Abstract
Vitamin E was proposed as treatment for Alzheimer’s disease many years ago. However, the effectiveness of the drug is not clear. Vitamin E is an antioxidant and neuroprotector and it has anti-inflammatory and hypocholesterolemic properties, driving to its importance for brain health. Moreover, the levels of vitamin E in Alzheimer’s disease patients are lower than in non-demented controls. Thus, vitamin E could be a good candidate to have beneficial effects against Alzheimer’s. However, evidence is consistent with a limited effectiveness of vitamin E in slowing progression of dementia; the information is mixed and inconclusive. The question is why does vitamin E fail to treat Alzheimer’s disease? In this paper we review the studies with and without positive results in Alzheimer’s disease and we discuss the reasons why vitamin E as treatment sometimes has positive results on cognition but at others, it does not.
1. Alzheimer’s Disease and the Hypothesis of Its Onset
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by a long evolution whose clinical symptoms appear late in life. However, in the last years the paradigm of AD has changed. In the past, researchers thought AD was an age-related disorder which begins during the aging process. Today we know that the onset of the disease occurs between 15 (for the genetic) and 20–30 years (for the sporadic) before any clinical symptom appears [1]. There is no a preventive or curative therapy for the disease and the lack of knowledge of when the disease begins greatly complicates the work of the physicians. Another added handicap is that neither do we know why the disease begins.
In this sense, there are several hypotheses trying to explain the beginning of AD. These hypotheses may not be exclusive, and they may well overlap and take place at the same time. We can divide the hypotheses into three groups: The hypotheses based on protein deposits. This group includes the beta-amyloid (Aβ) cascade hypothesis; and the tau hypothesis.
The deposits mainly formed by Aβ peptide are known as senile plaques [2]. Aβ comes from the proteolysis of a membrane protein called amyloid precursor protein (APP). In favor of the Aβ cascade theory we can say that mutations in genes involved in the genesis of Aβ cause AD [3,4]; mutations in the gene encoding the tau protein do not cause amyloid deposition [5,6]; the ApoE4 allele leads to a reduction in the clearance of the Aβ peptide and increases the risk of AD [7]; Aβ oligomers that are isolated from AD brains involve loss of synapses, neuronal density, and memory impairment [8]; Aβ peptide can induce hyper-phosphorylation of tau [9].
The deposits essentially formed by the tau protein are known as neurofibrillary tangles. Tau is a cytoskeleton protein which is very important for its stability. Tau changes to a hyper-phosphorylated state causing a disruption of the cytoskeleton in AD pathology. Neurons with a high content of hyper-phosphorylated tau enter into apoptosis and neurodegeneration takes place [10]. In favor of this theory, we can say that the severity of this type of dementia correlates well with the growing accumulation of neurofibrillary tangles in the brain [11,12,13]; there is a high correlation between hyper-phosphorylated tau species in the cerebrospinal fluid (CSF) in patients with AD and the degree of cognitive impairment [14]; a decrease in tau filaments by drugs directed against this therapeutic target alleviates cognitive deterioration [15].
The hypothesis of reactive processes which includes neuroinflammation as the first event in AD. An elevation of proinflammatory cytokines are found in AD [16,17]. High levels of tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) are also detected in the serum and in brain of patients when compared to controls. Multiple inflammatory markers are found in AD animal models, such as IL-1, IL-6, the granulocyte-macrophage colony-stimulating factor (GM-CFS), IL-12, IL-26, and TNF. Histologically, characteristic amyloid plaques are surrounded by microglia and reactive astrocytes appear in the brains of patients with AD [18]. Moreover, according to studies with mice with cerebral amyloidosis, the activation of astrocytes seems to occur very early in the pathogenic process [19]. Specifically, it has been seen that the elevation of both cells and proinflammatory cytokines appears before the deposit of Aβ [20].
The hypotheses based on loss of function: calcium misbalance hypothesis, vascular hypothesis and oxidative stress hypothesis. In favor of the calcium theory we can say that it has been confirmed that each mutation of early-onset AD alters the calcium balance in the cell [21]. In sporadic AD, before the presence of Aβ or any other histological alteration, there is evidence suggesting neuronal hyper-excitability due to an increase in calcium levels in neurons [22].
In favor of the vascular theory we can say that there is a clear relationship between vascular risk factors and AD; early vascular damage is present in individuals with AD; vascular damage alone causes neurodegeneration; there are intravascular deposits of Aβ in early phases of AD [23].
Lastly, one of the most important the accumulated experimental evidence is the oxidative stress theory.
Despite of the different theories about the onset of AD there is a common thread in all of them that occurs in the ageing cells causing, among other effects, a poor functioning of energy metabolism. This thread is oxidative stress and its role in this disease is central. We will discuss it in a broad manner in the following lines of this review and will interrelate its role with the role of vitamin E which is, mostly, tackling the effects of oxidative stress.
2. Oxidative Stress Theory and AD
Oxidative stress is the imbalance between high oxidant species formed and insufficient antioxidant defenses [24]. This causes an altered homeostatic balance resulting from oxidant insult [25]. The reduction of O2 to H2O in mitochondria. The reduction of O2 to H2O in mitochondria produces reactive oxygen species (also known as ROS) which are highly reactive and interact with several biological molecules nearby. On the other hand, the brain is the organ with the highest O2 consumption in our body. Despite composing the 2–3% of the total body weight, it uses about 20–25% of the basal metabolism. As a consequence, it is the highest ROS-generator organ. The brain metabolism is early reduced in AD and this could be associated with a decreasing metabolic regulation and increased ROS generation [26].
In AD, oxidation of all macromolecules is found very early in the brain of patients. Lipids, proteins, nucleic acids, and polysaccharides are all oxidized in AD.
Advanced glycation end products (AGE) are formed by glycation, a reaction between reduced sugars and protein side chains. Glycation products are very stable molecules and trend to accumulate inside neurons, in senile plaques and in neurofibrillary tangles [27].
Lipid peroxidation consists in the hydroxy radical attack of unsaturated lipids to generate highly reactive secondary products such as reactive carbonyls and reactive aldehydes, which are able to inactivate enzyme active sites overriding their physiological role. Furthermore, oxidized membranes have altered mobility. Aldehyde adducts of protein are common on senile plaques and neurofibrillary tangles and are most prominent in cell bodies of vulnerable neurons [28].
When proteins are oxidized, the peptide bond could be compromise and it can be cleaved. Moreover, reactive carbonyls are frequently generated and also protein nitration, a related phenomenon. All these protein modifications happen prominently in neuronal cell bodies [29].
Lastly, nucleic acids could also be affected by oxidation. ROS can alter both purinic and pyrimidinic bases with mutagenic and even deleterious results. Vulnerable neuronal cell bodies accumulate ostensibly oxidized nucleic acids [30].
Consequently, it is not surprising that antioxidant therapy has been proposed countless times as a treatment for Alzheimer’s disease.
3. Why Vitamin E as Treatment for AD?
3.1. Vitamin E Is an Antioxidant and Neuroprotector
Vitamin E is a term that includes a group of eight compounds and belongs to the fat-soluble vitamins. In this group we can find α-, β-, γ-, and δ-tocopherols and tocotrienols, their main characteristic is their antioxidant potential. However, vitamin E also has neuroprotective, anti-inflammatory, and hypocholesterolemic properties [31,32,33] driving to its importance for brain health.
