Deciphering the Code between Air Pollution and Disease: The Effect of Particulate Matter on Cancer Hallmarks
Abstract
:1. Introduction
2. Particulate Matter Generalities
2.1. Why Does Air Pollution Differ from Other Carcinogens?
2.2. If Air Pollution Is Classified as a Carcinogen by the IARC and Gives Rise to Many of Cancer’s Hallmarks, What Is Missing to Understand the Mechanism of Air Pollution as a Carcinogen?
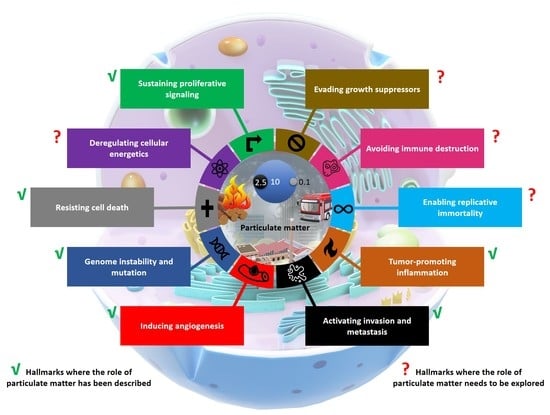
3. Evidence of PM Impacts on Different Cancer Hallmarks
3.1. Sustained Proliferative Signaling
3.2. Cell Death Resistance
3.3. Angiogenesis Induction
3.4. Activation of Invasion and Metastasis
3.5. Oxidative Stress and Inflammation
3.6. Genome Instability
4. Toward a Comprehensive Understanding of the Effect of PM during the Cancer Continuum
4.1. Absence of Mutational Fingerprints on DNA and Growth Suppression
4.2. Replicative Immortality, Cellular Energetics, and Immune Cell Destruction
4.3. Research to Be Conducted in the Immediate Future
4.3.1. Chronic Models
4.3.2. Air Pollution as a Mutagenic Agent Needs to Be Addressed Using Innovative Technology and Epigenetic Alterations
4.4. Beyond the Epidemiological Studies, Other Risk Factors May Be Missing in the Research Conducted to Reveal the Mechanism of Air Pollution Carcinogenicity
4.5. Air Pollution Exposure Is Not Considered as a Risk Factor for Failure in Lung Cancer Treatment
5. Final Remarks
6. Methods
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcon-Rodriguez, C.I.; Osornio-Vargas, A.R.; Sada-Ovalle, I.; Segura-Medina, P. Aeroparticles, Composition, and Lung Diseases. Front. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, R.M.; Yin, J. Particulate matter in the atmosphere: Which particle properties are important for its effects on health? Sci. Total Environ. 2000, 249, 85–101. [Google Scholar] [CrossRef]
- Gauderman, W.J.; Urman, R.; Avol, E.; Berhane, K.; McConnell, R.; Rappaport, E.; Chang, R.; Lurmann, F.; Gilliland, F. Association of improved air quality with lung development in children. N. Engl. J. Med. 2015, 372, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K.; et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- De Kok, T.M.; Driece, H.A.; Hogervorst, J.G.; Briede, J.J. Toxicological assessment of ambient and traffic-related particulate matter: A review of recent studies. Mutat. Res. 2006, 613, 103–122. [Google Scholar] [CrossRef]
- Yang, B.; Chen, D.; Zhao, H.; Xiao, C. The effects for PM2.5 exposure on non-small-cell lung cancer induced motility and proliferation. SpringerPlus 2016, 5, 2059. [Google Scholar] [CrossRef] [Green Version]
- Timblin, C.; BeruBe, K.; Churg, A.; Driscoll, K.; Gordon, T.; Hemenway, D.; Walsh, E.; Cummins, A.B.; Vacek, P.; Mossman, B. Ambient particulate matter causes activation of the c-jun kinase/stress-activated protein kinase cascade and DNA synthesis in lung epithelial cells. Cancer Res. 1998, 58, 4543–4547. [Google Scholar]
- Calderon-Garciduenas, L.; Rodriguez-Alcaraz, A.; Garcia, R.; Barragan, G.; Villarreal-Calderon, A.; Madden, M.C. Cell proliferation in nasal respiratory epithelium of people exposed to urban pollution. Carcinogenesis 1999, 20, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrysik, Z.; Vondracek, J.; Marvanova, S.; Ciganek, M.; Neca, J.; Pencikova, K.; Mahadevan, B.; Topinka, J.; Baird, W.M.; Kozubik, A.; et al. Activation of the aryl hydrocarbon receptor is the major toxic mode of action of an organic extract of a reference urban dust particulate matter mixture: The role of polycyclic aromatic hydrocarbons. Mutat. Res. 2011, 714, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Oya, E.; Ovrevik, J.; Arlt, V.M.; Nagy, E.; Phillips, D.H.; Holme, J.A. DNA damage and DNA damage response in human bronchial epithelial BEAS-2B cells following exposure to 2-nitrobenzanthrone and 3-nitrobenzanthrone: Role in apoptosis. Mutagenesis 2011, 26, 697–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Rui, W.; Zhang, F.; Ding, W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol. Toxicol. 