008, 180, 214–221. [Google Scholar] [CrossRef]
- Wakita, D.; Sumida, K.; Iwakura, Y.; Nishikawa, H.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Nishimura, T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 2010, 40, 1927–1937. [Google Scholar] [CrossRef]
- Makihara, S.; Okano, M.; Fujiwara, T.; Kariya, S.; Noda, Y.; Higaki, T.; Nishizaki, K. Regulation and characterization of IL-17A expression in patients with chronic rhinosinusitis and its relationship with eosinophilic inflammation. J. Allergy Clin. Immunol. 2010, 126, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Halwani, R.; Al-Muhsen, S.; Hamid, Q. T helper 17 cells in airway diseases: From laboratory bench to bedside. Chest 2013, 143, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [PubMed]
- Chung, K.F. Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2001, 34, 50s–59s. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, P.K. Comparison of the structural and inflammatory features of COPD and asthma. Giles, F. Filley Lecture. Chest 2000, 117 (Suppl 1.), 251S–260S. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) pathway. Sci. Signal 2010, 3, cm1. [Google Scholar] [CrossRef]
- Lewis, C.C.; Yang, J.Y.; Huang, X.; Banerjee, S.K.; Blackburn, M.R.; Baluk, P.; McDonald, D.M.; Blackwell, T.S.; Nagabhushanam, V.; Peters, W.; et al. Disease-specific gene expression profiling in multiple models of lung disease. Am. J. Respir. Crit. Care Med. 2008, 177, 376–387. [Google Scholar] [CrossRef]
- Ishida, A.; Ohta, N.; Suzuki, Y.; Kakehata, S.; Okubo, K.; Ikeda, H.; Shiraishi, H.; Izuhara, K. Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol. Int. 2012, 61, 589–595. [Google Scholar] [CrossRef]
- Yick, C.Y.; Zwinderman, A.H.; Kunst, P.W.; Grünberg, K.; Mauad, T.; Dijkhuis, A.; Bel, E.H.; Baas, F.; Lutter, R.; Sterk, P.J. Transcriptome sequencing (RNA-Seq) of human endobronchial biopsies: Asthma versus controls. Eur. Respir. J. 2013, 42, 662–670. [Google Scholar] [CrossRef]
- Clarke, L.A.; Sousa, L.; Barreto, C.; Amaral, M.D. Changes in transcriptome of native nasal epithelium expressing F508del-CFTR and intersecting data from comparable studies. Respir. Res. 2013, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Lu, X.; Purkey, M.R.; Homma, T.; Choi, A.W.; Carter, R.; Suh, L.; Norton, J.; Harris, K.E.; Conley, D.B.; et al. Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2015, 136, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, K.M.; Gau, Y.; Zhu, J.; Skerry, C.; Wall, S.M.; Soleimani, M.; Carbonetti, N.H. Epithelial anion transporter pendrin contributes to inflammatory lung pathology in mouse models of Bordetella pertussis infection. Infect. Immun. 2014, 82, 4212–4221. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.F.; Ostertag, P.; Pfrogner, E.; Rasp, G. Nasal interleukin-5, immunoglobulin E, eosinophilic cationic protein, and soluble intercellular adhesion molecule-1 in chronic sinusitis, allergic rhinitis, and nasal polyposis. Laryngoscope 2000, 110, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, E.O.; Hamilos, D.L.; Hadley, J.A.; Lanza, D.C.; Marple, B.F.; Nicklas, R.A.; Bachert, C.; Baraniuk, J.; Baroody, F.M.; Benninger, M.S.; et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. Otolaryngol. Head Neck Surg. 2004, 131, S1–S62. [Google Scholar] [CrossRef] [PubMed]
- Nofziger, C.; Dossena, S.; Suzuki, S.; Izuhara, K.; Paulmichl, M. Pendrin function in airway epithelia. Cell. Physiol. Biochem. 2011, 28, 571–578. [Google Scholar] [CrossRef]
