Nitrogen Regulating the Expression and Localization of Four Glutamine Synthetase Isoforms in Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Results
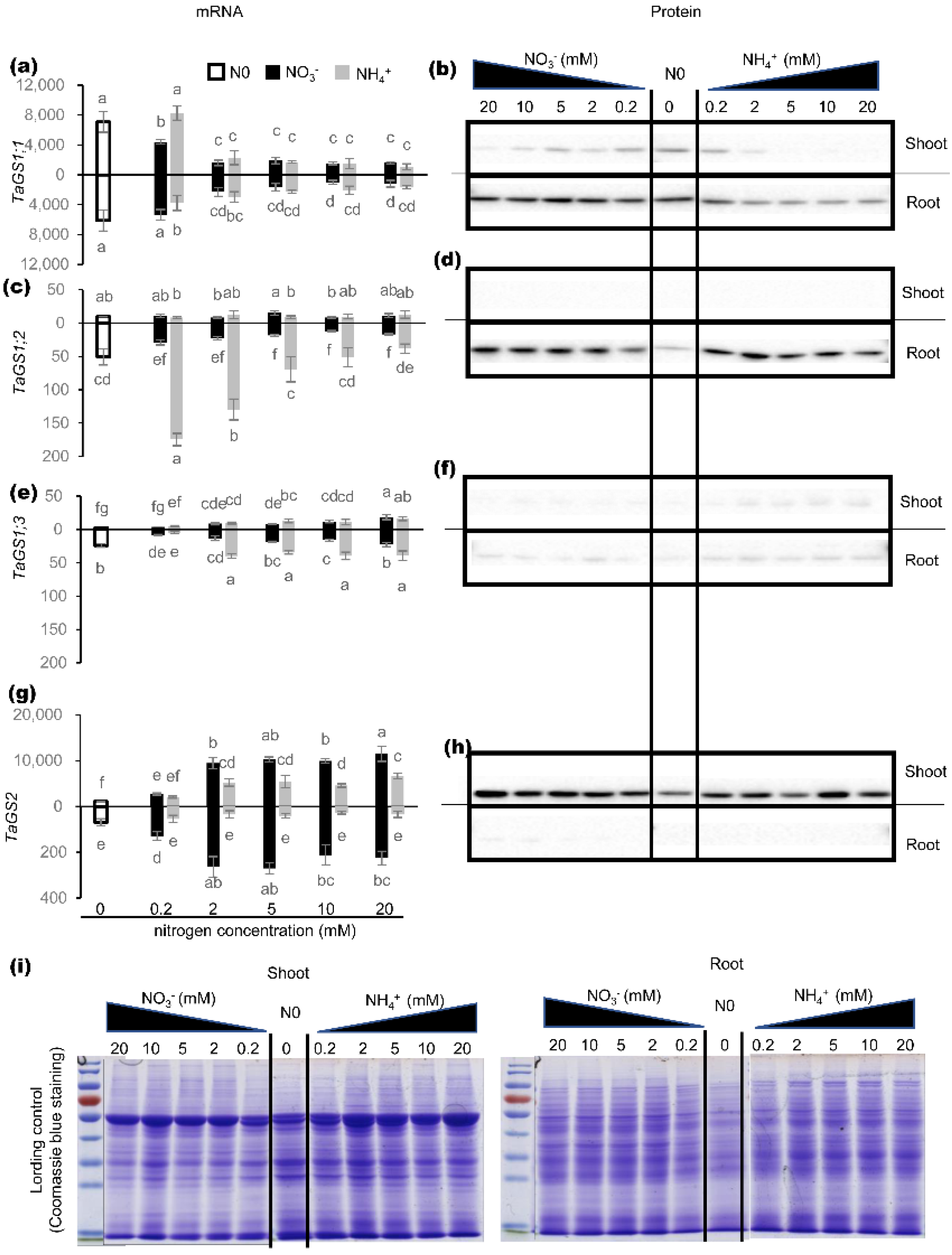
2.1. Effects of Nitrogen on Expression of TaGS Isozymes at mRNA and Protein Level
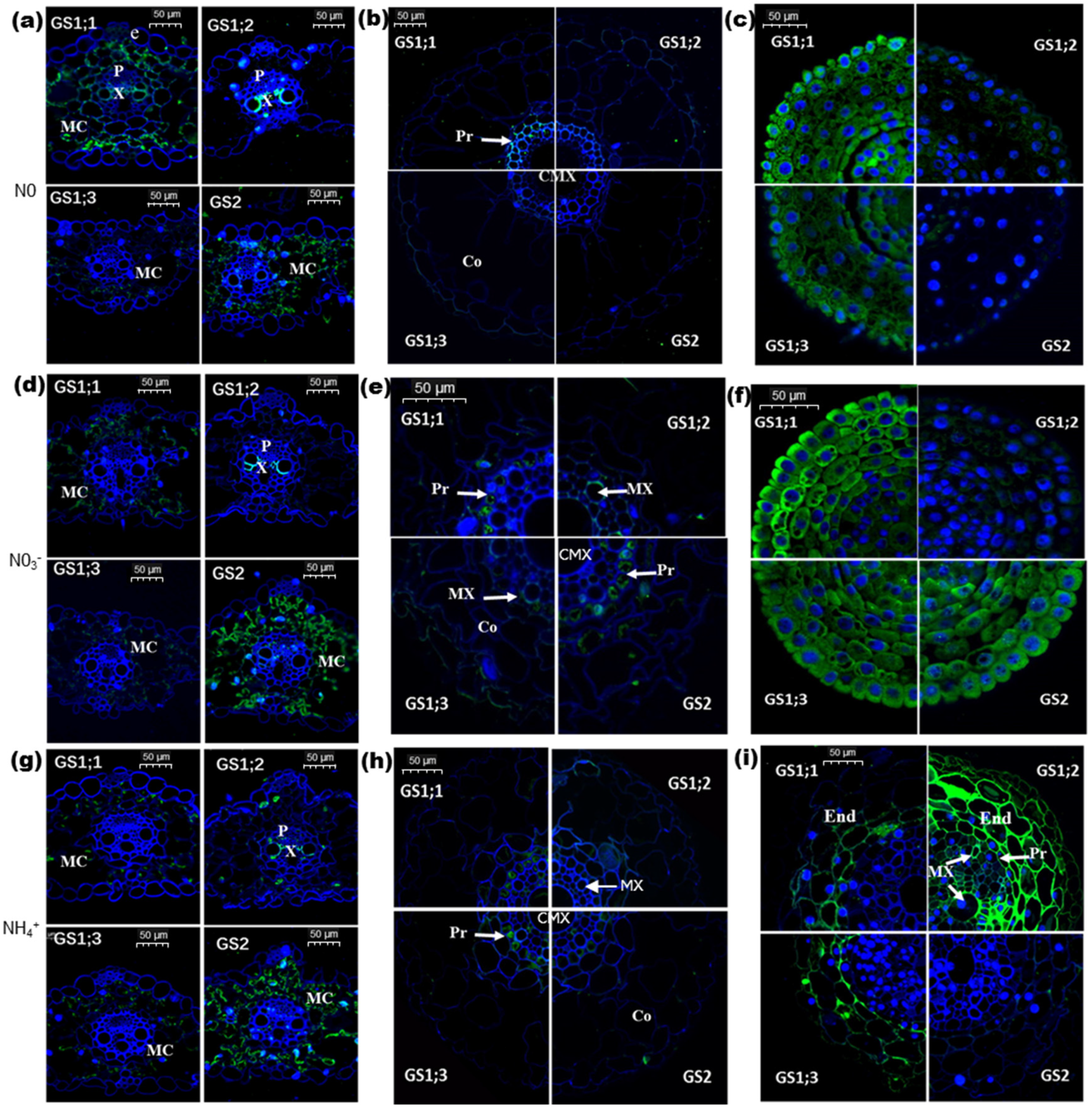
2.2. Effects of Nitrogen on Cellular Localization of Individual TaGS Isozymes at Protein Level
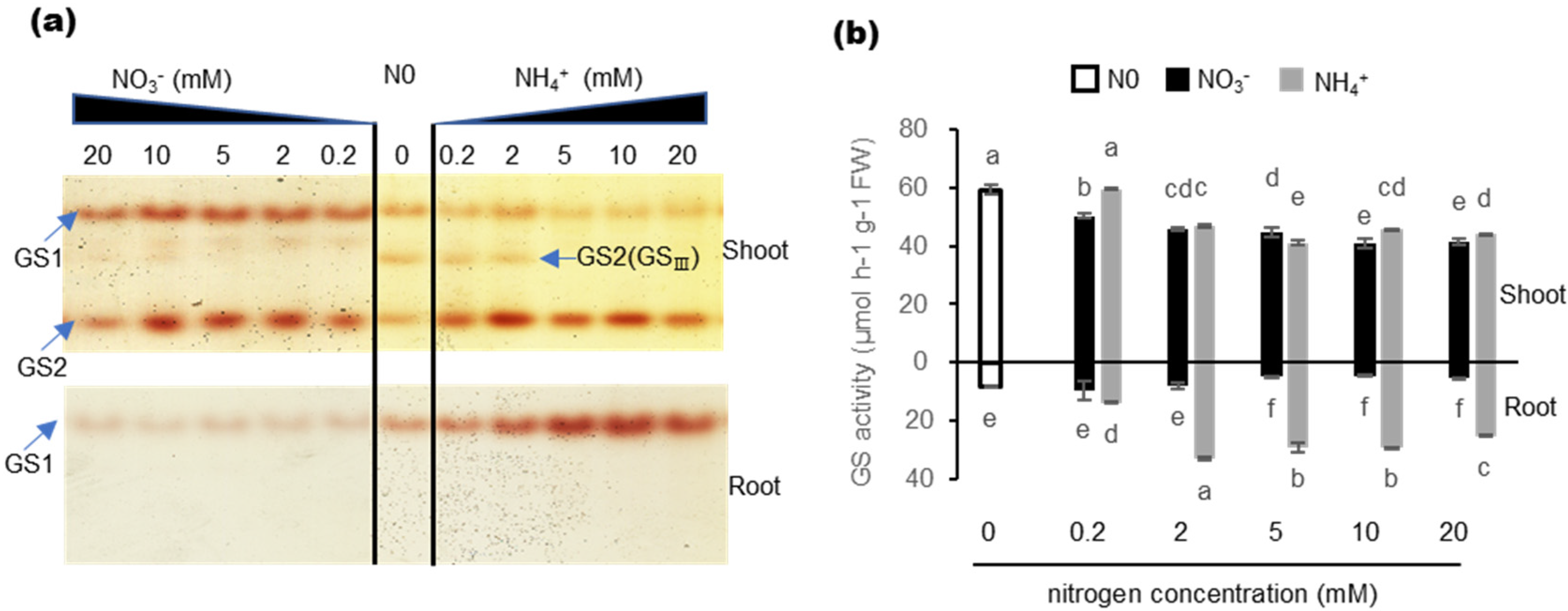
2.3. Effects of Nitrogen Nutrition on GS Isozyme and Total GS Activity
2.4. Kinetic Properties of Recombinant TaGS Isozymes
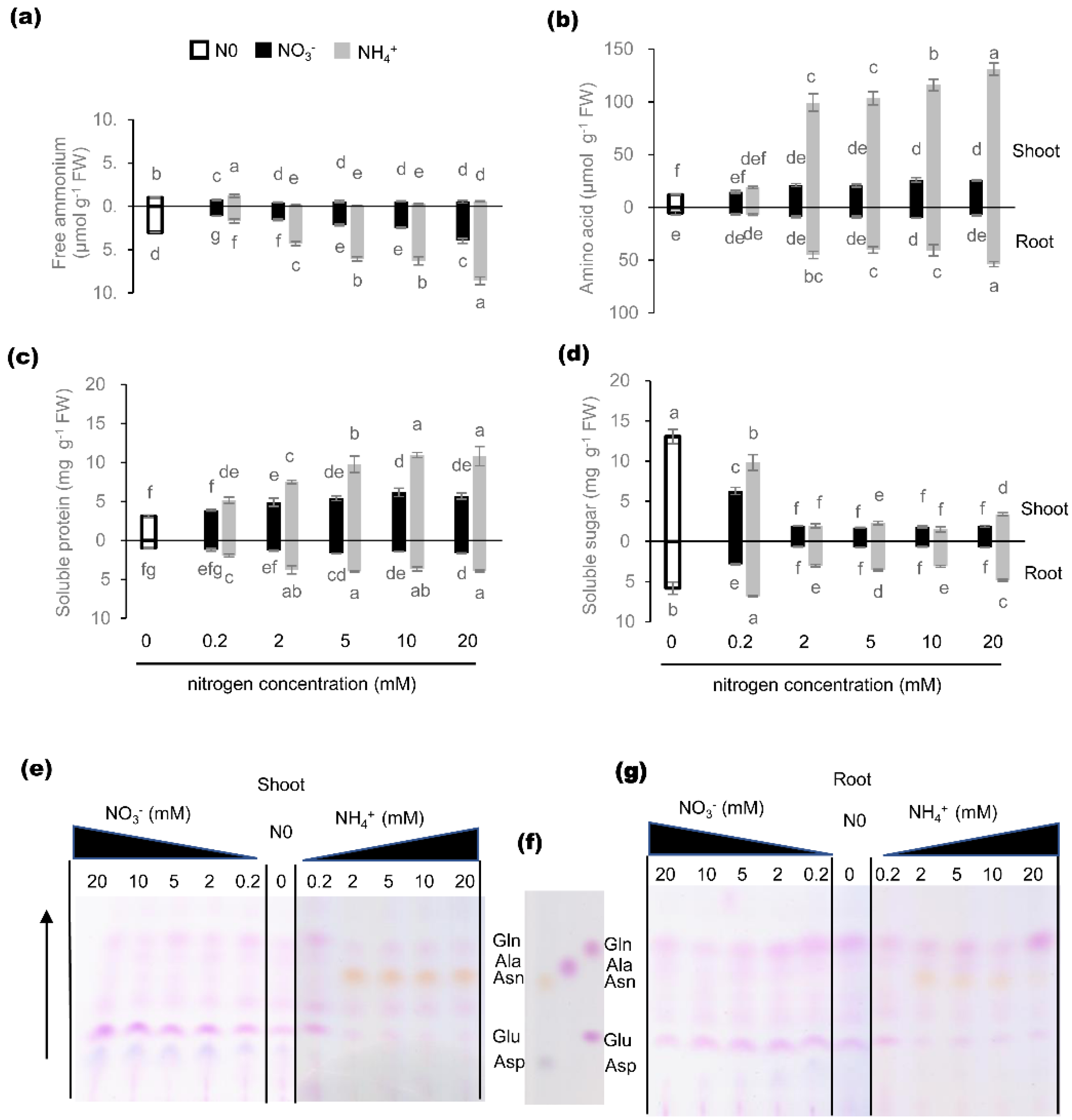
2.5. Effects of Nitrogen Nutrition on C/N Metabolite Status
3. Discussion
3.1. Nitrogen Nutrition Regulates Protein Accumulation of Individual TaGS Isozymes
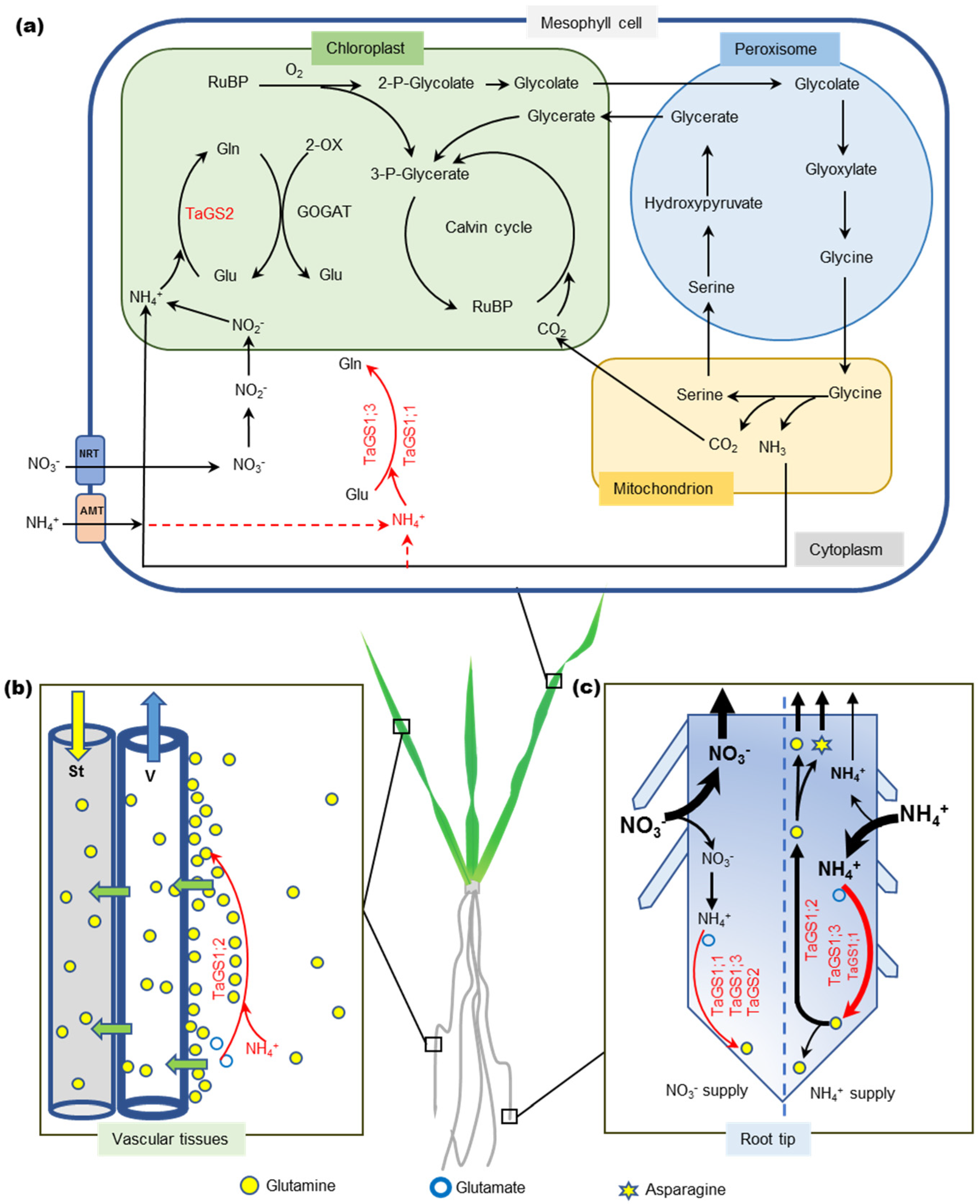
3.2. New Functions of TaGS Isozymes
3.3. TaGS Isozymes Synergistically Perform Nitrogen Assimilation and Translocation under Different N Nutrition Conditions
4. Materials and Methods
4.1. Plant Growth Conditions and Experimental Design
4.2. RNA Isolation and Quantitative Real-Time PCR
4.3. GS Activity Assay and Western Blotting
4.4. Immunolocalization Using Indirect Immunofluorescence Analysis
4.5. Expression of Recombinant Wheat GS Protein in E. coli
4.6. In Vitro Assay of Individual Recombinant Wheat GS Isozyme Activity
4.7. Metabolite Analysis
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GS | Glutamine synthetase |
| N | Nitrogen |
| NH4+ | Ammonium |
| NO3− | Nitrate |
| Gln | Glutamine |
| Asn | Asparagine |
| Glu | Glutamate |
| Asp | Aspartate |
| Ala | Alanine |
| AS | Asparagine synthase |
| NUE | Nitrogen use efficiency |
| CDS | Coding sequence |
| GMH | γ-glutamyl monohydroxamate |
| IPTG | Isopropyl-b-d-thiogalactoside |
| TLC | Thin layer chromatography |
References
