Potential Therapeutic Effects of Gut Hormones, Ghrelin and Obestatin in Oral Mucositis
Abstract
1. Physiological Mechanisms of Maintaining the Integrity of Oral Mucosa
1.1. Dynamics of Cell Renewal of Oral Mucosa
1.2. Protective and Healing Effect of Saliva
2. Oral Mucositis—Meet the Enemy
3. Management of Oral Mucositis
3.1. Prevention
3.2. Treatment
4. Ghrelin and Its Main Physiological Effects
5. Protective, Therapeutic and Anti-Inflammatory Effects of Ghrelin
6. Ghrelin as a New Weapon in the Treatment of Oral Mucositis
7. Obestatin and Its Protective and Therapeutic Effects
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jańczuk, Z. (Ed.) Choroby błony śluzowej jamy ustnej. Symptomatologia ogólna. In Zarys Kliniczny Stomatologii Zachowawczej; PZWL: Warszawa, Poland, 1981; pp. 487–575. [Google Scholar]
- Duncan, M.; Grant, G. Oral and intestinal mucositis—Causes and possible treatments. Aliment. Pharmacol. Ther. 2003, 18, 853–874. [Google Scholar] [CrossRef]
- Rodríguez-Caballero, A.; Torres-Lagares, D.; Robles-García, M.; Pachón-Ibáñez, J.; González-Padilla, D.; Gutiérrez-Pérez, J.L. Cancer treatment-induced oral mucositis: A critical review. Int. J. Oral Maxillofac. Surg. 2012, 41, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Eilers, J.; Harris, D.; Henry, K.; Johnson, L.A. Evidence-based interventions for cancer treatment-related mucositis: Putting evidence into practice. Clin. J. Oncol. Nurs. 2014, 18, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, A.; Oñate-Sánchez, R.-E.; Cabrerizo-Merino, M.C.; de Arriba-de la-Fuente, F.; Heras-Fernando, I.; Vicente-García, V. Influence of oral health on mucositis in patients undergoing hematopoietic progenitor cell transplantation (HPCT). Med. Oral Patol. Oral Cir. Bucal 2012, 17, e94–e101. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury. Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- López Castaño, F.; Oñate Sánchez, R.E.; Roldán Chicano, R.; Cabrerizo Merino, M.C. Valoración de la mucositis secundaria a tratamiento oncohematológico mediante distintas escalas. Revisión. Med. Oral Patol. Oral Cir. Bucal 2005, 10, 412–421. [Google Scholar]
- Nauntofte, B.; Jensen, J.L. Salivary secretion. In Textbook of Gastroenterology; Yamada, T., Alpers, D., Laine, L., Owang, C., Powell, D.W., Eds.; Lippincott, Wiliams & Wilkins Publishers: Philadelphia, PA, USA, 1999; pp. 263–278. [Google Scholar]
- Warzecha, Z.; Kownacki, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Dembinski, A. Ghrelin accelerates the healing of oral ulcers in non-sialoadenectomized and sialoadenectomized rats. J. Physiol. Pharmacol. 2013, 64, 657–668. [Google Scholar]
- Noguchi, S.; Ohba, Y.; Oka, T. Effect of salivary epidermal growth factor on wound healing of tongue in mice. Am. J. Physiol. 1991, 260, E620–E625. [Google Scholar] [CrossRef]
- Bodner, L.; Dayan, D.; Pinto, Y.; Hammel, I. Characteristics of palatal wound healing in desalivated rats. Arch. Oral Biol. 1993, 38, 17–21. [Google Scholar] [CrossRef]
- Caplan, D.J.; Hunt, R.J. Salivary flow and risk of tooth loss in an elderly population. Community Dent. Oral Epidemiol. 1996, 24, 68–71. [Google Scholar] [CrossRef]
- Samnieng, P.; Ueno, M.; Shinada, K.; Zaitsu, T.; Wright, F.A.; Kawaguchi, Y. Association of hyposalivation with oral function, nutrition and oral health in community-dwelling elderly Thai. Community Dent. Health 2012, 29, 117–123. [Google Scholar]
- Jones, L.R.; Toth, B.B.; Keene, H.J. Effects of total body irradiation on salivary gland function and caries-associated oral microflora in bone marrow transplant patients. Oral Sur. Oral Med. Oral Pathol. 1992, 73, 670–676. [Google Scholar] [CrossRef]
- Someya, M.; Sakata, K.I.; Nagakura, H.; Nakata, K.; Oouchi, A.; Hareyama, M. The changes in irradiated salivary gland function of patients with head and neck tumors treated with radiotherapy. Jpn. J. Clin. Oncol. 2003, 33, 336–340. [Google Scholar] [CrossRef]
- Möller, P.; Perrier, M.; Ozsahin, M.; Monnier, P. A prospective study of salivary gland function in patients undergoing radiotherapy for squamous cell carcinoma of the oropharynx. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 173–189. [Google Scholar] [CrossRef]
- Cohen, S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 1962, 237, 1555–1562. [Google Scholar] [PubMed]
- Gregory, H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature 1975, 257, 325–327. [Google Scholar] [CrossRef]
- Culmer, C.U.; Gray, J.S.; Adkison, J.L.; Ivy, A.C. On the origin of urogastrone. Science 1940, 91, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Kasselberg, A.G.; Orth, D.N.; Gray, M.E.; Stahlman, M.T. Immunocytochemical localization of human epidermal growth factor/urogastrone in several human tissues. J. Histochem. Cytochem. 1985, 33, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Gregory, H.; Walsh, S.; Hopkins, C.R. The identification of urogastrone in serum, saliva and gastric juice. Gastroenterology 1979, 77, 313–318. [Google Scholar]
- Jaworek, J.; Konturek, S.J.; Bielanski, W.; Bilski, J.; Hladij, M. Release and binding of epidermal growth factor in the pancreas of rats. Int. J. Pancreatol. 1992, 11, 9–17. [Google Scholar] [CrossRef]
- Soler, C.; Carpenter, G. The epidermal growth factor family. In Guidebook to Cytokines and Their Receptors; Nicola, N.A., Ed.; A Sambrook & Tooze Publication: Oxford, UK; New York, NY, USA; Tokyo, Japan, 1997; pp. 194–197. [Google Scholar]
- Playford, R.J.; Boulton, R.; Ghatei, M.A.; Bloom, S.R.; Wright, N.A.; Goodlad, R.A. Comparison of the effects of transforming growth factor alpha and epidermal growth factor on gastrointestinal proliferation and hormone release. Digestion 1996, 57, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Joh, T.; Imai, S.; Miyamoto, T.; Matsusako, K.; Iwai, A.; Katsumi, K.; Endo, K.; Goto, K.; Takeuchi, T. Experimental and clinical studies on epidermal growth factor for gastric mucosal protection and healing of gastric ulcers. J. Clin. Gastroenterol. 1988, 10 (Suppl. 1), S7–S12. [Google Scholar] [CrossRef]
- Konturek, S.J. Role of growth factors in gastroduodenal protection and healing of peptic ulcers. Gastroenterol. Clin. N. Am. 1990, 19, 41–65. [Google Scholar]
- Konturek, S.J.; Brzozowski, T.; Dembinski, A.; Warzecha, Z.; Konturek, P.K.; Yanaihara, N. Interaction of growth hormone-releasing factor and somatostatin on ulcer healing and mucosal growth in rats: Role of gastrin and epidermal growth factor. Digestion 1988, 41, 121–128. [Google Scholar] [CrossRef]
- Dembiński, A.; Drozdowicz, D.; Gregory, H.; Konturek, S.J.; Warzecha, Z. Inhibition of acid formation by epidermal growth factor in the isolated rabbit gastric glands. J. Physiol. 1986, 378, 347–357. [Google Scholar] [CrossRef]
- Konturek, S.J.; Dembinski, A.; Warzecha, Z.; Bielanski, W.; Brzozowski, T.; Drozdowicz, D. Epidermal growth factor (EGF) in the gastroprotective and ulcer healing actions of colloidal bismuth subcitrate (De-Nol) in rats. Gut 1988, 29, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Brzozowski, T.; Bielanski, W.; Warzecha, Z.; Drozdowicz, D. Epidermal growth factor in the gastroprotective and ulcer-healing actions of sucralfate in rats. Am. J. Med. 1989, 86, 32–37. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Konturek, P.C.; Ceranowicz, P.; Konturek, S.J. Epidermal growth factor protects against pancreatic damage in cerulein-induced pancreatitis. Digestion 1999, 60, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Dembiński, A.; Warzecha, Z.; Konturek, P.C.; Ceranowicz, P.; Stachura, J.; Tomaszewska, R.; Konturek, S.J. Epidermal growth factor accelerates pancreatic recovery after caerulein-induced pancreatitis. Eur. J. Pharmacol. 2000, 398, 159–168. [Google Scholar] [CrossRef]
- Tomaszewska, R.; Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Konturek, S.J.; Stachura, J. The influence of epidermal growth factor on the course of ischemia-reperfusion induced pancreatitis in rats. J. Physiol. Pharmacol. 2002, 53, 183–198. [Google Scholar] [PubMed]
- Konturek, P.C.; Dembinski, A.; Warzecha, Z.; Ihlm, A.; Ceranowicz, P.; Konturek, S.J.; Stachura, J.; Hahn, E.G. Comparison of Epidermal Growth Factor and Transforming Growth Factor-β1 Expression in Hormone-Induced Acute Pancreatitis in Rats. Digestion 1998, 59, 110–119. [Google Scholar] [CrossRef]
- Morris-Wiman, J.; Sego, R.; Brinkley, L.; Dolce, C. The effects of sialoadenectomy and exogenous EGF on taste bud morphology and maintenance. Chem. Sens. 2000, 25, 9–19. [Google Scholar] [CrossRef]
- Fujisawa, K.; Miyamoto, Y.; Nagayama, M. Basic fibroblast growth factor and epidermal growth factor reverse impaired ulcer healing of the rabbit oral mucosa. J. Oral Pathol. Med. 2003, 32, 358–366. [Google Scholar] [CrossRef]
- Ino, M.; Ushiro, K.; Ino, C.; Yamashita, T.; Kumazawa, T. Kinetics of epidermal growth factor in saliva. Acta Otolaryngol. Suppl. 1993, 500, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Azuma, N.; Katada, Y.; Sano, H. Deterioration in saliva quality in patients with Sjögren’s syndrome: Impact of decrease in salivary epidermal growth factor on the severity of intraoral manifestations. Inflamm. Regen. 2018, 38, 6. [Google Scholar] [CrossRef]
- Dumbrigue, H.B.; Sandow, P.L.; Nguyen, K.H.; Humphreys-Beher, M.G. Salivary epidermal growth factor levels decrease in patients receiving radiation therapy to the head and neck. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 89, 710–716. [Google Scholar] [CrossRef]
- Epstein, J.B.; Gorsky, M.; Guglietta, A.; Le, N.; Sonis, S.T. The correlation between epidermal growth factor levels in saliva and the severity of oral mucositis during oropharyngeal radiation therapy. Cancer 2000, 89, 2258–2265. [Google Scholar] [CrossRef]
- Wu, H.G.; Song, S.Y.; Kim, Y.S.; Oh, Y.T.; Lee, C.G.; Keum, K.C.; Ahn, Y.C.; Lee, S.W. Therapeutic effect of recombinant human epidermal growth factor (RhEGF) on mucositis in patients undergoing radiotherapy, with or without chemotherapy, for head and neck cancer: A double-blind placebo-controlled prospective phase 2 multi-institutional clinical trial. Cancer 2009, 115, 3699–3708. [Google Scholar] [CrossRef] [PubMed]
- Fahnestock, M. Structure and biosynthesis of nerve growth factor. Curr. Top. Microbiol. Immunol. 1991, 165, 1–26. [Google Scholar] [PubMed]
