RETRACTED: Contact/Release Coordinated Antibacterial Cotton Fabrics Coated with N-Halamine and Cationic Antibacterial Agent for Durable Bacteria-Killing Application
Abstract
1. Introduction
2. Results
2.1. Synthesis of Cotton Fabrics Treated with CDDA and Its Physical Properties
2.2. Preparation of N-Halamine-Containing Cotton Fabrics
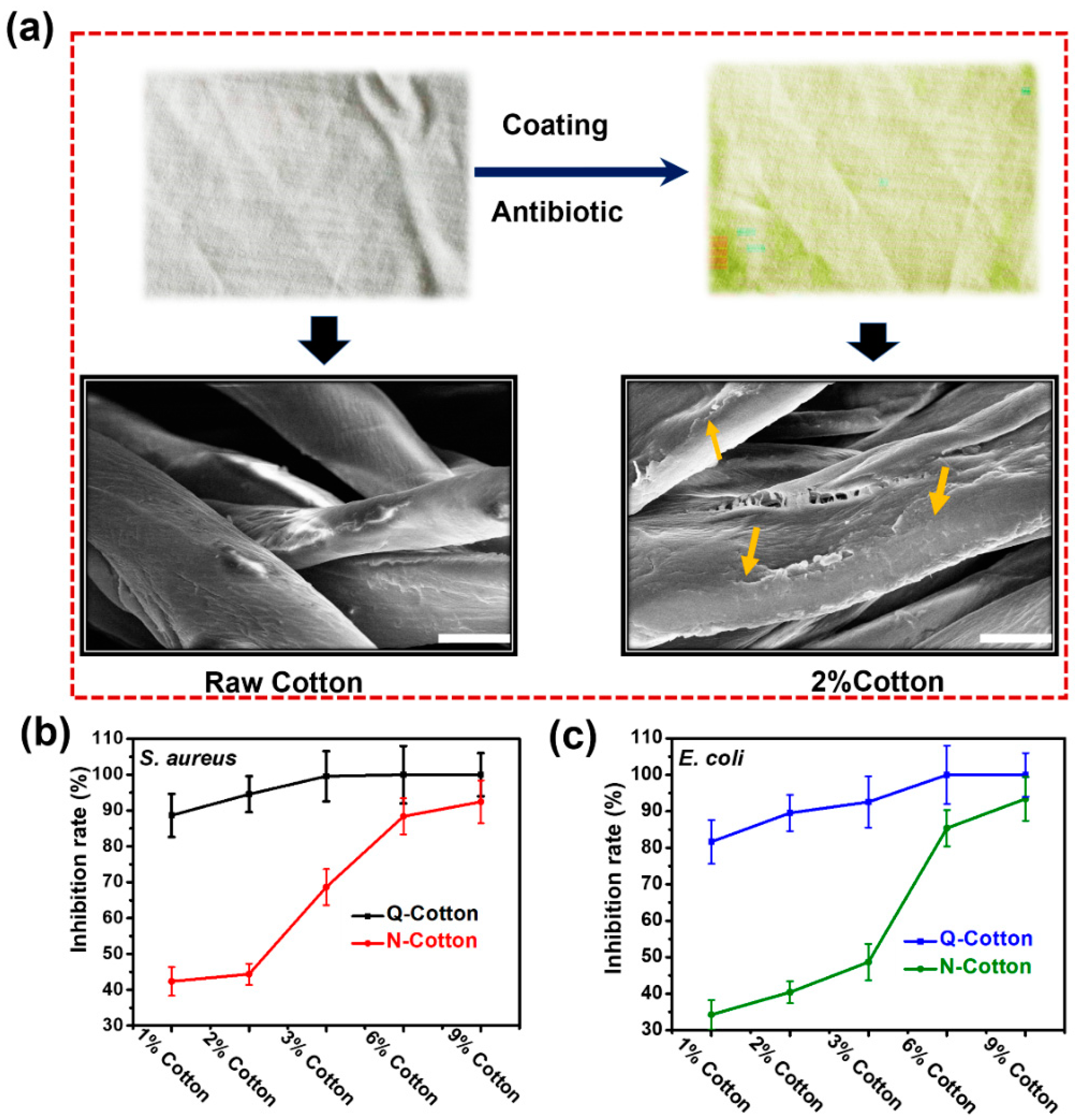
2.3. Contact/Release Coordinated Antibacterial Properties of Cotton Fabrics Containing N-Halamine
2.4. Antibacterial Performance of Modified Cotton Fabrics after Washing with Anionic Detergent in Water
3. Discussion
4. Materials and Method
4.1. Material
4.2. Synthesis of Modified L-Arginine with Carbon–Carbon Double Bond (M-Arg)
4.3. Preparation of Antibacterial Cotton Fabric with Dimethyl Dodecyl [3-(T Rimethoxy-Silyl) Propyl] Ammonium Chloride
4.4. Preparation of Antibacterial Cotton Fabrics Based on M-Arg Halogenation Reaction
4.5. Antibacterial Activity Assessment
4.5.1. Contact Killing Assay
4.5.2. Determining the Inhibition Rate of Antibacterial Fabrics
4.6. Bacteria Cells Morphology Observation with SEM
4.7. Confocal Laser Scanning Microscopy (CLSM) for Detection of Live/Dead Bacteria
4.8. The Test of Cl+ Ion Content on N-Halamine-Containing Cotton Fabrics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, M.; Wang, C.; Wang, H.; Jian, M.; Hao, X.; Zhang, Y. Carbonized Cotton Fabric for High-Performance Wearable Strain Sensors. Adv. Funct. Mater. 2017, 27, 1604795. [Google Scholar] [CrossRef]
- Sandin, G.; Peters, G.M. Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Gao, Y.; Cranston, R. Recent advances in antimicrobial treatments of textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar]
- Yang, L.; Zhan, C.D.; Huang, X.Y.; Hong, L.Z.; Fang, L.M.; Wang, W.; Su, J.Y. Durable Antibacterial Cotton Fabrics Based on Natural Borneol-Derived Anti-MRSA Agents. Adv. Healthc. Mater. 2020, 9, e2000186. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Cheng, X.L.; Ren, X.H.; Huang, T.S. Self-assembled antibacterial coating by N-halamine polyelectrolytes on a cellulose substrate. J. Mater. Chem. B 2015, 3, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Han, C.H.; Min, B.G. Superhydrophobic and Antibacterial Properties of Cotton Fabrics Coated with Copper Nanoparticles through Sonochemical Process. Fiber Polym. 2020, 21, 785–791. [Google Scholar] [CrossRef]
- Mikhailovskaya, A.P. Factors Determining the Effectiveness of Dyeing Fiber Materials Using Quaternary Ammonium Salts. Fibre Chem. 2018, 50, 188–192. [Google Scholar] [CrossRef]