- Kichey, T.; Heumez, E.; Pocholle, D.; Pageau, K.; Vanacker, H.; Dubois, F.; Le Gouis, J.; Hirel, B. Combined agronomic and physiological aspects of nitrogen management in wheat highlight a central role for glutamine synthetase. New Phytol. 2006, 169, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B.; Bains, N.S.; Farooq, M. Nitrogen Nutrition, its Assimilation and Remobilization in Diverse Wheat Genotypes. Int. J. Agric. Biol. 2015, 17, 531–538. [Google Scholar] [CrossRef]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in Agriculture: Balancing the Cost of an Essential Resource. Annu. Rev. Environ. Res. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Kant, S.; Bi, Y.; Rothstein, S.J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. J. Exp. Bot. 2010, 62, 1499–1509. [Google Scholar] [CrossRef]
- Thomsen, H.C.; Eriksson, D.; Møller, I.S.; Schjoerring, J.K. Cytosolic glutamine synthetase: A target for improvement of crop nitrogen use efficiency? Trends Plant Sci. 2014, 19, 656–663. [Google Scholar] [CrossRef]
- Fuentes, S.I.; Allen, D.J.; Ortiz-Lopez, A.; Hernández, G. Over-expression of cytosolic glutamine synthetase increases photosynthesis and growth at low nitrogen concentrations. J. Exp. Bot. 2001, 52, 1071–1081. [Google Scholar] [CrossRef]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two Cytosolic Glutamine Synthetase Isoforms of Maize Are Specifically Involved in the Control of Grain Production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef]
- Tobin, A.; Ridley, S.; Stewart, G. Changes in the activities of chloroplast and cytosolic isoenzymes of glutamine synthetase during normal leaf growth and plastid development in wheat. Planta 1985, 163, 544–548. [Google Scholar] [CrossRef]
- Bernard, S.M.; Møller, A.L.B.; Dionisio, G.; Kichey, T.; Jahn, T.P.; Dubois, F.; Baudo, M.; Lopes, M.S.; Tercé-Laforgue, T.; Foyer, C.H.; et al. Gene expression, cellular localisation and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant Mol. Biol. 2008, 67, 89–105. [Google Scholar] [CrossRef]