- Bracci-Laudiero, L.; De Stefano, M.E. NGF in early embryogenesis, differentiation, and pathology in the nervous and immune systems. Curr. Top. Behav. Neurosci. 2016, 29, 125–152. [Google Scholar] [CrossRef]
- Freeman, R.S.; Burch, R.L.; Crowder, R.J.; Lomb, D.J.; Schoell, M.C.; Straub, J.A.; Xie, L. NGF deprivation-induced gene expression: After ten years, where do we stand? Prog. Brain Res. 2004, 146, 111–126. [Google Scholar] [CrossRef]
- Lu, X.-M.; Shu, Y.-H.; Qiu, C.-H.; Chen, K.-T.; Wang, Y.-T. Protective effects and anti-apoptotic role of nerve growth factor on spinal cord neurons in sciatic nerve-injured rats. Neurol. Res. 2014, 36, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Koyama, H.; Sato, H.; Sawada, J.; Itakura, A.; Tanaka, A.; Matsumoto, M.; Konno, K.; Ushio, H.; Matsuda, K. Role of nerve growth factor in cutaneous wound healing: Accelerating effects in normal and healing-impaired diabetic mice. J. Exp. Med. 1998, 187, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Matsuda, H. Nerve growth factor and wound healing. Prog. Brain Res. 2004, 146, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Tirassa, P.; Lambiase, A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol. Res. 2008, 57, 253–258. [Google Scholar] [CrossRef]
- Næsse, E.P.; Schreurs, O.; Messelt, E.; Hayashi, K.; Schenck, K. Distribution of nerve growth factor, pro-nerve growth factor, and their receptors in human salivary glands. Eur. J. Oral Sci. 2013, 121, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Storesund, T.; Schreurs, O.; Khuu, C.; Husvik, C.; Karatsaidis, A.; Helgeland, K.; Martin-Zanca, D.; Schenck, K. Nerve growth factor β/pro-nerve growth factor and their receptors in normal human oral mucosa. Eur. J. Oral Sci. 2007, 115, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Karatsaidis, A.; Schreurs, O.; Bjørnland, T.; Sugisaki, M.; Schenck, K. NGF and its receptors TrkA and p75NTR in the epithelium of oral lichen. J. Oral Pathol. Med. 2008, 37, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Schenck, K.; Schreurs, O.; Hayashi, K.; Helgeland, K. The role of nerve growth factor (NGF) and its precursor forms in oral wound healing. Int. J. Mol. Sci. 2017, 18, 386. [Google Scholar] [CrossRef]
- Borelli, V.; Marchioli, A.; Di Taranto, R.; Romano, M.; Chiandussi, S.; Di Lenarda, R.; Biasotto, M.; Zabucchi, G. Neuropeptides in saliva of subjects with burning mouth syndrome: A pilot study. Oral Dis. 2010, 16, 365–374. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, REVIEWS3005. [Google Scholar] [CrossRef]
- Powers, C.J.; McLeskey, S.W.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 2000, 7, 165–197. [Google Scholar] [CrossRef]
- Van Setten, G.B. Basic fibroblast growth factor in human saliva: Detection and physiological implications. Laryngoscope 1995, 105, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Westermark, A.; Pyykkö, I.; Magnusson, M.; Ishizaki, H.; Jäntti, P.; Van Setten, G. Basic fibroblast growth factor in human saliva decreases with aging. Laryngoscope 2002, 112, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Jansma, J.; Spijkervet, F.K.L.; Burlage, F.R.; Coppes, R.P. Oral sequelae of head and neck radiotherapy. Crit. Rev. Oral Biol. Med. 2003, 14, 199–212. [Google Scholar] [CrossRef]
- Cotrim, A.P.; Sowers, A.; Mitchell, J.B.; Baum, B.J. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol. Ther. 2007, 15, 2101–2106. [Google Scholar] [CrossRef]
- Gorugantula, L.M.; Rees, T.; Plemons, J.; Chen, H.S.; Cheng, Y.S.L. Salivary basic fibroblast growth factor in patients with oral squamous cell carcinoma or oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 215–222. [Google Scholar] [CrossRef]
- Gupta, A.; Tripathi, A.; Patil, R.; Kumar, V.; Khanna, V.; Singh, V. Estimation of salivary and serum basic fibroblast growth factor in treated and untreated patients with oral squamous cell carcinoma. J. Oral Biol. Craniofac. Res. 2019, 9, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Castro, M.; Reyes, M.; Torres, V.A. Histatins, wound healing, and cell migration. Oral Dis. 2018, 24, 1150–1160. [Google Scholar] [CrossRef]
- Melino, S.; Santone, C.; Di Nardo, P.; Sarkar, B. Histatins: Salivary peptides with copper(II)- and zinc(II)-binding motifs Perspectives for biomedical applications. FEBS J. 2014, 281, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, L.M.; Azen, E.A. Histatins, a family of salivary histidine-rich proteins, are encoded by at least two loci (HIS1 and HIS2). Biochem. Biophys. Res. Commun. 1989, 160, 495–502. [Google Scholar] [CrossRef]
- Ahmad, M.; Piludu, M.; Oppenheim, F.G.; Helmerhorst, E.J.; Hand, A.R. Immunocytochemical Localization of Histatins in Human Salivary Glands. J. Histochem. Cytochem. 2004, 52, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Padovan, L.; Segat, L.; Pontillo, A.; Antcheva, N.; Tossi, A.; Crovella, S. Histatins in non-human primates: Gene variations and functional effects. Protein Pept. Lett. 2010, 17, 909–918. [Google Scholar] [CrossRef]
- Welling, M.M.; Brouwer, C.P.J.M.; Van’T Hof, W.; Veerman, E.C.I.; Amerongen, A.V. Histatin-derived monomeric and dimeric synthetic peptides show-strong bactericidal activity towards multidrug-resistant Staphylococcus aureus in vivo. Antimicrob. Agents Chemother. 2007, 51, 3416–3419. [Google Scholar] [CrossRef]
- Xu, T.; Levitz, S.M.; Diamond, R.D.; Oppenheim, F.G. Anticandidal activity of major human salivary histatins. Infect. Immun. 1991, 59, 2549–2554. [Google Scholar]
- Oudhoff, M.J.; Bolscher, J.G.M.; Nazmi, K.; Kalay, H.; van’t Hof, W.; Amerongen, A.V.N.; Veerman, E.C.I. Histatins are the major wound-closure stimulating factors in human saliva as identified in a cell culture assay. FASEB J. 2008, 22, 3805–3812. [Google Scholar] [CrossRef] [PubMed]
- Oudhoff, M.J.; Blaauboer, M.E.; Nazmi, K.; Scheres, N.; Bolscher, J.G.M.; Veerman, E.C.I. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol. Chem. 2010, 391, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, I.A.; Ferrando, M.L.; Van Der Wijk, A.E.; Hoebe, R.A.; Nazmi, K.; De Jonge, W.J.; Krawczyk, P.M.; Bolscher, J.G.M.; Veerman, E.C.I.; Stap, J. Human salivary peptide histatin-1 stimulates epithelial and endothelial cell adhesion and barrier function. FASEB J. 2017, 31, 3922–3933. [Google Scholar] [CrossRef]
- Scully, C.; Epstein, J.; Sonis, S. Oral mucositis: challenging complication of radiotherapy, chemotherapy, and radiochemotherapy. Part 2: diagnosis and management of mucositis. Head Neck 2004, 26, 77–84. [Google Scholar] [CrossRef]
- Stokman, M.A.; Spijkervet, F.K.L.; Boezen, H.M.; Schouten, J.P.; Roodenburg, J.L.N.; de Vries, E.G.E. Preventive Intervention Possibilities in Radiotherapy- and Chemotherapy-induced Oral Mucositis: Results of Meta-analyses. J. Dent. Res. 2006, 85, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.B.; Sonis, S.T.; Monopoli, M.M.; Sonis, A.L. A longitudinal study of oral ulcerative mucositis in bone marrow transplant recipients. Cancer 1993, 72, 1612–1617. [Google Scholar] [CrossRef]
- Pico, J.; Avila-Garavito, A.; Naccache, P. Mucositis: Its Occurrence, Consequences, and Treatment in the Oncology Setting. Oncologist 1998, 3, 446–451. [Google Scholar]
- Rubenstein, E.B.; Peterson, D.E.; Schubert, M.; Keefe, D.; McGuire, D.; Epstein, J.; Elting, L.S.; Fox, P.C.; Cooksley, C.; Sonis, S.T. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004, 100, 2026–2046. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, J.M.; Bensadoun, R.-J.; Tunèr, J.; Frigo, L.; Gjerde, K.; Lopes-Martins, R.A. A systematic review with meta-analysis of the effect of low-level laser therapy (LLLT) in cancer therapy-induced oral mucositis. Support. Care Cancer 2011, 19, 1069–1077. [Google Scholar] [CrossRef]
- McGuire, D.B.; Correa, M.E.P.; Johnson, J.; Wienandts, P. The role of basic oral care and good clinical practice principles in the management of oral mucositis. Support. Care Cancer 2006, 14, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Huebener, P.; Schwabe, R.F. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene 2016, 35, 5931–5941. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Gibson, R.J.; Bowen, J.M.; Stringer, A.M.; Sonis, S.T.; Keefe, D.M.K. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: Implications for the pathobiology of mucositis. Cancer Chemother. Pharmacol. 2008, 62, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Yeoh, A.S.-J.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: Pathobiology, animal models and cytotoxic drugs. Cancer Treat. Rev. 2007, 33, 448–460. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S.T. Mucositis. Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Sabater Recolons, M.; López López, J.; Rodríguez de Rivera Campillo, M.E.; Chimenos Küstner, E.; Conde Vidal, J.M. Buccodental health and oral mucositis. Clinical study in patients with hematological diseases. Med. Oral Patol. Oral Cir. 2006, 11, E497–E502. [Google Scholar]
- Maria, O.M.; Eliopoulos, N.; Muanza, T. Radiation-Induced Oral Mucositis. Front. Oncol. 2017, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Goyal, S.; Kim, M.M.; Cabrales, P.; Lybeck, M.; Caroen, S.; Oronsky, N.; Burbano, E.; Carter, C.; Oronsky, A. A Review of Clinical Radioprotection and Chemoprotection for Oral Mucositis. Transl. Oncol. 2018, 11, 771–778. [Google Scholar] [CrossRef]
- Shieh, S.H.; Wang, S.T.; Tsai, S.T.; Tseng, C.C. Mouth care for nasopharyngeal cancer patients undergoing radiotherapy. Eur. J. Cancer Part B Oral Oncol. 1997, 33, 36–41. [Google Scholar] [CrossRef]
- AAOM Clinical Practice Statement. Subject: Dental Evaluation Before Head and Neck Radiotherapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 564–565. [CrossRef]
- Spielberger, R.; Stiff, P.; Bensinger, W.; Gentile, T.; Weisdorf, D.; Kewalramani, T.; Shea, T.; Yanovich, S.; Hansen, K.; Noga, S.; McCarty, J.; LeMaistre, C.F.; Sung, E.C.; Blazar, B.R.; Elhardt, D.; Chen, M.G.; Emmanouilides, C. Palifermin for Oral Mucositis after Intensive Therapy for Hematologic Cancers. N. Engl. J. Med. 2004, 351, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Horsley, P.; Bauer, J.D.; Mazkowiack, R.; Gardner, R.; Bashford, J. Palifermin improves severe mucositis, swallowing problems, nutrition impact symptoms, and length of stay in patients undergoing hematopoietic stem cell transplantation. Support. Care Cancer 2006, 15, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125103s171s172lbl.pdf (accessed on 1 February 2019).
