ROS in Platelet Biology: Functional Aspects and Methodological Insights
Abstract
1. Introduction
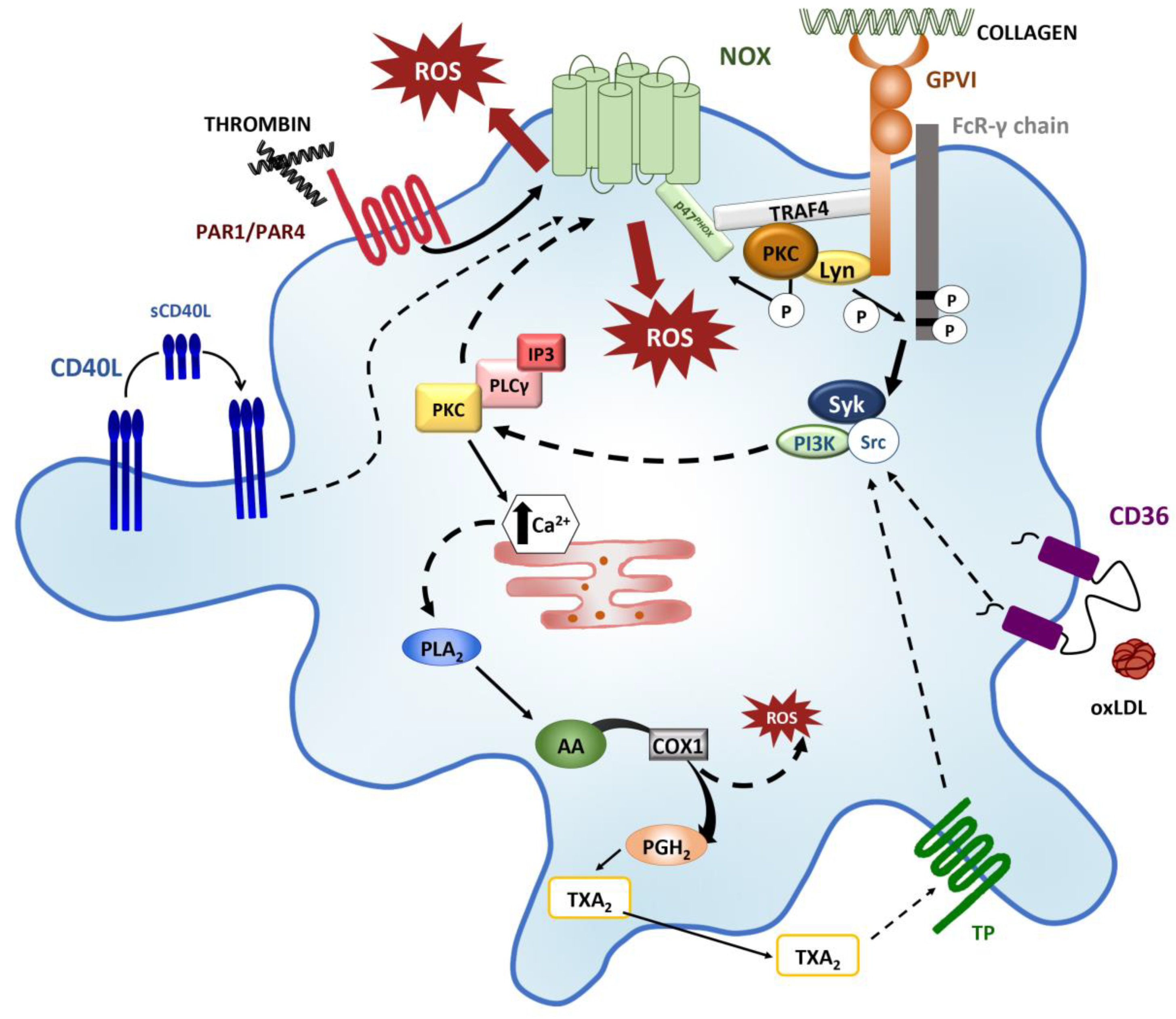
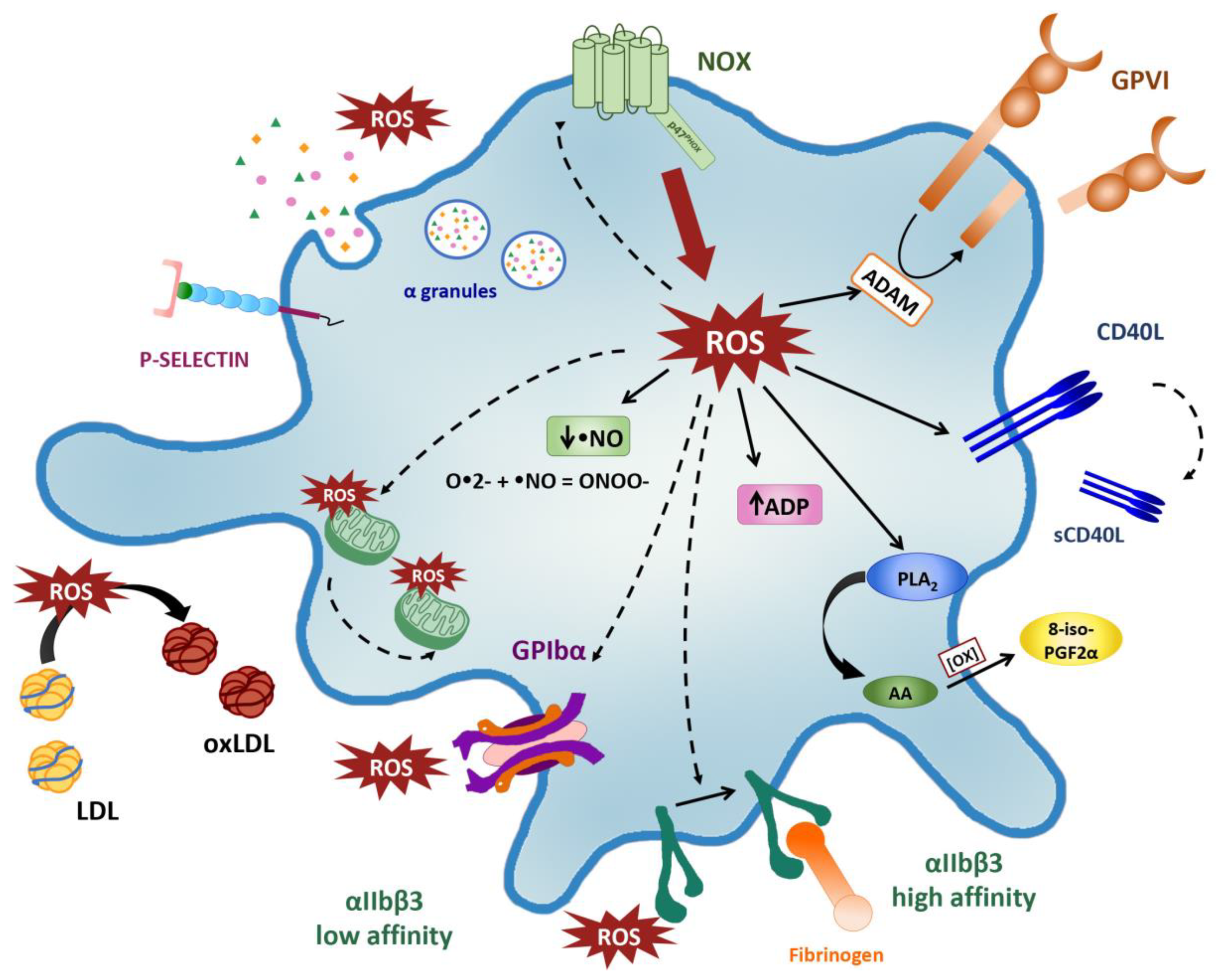
2. Sources of ROS in Platelets
3. Platelet Activation Triggers ROS/ Production
4. Endogenous and Exogenous Antioxidant Systems as Regulators of Platelet Function
4.1. Endogenous Platelet Antioxidant
4.2. Exogenous Redox Systems
5. Redox Control of Platelet Activation
6. Role of Platelet Mitochondria in Redox Balance
7. Methods to Assess Platelets Redox Biology
7.1. Detection of Reactive Oxygen Species Levels
7.2. Detection of Antioxidant Enzymes Activity
7.3. Detection of Protein Oxidation Products
7.4. Detection of Lipid Peroxidation
7.5. Analysis of Mitochondrial Function
8. Clinical Transferability
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| •NO | nitric oxide |
| •NO2 | nitrogen dioxide radical |
| •OH | hydroxyl radical |
| ∆Ψm | mitochondrial membrane potential |
| 2OH-Et+ | 2-hydroxy-ethidium |
| 3-NO-Tyr | 3-nitro-tyrosine |
| 8-iso-PGF2α | 8-iso-prostaglandin F2α |
| αIIbβ3 | glycoprotein GPIIb/IIIa |
| AA | arachidonic acid |
| ADP | adenosine diphosphate |
| ADAM | disintegrin and metalloproteinase |
| AMI | acute myocardial infarction |
| BHT | butyl hydroxytoluene |
| C11-BODIPY581/591 | 4,4-difluoro-5-(4-phenyl-1,3-butadienyl)-4-bora-3a, 4a-diaza-s-indacene-3-undecanoic acid |
| CAA | cerebrovascular amyloid angiopathy |
| CaM | calmodulin |
| CAT | catalase |
| CD40L | CD40 ligand |
| CD62p | P-selectin |
| COX | cyclooxygenase |
| DAF-FM | 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate |
| DCF | 2′,7′-dichlorofluorescein |
| DCFH-DA | dihydrochlorofluorescein diacetate |
| DHE | dihydroethidium |
| DNDPH | 2,4-dinitrophenylhydrazine |
| DNP | dinitrophenyl |
| DPI | diphenyleneiodonium cloride |
| DTNB | 5,5′-dithio-bis (2 nitrobenzoic acid) |
| ECAR | extracellualr acidification rate |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| eNOSIII | endothelial-like NO synthase III |
| EPR | electron paramagnetic resonance |
| ETC | electron transport chain |
| FAD | flavin adenine dinucleotide |
| FCCP | carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone |
| FCM | flow cytometry |
| FcRγ | Fc receptor γ-chain |
| GC-MS | gas chromatography coupled with mass spectroscopy |
| GPIbα | glycoprotein Ibα |
| GPVI | glycoprotein VI |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GSSG | glutathione disulfide |
| GST | glutathione S-transferase |
| H2O2 | hydrogen peroxide |
| HClO | hypochlorous acid |
| HE | hydroethidine |
| Hic-5 | focal adeshion adapter protein |
| HNE | 4-hydroxy-2-nonenal |
| HO-1 | heme oxygenase 1 |
| HPLC | high-performance liquid chromatography |
| IP3 | inositol 1,4,5-trisphosphate |
| ITAM | immunoreceptor tyrosine-based activation motif |
| LC-MS | liquid chromatography mass spectrometry |
| MAPKp38 | P38 mitogen-activated protein kinases |
| MDA | malondialdehyde |
| MDR | multidrug resistance |
| MKs | megakaryocytes |
| mPTP | mitochondrial permeability transition pore |
| NAC | N-acetylcysteine |
| NAD(P)H | nicotinamide adenine dinucleotide (phosphate) |
| NO-GC | NO-sensitive guanylyl cyclase |
| NOS | nitric oxide synthase |
| Nrf2-Keap1 | nuclear factor eruthroid-2 related factor 2/kelch-like ECH-associated protein |
| O•2- | superoxide anion |
| O2 | molecular oxygen |
| O2K | Oxygraph-2k |
| OCR | O2 consumption rate |
| OH− | hydroxyl ion |
| ONOO- | peroxynitrite |
| Ox-LDL | oxidized low-density lipoprotein |
| OXPHOS | oxidative phosphorylation |
| P2Y1 and P2Y12 | purinergic G protein-coupled receptors |
| PAR1 and PAR4 | protease activated receptors |
| PDIs | platelet disulfide isomerase |
| PGH2 | prostaglandin H2 |
| PI(3,4)P | phosphatidylinositol 3,4-biphosphate |
| PI3K | phosphatidylinositol 3-kinase |
| PKC | protein kinase C |
| PLA2 | phospholipase A2 |
| PLCγ2 | phospholipase Cγ2 |
| PLTs | platelets |
| PRP | platelet-rich plasma; |
| PrxII | peroxiredoxin II |
| PS | phosphatidylserine |
| Pyk2 | proline rich tyrosine kinase 2 |
| RNS | reactive nitrogen species |
| ROO• | peroxyl radicals |
| ROS | Reactive oxygen species |
| sCD40L | Soluble CD40 ligand |
| SH3 | Src homology-3 |
| SHP-2 | Src homology region 2-containing protein tyrosine phosphatases 2 |
| SOD1/2 | superoxide dismutase 1/2 |
| Syk | spleen tyrosine kinase |
| TBA | thiobarbituric acid |
| TNB | 5-thio-2-nitrobenzoic acid |
| TP | thromboxane receptor |
| TRAF4 | tumor nescrosis factor associated factor 4 |
| Trx | thioredoxin |
| TXA2 | thromboxane A2 |
| U46619 | thromboxane A2 analog |
| VWR | von Willebrand factor |
| X-linked CGD | X-linked chronic granulomatous disease |
| XF | extracellular flux analyzer |
| XO | xanthine oxidase |
References
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Varghese, S.; Vitseva, O.; Tanriverdi, K.; Freedman, J.E. CD40 Ligand Influences Platelet Release of Reactive Oxygen Intermediates. Arter. Thromb. Vasc. Biol. 2005, 25, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.S.; Oh, H.; Rhee, S.G.; Yoo, Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 2011, 32, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Bergendi, L.; Beneš, L.; Ďuračková, Z.; Ferenčik, M. Chemistry, physiology and pathology of free radicals. Life Sci. 1999, 65, 1865–1874. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Störz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simic, T.; Nicoletti, F.; Maksimović-Ivanić, D. The Double-Faced Role of Nitric Oxide and Reactive Oxygen Species in Solid Tumors. Antioxidants 2020, 9, 374. [Google Scholar] [CrossRef]
- Newsholme, P.; Haber, E.P.; Hirabara, S.M.; Rebelato, E.L.O.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.C.; Carpinelli, A.R.; Curi, R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007, 583, 9–24. [Google Scholar] [CrossRef]
- Nishikawa, T.; Araki, E. Impact of Mitochondrial ROS Production in the Pathogenesis of Diabetes Mellitus and Its Complications. Antioxidants Redox Signal. 2007, 9, 343–353. [Google Scholar] [CrossRef]
- Sottero, B.; Gargiulo, S.; Russo, I.; Barale, C.; Poli, G.; Cavalot, F. Postprandial Dysmetabolism and Oxidative Stress in Type 2 Diabetes: Pathogenetic Mechanisms and Therapeutic Strategies. Med. Res. Rev. 2015, 35, 968–1031. [Google Scholar] [CrossRef]
- Anfossi, G.; Russo, I.; Massucco, P.; Mattiello, L.; Trovati, M. Platelet resistance to the antiaggregating effect of N-acetyl-l-cysteine in obese, insulin-resistant subjects. Thromb. Res. 2003, 110, 39–46. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Gracia, K.C.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Moreira, P. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; Georgieva, M.G.; Ognyanov, I.V.; Kordos, K.; Jóźwik, A.; Kühl, T.; Perry, G.; Petralia, M.C.; Mazzon, E.; et al. Reactive Oxygen Species and Their Impact in Neurodegenerative Diseases: Literature Landscape Analysis. Antioxidants Redox Signal. 2020. [Google Scholar] [CrossRef]