- Nigro, D.; Fortunato, S.; Giove, S.L.; Paradiso, A.; Gu, Y.Q.; Blanco, A.; de Pinto, M.C.; Gadaleta, A. Glutamine synthetase in Durum Wheat: Genotypic Variation and Relationship with Grain Protein Content. Front. Plant Sci. 2016, 7, 971. [Google Scholar] [CrossRef]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Goodall, A.J.; Kumar, P.; Tobin, A.K. Identification and expression analyses of cytosolic glutamine synthetase genes in barley (Hordeum vulgare L.). Plant Cell Physiol. 2013, 54, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.F.; Wang, Z.; Mao, X.Y.; Zhang, C.W.; Wang, D.S.; Wang, X.; Hou, X.L. Overexpression of BcGS2 gene in non-heading Chinese cabbage (Brassica campestris) enhanced GS activity and total amino acid content in transgenic seedlings. Sci. Hortic. Amst. 2015, 186, 129–136. [Google Scholar] [CrossRef]
- Wallsgrove, R.M.; Turner, J.C.; Hall, N.P.; Kendall, A.C.; Bright, S.W. Barley mutants lacking chloroplast glutamine synthetase—Biochemical and genetic analysis. Plant Physiol. 1987, 83, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, A.; Takeba, G. Photorespiration protects C3 plants from photooxidation. Nature 1996, 384, 557–560. [Google Scholar] [CrossRef]
- Ishiyama, K.; Inoue, E.; Tabuchi, M.; Yamaya, T.; Takahashi, H. Biochemical background and compartmentalized functions of cytosolic glutamine synthetase for active ammonium assimilation in rice roots. Plant Cell Physiol. 2004, 45, 1640–1647. [Google Scholar] [CrossRef]
- Ishiyama, K.; Inoue, E.; Watanabe-Takahashi, A.; Obara, M.; Yamaya, T.; Takahashi, H. Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in Arabidopsis. J. Biol. Chem. 2004, 279, 16598–16605. [Google Scholar] [CrossRef]
- Guan, M.; Møller, I.; Schjoerring, J. Two cytosolic glutamine synthetase isoforms play specific roles for seed germination and seed yield structure in Arabidopsis. J. Exp. Bot. 2015, 66, 203–212. [Google Scholar] [CrossRef]