- European Medicines Agency, Public steatement, Kepivance. Available online: www.ema.europa.eu/en/documents/public-statement/public-statement-kepivance-withdrawal-marketing-authorisation-european-union_en.pdf (accessed on 1 February 2019).
- Kouvaris, J.R.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: The First Selective-Target and Broad-Spectrum Radioprotector. Oncologist 2007, 12, 738–747. [Google Scholar] [CrossRef]
- Anderson, C.M.; Sonis, S.T.; Lee, C.M.; Adkins, D.; Allen, B.G.; Sun, W.; Agarwala, S.S.; Venigalla, M.L.; Chen, Y.; Zhen, W.; Mould, D.R.; Holmlund, J.T.; Brill, J.M.; Buatti, J.M. Phase 1b/2a Trial of the Superoxide Dismutase Mimetic GC4419 to Reduce Chemoradiotherapy-Induced Oral Mucositis in Patients with Oral Cavity or Oropharyngeal Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Bensadoun, R.-J. Photobiomodulation or low-level laser therapy in the management of cancer therapy-induced mucositis, dermatitis and lymphedema. Curr. Opin. Oncol. 2018, 30, 226–232. [Google Scholar] [CrossRef]
- Bensadoun, R.J.; Franquin, J.C.; Ciais, G.; Darcourt, V.; Schubert, M.M.; Viot, M.; Dejou, J.; Tardieu, C.; Benezery, K.; Nguyen, T.D.; Laudyer, Y.; Dassonville, O.; Poissonnet, G.; Vallicioni, J.; Thyss, A.; Hamdi, M.; Chauvel, P.; Demard, F. Low-energy He/Ne laser in the prevention of radiation-induced mucositis. Support. Care Cancer 1999, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; Genot, M.T.; et al. Low-level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 2: proposed applications and treatment protocols. Support. Care Cancer 2016, 24, 2793–2805. [Google Scholar] [CrossRef] [PubMed]
- Zecha, J.A.; Raber-Durlacher, J.E.; Nair, R.G.; Epstein, J.B.; Sonis, S.T.; Elad, S.; Hamblin, M.R.; Barasch, A.; Migliorati, C.A.; Milstein, D.M.; et al. Low level laser therapy/photobiomodulation in the management of side effects of chemoradiation therapy in head and neck cancer: part 1: mechanisms of action, dosimetric, and safety considerations. Support. Care Cancer 2016, 24, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Wijers, O.B.; Levendag, P.C.; Harms, E.R.E.; Gan-Teng, A.M.; Schmitz, P.I.M.; Hendriks, W.D.H.; Wilms, E.B.; Van Der Est, H.; Visch, L.L. Mucositis reduction by selective elimination of oral flora in irradiated cancers of the head and neck: A placebo-controlled double-blind randomized study. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 343–352. [Google Scholar] [CrossRef]
- Stokman, M.A.; Spijkervet, F.K.L.; Burlage, F.R.; Dijkstra, P.U.; Manson, W.L.; de Vries, E.G.E.; Roodenburg, J.L.N. Oral mucositis and selective elimination of oral flora in head and neck cancer patients receiving radiotherapy: A double-blind randomised clinical trial. Br. J. Cancer 2003, 88, 1012–1016. [Google Scholar] [CrossRef]
- Symonds, R.P.; McIlroy, P.; Khorrami, J.; Paul, J.; Pyper, E.; Alcock, S.R.; McCallum, I.; Speekenbrink, A.B.J.; McMurray, A.; Lindemann, E.; et al. The reduction of radiation mucositis by selective decontamination antibiotic pastilles: A placebo-controlled double-blind trial. Br. J. Cancer 1996, 74, 312–317. [Google Scholar] [CrossRef]
- Bondi, E.; Baroni, C.; Prete, A.; Gatti, M.; Carrassi, A.; Lodi, G.; Porter, S.R. Local antimicrobial therapy of oral mucositis in paediatric patients undergoing bone marrow transplantation. Oral Oncol. 1997, 33, 322–326. [Google Scholar] [CrossRef]
- Nicolatou, O.; Sotiropoulou-Lontou, A.; Skarlatos, J.; Kyprianou, K.; Kolitsi, G.; Dardoufas, K. A pilot study of the effect of granulocyte-macrophage colony-stimulating factor on oral mucositis in head and neck cancer patients during X-radiation therapy: A preliminary report. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 551–556. [Google Scholar] [CrossRef]
- Rovirosa, A.; Ferre, J.; Biete, A. Granulocyte macrophage-colony-stimulating factor mouthwashes heal oral ulcers during head and neck radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 747–754. [Google Scholar] [CrossRef]
- Mantovani, G.; Massa, E.; Astara, G.; Murgia, V.; Gramignano, G.; Lusso, M.R.; Camboni, P.; Ferreli, L.; Mocci, M.; Perboni, S.; et al. Phase II clinical trial of local use of GM-CSF for prevention and treatment of chemotherapy- and concomitant chemoradiotherapy-induced severe oral mucositis in advanced head and neck cancer patients: An evaluation of effectiveness, safety and costs. Oncol. Rep. 2003, 10, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, X.-E.; Ji, Z.-Q.; Liu, M.-Y.; Qian, T.; Li, L. Safety and Efficacy of a Mouth-Rinse with Granulocyte Colony Stimulating Factor in Patients with Chemotherapy-Induced Oral Mucositis. Asian Pac. J. Cancer Prev. 2016, 17, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Du, W.; Ke, Q.; Huang, B.; Yang, J. The effects of recombinant human granulocyte colony-stimulating factor mouthwash on radiotherapy-induced oral mucositis in locally advanced nasopharyngeal carcinoma patients. Adv. Clin. Exp. Med. 2017, 26, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.K.; Swann, S.; LeVeque, F.; Scarantino, C.W.; Johnson, D.; Chen, A.; Fortin, A.; Pollock, J.D.; Kim, H.; Ang, K.K. The impact of concurrent granulocyte macrophage-colony stimulating factor on radiation-induced mucositis in head and neck cancer patients: A double-blind placebo-controlled prospective Phase III study by Radiation Therapy Oncology Group 9901. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.E.; Pugh, S.L.; James, J.L.; Scarantino, C.; Movsas, B.; Valicenti, R.K.; Fortin, A.; Pollock, J.; Kim, H.; Brachman, D.G.; et al. The impact of concurrent granulocyte-macrophage colony-stimulating factor on quality of life in head and neck cancer patients: Results of the randomized, placebo-controlled Radiation Therapy Oncology Group 9901 trial. Qual. Life Res. 2014, 23, 1841–1858. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, C.; Cariello, A.; Giovanis, P.; Monti, M.; Vertogen, B.; Leoni, M.; Tienghi, A.; Turci, D.; Rosti, G.; Nanni, O.; et al. Prophylaxis with GM-CSF mouthwashes does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high-dose chemotherapy with autologous peripheral blood stem cell transplantation rescue: A double blind, randomiz. Ann. Oncol. 2003, 14, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, D.; Sanz, M.; Sureda, A.; Sala, M.; Muñoz, L.; Subirá, M.; Laborda, R.; Clopés, A.; Sierra, J. Mouth-washings with recombinant human granulocyte–macrophage colony stimulating factor (rhGM-CSF) do not improve grade III–IV oropharyngeal mucositis (OM) in patients with hematological malignancies undergoing stem cell transplantation. Results of a randomized double-blind placebo-controlled study. Bone Marrow Transpl. 2002, 29, 783–787. [Google Scholar] [CrossRef]
- Su, Y.B.; Vickers, A.J.; Zelefsky, M.J.; Kraus, D.H.; Shaha, A.R.; Shah, J.P.; Serio, A.M.; Harrison, L.B.; Bosl, G.J.; Pfister, D.G. Double-Blind, Placebo-Controlled, Randomized Trial of Granulocyte-Colony Stimulating Factor During Postoperative Radiotherapy for Squamous Head and Neck Cancer. Cancer J. 2008, 12, 182–188. [Google Scholar] [CrossRef]
- Sharifi, H.; Heydari, A.; Salek, R.; Emami Zeydi, A. Oral cryotherapy for preventing chemotherapy-induced oral mucositis: An effective but yet neglected strategy. J Cancer Res Ther. 2017, 13, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Cascinu, S.; Fedeli, A.; Fedeli, S.L.; Catalano, G. Oral cooling (cryotherapy), an effective treatment for the prevention of 5-fluorouracil-induced stomatitis. Eur. J. Cancer. Part B Oral Oncol. 1994, 30, 234–236. [Google Scholar] [CrossRef]
- Mahood, D.J.; Dose, A.M.; Loprinzi, C.L.; Veeder, M.H.; Athmann, L.M.; Therneau, T.M.; Sorensen, J.M.; Gainey, D.K.; Mailliard, J.A.; Gusa, N.L. Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J. Clin. Oncol. 2017, 9, 449–452. [Google Scholar] [CrossRef]
- Lilleby, K.; Garcia, P.; Gooley, T.; McDonnnell, P.; Taber, R.; Holmberg, L.; Maloney, D.G.; Press, O.W.; Bensinger, W. A prospective, randomized study of cryotherapy during administration of high-dose melphalan to decrease the severity and duration of oral mucositis in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transpl. 2006, 37, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Ninomiya, I.; Yamaguchi, T.; Terai, S.; Nakanuma, S.; Kinoshita, J.; Makino, I.; Nakamura, K.; Miyashita, T.; Tajima, H.; et al. Oral cryotherapy for prophylaxis of oral mucositis caused by docetaxel, cisplatin, and fluorouracil chemotherapy for esophageal cancer. Esophagus 2019. [Google Scholar] [CrossRef]
- Bossola, M. Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: A narrative review. Nutrients 2015, 7, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Wędrychowicz, A.; Spodaryk, M.; Krasowska-Kwiecień, A.; Goździk, J. Total parenteral nutrition in children and adolescents treated with high-dose chemotherapy followed by autologous haematopoietic transplants. Br. J. Nutr. 2010, 103, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Beckerson, J.; Szydlo, R.M.; Hickson, M.; Mactier, C.E.; Innes, A.J.; Gabriel, I.H.; Palanicawandar, R.; Kanfer, E.J.; Macdonald, D.H.; Milojkovic, D.; et al. Impact of route and adequacy of nutritional intake on outcomes of allogeneic haematopoietic cell transplantation for haematologic malignancies. Clin. Nutr. 2018, 38, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S. Recent advances in topical anesthesia. J. Dent. Anesth. Pain Med. 2016, 16, 237–244. [Google Scholar] [CrossRef]
- Mogensen, S.; Treldal, C.; Kristensen, C.A.; Bentzen, J.; Lawson-Smith, L.; Petersen, J.; Andersen, O. Effect of bupivacaine lozenges on oral mucositis pain. Pain Rep. 2017, 2, e619. [Google Scholar] [CrossRef]
- Carnel, S.B.; Blakeslee, D.B.; Oswald, S.G.; Barnes, M. Treatment of Radiation- and Chemotherapy-Induced Stomatitis. Otolaryngol. Head Neck Surg. 1990, 102, 326–330. [Google Scholar] [CrossRef]
- Alfieri, S.; Ripamonti, C.I.; Marceglia, S.; Orlandi, E.; Iacovelli, N.A.; Granata, R.; Cavallo, A.; Pozzi, P.; Boffi, R.; Bergamini, C.; et al. Temporal course and predictive factors of analgesic opioid requirement for chemoradiation-induced oral mucositis in oropharyngeal cancer. Head Neck 2016, 38 (Suppl. 1), E1521–E1527. [Google Scholar] [CrossRef]
- Konopka-Filippow, M.; Zabrocka, E.; Wójtowicz, A.; Skalij, P.; Wojtukiewicz, M.Z.; Sierko, E. Pain management during radiotherapy and radiochemotherapy in oropharyngeal cancer patients: Single-institution experience. Int. Dent. J. 2015, 65, 242–248. [Google Scholar] [CrossRef]
- Vayne-Bossert, P.; Escher, M.; de Vautibault, C.G.; Dulguerov, P.; Allal, A.; Desmeules, J.; Herrmann, F.R.; Pautex, S. Effect of Topical Morphine (Mouthwash) on Oral Pain Due to Chemotherapy- and/or Radiotherapy-Induced Mucositis: A Randomized Double-Blinded Study. J. Palliat. Med. 2010, 13, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Sarvizadeh, M.; Hemati, S.; Meidani, M.; Ashouri, M.; Roayaei, M.; Shahsanai, A. Morphine mouthwash for the management of oral mucositis in patients with head and neck cancer. Adv. Biomed. Res. 2015, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Saroja, G.; Devi, S.; Namrata, R. Oral morphine solution as an oral rinse or mouth gargle for mucositis pain. Indian J. Palliat. Care 2010, 16, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Huang, H.; Sun, X.; Xia, Z.; Li, Y.; Lin, X.; Guo, Y. Efficacy and safety of transdermal fentanyl for treatment of oral mucositis pain caused by chemotherapy. Expert Opin. Pharmacother. 2008, 9, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Charbaji, N.; Schäfer-Korting, M.; Küchler, S. Morphine stimulates cell migration of oral epithelial cells by delta-opioid receptor activation. PLoS ONE 2012, 7, e42616. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.; Datta, G.; Ahuja, S.; Saxena, T.; Datta, A.G. Prevalence of Oral Complications occurring in a Population of Pediatric Cancer Patients receiving Chemotherapy. Int. J. Clin. Pediatr. Dent. 2017, 10, 166–171. [Google Scholar] [CrossRef]
- Campos, M.I.D.C.; Campos, C.N.; Aarestrup, F.M.; Aarestrup, B.J.V. Oral mucositis in cancer treatment: Natural history, prevention and treatment. Mol. Clin. Oncol. 2014, 2, 337–340. [Google Scholar] [CrossRef]
- Gelclair Bioadherent Oral Gel. Available online: www.gelclair.com (accessed on 22 February 2019).