2013, 29, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.; Garcon, G.; Saint-Georges, F.; Billet, S.; Verdin, A.; Gosset, P.; Mulliez, P.; Shirali, P. Occurrence of molecular abnormalities of cell cycle in L132 cells after in vitro short-term exposure to air pollution PM2.5. Chem.-Biol. Interact. 2010, 188, 558–565. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, B.; Xu, J.; Chen, D.M.; Xiao, C.L. PM2.5-induced alterations of cell cycle associated gene expression in lung cancer cells and rat lung tissues. Environ. Toxicol. Pharmacol. 2017, 52, 77–82. [Google Scholar] [CrossRef]
- Mavrofrydi, O.; Mavroeidi, P.; Papazafiri, P. Comparative assessment of HIF-1alpha and Akt responses in human lung and skin cells exposed to benzo[alpha]pyrene: Effect of conditioned medium from pre-exposed primary fibroblasts. Environ. Toxicol. 2016, 31, 1103–1112. [Google Scholar] [CrossRef]
- Sanchez-Perez, Y.; Chirino, Y.I.; Osornio-Vargas, A.R.; Morales-Barcenas, R.; Gutierrez-Ruiz, C.; Vazquez-Lopez, I.; Garcia-Cuellar, C.M. DNA damage response of A549 cells treated with particulate matter (PM10) of urban air pollutants. Cancer Lett. 2009, 278, 192–200. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, X.; Wu, Y.; Li, S.; Cao, L.; Dong, L. Exosomes derived from PM2.5treated lung cancer cells promote the growth of lung cancer via the Wnt3a/betacatenin pathway. Oncol. Rep. 2019, 41, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Liu, S.; Zhuang, G.; Xu, J.; Liu, Q.; Zhang, X.; Deng, C.; Guo, Z.; Zhao, W.; Liu, T.; et al. Signal Transductions of BEAS-2B Cells in Response to Carcinogenic PM2.5 Exposure Based on a Microfluidic System. Anal. Chem. 2017, 89, 5413–5421. [Google Scholar] [CrossRef]
- Zheng, L.; Dong, H.; Zhao, W.; Zhang, X.; Duan, X.; Zhang, H.; Liu, S.; Sui, G. An Air-Liquid Interface Organ-Level Lung Microfluidics Platform for Analysis on Molecular Mechanisms of Cytotoxicity Induced by Cancer-Causing Fine Particles. ACS Sens. 2019, 4, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Ferecatu, I.; Borot, M.C.; Bossard, C.; Leroux, M.; Boggetto, N.; Marano, F.; Baeza-Squiban, A.; Andreau, K. Polycyclic aromatic hydrocarbon components contribute to the mitochondria-antiapoptotic effect of fine particulate matter on human bronchial epithelial cells via the aryl hydrocarbon receptor. Part. Fibre Toxicol. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teranishi, M.; Toyooka, T.; Ohura, T.; Masuda, S.; Ibuki, Y. Benzo[a]pyrene exposed to solar-simulated light inhibits apoptosis and augments carcinogenicity. Chem.-Biol. Interact. 2010, 185, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chou, F.P.; Lin, H.H.; Wang, C.J. Gaseous nitrogen oxide repressed benzo[a]pyrene-induced human lung fibroblast cell apoptosis via inhibiting JNK1 signals. Arch. Toxicol. 2005, 79, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Lovera-Leroux, M.; Crobeddu, B.; Kassis, N.; Petit, P.X.; Janel, N.; Baeza-Squiban, A.; Andreau, K. The iron component of particulate matter is antiapoptotic: A clue to the development of lung cancer after exposure to atmospheric pollutants? Biochimie 2015, 118, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhou, Q.; Wang, T.; Jiang, Y.; Zhong, Y.; Qian, G.; Zhu, T.; Qiu, X.; An, J. Airborne nitro-PAHs induce Nrf2/ARE defense system against oxidative stress and promote inflammatory process by activating PI3K/Akt pathway in A549 cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2017, 44, 66–73. [Google Scholar] [CrossRef]