- Guan, M.; de Bang, T.C.; Pedersen, C.; Schjoerring, J.K. Cytosolic Glutamine Synthetase Gln1;2 Is the Main Isozyme Contributing to GS1 Activity and Can Be Up-Regulated to Relieve Ammonium Toxicity. Plant Physiol. 2016, 171, 1921–1933. [Google Scholar] [CrossRef]
- Lothier, J.; Gaufichon, L.; Sormani, R.; Lemaître, T.; Azzopardi, M.; Morin, H.; Chardon, F.; Reisdorf-Cren, M.; Avice, J.-C.; Masclaux-Daubresse, C. The cytosolic glutamine synthetase GLN1; 2 plays a role in the control of plant growth and ammonium homeostasis in Arabidopsis rosettes when nitrate supply is not limiting. J. Exp. Bot. 2011, 62, 1375–1390. [Google Scholar] [CrossRef]
- Konishi, N.; Ishiyama, K.; Beier, M.P.; Inoue, E.; Kanno, K.; Yamaya, T.; Takahashi, H.; Kojima, S. Contributions of two cytosolic glutamine synthetase isozymes to ammonium assimilation in Arabidopsis roots. J. Exp. Bot. 2017, 68, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Moison, M.; Marmagne, A.; Dinant, S.; Soulay, F.; Azzopardi, M.; Lothier, J.; Citerne, S.; Morin, H.; Legay, N.; Chardon, F.; et al. Three cytosolic glutamine synthetase isoforms localized in different-order veins act together for N remobilization and seed filling in Arabidopsis. J. Exp. Bot. 2018, 69, 4379–4393. [Google Scholar] [CrossRef] [PubMed]
- Hirel, B.; Martin, A.; Tercé-Laforgue, T.; Gonzalez-Moro, M.B.; Estavillo, J.M. Physiology of maize I: A comprehensive and integrated view of nitrogen metabolism in a C4 plant. Physiol. Plant. 2005, 124, 167–177. [Google Scholar] [CrossRef]
- Martin, A.; Belastegui-Macadam, X.; Quillere, I.; Floriot, M.; Valadier, M.H.; Pommel, B.; Andrieu, B.; Donnison, I.; Hirel, B. Nitrogen management and senescence in two maize hybrids differing in the persistence of leaf greenness: Agronomic, physiological and molecular aspects. New Phytol. 2005, 167, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, M.; Sugiyama, K.; Ishiyama, K.; Inoue, E.; Sato, T.; Takahashi, H.; Yamaya, T. Severe reduction in growth rate and grain filling of rice mutants lacking OsGS1;1, a cytosolic glutamine synthetase1;1. Plant J. 2005, 42, 641–651. [Google Scholar] [CrossRef]
