Cell Type-Specific p38δ Targeting Reveals a Context-, Stage-, and Sex-Dependent Regulation of Skin Carcinogenesis
Abstract
1. Introduction
2. Results
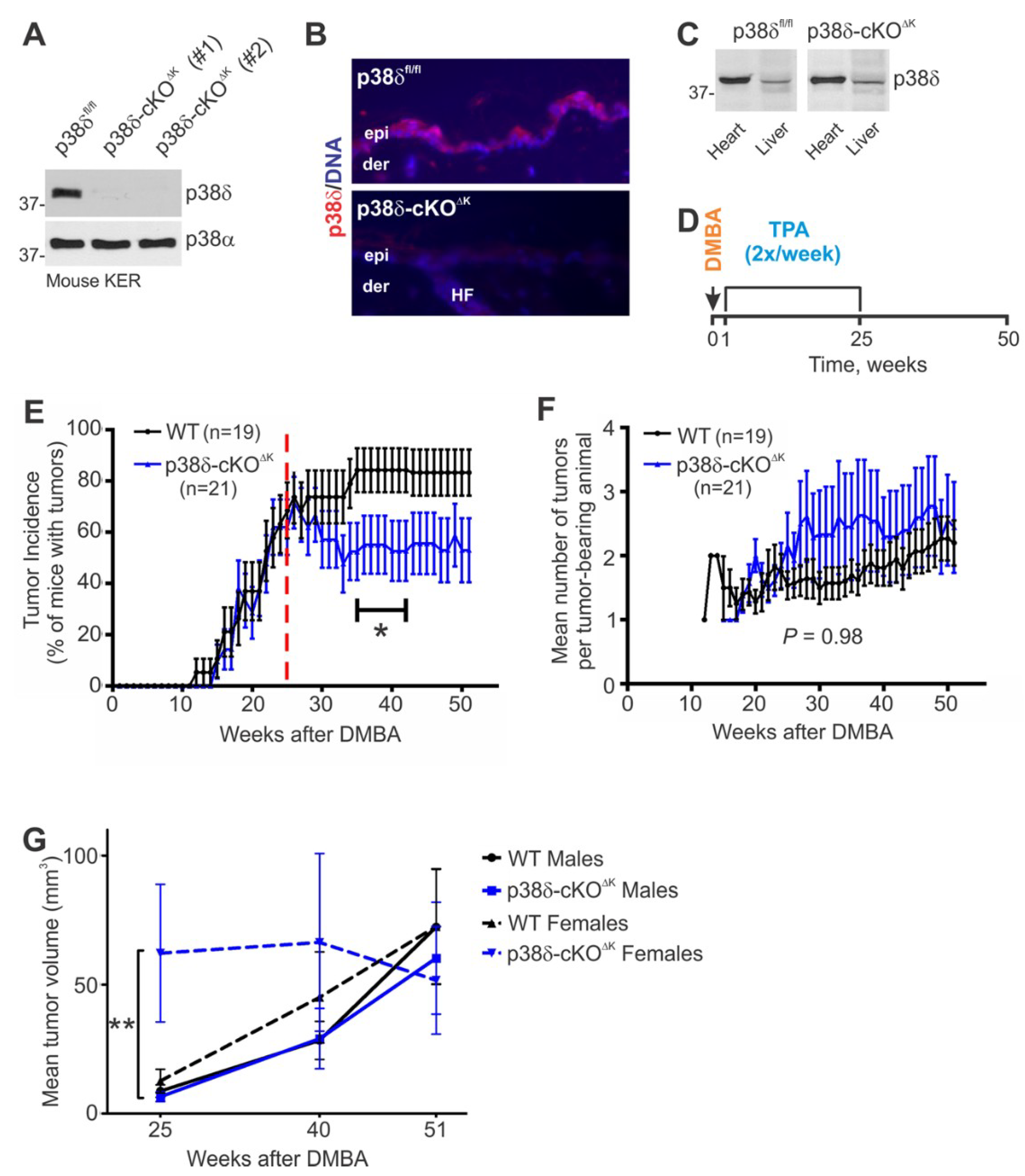
2.1. Mice Lacking Keratinocyte p38δ Exhibit a Normal Skin Phenotype
2.2. Keratinocyte-Specific Loss of p38δ Leads to Reduced Tumor Incidence during the Malignant Progression Stage of the DMBA/TPA Regimen and to Increased Tumor Growth in Females during the TPA Promotion Stage
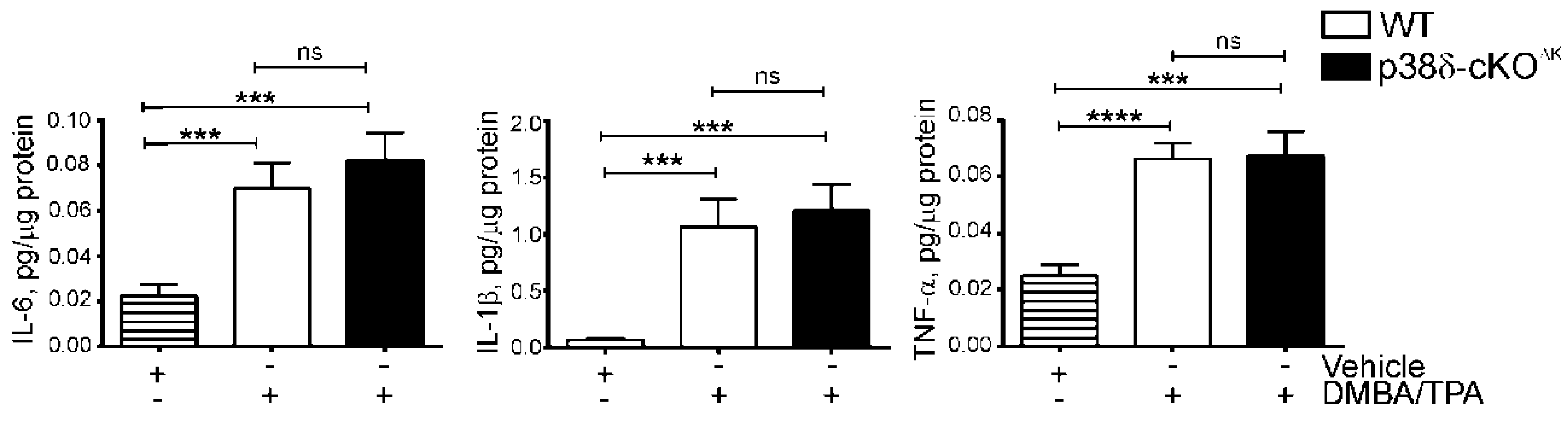
2.3. Keratinocyte p38δ Deletion Does Not Affect Induction of Inflammatory Cytokines in Response to a Short-Term DMBA/TPA Regimen in Mouse Skin
2.4. p38δ-cKO∆K Mice Exhibit a Reduced Incidence of Malignant Tumors
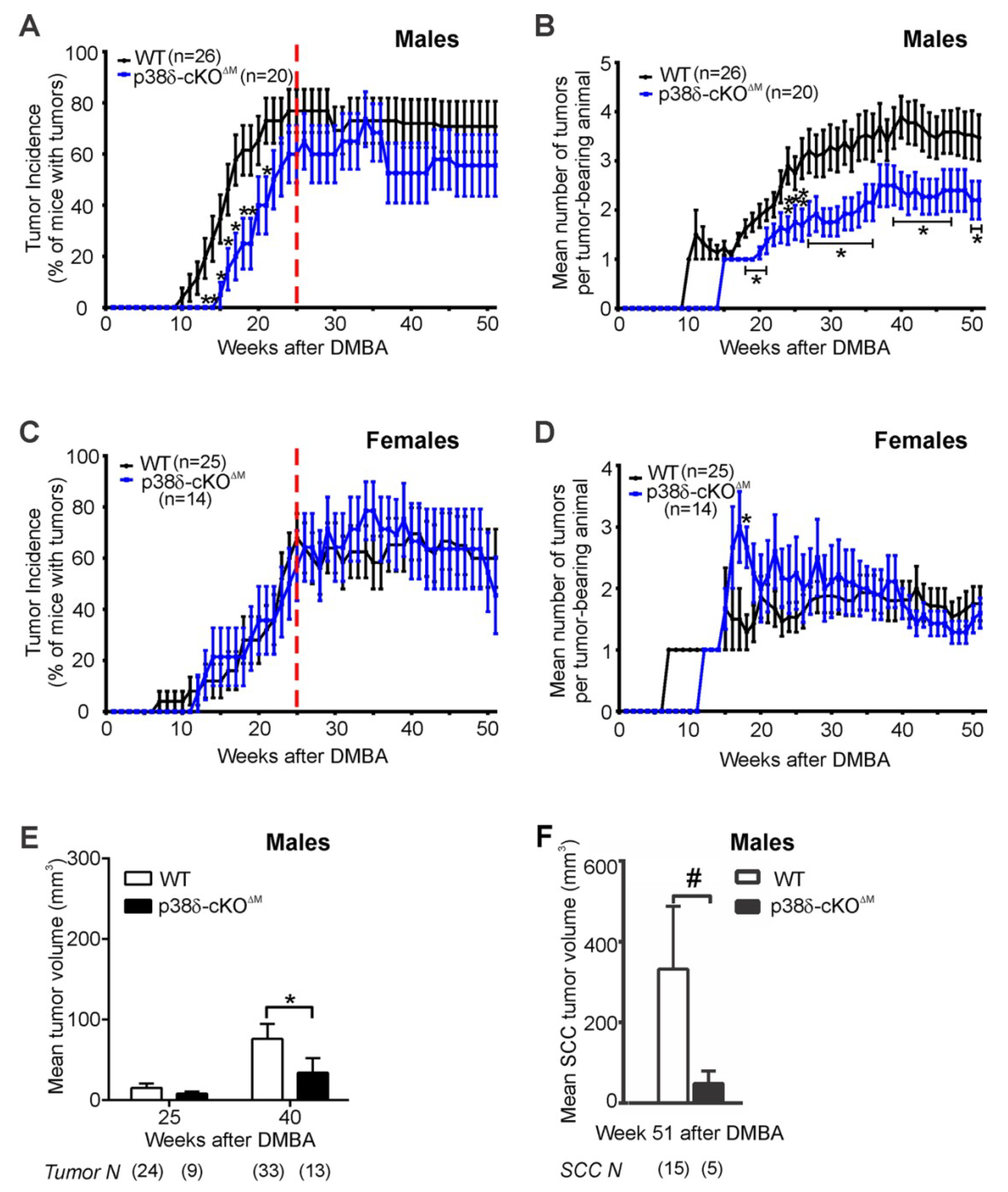
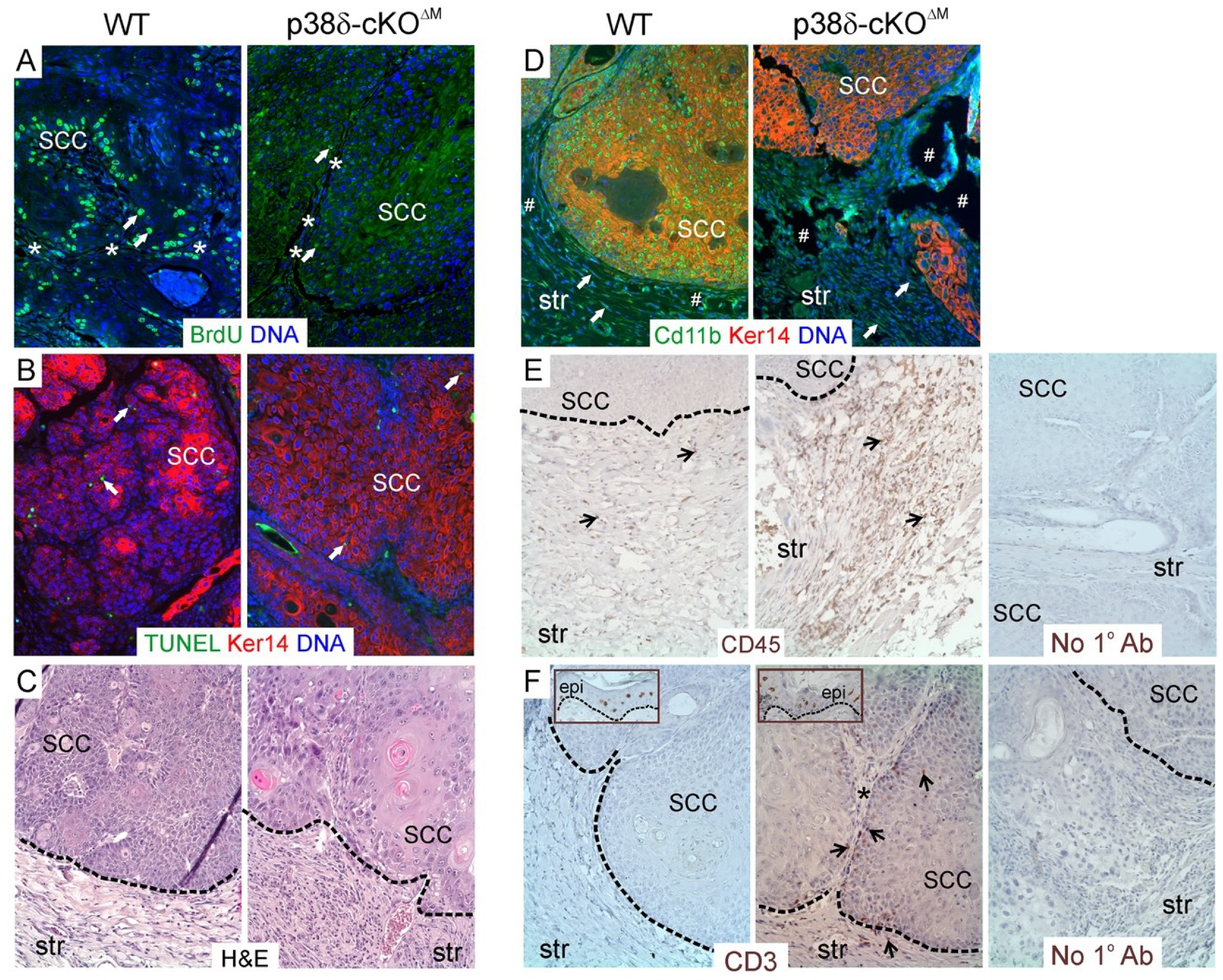
2.5. Loss of p38δ in Myeloid Cells Impairs Development of DMBA/TPA-Induced Skin Tumors in Male Mice
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Generation of Mice with Keratinocyte- and Myeloid Cell-Specific Ablation of p38δ
4.3. Chemical Skin Carcinogenesis
4.4. A Short-Term DMBA/TPA Treatment
4.5. Tissue Protein Isolation, Immunoblot Analysis and ELISA
4.6. Histology, Immunofluorescence and Immunohistochemistry
4.7. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SCC | squamous cell carcinoma |
| CSCC | cutaneous squamous cell carcinoma |
| KA | keratoacanthoma |
| DMBA | 7,12-dimethylbenz(a)anthracene |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| cKO | conditional knockout |
| ER | estrogen receptor |
References
- Karia, P.; Han, J.; Schmults, C. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J. Am. Acad. Dermatol. 