The antioxidant role of vitamin E is based on the presence of a hydroxyl group in its phenolic group on the chromanol ring that can donate a hydrogen atom and, in this way, neutralize a great variety of free radicals including reactive oxygen species (ROS) [34]. When this reaction takes place, a non-radical product and a vitamin E radical are obtained. Then the vitamin E radical can react with another free radical lipid or can be regenerated back to their native form by vitamin C [35,36,37,38]. By this process vitamin E neutralizes the peroxyl radicals and blocks lipid peroxidation [39], especially the polyunsaturated fatty acids peroxidation, which is essential for the protection of cellular membranes.
Vitamin E has more antioxidant potential against peroxyl radicals than other antioxidants like glutathione or β-carotene [40]. This antioxidant activity has been proved in several studies both in vitro and in vivo. One of the most important pieces of evidence of the antioxidant capacity of vitamin E was reported on 1997 by Ham and Liebler who gave supplementary vitamin E diet to rats. They evaluated the antioxidant properties of vitamin E inducing lipid peroxidation in liver cells by t-Bu-OOH. They showed a reduction in the metabolic changes in vitamin E-treated rats [41].
Moreover, vitamin E is considered one of the most important antioxidants in the brain and especially the α-tocopherol form. This is due to the high levels found in the brain of its transporter α-TTP (α-tocopherol transfer protein) whose functions include the regulation and distribution of levels of vitamin E in different tissues [42,43]. Its critical role in brain function is underscored by the fact that human carriers of a mutation in the α-TTP gene develop progressive spinocerebellar ataxia, areflexia, loss of proprioception, and extremely low vitamin E levels [44,45]. In the encephalon, α-TTP expression is highest in the cerebellum, specifically in astrocytes, which provide vitamin E to the neighboring neurons [46]. Importantly, α-TTP expression is increased in brains of patients with neurodegenerative diseases [42,47]. Therefore, we can conclude that vitamin E plays an important role in the neuroprotection through its antioxidant activity.
As mentioned previously, in AD there is an evident production of ROS that promotes the oxidation of all macromolecules. It may not be surprising the fact that Aβ is by itself an important inductor of oxidative stress inside cells since it involves generation of ROS by mitochondria and endoplasmic reticulum. Aβ also causes a disruption of cellular calcium homeostasis, crucial for the transmission of action potential. When Aβ aggregates and deposits near the cell membrane, it can promote lipid peroxidation to produce pro-oxidant species, including malondialdehyde (MDA) or 4-hydroxynonenal (4HNE). The last one, is an aldehyde able to covalently modify lipids and proteins on its vicinity and one of these proteins is tau [48,49].
Oxidative modification of tau can promote its aggregation in vitro [50] and may induce tau hyper-phosphorylation and the formation of neurofibrillary tangles [51]. Additionally, oxidative stress also favors tau hyper-phosphorylation by promoting glycogen synthase kinase-3 beta (GSK3β) activity. GSK3β is a ubiquitously expressed kinase and it phosphorylates tau in most serine and threonine residues in paired helical filaments [52]. Finally, 4HNE directly activates a mitogen-activated protein (MAP) kinase, p38 [53], which leads to tau hyper-phosphorylation. Truthfully; a correlation was found in transgenic mice exhibiting hyper-phosphorylated tau between activated p38 and the level of aggregated tau [54].
Lastly, vitamin E is considered one of the most important antioxidants in the brain and especially the α-tocopherol form. This is due to the high levels found in the brain of its transporter α-TTP (α-tocopherol transfer protein) whose functions include the regulation and distribution of levels of vitamin E in different tissues [42,43]. Its critical role in brain function is underscored by the fact that human carriers of a mutation in the α-TTP gene develop progressive spinocerebellar ataxia, areflexia, loss of proprioception, and extremely low vitamin E levels [44,45]. In the encephalon, α-TTP expression is highest in the cerebellum, specifically in astrocytes, which provide vitamin E to the neighboring neurons [46]. Importantly, α-TTP expression is increased in brains of patients with neurodegenerative diseases [42,47]. Therefore, we can conclude that vitamin E plays an important role in the neuroprotection through its antioxidant activity.
3.2. Vitamin E as an Anti-Inflammatory and Cell Signaling
Beyond its role as an antioxidant, vitamin E can enhance the immune response in elderly people. During aging and in some neurodegenerative diseases such as AD, a dysregulation of the immune system which triggers many inflammatory responses are produced. There are many studies that prove the anti-inflammatory property of vitamin E when elderly people take it as a diet supplement.
These studies show in vitro T cells proliferation, IL-2 production and E2 prostaglandin inhibition among other beneficial effects [32]. With 233 mg of vitamin E every day for 28 days, Lee and co-workers found an increase in IL-2 receptor levels in T-lymphocyte population [55]. With a similar dose during three months, De La Fuente et al. showed a beneficial effect of α-tocopherol in elderly people, specifically in the adherence capacity of lymphocytes, in increased IL-2 production, in NK activity and in lymphocytes proliferation [56].
However, this beneficial effect disappears with a concomitant disease, like allergic rhinitis [57] or causes a hypersensitivity response [58] or even harmful effects in adult smokers [59,60].
The action mechanism of vitamin E on the immune system involves the inhibition of prostaglandins E2 and D2 without inhibition of cyclo-oxygenase (COXs) and 5-lipoxygenase (5-LOX) activities [61,62,63]. On the one hand, vitamin E metabolites from the ω- and β-oxidation of its hydrophobic side chain inhibit COX activity [62]. On the other hand, vitamin E impedes membrane changes which trigger in the blockage of the calcium influx, through the inhibition of ionophores that produce the inhibition of 5-LOX activity [63]. Both inhibitions activate a signaling pathway that finally induces the inhibition of the prostaglandins. Then IL-2 production is generated and, therefore, an immune response (Figure 1).
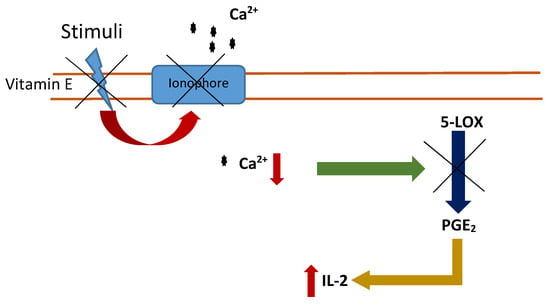
Figure 1.
Inhibition of 5-LOX activity by vitamin E. Vitamin E blocks calcium ionophores inducing a reduction in the intracellular calcium levels, triggering the inhibition of 5-LOX activity and inducing the inhibition of the prostaglandins (PGE2). Interleukin 2 (IL-2) levels increase and consequently an immune response.
It has been shown that vitamin E is an inhibitor of the protein kinase C (PKC) and this inhibition is independent of its antioxidant activity [64]. The mechanism is doubled: vitamin E can activate the phosphatase (PP)2A that inhibits the active form [65,66], and vitamin E can modulate the diacylglycerol kinase activity [65,66]. The result is different depending on the vitamin E isoform, α-tocopherol inhibits PKC in the vascular smooth muscle and cell growth is arrested whereas β-tocopherol prevents this effect [64,67,68].
By contrast, the inhibition of PKC by vitamin E could have non-beneficial effects in some aspects of AD pathology. For example, PKC plays an important role in immune response because T-cell subpopulation reactive to Aβ1-42 expresses different isoforms of PKC at different clinical stages of AD [69]. Moreover, PKC regulates α-secretase activity which is essential in the non-amyloidogenic APP processing. Thus, the amyloidogenic via is reduced and, therefore, the formation of amyloid beta toxic peptide [70].