- Kusano, M.; Tabuchi, M.; Fukushima, A.; Funayama, K.; Diaz, C.; Kobayashi, M.; Hayashi, N.; Tsuchiya, Y.N.; Takahashi, H.; Kamata, A.; et al. Metabolomics data reveal a crucial role of cytosolic glutamine synthetase 1;1 in coordinating metabolic balance in rice. Plant J. 2011, 66, 456–466. [Google Scholar] [CrossRef]
- Funayama, K.; Kojima, S.; Tabuchi-Kobayashi, M.; Sawa, Y.; Nakayama, Y.; Hayakawa, T.; Yamaya, T. Cytosolic Glutamine Synthetase1;2 is Responsible for the Primary Assimilation of Ammonium in Rice Roots. Plant Cell Physiol. 2013, 54, 934–943. [Google Scholar] [CrossRef]
- Yamaya, T.; Kusano, M. Evidence supporting distinct functions of three cytosolic glutamine synthetases and two NADH-glutamate synthases in rice. J. Exp. Bot. 2014, 65, 5519–5525. [Google Scholar] [CrossRef]
- Caputo, C.; Criado, M.V.; Roberts, I.N.; Gelso, M.A.; Barneix, A.J. Regulation of glutamine synthetase 1 and amino acids transport in the phloem of young wheat plants. Plant Physiol. Biochem. 2009, 47, 335–342. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, S.; Wei, Y.; Meng, X.; Wang, X.; Ma, X. The role of glutamine synthetase isozymes in enhancing nitrogen use efficiency of N-efficient winter wheat. Sci. Rep. 2017, 7, 1000. [Google Scholar] [CrossRef]
- Harper, C.J.; Hayward, D.; Kidd, M.; Wiid, I.; van Helden, P. Glutamate dehydrogenase and glutamine synthetase are regulated in response to nitrogen availability in Myocbacterium smegmatis. BMC Microbiol. 2010, 10, 1–12. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, A.; Jia, X.; Zhang, Z.; Ma, X.; Gu, M.; Meng, X.; Wang, X. Nitrogen Supply and Leaf Age Affect the Expression of TaGS1 or TaGS2 Driven by a Constitutive Promoter in Transgenic Tobacco. Genes 2018, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.L.; Temple, S.J.; Sengupta-Gopalan, C. Constitutive Overexpression of Cytosolic Glutamine Synthetase (GS1) Gene in Transgenic Alfalfa Demonstrates That GS1 May Be Regulated at the Level of RNA Stability and Protein Turnover. Plant Physiol. 