2013, 68, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Danés, A.; Blanpain, C. Deciphering the cells of origin of squamous cell carcinomas. Nat. Rev. Cancer 2018, 18, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, N.; Vasseur, R.; Van Seuningen, I. The cornerstone K-RAS mutation in pancreatic adenocarcinoma: From cell signaling network, target genes, biological processes to therapeutic targeting. Crit. Rev. Oncol. Hematol. 2017, 111, 7–19. [Google Scholar] [CrossRef]
- Dorard, C.; Vucak, G.; Baccarini, M. Deciphering the RAS/ERK pathway in vivo. Biochem. Soc. Trans. 2017, 45, 27. [Google Scholar] [CrossRef]
- Hotamisligil, G.; Davis, R. Cell Signaling and Stress Responses. Cold Spring Harb. Perspect. Biol. 2016, 8, a006072. [Google Scholar] [CrossRef]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403. [Google Scholar] [CrossRef]
- Escós, A.; Risco, A.; Alsina-Beauchamp, D.; Cuenda, A. p38γ and p38δ Mitogen Activated Protein Kinases (MAPKs), New Stars in the MAPK Galaxy. Front. Cell Dev. Biol. 2016, 4, 31. [Google Scholar] [CrossRef]
- Wagner, E.F.; Nebreda, Á.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537. [Google Scholar] [CrossRef]
- Efimova, T. p38δ mitogen-activated protein kinase regulates skin homeostasis and tumorigenesis. Cell Cycle 2010, 9, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Adhikary, G.; Kanade, S.; Rorke, E.; Eckert, R. p38δ regulates p53 to control p21Cip1 expression in human epidermal keratinocytes. J. Biol. Chem. 2014, 289, 11443–11453. [Google Scholar] [CrossRef]
- Schindler, E.M.; Hindes, A.; Gribben, E.L.; Burns, C.J.; Yin, Y.; Lin, M.-H.; Owen, R.J.; Longmore, G.D.; Kissling, G.E.; Arthur, J.S.C.; et al. p38δ Mitogen-Activated Protein Kinase Is Essential for Skin Tumor Development in Mice. Cancer Res. 2009, 69, 4648–4655. [Google Scholar] [CrossRef]
- Haider, A.S.; Peters, S.B.; Kaporis, H.; Cardinale, I.; Fei, J.; Ott, J.; Blumenberg, M.; Bowcock, A.M.; Krueger, J.G.; Carucci, J.A. Genomic Analysis Defines a Cancer-Specific Gene Expression Signature for Human Squamous Cell Carcinoma and Distinguishes Malignant Hyperproliferation from Benign Hyperplasia. J. Investig. Dermatol. 2006, 126, 869–881. [Google Scholar] [CrossRef]