Vitamin E can also regulate genes at a transcriptional level in a PKC-independent way, some of these genes are CD36 [71], SR class A [72] and intracellular adhesion molecule-1 [73].
To summarize, vitamin E not only acts as an antioxidant but also has cell signaling properties, both in a PKC-dependent or PK-independent way. Thus, the study of vitamin E treatment in AD should not only focus on its antioxidant properties, but also on anti-inflammatory and neuroprotection effects (Figure 2).
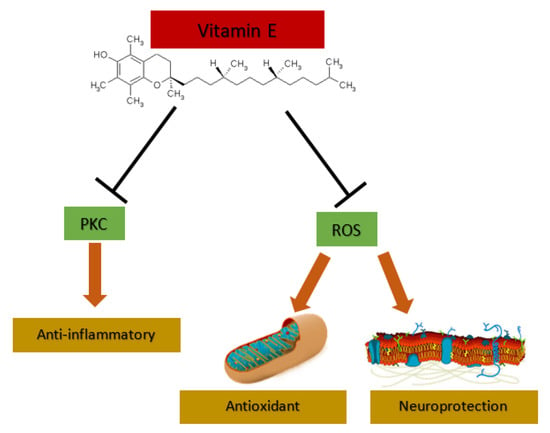
Figure 2.
Vitamin E effects. Vitamin E can act as anti-inflammatory through protein kinase C (PKC) inhibition and has antioxidant and neuroprotection properties through the attack to reactive oxygen species (ROS).
3.3. Levels of Vitamin E in AD Are Low
Nearly 30 years ago, it was published that vitamin E levels were decreased in 55 patients with AD compared to non-demented controls [74]. Since then, many works have corroborated these results. Sometimes with low number of patients (see Table 1), but always with significant results. A meta-analysis performed in 2014 reviewed 80 studies on micronutrients and AD. The authors concluded that vitamin E, among others, showed lower plasma levels in AD patients. Moreover, the authors did not find a relation between the levels of vitamin E and the malnourishment status of the patients and they suggest that micronutrient status may be compromise before malnutrition [75]. A recent meta-analysis of 116 selected publications has confirmed that vitamin E levels in AD patients are also significantly lower in CSF and in brain [76]. A more recent meta-analysis using 17 studies and including an overall of 904 AD patients and 1153 controls showed that AD patients had lower concentration of serum Vitamin E compared with healthy older controls [77].

Table 1.
Studies about the relation of Alzheimer’s disease and reduction of the vitamin E levels.
However, although they are less numerous, we can also find other studies reported no difference in vitamin E levels (Table 2). A very recent Mendelian randomized study investigated the relationship between circulating vitamin E and AD. For this purpose, the authors used the data from a large-scale vitamin E GWAS (genome-wide association study) with a total number of 7781 individuals of European descent and also a GWAS that includes 17,007 AD cases and 37,154 controls. This study showed no significant correlation between vitamin E levels and AD [88].
3.4. Vitamin E and Prevention of Cognitive Decline
Regarding epidemiological studies an association between supplementation with vitamin E and decreased risk of developing AD was also revealed. In 1998, a prospective study was published with 633 persons where none of the 27 vitamin E supplement users had AD after a period of 4.3 years of follow-up [89]. In 2002, Engerhalt et al. corroborated this result in a different cohort from The Netherlands, with a six-year follow up [90]. Another prospective study was published the same year, conducted from 1993 to 2000, of 815 residents aged 65 years and older, free of AD at baseline, and were followed up for a mean of 3.9 years. This study suggested that food containing vitamin E, but not other antioxidants, may be associated with a reduced risk of developing AD. However, this association was observed only among the individuals not carrying the APOE ɛ4 allele [91]. Another prospective study, the Cache Country Study from Utah (USA) concluded an evident lower AD risk in people supplemented with vitamin E and multivitamin complexes containing, among others, vitamin C. Interestingly, they showed no evidence of protective effect associated to the intake of these compounds alone [92].
Analyzed data from the Rotterdam Study (365 AD patients) also showed a modest reduction in the long-term risk of AD. Importantly, the positive results were only present in those participants with higher intake of foods rich in vitamin E. However, participants with average vitamin E intake did not have a lower risk of dementia [93]. Data from the Canadian Study of Health and Aging (1991–2002), a cohort study of dementia including 560 AD patients, suggested that the use of vitamin E supplements was associated with a reduced risk of cognitive decline [94].
On the other hand, three other studies did not show any association between vitamin E intake and risk of suffering AD. The first, with 2969 participants followed up biennially for 5.5 years, concluded that the use of supplemental vitamin E and C, alone or in combination, did not reduce risk of AD or overall dementia [95]. The second, with 3385 men from The Honolulu–Asia Aging Study, found that vitamin E and C supplements may improve cognitive function in late life, but no protective effect was found for Alzheimer’s dementia in particular [96]. Lastly, another study with 980 elderly subjects in the Washington Heights-Inwood Columbia Aging Project, found that neither dietary, supplemental nor total intake vitamin E was associated with a decreased risk of AD [97].

Table 2.
Studies that don’t show a relation between AD and vitamin E levels.
Table 2.
Studies that don’t show a relation between AD and vitamin E levels.
| Authors and Publication Year | Isoform | Method | Number of Patients and Diagnosis | Results |
|---|---|---|---|---|
| Schippling et al, 2000 [98] | α-tocopherol | HPLC | 29 patients | The difference of α-tocopherol levels among AD patients and controls were no significant |
| von Arnim et al., 2012 [99] | α-tocopherol | HPLC | 74 MCI patients | No association was found for vitamin E levels and dementia |
| Charlton el at., 2004 [100] | α-tocopherol | HPLC | 15 AD patients | No differences in the vitamin E levels between AD patients and controls |
| Ryglewicz et al., 2002 [101] | α-tocopherol | HPLC | 26 AD patients | Levels of vitamin E was significantly lower in patients with vascular dementia in comparison to patients with AD and controls |
4. Is Vitamin E Effective as Treatment for AD? An Approach to Main Trials
An early study published in 1997 by Sano et al. showed the efficacy of vitamin E as treatment in AD for the first time. This double-blind, randomized multicenter clinical trial was also placebo-controlled and mainly focused on vitamin E supplementation in AD patients with moderately severe impairment [102]. Three hundred and forty-one patients were recruited and received 2000 IU/d vitamin E or placebo for two years [102]. As results, they measured time to occurrence of death, institutionalization, loss of ability to perform basic daily living activities, or severe dementia [102]. They concluded that this dose of vitamin E slows AD progression. Twenty-two years later the controversy still exists. Table 3 summarizes Clinical trials about the effectiveness of vitamin E in AD treatment.

Table 3.
Clinical trials about the effectiveness of vitamin E in the AD treatment.
In 2005, Petersen et al. selected 769 subjects and performed a double-blind study [103]. The subjects received 2000 IU/d of vitamin E or placebo for three years, but the authors found that vitamin E supplementation had no benefit [103]. Interestingly, a study published in 2009 (800 IU/d, six months) found two different subgroups in the vitamin E supplemented group. In one group, called “respondents” to vitamin E, oxidative stress parameters were lower after the treatment and scores on the cognitive tests were maintained. However, the authors found a second group called “non-respondents”, in which vitamin E was not effective in preventing oxidative stress and cognition decreased to levels even lower than those of patients taking placebo. They concluded that when vitamin E lowers oxidative stress cognitive status is maintained in patients. However, when vitamin E does not prevent oxidative stress, it is detrimental in terms of cognition [104].