- Ware, J.; Corken, A.; Khetpal, R. Platelet function beyond hemostasis and thrombosis. Curr. Opin. Hematol. 2013, 20, 451–456. [Google Scholar] [CrossRef]
- Rondina, M.T.; Weyrich, A.; Zimmerman, G.A. Platelets as cellular effectors of inflammation in vascular diseases. Circ. Res. 2013, 112, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Carubbi, C.; Tagliazucchi, G.M.; Masselli, E.; Mirandola, P.; Pigazzani, F.; Crocamo, A.; Notarangelo, M.F.; Suma, S.; Paraboschi, E.; et al. Sighting acute myocardial infarction through platelet gene expression. Sci. Rep. 2019, 9, 19574–19578. [Google Scholar] [CrossRef]
- Geddis, A.E. The regulation of proplatelet production. Haematologica 2009, 94, 756–759. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Italiano, J.E.; Lecine, P.; Shivdasani, R.A.; Hartwig, J.H. Blood Platelets Are Assembled Principally at the Ends of Proplatelet Processes Produced by Differentiated Megakaryocytes. J. Cell Biol. 1999, 147, 1299–1312. [Google Scholar] [CrossRef]
- Carubbi, C.; Masselli, E.; Martini, S.; Galli, D.; Aversa, F.; Mirandola, P.; Italiano, J.E., Jr.; Gobbi, G.; Vitale, M. Human thrombopoiesis depends on Protein kinase Cdelta/protein kinase Cepsilon functional couple. Haematologica 2016, 101, 812–820. [Google Scholar] [CrossRef]
- Bassini, A.; Zauli, G.; Migliaccio, G.; Migliaccio, A.R.; Pascuccio, M.; Pierpaoli, S.; Guidotti, L.; Capitani, S.; Vitale, M. Lineage-restricted expression of protein kinase C isoforms in hematopoiesis. Blood 1999, 93, 1178–1188. [Google Scholar] [CrossRef]
- Gobbi, G.; Mirandola, P.; Carubbi, C.; Galli, D.; Vitale, M. Protein kinase C epsilon in hematopoiesis: Conductor or selector? Semin Thromb Hemost 2013, 39, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Masselli, E.; Carubbi, C.; Gobbi, G.; Mirandola, P.; Galli, D.; Martini, S.; Bonomini, S.; Crugnola, M.; Craviotto, L.; Aversa, F.; et al. Protein kinase Cvarepsilon inhibition restores megakaryocytic differentiation of hematopoietic progenitors from primary myelofibrosis patients. Leukemia 2015, 29, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Masselli, E.; Carubbi, C.; Pozzi, G.; Martini, S.; Aversa, F.; Galli, D.; Gobbi, G.; Mirandola, P.; Vitale, M. Platelet expression of PKCepsilon oncoprotein in myelofibrosis is associated with disease severity and thrombotic risk. Ann. Transl. Med. 2017, 5, 273. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Franchini, M.; Targher, G. Arterial thrombus formation in cardiovascular disease. Nat. Rev. Cardiol. 2011, 8, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Gesi, M.; Galli, D.; Mirandola, P.; Carubbi, C.; Masselli, E.; Vitale, M.; Gobbi, G. Cytofluorimetric Platelet Analysis. Semin. Thromb. Hemost. 2013, 40, 088–098. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, R.B.; Sathler, P.C.; Lourenço, A.L.; Saito, M.; Cabral, L.M.; Rampelotto, P.H.; Castro, H.C. Platelets: Still a Therapeutical Target for Haemostatic Disorders. Int. J. Mol. Sci. 2014, 15, 17901–17919. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Arthur, J.F.; Gardiner, E.E.; Andrews, R.K.; Zeng, L.; Xu, K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2018, 14, 126–130. [Google Scholar] [CrossRef]
- Zharikov, S.; Shiva, S. Platelet mitochondrial function: From regulation of thrombosis to biomarker of disease. Biochem. Soc. Trans. 2013, 41, 118–123. [Google Scholar] [CrossRef]
- Kiyuna, L.A.; E Albuquerque, R.P.; Chen, C.-H.; Mochly-Rosen, D.; Ferreira, J.C. Targeting mitochondrial dysfunction and oxidative stress in heart failure: Challenges and opportunities. Free Radic. Biol. Med. 2018, 129, 155–168. [Google Scholar] [CrossRef]
- Chen, S.; Su, Y.; Wang, J. ROS-mediated platelet generation: A microenvironment-dependent manner for megakaryocyte proliferation, differentiation, and maturation. Cell Death Dis. 2013, 4, e722. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Pignatelli, P. Platelet NOX, a novel target for anti-thrombotic treatment. Thromb. Haemost. 2014, 111, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Araya-Maturana, R.; Urra, F.A. Regulation of mitochondrial function as a promising target in platelet activation-related diseases. Free Radic. Biol. Med. 2019, 136, 172–182. [Google Scholar] [CrossRef]
- Iuliano, L.; Colavita, A.R.; Leo, R.; Praticò, M.; Violi, F. Oxygen Free Radicals and Platelet Activation. Free Radic. Biol. Med. 1997, 22, 999–1006. [Google Scholar] [CrossRef]
- Freedman, J.E. Oxidative stress and platelets. Arter. Thromb. Vasc. Biol. 2008, 28, s11–s16. [Google Scholar] [CrossRef] [PubMed]
- Eitan, F.; Mutaz, D. Oxidative Stress and Platelet Dysfunction. Thromb. Haemost. Res. 2018, 2, 1017. [Google Scholar]
- Wachowicz, B.; Olas, B.; Zbikowska, H.; Buczyński, A. Generation of reactive oxygen species in blood platelets. Platelets 2002, 13, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M. NADPH oxidase. Curr. Opin. Immunol. 2004, 16, 42–47. [Google Scholar] [CrossRef]
- Fuentes, E.; Gibbins, J.M.; Holbrook, L.-M.; Palomo, I. NADPH oxidase 2 (NOX2): A key target of oxidative stress-mediated platelet activation and thrombosis. Trends Cardiovasc. Med. 2018, 28, 429–434. [Google Scholar] [CrossRef]
- Leto, T.L.; Morand, S.; Hurt, D.; Ueyama, T. Targeting and Regulation of Reactive Oxygen Species Generation by Nox Family NADPH Oxidases. Antioxidants Redox Signal. 2009, 11, 2607–2619. [Google Scholar] [CrossRef]
- Koga, H.; Terasawa, H.; Nunoi, H.; Takeshige, K.; Inagaki, F.; Sumimoto, H. Tetratricopeptide Repeat (TPR) Motifs of p67phoxParticipate in Interaction with the Small GTPase Rac and Activation of the Phagocyte NADPH Oxidase. J. Biol. Chem. 1999, 274, 25051–25060. [Google Scholar] [CrossRef]
- Akbar, H.; Duan, X.; Piatt, R.; Saleem, S.; Davis, A.K.; Tandon, N.N.; Bergmeier, W.; Zheng, Y. Small molecule targeting the Rac1-NOX2 interaction prevents collagen-related peptide and thrombin-induced reactive oxygen species generation and platelet activation. J. Thromb. Haemost. 2018, 16, 2083–2096. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Sanguigni, V.; Lenti, L.; Ferro, D.; Finocchi, A.; Rossi, P.; Violi, F. gp91phox-Dependent Expression of Platelet CD40 Ligand. Circulation 2004, 110, 1326–1329. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Loffredo, L.; Nocella, C.; Bartimoccia, S.; Sanguigni, V.; Soresina, A.; Plebani, A.; Azzari, C.; Martire, B.; Pignata, C.; et al. Impaired platelet activation in patients with hereditary deficiency of p47phox. Br. J. Haematol. 2016, 180, 454–456. [Google Scholar] [CrossRef]