- Peixoto, M.S.; de Oliveira Galvao, M.F.; Batistuzzo de Medeiros, S.R. Cell death pathways of particulate matter toxicity. Chemosphere 2017, 188, 32–48. [Google Scholar] [CrossRef]
- Bhargava, A.; Tamrakar, S.; Aglawe, A.; Lad, H.; Srivastava, R.K.; Mishra, D.K.; Tiwari, R.; Chaudhury, K.; Goryacheva, I.Y.; Mishra, P.K. Ultrafine particulate matter impairs mitochondrial redox homeostasis and activates phosphatidylinositol 3-kinase mediated DNA damage responses in lymphocytes. Environ. Pollut. (Barking, Essex: 1987) 2018, 234, 406–419. [Google Scholar] [CrossRef]
- Kafoury, R.M.; Madden, M.C. Diesel exhaust particles induce the over expression of tumor necrosis factor-alpha (TNF-alpha) gene in alveolar macrophages and failed to induce apoptosis through activation of nuclear factor-kappaB (NF-kappaB). Int. J. Environ. Res. Public Health 2005, 2, 107–113. [Google Scholar] [CrossRef]
- Arenz, A.; Hellweg, C.E.; Stojicic, N.; Baumstark-Khan, C.; Grotheer, H.H. Gene expression modulation in A549 human lung cells in response to combustion-generated nano-sized particles. Ann. N. Y. Acad. Sci. 2006, 1091, 170–183. [Google Scholar] [CrossRef]
- Montiel-Davalos, A.; Ibarra-Sanchez Mde, J.; Ventura-Gallegos, J.L.; Alfaro-Moreno, E.; Lopez-Marure, R. Oxidative stress and apoptosis are induced in human endothelial cells exposed to urban particulate matter. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2010, 24, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, B.; Owens, J.W. The influence of Hurricanes Katrina and Rita on the inflammatory cytokine response and protein expression in A549 cells exposed to PM2.5 collected in the Baton Rouge-Port Allen industrial corridor of Southeastern Louisiana in 2005. Toxicol. Mech. Methods 2014, 24, 220–242. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Yang, Y.P.; Liu, Z.Y. Study of Berberine on Attenuating PM2.5-Induced Vascular Endothelial Cells Injury by ERK1/2 Signal Pathway. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2016, 39, 1623–1627. [Google Scholar]
- Wan, Q.; Yang, Y.P.; Liu, Z.Y. Puerarin attenuates PM2.5-induced vascular endothelial cells injury via ERK1/2 signaling pathway. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2016, 41, 2309–2314. [Google Scholar] [CrossRef]
- Tas, I.; Zhou, R.; Park, S.Y.; Yang, Y.; Gamage, C.D.B.; Son, Y.J.; Paik, M.J.; Kim, H. Inflammatory and tumorigenic effects of environmental pollutants found in particulate matter on lung epithelial cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2019, 59, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Perez, Y.; Chirino, Y.I.; Osornio-Vargas, A.R.; Herrera, L.A.; Morales-Barcenas, R.; Lopez-Saavedra, A.; Gonzalez-Ramirez, I.; Miranda, J.; Garcia-Cuellar, C.M. Cytoplasmic p21(CIP1/WAF1), ERK1/2 activation, and cytoskeletal remodeling are associated with the senescence-like phenotype after airborne particulate matter (PM(10)) exposure in lung cells. Toxicol. Lett. 2014, 225, 12–19. [Google Scholar] [CrossRef]