Nonetheless, in 2014, Dysken et al. in a double-blind, placebo-controlled, parallel-group, randomized clinical trial (The TEAM-AD VA Cooperative Randomized Trial) recruited 613 patients with mild to moderate AD and with the same dose (2000 IU/d of alpha-tocopherol or placebo) found that α-tocopherol supplementation reduced functional decline in patients with mild to moderate AD [105]. Curiously, these results were not observed with memantine or with the combination of memantine and vitamin E treatment, so it could be that memantine interferes in the vitamin E effect [105].
In 2017 in the PREADViSE study, Kryscio et al. with a low dose of vitamin E (400 IU/d) found that vitamin E supplementation in selected 7540 asymptomatic older men did not prevent dementia [106]. They evaluated the effect of the vitamin E and selenium supplementation in the prevention of AD and concluded that neither vitamin E, nor selenium, nor even a combination of both prevented dementia [106].
However, the attempts to obtain an effective treatment for AD based on vitamin E has continued. There are still many studies that assess the effectiveness of different forms of vitamin E or the combination of vitamin E with another compound that could have a beneficial effect against the evolution of dementia. Last year, the group headed by Ikuo Tooyama evaluated the beneficial effect of the tocotrienol-rich fraction, a combination of different vitamin E analogues from palm oil, in a transgenic mice model of AD [107]. The results showed that the supplementation rescued the cognition function in the transgenic mice and reduced the Aβ deposition although the Aβ oligomers levels did not change [100]. Finally, another study with an AD animal model evaluated a combination of vitamin E with fish oil supplementation [108]. Their results showed that only a low vitamin E diet and fish oil supplement rescued the cognitive function in the transgenic mice. Higher doses of vitamin E were not beneficial suggesting that high levels of vitamin E act as a prooxidant causing oxidation [108].
5. Why Does Vitamin E Fail to Treat AD?
Since oxidative stress is an early hallmark of AD, many efforts have been made by the scientific community to try antioxidant treatment as a possible therapy. However, as we have discussed above, evidence is consistent with a limited effectiveness of vitamin E in slowing progression of dementia; the information is mixed and inconclusive. However, why does vitamin E with other antioxidants fail to improve the course of cognitive decline in AD? This highlights a paradox in oxidative stress: the efforts to increase oxidative defenses have not proved beneficial for human diseases [109] which has been called “antioxidant paradox”. Halliwell points out three possibilities: the extrapolation of results obtained in animals to humans, the lack of measurements of baseline nutritional status in human cohorts and some antioxidants can have pro-oxidant effects [110]. As we have discussed above, plasma levels of vitamin E are low in the patients, so we have evidence about the “baseline nutritional status”. However, it is true that the measurement of baseline vitamin E levels is often not check in the main trials before beginning.
On the other hand, the extrapolation of results from animals to humans is not trivial. The correct methods to measure oxidative stress are still under debate and sometimes are not specific or appropriated [111,112,113,114]. Many studies of oxidative stress in AD are old and methods could not be up-to-date. To follow the antioxidant response by measuring an oxidative stress biomarker is very recommendable. However, to choose a good oxidative stress biomarker could not be easy. The specificity of some used biomarkers of oxidative stress, as in the case of oxidized low-density lipoprotein antibodies, could be questionable. In this regard, it has been recommended the analysis of oxidative stress in specific proteins involved in the disease. These markers could better represent a specific pathological pathway and a way for therapeutic monitoring and a prediction of results [115]. An example of the importance of monitoring oxidation in patients is the ineffectiveness of vitamin E supplements in modifying the oxidative balance, as in the non-respondents group found by Lloret et al. [104]. In this work was found that only patients in whom oxidative stress had decreased showed a cognitive improvement, but non-respondents worsened in their results of cognitive tests.
Another important point is bioavailability of vitamin E, which is complex and influenced by some important factors, such as the intake of competing nutrients, intestinal differences in absorption, age, gender, smoking, obesity and genetic polymorphisms. Sterols from plants, eicosapentaenoic and retinoic acids, and even dietary fiber, are described as competing nutrients that decrease the absorption of vitamin E [116,117,118,119]. Moreover, the rate of absorption into the bloodstream differs between 20% and 80% in humans, mainly due to the differences in food contents and the form of vitamin E. The transport of vitamin E in blood circulation is done by lipoproteins [120]. In particular, the entrance to the central nervous system of α-tocopherol in vivo is provided by high-density lipoprotein (HDL) particles and HDL levels vary greatly among people [121]. In cells, vitamin E is bound to α-TTP, a transport protein highly expressed in AD brains and especially under oxidative stress conditions [47].
Age and gender also influence vitamin E bioavailability. After 60 years the levels of vitamin E increase in plasma but decrease after 80 years [122]. Women seem to have higher maximum plasma concentrations of α-tocopherol than men maybe due to differences in HDL levels between genders. [123].
On the other hand, smokers have lower levels of a-tocopherol in serum than non-smokers, although the variances could be attributed to the differences in dietary patterns [124,125,126]. And obesity has an inverse relationship with serum α-tocopherol levels [127]. In particular, the waist-to-hip ratio and waist circumference were associated with α-tocopherol serum concentrations independently of gender. [128,129,130].
Lastly, genetic polymorphisms in genes encoding for proteins involved in vitamin E metabolism could also explain interindividual differences in bioavailability [131,132,133,134]. More than 50 different single-nucleotide polymorphisms (SNPs) has been related to vitamin E homeostasis.
Another point is the efficacy of vitamin E to pass through the blood-brain barrier and the raise of levels in brain. Therefore, demonstrating the efficacy of vitamin E in reducing brain oxidative damage is very important [135].
Many other studies can be confusing due to the form of vitamin E supplemented or due to the combination with other antioxidants, nutrients and pharmaceuticals used [136].
The Mediterranean diet along with moderate physical activity is nowadays believed to be a first line of defense against the development and progression of AD. However, in most cases the studies defending this idea were observational. As a consequence, results from large, multicenter randomized clinical trials clarifying the actual link between moderate exercise, physical activity and healthy Mediterranean diet on cognition in the elderly are awaited. It is true that an antioxidant-enriched diet may be better than pure antioxidants intake in a pharmaceutical form, because their bioavailability could be different [137].
Another crucial fact is the complexity of the brain. Oxidative stress leads to neuronal damage and even apoptosis cell death and neurodegeneration, so neuronal networks change, and compensatory response is induced. Reductive stress rather than oxidative stress was found in healthy young person ApoE 4/4 [138]. This scenario complicates enormously the successful efficacy of antioxidant therapy. Remodel or regenerate the lost neuronal network properly is highly difficult and ever more so if we consider that it is an aging brain or a compensating brain.
6. Conclusions
At the present time, clinical studies have revealed unreliable findings on the effect of vitamin E on AD-developing risk. Thus, it remains unclear whether vitamin E levels are genetically associated with AD risk or if the supplementation with this compound could be beneficial in delaying the progression of dementia. In this review we can find that the number of studies showing a decrease in the levels of plasma vitamin E in AD patients is higher than the studies with contrary results. However, vitamin E as treatment sometimes has positive results on cognition but at others, it does not. The loss of neuronal networks and its replacement, the very different nutritional status of the patients at baseline, the range of time of brain compensation in each person, the antioxidant effect of vitamin E in each person among others could be the reason for the failure in the treatment.