- Vanoni, S.; Nofziger, C.; Dossena, S.; Soyal, S.M.; Patsch, W.; Plevani, P.; Duschl, A.; Paulmichl, M. The human pendrin promoter contains two N(4) GAS motifs with different functional relevance. Cell. Physiol. Biochem. 2013, 32, 238–248. [Google Scholar] [CrossRef]
- Lewis, J.D.; Meehan, R.R.; Henzel, W.J.; Maurer-Fogy, I.; Jeppesen, P.; Klein, F.; Bird, A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 1992, 69, 905–914. [Google Scholar] [CrossRef]
- Robertson, K.D.; Wolffe, A.P. DNA methylation in health and disease. Nat. Rev. Genet. 2000, 1, 11–19. [Google Scholar] [CrossRef]
- Kaludov, N.K.; Wolffe, A.P. MeCP2 driven transcriptional repression in vitro: Selectivity for methylated DNA, action at a distance and contacts with the basal transcription machinery. Nucleic Acids Res. 2000, 28, 1921–1928. [Google Scholar] [CrossRef]
- Nan, X.; Ng, H.H.; Johnson, C.A.; Laherty, C.D.; Turner, B.M.; Eisenman, R.N.; Bird, A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 1998, 393, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.L.; Veenstra, G.J.; Wade, P.A.; Vermaak, D.; Kass, S.U.; Landsberger, N.; Strouboulis, J.; Wolffe, A.P. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 1998, 19, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Nofziger, C.; Dossena, S.; Vanoni, S.; Diasio, R.M. Methylation of the human pendrin promoter. Cell. Physiol. Biochem. 2011, 28, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Scantamburlo, G.; Vanoni, S.; Dossena, S.; Soyal, S.M.; Bernardinelli, E.; Civello, D.A.; Patsch, W.; Paulmichl, M.C. Interleukin-4 Induces CpG Site-Specific Demethylation of the Pendrin Promoter in Primary Human Bronchial Epithelial Cells. Cell. Physiol. Biochem. 2017, 41, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Vento-Tormo, R.; Company, C.; Rodríguez-Ubreva, J.; de la Rica, L.; Urquiza, J.M.; Javierre, B.M.; Sabarinathan, R.; Luque, A.; Esteller, M.; Aran, J.M.; et al. IL-4 orchestrates STAT6-mediated DNA demethylation leading to dendritic cell differentiation. Genome Biol. 2016, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Connelly, C.E.; Sun, Y.; Carbonetti, N.H. Pertussis toxin exacerbates and prolongs airway inflammatory responses during Bordetella pertussis infection. Infect. Immun. 2012, 80, 4317–4332. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.; Powell, D.A.; Carbonetti, N.H. Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS ONE 2009, 4, e7079. [Google Scholar] [CrossRef]
- Kreindler, J.L.; Bertrand, C.A.; Lee, R.J.; Karasic, T.; Aujla, S.; Pilewski, J.M.; Frizzell, R.A.; Kolls, J.K. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 296, L257–L266. [Google Scholar] [CrossRef] [Green Version]
- Lindén, A.; Dahlén, B. Interleukin-17 cytokine signalling in patients with asthma. Eur. Respir. J. 2014, 44, 1319–1331. [Google Scholar] [CrossRef] [Green Version]
- Caramori, G.; Adcock, I.M.; di Stefano, A.; Chung, K.F. Cytokine inhibition in the treatment of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 397–412. [Google Scholar] [Green Version]
- Bel, E.H.; Ten Brinke, A. New Anti-Eosinophil Drugs for Asthma and COPD: Targeting the Trait! Chest 2017, 152, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Hogmalm, A.; Bry, M.; Strandvik, B.K. IL-1β expression in the distal lung epithelium disrupts lung morphogenesis and epithelial cell differentiation in fetal mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L23–L34. [Google Scholar] [CrossRef]