- Barber, C.; Powell, R.; Ellis, A.; Hewett, J. Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: A preliminary, double centre, randomised controlled trial on patients with radiotherapy-induced oral mucositis. Support. Care Cancer 2007, 15, 427–440. [Google Scholar] [CrossRef]
- Vokurka, S.; Skardova, J.; Hruskova, R.; Kabatova-Maxova, K.; Svoboda, T.; Bystricka, E.; Steinerova, K.; Koza, V. The effect of polyvinylpyrrolidone-sodium hyaluronate gel (Gelclair) on oral microbial colonization and pain control compared with other rinsing solutions in patients with oral mucositis after allogeneic stem cells transplantation. Med. Sci. Monit. 2011, 17, CR572–CR576. [Google Scholar] [CrossRef]
- Rasero, L.; Marsullo, M.; Dal Molin, A. Assessing the effectiveness of Gelclair(R) in the prevention and therapy of stomatitis in patients undergoing hematopoietic stem-cell transplantation: A randomized trial. Prof. Inferm. 2014, 67, 15–20. [Google Scholar] [CrossRef]
- Yu, S.Y.; Sun, X.D.; Wu, S.K.; Dong, L.H.; Qin, S.K.; Chen, Y.P.; Cheng, Y. Local analgesic effect of a bioadhesive barrier-forming oral liquid in cancer patients with oral mucositis caused by chemotherapy and/or radiotherapy: A randomized multicenter, single-use, positive-controlled, open-label study. OncoTargets Ther. 2018, 11, 8555–8564. [Google Scholar] [CrossRef]
- Allison, R.R.; Ambrad, A.A.; Arshoun, Y.; Carmel, R.J.; Ciuba, D.F.; Feldman, E.; Finkelstein, S.E.; Gandhavadi, R.; Heron, D.E.; Lane, S.C.; et al. Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer 2014, 120, 1433–1440. [Google Scholar] [CrossRef]
- Chaitanya, N.C.; Muthukrishnan, A.; Babu, D.B.G.; Kumari, C.S.; Lakshmi, M.A.; Palat, G.; Alam, K.S. Role of Vitamin E and Vitamin A in Oral Mucositis Induced by Cancer Chemo/Radiotherapy- A Meta-analysis. J. Clin. Diagn. Res. 2017, 11, ZE06–ZE09. [Google Scholar] [CrossRef]
- Nagi, R.; Patil, D.J.; Rakesh, N.; Jain, S.; Sahu, S. Natural agents in the management of oral mucositis in cancer patients-systematic review. J. Oral Biol. Craniofac. Res. 2018, 8, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Motallebnejad, M.; Akram, S.; Moghadamnia, A.; Moulana, Z.; Omidi, S. The effect of topical application of pure honey on radiation-induced mucositis: A randomized clinical trial. J. Contemp. Dent. Pract. 2008, 9, 40–47. [Google Scholar]
- Puataweepong, P.; Dhanachai, M.; Dangprasert, S.; Sithatani, C.; Sawangsilp, T.; Narkwong, L.; Puttikaran, P.; Intagumtornchai, T. The efficacy of oral aloe vera juice for radiation induced mucositis in head and neck cancer patients: A double-blind placebo-controlled study. Asian Biomed. 2009, 3, 375–382. [Google Scholar]
- Elad, S.; Meidan, I.; Sellam, G.; Simaan, S.; Zeevi, I.; Waldman, E.; Weintraub, M.; Revel-Vilk, S. Topical curcumin for the prevention of oral mucositis in pediatric patients: Case series. Altern. Ther. Health Med. 2013, 19, 21–24. [Google Scholar]
- Patil, K.; Guledgud, M.V.; Kulkarni, P.K.; Keshari, D.; Tayal, S. Use of curcumin mouthrinse in radio-chemotherapy induced oral mucositis patients: A pilot study. J. Clin. Diagn. Res. 2015, 9, ZC59–ZC62. [Google Scholar] [CrossRef]
- Ahmed, K.M. The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer. A randomized clinical trial. Saudi Dent. J. 2013, 25, 141–147. [Google Scholar] [CrossRef]
- Akhavan Karbassi, M.H.; Yazdi, M.F.; Ahadian, H.; SadrAbad, M.J. Randomized DoubleBlind PlaceboControlled Trial of Propolis for Oral Mucositis in Patients Receiving Chemotherapy for Head and Neck Cancer. Asian Pacific J. Cancer Prev. 2016, 17, 3611–3614. [Google Scholar]
- Piredda, M.; Facchinetti, G.; Biagioli, V.; Giannarelli, D.; Armento, G.; Tonini, G.; De Marinis, M.G. Propolis in the prevention of oral mucositis in breast cancer patients receiving adjuvant chemotherapy: A pilot randomised controlled trial. Eur. J. Cancer Care (Engl.) 2017, 26, e12757. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, P.E.; Ciol, M.A.; de Melo, N.S.; de Souza Figueiredo, P.T.; Leite, A.F.; de Melo Manzi, N. Chamomile infusion cryotherapy to prevent oral mucositis induced by chemotherapy: A pilot study. Support. Care Cancer 2016, 24, 4393–4398. [Google Scholar] [CrossRef]
- Gomes, V.T.S.; Nonato Silva Gomes, R.; Gomes, M.S.; Joaquim, W.M.; Lago, E.C.; Nicolau, R.A. Effects of Matricaria recutita (L.) in the Treatment of Oral Mucositis. Sci. World J. 2018, 2018, 4392184. [Google Scholar] [CrossRef]
- Jaworek, J.; Leja-Szpak, A.; Nawrot-Porąbka, K.; Szklarczyk, J.; Kot, M.; Pierzchalski, P.; Góralska, M.; Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; et al. Effects of melatonin and its analogues on pancreatic inflammation, enzyme secretion, and tumorigenesis. Int. J. Mol. Sci. 2017, 18, 1014. [Google Scholar] [CrossRef]
- Abdel Moneim, A.E.; Guerra-Librero, A.; Florido, J.; Shen, Y.Q.; Fernández-Gil, B.; Acuña-Castroviejo, D.; Escames, G. Oral Mucositis: Melatonin Gel an Effective New Treatment. Int. J. Mol. Sci. 2017, 18, 1003. [Google Scholar] [CrossRef]
- Leja-Szpak, A.; Nawrot-Porąbka, K.; Góralska, M.; Jastrzębska, M.; Link-Lenczowski, P.; Bonior, J.; Pierzchalski, P.; Jaworek, J. Effects of Melatonin and Its Analogues on Pancreatic Inflammation, Enzyme Secretion, and Tumorigenesis. Pharmacol Rep. 2018, 70, 1079–1088. [Google Scholar] [CrossRef]
- Lissoni, P.; Barni, S.; Mandalà, M.; Ardizzoia, A.; Paolorossi, F.; Vaghi, M.; Longarini, R.; Malugani, F.; Tancini, G. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur. J. Cancer 1999, 35, 1688–1692. [Google Scholar] [CrossRef]
- Lissoni, P.; Tancini, G.; Barni, S.; Paolorossi, F.; Ardizzoia, A.; Conti, A.; Maestroni, G. Treatment of cancer chemotherapy-induced toxicity with the pineal hormone melatonin. Support. Care Cancer 1997, 5, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Onseng, K.; Johns, N.P.; Khuayjarernpanishk, T.; Subongkot, S.; Priprem, A.; Hurst, C. Beneficial Effects of Adjuvant Melatonin in Minimizing Oral Mucositis Complications in Head and Neck Cancer Patients Receiving Concurrent Chemoradiation. J. Altern. Complement. Med. 2017, 23, 957–963. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- McKee, K.K.; Palyha, O.C.; Feighner, S.D.; Hreniuk, D.L.; Tan, C.P.; Phillips, M.S.; Smith, R.G.; Van der Ploeg, L.H.T.; Howard, A.D. Molecular Analysis of Rat Pituitary and Hypothalamic Growth Hormone Secretagogue Receptors. Mol. Endocrinol. 1997, 11, 415–423. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembinski, A. Protective and Therapeutic Effects of Ghrelin in the Gut. Curr. Med. Chem. 2012, 19, 118–125. [Google Scholar] [CrossRef]
- Davenport, A.P.; Bonner, T.I.; Foord, S.M.; Harmar, A.J.; Neubig, R.R.; Pin, J.P.; Spedding, M.; Kojima, M.; Kangawa, K. International Union of Pharmacology. LVI. Ghrelin Receptor Nomenclature, Distribution, and Function. Pharmacol. Rev. 2005, 57, 541–546. [Google Scholar] [CrossRef]
- Hattori, N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm. IGF Res. 2009, 19, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and Function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef]
- Seim, I.; Collet, C.; Herington, A.C.; Chopin, L.K. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genomisc 2007, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the Acyltransferase that Octanoylates Ghrelin, an Appetite-Stimulating Peptide Hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Akamizu, T.; Takaya, K.; Irako, T.; Hosoda, H.; Teramukai, S.; Matsuyama, A.; Tada, H.; Miura, K.; Shimizu, A.; Fukushima, M.; et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur. J. Endocrinol. 2004, 150, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988–2991. [Google Scholar] [CrossRef]
- Sakata, I.; Sakai, T. Ghrelin Cells in the Gastrointestinal Tract. Int. J. Pept. 2010, 2010, 945056. [Google Scholar] [CrossRef]
- Yokote, R.; Sato, M.; Matsubara, S.; Ohye, H.; Niimi, M.; Murao, K.; Takahara, J. Molecular cloning and gene expression of growth hormone-releasing peptide receptor in rat tissues. Peptides 1998, 19, 15–20. [Google Scholar] [CrossRef]
- Dixit, V.D.; Schaffer, E.M.; Pyle, R.S.; Collins, G.D.; Sakthivel, S.K.; Palaniappan, R.; Lillard, J.W., Jr.; Taub, D.D. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Investig. 2004, 114, 57–66. [Google Scholar] [CrossRef]
- Tannenbaum, G.S.; Lapointe, M.; Beaudet, A.; Howard, A.D. Expression of growth hormone secretagogue-receptors by growth hormone- releasing hormone neurons in the mediobasal hypothalamus. Endocrinology 1998, 139, 4420–4423. [Google Scholar] [CrossRef]
- Takaya, K.; Ariyasu, H.; Kanamoto, N.; Iwakura, H.; Yoshimoto, A.; Harada, M.; Mori, K.; Komatsu, Y.; Usui, T.; Shimatsu, A.; et al. Ghrelin strongly stimulates growth hormone (GH) release in humans. J. Clin. Endocrinol. Metab. 2000, 85, 4908–4911. [Google Scholar] [CrossRef]