2001, 126, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Y.; Shi, L.; Ma, X.; Theg, S.M. New isoforms and assembly of glutamine synthetase in the leaf of wheat (Triticum aestivum L.). J. Exp. Bot. 2015, 66, 6827–6834. [Google Scholar] [CrossRef] [PubMed]
- Migge, A.; Carrayol, E.; Hirel, B.; Becker, T.W. Leaf-specific overexpression of plastidic glutamine synthetase stimulates the growth of transgenic tobacco seedlings. Planta 2000, 210, 252–260. [Google Scholar] [CrossRef]
- Oliveira, I.C.; Brears, T.; Knight, T.J.; Clark, A.; Coruzzi, G.M. Overexpression of cytosolic glutamine synthetase. Relation to nitrogen, light, and photorespiration. Plant Physiol. 2002, 129, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Setién, I.; Fuertes-Mendizabal, T.; González, A.; Aparicio-Tejo, P.M.; González-Murua, C.; González-Moro, M.B.; Estavillo, J.M. High irradiance improves ammonium tolerance in wheat plants by increasing N assimilation. J. Plant Physiol. 2013, 170, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; He, Z.; Han, B. Composition and Transport of Amino Acids in Wheat Plant during Grain Development. J. Triticeae Crop. 2000, 20, 17–22. [Google Scholar]
- Peeters, K.M.U.; Van Laere, A.J. Amino acid metabolism associated with N-mobilization from the flag leaf of wheat (Triticum aestivum L.) during grain development. Plant Cell Environ. 1994, 17, 131–141. [Google Scholar] [CrossRef]
- Sivasankar, S.; Oaks, A. Nitrate assimilation in higher plants: The effect of metabolites and light. Plant Physiol. Biochem. 1996, 34, 609–620. [Google Scholar]
- Han, J.; Wang, R.; Jia, Z. The Transport of Assimilates in Plants; Science Press: Beijing, China, 1986. [Google Scholar]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, J.; Liu, Y.; Tian, Z.; Muhammad, A.; Zhang, Y.; Jiang, D.; Cao, W.; Dai, T. Higher Ammonium Transamination Capacity Can Alleviate Glutamate Inhibition on Winter Wheat (Triticum aestivum L.) Root Growth under High Ammonium Stress. PLoS ONE 2016, 11, e0160997. [Google Scholar] [CrossRef] [PubMed]