- Reyes-Zarate, E.; Sanchez-Perez, Y.; Gutierrez-Ruiz, M.C.; Chirino, Y.I.; Osornio-Vargas, A.R.; Morales-Barcenas, R.; Souza-Arroyo, V.; Garcia-Cuellar, C.M. Atmospheric particulate matter (PM10) exposure-induced cell cycle arrest and apoptosis evasion through STAT3 activation via PKCzeta and Src kinases in lung cells. Environ. Pollut. (Barking, Essex: 1987) 2016, 214, 646–656. [Google Scholar] [CrossRef]
- Liu, T.; Wu, B.; Wang, Y.; He, H.; Lin, Z.; Tan, J.; Yang, L.; Kamp, D.W.; Zhou, X.; Tang, J.; et al. Particulate matter 2.5 induces autophagy via inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin kinase signaling pathway in human bronchial epithelial cells. Mol. Med. Rep. 2015, 12, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Zhang, F.; Wang, L.; Rui, W.; Long, F.; Zhao, Y.; Chen, D.; Ding, W. Airborne fine particulate matter induces multiple cell death pathways in human lung epithelial cells. Apoptosis Int. J. Program. Cell Death 2014, 19, 1099–1112. [Google Scholar] [CrossRef]
- Deng, X.; Feng, N.; Zheng, M.; Ye, X.; Lin, H.; Yu, X.; Gan, Z.; Fang, Z.; Zhang, H.; Gao, M.; et al. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 112–125. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, X.; Feng, N.; Wang, R.; Zhang, W.; Deng, X.; Wang, Y.; Yu, X.; Ye, X.; Li, L.; et al. LncRNA LCPAT1 Mediates Smoking/ Particulate Matter 2.5-Induced Cell Autophagy and Epithelial-Mesenchymal Transition in Lung Cancer Cells via RCC2. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Catino, S.; Tutino, M.; Ruggieri, S.; Marinaccio, C.; Giua, R.; de Gennaro, G.; Corsi, P.; Assennato, G.; Ribatti, D. Angiogenic activity in vivo of the particulate matter (PM10). Ecotoxicol. Environ. Saf. 2017, 140, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shimizu, Y.; Wu, X.; Kelly, G.T.; Xu, X.; Wang, L.; Qian, Z.; Chen, Y.; Garcia, J.G.N. Particulate matter disrupts human lung endothelial cell barrier integrity via Rho-dependent pathways. Pulm. Circ. 2017, 7, 617–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Xiao, C. PM2.5 exposure significantly improves the exacerbation of A549 tumor-bearing CB17-SCID mice. Environ. Toxicol. Pharmacol. 2018, 60, 169–175. [Google Scholar] [CrossRef]
- Hesselbach, K.; Kim, G.J.; Flemming, S.; Haupl, T.; Bonin, M.; Dornhof, R.; Gunther, S.; Merfort, I.; Humar, M. Disease relevant modifications of the methylome and transcriptome by particulate matter (PM2.5) from biomass combustion. Epigenetics 2017, 12, 779–792. [Google Scholar] [CrossRef] [Green Version]
- Libalova, H.; Krckova, S.; Uhlirova, K.; Klema, J.; Ciganek, M.; Rossner, P., Jr.; Sram, R.J.; Vondracek, J.; Machala, M.; Topinka, J. Analysis of gene expression changes in A549 cells induced by organic compounds from respirable air particles. Mutat. Res. 2014, 770, 94–105. [Google Scholar] [CrossRef]
- Yang, D.; Ma, M.; Zhou, W.; Yang, B.; Xiao, C. Inhibition of miR-32 activity promoted EMT induced by PM2.5 exposure through the modulation of the Smad1-mediated signaling pathways in lung cancer cells. Chemosphere 2017, 184, 289–298. [Google Scholar] [CrossRef]
- Wei, H.; Liang, F.; Cheng, W.; Zhou, R.; Wu, X.; Feng, Y.; Wang, Y. The mechanisms for lung cancer risk of PM2.5: Induction of epithelial-mesenchymal transition and cancer stem cell properties in human non-small cell lung cancer cells. Environ. Toxicol. 2017, 32, 2341–2351. [Google Scholar] [CrossRef]
- Chi, Y.; Huang, Q.; Lin, Y.; Ye, G.; Zhu, H.; Dong, S. Epithelial-mesenchymal transition effect of fine particulate matter from the Yangtze River Delta region in China on human bronchial epithelial cells. J. Environ. Sci. 2018, 66, 155–164. [Google Scholar] [CrossRef]