- Niu, J.; Ma, L.; Zhang, Q.; Bu, D. Effect of peptides and corn processing on in vitro rumen fermentation and microbial protein synthesis. J. Anim. Sci. 2018, 96, 418. [Google Scholar] [CrossRef]
- Musaev, Y.I.; Khashirova, S.Y.; Musaeva, E.B.; Kirzhinova, I.K. New Nanocomposites: Cotton-Cellulose-Ketimines Based on Guanidine and Aminoguanidine. Fibre Chem. 2018, 50, 60–63. [Google Scholar] [CrossRef]
- Ding, X.; Duan, S.; Ding, X.; Liu, R.; Xu, F.-J. Versatile Antibacterial Materials: An Emerging Arsenal for Combatting Bacterial Pathogens. Adv. Funct. Mater. 2018, 28, 1802140. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, J.; Lyu, B.; Lyu, L.; Ma, J.; Yang, L. Poly(quaternary ammonium salt-epoxy) grafted onto Ce doped ZnO composite: An enhanced and durable antibacterial agent. Carbohyd. Polym. 2018, 200, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hanczvikkel, A.; Vig, A.; Toth, A. Survival capability of healthcare-associated, multidrug-resistant bacteria on untreated and on antimicrobial textiles. J. Ind. Text. 2019, 48, 1113–1135. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, S.; Galluzzi, M.; Li, F.; Zhang, X.; Yang, X.; Liu, X.; Cai, X.; Zhu, X.; Du, B.; et al. Insight into multifunctional polyester fabrics finished by one-step eco-friendly strategy. Chem. Eng. J. 2019, 358, 634–642. [Google Scholar] [CrossRef]
- Simoncic, B.; Tomsic, B. Structures of Novel Antimicrobial Agents for Textiles—A Review. Text. Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Song, J.; Jang, J. Antimicrobial polymer nanostructures: Synthetic route, mechanism of action and perspective. Adv. Colloid Interface Sci. 2014, 203, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Bulut, O.; Kamali, A.R.; Ege, D. L-Arginine modified multi-walled carbon nanotube/sulfonated poly(ether Ego ketone) nanocomposite films for biomedical applications. Appl. Surf. Sci. 2018, 444, 168–176. [Google Scholar] [CrossRef]
- Kang, J.K.; Lee, S.C.; Kim, S.B. Synthesis of quaternary ammonium functionalized silica gel through grafting of dimethyl dodecyl 3-(trimethoxysilyl) propyl ammonium chloride for nitrate removal in batch and column studies. J. Taiwan Inst. Chem. Eng. 2019, 102, 153–162. [Google Scholar] [CrossRef]
- Eknoian, M.W.; Putman, J.H.; Worley, S.D. Monomeric and polymeric N-halamine disinfectants. Ind. Eng. Chem. Res. 1998, 37, 2873–2877. [Google Scholar] [CrossRef]