- Minty, A.; Chalon, P.; Derocq, J.M.; Dumont, X.; Guillemot, J.C.; Kaghad, M.; Labit, C.; Leplatois, P.; Liauzun, P.B. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature 1993, 362, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Izuhara, K.; Suzuki, S.; Nofziger, C.; Ogawa, M.; Ohta, S.; Nanri, Y.; Mitamura, Y.; Yoshihara, T.; Pedemonte, N.; Galietta, L.J.V.; et al. The role of pendrin in the airways: Links with asthma and COPD. In The Role of Pendrin in Health and Disease; Springer: Cham, Switzerland, 2017; pp. 141–154. [Google Scholar]
- Zünd, G.; Madara, J.L.; Dzus, A.L.; Awtrey, C.S.; Colgan, S.P. Interleukin-4 and interleukin-13 differentially regulate epithelial chloride secretion. J. Biol. Chem. 1996, 271, 7460–7464. [Google Scholar] [CrossRef] [PubMed]
- Danahay, H.; Atherton, H.; Jones, G.; Bridges, R.J.; Poll, C.T. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L226–L236. [Google Scholar] [CrossRef]
- Galietta, L.J.; Pagesy, P.; Folli, C.; Caci, E.; Romio, L.; Costes, B.; Nicolis, E.; Cabrini, G.; Goossens, M.R.; et al. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J. Immunol. 2002, 168, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dong, Q.; Louahed, J.; Dragwa, C.; Savio, D.; Huang, M.; Weiss, C.; Tomer, Y.; McLane, M.P.; Nicolaides, N.C.; et al. Characterization of a calcium-activated chloride channel as a shared target of Th2 cytokine pathways and its potential involvement in asthma. Am. J. Respir. Cell Mol. Biol. 2001, 25, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Garnett, J.P.; Hickman, E.; Burrows, R.; Hegyi, P.; Tiszlavicz, L.; Cuthbert, A.W.; Fong, P.; Gray, M.A. Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J. Biol. Chem. 2011, 286, 41069–41082. [Google Scholar] [CrossRef]
- Klocke, R.A. Catalysis of CO2 reactions by lung carbonic anhydrase. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 882–888. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoo, J.E.; Namkung, W.; Cho, H.J.; Kim, K.; Kang, J.W.; Yoon, J.H.; Choi, J.Y. Thick airway surface liquid volume and weak mucin expression in pendrin-deficient human airway epithelia. Physiol. Rep. 2015, 3, e12480. [Google Scholar] [CrossRef] [Green Version]
- Boucher, R.C. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu. Rev. Med. 2007, 58, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.F. Mucus hypersecretion in chronic obstructive pulmonary disease. Novartis Found. Symp. 2001, 234, 65–77. [Google Scholar] [PubMed]
- Rogers, D.F. Airway mucus hypersecretion in asthma: An undervalued pathology? Curr. Opin. Pharmacol. 2004, 4, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.C.; Voynow, J.A. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 2006, 86, 245–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Kim, K.E.; Yoon, J.H.; Song, K.S. Upregulation of MUC5AC gene expression by IL-4 through CREB in human airway epithelial cells. J. Cell. Biochem. 2009, 108, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz-Scroggins, M.E.; Boushey, H.A.; Finkbeiner, W.E.; Widdicombe, J.H. Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 2010, 43, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Roger, J.; Fitau, J.; Combe, D.; Giddings, J.; Heeke, G.V.; Jones, C.E. Goblet cells are derived from a FOXJ1-expressing progenitor in a human airway epithelium. Am. J. Respir Cell Mol. Biol. 2011, 44, 276–284. [Google Scholar] [CrossRef]
- Garcia, M.A.; Yang, N.; Quinton, P.M. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J. Clin. Investig. 2009, 119, 2613–2622. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Ermund, A.; Ambort, D.; Johansson, M.E.; Nilsson, H.E.; Thorell, K.; Hebert, H.; Sjövall, H.; Hansson, G.C. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 2012, 209, 1263–1272. [Google Scholar] [CrossRef] [Green Version]