Author Contributions
All authors contributed to the writing of the manuscript. D.E. made the tables and figures. A.L. supervised its preparation and made the final edition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.J.; Visser, P.J. Prevalence of Cerebral Amyloid Pathology in Persons Without Dementia A Meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Multhaup, G.; Simms, G.; Pottgiesser, J.; Martins, R.N.; Beyreuther, K. Neuronal origin of a cerebral amyloid: Neurofibrillary tangles of Alzheimer’s disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985, 4, 2757–2763. [Google Scholar] [CrossRef] [PubMed]
- Citron, M.; Oltersdorf, T.; Haass, C.; McConlogue, L.; Hung, A.Y.; Seubert, P.; Vigo-Pelfrey, C.; Lieberburg, I.; Selkoe, D.J. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 1992, 360, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.D.; Golde, T.E.; Younkin, S.G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science 1993, 259, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Poorkaj, P.; Bird, T.D.; Wijsman, E.; Nemens, E.; Garruto, R.M.; Anderson, L.; Andreadis, A.; Wiederholt, W.C.; Raskind, M.; Schellenberg, G.D. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 1998, 43, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Hutton, M.; Lendon, C.L.; Rizzu, P.; Baker, M.; Froelich, S.; Houlden, H.; Pickering-Brown, S.; Chakraverty, S.; Isaacs, A.; Grover, A.; et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011, 3, 89ra57. [Google Scholar] [CrossRef] [PubMed]
- Müller-Schiffmann, A.; Herring, A.; Abdel-Hafiz, L.; Chepkova, A.N.; Schäble, S.; Wedel, D.; Horn, A.H.; Sticht, H.; de Souza Silva, M.A.; Gottmann, K.; et al. Amyloid-β dimers in the absence of plaque pathology impair learning and synaptic plasticity. Brain 2016, 139, 509–525. [Google Scholar] [CrossRef]
- Jin, M.; Shepardson, N.; Yang, T.; Chen, G.; Walsh, D.; Selkoe, D.J. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B.; Farías, G.; Morales, I.; Navarrete, L. The revitalized tau hypothesis on Alzheimer’s disease. Arch. Med. Res. 2010, 41, 226–231. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Muñoz, J.P.; Barbeito, L. The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch. Med. Res. 2001, 32, 367–381. [Google Scholar] [CrossRef]
- Ghoshal, N.; García-Sierra, F.; Wuu, J.; Leurgans, S.; Bennett, D.A.; Berry, R.W.; Binder, L.I. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Exp. Neurol. 2002, 177, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Bierer, L.M.; Haroutunian, V.; Gabriel, S.; Knott, P.J.; Carlin, L.S.; Purohit, D.P.; Perl, D.P.; Schmeidler, J.; Kanof, P.; Davis, K.L. Neurochemical correlates of dementia severity in Alzheimer’s disease: Relative importance of the cholinergic deficits. J. Neurochem. 1995, 64, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B.; Lavados, M.; Guillón, M.; Mujica, C.; Bosch, R.; Farías, G.; Fuentes, P. Anomalously phosphorylated tau and Abeta fragments in the CSF correlates with cognitive impairment in MCI subjects. Neurobiol. Aging 2006, 27, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Duff, K.; Kuret, J.; Congdon, E.E. Disaggregation of tau as a therapeutic approach to tauopathies. Curr. Alzheimer Res. 2010, 7, 40. [Google Scholar] [CrossRef]
- Fillit, H.; Ding, W.H.; Buee, L.; Kalman, J.; Altstiel, L.; Lawlor, B.; Wolf-Klein, G. Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci. Lett. 1991, 129, 318–320. [Google Scholar] [CrossRef]
- Strauss, S.; Bauer, J.; Ganter, U.; Jonas, U.; Berger, M.; Volk, B. Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab. Invest. 1992, 66, 223–230. [Google Scholar] [PubMed]
- Sastre, M.; Klockgether, T.; Heneka, M.T. Contribution of inflammatory processes to Alzheimer’s disease: Molecular mechanisms. Int. J. Dev. Neurosci. 2006, 24, 167–176. [Google Scholar] [CrossRef]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Dewachter, I.; Walter, J.; Klockgether, T.; Van Leuven, F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflamm. 2005, 2. [Google Scholar] [CrossRef]
- Kitazawa, M.; Oddo, S.; Yamasaki, T.R.; Green, K.N.; LaFerla, F.M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 8843–8853. [Google Scholar] [CrossRef]
- Alzheimer’s Association Calcium Hypothesis Workgroup. Calcium Hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement. 2017, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Fasolato, C. When, where and how? Focus on neuronal calcium dysfunctions in Alzheimer’s Disease. Cell Calcium 2016, 60, 289–298. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.C. The vascular hypothesis of Alzheimer’s disease: Bench to bedside and beyond. Neurodegener. Dis. 2010, 7, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Biochemistry of Oxidative Stress. Angew. Chem. Int. Ed. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Perry, G.; Nunomura, A.; Siedlak, S.L.; Harris, P.L.R.; Zhu, X.; Castellani, R.J.; Aliev, G.; Smith, M.A. Oxidant and antioxidant responses in Alzheimer disease. Recent Res. Dev. Biophys. Biochem. 2001, 1, 35–41. [Google Scholar]
- Mosconi, L.; Pupi, A.; De Leon, M.J. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2008, 1147, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Harris, P.L.R.; Sayre, L.M.; Fujii, J.; Taniguchi, N.; Vitek, M.P.; Founds, H.; Atwood, C.S.; Perry, G.; Smith, M.A. Advanced glycation in neurofibrillary pathology of Alzheimer disease: Ne-(carboxymethyl) lysine and hexitol-lysine. Free Radic. Biol. Med. 2001, 31, 175–180. [Google Scholar] [CrossRef]
- Sayre, L.M.; Zelasko, D.A.; Harris, P.L.R.; Perry, G.; Salomon, R.G.; Smith, M.A. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem. 1997, 68, 2092–2097. [Google Scholar] [CrossRef]
- Smith, M.A.; Sayre, L.M.; Anderson, V.E.; Harris, P.L.; Beal, M.F.; Kowall, N.; Perry, G. Cytochemical demonstration of oxidative damage in Alzheimer disease by immunochemical enhancement of the carbonyl reaction with 2,4-dinitrophenylhydrazine. J. Histochem. Cytochem. 1998, 46, 731–735. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Pappolla, M.A.; Wade, R.; Hirai, K.; Chiba, S.; Smith, M.A. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci. 1999, 19, 1959–1964. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-inflammatory properties of α- and γ-tocopherol. Mol. Asp. Med. 2007, 28, 668–691. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P. Vitamin E and its Function in Membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, A.; Han, D.; Packer, L. Vitamin E recycling in Human Erythrocyte Membranes. J. Biol. Chem. 1993, 268, 10906–10913. [Google Scholar]
- Maguire, J.J.; Wilson, D.S.; Packer, L. Mitochondrial Electron Transport-Linked Tocopheroxyl Radical Reduction. J. Biol. Chem. 1989, 264, 21462–21465. [Google Scholar] [PubMed]
- Thiele, J.J.; Schroeter, C.; Hsieh, S.N.; Podda, M.; Packer, L. Antixoidant Network of the Stratum Corneum. Curr. Probl. Dermatol. 2001, 29, 26–42. [Google Scholar] [PubMed]