- Tan, F.L.-S.; Ooi, A.; Huang, D.; Wong, J.C.; Qian, C.-N.; Chao, C.; Ooi, L.; Tan, Y.-M.; Chung, A.; Cheow, P.-C.; et al. p38delta/MAPK13 as a diagnostic marker for cholangiocarcinoma and its involvement in cell motility and invasion. Int. J. Cancer 2010, 126, 2353–2361. [Google Scholar] [CrossRef]
- Yasuda, K.; Hirohashi, Y.; Kuroda, T.; Takaya, A.; Kubo, T.; Kanaseki, T.; Tsukahara, T.; Hasegawa, T.; Saito, T.; Sato, N.; et al. MAPK13 is preferentially expressed in gynecological cancer stem cells and has a role in the tumor-initiation. Biochem. Biophys. Res. Commun. 2016, 472, 643–647. [Google Scholar] [CrossRef]
- Wada, M.; Canals, D.; Adada, M.; Coant, N.; Salama, M.F.; Helke, K.L.; Arthur, J.S.; Shroyer, K.R.; Kitatani, K.; Obeid, L.M.; et al. P38 delta MAPK promotes breast cancer progression and lung metastasis by enhancing cell proliferation and cell detachment. Oncogene 2017, 36, 6649. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Ala-Aho, R.; Jokilehto, T.; Peltonen, J.; Kallajoki, M.; Grenman, R.; Jaakkola, P.; Westermarck, J.; Kähäri, V.-M. p38alpha and p38delta mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene 2007, 26, 5267–5279. [Google Scholar] [CrossRef]
- Zur, R.; Garcia-Ibanez, L.; Nunez-Buiza, A.; Aparicio, N.; Liappas, G.; Escós, A.; Risco, A.; Page, A.; Saiz-Ladera, C.; Alsina-Beauchamp, D.; et al. Combined deletion of p38γ and p38δ reduces skin inflammation and protects from carcinogenesis. Oncotarget 2015, 6, 12920–12935. [Google Scholar] [CrossRef]