- Hoegger, M.J.; Fischer, A.J.; McMenimen, J.D.; Ostedgaard, L.S.; Tucker, A.J.; Awadalla, M.A.; Moninger, T.O.; Michalski, A.S.; Hoffman, E.A.; Welsh, M.J.; et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 2014, 345, 818–822. [Google Scholar] [CrossRef] [Green Version]
- Gorrieri, G.; Scudieri, P.; Caci, E.; Schiavon, M.; Tomati, V.; Sirci, F.; Napolitano, F.; Carrella, D.; Gianotti, A.; Musante, I.; et al. Goblet Cell Hyperplasia Requires High Bicarbonate Transport to Support Mucin Release. Sci. Rep. 2016, 6, 36016. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Kusunoki, T.; Ikeda, K. Relationships between IL-17A and macrophages or MUC5AC in eosinophilic chronic rhinosinusitis and proposed pathological significance. Allergy Rhinol. (Provid.) 2012, 3, e50–e54. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ikeda, S.; Asamori, T.; Honda, K.; Kawashima, Y.; Kitamura, K.; Suzuki, K.; Tsutsumi, T. Increased expression of pendrin in eosinophilic chronic rhinosinusitis with nasal polyps. Braz. J. Otorhinolaryngol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ratner, A.J.; Prince, A. Lactoperoxidase. New recognition of an “old” enzyme in airway defenses. Am. J. Respir. Cell Mol. Biol. 2000, 22, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J.; Hawkins, C.L. Hypothiocyanous acid: Benign or deadly? Chem. Res. Toxicol. 2012, 25, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Izuhara, K.; Suzuki, S.; Ogawa, M.; Nunomura, S.; Nanri, Y.; Mitamura, Y.; Yoshihara, T. The Significance of Hypothiocyanite Production via the Pendrin/DUOX/Peroxidase Pathway in the Pathogenesis of Asthma. Oxid. Med. Cell. Longev. 2017, 2017, 1054801. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, M.A.; Fernandez, V.; Forteza, R.; Randell, S.H.; Salathe, M.; Conner, G.E. Transcellular thiocyanate transport by human airway epithelia. J. Physiol. 2004, 561, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Moskwa, P.; Lorentzen, D.; Excoffon, K.J.; Zabner, J.; McCray, P.B.; Nauseef, W.M.; Dupuy, C.; Bánfi, B. A novel host defense system of airways is defective in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007, 175, 174–183. [Google Scholar] [CrossRef]
- Harper, R.W.; Xu, C.; Eiserich, J.P.; Chen, Y.; Kao, C.Y.; Thai, P.; Setiadi, H.; Wu, R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 2005, 579, 4911–4917. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Ogawa, M.; Ohta, S.; Nunomura, S.; Nanri, Y.; Shiraishi, H.; Mitamura, Y.; Yoshihara, T.; Lee, J.J.; Izuhara, K. Induction of Airway Allergic Inflammation by Hypothiocyanite via Epithelial Cells. J. Biol. Chem. 2016, 291, 27219–27227. [Google Scholar] [CrossRef]
- Suzuki, S.; Ogawa, M.; Ohta, S.; Arima, K.; Nunomura, S.; Nanri, Y.; Mitamura, Y.; Yoshihara, T.; Nakamura, Y.; Yamauchi, K.; et al. The potential for repositioning antithyroid agents as antiasthma drugs. J. Allergy Clin. Immunol. 2016, 138, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Liacouras, C.A.; Ruchelli, E. Eosinophilic esophagitis. Curr. Opin. Pediatr. 2004, 16, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Hogan, S.P.; Brandt, E.B.; Rothenberg, M.E. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J. Clin. Investig. 2001, 107, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Rothenberg, M.E. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology 2003, 125, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Tobey, N.A.; Reddy, S.P.; Khalbuss, W.E.; Silvers, S.M.; Cragoe, E.J.; Orlando, R.C. Na+-dependent and -independent Cl−/HCO3− exchangers in cultured rabbit esophageal epithelial cells. Gastroenterology 1993, 104, 185–195. [Google Scholar] [CrossRef]