- Broglio, F.; Benso, A.; Castiglioni, C.; Gottero, C.; Prodam, F.; Destefanis, S.; Gauna, C.; Van Der Lely, A.J.; Deghenghi, R.; Bo, M.; et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J. Clin. Endocrinol. Metab. 2003, 88, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Small, C.J.; Abbott, C.R.; Dhillo, W.S.; Seal, L.J.; Cohen, M.A.; Batterham, R.L.; Taheri, S.; Stanley, S.A.; Ghatei, M.A.; et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001, 50, 2540–2547. [Google Scholar] [CrossRef]
- Shimbara, T.; Mondal, M.S.; Kawagoe, T.; Toshinai, K.; Koda, S.; Yamaguchi, H.; Date, Y.; Nakazato, M. Central administration of ghrelin preferentially enhances fat ingestion. Neurosci. Lett. 2004, 369, 75–79. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Bielański, W.; Pawlik, W.W.; Kuwahara, A.; Kato, I. Dual age-dependent effect of ghrelin administration on serum level of insulin-like growth factor-1 and gastric growth in young rats. Eur. J. Pharmacol. 2006, 529, 145–150. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Konturek, S.J.; Polus, A.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Influence of ghrelin on gastric and duodenal growth and expression of digestive enzymes. J. Physiol. Pharmacol. 2006, 57, 425–437. [Google Scholar]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin Enhances Appetite and Increases Food Intake in Humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992. [Google Scholar] [CrossRef] [PubMed]
- Park, A.J.; Patterson, M.; Bloom, S.R.; Druce, M.R.; Milton, J.E.; Ghatei, M.A.; Wren, A.M.; Frost, G.; Small, C. Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. (London) 2005, 29, 1130–1136. [Google Scholar] [CrossRef]
- Druce, M.R.; Wren, A.M.; Park, A.J.; Milton, J.E.; Patterson, M.; Frost, G.; Ghatei, M.A.; Small, C.; Bloom, S.R. Subcutaneous administration of ghrelin stimulates energy intake in healthy lean human volunteers. Int. J. Obes. (London) 2006, 30, 293–296. [Google Scholar] [CrossRef]
- Hotta, M.; Ohwada, R.; Akamizu, T.; Shibasaki, T.; Takano, K.; Kangawa, K. Ghrelin increases hunger and food intake in patients with restricting-type anorexia nervosa: A pilot study. Endocr. J. 2009, 56, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Tschöp, M.H. Ghrelin—A key pleiotropic hormone-regulating systemic energy metabolism. Endocr. Dev. 2013, 25, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A. Peptidyl hormones of endocrine cells origin in the gut—Their discovery and physiological relevance. J. Physiol. Pharmacol. 2015, 66, 11–27. [Google Scholar]
- Ariyasu, H.; Takaya, K.; Tagami, T.; Ogawa, Y.; Hosoda, K.; Akamizu, T.; Suda, M.; Koh, T.; Natsui, K.; Toyooka, S.; et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4753–4758. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nagaya, N.; Isobe, T.; Imazu, M.; Okumura, H.; Hosoda, H.; Kojima, M.; Kangawa, K.; Kohno, N. Increased plasma ghrelin level in lung cancer cachexia. Clin. Cancer Res. 2003, 9, 774–778. [Google Scholar] [PubMed]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef]
- Foster-Schubert, K.E.; Overduin, J.; Prudom, C.E.; Liu, J.; Callahan, H.S.; Gaylinn, B.D.; Thorner, M.O.; Cummings, D.E. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J. Clin. Endocrinol. Metab. 2008, 93, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Riediger, T.; Traebert, M.; Schmid, H.A.; Scheel, C.; Lutz, T.A.; Scharrer, E. Site-specific effects of ghrelin on the neuronal activity in the hypothalamic arcuate nucleus. Neurosci. Lett. 2003, 341, 151–155. [Google Scholar] [CrossRef]
- Kamegai, J.; Tamura, H.; Shimizu, T.; Ishii, S.; Sugihara, H.; Wakabayashi, I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 2001, 50, 2438–2443. [Google Scholar] [CrossRef] [PubMed]
- Toshinai, K.; Date, Y.; Murakami, N.; Shimada, M.; Mondal, M.S.; Shimbara, T.; Guan, J.L.; Wang, Q.P.; Funahashi, H.; Sakurai, T.; Shioda, S.; Matsukura, S.; Kangawa, K.; Nakazato, M. Ghrelin-Induced Food Intake Is Mediated via the Orexin Pathway. Endocrinology 2003, 144, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Toshinai, K.; Koda, S.; Miyazato, M.; Shimbara, T.; Tsuruta, T.; Niijima, A.; Kangawa, K.; Nakazato, M. Peripheral Interaction of Ghrelin with Cholecystokinin on Feeding Regulation. Endocrinology 2005, 146, 3518–3525. [Google Scholar] [CrossRef]
- Date, Y.; Shimbara, T.; Koda, S.; Toshinai, K.; Ida, T.; Murakami, N.; Miyazato, M.; Kokame, K.; Ishizuka, Y.; Ishida, Y.; et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006, 4, 323–331. [Google Scholar] [CrossRef]
- Bellone, S.; Castellino, N.; Broglio, F.; Rapa, A.; Vivenza, D.; Radetti, G.; Bellone, J.; Gottero, C.; Ghigo, E.; Bona, G. Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. J. Clin. Endocrinol. Metab. 2004, 89, 1662–1665. [Google Scholar] [CrossRef] [PubMed]
- Prodam, F.; Monzani, A.; Ricotti, R.; Marolda, A.; Bellone, S.; Aimaretti, G.; Roccio, M.; Bona, G. Systematic review of ghrelin response to food intake in pediatric age, from neonates to adolescents. J. Clin. Endocrinol. Metab. 2014, 99, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Saito, E.S.; Kaiya, H.; Takagi, T.; Yamasaki, I.; Denbow, D.M.; Kangawa, K.; Furuse, M. Chicken ghrelin and growth hormone-releasing peptide-2 inhibit food intake of neonatal chicks. Eur. J. Pharmacol. 2002, 453, 75–79. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Bielański, W.; Cieszkowski, J.; Dembiński, M.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Variable effect of ghrelin administration on pancreatic development in young rats. Role of insulin-like growth factor-1. J. Physiol. Pharmacol. 2005, 56, 555–570. [Google Scholar] [PubMed]
- Masuda, Y.; Tanaka, T.; Inomata, N.; Ohnuma, N.; Tanaka, S.; Itoh, Z.; Hosoda, H.; Kojima, M.; Kangawa, K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem. Biophys. Res. Commun. 2000, 276, 905–908. [Google Scholar] [CrossRef] [PubMed]
- De la Cour, C.D.; Lindström, E.; Norlén, P.; Håkanson, R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul. Pept. 2004, 120, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Nakazato, M.; Murakami, N.; Kojima, M.; Kangawa, K.; Matsukura, S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem. Biophys. Res. Commun. 2001, 280, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Sibilia, V.; Pagani, F.; Guidobono, F.; Locatelli, V.; Torsello, A.; Deghenghi, R.; Netti, C. Evidence for a central inhibitory role of growth hormone secretagogues and ghrelin on gastric acid secretion in conscious rats. Neuroendocrinology 2002, 75, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, M.; Chen, X.; Segura, B.J.; Mulholland, M.W. Inhibition of pancreatic protein secretion by ghrelin in the rat. J. Physiol. 2001, 537, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Wang, G.; Englander, E.W.; Kojima, M.; Greeley, G.H. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: Enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002, 143, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Nakazato, M.; Hashiguchi, S.; Dezaki, K.; Mondal, M.S.; Hosoda, H.; Kojima, M.; Kangawa, K.; Arima, T.; Matsuo, H.; et al. Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes 2002, 51, 124–129. [Google Scholar] [CrossRef]
- Reimer, M.K.; Pacini, G.; Ahrén, B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 2003, 144, 916–921. [Google Scholar] [CrossRef]
- Broglio, F.; Arvat, E.; Benso, A.; Gottero, C.; Muccioli, G.; Papotti, M.; van der Lely, A.J.; Deghenghi, R.; Ghigo, E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5083–5086. [Google Scholar] [CrossRef] [PubMed]
- Dezaki, K. Ghrelin function in insulin release and glucose metabolism. Endocr. Dev. 2013, 25, 135–143. [Google Scholar]
- Frascarelli, S.; Ghelardoni, S.; Ronca-Testoni, S.; Zucchi, R. Effect of ghrelin and synthetic growth hormone secretagogues in normal and ischemic rat heart. Basic Res. Cardiol. 2003, 98, 401–405. [Google Scholar] [CrossRef]
- Takeda, R.; Nishimatsu, H.; Suzuki, E.; Satonaka, H.; Nagata, D.; Oba, S.; Sata, M.; Takahashi, M.; Yamamoto, Y.; Terauchi, Y.; et al. Ghrelin improves renal function in mice with ischemic acute renal failure. J. Am. Soc. Nephrol. 2006, 17, 113–121. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Xie, D.; Liu, K.; Chen, L. Ghrelin reduces injury of hippocampal neurons in a rat model of cerebral ischemia/reperfusion. Chin. J. Physiol. 2006, 49, 244–250. [Google Scholar]