- Kiss, A.; Koppel, A.C.; Anders, J.; Cataisson, C.; Yuspa, S.H.; Blumenberg, M.; Efimova, T. Keratinocyte p38δ loss inhibits Ras-induced tumor formation, while systemic p38δ loss enhances skin inflammation in the early phase of chemical carcinogenesis in mouse skin. Mol. Carcinog. 2016, 55, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Demehri, S.; Turkoz, A.; Manivasagam, S.; Yockey, L.J.; Turkoz, M.; Kopan, R. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell 2012, 22, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Iversen, L.; Kragballe, K.; Arthur, J.S.C.; Johansen, C. Mice lacking MSK1 and MSK2 show reduced skin tumor development in a two-stage chemical carcinogenesis model. Cancer Investig. 2011, 29, 240–245. [Google Scholar] [CrossRef]
- Scortegagna, M.; Cataisson, C.; Martin, R.J.; Hicklin, D.J.; Schreiber, R.D.; Yuspa, S.H.; Arbeit, J.M. HIF-1alpha regulates epithelial inflammation by cell autonomous NFkappaB activation and paracrine stromal remodeling. Blood 2008, 111, 3343–3354. [Google Scholar] [CrossRef]
- Scortegagna, M.; Martin, R.J.; Kladney, R.D.; Neumann, R.G.; Arbeit, J.M. Hypoxia-inducible factor-1alpha suppresses squamous carcinogenic progression and epithelial-mesenchymal transition. Cancer Res. 2009, 69, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Cipolat, S.; Hoste, E.; Natsuga, K.; Quist, S.R.; Watt, F.M. Epidermal barrier defects link atopic dermatitis with altered skin cancer susceptibility. Elife 2014, 3, e01888. [Google Scholar] [CrossRef] [PubMed]
- Di Piazza, M.; Nowell, C.S.; Koch, U.; Durham, A.-D.; Radtke, F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell 2012, 22, 479–493. [Google Scholar] [CrossRef]
- Biozzi, G.; Ribeiro, O.G.; Saran, A.; Araujo, M.L.; Maria, D.A.; De Franco, M.; Cabrera, W.K.; Sant’anna, O.A.; Massa, S.; Covelli, V.; et al. Effect of genetic modification of acute inflammatory responsiveness on tumorigenesis in the mouse. Carcinogenesis 1998, 19, 337–346. [Google Scholar] [CrossRef]