- Tobey, N.A.; Koves, G.; Orlando, R.C. Human esophageal epithelial cells possess an Na+/H+ exchanger for H+ extrusion. Am. J. Gastroenterol. 1998, 93, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Shallat, S.; Schmidt, L.; Reaka, A.; Rao, D.; Chang, E.B.; Rao, M.C.; Ramaswamy, K.; Layden, T.J. NHE-1 isoform of the Na+/H+ antiport is expressed in the rat and rabbit esophagus. Gastroenterology 1995, 109, 1421–1428. [Google Scholar] [CrossRef]
- Layden, T.J.; Schmidt, L.; Agnone, L.; Lisitza, P.; Brewer, J.; Goldstein, J.L. Rabbit esophageal cell cytoplasmic pH regulation: Role of Na+-H+ antiport and Na+-dependent HCO3− transport systems. Am. J. Physiol. 1992, 263, G407–G413. [Google Scholar] [CrossRef]
- Schreiber, R. 22+ signaling, intracellular pH and cell volume in cell proliferation. J. Membr. Biol. 2005, 205, 129–137. [Google Scholar] [CrossRef]
- Shrode, L.D.; Tapper, H.; Grinstein, S. Role of intracellular pH in proliferation, transformation, and apoptosis. J. Bioenerg. Biomembr. 1997, 29, 393–399. [Google Scholar] [CrossRef]
- Zeng, C.; Vanoni, S.; Wu, D.; Caldwell, J.M.; Wheeler, J.C.; Arora, K.; Noah, T.K.; Waggoner, L.; Besse, J.A.; Yamani, A.N.; et al. Solute carrier family 9, subfamily A, member 3 (SLC9A3)/sodium-hydrogen exchanger member 3 (NHE3) dysregulation and dilated intercellular spaces in patients with eosinophilic esophagitis. J. Allergy Clin. Immunol. 2018, 142, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Sodikoff, J.; Hirano, I. Therapeutic strategies in eosinophilic esophagitis: Induction, maintenance and refractory disease. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 829–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holgate, S.T. Pathophysiology of asthma: What has our current understanding taught us about new therapeutic approaches? J. Allergy Clin. Immunol. 2011, 128, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Lemanske, R.F.; Hanania, N.A.; Korenblat, P.E.; Parsey, M.V.; Arron, J.R.; Harris, J.M.; Scheerens, H.; Wu, L.C.; Su, Z.; et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011, 365, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Wilbraham, D.; Fuller, R.; Getz, E.B.; Longphre, M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: Results of two phase 2a studies. Lancet 2007, 370, 1422–1431. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Wen, T.; Greenberg, A.; Alpan, O.; Enav, B.; Hirano, I.; Nadeau, K.; Kaiser, S.; Peters, T.; Perez, A.; et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2015, 135, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Vitor, A.B.; Trindade, E.; Lima, R.; Tavares, M.; Lopes, J.; Dias, J.A. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. Eur. J. Pediatr. 2011, 170, 1471–1474. [Google Scholar] [CrossRef]
- Tieu, D.D.; Peters, A.T.; Carter, R.G.; Carter, R.T.; Suh, L.; Conley, D.B.; Chandra, R.; Norton, J.; Grammer, L.C.; Harris, K.E.; et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2010, 125, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Rochman, M.; Azouz, N.P.; Rothenberg, M.E. Epithelial origin of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2018, 142, 10–23. [Google Scholar] [CrossRef]
- Holgate, S.T. Epithelium dysfunction in asthma. J. Allergy Clin. Immunol. 2007, 120, 1233–1244. [Google Scholar] [CrossRef]