- Brigelius-Flohe, R. Vitamin E: The shrew waiting to be tamed. Free Radic. Biol. Med. 2009, 46, 543–554. [Google Scholar] [CrossRef]
- Xu, L.; Davis, T.A.; Porter, N.A. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 2009, 131, 13037–13044. [Google Scholar] [CrossRef] [PubMed]
- Ham, A.J.; Liebler, D.C. Antioxidant reactions of vitamin E in the perfused rat liver: Product distribution and effect of dietary vitamin E supplementation. Arch. Biochem. Biophys. 1997, 339, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Copp, R.P.; Wisniewski, T.; Hentati, F.; Larnaout, A.; Ben Hamida, M.; Kayden, H.J. Localization of alpha-tocopherol transfer protein in the brains of patients with ataxia with vitamin e deficiency and other oxidative stress related neurodegenerative disorders. Brain Res. 1999, 822, 80–87. [Google Scholar] [CrossRef]
- Ulatowski, L.M.; Manor, D. Vitamin E and neurodegeneration. Neurobiol. Dis. 2015, 84, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.E.; Matthews, S.; Jones, S.; Ellis, C.J.; Booth, I.W.; Muller, D.P. Spinocerebellar degeneration associated with a selective defect of vitamin E absorption. N. Engl. J. Med. 1985, 313, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, L.; Ouahchi, K.; Kayden, H.J.; Di Donato, S.; Reutenauer, L.; Mandel, J.L.; Koenig, M. Ataxia with isolated vitamin E deficiency: Heterogeneity of mutations and phenotypic variability in a large number of families. Am. J. Hum. Genet. 1998, 62, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Ulatowski, L.; Manor, D. Vitamin E trafficking in neurologic health and disease. Annu. Rev. Nutr. 2013, 33, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Ulatowski, L.; Dreussi, C.; Noy, N.; Barnholtz-Sloan, J.; Klein, E.; Manor, D. Expression of the α-tocopherol transfer protein gene is regulated by oxidative stress and common single-nucleotide polymorphisms. Free Radic. Biol. Med. 2012, 53, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef]
- Alavi Naini, S.M.; Soussi-Yanicostas, N. Tau hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative tauopathies? Oxid. Med. Cell. Longev. 2015, 151979. [Google Scholar] [CrossRef]
- Gamblin, T.C.; King, M.E.; Kuret, J.; Berry, R.W.; Binder, L.I. Oxidative regulation of fatty acid-induced tau polymerization. Biochemistry 2000, 39, 14203–14210. [Google Scholar] [CrossRef]
- Pérez, M.; Cuadros, R.; Smith, M.A.; Perry, G.; Avila, J. Phosphorylated, but not native, tau protein assembles following reaction with the lipid peroxidation product, 4-hydroxy-2-nonenal. FEBS Lett. 2000, 486, 270–274. [Google Scholar] [CrossRef]
- Hernandez, F.; Lucas, J.J.; Avila, J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, S141–S144. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, E.; Lloret, A.; Fuchsberger, T.; Viña, J. Aβ and tau toxicities in Alzheimer’s are linked via oxidative stress-induced p38 activation: Protective role of vitamin E. Redox Biol. 2014, 2, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, I.; Garwood, C.; Hanger, D.P.; Anderton, B.H.; Noble, W. Kinase activities increase during the development of tauopathy in htau mice. J. Neurochem. 2007, 103, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Man-Fan Wan, J. Vitamin E Supplementation Improves Cell-Mediated Immunity and Oxidative Stress of Asian Men and Women. J. Nutr. 2000, 130, 2932–2937. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Hernanz, A.; Guayerbas, N.; Victor, V.M.; Arnalich, F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic. Res. 2008, 42, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Montano Velazquez, B.B.; Jauregui-Renaud, K.; Banuelos Arias Adel, C.; Ayala, J.C.; Martinez, M.D.; Campillo Navarrete, R.; Rosalia, I.S.; Salazar Mdel, R.; Serrano, H.A.; Mondragon, A.O.; et al. Vitamin E effects on nasal symptoms and serum specific IgE levels in patients with perennial allergic rhinitis. Ann. Allergy Asthma Immunol. 2006, 96, 45–50. [Google Scholar] [CrossRef]
- Wolvers, D.A.; van Herpen-Broekmans, W.M.; Logman, M.H.; van der Wielen, R.P.; Albers, R. Effect of a mixture of micronutrients, but not of bovine colostrum concentrate, on immune function parameters in healthy volunteers: A randomized placebo-controlled study. Nutr. J. 2006, 5, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hemila, H.; Kaprio, J. Modification of the effect of vitamin E supplementation on the mortality of male smokers by age and dietary vitamin C. Am. J. Epidemiol. 2009, 169, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Hemila, H.; Kaprio, J. Vitamin E may affect the life expectancy of men, depending on dietary vitamin C intake and smoking. Age Ageing 2011, 40, 215–220. [Google Scholar] [CrossRef]
- Jiang, Q.; Elson-Schwab, I.; Courtemanche, C.; Ames, B.N. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11494–11499. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, X.; Lill, M.A.; Danielson, M.L.; Freiser, H.; Huang, J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA 2008, 105, 20464–20469. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yin, X.; Jiang, Q. Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J. Immunol. 2011, 186, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Tasinato, A.; Boscoboinik, D.; Bartoli, G.M.; Maroni, P.; Azzi, A. d-α-Tocopherol Inhibition of Vascular Smooth Muscle Cell Proliferation Occurs at Physiological Correlates with Protein Kinase C Inhibition and Is Independent of Its Antioxidant properties. Proc. Natl. Acad. Sci. USA 1995, 92, 12190–12194. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Tasinato, A.; Clement, S.; Ozer, N.K.; Boscoboinik, D.; Azzi, A. α-Tocopherol Specifically Inactivates Cellular Protein Kinase C Alpha by Changing Its Phosphorylation State. Biochem. J. 1998, 334, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.; Tasinato, A.; Boscoboinik, D. The Effect of Alpha Tocopherol on the Synthesis, Phosphorylation and Activity of Protein Kinase C in Smooth Muscle Cells After Phorbol 12-Myristate 13-Acetate Down-Regulation. Eur. J. Biochem. 1997, 246, 745–749. [Google Scholar] [CrossRef]
- Boscoboinik, D.; Szewczyk, A.; Azzi, A. α-Tocopherol (Vitamin E) regulates vascular smooth muscle cell proliferation and protein kinase C activity. Arch. Biochem. Biophys. 1991, 286, 264–269. [Google Scholar] [CrossRef]
- Boscoboinik, D.; Szewczyk, A.; Hensey, C.; Azzi, A. Inhibition of cell proliferation by α-Tocopherol. Role of protein kinase C. J. Biol. Chem. 1991, 266, 6188–6194. [Google Scholar]
- Miscia, S.; Ciccocioppo, F.; Lanuti, P.; Velluto, L.; Bascelli, A.; Pierdomenico, L.; Genovesi, D.; Di Siena, A.; Santavenere, E.; Gambi, F.; et al. Aβ1–42 stimulated T cells express P-PKC-δ and P-PKC-ζ in Alzheimer disease. Neurobiol. Aging 2009, 30, 394–406. [Google Scholar] [CrossRef]