- Del Reino, P.; Alsina-Beauchamp, D.; Escós, A.; Cerezo-Guisado, M.; Risco, A.; Aparicio, N.; Zur, R.; Fernandez-Estévez, M.; Collantes, E.; Montans, J.; et al. Pro-oncogenic role of alternative p38 mitogen-activated protein kinases p38γ and p38δ, linking inflammation and cancer in colitis-associated colon cancer. Cancer Res. 2014, 74, 6150–6160. [Google Scholar] [CrossRef] [PubMed]
- Cotechini, T.; Medler, T.; Coussens, L. Myeloid Cells as Targets for Therapy in Solid Tumors. Cancer J. 2015, 21, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Hale, K.; Trollinger, D.; Rihanek, M.; Manthey, C. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J. Immunol. 1999, 162, 4246–4252. [Google Scholar]
- Ittner, A.; Block, H.; Reichel, C.; Varjosalo, M.; Gehart, H.; Sumara, G.; Gstaiger, M.; Krombach, F.; Zarbock, A.; Ricci, R. Regulation of PTEN activity by p38δ-PKD1 signaling in neutrophils confers inflammatory responses in the lung. J. Exp. Med. 2012, 209, 2229–2246. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Endoh, H.; Masuhiro, Y.; Kitamoto, T.; Uchiyama, S.; Sasaki, H.; Masushige, S.; Gotoh, Y.; Nishida, E.; Kawashima, H.; et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995, 270, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Frigo, D.E.; Basu, A.; Nierth-Simpson, E.N.; Weldon, C.B.; Dugan, C.M.; Elliott, S.; Collins-Burow, B.M.; Salvo, V.A.; Zhu, Y.; Melnik, L.I.; et al. p38 mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol. Endocrinol. 2006, 20, 971–983. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bai, W. Regulation of Estrogen Receptor Nuclear Export by Ligand-Induced and p38-Mediated Receptor Phosphorylation. Mol. Cell. Biol. 2002, 22, 5835–5845. [Google Scholar] [CrossRef] [PubMed]