- Li, W.; Liu, T.; Xiong, Y.; Lv, J.; Cui, X.; He, R. Diesel exhaust particle promotes tumor lung metastasis via the induction of BLT1-mediated neutrophilic lung inflammation. Cytokine 2018, 111, 530–540. [Google Scholar] [CrossRef]
- Yue, H.; Yun, Y.; Gao, R.; Li, G.; Sang, N. Winter Polycyclic Aromatic Hydrocarbon-Bound Particulate Matter from Peri-urban North China Promotes Lung Cancer Cell Metastasis. Environ. Sci. Technol. 2015, 49, 14484–14493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, Y.; Hou, T.; Liao, J.; Zhang, C.; Sun, C.; Wang, G. PM2.5 induces EMT and promotes CSC properties by activating Notch pathway in vivo and vitro. Ecotoxicol. Environ. Saf. 2019, 178, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, C.; Fu, Y.; Guo, L.; Kong, X.; Cai, H. Methylation analysis for multiple gene promoters in non-small cell lung cancers in high indoor air pollution region in China. Bull. Cancer 2018, 105, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, X.; Chen, D.; Xiao, C. Effects of fine air particulates on gene expression in non-small-cell lung cancer. Adv. Med. Sci. 2017, 62, 295–301. [Google Scholar] [CrossRef]
- Morales-Barcenas, R.; Chirino, Y.I.; Sanchez-Perez, Y.; Osornio-Vargas, A.R.; Melendez-Zajgla, J.; Rosas, I.; Garcia-Cuellar, C.M. Particulate matter (PM10) induces metalloprotease activity and invasion in airway epithelial cells. Toxicol. Lett. 2015, 237, 167–173. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Q.; Yuan, X.; Chai, L.; Li, D.; Liu, J.; Lv, Z. Atmospheric particulate matter2.5 promotes the migration and invasion of hepatocellular carcinoma cells. Oncol. Lett. 2017, 13, 3445–3450. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ji, N.; Wang, Z.; Wu, C.; Sun, Z.; Li, Y.; Hu, F.; Wang, Z.; Huang, M.; Zhang, M. Fine Particulate Matter (PM2.5) Promoted the Invasion of Lung Cancer Cells via an ARNT2/PP2A/STAT3/MMP2 Pathway. J. Biomed. Nanotechnol. 2018, 14, 2172–2184. [Google Scholar] [CrossRef]
- Moller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res. Rev. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [CrossRef]
- Lee, C.W.; Chi, M.C.; Hsu, L.F.; Yang, C.M.; Hsu, T.H.; Chuang, C.C.; Lin, W.N.; Chu, P.M.; Lee, I.T. Carbon monoxide releasing molecule-2 protects against particulate matter-induced lung inflammation by inhibiting TLR2 and 4/ROS/NLRP3 inflammasome activation. Mol. Immunol. 2019, 112, 163–174. [Google Scholar] [CrossRef]
- Guan, L.; Geng, X.; Stone, C.; Cosky, E.E.P.; Ji, Y.; Du, H.; Zhang, K.; Sun, Q.; Ding, Y. PM2.5 exposure induces systemic inflammation and oxidative stress in an intracranial atherosclerosis rat model. Environ. Toxicol. 2019, 34, 530–538. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Li, Z.; Yue, J.; Xu, M.; Zhang, Y.; Yung, K.K.L.; Li, R. Fine particulate matter induces mitochondrial dysfunction and oxidative stress in human SH-SY5Y cells. Chemosphere 2019, 218, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcabrini, A.; Meschini, S.; Marra, M.; Falzano, L.; Colone, M.; De Berardis, B.; Paoletti, L.; Arancia, G.; Fiorentini, C. Fine environmental particulate engenders alterations in human lung epithelial A549 cells. Environ. Res. 2004, 95, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Rosas Perez, I.; Serrano, J.; Alfaro-Moreno, E.; Baumgardner, D.; Garcia-Cuellar, C.; Martin Del Campo, J.M.; Raga, G.B.; Castillejos, M.; Colin, R.D.; Osornio Vargas, A.R. Relations between PM10 composition and cell toxicity: A multivariate and graphical approach. Chemosphere 2007, 67, 1218–1228. [Google Scholar] [CrossRef]