- Skovronsky, D.M.; Moore, D.B.; Milla, M.E.; Doms, R.W.; Lee, V.M. Protein kinase C-dependent alpha-secretase competes with beta-secretase for cleavage of amyloid-beta precursor protein in the trans-golgi network. J. Biol. Chem. 2000, 275, 2568–2575. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Thiery, J.; Seidel, D. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation 1999, 102, 82–87. [Google Scholar] [CrossRef]
- Teupser, D.; Stein, O.; Burkhardt, R.; Nebendahl, K.; Stein, Y.; Thiery, J. Scavenger receptor activity is increased in macrophages from rabbit with low atherosclerotic response: Studies in normocholesterolemic high and low atherosclerotic response rabbits. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Koga, T.; Martin, K.R.; Meydani, M. Effect of Vitamin E on human aortic endothelial cell production of chemokines and adhesion to monocytes. Atherosclerosis 1999, 147, 297–307. [Google Scholar] [CrossRef]
- Jeandel, C.; Nicolas, M.B.; Dubois, F.; Nabet-Belleville, F.; Penin, F.; Cuny, G. Lipid peroxidation and free radical scavengers in Alzheimer’s disease. Gerontology 1989, 35, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with Alzheimer’s disease: Systematic review and meta-analysis. Alzheimers Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef] [PubMed]
- de Wilde, M.C.; Vellas, B.; Girault, E.; Yavuz, A.C.; Sijben, J.W. Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimers Dement. 2017, 3, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, X.; Liu, Y.; Shu, Y.; Chen, T.; Xu, L.; Li, M.; Guan, X. Do low-serum vitamin E levels increase the risk of Alzheimer disease in older people? Evidence from a meta-analysis of case-control studies. Int. J. Geriatr. Psychiatry 2018, 33, e257–e263. [Google Scholar] [CrossRef]
- Zaman, Z.; Roche, S.; Fielden, P.; Frost, P.G.; Niriella, D.C.; Cayley, A.C. Plasma concentrations of vitamins A and E and carotenoids in Alzheimer’s disease. Age Ageing 1992, 21, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; de Bustos, F.; Molina, J.A.; Benito-León, J.; Tallón-Barranco, A.; Gasalla, T.; Ortí-Pareja, M.; Guillamón, F.; Rubio, J.C.; Arenas, J.; et al. Cerebrospinal fluid levels of alpha-tocopherol (vitamin E) in Alzheimer’s disease. J. Neural Transm. 1997, 104, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.J.; Bayer, A.J.; Johnston, J.; Warner, C.; Maxwell, S.R. Altered plasma antioxidant status in subjects with Alzheimer’s disease and vascular dementia. Int. J. Geriatr. Psychiatry 1998, 13, 840–845. [Google Scholar] [CrossRef]
- Foy, C.J.; Passmore, A.P.; Vahidassr, M.D.; Young, I.S.; Lawson, J.T. Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. Q. J. Med. 1999, 92, 39–45. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Delmas-Beauvieux, M.C.; Peuchant, E.; Richard-Harston, S.; Decamps, A.; Reignier, B.; Emeriau, J.P.; Rainfray, M. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing 2001, 30, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Polidori, M.C.; Mecocci, P. Plasma susceptibility to free radical-induced antioxidant consumption and lipid peroxidation is increased in very old subjects with Alzheimer disease. J. Alzheimers Dis. 2002, 4, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.; Cherubini, A.; Ingegni, T.; Mattioli, P.; Catani, M.; Rinaldi, P.; Cecchetti, R.; Stahl, W.; Senin, U.; et al. Lymphocyte oxidative DNA damage and plasma antioxidants in Alzheimer disease. Arch. Neurol. 2002, 59, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Giavarotti, L.; Simon, K.A.; Azzalis, L.A.; Fonseca, F.L.A.; Lima, A.F.; Freitas, M.C.V.; Brunialti, M.K.C.; Salomao, R.; Moscardi, A.A.V.S.; Montano, M.B.M.M.; et al. Mild systemic oxidative stress in the subclinical stage of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 609019. [Google Scholar] [CrossRef]
- Mullan, K.; Williams, M.A.; Cardwell, C.R.; McGuinness, B.; Passmore, P.; Silvestri, G.; Woodside, J.V.; McKay, G.J. Serum concentrations of vitamin E and carotenoids are altered in Alzheimer’s disease: A case-control study. Alzheimers. Dement. Trans. Res. Clin. Interv. 2017, 3, 432–439. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Jin, S.; Hu, Y.; Wang, T.; Tian, R.; Han, Z.; Xu, D.; Jiang, Q. Circulating vitamin E levels and Alzheimer’s disease: A Mendelian randomization study. Neurobiol. Aging 2018, 72, e1–e189. [Google Scholar] [CrossRef]
- Morris, M.C.; Beckett, L.A.; Scherr, P.A.; Hebert, L.E.; Bennett, D.A.; Field, T.S.; Evans, D.A. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1998, 12, 121–126. [Google Scholar] [CrossRef]
- Engelhart, M.J.; Geerlings, M.I.; Ruitenberg, A.; van Swieten, J.C.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 2002, 287, 3223–3229. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Wilson, R.S.; Scherr, P.A. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA 2002, 287, 3230–3237. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch. Neurol. 2004, 61, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Grodstein, F.; van Rooij, F.J.; Hofman, A.; Stampfer, M.J.; Witteman, J.C.; Breteler, M.M. Dietary antioxidants and long-term risk of dementia. Arch. Neurol. 2010, 67, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Basambombo, L.L.; Carmichael, P.H.; Côté, S.; Laurin, D. Use of vitamin E and C supplements for the prevention of cognitive decline. Ann. Pharmacother. 2017, 51, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.L.; Anderson, M.L.; Crane, P.K.; Breitner, J.C.; McCormick, W.; Bowen, J.D.; Teri, L.; Larson, E. Antioxidant vitamin supplement use and risk of dementia or Alzheimer’s disease in older adults. J. Am. Geriatr. Soc. 2008, 56, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Masaki, K.H.; Losonczy, K.G.; Izmirlian, G.; Foley, D.J.; Ross, G.W.; Petrovitch, H.; Havlik, R.; White, L.R. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology 2000, 54, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.X.; Shea, S.; Mayeux, R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch. Neurol. 2003, 60, 203–208. [Google Scholar] [CrossRef]
- Schippling, S.; Kontush, A.; Arlt, S.; Buhmann, C.; Sturenburg, H.J.; Mann, U.; Muller-Thomsen, T.; Beisiegel, U. Increased lipoprotein oxidation in Alzheimer’s disease. Free Radic. Biol. Med. 2000, 28, 351–360. [Google Scholar] [CrossRef]
- von Arnim, C.A.; Herbolsheimer, F.; Nikolaus, T.; Peter, R.; Biesalski, H.K.; Ludolph, A.C.; Riepe, M.; Nagel, G. Dietary antioxidants and dementia in a population-based case-control study among older people in South Germany. J. Alzheimers Dis. 2012, 31, 717–724. [Google Scholar] [CrossRef]
- Charlton, K.E.; Rabinowitz, T.L.; Geffen, L.N.; Dhansay, M.A. Lowered plasma vitamin C, but not vitamin E, concentration in dementia patients. J. Nutr. Health Aging 2004, 8, 99–107. [Google Scholar]