- Begonja, A.J.; Gambaryan, S.; Geiger, J.; Aktas, B.; Pozgajova, M.; Nieswandt, B.; Walter, U. Platelet NAD(P)H-oxidase-generated ROS production regulates alphaIIbbeta3-integrin activation independent of the NO/cGMP pathway. Blood 2005, 106, 2757–2760. [Google Scholar] [CrossRef] [PubMed]
- Krötz, F.; Sohn, H.Y.; Gloe, T.; Zahler, S.; Riexinger, T.; Schiele, T.M.; Becker, B.F.; Theisen, K.; Klauss, V.; Pohl, U. NAD(P)H oxidase–dependent platelet superoxide anion release increases platelet recruitment. Blood 2002, 100, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.K.; Kim, K.; Estevez, B.; Xu, Z.; Stojanovic-Terpo, A.; Shen, B.; Ushio-Fukai, M.; Cho, J.; Du, X. Differential Roles of the NADPH-Oxidase 1 and 2 in Platelet Activation and Thrombosis. Arter. Thromb. Vasc. Biol. 2016, 36, 846–854. [Google Scholar] [CrossRef]
- Walsh, T.; Berndt, M.; Carrim, N.; Cowman, J.; Kenny, D.; Metharom, P. The role of Nox1 and Nox2 in GPVI-dependent platelet activation and thrombus formation. Redox Biol. 2014, 2, 178–186. [Google Scholar] [CrossRef]
- Vara, D.; Cifuentes-Pagano, E.; Pagano, P.J.; Pula, G. A novel combinatorial technique for simultaneous quantification of oxygen radicals and aggregation reveals unexpected redox patterns in the activation of platelets by different physiopathological stimuli. Haematologica 2019, 104, 1879–1891. [Google Scholar] [CrossRef]
- Obermayer, G.; Afonyushkin, T.; Binder, C.J. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J. Thromb. Haemost. 2018, 16, 418–428. [Google Scholar] [CrossRef]
- Jaunmuktane, Z.; Mead, S.; Ellis, M.; Wadsworth, J.D.; Nicoll, A.J.; Kenny, J.; Launchbury, F.; Linehan, J.; Richard-Loendt, A.; Walker, A.S.; et al. Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature 2015, 525, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Matarrese, P.; Straface, E.; Palumbo, G.; Anselmi, M.; Gambardella, L.; Ascione, B.; Del Principe, D.; Malorni, W. Mitochondria regulate platelet metamorphosis induced by opsonized zymosan A - activation and long-term commitment to cell death. FEBS J. 2009, 276, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.-I.; Edelstein, D.; Du, X.-L.; Brownlee, M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes 2001, 50, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Jesel, L.; Freyssinet, J.-M.; Toti, F. Cellular Mechanisms Underlying the Formation of Circulating Microparticles. Arter. Thromb. Vasc. Biol. 2011, 31, 15–26. [Google Scholar] [CrossRef]
- Nurden, P.; Gobbi, G.; Nurden, A.; Enouf, J.; Youlyouz-Marfak, I.; Carubbi, C.; La Marca, S.; Punzo, M.; Baronciani, L.; De Marco, L.; et al. Abnormal VWF modifies megakaryocytopoiesis: Studies of platelets and megakaryocyte cultures from patients with von Willebrand disease type 2B. Blood 2010, 115, 2649–2656. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling During Platelet Adhesion and Activation. Arter. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef]
- Bennett, J.S. Structure and function of the platelet integrin alphaIIbbeta3. J. Clin. Invest. 2005, 115, 3363–3369. [Google Scholar] [CrossRef]
- Bakdash, N.; Williams, M.S. Spatially distinct production of reactive oxygen species regulates platelet activation. Free Radic. Biol. Med. 2008, 45, 158–166. [Google Scholar] [CrossRef]
- Pignatelli, P.; Pulcinelli, F.M.; Lenti, L.; Gazzaniga, P.P.; Violi, F. Hydrogen peroxide is involved in collagen-induced platelet activation. Blood 1998, 91, 484–490. [Google Scholar] [CrossRef]
- Nieswandt, B.; Watson, S.P. Platelet-collagen interaction: Is GPVI the central receptor? Blood 2003, 102, 449–461. [Google Scholar] [CrossRef]
- Suzuki-Inoue, K.; Tulasne, D.; Shen, Y.; Bori-Sanz, T.; Inoue, O.; Jung, S.M.; Moroi, M.; Andrews, R.K.; Berndt, M.C.; Watson, S.P. Association of Fyn and Lyn with the Proline-rich Domain of Glycoprotein VI Regulates Intracellular Signaling. J. Biol. Chem. 2002, 277, 21561–21566. [Google Scholar] [CrossRef]
- Stegner, D.; Haining, E.J.; Nieswandt, B. Targeting Glycoprotein VI and the Immunoreceptor Tyrosine-Based Activation Motif Signaling Pathway. Arter. Thromb. Vasc. Biol. 2014, 34, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.F.; Shen, Y.; Gardiner, E.E.; Coleman, L.; Kenny, D.; Andrews, R.K.; Berndt, M.C. TNF receptor-associated factor 4 (TRAF4) is a novel binding partner of glycoprotein Ib and glycoprotein VI in human platelets. J. Thromb. Haemost. 2011, 9, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Canobbio, I.; Cipolla, L.; Guidetti, G.F.; Manganaro, D.; Visconte, C.; Kim, S.; Okigaki, M.; Falasca, M.; Kunapuli, S.P.; Torti, M. The focal adhesion kinase Pyk2 links Ca2+ signalling to Src family kinase activation and protein tyrosine phosphorylation in thrombin-stimulated platelets. Biochem. J. 2015, 469, 199–210. [Google Scholar] [CrossRef]
- Arthur, J.F.; Qiao, J.; Shen, Y.; Davis, A.K.; Dunne, E.; Berndt, M.C.; Gardiner, E.E.; Andrews, R.K. ITAM receptor-mediated generation of reactive oxygen species in human platelets occurs via Syk-dependent and Syk-independent pathways. J. Thromb. Haemost. 2012, 10, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Durrant, T.N.; Hers, I. PI3K inhibitors in thrombosis and cardiovascular disease. Clin. Transl. Med. 2020, 9, 1–21. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, M.; Luo, D.; Yue, M.; Wang, S.; Chen, X.; Zhou, Y.; Wang, Y.; Cai, Y.; Hu, X.; et al. Class III PI3K Positively Regulates Platelet Activation and Thrombosis via PI(3)P-Directed Function of NADPH Oxidase. Arter. Thromb. Vasc. Biol. 2017, 37, 2075–2086. [Google Scholar] [CrossRef]
- Carrim, N.; Arthur, J.F.; Hamilton, J.R.; Gardiner, E.E.; Andrews, R.K.; Moran, N.; Berndt, M.C.; Metharom, P. Thrombin-induced reactive oxygen species generation in platelets: A novel role for protease-activated receptor 4 and GPIbα. Redox Biol. 2015, 6, 640–647. [Google Scholar] [CrossRef]
- Wilson, S.J.; Cavanagh, C.C.; Lesher, A.M.; Frey, A.J.; Russell, S.E.; Smyth, E.M. Activation-dependent stabilization of the human thromboxane receptor: Role of reactive oxygen species. J. Lipid Res. 2009, 50, 1047–1056. [Google Scholar] [CrossRef]