- Li, C.; Xue, L.; Cai, Q.; Bao, S.; Zhao, T.; Xiao, L.; Gao, G.; Harnoode, C.; Dong, A. Design, synthesis and biocidal effect of novel amine N-halamine microspheres based on 2,2,6,6-tetramethyl-4-piperidinol as promising antibacterial agents. RSC Adv. 2014, 4, 47853–47864. [Google Scholar] [CrossRef]
- Dong, A.; Xue, M.; Lan, S.; Wang, Q.; Zhao, Y.; Wang, Y.; Zhang, Y.; Gao, G.; Liu, F.; Harnoode, C. Bactericidal evaluation of N-halamine-functionalized silica nanoparticles based on barbituric acid. Colloid Surf. B 2014, 113, 450–457. [Google Scholar] [CrossRef]
- Ganesan, R.M.; Prabu, H.G. Synthesis of gold nanoparticles using herbal Acorus calamus rhizome extract and coating on cotton fabric for antibacterial and UV blocking applications. Arab. J. Chem. 2019, 12, 2166–2174. [Google Scholar] [CrossRef]
- Wu, M.; Ma, B.; Pan, T.; Chen, S.; Sun, J. Silver-Nanoparticle-Colored Cotton Fabrics with Tunable Colors and Durable Antibacterial and Self-Healing Superhydrophobic Properties. Adv. Funct. Mater. 2016, 26, 569–576. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Chen, C.; Hu, J.; Zhou, C.; Cai, X.; Wang, W.; Zheng, C.; Zhang, P.; Cheng, J.; et al. Durably Antibacterial and Bacterially Antiadhesive Cotton Fabrics Coated by Cationic Fluorinated Polymers. ACS Appl. Mater. Inter. 2018, 10, 6124–6136. [Google Scholar] [CrossRef]
- Cruz-Romero, M.C.; Murphy, T.; Morris, M.; Cummins, E.; Kerry, J.P. Antimicrobial activity of chitosan, organic acids and nano-sized solubilisates for potential use in smart antimicrobially-active packaging for potential food applications. Food Control 2013, 34, 393–397. [Google Scholar] [CrossRef]
- Zamani, E.; Chatterjee, S.; Changa, T.; Immethun, C.; Sarella, A.; Saha, R.; Dishari, S.K. Mechanistic Understanding of the Interactions of Cationic Conjugated Oligo- and Polyelectrolytes with Wild-type and Ampicillin-resistant Escherichia coli. Sci. Rep. 2019, 9, 20411. [Google Scholar] [CrossRef]
- He, Y.; Wan, X.; Xiao, K.; Lin, W.; Li, J.; Li, Z.; Luo, F.; Tan, H.; Li, J.; Fu, Q. Anti-biofilm surfaces from mixed dopamine-modified polymer brushes: Synergistic role of cationic and zwitterionic chains to resist staphyloccocus aureus. Biomater. Sci. 2019, 7, 5369–5382. [Google Scholar] [CrossRef] [PubMed]
- Breloy, L.; Ouarabi, C.A.; Brosseau, A.; Dubot, P.; Brezova, V.; Andaloussi, S.A.; Malval, J.-P.; Versace, D.-L. Beta-Carotene/Limonene Derivatives/Eugenol: Green Synthesis of Antibacterial Coatings under Visible-Light Exposure. ACS Sustain. Chem. Eng. 2019, 7, 19591–19604. [Google Scholar] [CrossRef]