- Ryglewicz, D.; Rodo, M.; Kunicki, P.K.; Bednarska-Makaruk, M.; Graban, A.; Lojkowska, W.; Wehr, H. Plasma antioxidant activity and vascular dementia. J. Neurol. Sci. 2002, 203–204, 195–197. [Google Scholar] [CrossRef]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A.; et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Badía, M.C.; Mora, N.J.; Pallardó, F.V.; Alonso, M.D.; Viña, J. Vitamin E paradox in Alzheimer’s disease: It does not prevent loss of cognition and may even be detrimental. J. Alzheimers Dis. 2009, 17, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of Antioxidant Supplement Use and Dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol. 2017, 74, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Ibrahima, N.F.; Yanagisawa, D.; Durani, L.W.; Hamezah, H.S.; Damanhuri, H.A.; Ngah, W.Z.W.; Tsuji, M.; Kiuchi, Y.; Ono, K.; Tooyama, I. Tocotrienol-Rich Fraction Modulates Amyloid Pathology and Improves Cognitive Function in AβPP/PS1 Mice. J. Alzheimers Dis. 2017, 55, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Huang, X.; Zhen, J.; Van Halm-Lutterodt, N.; Wang, J.; Zhou, C.; Yuan, L. Dietary Vitamin E Status Dictates Oxidative Stress Outcomes by Modulating Effects of Fish Oil Supplementation in Alzheimer Disease Model APPswe/PS1dE9 Mice. Mol. Neurobiol. 2018, 55, 9204–9219. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., 2nd; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Biophys. Acta 2014, 1840, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Hardy, M.; Michalski, R.; Sikora, A.; Zielonka, M.; Cheng, G.; Ouari, O.; Podsiadły, R.; Kalyanaraman, B. Recent developments in the probes and assays for measurement of the activity of NADPH oxidases. Cell Biochem. Biophys. 2017, 75, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Ribou, A.C. Synthetic Sensors for Reactive Oxygen Species Detection and Quantification: A Critical Review of Current Methods. Antioxid. Redox Signal. 2016, 25, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Richelle, M.; Enslen, M.; Hager, C.; Groux, M.; Tavazzi, I.; Godin, J.P.; Berger, A.; Métairon, S.; Quaile, S.; Piguet-Welsch, C.; et al. Both free and esterified plant sterols reduce cholesterol absorption and the bioavailability of beta-carotene and alpha-tocopherol in normocholesterolemic humans. Am. J. Clin. Nutr. 2004, 80, 171–177. [Google Scholar] [CrossRef]
- Bjørneboe, A.; Bjørneboe, G.E.; Drevon, C.A. Absorption, transport and distribution of vitamin E. J. Nutr. 1990, 120, 233–242. [Google Scholar] [CrossRef]
- Bieri, J.G.; Wu, A.L.; Tolliver, T.J. Reduced intestinal absorption of vitamin E by low dietary levels of retinoic acid in rats. J. Nutr. 1981, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.L.; Vuksan, V.; Jenkins, D.J. Fiber in the treatment of hyperlipidemia. In CRC Handbook of Dietary Fiber in Human Nutrition, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 358–380. [Google Scholar]
- Kolleck, I.; Schlame, M.; Fechner, H.; Looman, A.C.; Wissel, H.; Rüstow, B. HDL is the major source of vitamin E for type II pneumocytes. Free Radic. Biol. Med. 1999, 27, 882–890. [Google Scholar] [CrossRef]
- Goti, D.; Hammer, A.; Galla, H.J.; Malle, E.; Sattler, W. Uptake of lipoprotein-associated alpha-tocopherol by primary porcine brain capillary endothelial cells. J. Neurochem. 2000, 74, 1374–1383. [Google Scholar] [CrossRef]
- Campbell, D.; Bunker, V.W.; Thomas, A.J.; Clayton, B.E. Selenium and vitamin E status of healthy and institutionalized elderly subjects: Analysis of plasma, erythrocytes and platelets. Br. J. Nutr. 1989, 62, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.W.; Paterson, E.; Atkinson, J.K.; Ramakrishnan, R.; Cross, C.E.; Traber, M.G. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic. Biol. Med. 2005, 38, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Al-Azemi, M.K.; Omu, A.E.; Fatinikun, T.; Mannazhath, N.; Abraham, S. Factors contributing to gender differences in serum retinol and alpha-tocopherol in infertile couples. Reprod. Biomed. Online 2009, 19, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Khand, F.; Khand, T.U. Effect of smoking on serum xanthine oxidase, malondialdehyde, ascorbic acid and α-tocopherol levels in healthy male subjects. Pak. J. Med. Sci. 2015, 31, 146–149. [Google Scholar] [CrossRef]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Gunanti, I.R.; Marks, G.C.; Al-Mamun, A.; Long, K.Z. Low serum concentrations of carotenoids and vitamin E are associated with high adiposity in Mexican-American children. J. Nutr. 2014, 144, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Wallström, P.; Wirfält, E.; Lahmann, P.H.; Gullberg, B.; Janzon, L.; Berglund, G. Serum concentrations of beta-carotene and alpha-tocopherol are associated with diet, smoking, and general and central adiposity. Am. J. Clin. Nutr. 2001, 73, 777–785. [Google Scholar] [CrossRef]
- Ohrvall, M.; Tengblad, S.; Vessby, B. Lower tocopherol serum levels in subjects with abdominal adiposity. J. Intern. Med. 1993, 234, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.M.; van’t Veer, P.; Kok, F.; Kardinaal, A.F.; Aro, A. Predictors of adipose tissue tocopherol and toenail selenium levels in nine countries: The EURAMIC study. European Multicentre Case-Control Study on Antioxidants, Myocardial Infarction, and Cancer of the Breast. Eur. J. Clin. Nutr. 1996, 50, 599–606. [Google Scholar]
- Döring, F.; Rimbach, G.; Lodge, J.K. In silico search for single nucleotide polymorphisms in genes important in vitamin E homeostasis. IUBMB Life 2004, 56, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R.; Tourniaire, F. Can genetic variability in α-tocopherol bioavailability explain the heterogeneous response to α-tocopherol supplements? Antioxid. Redox Signal. 2015, 22, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Moussa, M.; Reboul, E.; Lyan, B.; Defoort, C.; Vincent-Baudry, S.; Maillot, M.; Gastaldi, M.; Darmon, M.; Portugal, H.; et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J. Nutr. 2007, 137, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Major, J.M.; Yu, K.; Wheeler, W.; Zhang, H.; Cornelis, M.C.; Wright, M.E.; Yeager, M.; Snyder, K.; Weinstein, S.J.; Mondul, A.; et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum. Mol. Genet. 2011, 20, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G.; et al. Antioxidants for Alzheimer disease: A randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012, 69, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, B.; Ulatowski, L.M. Vitamin E and Alzheimer’s Disease-Is It Time for Personalized Medicine? Antioxidants (Basel) 2017, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Panza, F.; Frisardi, V.; Seripa, D.; Logroscino, G.; Imbimbo, B.P.; Pilotto, A. Diet and Alzheimer’s disease risk factors or prevention: The current evidence. Expert Rev. Neurother. 2011, 11, 677–708. [Google Scholar] [CrossRef]
- Badia, M.C.; Lloret, A.; Giraldo, E.; Dasí, F.; Olaso, G.; Alonso, M.D.; Viña, J. Lymphocytes from young healthy persons carrying the ApoE4 allele overexpress stress-related proteins involved in the pathophysiology of Alzheimer’s disease. J. Alzheimers Dis. 2012, 33, 77–83. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).