- Morel, A.; Miller, E.; Bijak, M.; Saluk, J. The increased level of COX-dependent arachidonic acid metabolism in blood platelets from secondary progressive multiple sclerosis patients. Mol. Cell. Biochem. 2016, 420, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Minuz, P.; Meneguzzi, A.; Fumagalli, L.; Degan, M.; Calabria, S.; Ferraro, R.; Ricci, M.; Veneri, D.; Berton, G. Calcium-Dependent Src Phosphorylation and Reactive Oxygen Species Generation Are Implicated in the Activation of Human Platelet Induced by Thromboxane A2 Analogs. Front. Pharmacol. 2018, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Aloui, C.; Prigent, A.; Sut, C.; Tariket, S.; Hamzeh-Cognasse, H.; Pozzetto, B.; Richard, Y.; Cognasse, F.; Laradi, S.; Garraud, O.; et al. The Signaling Role of CD40 Ligand in Platelet Biology and in Platelet Component Transfusion. Int. J. Mol. Sci. 2014, 15, 22342–22364. [Google Scholar] [CrossRef] [PubMed]
- Inwald, D.; McDowall, A.; Peters, M.J.; E Callard, R.; Klein, N. CD40 Is Constitutively Expressed on Platelets and Provides a Novel Mechanism for Platelet Activation. Circ. Res. 2003, 92, 1041–1048. [Google Scholar] [CrossRef]
- Trpkovic, A.; Resanović, I.; Stanimirovic, J.; Radak, D.; Mousa, S.; Cenic-Milosevic, D.; Jevremović, D.; Isenović, E. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 2014, 52, 70–85. [Google Scholar] [CrossRef]
- A Podrez, E.; Byzova, T.V.; Febbraio, M.; Salomon, R.G.; Ma, Y.; Valiyaveettil, M.; Poliakov, E.; Sun, M.; Finton, P.J.; Curtis, B.R.; et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 2007, 13, 1086–1095. [Google Scholar] [CrossRef]
- Mehta, J.L.; Sanada, N.; Hu, C.-P.; Chen, J.; Dandapat, A.; Sugawara, F.; Satoh, H.; Inoue, K.; Kawase, Y.; Jishage, K.-I.; et al. Deletion of LOX-1 Reduces Atherogenesis in LDLR Knockout Mice Fed High Cholesterol Diet. Circ. Res. 2007, 100, 1634–1642. [Google Scholar] [CrossRef]
- Magwenzi, S.; Woodward, C.; Wraith, K.S.; Aburima, A.; Raslan, Z.; Jones, H.; McNeil, C.; Wheatcroft, S.; Yuldasheva, N.; Febbriao, M.; et al. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signaling cascade. Blood 2015, 125, 2693–2703. [Google Scholar] [CrossRef]
- Bartimoccia, S.; Nocella, C.; Pastori, D.; Pignatelli, P.; Carnevale, R. Platelet Oxidative Stress and Antioxidant Nutrients. J. Vasc. Med. Surg. 2014, 2, 164. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Trachtenburg, J.; Ryan, U.S.; Abendschein, D.R. Potentiation of endogenous nitric oxide with superoxide dismutase inhibits platelet-mediated thrombosis in injured and stenotic arteries. J. Am. Coll. Cardiol. 1995, 25, 269–275. [Google Scholar] [CrossRef]
- Fidler, T.P.; Campbell, R.A.; Funari, T.; Dunne, N.; Balderas Angeles, E.; Middleton, E.A.; Chaudhuri, D.; Weyrich, A.S.; Abel, E.D. Deletion of GLUT1 and GLUT3 Reveals Multiple Roles for Glucose Metabolism in Platelet and Megakaryocyte Function. Cell. Rep. 2017, 20, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Fidler, T.P.; Rowley, J.W.; Araujo, C.; Boudreau, L.H.; Marti, A.; Souvenir, R.; Dale, K.; Boilard, E.; Weyrich, A.; Abel, E.D. Superoxide Dismutase 2 is dispensable for platelet function. Thromb. Haemost. 2017, 117, 1859–1867. [Google Scholar] [CrossRef]
- Cardoso’, A.R.; Chausse, B.; Da Cunha, F.M.; Luévano-Martínez, L.A.; Marazzi, T.B.; Pessoa, P.S.; Queliconi, B.B.; Kowaltowski, A.J. Mitochondrial compartmentalization of redox processes. Free Radic. Biol. Med. 2012, 52, 2201–2208. [Google Scholar] [CrossRef]
- Jang, J.Y.; Min, J.H.; Chae, Y.H.; Baek, J.Y.; Bin Wang, S.; Park, S.J.; Oh, G.T.; Lee, S.-H.; Ho, Y.-S.; Chang, T.-S. Reactive Oxygen Species Play a Critical Role in Collagen-Induced Platelet Activation via SHP-2 Oxidation. Antioxidants Redox Signal. 2014, 20, 2528–2540. [Google Scholar] [CrossRef] [PubMed]
- Raes, M.; Michiels, C.; Remacle, J. Comparative study of the enzymatic defense systems against oxygen-derived free radicals: The key role of glutathione peroxidase. Free Radic. Biol. Med. 1987, 3, 3–7. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J.; et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Bin Wang, S.; Min, J.H.; Chae, Y.H.; Baek, J.Y.; Yu, D.-Y.; Chang, T.-S. Peroxiredoxin II Is an Antioxidant Enzyme That Negatively Regulates Collagen-stimulated Platelet Function. J. Biol. Chem. 2015, 290, 11432–11442. [Google Scholar] [CrossRef]
- Metcalfe, C.; Ramasubramoni, A.; Pula, G.; Harper, M.T.; Mundell, S.J.; Coxon, C.H. Thioredoxin Inhibitors Attenuate Platelet Function and Thrombus Formation. PLoS ONE 2016, 11, e0163006. [Google Scholar] [CrossRef]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Thomas, G.; Skrinska, V.; Lucas, F.V.; Schumacher, O.P. Platelet glutathione and thromboxane synthesis in diabetes. Diabetes 1985, 34, 951–954. [Google Scholar] [CrossRef]
- Van Gorp, R.M.; Van Dam-Mieras, M.C.; Hornstra, G.; Heemskerk, J.W. Effect of membrane-permeable sulfhydryl reagents and depletion of glutathione on calcium mobilisation in human platelets. Biochem. Pharmacol. 1997, 53, 1533–1542. [Google Scholar] [CrossRef]
- Hamilos, M.; Petousis, S.; Parthenakis, F. Interaction between platelets and endothelium: From pathophysiology to new therapeutic options. Cardiovasc. Diagn. Ther. 2018, 8, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Essex, D.W. The Role of Thiols and Disulfides in Platelet Function. Antioxidants Redox Signal. 2004, 6, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Takebe, G.; Yarimizu, J.; Saito, Y.; Hayashi, T.; Nakamura, H.; Yodoi, J.; Nagasawa, S.; Takahashi, K. A Comparative Study on the Hydroperoxide and Thiol Specificity of the Glutathione Peroxidase Family and Selenoprotein P. J. Biol. Chem. 2002, 277, 41254–41258. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.C.; Mahoney, C.E.; Anderson, L.; Ottaviano, F.; Croce, K.; Leopold, J.A.; Zhang, Y.-Y.; Tang, S.-S.; Handy, D.E.; Loscalzo, J. Glutathione Peroxidase-3 Deficiency Promotes Platelet-Dependent Thrombosis In Vivo. Circulation 2011, 123, 1963–1973. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Dragan, S.; Andrica, F.; Serban, M.-C.; Timar, R. Polyphenols-rich natural products for treatment of diabetes. Curr. Med. Chem. 2015, 22, 14–22. [Google Scholar] [CrossRef]
- Albarracin, S.L.; Stab, B.; Casas, Z.; Sutachan, J.J.; Samudio, I.; Gonzalez, J.; Gonzalo, L.; Capani, F.; Morales, L.; Barreto, G.E. Effects of natural antioxidants in neurodegenerative disease. Nutr. Neurosci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Bulmer, A.C.; Singh, I. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J. Hum. Nutr. Diet. 2013, 27, 1–21. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P.; Basili, S.; Grum, F.; Hufendiek, K.; Gamulescu, M.; Rümmele, P.; Schlachetzki, F.; Franz, S.; Bogdahn, U. Nutrition, Supplements, and Vitamins in Platelet Function and Bleeding. Circulation 2010, 121, 1033–1044. [Google Scholar] [CrossRef]