- Wu, R.; Dong, W.; Zhou, M.; Zhang, F.; Marini, C.P.; Ravikumar, T.S.; Wang, P. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am. J. Respir. Crit. Care Med. 2007, 176, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Souza-Moreira, L.; Delgado-Maroto, V.; Morell, M.; O’Valle, F.; Del Moral, R.G.; Gonzalez-Rey, E. Therapeutic effect of ghrelin in experimental autoimmune encephalomyelitis by inhibiting antigen-specific Th1/Th17 responses and inducing regulatory T cells. Brain. Behav. Immun. 2013, 30, 54–60. [Google Scholar] [CrossRef]
- Hernández-Cortés, P.; Toledo-Romero, M.A.; Delgado, M.; Gonzalez-Rey, E.; Sánchez, R.G.; Prados-Olleta, N.; Aneiros-Fernández, J.; Crespo-Lora, V.; Aguilar, M.; Galindo-Moreno, P.; et al. Ghrelin and adipose-derived mesenchymal stromal cells improve nerve regeneration in a rat model of epsilon-caprolactone conduit reconstruction. Histol. Histopathol. 2017, 32, 627–637. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Li, H.; Yang, Z.; Zeng, Y.; Liu, J.; Hao, Y.; Li, R. Ghrelin accelerates wound healing through GHS-R1a-mediated MAPK-NF-κ B/GR signaling pathways in combined radiation and burn injury in rats. Sci. Rep. 2016, 6, 27499. [Google Scholar] [CrossRef] [PubMed]
- Sibilia, V.; Rindi, G.; Pagani, F.; Rapetti, D.; Locatelli, V.; Torsello, A.; Campanini, N.; Deghenghi, R.; Netti, C. Ghrelin protects against ethanol-induced gastric ulcers in rats: Studies on the mechanisms of action. Endocrinology 2003, 144, 353–359. [Google Scholar] [CrossRef]
- Brzozowski, T.; Konturek, P.C.; Konturek, S.J.; Kwiecień, S.; Drozdowicz, D.; Bielanski, W.; Pajdo, R.; Ptak, A.; Nikiforuk, A.; Pawlik, W.W.; et al. Exogenous and endogenous ghrelin in gastroprotection against stress-induced gastric damage. Regul. Pept. 2004, 120, 39–51. [Google Scholar] [CrossRef]
- Íşeri, S.Ö.; Şener, G.; Yüksel, M.; Contuk, G.; Çetinel, Ş.; Gedik, N.; Yeǧen, B.Ç. Ghrelin against alendronate-induced gastric damage in rats. J. Endocrinol. 2005, 187, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Sendur, R.; Cieszkowski, J.; Ceranowicz, D.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J. Physiol. Pharmacol. 2009, 60, 87–98. [Google Scholar]
- Warzecha, Z.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Ginter, G.; Ptak-Belowska, A.; Dembinski, A. Involvement of cyclooxygenase-1 and cyclooxygenase-2 activity in the therapeutic effect of ghrelin in the course of ethanol-induced gastric ulcers in rats. J. Physiol. Pharmacol. 2014, 65, 95–106. [Google Scholar] [PubMed]
- Warzecha, Z.; Ceranowicz, D.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Kuwahara, A.; Kato, I.; Konturek, P.C. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med. Sci. Monit. 2012, 18, BR181–BR187. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Dong, W.; Ji, Y.; Zhou, M.; Marini, C.P.; Ravikumar, T.S.; Wang, P. Orexigenic hormone Ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS ONE 2008, 3, e2026. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Chorny, A.; Delgado, M. Therapeutic Action of Ghrelin in a Mouse Model of Colitis. Gastroenterology 2006, 130, 1707–1720. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Engel, M.; Burnat, G.; Gaca, P.; Kwiecien, S.; Pajdo, R.; Konturek, S.J. Ghrelin ameliorates colonic inflammation. Role of nitric oxide and sensory nerves. J. Physiol. Pharmacol. 2009, 60, 41–47. [Google Scholar]
- Maduzia, D.; Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Galazka, K.; Dembinski, A. The influence of pretreatment with ghrelin on the development of acetic-acid-induced colitis in rats. J. Physiol. Pharmacol. 2015, 66, 875–885. [Google Scholar] [PubMed]
- Pamukcu, O.; Kumral, Z.N.O.; Ercan, F.; Yegen, B.Ç.; Ertem, D. Anti-inflammatory effect of obestatin and ghrelin in dextran sulfate sodium-induced colitis in rats. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 211–218. [Google Scholar] [CrossRef]
- Matuszyk, A.; Ceranowicz, D.; Warzecha, Z.; Ceranowicz, P.; Fyderek, K.; Gałązka, K.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Pihut, M.; et al. The Influence of Ghrelin on the Development of Dextran Sodium Sulfate-Induced Colitis in Rats. BioMed Res. Int. 2015, 2015, 718314. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, L.; Dai, W.; Mao, Y.; Li, S.; Wang, J.; Li, H.; Guo, C.; Fan, X. Ghrelin ameliorates intestinal barrier dysfunction in experimental colitis by inhibiting the activation of nuclear factor-kappa B. Biochem. Biophys. Res. Commun. 2015, 458, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Gałązka, K.; Bonior, J.; Jaworek, J.; Bartuś, K.; Gil, K.; et al. Exogenous ghrelin accelerates the healing of acetic acid-induced colitis in rats. Int. J. Mol. Sci. 2016, 17, 1455. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Ceranowicz, D.; Kuśnierz-Cabala, B.; Bonior, J.; Jaworek, J.; Ambroży, T.; Gil, K.; Olszanecki, R.; et al. Essential role of growth hormone and IGF-1 in therapeutic effect of ghrelin in the course of acetic acid-induced colitis. Int. J. Mol. Sci. 2017, 18, 1118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhan, Y.; Zeng, H.; Moyer, M.P.; Mantzoros, C.S.; Pothoulakis, C. Ghrelin stimulates interleukin-8 gene expression through protein kinase C-mediated NF-κB pathway in human colonic epithelial cells. J. Cell. Biochem. 2006, 97, 1317–1327. [Google Scholar] [CrossRef]
- Kasimay, Ö.; Işeri, S.Ö.; Barlas, A.; Bangir, D.; Yegen, C.; Arbak, S.; Yegen, B.Ç. Ghrelin ameliorates pancreaticobiliary inflammation and associated remote organ injury in rats. Hepatol. Res. 2006, 36, 11–19. [Google Scholar] [CrossRef]
- Dembinski, A.; Warzecha, Z.; Ceranowicz, P.; Tomaszewska, R.; Stachura, J.; Konturek, S.J.; Konturek, P.C. Ghrelin attenuates the development of acute pancreatitis in rat. J. Physiol. Pharmacol. 2003, 54, 561–573. [Google Scholar] [PubMed]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Cieszkowski, J.; Pawlik, W.W.; Tomaszewska, R.; Kuśnierz-Cabala, B.; Naskalski, J.W.; Kuwahara, A.; Kato, I. Role of growth hormone and insulin-like growth factor-1 in the protective effect of ghrelin in ischemia/reperfusion-induced acute pancreatitis. Growth Horm. IGF Res. 2006, 16, 348–356. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, C. Ghrelin inhibits the development of acute pancreatitis and nuclear factor κB activation in pancreas and liver. Pancreas 2009, 38, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xue, C. Ghrelin attenuates acute pancreatitis-induced lung injury and inhibits substance P expression. Am. J. Med. Sci. 2010, 339, 49–54. [Google Scholar] [CrossRef]
- Warzecha, Z.; Ceranowicz, P.; Dembinski, A.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis in rats. J. Physiol. Pharmacol. 2010, 61, 419–427. [Google Scholar]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Therapeutic Effect of Ghrelin in the Course of Ischemia/Reperfusion-Induced Acute Pancreatitis. Curr. Pharm. Des. 2015, 21, 2284–2290. [Google Scholar] [CrossRef]
- Ceranowicz, D.; Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Cieszkowski, J.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Role of hormonal axis, growth hormone—IGF-1, in therapeutic effect of ghrelin in the course of cerulein-induced acute pancreatitis. J. Physiol. Pharmacol. 2010, 61, 599–606. [Google Scholar]
- Bonior, J.; Ceranowicz, P.; Gajdosz, R.; Kuśnierz-Cabala, B.; Pierzchalski, P.; Warzecha, Z.; Dembiński, A.; Pędziwiatr, M.; Kot, M.; Szpak, A.L.; et al. Molecular ghrelin system in the pancreatic acinar cells: The role of the polypeptide, caerulein and sensory nerves. Int. J. Mol. Sci. 2017, 18, 929. [Google Scholar] [CrossRef] [PubMed]
- Bonior, J.; Warzecha, Z.; Ceranowicz, P.; Gajdosz, R.; Pierzchalski, P.; Kot, M.; Leja-Szpak, A.; Nawrot-Porąbka, K.; Link-Lenczowski, P.; Pędziwiatr, M.; et al. Capsaicin-sensitive sensory nerves are necessary for the protective effect of ghrelin in cerulein-induced acute pancreatitis in rats. Int. J. Mol. Sci. 2017, 18, 1402. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, X.; Tang, C. Change of plasma ghrelin level in acute pancreatitis. Pancreatology 2006, 6, 531–535. [Google Scholar] [CrossRef]
- Daniel, P.; Lesńiowski, B.; Jasińska, A.; Pietruczuk, M.; Malecka-Panas, E. Usefulness of assessing circulating levels of resistin, ghrelin, and IL-18 in alcoholic acute pancreatitis. Dig. Dis. Sci. 2010, 55, 2982–2987. [Google Scholar] [CrossRef] [PubMed]
- Ulger, B.V.; Gül, M.; Uslukaya, O.; Oguz, A.; Bozdag, Z.; Yüksel, H.; Böyük, A. New hormones to predict the severity of gallstone-induced acute pancreatitis. Turk. J. Gastroenterol. 2014, 25, 714–717. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.D.; Kong, Y.H.; Han, K.H.; Jeong, W.J.; Lee, S.J.; Cheon, G.J. The Relevance of Serum Ghrelin Concentration to Severity of Acute Pancreatitis. Gut Liver 2010, 4, 234–240. [Google Scholar] [CrossRef]