- Osornio-Vargas, A.R.; Bonner, J.C.; Alfaro-Moreno, E.; Martinez, L.; Garcia-Cuellar, C.; Ponce-de-Leon Rosales, S.; Miranda, J.; Rosas, I. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ. Health Perspect. 2003, 111, 1289–1293. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Chen, Y.; Li, X.; Zhang, Z.; Chu, H. Ambient fine particulate matter (PM2.5) induces oxidative stress and pro-inflammatory response via up-regulating the expression of CYP1A1/1B1 in human bronchial epithelial cells in vitro. Mutat. Res. Gen. Toxicol. Environ. Mutagen. 2019, 839, 40–48. [Google Scholar] [CrossRef]
- Longhin, E.; Capasso, L.; Battaglia, C.; Proverbio, M.C.; Cosentino, C.; Cifola, I.; Mangano, E.; Camatini, M.; Gualtieri, M. Integrative transcriptomic and protein analysis of human bronchial BEAS-2B exposed to seasonal urban particulate matter. Environ. Pollut. (Barking Essex 1987) 2016, 209, 87–98. [Google Scholar] [CrossRef]
- Pan, X.; Yuan, X.; Li, X.; Gao, S.; Sun, H.; Zhou, H.; Hou, L.; Peng, X.; Jiang, Y.; Yan, B. Induction of Inflammatory Responses in Human Bronchial Epithelial Cells by Pb(2+)-Containing Model PM2.5 Particles via Downregulation of a Novel Long Noncoding RNA lnc-PCK1-2:1. Environ. Sci. Technol. 2019, 53, 4566–4578. [Google Scholar] [CrossRef]
- Atafar, Z.; Pourpak, Z.; Yunesian, M.; Nicknam, M.H.; Hassanvand, M.S.; Soleimanifar, N.; Saghafi, S.; Alizadeh, Z.; Rezaei, S.; Ghanbarian, M.; et al. Proinflammatory effects of dust storm and thermal inversion particulate matter (PM10) on human peripheral blood mononuclear cells (PBMCs) in vitro: A comparative approach and analysis. J. Environ. Health Sci. Eng. 2019, 17, 433–444. [Google Scholar] [CrossRef]
- Muller, L.; Chehrazi, C.V.; Henderson, M.W.; Noah, T.L.; Jaspers, I. Diesel exhaust particles modify natural killer cell function and cytokine release. Part. Fibre Toxicol. 2013, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.Y.; Huang, D.Y.; Zhang, H.J.; Wang, S.; Chen, X.F. Exposure to particulate matter 2.5 (PM2.5) induced macrophage-dependent inflammation, characterized by increased Th1/Th17 cytokine secretion and cytotoxicity. Int. Immunopharmacol. 2017, 50, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.P.; Chen, C.H.; Lin, W.Y.; Liu, C.S.; Liu, K.J.; Hsiao, M.; Chang, Y.C.; Hung, S.C. Ambient particulate matter attenuates Sirtuin1 and augments SREBP1-PIR axis to induce human pulmonary fibroblast inflammation: Molecular mechanism of microenvironment associated with COPD. Aging 2019, 11, 4654–4671. [Google Scholar] [CrossRef]
- Fashi, M.; Agha Alinejad, H.; Asilian Mahabadi, H. The Effect of Aerobic Exercise in Ambient Particulate Matter on Lung Tissue Inflammation and Lung Cancer. Iran. J. Cancer Prev. 2015, 8, e2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farina, F.; Lonati, E.; Milani, C.; Massimino, L.; Ballarini, E.; Donzelli, E.; Crippa, L.; Marmiroli, P.; Botto, L.; Corsetto, P.A.; et al. In Vivo Comparative Study on Acute and Sub-acute Biological Effects Induced by Ultrafine Particles of Different Anthropogenic Sources in BALB/c Mice. Int. J. Mol. Sci. 2019, 20, 2805. [Google Scholar] [CrossRef] [Green Version]
- Motykiewicz, G.; Michalska, J.; Pendzich, J.; Perera, F.P.; Chorazy, M. A cytogenetic study of men environmentally and occupationally exposed to airborne pollutants. Mutat. Res. 1992, 280, 253–259. [Google Scholar] [CrossRef]