- Antoon, J.W.; Bratton, M.R.; Guillot, L.M.; Wadsworth, S.; Salvo, V.A.; Burow, M.E. Inhibition of p38-MAPK alters SRC coactivation and estrogen receptor phosphorylation. Cancer Biol. Ther. 2012, 13, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Palucka, A.K.; Coussens, L.M. The Basis of Oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Andreu, P.; Coussens, L.M. Interactions between lymphocytes and myeloid cells regulate pro-versus anti-tumor immunity. Cancer Metastasis Rev. 2010, 29, 309–316. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Marone, G.; Mantovani, A. Cancer Inflammation and Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028662. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.; Novitskiy, S.; Moses, H.L. The roles of TGFβ in the tumour microenvironment. Nat. Rev. Cancer 2013, 13, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Andreu, P.; Johansson, M.; Affara, N.I.; Pucci, F.; Tan, T.; Junankar, S.; Korets, L.; Lam, J.; Tawfik, D.; DeNardo, D.G.; et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 2010, 17, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Reuver, S.; Feijoo, C.; Hasegawa, M.; Thomas, G.M.; Centeno, F.; Kuhlendahl, S.; Leal-Ortiz, S.; Goedert, M.; Garner, C.; et al. Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2. Biochem. J. 2004, 380, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Sumara, G.; Formentini, I.; Collins, S.; Sumara, I.; Windak, R.; Bodenmiller, B.; Ramracheya, R.; Caille, D.; Jiang, H.; Platt, K.A.; et al. Regulation of PKD by the MAPK p38δ in Insulin Secretion and Glucose Homeostasis. Cell 2009, 136, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Dassule, H.R.; Lewis, P.; Bei, M.; Maas, R.; McMahon, A.P. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 2000, 127, 4775. [Google Scholar] [PubMed]
- Clausen, B.; Burkhardt, C.; Reith, W.; Renkawitz, R.; Förster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999, 8, 265–277. [Google Scholar] [CrossRef] [PubMed]