- Carnevale, R.; Loffredo, L.; Pignatelli, P.; Nocella, C.; Bartimoccia, S.; Di Santo, S.; Martino, F.; Catasca, E.; Perri, L.; Violi, F. Dark chocolate inhibits platelet isoprostanes via NOX2 down-regulation in smokers. J. Thromb. Haemost. 2012, 10, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Pignatelli, P.; Nocella, C.; Loffredo, L.; Pastori, D.; Vicario, T.; Petruccioli, A.; Bartimoccia, S.; Violi, F. Extra virgin olive oil blunt post-prandial oxidative stress via NOX2 down-regulation. Atherosclerosis 2014, 235, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Cavarretta, E.; Peruzzi, M.; Del Vescovo, R.; Di Pilla, F.; Gobbi, G.; Serdoz, A.; Ferrara, R.; Schirone, L.; Sciarretta, S.; Nocella, C.; et al. Dark Chocolate Intake Positively Modulates Redox Status and Markers of Muscular Damage in Elite Football Athletes: A Randomized Controlled Study. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Pastori, D.; Carnevale, R.; Cangemi, R.; Saliola, M.; Nocella, C.; Bartimoccia, S.; Vicario, T.; Farcomeni, A.; Violi, F.; Pignatelli, P. Vitamin E Serum Levels and Bleeding Risk in Patients Receiving Oral Anticoagulant Therapy: A Retrospective Cohort Study. J. Am. Hear. Assoc. 2013, 2, 000364. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.P.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Kim, K.; Li, J.; Tseng, A.; Andrews, R.K.; Cho, J. NOX2 is critical for heterotypic neutrophil-platelet interactions during vascular inflammation. Blood 2015, 126, 1952–1964. [Google Scholar] [CrossRef]
- Hartmann, M.; Herrlich, A.; Herrlich, P. Who decides when to cleave an ectodomain? Trends Biochem. Sci. 2013, 38, 111–120. [Google Scholar] [CrossRef]
- Brill, A.; Chauhan, A.K.; Canault, M.; Walsh, M.T.; Bergmeier, W.; Wagner, D.D. Oxidative stress activates ADAM17/TACE and induces its target receptor shedding in platelets in a p38-dependent fashion. Cardiovasc. Res. 2009, 84, 137–144. [Google Scholar] [CrossRef]
- Baaten, C.C.F.M.J.; Swieringa, F.; Misztal, T.; Mastenbroek, T.G.; Feijge, M.A.H.; Bock, P.E.; Donners, M.M.P.C.; Collins, P.W.; Li, R.; Van Der Meijden, P.E.J.; et al. Platelet heterogeneity in activation-induced glycoprotein shedding: Functional effects. Blood Adv. 2018, 2, 2320–2331. [Google Scholar] [CrossRef]
- Bergmeier, W.; Piffath, C.L.; Cheng, G.; Dole, V.S.; Zhang, Y.; von Andrian, U.H.; Wagner, D.D. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates GPIbalpha shedding from platelets in vitro and in vivo. Circ. Res. 2004, 95, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E.; Arthur, J.F.; Kahn, M.L.; Berndt, M.C.; Andrews, R.K. Regulation of platelet membrane levels of glycoprotein VI by a platelet-derived metalloproteinase. Blood 2004, 104, 3611–3617. [Google Scholar] [CrossRef]
- Hosseini, E.; Solouki, A.; Roudsari, Z.O.; Kargar, F.; Ghasemzadeh, M. Reducing state attenuates ectodomain shedding of GPVI while restoring adhesion capacities of stored platelets: Evidence addressing the controversy around the effects of redox condition on thrombosis. J. Thromb. Thrombolysis 2020, 50, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Sanguigni, V.; Paola, S.G.; Coco, E.L.; Lenti, L.; Violi, F. Vitamin C inhibits platelet expression of CD40 ligand. Free Radic. Biol. Med. 2005, 38, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Dangel, O.; Mergia, E.; Karlisch, K.; Groneberg, D.; Koesling, D.; Friebe, A. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J. Thromb. Haemost. 2010, 8, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Carnevale, R.; Di Santo, S.; Bartimoccia, S.; Sanguigni, V.; Lenti, L.; Finocchi, A.; Mendolicchio, L.; Soresina, A.R.; Plebani, A.; et al. Inherited Human gp91phoxDeficiency Is Associated With Impaired Isoprostane Formation and Platelet Dysfunction. Arter. Thromb. Vasc. Biol. 2011, 31, 423–434. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Bartling, A.; Lenzen, H.; Tsikas, D.; Maas, R.; Brummer, J.; Gutzki, F.M.; Berger, J.; Frolich, J.C.; Boger, R.H. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: A matched case-control study. Circulation 2004, 109, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Botto, N.; Andreassi, M.G.; Berti, S.; Biagini, A. Evidence for enhanced 8-isoprostane plasma levels, as index of oxidative stress in vivo, in patients with coronary artery disease. Coron. Artery Dis. 2003, 14, 213–218. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Bierend, A.; Maas, R.; Trinks, R.; Kom, G.D.; Tsikas, D.; Böger, R.H. Redox-generated isoprostanes are associated with residual platelet activity in aspirin-treated patients with stable coronary heart disease. J. Thromb. Haemost. 2010, 8, 2662–2670. [Google Scholar] [CrossRef]
- Praticò, D.; Iuliano, L.; Mauriello, A.; Spagnoli, L.G.; A Lawson, J.; Rokach, J.; Maclouf, J.; Violi, F.; A Fitzgerald, G. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J. Clin. Investig. 1997, 100, 2028–2034. [Google Scholar] [CrossRef]
- Becatti, M.; Fiorillo, C.; Gori, A.M.; Marcucci, R.; Paniccia, R.; Giusti, B.; Violi, F.; Pignatelli, P.; Gensini, G.F.; Abbate, R. Platelet and leukocyte ROS production and lipoperoxidation are associated with high platelet reactivity in Non-ST elevation myocardial infarction (NSTEMI) patients on dual antiplatelet treatment. Atherosclerosis 2013, 231, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Gopaul, N.K.; Änggård, E.; Mallet, A.; Betteridge, D.; Wolff, S.; Nourooz-Zadeh, J. Plasma 8-epi-PGF2α levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995, 368, 225–229. [Google Scholar] [CrossRef]
- Mezzetti, A.; Cipollone, F.; Cuccurullo, F. Oxidative stress and cardiovascular complications in diabetes: Isoprostanes as new markers on an old paradigm. Cardiovasc. Res. 2000, 47, 475–488. [Google Scholar] [CrossRef]
- Minuz, P.; Patrignani, P.; Gaino, S.; Degan, M.; Menapace, L.; Tommasoli, R.; Seta, F.; Capone, M.L.; Tacconelli, S.; Palatresi, S.; et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation 2002, 106, 2800–2805. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Basili, S.; Falco, A.; Davì, G. Oxidant Stress and Platelet Activation in Hypercholesterolemia. Antioxidants Redox Signal. 2004, 6, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, N.; Formoso, G.; Pandolfi, A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc. Pharmacol. 2016, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Bartimoccia, S.; Nocella, C.; Di Santo, S.; Loffredo, L.; Illuminati, G.; Lombardi, E.; Boz, V.; Del Ben, M.; De Marco, L.; et al. LDL oxidation by platelets propagates platelet activation via an oxidative stress-mediated mechanism. Atherosclerosis 2014, 237, 108–116. [Google Scholar] [CrossRef]
- Chacko, B.K.; A Kramer, P.; Ravi, S.; Johnson, M.S.; Hardy, R.W.; Ballinger, S.W.; Darley-Usmar, V.M. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Investig. 2013, 93, 690–700. [Google Scholar] [CrossRef]