- Belastegui-Macadam, X.M.; Estavillo, J.M.; García-Mina, J.M.; González, A.; Bastias, E.; Gónzalez-Murua, C. Clover and ryegrass are tolerant species to ammonium nutrition. J. Plant Physiol. 2007, 164, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Bio, A.F.M.; Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Martins-Loução, M.A. How does glutamine synthetase activity determine plant tolerance to ammonium? Planta 2006, 223, 1068–1080. [Google Scholar] [CrossRef]
- Hollstein, M.; Montesano, R.; Yamasaki, H. Ammonium tolerance and the regulation of two cytosolic glutamine synthetases in the roots of Sorghum. Funct. Plant Biol. 2010, 37, 55–63. [Google Scholar]
- Aarnes, H.; Eriksen, A.B.; Petersen, D.; Rise, F. Accumulation of ammonium in Norway spruce (Picea abies) seedlings measured by in vivo14N-NMR. J. Exp. Bot. 2007, 58, 929–934. [Google Scholar] [CrossRef][Green Version]
- Ikeda, M.; Kusano, T.; Koga, N. Carbon skeletons for amide synthesis during ammonium nutrition in tomato and wheat roots. Soil Sci. Plant Nutr. 2004, 50, 141–147. [Google Scholar] [CrossRef]
- Sakurai, N.; Hayakawa, T.; Nakamura, T.; Yamaya, T. Changes in the cellular localization of cytosolic glutamine synthetase protein in vascular bundles of rice leaves at various stages of development. Planta 1996, 200, 306–311. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Zhao, P.; Xiong, S.; Guo, F. Effect of water control on activities of nitrogen assimilation enzymes and grain quality in winter wheat. Acta Phytoecol. Sin. 2005, 29, 48–53. [Google Scholar]
- Németh, E.; Nagy, Z.; Pécsváradi, A. Chloroplast Glutamine Synthetase, the Key Regulator of Nitrogen Metabolism in Wheat, Performs Its Role by Fine Regulation of Enzyme Activity via Negative Cooperativity of Its Subunits. Front. Plant Sci. 2018, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z. Guidelines for Modern Plant Physiology Experiments, 1st ed.; Science Press: Beijing, China, 1999; p. 1. [Google Scholar]