- Mao, T.; Wei, Y.; Zheng, C.; Cheng, W.; Zhang, Z.; Zhu, Y.; Wang, R.; Zeng, Z. Antibacterial Cotton Fabrics Coated by Biodegradable Cationic Silicone Softeners. J. Surfactants Deterg. 2019, 22, 1429–1443. [Google Scholar] [CrossRef]
- Liu, J.; Dong, C.H.; Wei, D.D.; Zhang, Z.; Xie, W.H.; Li, Q.; Lu, Z. Multifunctional Antibacterial and Hydrophobic Cotton Fabrics Treated with Cyclic Polysiloxane Quaternary Ammonium Salt. Fiber Polym. 2019, 20, 1368–1374. [Google Scholar] [CrossRef]
- Gao, D.G.; Li, Y.J.; Lyu, B.; Jin, D.N.; Ma, J.Z. Silicone quaternary ammonium saltbased nanocomposite: A long-acting antibacterial cotton fabric finishing agent with good softness and air permeability. Cellulose. 2020, 27, 1055T1069. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Z.; Lin, W.; Moon, K.S.; Wong, C.P. Reversible Superhydrophobic-Superhydrophilic Transition of ZnO Nanorod/Epoxy Composite Films. ACS Appl. Mater. Interfaces 2012, 4, 3959–3964. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gao, Q.; Xu, L.; Zhang, M.; Xing, Z.; Guo, X.; Zhang, K.; Wu, G. Significant Improvement in Thermal and UV Resistances of UHMWPE Fabric through in Situ Formation of Polysiloxane-TiO2 Hybrid Layers. ACS Appl. Mater. Interfaces 2016, 8, 23311–23320. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Zhang, Q.; Li, L.; Li, P.; Wang, Y.-J.; Simalou, O.; Zhang, Y.; Gao, G.; Dong, A. N-Halamine-Containing Electrospun Fibers Kill Bacteria via a Contact/Release Co-Determined Antibacterial Pathway. ACS Appl. Mater. Interfaces 2016, 8, 31530–31540. [Google Scholar] [CrossRef]
- Qian, L.; Xiao, H.; Zhao, G.; He, B. Synthesis of Modified Guanidine-Based Polymers and their Antimicrobial Activities Revealed by AFM and CLSM. ACS Appl. Mater. Interfaces 2011, 3, 1895–1901. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-H. Antibacterial Actions of Ginkgolic Acids and Related Mechanisms. In New Biocides Development: Combined Approach of Chemistry and Microbiology; Zhu, P.C., Ed.; ACS Publications: Washington, DC, USA, 2007; Volume 967, pp. 389–410. [Google Scholar]
- Zhao, Y.; Wei, B.; Wu, M.; Zhang, H.; Yao, J.; Chen, X.; Shao, Z. Preparation and characterization of antibacterial poly(lactic acid) nanocomposites with N-halamine modified silica. Int. J. Biol. Macromol. 2020, 155, 1468–1477. [Google Scholar] [CrossRef]