- Ravi, S.; Chacko, B.; Sawada, H.; Kramer, P.A.; Johnson, M.S.; Benavides, G.A.; O’Donnell, V.; Marques, M.; Darley-Usmar, V. Metabolic Plasticity in Resting and Thrombin Activated Platelets. PLoS ONE 2015, 10, e0123597. [Google Scholar] [CrossRef]
- Verhoeven, A.J.; Mommersteeg, M.E.; Akkerman, J.-W.N. Metabolic energy is required in human platelets at any stage during optical aggregation and secretion. Biochim. Biophys. Acta (BBA) Gen. Subj. 1984, 800, 242–250. [Google Scholar] [CrossRef]
- Akahori, M.; Uedono, Y.; Yamagami, K.; Takeyama, N.; Kitazawa, Y.; Tanaka, T. Hypoxia alters the energy metabolism and aggregation of washed human platelets. Haematologica 1995, 26, 191–198. [Google Scholar]
- De La Peña, N.C.; Gutiérrez-Aguilar, M.; Hernández-Reséndiz, I.; Marín-Hernández, A.; Rodríguez-Enríquez, S. Glycoprotein Ib activation by thrombin stimulates the energy metabolism in human platelets. PLoS ONE 2017, 12, e0182374. [Google Scholar] [CrossRef] [PubMed]
- Tomasiak, M.; Stelmach, H.; Rusak, T.; Wysocka, J. Nitric oxide and platelet energy metabolism. Acta Biochim. Pol. 2004, 51, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Muntean, D.M.; Sturza, A.; Dănilă, M.D.; Borza, C.; Duicu, O.M.; Mornoș, C. The Role of Mitochondrial Reactive Oxygen Species in Cardiovascular Injury and Protective Strategies. Oxidative Med. Cell. Longev. 2016, 2016, 1–19. [Google Scholar] [CrossRef]
- Sowton, A.; Millington-Burgess, S.L.; Murray, A.J.; Harper, M.T. Rapid kinetics of changes in oxygen consumption rate in thrombin-stimulated platelets measured by high-resolution respirometry. Biochem. Biophys. Res. Commun. 2018, 503, 2721–2727. [Google Scholar] [CrossRef]
- Lopez, J.J.; Salido, G.M.; Gómez-Arteta, E.; Rosado, J.A.; Pariente, J. Thrombin induces apoptotic events through the generation of reactive oxygen species in human platelets. J. Thromb. Haemost. 2007, 5, 1283–1291. [Google Scholar] [CrossRef]
- Girish, K.S.; Paul, M.; Thushara, R.M.; Hemshekhar, M.; Sundaram, M.S.; Rangappa, K.S.; Kemparaju, K. Melatonin elevates apoptosis in human platelets via ROS mediated mitochondrial damage. Biochem. Biophys. Res. Commun. 2013, 438, 198–204. [Google Scholar] [CrossRef]
- Zinkevich, N.S.; Gutterman, D.D. ROS-induced ROS release in vascular biology: Redox-redox signaling. Am. J. Physiol. Circ. Physiol. 2011, 301, H647–H653. [Google Scholar] [CrossRef]
- Zorov, D.B.; Filburn, C.R.; Klotz, L.-O.; Zweier, J.L.; Sollott, S.J. Reactive Oxygen Species (Ros-Induced) Ros Release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000, 192, 1001–1014. [Google Scholar] [CrossRef]
- McStay, G.P.; Clarke, S.J.; Halestrap, A.P. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem. J. 2002, 367 Pt 2, 541–548. [Google Scholar] [CrossRef]
- Lee, S.B.; Bae, I.H.; Bae, Y.S.; Um, H.D. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J. Biol. Chem. 2006, 281, 36228–36235. [Google Scholar] [CrossRef] [PubMed]
- Doughan, A.K.; Harrison, D.G.; Dikalov, S.I. Molecular Mechanisms of Angiotensin II–Mediated Mitochondrial Dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008, 102, 488–496. [Google Scholar] [CrossRef]
- Kimura, S.; Zhang, G.-X.; Nishiyama, A.; Shokoji, T.; Yao, L.; Fan, Y.-Y.; Rahman, M.; Suzuki, T.; Maeta, H.; Abe, Y. Role of NAD(P)H Oxidase- and Mitochondria-Derived Reactive Oxygen Species in Cardioprotection of Ischemic Reperfusion Injury by Angiotensin II. Hypertension 2005, 45, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Yu, S.; Li, Q.; He, Y.; Liang, W.; Yu, L.; Xu, D.; Sun, T.; Zhang, R.; Li, Q. Investigation of platelet apoptosis in adult patients with chronic immune thrombocytopenia. Hematology 2016, 22, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tonon, G.; Luo, X.; Greco, N.J.; Chen, W.; Shi, Y.; A Jamieson, G. Weak platelet agonists and U46619 induce apoptosis-like events in platelets, in the absence of phosphatidylserine exposure. Thromb. Res. 2002, 107, 345–350. [Google Scholar] [CrossRef]
- Jobe, S.M.; Wilson, K.M.; Leo, L.; Raimondi, A.; Molkentin, J.D.; Lentz, S.R.; Di Paola, J. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood 2008, 111, 1257–1265. [Google Scholar] [CrossRef]
- Lopez, J.J.; Salido, G.M.; Pariente, J.; Rosado, J.A. Thrombin induces activation and translocation of Bid, Bax and Bak to the mitochondria in human platelets. J. Thromb. Haemost. 2008, 6, 1780–1788. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, F.; Chen, X.; Luo, M.; Hu, L.; Lu, Y. The Role of Mitochondria-Derived Reactive Oxygen Species in Hyperthermia-Induced Platelet Apoptosis. PLoS ONE 2013, 8, e75044. [Google Scholar] [CrossRef]
- Ho, E.; Galougahi, K.K.; Liu, C.-C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Dikalov, S.; Harrison, D.G. Methods for Detection of Mitochondrial and Cellular Reactive Oxygen Species. Antioxidants Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Hickerson, D.H.M.; Bode, A.P. Flow cytometry of platelets for clinical analysis. Hematol. Clin. N. Am. 2002, 16, 421–454. [Google Scholar] [CrossRef]
- Pasalic, L.; Pennings, G.; Connor, D.E.; Campbell, H.; Kritharides, L.; Chen, V.M. Flow Cytometry Protocols for Assessment of Platelet Function in Whole Blood. Adv. Struct. Saf. Stud. 2017, 1646, 369–389. [Google Scholar] [CrossRef]
- Carubbi, C.; Masselli, E.; Nouvenne, A.; Russo, M.; Galli, D.; Mirandola, P.; Gobbi, G.; Vitale, M. Laboratory diagnostics of inherited platelet disorders. Clin. Chem. Lab. Med. 2014, 52, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Mirandola, P.; Tazzari, P.L.; Ricci, F.; Caimi, L.; Cacchioli, A.; Papa, S.; Conte, R.; Vitale, M. Flow cytometry detection of serotonin content and release in resting and activated platelets. Br. J. Haematol. 2003, 121, 892–896. [Google Scholar] [CrossRef]
- Carubbi, C.; Masselli, E.; Pozzi, G.; Mattioli, M.; Martini, S.; Goldoni, M.; Aloe, R.; Cervellin, G.; Vitale, M.; Gobbi, G. Combination of Platelet expression of PKCepsilon and cardiac troponin-I for early diagnosis of chest pain patients in the emergency department. Sci. Rep. 2019, 9, 2125. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.E.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2011, 52, 1–6. [Google Scholar] [CrossRef]
- Komosa, A.; Rzymski, P.; Perek, B.; Ropacka-Lesiak, M.; Lesiak, M.; Siller-Matula, J.M.; Poniedziałek, B. Platelets redox balance assessment: Current evidence and methodological considerations. Vasc. Pharmacol. 2017, 93, 6–13. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 1–32. [Google Scholar] [CrossRef]
- Drummen, G.; Van Liebergen, L.C.M.; Kamp, J.A.F.O.D.; A Post, J. C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef]