- Simon, D.; Radonjic-Hösli, S.; Straumann, A.; Yousefi, S.; Simon, H.U. Active eosinophilic esophagitis is characterized by epithelial barrier defects and eosinophil extracellular trap formation. Allergy 2015, 70, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Madeo, A.C.; Manichaikul, A.; Pryor, S.P.; Griffith, A.J. Do mutations of the Pendred syndrome gene, SLC26A4, confer resistance to asthma and hypertension? J. Med. Genet. 2009, 46, 405–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumai, Y.; Eladari, D. An emerging role of pendrin in health and disease. Physiol. Rep. 2015, 3, e12503. [Google Scholar] [CrossRef] [PubMed]
- Haggie, P.M.; Phuan, P.W.; Tan, J.A.; Zlock, L.; Finkbeiner, W.E.; Verkman, A.S. Inhibitors of pendrin anion exchange identified in a small molecule screen increase airway surface liquid volume in cystic fibrosis. FASEB J. 2016, 30, 2187–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardinelli, E.; Costa, R.; Nofziger, C.; Paulmichl, M.; Dossena, S. Effect of Known Inhibitors of Ion Transport on Pendrin (SLC26A4) Activity in a Human Kidney Cell Line. Cell. Physiol. Biochem. 2016, 38, 1984–1998. [Google Scholar] [CrossRef] [PubMed]
- Dossena, S.; Vezzoli, V.; Cerutti, N.; Bazzini, C.; Tosco, M.; Sironi, C.; Rodighiero, S.; Meyer, G.; Fascio, U.; Fürst, J.; et al. Functional characterization of wild-type and a mutated form of SLC26A4 identified in a patient with Pendred syndrome. Cell. Physiol. Biochem. 2006, 17, 245–256. [Google Scholar] [CrossRef]
- Nakano, T.; Vezzoli, V.; Cerutti, N.; Bazzini, C.; Tosco, M.; Sironi, C.; Rodighiero, S.; Meyer, G.; Fascio, U.; Fürst, J. Niflumic acid suppresses interleukin-13-induced asthma phenotypes. Am. J. Respir. Crit. Care Med. 2006, 173, 1216–1221. [Google Scholar] [CrossRef]
- Kondo, M.; Nakata, J.; Arai, N.; Izumo, T.; Tagaya, E.; Takeyama, K.; Tamaoki, J.; Nagai, A. Niflumic acid inhibits goblet cell degranulation in a guinea pig asthma model. Allergol. Int. 2012, 61, 133–142. [Google Scholar] [CrossRef]
- Wylie, G.; Appelboom, T.; Bolten, W.; Breedveld, F.C.; Feely, J.; Leeming, M.R.; Le Loët, X.; Manthorpe, R.; Marcolongo, R.; Smolen, J. A comparative study of tenidap, a cytokine-modulating anti-rheumatic drug, and diclofenac in rheumatoid arthritis: A 24-week analysis of a 1-year clinical trial. Br. J. Rheumatol. 1995, 34, 554–563. [Google Scholar] [CrossRef]
- Ayral, X.; Mackillop, N.; Genant, H.K.; Kirkpatrick, J.; Beaulieu, A.; Pippingskiöld, P.; Will, R.K.; Alava, S.; Dougados, M. Arthroscopic evaluation of potential structure-modifying drug in osteoarthritis of the knee. A multicenter, randomized, double-blind comparison of tenidap sodium vs piroxicam. Osteoarthr. Cartil. 2003, 11, 198–207. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanoni, S.; Scantamburlo, G.; Dossena, S.; Paulmichl, M.; Nofziger, C. Interleukin-Mediated Pendrin Transcriptional Regulation in Airway and Esophageal Epithelia. Int. J. Mol. Sci. 2019, 20, 731. https://doi.org/10.3390/ijms20030731
Vanoni S, Scantamburlo G, Dossena S, Paulmichl M, Nofziger C. Interleukin-Mediated Pendrin Transcriptional Regulation in Airway and Esophageal Epithelia. International Journal of Molecular Sciences. 2019; 20(3):731. https://doi.org/10.3390/ijms20030731
Chicago/Turabian StyleVanoni, Simone, Giada Scantamburlo, Silvia Dossena, Markus Paulmichl, and Charity Nofziger. 2019. "Interleukin-Mediated Pendrin Transcriptional Regulation in Airway and Esophageal Epithelia" International Journal of Molecular Sciences 20, no. 3: 731. https://doi.org/10.3390/ijms20030731
APA StyleVanoni, S., Scantamburlo, G., Dossena, S., Paulmichl, M., & Nofziger, C. (2019). Interleukin-Mediated Pendrin Transcriptional Regulation in Airway and Esophageal Epithelia. International Journal of Molecular Sciences, 20(3), 731. https://doi.org/10.3390/ijms20030731