- Wang, H.; Qin, M.; Liang, Z.; Chang, R.; Fu, H.; Wei, Y.; Tang, G. Serum ghrelin, but not obestatin, is a potential predictor of acute pancreatitis severity. Medicine (Baltimore) 2017, 96, e7963. [Google Scholar] [CrossRef]
- Gröschl, M.; Topf, H.G.; Bohlender, J.; Zenk, J.; Klussmann, S.; Dötsch, J.; Rascher, W.; Rauh, M. Identification of ghrelin in human saliva: Production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin. Chem. 2005, 51, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Li, B.B.; Chen, Z.B.; Li, B.C.; Lin, Q.; Li, X.X.; Li, S.L.; Ding, C.; Wu, L.L.; Yu, G.Y. Expression of ghrelin in human salivary glands and its levels in saliva and serum in Chinese obese children and adolescents. Arch. Oral Biol. 2011, 56, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Labordè, N.J.; Kajiya, M.; Shin, J.; Zhu, T.; Thondukolam, A.K.; Min, C.; Kamata, N.; Karimbux, N.Y.; Stashenko, P.; et al. Expression and possible immune-regulatory function of ghrelin in oral epithelium. J. Dent. Res. 2011, 90, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ozercan, I.H.; Geckil, H.; Dagli, F.; Aydin, S.; Kumru, S.; Kilic, N.; Sahin, I.; Ozercan, M.R. Ghrelin is present in teeth. J. Biochem. Mol. Biol. 2007, 40, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Martin, B.; Kim, W.; White, C.M.; Ji, S.; Sun, Y.; Smith, R.G.; Sévigny, J.; Tschöp, M.H.; Maudsley, S.; et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (Citric Acid) tastants. PLoS ONE 2010, 5, e12729. [Google Scholar] [CrossRef]
- Liu, B.; Han, X.; Feng, W.; Cui, J.; Hasegawa, T.; Amizuka, N.; Xu, X.; Li, M. Altered distribution of Ghrelin protein in mice molar development. Arch. Oral Biol. 2016, 65, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Nokhbehsaim, M.; Memmert, S.; Damanaki, A.; Nanayakkara, S.; Zhou, X.; Jäger, A.; Deschner, J. Effect of interleukin-1β on ghrelin receptor in periodontal cells. Clin. Oral Investig. 2019, 23, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dynesen, A.W.; Bardow, A.; Astrup, A.; Petersson, B.; Holst, J.J.; Nauntofte, B. Meal-induced compositional changes in blood and saliva in persons with bulimia nervosa. Am. J. Clin. Nutr. 2008, 87, 12–22. [Google Scholar] [CrossRef]
- Aydin, S.; Halifeoglu, I.; Ozercan, I.H.; Erman, F.; Kilic, N.; Aydin, S.; Ilhan, N.; Ilhan, N.; Ozkan, Y.; Akpolat, N.; et al. A comparison of leptin and ghrelin levels in plasma and saliva of young healthy subjects. Peptides 2005, 26, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.G.; Idris, S.B.; Mustafa, M.; Ahmed, M.F.; Åstrøm, A.N.; Mustafa, K.; Ibrahim, S.O. Impact of chronic periodontitis on levels of glucoregulatory biomarkers in gingival crevicular fluid of adults with and without type 2 diabetes. PLoS ONE 2015, 10, e0127660. [Google Scholar] [CrossRef] [PubMed]
- Nokhbehsaim, M.; Damanaki, A.; Nogueira, A.V.B.; Eick, S.; Memmert, S.; Zhou, X.; Nanayakkara, S.; Götz, W.; Cirelli, J.A.; Jäger, A.; et al. Regulation of Ghrelin Receptor by Periodontal Bacteria in Vitro and in Vivo. Med. Inflamm. 2017, 2017. [Google Scholar] [CrossRef]
- Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Ceranowicz, D.; Kusnierz-Cabala, B.; Pedziwiatr, M.; Dembinski, M.; Ambrozy, T.; Kaczmarzyk, T.; Pihut, M.; et al. Therapeutic Effect of Exogenous Ghrelin in the Healing of Gingival Ulcers Is Mediated by the Release of Endogenous Growth Hormone and Insulin-Like Growth Factor-1. J. Physiol. Pharmacol. 2017, 68, 609–617. [Google Scholar]
- Acharya, S.; Pai, K.M.; Bhat, S.; Mamatha, B.; Bejadi, V.M.; Acharya, S. Oral changes in patients undergoing chemotherapy for breast cancer. Indian J. Dent. Res. 2017, 28, 261–268. [Google Scholar] [CrossRef]
- Bogusławska-Kapała, A.; Cackowska-Lass, A.; Balon, J.; Hellmann, A.; Kochańska, B. Saliva secretion and abnormal moistening of oral mucosa after bone marrow transplantation. Bull. Group. Int. Rech. Sci. Stomatol. Odontol. 2006, 47, 1–5. [Google Scholar]
- Jensen, S.B.; Mouridsen, H.T.; Reibel, J.; Brünner, N.; Nauntofte, B. Adjuvant chemotherapy in breast cancer patients induces temporary salivary gland hypofunction. Oral Oncol. 2008, 44, 162–173. [Google Scholar] [CrossRef]
- Harrison, T.; Bigler, L.; Tucci, M.; Pratt, L.; Malamud, F.; Thigpen, J.T.; Streckfus, C.; Younger, H. Salivary sIgA concentrations and stimulated whole saliva flow rates among women undergoing chemotherapy for breast cancer: An exploratory study. Spec. Care Dent. 1998, 18, 109–112. [Google Scholar] [CrossRef]
- Khatib, M.N.; Gaidhane, A.; Gaidhane, S.; Quazi, Z.S. Ghrelin as a Promising Therapeutic Option for Cancer Cachexia. Cell. Physiol. Biochem. 2018, 48, 2172–2188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.V.; Ren, P.G.; Avsian-Kretchmer, O.; Luo, C.W.; Rauch, R.; Klein, C.; Hsueh, A.J.W. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Scrima, M.; Campiglia, P.; Esposito, C.; Gomez-Monterrey, I.; Novellino, E.; D’Ursi, A.M. Obestatin conformational features: A strategy to unveil obestatin’s biological role? Biochem. Biophys. Res. Commun. 2007, 363, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Subasinghage, A.P.; Green, B.D.; Flatt, P.R.; Irwin, N.; Hewage, C.M. Metabolic and structural properties of human obestatin {1-23} and two fragment peptides. Peptides 2010, 31, 1697–1705. [Google Scholar] [CrossRef]
- Alén, B.O.; Nieto, L.; Gurriarán-Rodríguez, U.; Mosteiro, C.S.; Álvarez-Pérez, J.C.; Otero-Alén, M.; Camiña, J.P.; Gallego, R.; García-Caballero, T.; Martín-Pastor, M.; et al. The NMR Structure of Human Obestatin in Membrane-Like Environments: Insights into the Structure-Bioactivity Relationship of Obestatin. PLoS ONE 2012, 7, e45434. [Google Scholar] [CrossRef]
- Zhao, C.M.; Furnes, M.W.; Stenström, B.; Kulseng, B.; Chen, D. Characterization of obestatin- and ghrelin-producing cells in the gastrointestinal tract and pancreas of rats: an immunohistochemical and electron-microscopic study. Cell Tissue Res. 2008, 331, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Dun, S.L.; Brailoiu, G.C.; Brailoiu, E.; Yang, J.; Chang, J.K.; Dun, N.J. Distribution and biological activity of obestatin in the rat. J. Endocrinol. 2006, 191, 481–489. [Google Scholar] [CrossRef]
- Grönberg, M.; Tsolakis, A.V.; Magnusson, L.; Janson, E.T.; Saras, J. Distribution of obestatin and ghrelin in human tissues: immunoreactive cells in the gastrointestinal tract, pancreas, and mammary glands. J. Histochem. Cytochem. 2008, 56, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Vindigni, C.; Tripodi, S.A.; Mazzi, L.; Nuti, R.; Figura, N.; Collodel, G. Immunolocalisation of ghrelin and obestatin in human testis, seminal vesicles, prostate and spermatozoa. Andrologia 2014, 46, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Rosas, R.; Ceppi, P.; Rapa, I.; Cassoni, P.; Wiedenmann, B.; Settanni, F.; Granata, R.; Papotti, M. Obestatin in human neuroendocrine tissues and tumours: Expression and effect on tumour growth. J. Pathol. 2009, 218, 458–466. [Google Scholar] [CrossRef]
- Alnema, M.M.; Aydin, S.; Ozkan, Y.; Dagli, A.F.; Ozercan, H.I.; Yildirim, N.; Sahin, I.; Karaoglu, A.; Kilic, N.; Yilmaz, M.; et al. Ghrelin and obestatin expression in oral squamous cell carcinoma: An immunohistochemical and biochemical study. Mol. Cell. Biochem. 2010, 339, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.V.; Jahr, H.; Luo, C.-W.; Klein, C.; Van Kolen, K.; Ver Donck, L.; De, A.; Baart, E.; Li, J.; Moechars, D.; Hsueh, A.J.W. Obestatin Induction of Early-Response Gene Expression in Gastrointestinal and Adipose Tissues and the Mediatory Role of G Protein-Coupled Receptor, GPR39. Mol. Endocrinol. 2008, 22, 1464–1475. [Google Scholar] [CrossRef]
- Alen, B.O.; Leal-Lopez, S.; Alen, M.O.; Viano, P.; Garcia-Castro, V.; Mosteiro, C.S.; Beiras, A.; Casanueva, F.F.; Gallego, R.; Garcia-Caballero, T.; et al. The role of the obestatin/GPR39 system in human gastric adenocarcinomas. Oncotarget 2016, 7, 5957–5971. [Google Scholar] [CrossRef]
- Moechars, D.; Depoortere, I.; Moreaux, B.; de Smet, B.; Goris, I.; Hoskens, L.; Daneels, G.; Kass, S.; Ver Donck, L.; Peeters, T.; et al. Altered Gastrointestinal and Metabolic Function in the GPR39-Obestatin Receptor-Knockout Mouse. Gastroenterology 2006, 131, 1131–1141. [Google Scholar] [CrossRef]
- Santos-Zas, I.; Gurriarán-Rodríguez, U.; Cid-Díaz, T.; Figueroa, G.; González-Sánchez, J.; Bouzo-Lorenzo, M.; Mosteiro, C.S.; Señarís, J.; Casanueva, F.F.; Casabiell, X.; et al. β-Arrestin scaffolds and signaling elements essential for the obestatin/GPR39 system that determine the myogenic program in human myoblast cells. Cell. Mol. Life Sci. 2016, 73, 617–635. [Google Scholar] [CrossRef]