- Kojima, H.; Nakatsubo, N.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal. Chem. 1998, 70, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Radziwon-Balicka, A.; Lesyk, G.; Back, V.; Fong, T.; Loredo-Calderon, E.L.; Dong, B.; El-Sikhry, H.; El-Sherbeni, A.; El-Kadi, A.; Ogg, S.; et al. Differential eNOS-signalling by platelet subpopulations regulates adhesion and aggregation. Cardiovasc. Res. 2017, 113, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.L.; Buettner, G.R.; O’Malley, Y.Q.; A Wessels, D.; Flaherty, D.M. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: Comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic. Biol. Med. 1999, 27, 146–159. [Google Scholar] [CrossRef]
- Peluso, I.; Manafikhi, H.; Reggi, R.; Palmery, M. Interference of flavonoids with fluorescent intracellular probes: Methodological implications in the evaluation of the oxidative burst by flow cytometry. Cytom. Part A 2014, 85, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kalivendi, S.; Zhang, H.; Joseph, J.; Nithipatikom, K.; Vásquez-Vivar, J.; Kalyanaraman, B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: Potential implications in intracellular fluorescence detection of superoxide. Free Radic. Biol. Med. 2003, 34, 1359–1368. [Google Scholar] [CrossRef]
- Abubaker, A.A.; Vara, D.; Eggleston, I.; Canobbio, I.; Pula, G. A novel flow cytometry assay using dihydroethidium as redox-sensitive probe reveals NADPH oxidase-dependent generation of superoxide anion in human platelets exposed to amyloid peptide beta. Platelets 2019, 30, 181–189. [Google Scholar] [CrossRef]
- Robinson, K.M.; Janes, M.S.; Pehar, M.; Monette, J.S.; Ross, M.; Hagen, T.M.; Murphy, M.P.; Beckman, J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 2006, 103, 15038–15043. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Haskó, G.; Hawkins, B.J.; Madesh, M.; Pacher, P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2007, 2, 2295–2301. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Krötz, F.; Sohn, H.-Y.; Pohl, U. Reactive Oxygen Species: Players in the platelet game. Arter. Thromb. Vasc. Biol. 2004, 24, 1988–1996. [Google Scholar] [CrossRef]
- Sonego, G.; Abonnenc, M.; Tissot, J.-D.; Prudent, M.; Lion, N. Redox Proteomics and Platelet Activation: Understanding the Redox Proteome to Improve Platelet Quality for Transfusion. Int. J. Mol. Sci. 2017, 18, 387. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, A.; Jordan, J.; Tsikas, D. High-performance liquid chromatography ultraviolet assay for human erythrocytic catalase activity by measuring glutathione as o-phthalaldehyde derivative. Anal. Biochem. 2011, 410, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Segura-Aguilar, J. A new direct method for determining superoxide dismutase activity by measuring hydrogen peroxide formation. Chem. Interact. 1993, 86, 69–78. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. Assay of superoxide dismutase activity in a plate assay using WST-1. Free Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Vives-Bauza, C.; Starkov, A.A.; Garcia-Arumi, E. Measurements of the Antioxidant Enzyme Activities of Superoxide Dismutase, Catalase, and Glutathione Peroxidase. Methods Cell Biol. 2007, 80, 379–393. [Google Scholar] [CrossRef]
- Slaughter, M.R.; O’Brien, P.J. Fully-automated spectrophotometric method for measurement of antioxidant activity of catalase. Clin. Biochem. 2000, 33, 525–534. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. [12] Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Alexandru, N.; Constantin, A.; Popov, L.-D. Carbonylation of platelet proteins occurs as consequence of oxidative stress and thrombin activation, and is stimulated by ageing and type 2 diabetes. Clin. Chem. Lab. Med. 2008, 46, 528–536. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Ferrer-Sueta, G.; Peluffo, G.; Valez, V.; Zhang, H.; Kalyanaraman, B.; Radi, R. Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids 2006, 32, 501–515. [Google Scholar] [CrossRef]
- Schopfer, F. NO-dependent protein nitration: A cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003, 28, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.M.; Peluffo, G.; Radi, R. Protein tyrosine nitration—Functional alteration or just a biomarker? Free Radic. Biol. Med. 2008, 45, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ying, J.; Jiang, B.; Guo, W.; Adachi, T.; Sharov, V.; Lazar, H.; Menzoian, J.; Knyushko, T.V.; Bigelow, D.; et al. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am. J. Physiol. Circ. Physiol. 2006, 290, H2220–H2227. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, S.; Ikeda, H.; Haramaki, N.; Murohara, T.; Imaizumi, T. Intraplatelet tetrahydrobiopterin plays an important role in regulating canine coronary arterial thrombosis by modulating intraplatelet nitric oxide and superoxide generation. Circulation 2001, 104, 2478–2484. [Google Scholar] [CrossRef][Green Version]
- Watt, J.; Ewart, M.-A.; Greig, F.H.; Oldroyd, K.G.; Wadsworth, R.M.; Kennedy, S. The effect of reactive oxygen species on whole blood aggregation and the endothelial cell-platelet interaction in patients with coronary heart disease. Thromb. Res. 2012, 130, 210–215. [Google Scholar] [CrossRef][Green Version]
- Vazzana, N.; Ganci, A.; Cefalu, A.B.; Lattanzio, S.; Noto, D.; Santoro, N.; Saggini, R.; Puccetti, L.; Averna, M.; Davi, G. Enhanced lipid peroxidation and platelet activation as potential contributors to increased cardiovascular risk in the low-HDL phenotype. J. Am. Heart Assoc. 2013, 2, e000063. [Google Scholar] [CrossRef]
- Longmire, A.W.; Roberts, L.; Morrow, J.D. Actions of the E2-isoprostane, 8-ISO-PGE2, on the platelet thromboxane/ endoperoxide receptor in humans and rats: Additional evidence for the existence of a unique isoprostane receptor. Prostaglandins 1994, 48, 247–256. [Google Scholar] [CrossRef]
- Sousa, B.; Pitt, A.R.; Spickett, C.M. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 2017, 111, 294–308. [Google Scholar] [CrossRef]
- Gasparovic, A.C.; Jaganjac, M.; Mihaljevic, B.; Sunjic, S.B.; Zarkovic, N. Assays for the Measurement of Lipid Peroxidation. Methods Mol. Biol. 2013, 965, 283–296. [Google Scholar]
- Wang, L.; Wu, Q.; Fan, Z.; Xie, R.; Wang, Z.; Lu, Y. Platelet mitochondrial dysfunction and the correlation with human diseases. Biochem. Soc. Trans. 2017, 45, 1213–1223. [Google Scholar] [CrossRef]
- Sjövall, F.; Morota, S.; Hansson, M.J.; Friberg, H.; Gnaiger, E.; Elmér, E. Temporal increase of platelet mitochondrial respiration is negatively associated with clinical outcome in patients with sepsis. Crit. Care 2010, 14, R214. [Google Scholar] [CrossRef]