- Ma, W.; Li, L.; Lin, X.; Wang, Y.; Ren, X.; Huang, T.-S. Novel ZnO/N-halamine-Mediated Multifunctional Dressings as Quick Antibacterial Agent for Biomedical Applications. ACS Appl. Mater. Interfaces 2019, 11, 31411–31420. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, S.; Wang, Y.; Liu, Y.; Li, R.; Ren, X.; Huang, T.-S. Antibacterial polyvinyl alcohol films incorporated with N-halamine grafted oxidized microcrystalline cellulose. Compos. Commun. 2019, 15, 25–29. [Google Scholar] [CrossRef]
- Kim, Y.; Binauld, S.; Stenzel, M.H. Zwitterionic Guanidine-Based Oligomers Mimicking Cell-Penetrating Peptides as a Nontoxic Alternative to Cationic Polymers to Enhance the Cellular Uptake of Micelles. Biomacromolecules 2012, 13, 3418–3426. [Google Scholar] [CrossRef]






| Name Code | M-Arg (g) | DDA (g) | Cotton Fabrics (g) | NaClO (mL) | H2O (mL) |
|---|---|---|---|---|---|
| Cotton-1 | 0.1 | 10 | 20 | 250 | |
| Cotton-2 | 0.1 | 0.1 | 10 | 20 | 250 |
| Cotton-3 | 0.2 | 0.1 | 10 | 20 | 250 |
| Cotton-4 | 0.3 | 0.1 | 10 | 20 | 250 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, H.; Liu, C.; Zhu, J.; Li, F.-X.; Wang, X.-L.; Yu, J.-Y.; Qin, X.-H.; Wu, D.-Q. RETRACTED: Contact/Release Coordinated Antibacterial Cotton Fabrics Coated with N-Halamine and Cationic Antibacterial Agent for Durable Bacteria-Killing Application. Int. J. Mol. Sci. 2020, 21, 6531. https://doi.org/10.3390/ijms21186531
Han H, Liu C, Zhu J, Li F-X, Wang X-L, Yu J-Y, Qin X-H, Wu D-Q. RETRACTED: Contact/Release Coordinated Antibacterial Cotton Fabrics Coated with N-Halamine and Cationic Antibacterial Agent for Durable Bacteria-Killing Application. International Journal of Molecular Sciences. 2020; 21(18):6531. https://doi.org/10.3390/ijms21186531
Chicago/Turabian StyleHan, Hua, Chang Liu, Jie Zhu, Fa-Xue Li, Xue-Li Wang, Jian-Yong Yu, Xiao-Hong Qin, and De-Qun Wu. 2020. "RETRACTED: Contact/Release Coordinated Antibacterial Cotton Fabrics Coated with N-Halamine and Cationic Antibacterial Agent for Durable Bacteria-Killing Application" International Journal of Molecular Sciences 21, no. 18: 6531. https://doi.org/10.3390/ijms21186531
APA StyleHan, H., Liu, C., Zhu, J., Li, F.-X., Wang, X.-L., Yu, J.-Y., Qin, X.-H., & Wu, D.-Q. (2020). RETRACTED: Contact/Release Coordinated Antibacterial Cotton Fabrics Coated with N-Halamine and Cationic Antibacterial Agent for Durable Bacteria-Killing Application. International Journal of Molecular Sciences, 21(18), 6531. https://doi.org/10.3390/ijms21186531