- Lauwers, E.; Landuyt, B.; Arckens, L.; Schoofs, L.; Luyten, W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem. Biophys. Res. Commun. 2006, 351, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Egerod, K.L.; Schild, E.; Vickers, S.P.; Cheetham, S.; Gerlach, L.O.; Storjohann, L.; Stidsen, C.E.; Jones, R.; Beck-Sickinger, A.G.; et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 2007, 148, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Perreault, M.; Klaman, L.D.; Tobin, J.F.; Smith, E.; Gimeno, R.E. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology 2007, 148, 501–506. [Google Scholar] [CrossRef]
- Gargantini, E.; Lazzari, L.; Settanni, F.; Taliano, M.; Trovato, L.; Gesmundo, I.; Ghigo, E.; Granata, R. Obestatin promotes proliferation and survival of adult hippocampal progenitors and reduces amyloid-β-induced toxicity. Mol. Cell. Endocrinol. 2016, 422, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Granata, R.; Settanni, F.; Gallo, D.; Trovato, L.; Biancone, L.; Cantaluppi, V.; Nano, R.; Annunziata, M.; Campiglia, P.; Arnoletti, E.; et al. Obestatin promotes survival of pancreatic β-cells and human islets and induces expression of genes involved in the regulation of β-cell mass and function. Diabetes 2008, 57, 967–979. [Google Scholar] [CrossRef]
- Dong, X.Y.; He, J.M.; Tang, S.Q.; Li, H.Y.; Jiang, Q.Y.; Zou, X.T. Is GPR39 the natural receptor of obestatin? Peptides 2009, 30, 431–438. [Google Scholar] [CrossRef]
- Ataka, K.; Inui, A.; Asakawa, A.; Kato, I.; Fujimiya, M. Obestatin inhibits motor activity in the antrum and duodenum in the fed state of conscious rats. Am. J. Physiol. Liver Physiol. 2008, 294, G1210–G1218. [Google Scholar] [CrossRef]
- Fujimiya, M.; Ataka, K.; Asakawa, A.; Chen, C.Y.; Kato, I.; Inui, A. Ghrelin, des-acyl ghrelin and obestatin on the gastrointestinal motility. Peptides 2011, 32, 2348–2351. [Google Scholar] [CrossRef]
- Samson, W.K.; Yosten, G.L.; Chang, J.K.; Ferguson, A.V.; White, M.M. Obestatin inhibits vasopressin secretion: Evidence for a physiological action in the control of fluid homeostasis. J. Endocrinol. 2008, 196, 559–564. [Google Scholar] [CrossRef]
- Samson, W.K.; White, M.M.; Price, C.; Ferguson, A.V. Obestatin acts in brain to inhibit thirst. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R637–R643. [Google Scholar] [CrossRef]
- Kapica, M.; Zabielska, M.; Puzio, I.; Jankowska, A.; Kato, I.; Kuwahara, A.; Zabielski, R. Obestatin stimulates the secretion of pancreatic juice enzymes through a vagal pathway in anaesthetized rats—Preliminary results. J. Physiol. Pharmacol. 2007, 58, 123–130. [Google Scholar] [PubMed]
- Carlini, V.P.; Schiöth, H.B.; DeBarioglio, S.R. Obestatin improves memory performance and causes anxiolytic effects in rats. Biochem. Biophys. Res. Commun. 2007, 352, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Szentirmai, E.; Krueger, J.M. Obestatin alters sleep in rats. Neurosci. Lett. 2006, 404, 222–226. [Google Scholar] [CrossRef]
- Alloatti, G.; Arnoletti, E.; Bassino, E.; Penna, C.; Perrelli, M.G.; Ghé, C.; Muccioli, G. Obestatin affords cardioprotection to the ischemic-reperfused isolated rat heart and inhibits apoptosis in cultures of similarly stressed cardiomyocytes. Am. J. Physiol. Circ. Physiol. 2010, 299, H470–H481. [Google Scholar] [CrossRef] [PubMed]
- Penna, C.; Tullio, F.; Femminò, S.; Rocca, C.; Angelone, T.; Cerra, M.C.; Gallo, M.P.; Gesmundo, I.; Fanciulli, A.; Brizzi, M.F.; Pagliaro, P.; Alloatti, G.; Granata, R. Obestatin regulates cardiovascular function and promotes cardioprotection through the nitric oxide pathway. J. Cell. Mol. Med. 2017, 21, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dong, X.-W.; Xia, J.-Y.; Xu, K.; Xu, Z.-R. Obestatin Plays Beneficial Role in Cardiomyocyte Injury Induced by Ischemia-Reperfusion In Vivo and In Vitro. Med. Sci. Monit. 2017, 23, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Koç, M.; Kumral, Z.N.; Özkan, N.; Memi, G.; Kaçar, Ö.; Bilsel, S.; Çetinel, Ş.; Yeğen, B.Ç. Obestatin improves ischemia/reperfusion-induced renal injury in rats via its antioxidant and anti-apoptotic effects: Role of the nitric oxide. Peptides 2014, 60, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Gurriarán-Rodríguez, U.; Santos-Zas, I.; González-Sánchez, J.; Beiroa, D.; Moresi, V.; Mosteiro, C.S.; Lin, W.; Viñuela, J.E.; Señarís, J.; García-Caballero, T.; et al. Action of obestatin in skeletal muscle repair: Stem cell expansion, muscle growth, and microenvironment remodeling. Mol. Ther. 2015, 23, 1003–1021. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Cieszkowski, J.; Dembiński, M.; Ptak-Belowska, A.; Kuwahara, A.; Kato, I. Administration of obestatin accelerates the healing of chronic gastric ulcers in rats. Med. Sci. Monit. 2011, 17, BR196–BR200. [Google Scholar] [CrossRef]
- Şen, L.S.; Karakoyun, B.; Yegen, C.; Akkiprik, M.; Yüksel, M.; Ercan, F.; Özer, A.; Yegen, B. Treatment with either obestatin or ghrelin attenuates mesenteric ischemia-reperfusion-induced oxidative injury of the ileum and the remote organ lung. Peptides 2015, 71, 8–19. [Google Scholar] [CrossRef]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Gałązka, K.; Bonior, J.; Jaworek, J.; Konturek, P.C.; Gil, K.; Dembiński, A. Pretreatment with obestatin inhibits the development of acetic acid-induced colitis in rats. Arch. Med. Sci. 2018, 14, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Konarska, K.; Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Chmura, A.; Kuśnierz-Cabala, B.; Gałazka, K.; Kowalczyk, P.; Miskiewicz, A.; Konturek, T.J.; et al. Treatment with obestatin—A ghrelin gene-encoded peptide—Reduces the severity of experimental colitis evoked by trinitrobenzene sulfonic acid. Int. J. Mol. Sci. 2018, 19, 1643. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Kuśnierz-Cabala, B.; Konturek, P.; Ambroży, T.; Dembiński, A. Obestatin Accelerates the Healing of Acetic Acid-Induced Colitis in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 2834386. [Google Scholar] [CrossRef]
- Baragli, A.; Grande, C.; Gesmundo, I.; Settanni, F.; Taliano, M.; Gallo, D.; Gargantini, E.; Ghigo, E.; Granata, R. Obestatin Enhances In Vitro Generation of Pancreatic Islets through Regulation of Developmental Pathways. PLoS ONE 2013, 8, e64374. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Cieszkowski, J.; Dembinski, M.; Sendur, R.; Kusnierz-Cabala, B.; Tomaszewska, R.; Kuwahara, A.; Kato, I. Pretreatment with obestatin inhibits the development of cerulein-induced pancreatitis. J. Physiol. Pharmacol. 2009, 60, 95–101. [Google Scholar]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kuśnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Pretreatment with obestatin reduces the severity of ischemia/reperfusion-induced acute pancreatitis in rats. Eur. J. Pharmacol. 2015, 760, 113–121. [Google Scholar] [CrossRef]
- Bukowczan, J.; Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Kusnierz-Cabala, B.; Tomaszewska, R.; Dembinski, A. Therapeutic effect of obestatin in the course of cerulein-induced acute pancreatitis. Pancreas 2016, 45, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kuśnierz-Cabala, B.; Tomaszewska, R. Obestatin accelerates the recovery in the course of ischemia/reperfusion-induced acute pancreatitis in rats. PLoS ONE 2015, 10, e0134380. [Google Scholar] [CrossRef]
- El-Gohary, O.A. Obestatin improves hepatic injury induced by ischemia/reperfusion in rats: Role of nitric oxide. Gen. Physiol. Biophys. 2017, 36, 109–115. [Google Scholar] [CrossRef]
- Khaleel, E.F.; Abdel-Aleem, G.A. Obestatin protects and reverses nonalcoholic fatty liver disease and its associated insulin resistance in rats via inhibition of food intake, enhancing hepatic adiponectin signaling, and blocking ghrelin acylation. Arch. Physiol. Biochem. 2018, 125, 64–78. [Google Scholar] [CrossRef] [PubMed]
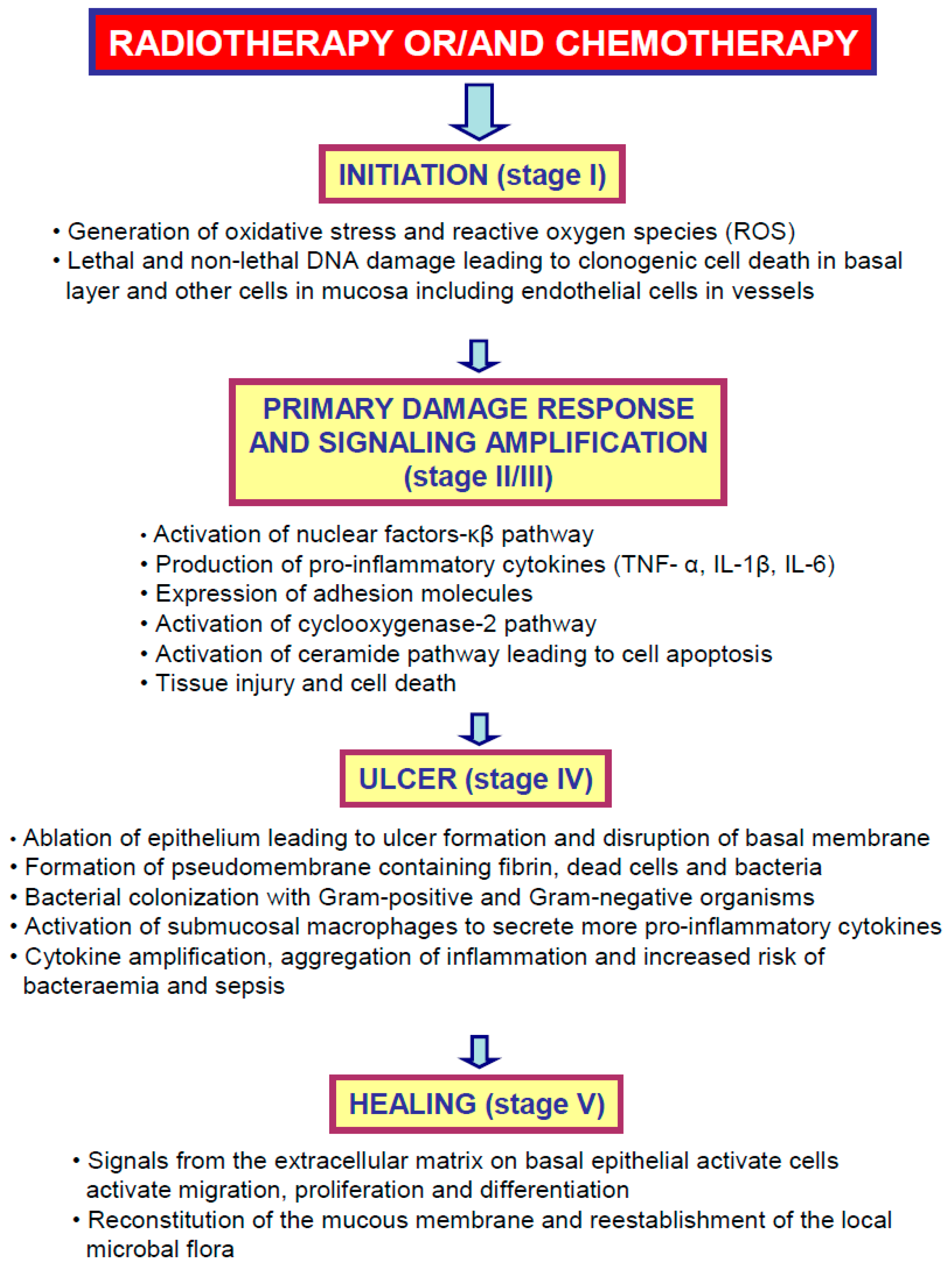


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stempniewicz, A.; Ceranowicz, P.; Warzecha, Z. Potential Therapeutic Effects of Gut Hormones, Ghrelin and Obestatin in Oral Mucositis. Int. J. Mol. Sci. 2019, 20, 1534. https://doi.org/10.3390/ijms20071534
Stempniewicz A, Ceranowicz P, Warzecha Z. Potential Therapeutic Effects of Gut Hormones, Ghrelin and Obestatin in Oral Mucositis. International Journal of Molecular Sciences. 2019; 20(7):1534. https://doi.org/10.3390/ijms20071534
Chicago/Turabian StyleStempniewicz, Agnieszka, Piotr Ceranowicz, and Zygmunt Warzecha. 2019. "Potential Therapeutic Effects of Gut Hormones, Ghrelin and Obestatin in Oral Mucositis" International Journal of Molecular Sciences 20, no. 7: 1534. https://doi.org/10.3390/ijms20071534
APA StyleStempniewicz, A., Ceranowicz, P., & Warzecha, Z. (2019). Potential Therapeutic Effects of Gut Hormones, Ghrelin and Obestatin in Oral Mucositis. International Journal of Molecular Sciences, 20(7), 1534. https://doi.org/10.3390/ijms20071534