| Name | Km * (mM) | Vmax ** (nKat/1 Unit Protein) | ||
|---|---|---|---|---|
| Glu | Hydroxylamine | Glu | Hydroxylamine | |
| TaGS1;1 | 0.65 ± 0.01 d | 0.26 ± 0.02 c | 0.13 ± 0.001 d | 0.1 ± 0.002 d |
| TaGS1;2 | 0.87 ± 0.01 c | 0.66 ± 0.003 a | 0.5 ± 0.002 c | 0.36 ± 0.003 c |
| TaGS1;3 | 4.13 ± 0.35 a | 0.64 ± 0.04 a | 1.95 ± 0.07 a | 1.63 ± 0.08 a |
| TaGS2 | 2.43 ± 0.27 b | 0.36 ± 0.04 b | 0.92 ± 0.04 b | 1.00 ± 0.04 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Wang, X.; Zhang, Z.; Xiong, S.; Meng, X.; Zhang, J.; Wang, L.; Zhang, X.; Yu, M.; Ma, X. Nitrogen Regulating the Expression and Localization of Four Glutamine Synthetase Isoforms in Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2020, 21, 6299. https://doi.org/10.3390/ijms21176299
Wei Y, Wang X, Zhang Z, Xiong S, Meng X, Zhang J, Wang L, Zhang X, Yu M, Ma X. Nitrogen Regulating the Expression and Localization of Four Glutamine Synthetase Isoforms in Wheat (Triticum aestivum L.). International Journal of Molecular Sciences. 2020; 21(17):6299. https://doi.org/10.3390/ijms21176299
Chicago/Turabian StyleWei, Yihao, Xiaochun Wang, Zhiyong Zhang, Shuping Xiong, Xiaodan Meng, Jie Zhang, Lulu Wang, Xiaojiao Zhang, Meiqin Yu, and Xinming Ma. 2020. "Nitrogen Regulating the Expression and Localization of Four Glutamine Synthetase Isoforms in Wheat (Triticum aestivum L.)" International Journal of Molecular Sciences 21, no. 17: 6299. https://doi.org/10.3390/ijms21176299
APA StyleWei, Y., Wang, X., Zhang, Z., Xiong, S., Meng, X., Zhang, J., Wang, L., Zhang, X., Yu, M., & Ma, X. (2020). Nitrogen Regulating the Expression and Localization of Four Glutamine Synthetase Isoforms in Wheat (Triticum aestivum L.). International Journal of Molecular Sciences, 21(17), 6299. https://doi.org/10.3390/ijms21176299