| Title | Males and Females | Males | Females | |||
|---|---|---|---|---|---|---|
| WT | p38δ-cKOΔM | WT | p38δ-cKOΔM | WT | p38δ-cKOΔM | |
| Number of animals | 51 | 34 | 26 | 20 | 25 | 14 |
| Median time to tumor (weeks) | 21 | 23 | 16.5 | 22.5 | 23 | 23.5 |
| Ratio of median time until tumor | 0.913 | 0.7333 | 0.9787 | |||
| 95% confidence interval of ratio of median time until tumor | 0.2985–1.528 | 0.2146–1.252 | 0.4966–1.461 | |||
| Mean time to tumor ± SE | 24.4 ± 1.9 | 28.3 ± 2.4 | 21.9 ± 2.6 | 28.8 ± 3.1 | 26.9 ± 2.6 | 27.5 ± 3.7 |
| Log-rank test p-values (one-sided) | 0.0949 | 0.0291 (*) | 0.4371 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, A.; Koppel, A.C.; Murphy, E.; Sall, M.; Barlas, M.; Kissling, G.; Efimova, T. Cell Type-Specific p38δ Targeting Reveals a Context-, Stage-, and Sex-Dependent Regulation of Skin Carcinogenesis. Int. J. Mol. Sci. 2019, 20, 1532. https://doi.org/10.3390/ijms20071532
Kiss A, Koppel AC, Murphy E, Sall M, Barlas M, Kissling G, Efimova T. Cell Type-Specific p38δ Targeting Reveals a Context-, Stage-, and Sex-Dependent Regulation of Skin Carcinogenesis. International Journal of Molecular Sciences. 2019; 20(7):1532. https://doi.org/10.3390/ijms20071532
Chicago/Turabian StyleKiss, Alexi, Aaron C. Koppel, Emily Murphy, Maxwell Sall, Meral Barlas, Grace Kissling, and Tatiana Efimova. 2019. "Cell Type-Specific p38δ Targeting Reveals a Context-, Stage-, and Sex-Dependent Regulation of Skin Carcinogenesis" International Journal of Molecular Sciences 20, no. 7: 1532. https://doi.org/10.3390/ijms20071532
APA StyleKiss, A., Koppel, A. C., Murphy, E., Sall, M., Barlas, M., Kissling, G., & Efimova, T. (2019). Cell Type-Specific p38δ Targeting Reveals a Context-, Stage-, and Sex-Dependent Regulation of Skin Carcinogenesis. International Journal of Molecular Sciences, 20(7), 1532. https://doi.org/10.3390/ijms20071532