- Tan, C.; Lu, S.; Wang, Y.; Zhu, Y.; Shi, T.; Lin, M.; Deng, Z.; Wang, Z.; Song, N.; Li, S.; et al. Long-term exposure to high air pollution induces cumulative DNA damages in traffic policemen. Sci. Total Environ. 2017, 593–594, 330–336. [Google Scholar] [CrossRef]
- Zhou, B.; Liang, G.; Qin, H.; Peng, X.; Huang, J.; Li, Q.; Qing, L.; Zhang, L.; Chen, L.; Ye, L.; et al. p53-Dependent apoptosis induced in human bronchial epithelial (16-HBE) cells by PM(2.5) sampled from air in Guangzhou, China. Toxicol. Mechan. Methods 2014, 24, 552–559. [Google Scholar] [CrossRef]
- Quezada-Maldonado, E.M.; Sanchez-Perez, Y.; Chirino, Y.I.; Vaca-Paniagua, F.; Garcia-Cuellar, C.M. miRNAs deregulation in lung cells exposed to airborne particulate matter (PM10) is associated with pathways deregulated in lung tumors. Environ. Pollut. (Barking Essex 1987) 2018, 241, 351–358. [Google Scholar] [CrossRef]
- Silvani, S.; Figliuzzi, M.; Remuzzi, A. Toxicological evaluation of airborne particulate matter. Are cell culture technologies ready to replace animal testing? J. Appl. Toxicol. 2019, 39, 1484–1491. [Google Scholar] [CrossRef]
- Li, Z.; Ma, J.; Li, X.; Chan, M.T.V.; Wu, W.K.K.; Wu, Z.; Shen, J. Aberrantly expressed long non-coding RNAs in air pollution-induced congenital defects. J. Cell. Mol. Med. 2019, 23, 7717–7725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, J.E.; Jorge, S.; Santos, H.M.; Chiechi, A.; Galstyan, A.; Lodeiro, C.; Diniz, M.; Kleinman, M.T.; Ljubimova, J.Y.; Capelo, J.L. Proteomic changes driven by urban pollution suggest particulate matter as a deregulator of energy metabolism, mitochondrial activity, and oxidative pathways in the rat brain. Sci. Total Environ. 2019, 687, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Caballero, H.; Rao, X.; Sun, Q.; Warmoes, M.O.; Penghui, L.; Sussan, T.E.; Park, B.; Fan, T.W.; Maiseyeu, A.; Rajagopalan, S.; et al. Air pollution-derived particulate matter dysregulates hepatic Krebs cycle, glucose and lipid metabolism in mice. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahay, D.; Terry, M.B.; Miller, R. Is breast cancer a result of epigenetic responses to traffic-related air pollution? A review of the latest evidence. Epigenomics 2019, 11, 701–714. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santibáñez-Andrade, M.; Chirino, Y.I.; González-Ramírez, I.; Sánchez-Pérez, Y.; García-Cuellar, C.M. Deciphering the Code between Air Pollution and Disease: The Effect of Particulate Matter on Cancer Hallmarks. Int. J. Mol. Sci. 2020, 21, 136. https://doi.org/10.3390/ijms21010136
Santibáñez-Andrade M, Chirino YI, González-Ramírez I, Sánchez-Pérez Y, García-Cuellar CM. Deciphering the Code between Air Pollution and Disease: The Effect of Particulate Matter on Cancer Hallmarks. International Journal of Molecular Sciences. 2020; 21(1):136. https://doi.org/10.3390/ijms21010136
Chicago/Turabian StyleSantibáñez-Andrade, Miguel, Yolanda I. Chirino, Imelda González-Ramírez, Yesennia Sánchez-Pérez, and Claudia M. García-Cuellar. 2020. "Deciphering the Code between Air Pollution and Disease: The Effect of Particulate Matter on Cancer Hallmarks" International Journal of Molecular Sciences 21, no. 1: 136. https://doi.org/10.3390/ijms21010136
APA StyleSantibáñez-Andrade, M., Chirino, Y. I., González-Ramírez, I., Sánchez-Pérez, Y., & García-Cuellar, C. M. (2020). Deciphering the Code between Air Pollution and Disease: The Effect of Particulate Matter on Cancer Hallmarks. International Journal of Molecular Sciences, 21(1), 136. https://doi.org/10.3390/ijms21010136