- Cardenes, N.; Corey, C.; Geary, L.; Jain, S.; Zharikov, S.; Barge, S.; Novelli, E.M.; Shiva, S. Platelet bioenergetic screen in sickle cell patients reveals mitochondria complex V inhibition, which contributes to platelet activation. Blood 2014, 123, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Stitham, J.; Jin, Y.; Liu, R.; Lee, S.H.; Du, J.; Atteya, G.; Gleim, S.; Spollett, G.; Martin, K.; et al. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation 2014, 129, 1598–1609. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.; Runge, M.S. Mitochondrial Dysfunction in Atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Guo, X.; Li, Q.; Mei, G.; Lao, G. Platelets of type 2 diabetic patients are characterized by high ATP content and low mitochondrial membrane potential. Platelets 2009, 20, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef]
- Miniaev, M.V.; Belyakova, M.; Kostiuk, N.V.; Leshchenko, D.V.; Fedotova, T.A. Non-obvious Problems in Clark Electrode Application at Elevated Temperature and Ways of Their Elimination. J. Anal. Methods Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Dmitriev, R.I.; Papkovsky, D.B. Optical probes and techniques for O2 measurement in live cells and tissue. Cell. Mol. Life Sci. 2012, 69, 2025–2039. [Google Scholar] [CrossRef]
- Kramer, P.A.; Ravi, S.; Chacko, B.; Johnson, M.S.; Darley-Usmar, V. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2014, 2, 206–210. [Google Scholar] [CrossRef]
- Ferrick, D.A.; Neilson, A.; Beeson, C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today 2008, 13, 268–274. [Google Scholar] [CrossRef]
- Horan, M.P.; Pichaud, N.; Ballard, J.W.O. Review: Quantifying Mitochondrial Dysfunction in Complex Diseases of Aging. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2012, 67, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Colas, R.; Sassolas, A.; Guichardant, M.; Cugnet-Anceau, C.; Moret, M.; Moulin, P.; Lagarde, M.; Calzada, C. LDL from obese patients with the metabolic syndrome show increased lipid peroxidation and activate platelets. Diabetologia 2011, 54, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.B.; A Siqueira, M.; E Mann, G.; Mc Brunini, T.; Mendes-Ribeiro, A.C. Platelet aggregation in arterial hypertension: Is there a nitric oxide-urea connection? Clin. Exp. Pharmacol. Physiol. 2010, 37, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Redondo, P.C.; Jardin, I.; Hernández-Cruz, J.M.; Pariente, J.; Salido, G.M.; Rosado, J.A. Hydrogen peroxide and peroxynitrite enhance Ca2+ mobilization and aggregation in platelets from type 2 diabetic patients. Biochem. Biophys. Res. Commun. 2005, 333, 794–802. [Google Scholar] [CrossRef]
- Kedzierska, M.; Olas, B.; Wachowicz, B.; Stochmal, A.; Oleszek, W.; Jeziorski, A.; Piekarski, J. The nitrative and oxidative stress in blood platelets isolated from breast cancer patients: The protectory action ofaronia melanocarpaextract. Platelets 2010, 21, 541–548. [Google Scholar] [CrossRef]
- Olas, B.; Kedzierska, M.; Wachowicz, B.; Stochmal, A.; Oleszek, W. Effects of polyphenol-rich extract from berries ofAronia melanocarpaon the markers of oxidative stress and blood platelet activation. Platelets 2010, 21, 274–281. [Google Scholar] [CrossRef]
- Sikora, J.; Broncel, M.; Markowicz, M.; Chałubiński, M.; Wojdan, K.; Mikiciuk-Olasik, E. Short-term supplementation with Aronia melanocarpa extract improves platelet aggregation, clotting, and fibrinolysis in patients with metabolic syndrome. Eur. J. Nutr. 2011, 51, 549–556. [Google Scholar] [CrossRef]
- Haimeur, A.; Messaouri, H.; Ulmann, L.; Mimouni, V.; Masrar, A.; Chraïbi, A.; Tremblin, G.; Meskini, N. Argan oil prevents prothrombotic complications by lowering lipid levels and platelet aggregation, enhancing oxidative status in dyslipidemic patients from the area of Rabat (Morocco). Lipids Health Dis. 2013, 12, 107. [Google Scholar] [CrossRef]
- Mekhfi, H.; Gadi, D.; Bnouham, M.; Ziyyat, A.; Legssyer, A.; Aziz, M. Effect of Argan Oil on Platelet Aggregation and Bleeding Time: A Beneficial Nutritional Property. J. Complement. Integr. Med. 2008, 5, 1553–3840. [Google Scholar] [CrossRef]
- Olas, B.; Saluk, J.; Pawlaczyk, I.; Nowak, P.; Kolodziejczyk-Czepas, J.; Gancarz, R.; Wachowicz, B. Antioxidant and antiaggregatory effects of an extract fromConyza canadensison blood plateletsin vitro. Platelets 2006, 17, 354–360. [Google Scholar] [CrossRef]
- Bouaziz, A.; Salido, S.; Palomino, P.L.; Sanchez, A.; Altarejos, J.; Bartegi, A.; Salido, G.M.; Rosado, J.A. Cinnamtannin B-1 from bay wood reduces abnormal intracellular Ca2+ homeostasis and platelet hyperaggregability in type 2 diabetes mellitus patients. Arch. Biochem. Biophys. 2007, 457, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.J.; Jardin, I.; Salido, G.M.; Rosado, J.A. Cinnamtannin B-1 as an antioxidant and platelet aggregation inhibitor. Life Sci. 2008, 82, 977–982. [Google Scholar] [CrossRef] [PubMed]


| Probe | Detected ROS | Maximum Excitation Spectra (nm) | Maximum Emission Spectra (nm) | Limitations and Artefacts | References |
|---|---|---|---|---|---|
| CellROX® Green | H2O2 NO ONOO− O•2- | 485 | 520 | Antioxidants | [158] |
| CellROX® Orange | H2O2 HO• NO ONOO− O•2- | 545 | 565 | Antioxidants | [158,159] |
| CellROX® Deep Red | O•2- HO• | 644 | 655 | Antioxidants | [159] |
| C11-BODIPY581/591 (membrane) | HO• ROO• | 488 | 520 | Hemolysis Antioxidants | [159,160] |
| DAF-FM | NO | 488 | 520 | [161,162] | |
| DCFH-DA/ DCF | HO• ROO• •NO ONOO− Indirectly H2O2 | 495 | 529 | Hemolysis Self-propagation of DCF radicals Esterase inhibitors Plasma esterase in whole blood or PRP EDTA and citrate Antioxidants | [157,163,164] |
| DHE/2OH-Et+ | O•2- | 400 | 580 | Heme enzymes interference Redox-cycling Auto-oxidation | [151,165,166] |
| MitoSOXTM Red | Mitochindrial O•2- | 510 | 580 | Mitochondria overload | [151,167,168] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 2020, 21, 4866. https://doi.org/10.3390/ijms21144866
Masselli E, Pozzi G, Vaccarezza M, Mirandola P, Galli D, Vitale M, Carubbi C, Gobbi G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. International Journal of Molecular Sciences. 2020; 21(14):4866. https://doi.org/10.3390/ijms21144866
Chicago/Turabian StyleMasselli, Elena, Giulia Pozzi, Mauro Vaccarezza, Prisco Mirandola, Daniela Galli, Marco Vitale, Cecilia Carubbi, and Giuliana Gobbi. 2020. "ROS in Platelet Biology: Functional Aspects and Methodological Insights" International Journal of Molecular Sciences 21, no. 14: 4866. https://doi.org/10.3390/ijms21144866
APA StyleMasselli, E., Pozzi, G., Vaccarezza, M., Mirandola, P., Galli, D., Vitale, M., Carubbi, C., & Gobbi, G. (2020). ROS in Platelet Biology: Functional Aspects and Methodological Insights. International Journal of Molecular Sciences, 21(14), 4866. https://doi.org/10.3390/ijms21144866






